Abstract
Altered inflammatory cytokine profiles are often observed in individuals suffering from major depression. Recent clinical work reports on elevated IL-6 and decreased IL-10 in depression. Elevated IL-6 has served as a consistent biomarker of depression and IL-10 is proposed to influence depressive behavior through its ability to counterbalance pro-inflammatory cytokine expression. Clinical and animal studies suggest a role for IL-10 in modifying depressive behavior. Murine restraint stress (RST) is regularly employed in the study of behavioral and biological symptoms associated with depressive disorders. While responses to acute RST exposure have been widely characterized, few studies have examined the ongoing and longitudinal effects of extended RST and fewer still have examined the lasting impact during the post-stress period. Consistent with clinical data, we report that a protocol of prolonged murine RST produced altered cytokine profiles similar to those observed in major depressive disorder. Parallel to these changes in circulating cytokines, IL-10 mRNA expression was diminished in the cortex and hippocampus throughout the stress period and following cessation of RST. Moreover, chronic RST promoted depressive-like behavior throughout the 28-day stress period and these depressive-like complications were maintained weeks after cessation of RST. Because of the correlation between IL-10 suppression and depressive behavior and because many successful antidepressant therapies yield increases in IL-10, we examined the effects of IL-10 treatment on RST-induced behavioral changes. Behavioral deficits induced by RST were reversed by exogenous administration of recombinant IL-10. This work provides one of the first reports describing the biological and behavioral impact following prolonged RST and, taken together, this study provides details on the correlation between responses to chronic RST and those seen in depressive disorders.
Introduction
Major depression currently ranks as the fourth leading cause of disability worldwide [1], [2]. Altered inflammatory cytokine profiles are often observed in depressed individuals [3]–[6]. For example, one of the most consistent biomarkers of depression is elevations in circulating IL-6 [7], [8] and is associated with treatment-resistance [9], [10]. Some animal studies indicate that overexpression of IL-6 promotes depressive-like behavior [11], [12] whereas others are unable to elicit such responses [13], [14]. Recent literature describes concurrent increases in IL-6 and decreases in IL-10 in individuals suffering from major depression [15], [16]. Fluctuations in anti-inflammatory IL-10 are similarly associated with depressive symptoms in humans and are proposed to influence depressive behavior when reduced anti-inflammatory expression is unable to counterbalance the expression of pro-inflammatory cytokines [17], [18]. Additionally, IL-10 plays a role in regulating hypothalamic-pituitary-adrenal (HPA) axis homeostasis by suppressing adrenocorticotropic hormone-induced steroid production and diminished IL-10 expression can affect HPA hyperactivity and glucocorticoid resistance seen in depressed patients [19]–[22]. Importantly, IL-10 treatment ameliorates LPS-induced sickness behavior and depressive symptoms in transgenic mice [23]–[25]. This coincides with both clinical and animal studies indicating that multiple classes of antidepressants elevate IL-10 levels upon successful treatment [26]–[29] and supports the role of IL-10 in affecting depressive behavior.
Rodent restraint stress has been used in modeling human disease for over 85 years [30] and in modeling psychological disease for 35 years [31]. This model is regularly employed in studying behavioral and biological symptoms associated with human depressive disorders [32]–[36]. Although a great deal of work has been done to characterize the biological and immunological events following acute psychological stress exposure, the vast majority of research utilizing RST is conducted over short experimental windows and conveys results obtained at single time points following completion of stress exposure [37]–[41]. Consequently, few studies examine the ongoing and longitudinal effects of extended restraint stress on physiology and behavior. In response, this study was undertaken to provide an examination of biological and behavioral responses to prolonged restraint stress and in the process examine the link between altered peripheral and central cytokine profiles and depressive behavior not only throughout, but also following chronic psychological stress. Here we report that this model of RST (6 hours daily for 28 days) in mice evoked depressive symptoms and cytokine profiles similar to those seen in human depression. This chronic RST resulted in depressive-like complications and altered cytokine expression that persisted for two weeks following stress cessation. Moreover, depressive symptoms induced by RST were rescued by peripheral treatment with recombinant IL-10. Together, this work deepens our understanding of the effects of chronic psychological stress and further supports chronic restraint stress in modeling depressive disorders.
