Abstract
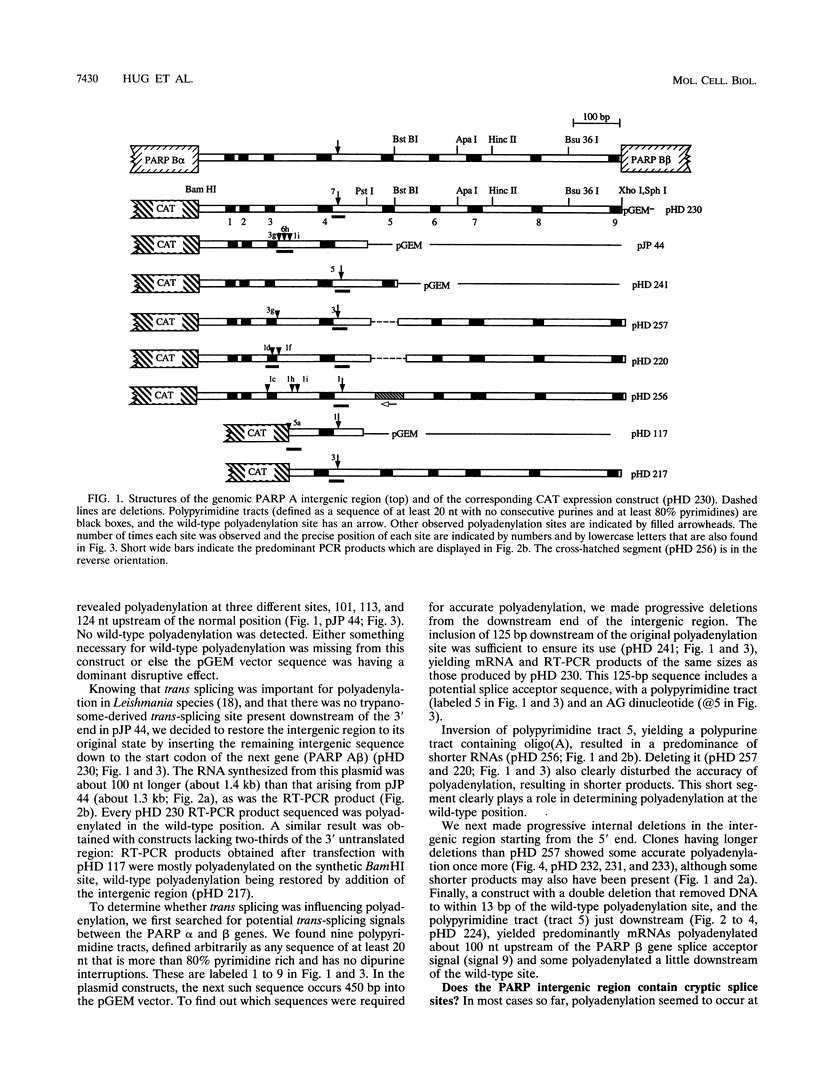
Nearly all trypanosome mRNAs are synthesized as polycistronic precursors, from which mature mRNAs are excised by trans splicing and polyadenylation. Polyadenylation of a procyclic acidic repetitive protein (PARP, or procyclin) transcript was studied by transient transfection of constructs bearing a chloramphenicol acetyltransferase gene linked to the PARP intergenic region. Polyadenylation usually occurred at A residues, about 100 bases upstream of a trans-splicing acceptor signal. The wild-type polyadenylation site has a cryptic trans-splicing signal about 100 bp downstream: deletion or inversion of this signal results in polyadenylation at multiple sites, upstream of other cryptic trans-splicing signals. The PARP mRNA precursor appears to contain a hierarchy of possible processing signals, the function of cryptic ones being revealed only when the dominant ones are deleted or moved. Correct polyadenylation can be restored by addition of trans-splicing signals from other loci. The results indicate that polyadenylation is coupled to downstream trans splicing but that the products of the trans-splicing reaction are not necessarily functional mRNAs.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agabian N. Trans splicing of nuclear pre-mRNAs. Cell. 1990 Jun 29;61(7):1157–1160. doi: 10.1016/0092-8674(90)90674-4. [DOI] [PubMed] [Google Scholar]
- Aline R. F., Jr, Stuart K. Trypanosoma brucei: conserved sequence organization 3' to telomeric variant surface glycoprotein genes. Exp Parasitol. 1989 Jan;68(1):57–66. doi: 10.1016/0014-4894(89)90008-8. [DOI] [PubMed] [Google Scholar]
- Atwater J. A., Wisdom R., Verma I. M. Regulated mRNA stability. Annu Rev Genet. 1990;24:519–541. doi: 10.1146/annurev.ge.24.120190.002511. [DOI] [PubMed] [Google Scholar]
- Beverley S. M., Clayton C. E. Transfection of Leishmania and Trypanosoma brucei by electroporation. Methods Mol Biol. 1993;21:333–348. doi: 10.1385/0-89603-239-6:333. [DOI] [PubMed] [Google Scholar]
