Abstract
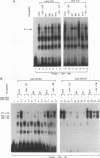
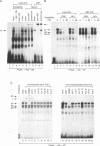
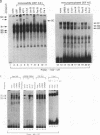
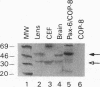
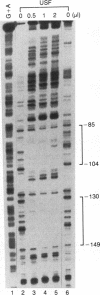
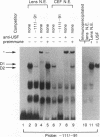
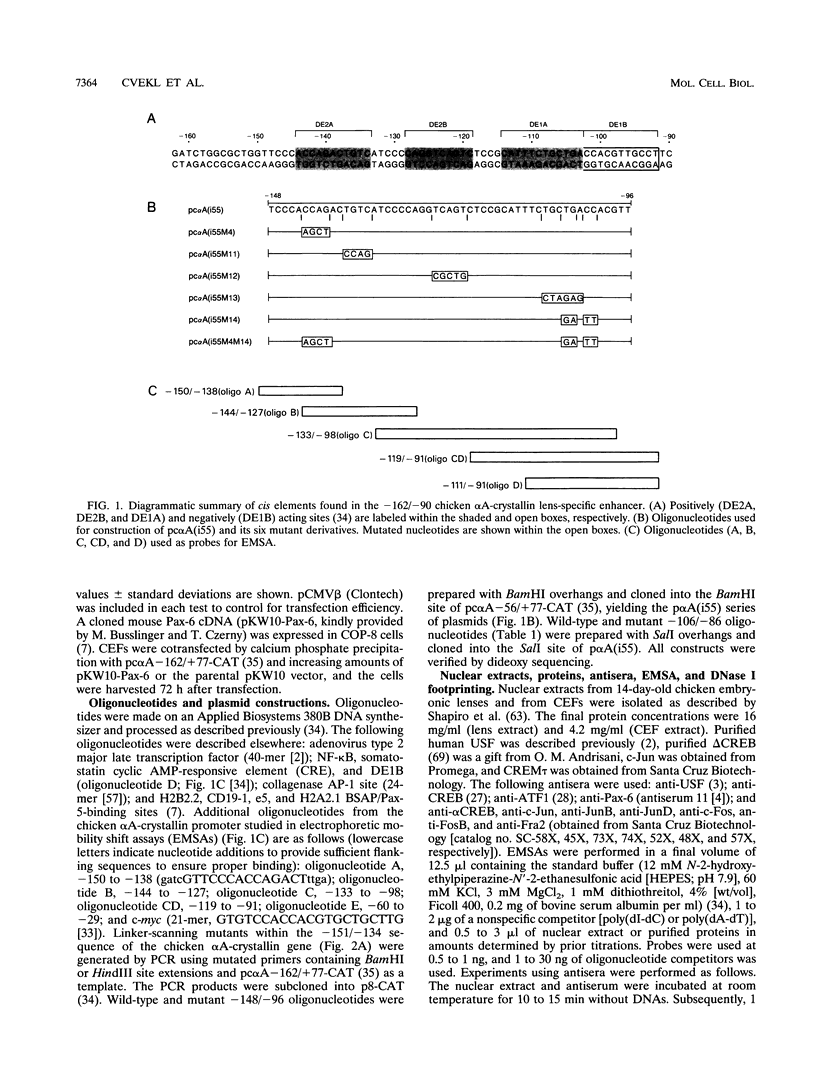
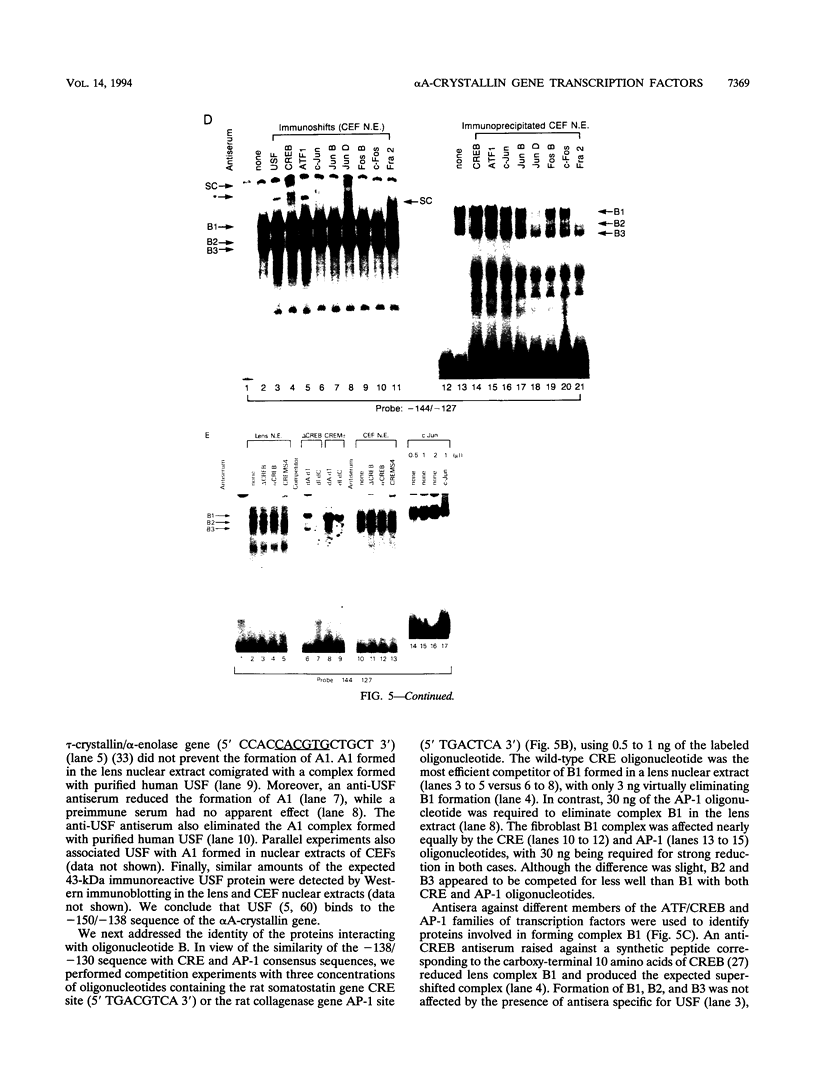
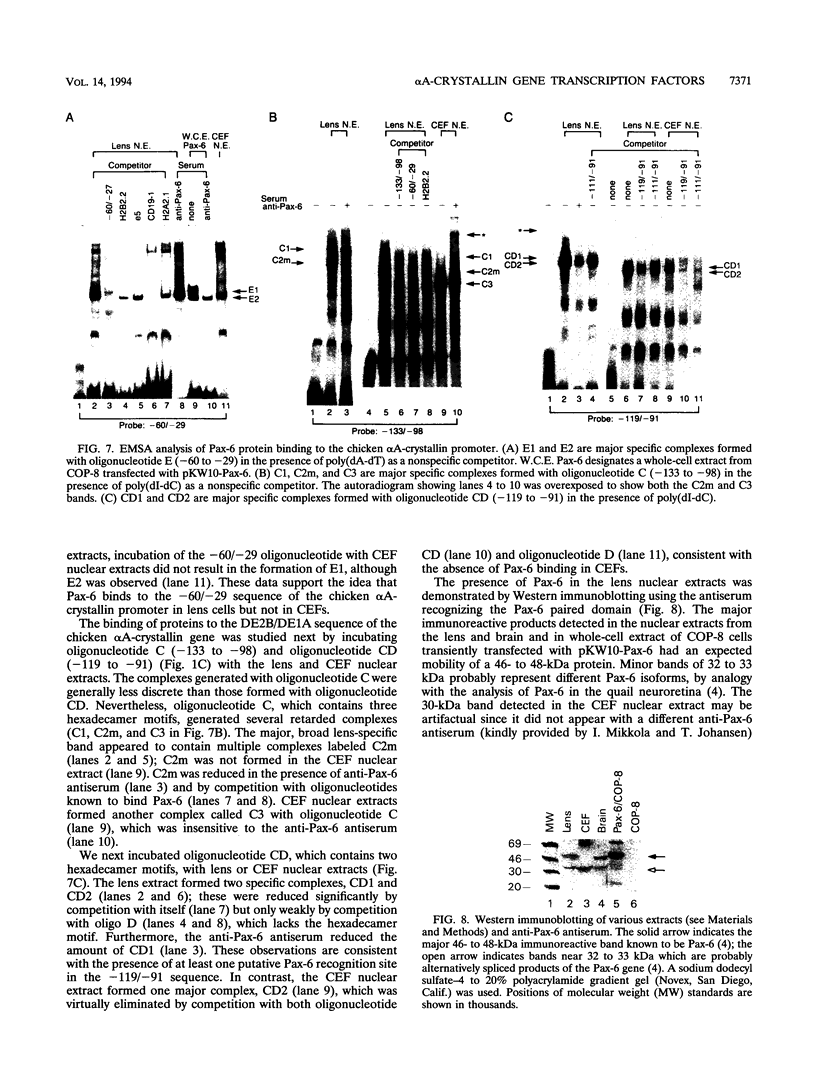
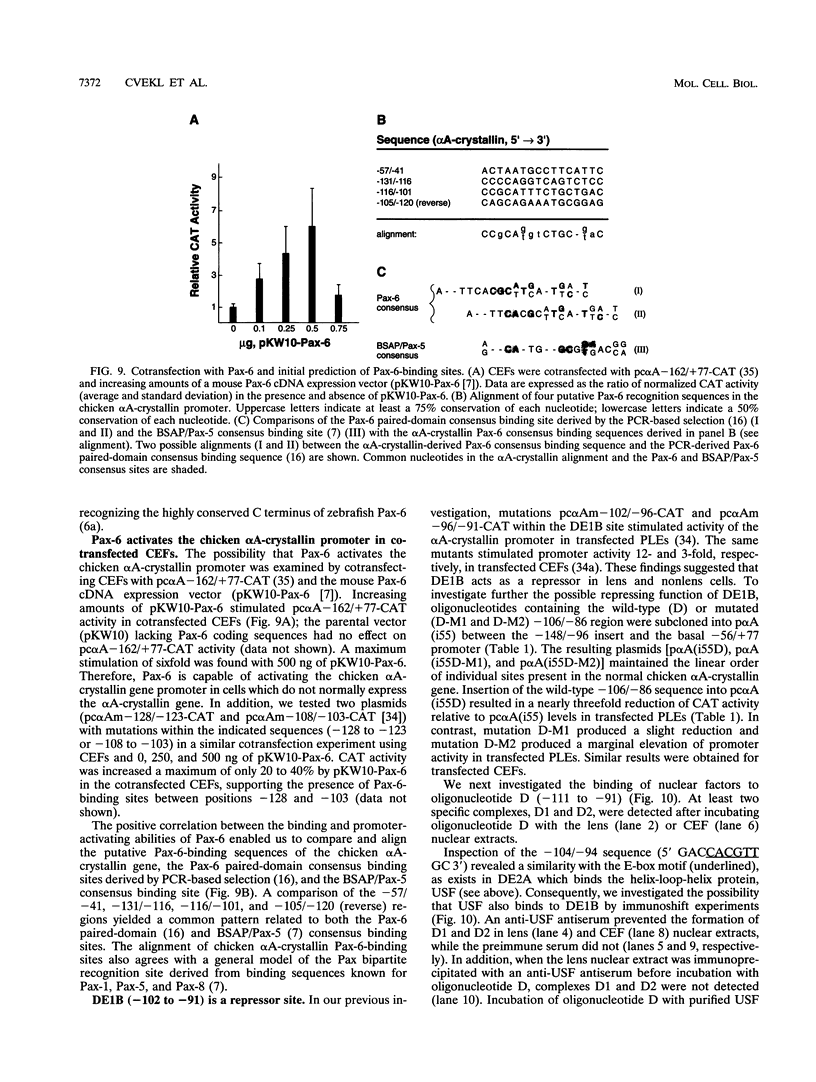
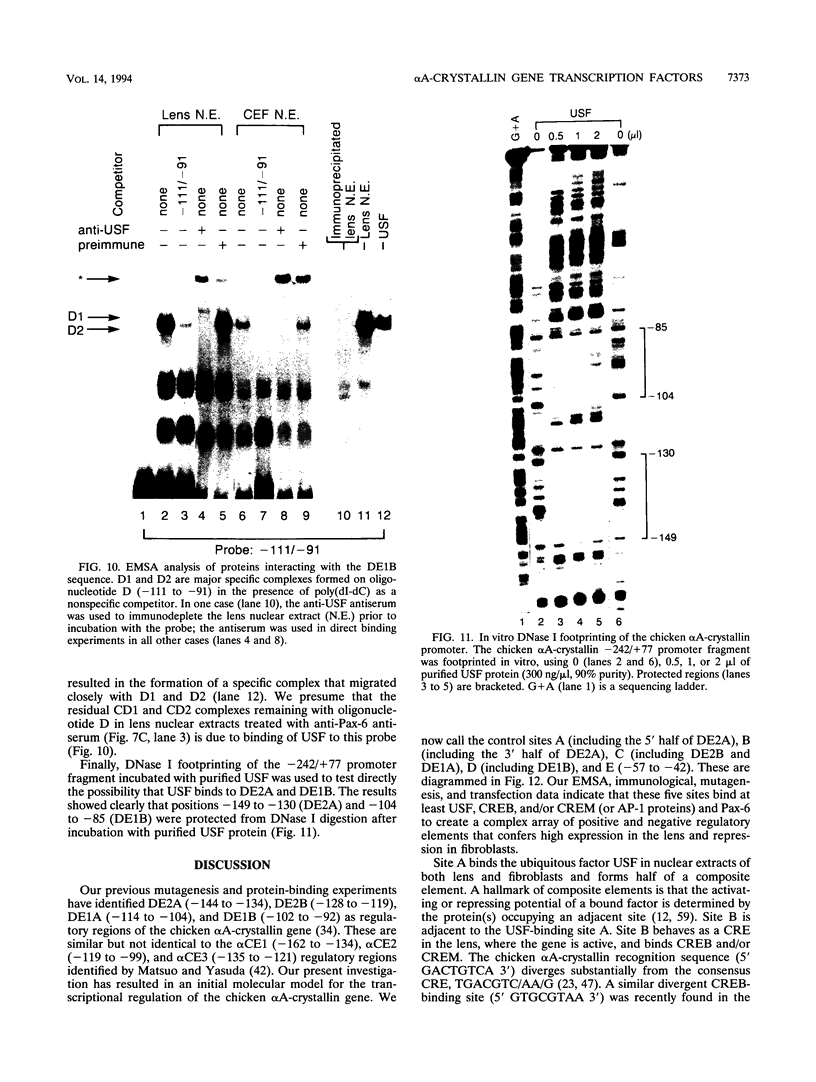
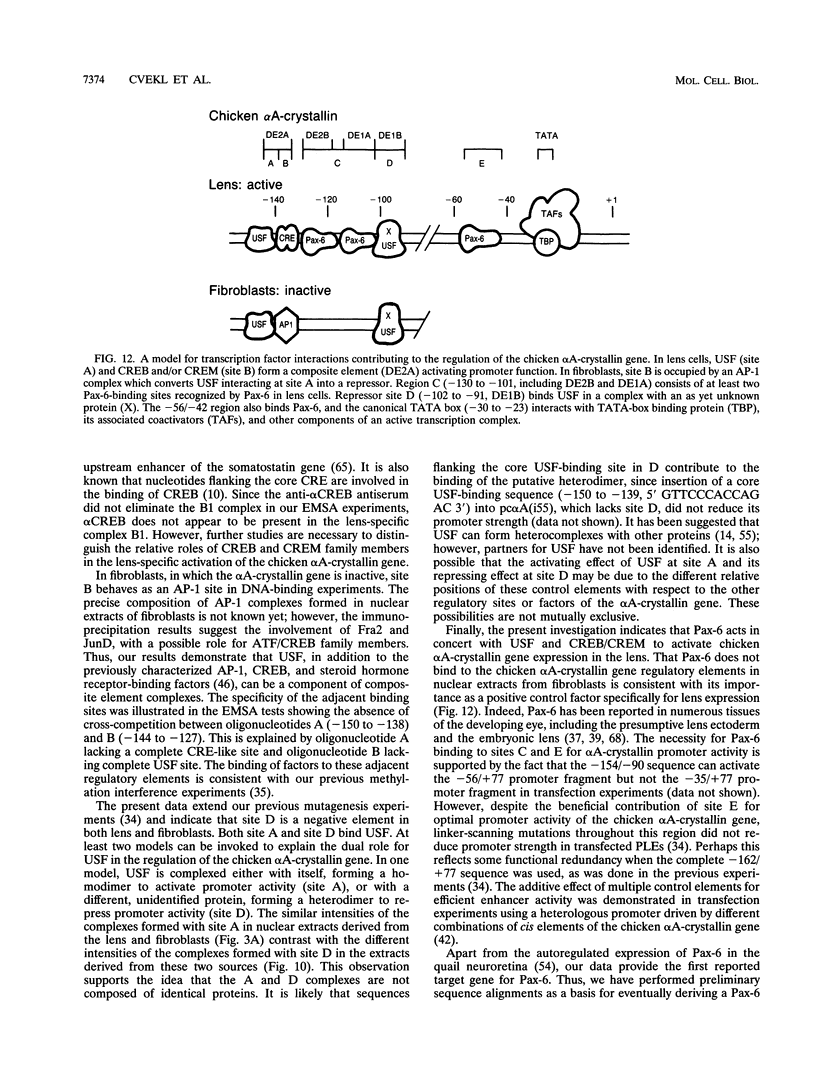
The abundance of crystallins (> 80% of the soluble protein) in the ocular lens provides advantageous markers for selective gene expression during cellular differentiation. Here we show by functional and protein-DNA binding experiments that the chicken alpha A-crystallin gene is regulated by at least five control elements located at sites A (-148 to -139), B (-138 to -132), C (-128 to -101), D (-102 to -93), and E (-56 to -41). Factors interacting with these sites were characterized immunologically and by gel mobility shift experiments. The results are interpreted with the following model. Site A binds USF and is part of a composite element with site B. Site B binds CREB and/or CREM to enhance expression in the lens and binds an AP-1 complex including CREB, Fra2 and/or JunD which interacts with USF on site A to repress expression in fibroblasts. Sites C and E (which is conserved across species) bind Pax-6 in the lens to stimulate alpha A-crystallin promoter activity. These experiments provide the first direct data that Pax-6 contributes to the lens-specific expression of a crystallin gene. Site D (-104 to -93) binds USF and is a negative element. Thus, the data indicate that USF, CREB and/or CREM (or AP-1 factors), and Pax-6 bind a complex array of positive and negative cis-acting elements of the chicken alpha A-crystallin gene to control high expression in the lens and repression in fibroblasts.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adya N., Zhao L. J., Huang W., Boros I., Giam C. Z. Expansion of CREB's DNA recognition specificity by Tax results from interaction with Ala-Ala-Arg at positions 282-284 near the conserved DNA-binding domain of CREB. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5642–5646. doi: 10.1073/pnas.91.12.5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresnick E. H., Felsenfeld G. Evidence that the transcription factor USF is a component of the human beta-globin locus control region heteromeric protein complex. J Biol Chem. 1993 Sep 5;268(25):18824–18834. [PubMed] [Google Scholar]