Methods
Mice
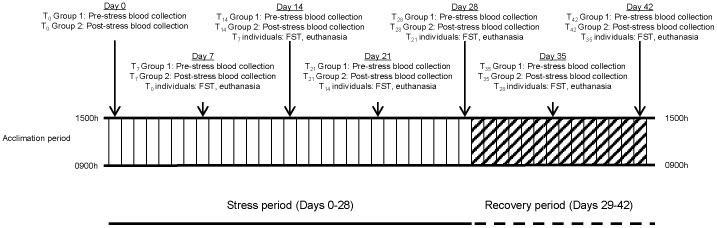
Female C57BL/6J mice age 6–8 weeks were purchased from The Jackson Laboratory (Bar Harbor, ME) and housed in an all-female room in groups of five per cage in an AAALAC-accredited facility on a 12-hour (0600/1800 h) light/dark cycle with ad libitum access to standard rodent chow and water. Female mice were selected due to lower incidences of injurious physical interactions. Mice were allowed to acclimate for 7–10 days before exposure to experimental procedures outlined in a protocol approved by The Ohio State University’s Institutional Animal Care and Use Committee and Office of Responsible Research Practices. Mice were handled minimally and humanely throughout the study and no signs of hypothermia or irregular grooming were noted. Mice were humanely sacrificed by CO2 asphyxiation. Data included in this report were collected without repeated measurement, sampling, or testing of any individuals (see Figure 1 for graphical representation).
Figure 1. Experimental timeline.
Experiment 1– Restraint Stress
RST stress experiments were designed and conducted in line with previous reports [42]–[44]. Following the acclimation period, individual cages of animals were randomly assigned to control or RST groups. Beginning the morning of day 0 and concluding on day 28, each animal assigned to the RST group was placed in an individual well-ventilated 50 mL polystyrene tube at 0900 h and returned to its respective cage in a horizontal resting position. At 1500 h RST animals were removed from restraint tubes and allowed to freely move until the next restraint cycle. Control animals were denied access to food and water during the RST period (0900–1500 h) and were otherwise not disturbed. Following the conclusion of the stress period on Day 28, RST and control animals were permitted access to food and water ad libitum.
Experiment 2– Recombinant IL-10 Treatment
Following the acclimation period, individual cages of animals were randomly assigned to control, RST with vehicle treatment, or RST with recombinant murine IL-10 (rIL-10). Beginning on day 1 and concluding on day 21, mice assigned to respective control or RST groups were exposed to restraint stress and treated as described in Experiment 1. Prior to RST on days 14–20, mice were treated by subcutaneous injection with either 50 µL PBS (control and RST group) or 2 µg of rIL-10 (eBiosciences, Sand Diego, CA) in 50 µL PBS.
Blood Collection
On days 0, 7, 14, 21, 28, 35, and 42, approximately 100 µL of blood was collected from the retro-orbital plexus of experimentally naïve RST and control animals under isoflurane anesthesia (Vedco, St. Joseph, MO) at either 0900 h or 1500 h and blood collection from all animals was completed within five minutes of first handling respective cages in line with previous studies [45]–[47]. Mice were returned to their respective cages following blood collection. Serum was isolated using BD Microtainer Serum Separator tubes (BD, Franklin Lakes, NJ) and stored at −80°C until analysis.
Forced Swim Test
On Days 7, 14, 21, 28, 35, and 42, approximately 18 hours following completion of the most recent restraint exposure, mice from which blood had been collected one week previously were subjected to a single forced swim test (FST) [48]–[51]. Individual animals were placed in a glass cylinder (43 cm tall, 22 cm in diameter) containing room temperature water (23±1°) filled to a depth of 15 cm. Mice were permitted to move freely for 8 minutes and their movements were recorded. Time to first immobility (defined as 2 consecutive seconds of stationary posture) was recorded as well as total time spent swimming or struggling. Cylinders were cleaned and water changed throughout the swim tests.