- Bruzik J. P., Steitz J. A. Spliced leader RNA sequences can substitute for the essential 5' end of U1 RNA during splicing in a mammalian in vitro system. Cell. 1990 Sep 7;62(5):889–899. doi: 10.1016/0092-8674(90)90264-f. [DOI] [PubMed] [Google Scholar]
- Chung H. M., Lee M. G., Dietrich P., Huang J., Van der Ploeg L. H. Disruption of largest subunit RNA polymerase II genes in Trypanosoma brucei. Mol Cell Biol. 1993 Jun;13(6):3734–3743. doi: 10.1128/mcb.13.6.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton C. E., Mowatt M. R. The procyclic acidic repetitive proteins of Trypanosoma brucei. Purification and post-translational modification. J Biol Chem. 1989 Sep 5;264(25):15088–15093. [PubMed] [Google Scholar]
- Clayton C. Developmental regulation of nuclear gene expression in Trypanosoma brucei. Prog Nucleic Acid Res Mol Biol. 1992;43:37–66. doi: 10.1016/s0079-6603(08)61043-0. [DOI] [PubMed] [Google Scholar]
- De Lange T., Kooter J. M., Michels P. A., Borst P. Telomere conversion in trypanosomes. Nucleic Acids Res. 1983 Dec 10;11(23):8149–8165. doi: 10.1093/nar/11.23.8149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goguel V., Rosbash M. Splice site choice and splicing efficiency are positively influenced by pre-mRNA intramolecular base pairing in yeast. Cell. 1993 Mar 26;72(6):893–901. doi: 10.1016/0092-8674(93)90578-e. [DOI] [PubMed] [Google Scholar]
- Gunderson S. I., Beyer K., Martin G., Keller W., Boelens W. C., Mattaj L. W. The human U1A snRNP protein regulates polyadenylation via a direct interaction with poly(A) polymerase. Cell. 1994 Feb 11;76(3):531–541. doi: 10.1016/0092-8674(94)90116-3. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Huang J., Van der Ploeg L. H. Requirement of a polypyrimidine tract for trans-splicing in trypanosomes: discriminating the PARP promoter from the immediately adjacent 3' splice acceptor site. EMBO J. 1991 Dec;10(12):3877–3885. doi: 10.1002/j.1460-2075.1991.tb04957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., van der Ploeg L. H. Maturation of polycistronic pre-mRNA in Trypanosoma brucei: analysis of trans splicing and poly(A) addition at nascent RNA transcripts from the hsp70 locus. Mol Cell Biol. 1991 Jun;11(6):3180–3190. doi: 10.1128/mcb.11.6.