- Carriere C., Plaza S., Martin P., Quatannens B., Bailly M., Stehelin D., Saule S. Characterization of quail Pax-6 (Pax-QNR) proteins expressed in the neuroretina. Mol Cell Biol. 1993 Dec;13(12):7257–7266. doi: 10.1128/mcb.13.12.7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carthew R. W., Chodosh L. A., Sharp P. A. The major late transcription factor binds to and activates the mouse metallothionein I promoter. Genes Dev. 1987 Nov;1(9):973–980. doi: 10.1101/gad.1.9.973. [DOI] [PubMed] [Google Scholar]
- Chepelinsky A. B., Sommer B., Piatigorsky J. Interaction between two different regulatory elements activates the murine alpha A-crystallin gene promoter in explanted lens epithelia. Mol Cell Biol. 1987 May;7(5):1807–1814. doi: 10.1128/mcb.7.5.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerny T., Schaffner G., Busslinger M. DNA sequence recognition by Pax proteins: bipartite structure of the paired domain and its binding site. Genes Dev. 1993 Oct;7(10):2048–2061. doi: 10.1101/gad.7.10.2048. [DOI] [PubMed] [Google Scholar]
- Deutsch P. J., Hoeffler J. P., Jameson J. L., Lin J. C., Habener J. F. Structural determinants for transcriptional activation by cAMP-responsive DNA elements. J Biol Chem. 1988 Dec 5;263(34):18466–18472. [PubMed] [Google Scholar]
- Diamond M. I., Miner J. N., Yoshinaga S. K., Yamamoto K. R. Transcription factor interactions: selectors of positive or negative regulation from a single DNA element. Science. 1990 Sep 14;249(4974):1266–1272. doi: 10.1126/science.2119054. [DOI] [PubMed] [Google Scholar]
- Donovan D. M., Sax C. M., Klement J. F., Li X., Chepelinsky A. B., Piatigorsky J. Conservation of mouse alpha A-crystallin promoter activity in chicken lens epithelial cells. J Mol Evol. 1992 Oct;35(4):337–345. doi: 10.1007/BF00161171. [DOI] [PubMed] [Google Scholar]