Tissue Collection
On days 7, 14, 21, 28, 35, and 42 mice were euthanized by CO2 asphyxiation and immediately weighed. Whole blood was collected by cardiac puncture and serum was isolated using serum separator tubes (BD, Franklin Lakes, NJ) and stored at −80°C until assayed. Spleens, thymuses, and adrenal glands were removed and weighed. The cortex and hippocampus of each individual was isolated and flash frozen in liquid nitrogen before storage at −80°C.
Corticosterone Measurement
Serum corticosterone levels were assessed using a corticosterone double antibody 125I RIA kit (MP Biomedicals, Costa Mesa, CA) according to manufacturer’s instructions. Samples were measured using a Packard Cobra II Auto Gamma counter (Perkin-Elmer Wellesley, MA).
Serum Cytokine Assay
Serum cytokine levels were measured using a custom BioRad Bioplex assay (Bio-Rad, Hercules, CA) including IL-1β, IL-4, IL-6, IL-10, TNFα, IFNγ with lower detection limits of, respectively, 9.4, 2.1, 0.2, 1.0, 1.2, and 1.4 pg/mL and analyzed using a BioPlex 200 Analysis System (Bio-Rad, Hercules, CA). Serum from RST and control animals from each time point was assayed in duplicate per manufacturer’s instructions. Samples failing to meet internal control standards or below detection limits were omitted. Cytokine levels of control individuals did not vary over time and data presented is relative to control expression levels of each cytokine at each time point.
RT-PCR
Total RNA was extracted from cortices and hippocampi using standard methods and Tri-reagent (Sigma, St. Louis, MO) according to manufacturer’s recommendations. RNA was assessed for quantity and integrity using a NanoDrop ND-100 Spectrophotometer (NanoDrop, Wilmington, DE). cDNA was produced using Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA). Quantitative PCR was performed using an Applied Biosystems Assay-on-Demand Gene Expression protocol specific for murine samples as previously described [52]. In brief, cDNA was amplified by real-time PCR where target cDNA (IL-1β, IL-4, IL-6, IL-10, TNFα, IFNγ) and reference cDNA (glyceraldehyde-3-phosphate dehydrogenase) were amplified simultaneously using an oligonucleotide probe with a 5′-fluorescent reporter dye (6-FAM) and a 3′-quencher dye (NFQ or TAMRA). Fluorescence was determined on an ABI PRISM 7300 sequence detection system (Applied Biosystems). Data were analyzed using the comparative threshold method and results were expressed as fold-difference.
Statistics
All data described in this work were collected and analyzed within the structure of a between-subjects design. Data were collected and analyzed without repeated measures and analyzed by ANOVA with two factors (RST and day) except for measurements of corticosterone levels which were analyzed by ANOVA with three factors (RST, day, and AM/PM). The primary comparison was response of RST mice across the stress or non-stress period and comparisons at each specific day and time were further examined. Holm’s method was applied to adjust for multiplicity of the primary outcomes and control the overall family-wise error rate at α = 0.05 [53]. PCR data were subjected to Shapiro-Wilk test using Statistical Analysis Systems (SAS Institute, Inc., Cary, NC) statistical software. Observations greater than three interquartile ranges from the first and third quartile were considered outliers and were excluded in the subsequent analysis [52]. Sample numbers analyzed for each time point are provided as a supplementary table (Fig. S1).