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hug M., Carruthers V. B., Hartmann C., Sherman D. S., Cross G. A., Clayton C. A possible role for the 3'-untranslated region in developmental regulation in Trypanosoma brucei. Mol Biochem Parasitol. 1993 Sep;61(1):87–95. doi: 10.1016/0166-6851(93)90161-p. [DOI] [PubMed] [Google Scholar]
- Jackson R. J. Cytoplasmic regulation of mRNA function: the importance of the 3' untranslated region. Cell. 1993 Jul 16;74(1):9–14. doi: 10.1016/0092-8674(93)90290-7. [DOI] [PubMed] [Google Scholar]
- Kapotas N., Bellofatto V. Differential response to RNA trans-splicing signals within the phosphoglycerate kinase gene cluster in Trypanosoma brucei. Nucleic Acids Res. 1993 Aug 25;21(17):4067–4072. doi: 10.1093/nar/21.17.4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBowitz J. H., Smith H. Q., Rusche L., Beverley S. M. Coupling of poly(A) site selection and trans-splicing in Leishmania. Genes Dev. 1993 Jun;7(6):996–1007. doi: 10.1101/gad.7.6.996. [DOI] [PubMed] [Google Scholar]
- Lou H., McCullough A. J., Schuler M. A. 3' splice site selection in dicot plant nuclei is position dependent. Mol Cell Biol. 1993 Aug;13(8):4485–4493. doi: 10.1128/mcb.13.8.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz C. S., Alwine J. C. Direct interaction of the U1 snRNP-A protein with the upstream efficiency element of the SV40 late polyadenylation signal. Genes Dev. 1994 Mar 1;8(5):576–586. doi: 10.1101/gad.8.5.576. [DOI] [PubMed] [Google Scholar]
- Maniatis T. Mechanisms of alternative pre-mRNA splicing. Science. 1991 Jan 4;251(4989):33–34. doi: 10.1126/science.1824726. [DOI] [PubMed] [Google Scholar]
- Matthews K. R., Tschudi C., Ullu E. A common pyrimidine-rich motif governs trans-splicing and polyadenylation of tubulin polycistronic pre-mRNA in trypanosomes. Genes Dev. 1994 Feb 15;8(4):491–501. doi: 10.1101/gad.8.4.491. [DOI] [PubMed] [Google Scholar]
- McKeown M. Alternative mRNA splicing. Annu Rev Cell Biol. 1992;8:133–155. doi: 10.1146/annurev.cb.08.110192.001025. [DOI] [PubMed] [Google Scholar]