- Du H., Roy A. L., Roeder R. G. Human transcription factor USF stimulates transcription through the initiator elements of the HIV-1 and the Ad-ML promoters. EMBO J. 1993 Feb;12(2):501–511. doi: 10.1002/j.1460-2075.1993.tb05682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin R. A., Wawrousek E. F., Piatigorsky J. Expression of the murine alpha B-crystallin gene is not restricted to the lens. Mol Cell Biol. 1989 Mar;9(3):1083–1091. doi: 10.1128/mcb.9.3.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein J., Cai J., Glaser T., Jepeal L., Maas R. Identification of a Pax paired domain recognition sequence and evidence for DNA-dependent conformational changes. J Biol Chem. 1994 Mar 18;269(11):8355–8361. [PubMed] [Google Scholar]
- Foulkes N. S., Mellström B., Benusiglio E., Sassone-Corsi P. Developmental switch of CREM function during spermatogenesis: from antagonist to activator. Nature. 1992 Jan 2;355(6355):80–84. doi: 10.1038/355080a0. [DOI] [PubMed] [Google Scholar]
- Fujii M., Tsuchiya H., Chuhjo T., Akizawa T., Seiki M. Interaction of HTLV-1 Tax1 with p67SRF causes the aberrant induction of cellular immediate early genes through CArG boxes. Genes Dev. 1992 Nov;6(11):2066–2076. doi: 10.1101/gad.6.11.2066. [DOI] [PubMed] [Google Scholar]
- Glaser T., Walton D. S., Maas R. L. Genomic structure, evolutionary conservation and aniridia mutations in the human PAX6 gene. Nat Genet. 1992 Nov;2(3):232–239. doi: 10.1038/ng1192-232. [DOI] [PubMed] [Google Scholar]
- Grainger R. M. Embryonic lens induction: shedding light on vertebrate tissue determination. Trends Genet. 1992 Oct;8(10):349–355. doi: 10.1016/0168-9525(92)90280-h. [DOI] [PubMed] [Google Scholar]
- Gruss P., Walther C. Pax in development. Cell. 1992 May 29;69(5):719–722. doi: 10.1016/0092-8674(92)90281-g. [DOI] [PubMed] [Google Scholar]
- Habener J. F. Cyclic AMP response element binding proteins: a cornucopia of transcription factors. Mol Endocrinol. 1990 Aug;4(8):1087–1094. doi: 10.1210/mend-4-8-1087. [DOI] [PubMed] [Google Scholar]
- Hayashi S., Goto K., Okada T. S., Kondoh H. Lens-specific enhancer in the third intron regulates expression of the chicken delta 1-crystallin gene. Genes Dev. 1987 Oct;1(8):818–828. doi: 10.1101/gad.1.8.818. [DOI] [PubMed] [Google Scholar]