Results
Prolonged RST Elevated Corticosterone Levels, Decreased Body Weight, Spleen Weight and Evoked a Sustained Stress Response
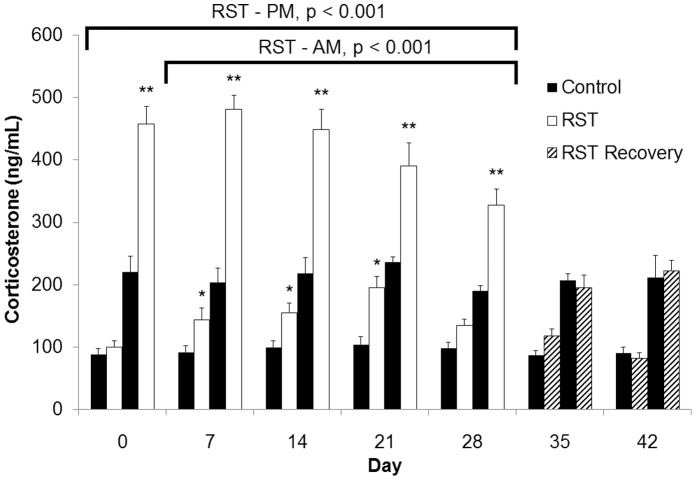
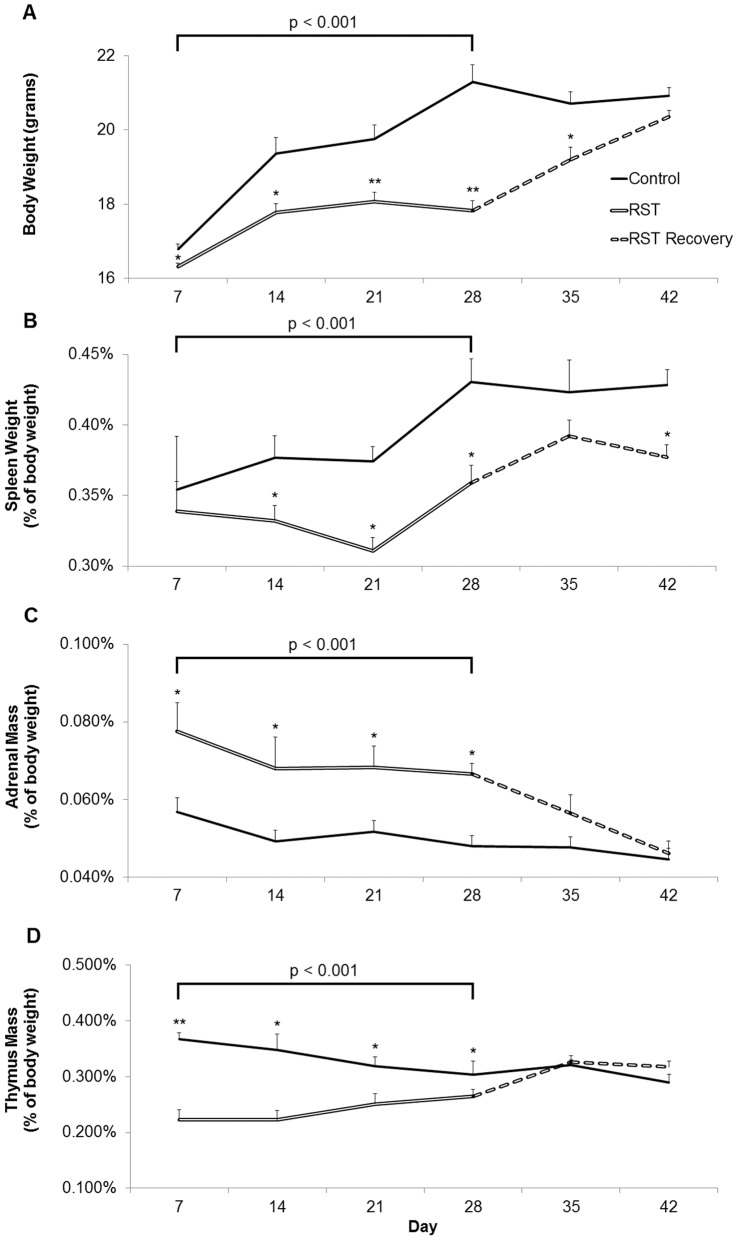
To assess the effects of restraint stress on HPA axis and diurnal corticosterorne rhythm, serum samples were collected from experimentally naïve mice at either 0900 h or 1500 h and assessed for serum corticosterone concentration. Baseline corticosterone measures of control mice were within or below reported ranges [45]–[47]. Prior to initial stress exposure on Day 1, RST and control mice showed no difference in AM baseline corticosterone levels (Fig. 2). After one week, RST increased morning baseline corticosterone levels at 18 hours following the previous stress exposure. Morning corticosterone levels were elevated throughout the stress period (RST 0900 h Day 1–28, p<0.001) and returned to the level of control mice following stress cessation. Corticosterone levels measured immediately following restraint (1500 h) were elevated by RST throughout the stress period (Days 1–28, p<0.001). Corticosterone levels returned to normal afternoon levels one week after the cessation of RST. Body weight was determined over the 42 day time course. RST decreased body weight starting at one week and this reduction was maintained throughout the stress period (Fig. 3A, p<0.001). Following cessation of stress, mice subjected to RST returned to that of control mice. Because stress-induced elevations in glucocorticoid levels induces splenic atrophy in RST mice (Wang et al. 2008), splenic mass was determined during the stress period and following stress cessation. RST decreased spleen weight during the stress period (Day 7–28) compared to controls (Fig. 3B; p<0.001). Spleen proportions increased after the conclusion of RST but remained significantly smaller than control mice two weeks after stress cessation. Previous reports associate restraint stress with increased adrenal and decreased thymus mass (Toth et al. 2008), therefore these parameters were determined during and following the stress period. In accordance with earlier reports, RST increased adrenal gland mass (Fig. 3C; p<0.001) and decreased thymic mass (Fig. 3D; p<0.001) during the stress period (Day 7–28). After a maximum at Day 7, adrenal gland mass decreased throughout the remainder of the stress and post-stress period (Day 29–42). In contrast, thymuses of RST animals recovered rapidly upon completion of the stress protocol (Day 35).