- Michels P. A., Liu A. Y., Bernards A., Sloof P., Van der Bijl M. M., Schinkel A. H., Menke H. H., Borst P., Veeneman G. H., Tromp M. C. Activation of the genes for variant surface glycoproteins 117 and 118 in Trypanosoma brucei. J Mol Biol. 1983 Jun 5;166(4):537–556. doi: 10.1016/s0022-2836(83)80283-6. [DOI] [PubMed] [Google Scholar]
- Mowatt M. R., Clayton C. E. Polymorphism in the procyclic acidic repetitive protein gene family of Trypanosoma brucei. Mol Cell Biol. 1988 Oct;8(10):4055–4062. doi: 10.1128/mcb.8.10.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowatt M. R., Wisdom G. S., Clayton C. E. Variation of tandem repeats in the developmentally regulated procyclic acidic repetitive proteins of Trypanosoma brucei. Mol Cell Biol. 1989 Mar;9(3):1332–1335. doi: 10.1128/mcb.9.3.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa M., Berget S. M. Mutation of the AAUAAA polyadenylation signal depresses in vitro splicing of proximal but not distal introns. Genes Dev. 1991 Nov;5(11):2086–2095. doi: 10.1101/gad.5.11.2086. [DOI] [PubMed] [Google Scholar]
- Niwa M., MacDonald C. C., Berget S. M. Are vertebrate exons scanned during splice-site selection? Nature. 1992 Nov 19;360(6401):277–280. doi: 10.1038/360277a0. [DOI] [PubMed] [Google Scholar]
- Pays E., Coquelet H., Tebabi P., Pays A., Jefferies D., Steinert M., Koenig E., Williams R. O., Roditi I. Trypanosoma brucei: constitutive activity of the VSG and procyclin gene promoters. EMBO J. 1990 Oct;9(10):3145–3151. doi: 10.1002/j.1460-2075.1990.tb07512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholler J. K., Aline R. F., Jr, Stuart K. D. Variant specific transcripts from the co-transposed segments of variant surface glycoprotein genes in Trypanosoma brucei. Mol Biochem Parasitol. 1988 May;29(1):89–103. doi: 10.1016/0166-6851(88)90123-5. [DOI] [PubMed] [Google Scholar]
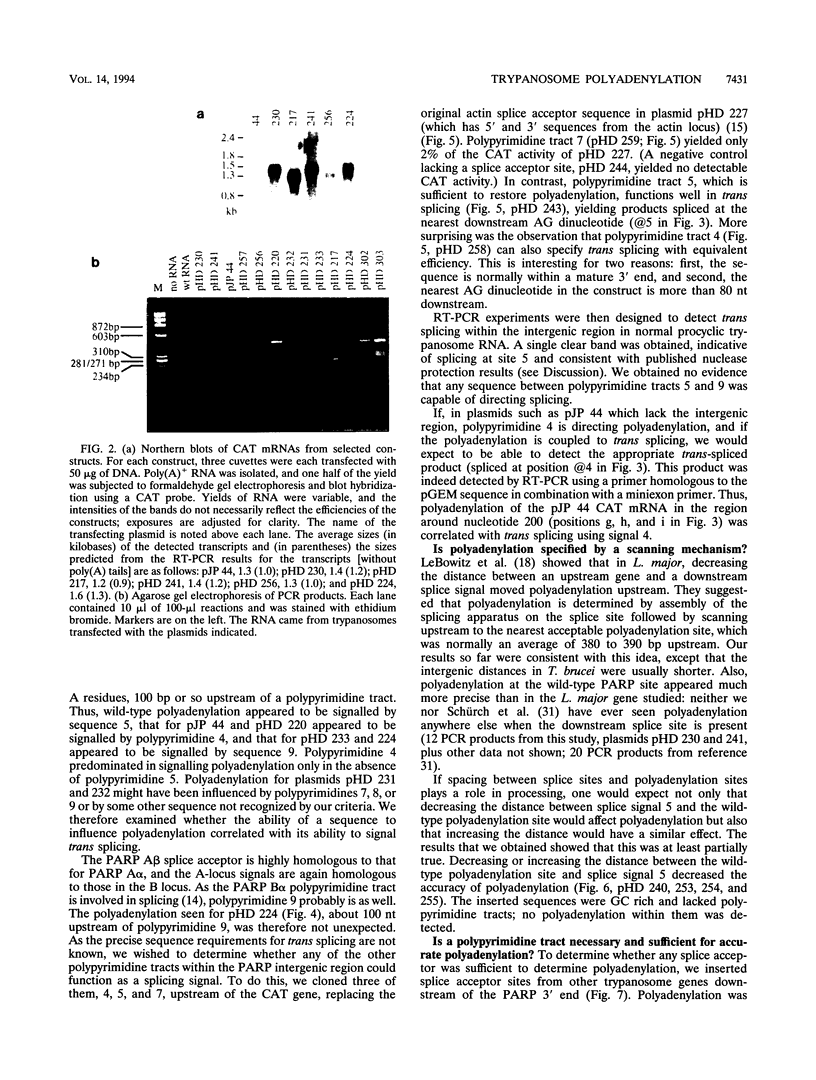