- Hill R. E., Favor J., Hogan B. L., Ton C. C., Saunders G. F., Hanson I. M., Prosser J., Jordan T., Hastie N. D., van Heyningen V. Mouse small eye results from mutations in a paired-like homeobox-containing gene. Nature. 1991 Dec 19;354(6354):522–525. doi: 10.1038/354522a0. [DOI] [PubMed] [Google Scholar]
- Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst H. C., Masson N., Jones N. C., Lee K. A. The cellular transcription factor CREB corresponds to activating transcription factor 47 (ATF-47) and forms complexes with a group of polypeptides related to ATF-43. Mol Cell Biol. 1990 Dec;10(12):6192–6203. doi: 10.1128/mcb.10.12.6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia T. D., Craig E. A. Four small Drosophila heat shock proteins are related to each other and to mammalian alpha-crystallin. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2360–2364. doi: 10.1073/pnas.79.7.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski C. J., Chepelinsky A. B., Piatigorsky J. The alpha A-crystallin gene: conserved features of the 5'-flanking regions in human, mouse, and chicken. J Mol Evol. 1991 Dec;33(6):495–505. doi: 10.1007/BF02102802. [DOI] [PubMed] [Google Scholar]
- Kadesch T. Consequences of heteromeric interactions among helix-loop-helix proteins. Cell Growth Differ. 1993 Jan;4(1):49–55. [PubMed] [Google Scholar]
- Kantorow M., Cvekl A., Sax C. M., Piatigorsky J. Protein-DNA interactions of the mouse alpha A-crystallin control regions. Differences between expressing and non-expressing cells. J Mol Biol. 1993 Mar 20;230(2):425–435. doi: 10.1006/jmbi.1993.1160. [DOI] [PubMed] [Google Scholar]
- Kim R. Y., Lietman T., Piatigorsky J., Wistow G. J. Structure and expression of the duck alpha-enolase/tau-crystallin-encoding gene. Gene. 1991 Jul 22;103(2):193–200. doi: 10.1016/0378-1119(91)90273-e. [DOI] [PubMed] [Google Scholar]
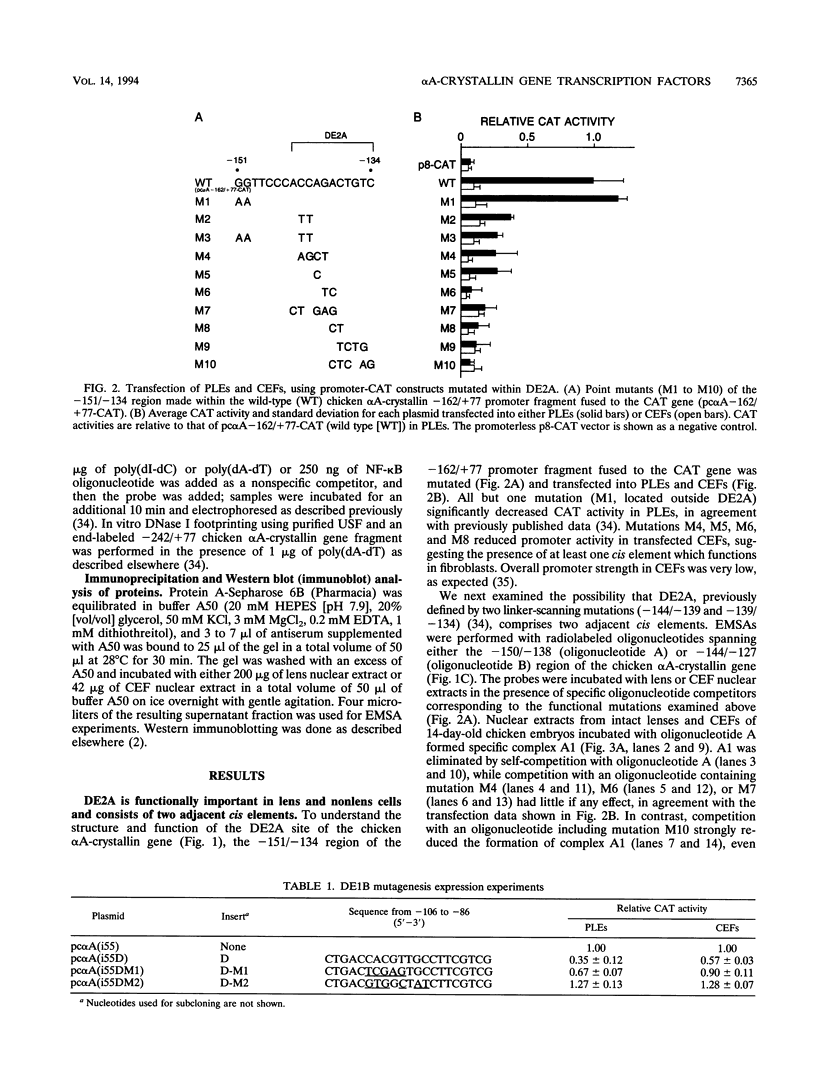
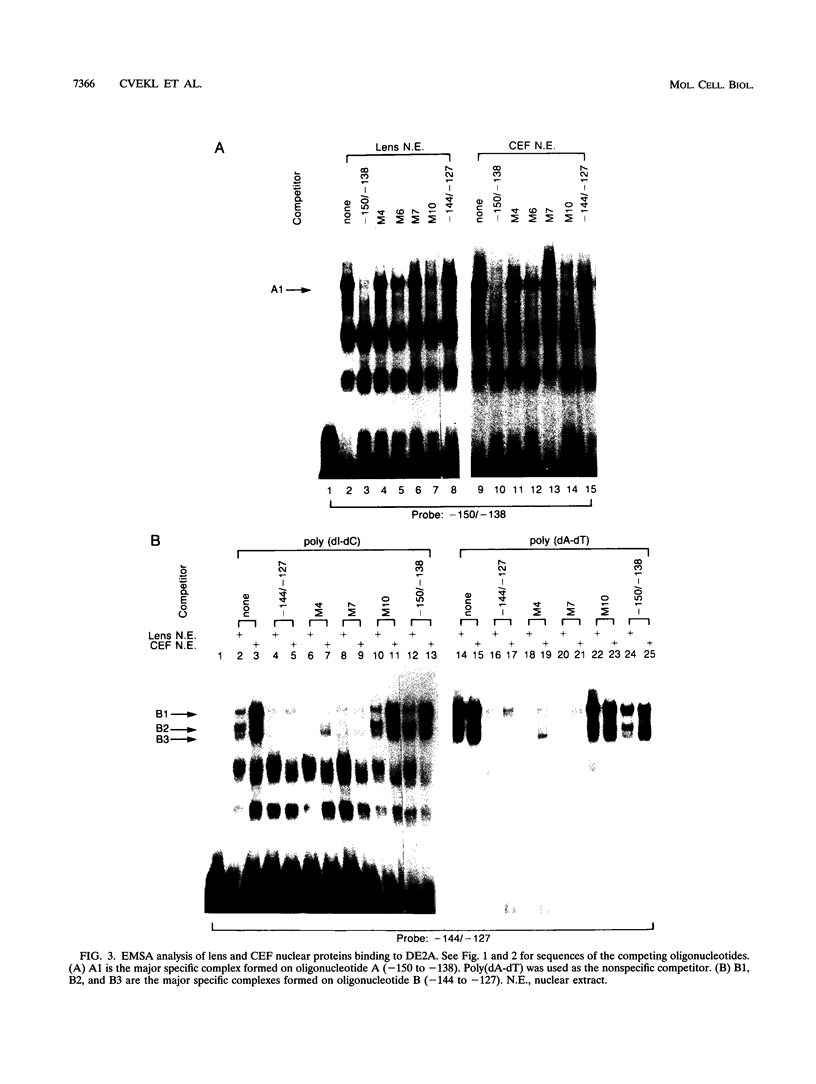
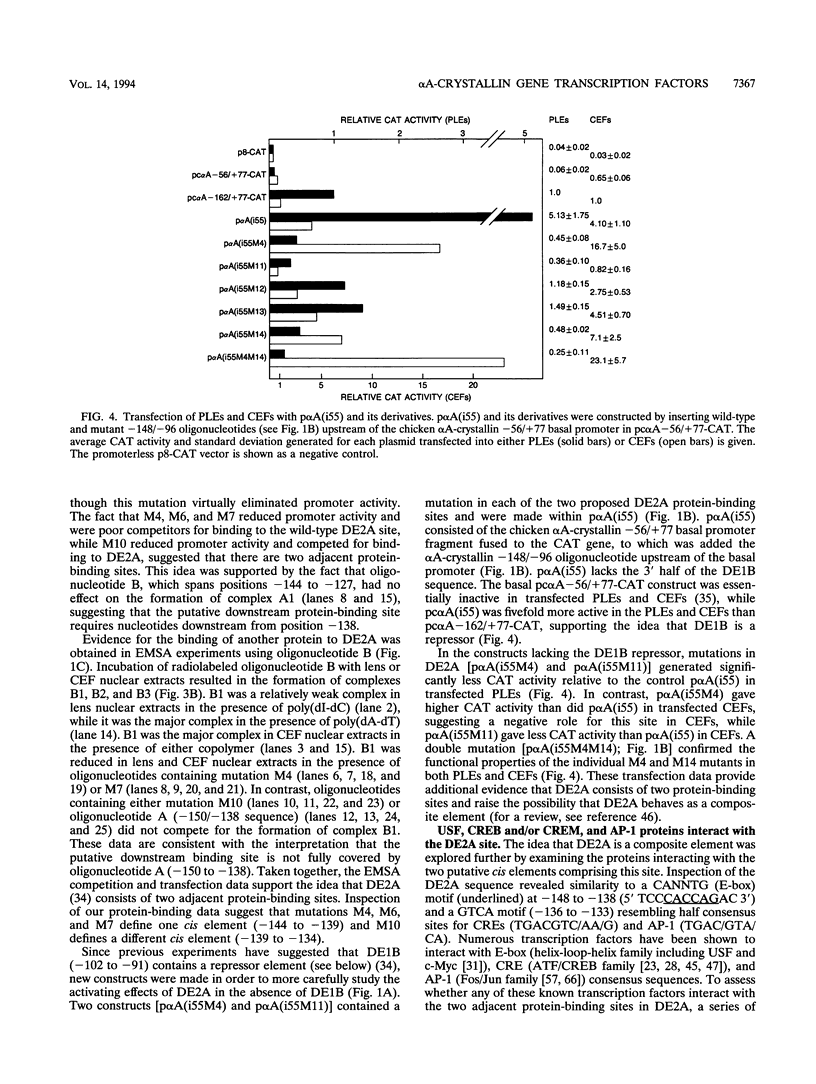
- Klement J. F., Cvekl A., Piatigorsky J. Functional elements DE2A, DE2B, and DE1A and the TATA box are required for activity of the chicken alpha A-crystallin gene in transfected lens epithelial cells. J Biol Chem. 1993 Mar 25;268(9):6777–6784. [PubMed] [Google Scholar]