Figure 2. Prolonged RST elevated corticosterone levels throughout the stress period.
Corticosterone levels were determined during the stress period (solid bars, Day 0–28) and following stress cessation (hashed bars, Day 29–42). For each data point, n = 5–15 individuals. Data shown is mean +SEM. *p<.05; **p<.0001. Data were collected without repeated sampling of individuals.
Figure 3. Prolonged RST evoked sustained elevations in stress response.
Bodyweight (A), splenic mass (B), adrenal mass (C), and thymic mass (D) were examined during (solid lines, Day 7–28) and following stress cessation (broken lines, Day 29–42). For each data point n = 10 individuals. Data shown is mean +SEM. *p<0.05; **p<.0001. Data were collected without repeated sampling of individuals.
Restraint Stress Produces a Depressive-like Phenotype both During and following the Stress Period
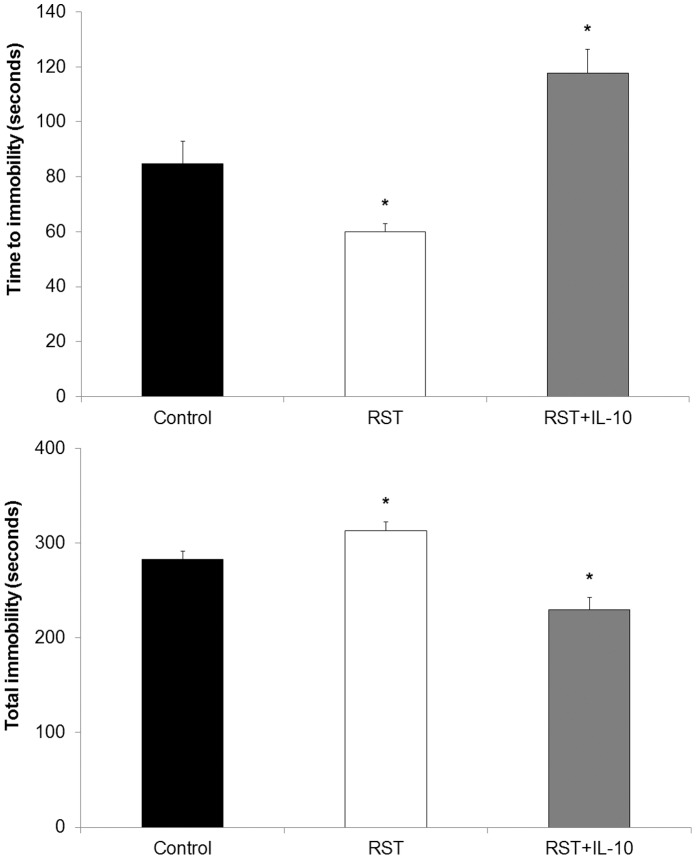
Previous work associates rodent restraint stress with the development of depressive-like behavior. Time spent immobile [33], [36], [54], [55] and latency to first immobility [55]–[57] are used in quantifying a depressive behavioral phenotype in forced swim tests. RST decreased time to first immobility throughout the stress period (Fig. 4; p<0.001). This was resolved following the completion of RST. Moreover, immobility in the forced swim test was increased throughout the stress period overall (p<0.001) and remained increased seven days following the cessation of RST (Days 35; p<0.05). In addition, RST appears to increase immobility during the post stress period, an effect that was marginally significant (p = 0.056). These data indicate that chronic RST promotes a depressive-like behavior that persists weeks after the cessation of stress.