- Schürch N., Hehl A., Vassella E., Braun R., Roditi I. Accurate polyadenylation of procyclin mRNAs in Trypanosoma brucei is determined by pyrimidine-rich elements in the intergenic regions. Mol Cell Biol. 1994 Jun;14(6):3668–3675. doi: 10.1128/mcb.14.6.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman D. R., Janz L., Hug M., Clayton C. Anatomy of the parp gene promoter of Trypanosoma brucei. EMBO J. 1991 Nov;10(11):3379–3386. doi: 10.1002/j.1460-2075.1991.tb04902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogin M. L., Gunderson J. H., Elwood H. J., Alonso R. A., Peattie D. A. Phylogenetic meaning of the kingdom concept: an unusual ribosomal RNA from Giardia lamblia. Science. 1989 Jan 6;243(4887):75–77. doi: 10.1126/science.2911720. [DOI] [PubMed] [Google Scholar]
- Spieth J., Brooke G., Kuersten S., Lea K., Blumenthal T. Operons in C. elegans: polycistronic mRNA precursors are processed by trans-splicing of SL2 to downstream coding regions. Cell. 1993 May 7;73(3):521–532. doi: 10.1016/0092-8674(93)90139-h. [DOI] [PubMed] [Google Scholar]
- Ullu E., Matthews K. R., Tschudi C. Temporal order of RNA-processing reactions in trypanosomes: rapid trans splicing precedes polyadenylation of newly synthesized tubulin transcripts. Mol Cell Biol. 1993 Jan;13(1):720–725. doi: 10.1128/mcb.13.1.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
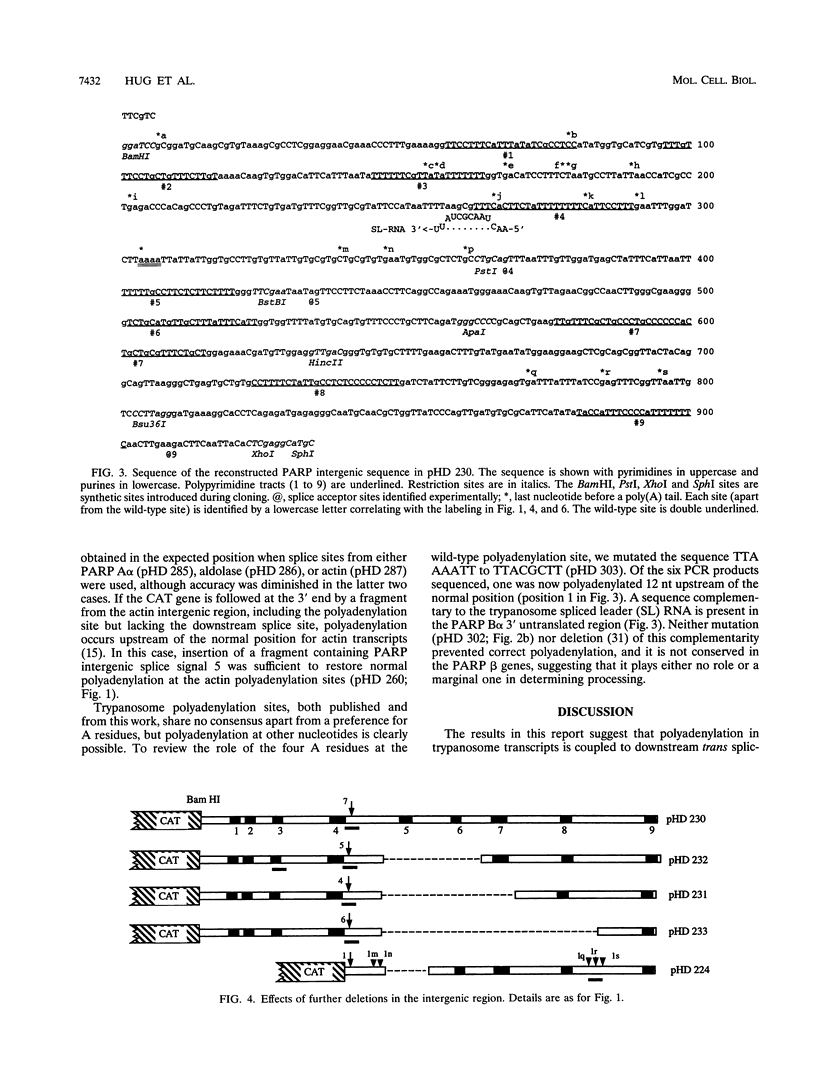
- Vassella E., Braun R., Roditi I. Control of polyadenylation and alternative splicing of transcripts from adjacent genes in a procyclin expression site: a dual role for polypyrimidine tracts in trypanosomes? Nucleic Acids Res. 1994 Apr 25;22(8):1359–1364. doi: 10.1093/nar/22.8.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassarman K. M., Steitz J. A. Association with terminal exons in pre-mRNAs: a new role for the U1 snRNP? Genes Dev. 1993 Apr;7(4):647–659. doi: 10.1101/gad.7.4.647. [DOI] [PubMed] [Google Scholar]
- Zomerdijk J. C., Ouellette M., ten Asbroek A. L., Kieft R., Bommer A. M., Clayton C. E., Borst P. The promoter for a variant surface glycoprotein gene expression site in Trypanosoma brucei. EMBO J. 1990 Sep;9(9):2791–2801. doi: 10.1002/j.1460-2075.1990.tb07467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]