- Klement J. F., Wawrousek E. F., Piatigorsky J. Tissue-specific expression of the chicken alpha A-crystallin gene in cultured lens epithelia and transgenic mice. J Biol Chem. 1989 Nov 25;264(33):19837–19844. [PubMed] [Google Scholar]
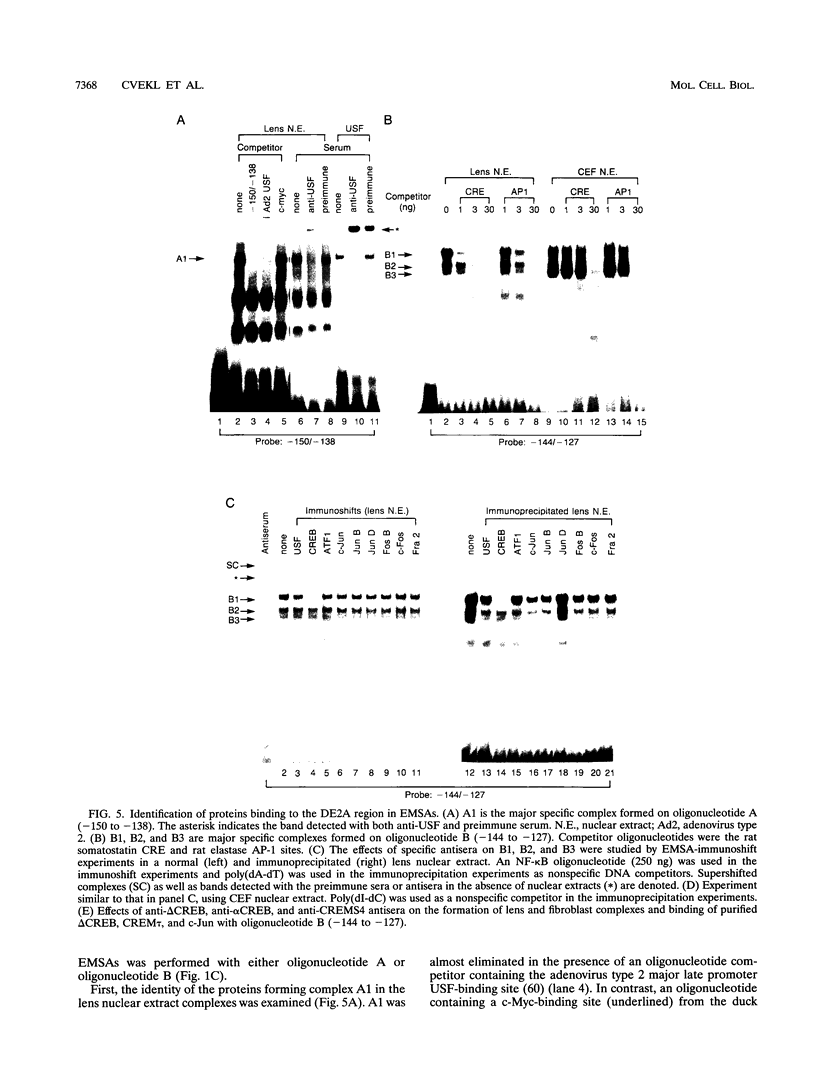
- Krauss S., Johansen T., Korzh V., Fjose A. Expression pattern of zebrafish pax genes suggests a role in early brain regionalization. Nature. 1991 Sep 19;353(6341):267–270. doi: 10.1038/353267a0. [DOI] [PubMed] [Google Scholar]
- Krauss S., Johansen T., Korzh V., Moens U., Ericson J. U., Fjose A. Zebrafish pax[zf-a]: a paired box-containing gene expressed in the neural tube. EMBO J. 1991 Dec;10(12):3609–3619. doi: 10.1002/j.1460-2075.1991.tb04927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D. C., Gonzalez P., Wistow G. Zeta-crystallin: a lens-specific promoter and the gene recruitment of an enzyme as a crystallin. J Mol Biol. 1994 Feb 25;236(3):669–678. doi: 10.1006/jmbi.1994.1178. [DOI] [PubMed] [Google Scholar]
- Li H. S., Yang J. M., Jacobson R. D., Pasko D., Sundin O. Pax-6 is first expressed in a region of ectoderm anterior to the early neural plate: implications for stepwise determination of the lens. Dev Biol. 1994 Mar;162(1):181–194. doi: 10.1006/dbio.1994.1077. [DOI] [PubMed] [Google Scholar]
- Martha A., Ferrell R. E., Mintz-Hittner H., Lyons L. A., Saunders G. F. Paired box mutations in familial and sporadic aniridia predicts truncated aniridia proteins. Am J Hum Genet. 1994 May;54(5):801–811. [PMC free article] [PubMed] [Google Scholar]
- Matsuo I., Kitamura M., Okazaki K., Yasuda K. Binding of a factor to an enhancer element responsible for the tissue-specific expression of the chicken alpha A-crystallin gene. Development. 1991 Oct;113(2):539–550. doi: 10.1242/dev.113.2.539. [DOI] [PubMed] [Google Scholar]
- Matsuo I., Yasuda K. The cooperative interaction between two motifs of an enhancer element of the chicken alpha A-crystallin gene, alpha CE1 and alpha CE2, confers lens-specific expression. Nucleic Acids Res. 1992 Jul 25;20(14):3701–3712. doi: 10.1093/nar/20.14.3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAvoy J. W. Induction of the eye lens. Differentiation. 1980;17(3):137–149. doi: 10.1111/j.1432-0436.1980.tb01091.x. [DOI] [PubMed] [Google Scholar]