Figure 4. Mice exposed to prolonged RST demonstrated sustained depressive-like behavior.
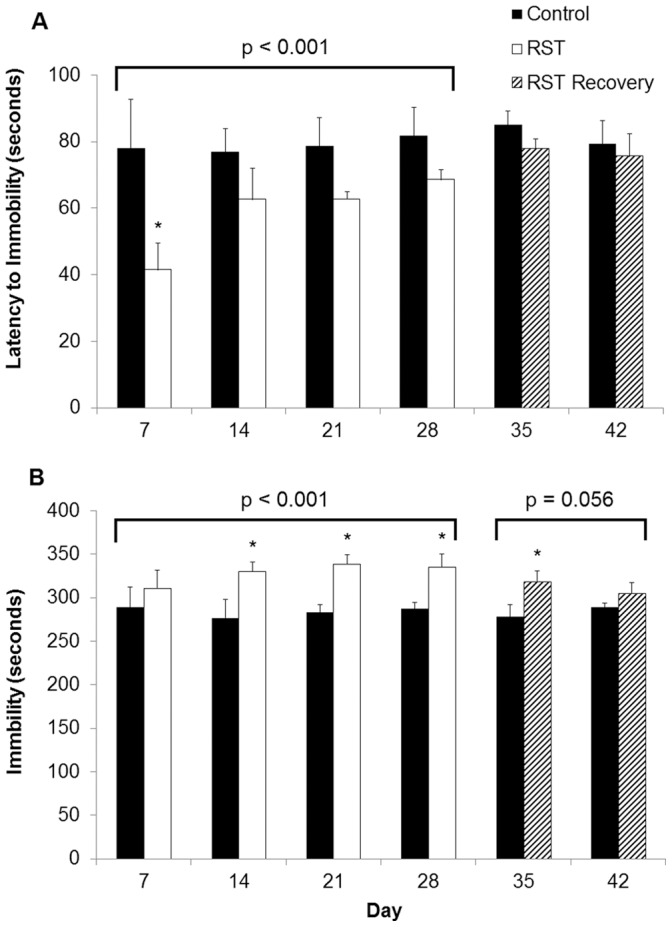
Mice were exposed to FST throughout the experimental period. Time to first immobility (A) as well as total immobility (B) were recorded. For each data point n = 10 individuals. Data shown is mean +SEM. *p<0.05. Data were collected without sampling of individuals.
Prolonged Restraint Stress Increases Circulating IL-6, Decreased IL-4 and IL-10
Depressive disorders are associated with altered circulating cytokine profiles [3], [4]. To investigate the role of inflammatory response in depressive-like behavior, serum levels of IL-1β, IL-4, IL-6, IL-10, TNFα, and IFNγ were determined throughout and following the stress period. Control values of cytokines were consistent with previous reports [58]–[60]. RST increased IL-6 (p<0.05) and decreased IL-4 (p<0.05) and IL-10 (p<0.05) in the serum during the stress period (Day 7–28). Moreover, these RST-induced reductions in the serum levels of IL-4 and IL-10 were sustained during the post-stress period (Fig. 5, Day 29–42, p<0.05, and p<0.05, respectively). Despite reduced circulating anti-inflammatory cytokines, inflammatory cytokines including IL-1β, TNFα and IFNγ were not increased in the serum by RST. Data is represented relative to levels of corresponding cytokines of control mice at each time point and cytokines levels of control mice were consistent over time.
Figure 5. RST elevated circulating IL-6 and decreased IL4 and IL-10.