- McDermott J. B., Peterson C. A., Piatigorsky J. Structure and lens expression of the gene encoding chicken beta A3/A1-crystallin. Gene. 1992 Aug 15;117(2):193–200. doi: 10.1016/0378-1119(92)90729-9. [DOI] [PubMed] [Google Scholar]
- Meyer T. E., Habener J. F. Cyclic adenosine 3',5'-monophosphate response element binding protein (CREB) and related transcription-activating deoxyribonucleic acid-binding proteins. Endocr Rev. 1993 Jun;14(3):269–290. doi: 10.1210/edrv-14-3-269. [DOI] [PubMed] [Google Scholar]
- Miner J. N., Diamond M. I., Yamamoto K. R. Joints in the regulatory lattice: composite regulation by steroid receptor-AP1 complexes. Cell Growth Differ. 1991 Oct;2(10):525–530. [PubMed] [Google Scholar]
- Montminy M. R., Gonzalez G. A., Yamamoto K. K. Regulation of cAMP-inducible genes by CREB. Trends Neurosci. 1990 May;13(5):184–188. doi: 10.1016/0166-2236(90)90045-c. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Donovan D. M., Hamada K., Sax C. M., Norman B., Flanagan J. R., Ozato K., Westphal H., Piatigorsky J. Regulation of the mouse alpha A-crystallin gene: isolation of a cDNA encoding a protein that binds to a cis sequence motif shared with the major histocompatibility complex class I gene and other genes. Mol Cell Biol. 1990 Jul;10(7):3700–3708. doi: 10.1128/mcb.10.7.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerenberg M., Hinrichs S. H., Reynolds R. K., Khoury G., Jay G. The tat gene of human T-lymphotropic virus type 1 induces mesenchymal tumors in transgenic mice. Science. 1987 Sep 11;237(4820):1324–1329. doi: 10.1126/science.2888190. [DOI] [PubMed] [Google Scholar]
- Paca-Uccaralertkun S., Zhao L. J., Adya N., Cross J. V., Cullen B. R., Boros I. M., Giam C. Z. In vitro selection of DNA elements highly responsive to the human T-cell lymphotropic virus type I transcriptional activator, Tax. Mol Cell Biol. 1994 Jan;14(1):456–462. doi: 10.1128/mcb.14.1.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatigorsky J. Lens crystallins. Innovation associated with changes in gene regulation. J Biol Chem. 1992 Mar 5;267(7):4277–4280. [PubMed] [Google Scholar]
- Piatigorsky J. Lens differentiation in vertebrates. A review of cellular and molecular features. Differentiation. 1981;19(3):134–153. doi: 10.1111/j.1432-0436.1981.tb01141.x. [DOI] [PubMed] [Google Scholar]
- Pognonec P., Roeder R. G. Recombinant 43-kDa USF binds to DNA and activates transcription in a manner indistinguishable from that of natural 43/44-kDa USF. Mol Cell Biol. 1991 Oct;11(10):5125–5136. doi: 10.1128/mcb.11.10.5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth H. J., Das G. C., Piatigorsky J. Chicken beta B1-crystallin gene expression: presence of conserved functional polyomavirus enhancer-like and octamer binding-like promoter elements found in non-lens genes. Mol Cell Biol. 1991 Mar;11(3):1488–1499. doi: 10.1128/mcb.11.3.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryseck R. P., Bravo R. c-JUN, JUN B, and JUN D differ in their binding affinities to AP-1 and CRE consensus sequences: effect of FOS proteins. Oncogene. 1991 Apr;6(4):533–542. [PubMed] [Google Scholar]
- Saha M. S., Servetnick M., Grainger R. M. Vertebrate eye development. Curr Opin Genet Dev. 1992 Aug;2(4):582–588. doi: 10.1016/s0959-437x(05)80176-5. [DOI] [PubMed] [Google Scholar]
- Sakai D. D., Helms S., Carlstedt-Duke J., Gustafsson J. A., Rottman F. M., Yamamoto K. R. Hormone-mediated repression: a negative glucocorticoid response element from the bovine prolactin gene. Genes Dev. 1988 Sep;2(9):1144–1154. doi: 10.1101/gad.2.9.1144. [DOI] [PubMed] [Google Scholar]
- Sawadogo M., Roeder R. G. Interaction of a gene-specific transcription factor with the adenovirus major late promoter upstream of the TATA box region. Cell. 1985 Nov;43(1):165–175. doi: 10.1016/0092-8674(85)90021-2. [DOI] [PubMed] [Google Scholar]
- Sax C. M., Farrell F. X., Zehner Z. E., Piatigorsky J. Regulation of vimentin gene expression in the ocular lens. Dev Biol. 1990 May;139(1):56–64. doi: 10.1016/0012-1606(90)90278-q. [DOI] [PubMed] [Google Scholar]
- Sax C. M., Piatigorsky J. Expression of the alpha-crystallin/small heat-shock protein/molecular chaperone genes in the lens and other tissues. Adv Enzymol Relat Areas Mol Biol. 1994;69:155–201. doi: 10.1002/9780470123157.ch5. [DOI] [PubMed] [Google Scholar]
- Seigel G. M., Notter M. F. Differentiation of Y79 retinoblastoma cells induced by succinylated concanavalin A. Cell Growth Differ. 1993 Jan;4(1):1–7. [PubMed] [Google Scholar]
- Shapiro D. J., Sharp P. A., Wahli W. W., Keller M. J. A high-efficiency HeLa cell nuclear transcription extract. DNA. 1988 Jan-Feb;7(1):47–55. doi: 10.1089/dna.1988.7.47. [DOI] [PubMed] [Google Scholar]
- Ton C. C., Hirvonen H., Miwa H., Weil M. M., Monaghan P., Jordan T., van Heyningen V., Hastie N. D., Meijers-Heijboer H., Drechsler M. Positional cloning and characterization of a paired box- and homeobox-containing gene from the aniridia region. Cell. 1991 Dec 20;67(6):1059–1074. doi: 10.1016/0092-8674(91)90284-6. [DOI] [PubMed] [Google Scholar]
- Vallejo M., Penchuk L., Habener J. F. Somatostatin gene upstream enhancer element activated by a protein complex consisting of CREB, Isl-1-like, and alpha-CBF-like transcription factors. J Biol Chem. 1992 Jun 25;267(18):12876–12884. [PubMed] [Google Scholar]
- Vogt P. K., Bos T. J. jun: oncogene and transcription factor. Adv Cancer Res. 1990;55:1–35. doi: 10.1016/s0065-230x(08)60466-2. [DOI] [PubMed] [Google Scholar]
- Wagner S., Green M. R. HTLV-I Tax protein stimulation of DNA binding of bZIP proteins by enhancing dimerization. Science. 1993 Oct 15;262(5132):395–399. doi: 10.1126/science.8211160. [DOI] [PubMed] [Google Scholar]
- Walther C., Gruss P. Pax-6, a murine paired box gene, is expressed in the developing CNS. Development. 1991 Dec;113(4):1435–1449. doi: 10.1242/dev.113.4.1435. [DOI] [PubMed] [Google Scholar]
- Williams J. S., Dixon J. E., Andrisani O. M. Binding constant determination studies utilizing recombinant delta CREB protein. DNA Cell Biol. 1993 Mar;12(2):183–190. doi: 10.1089/dna.1993.12.183. [DOI] [PubMed] [Google Scholar]
- Wistow G. J., Piatigorsky J. Lens crystallins: the evolution and expression of proteins for a highly specialized tissue. Annu Rev Biochem. 1988;57:479–504. doi: 10.1146/annurev.bi.57.070188.002403. [DOI] [PubMed] [Google Scholar]
- de Groot R. P., Sassone-Corsi P. Hormonal control of gene expression: multiplicity and versatility of cyclic adenosine 3',5'-monophosphate-responsive nuclear regulators. Mol Endocrinol. 1993 Feb;7(2):145–153. doi: 10.1210/mend.7.2.8385737. [DOI] [PubMed] [Google Scholar]
- de Jong W. W., Leunissen J. A., Voorter C. E. Evolution of the alpha-crystallin/small heat-shock protein family. Mol Biol Evol. 1993 Jan;10(1):103–126. doi: 10.1093/oxfordjournals.molbev.a039992. [DOI] [PubMed] [Google Scholar]