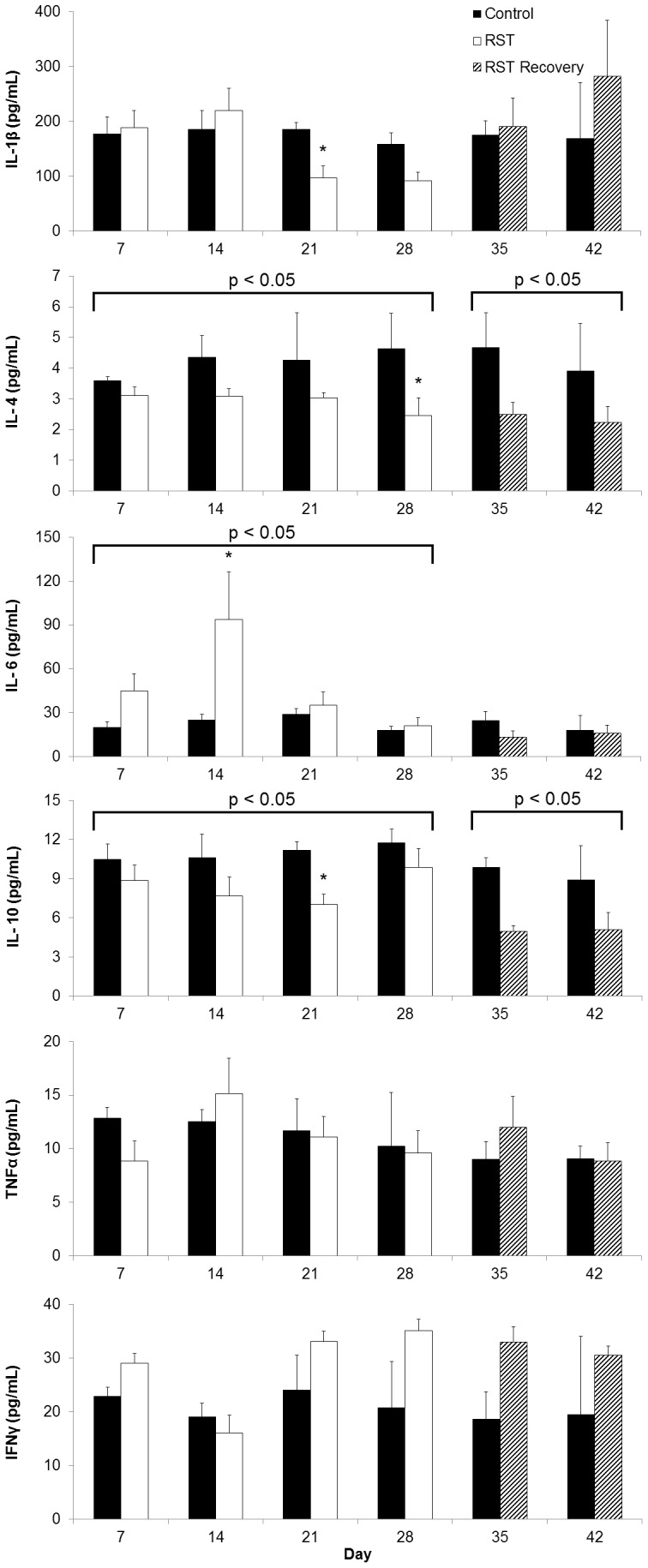
Circulating levels of pro- and anti-inflammatory cytokines were measured throughout the experimental period. For each data point n = 3–9 individuals. Data shown is mean +SEM. *p<0.05. Data were collected without repeated sampling of individuals.
Prolonged Restraint Stress Elicits Suppression of IL-10 in the Cortex and Hippocampus
To determine if the effect of RST on serum cytokines was paralleled in the brain, IL-1β, IL-4, IL-6, IL-10, TNFα, and IFNγ mRNA expression levels were determined in the cortex and hippocampus throughout and following the restraint period. RST decreased mRNA IL-10 expression in the cortex and hippocampus during the stress period (Fig. 6; p<0.001 and p<0.01, respectively; Day 7–28). This RST associated decrease in IL-10 mRNA expression was maintained during the weeks following the cessation of stress (Fig. 6, Day 29–42; p<0.001 and p<0.01, respectively). Similar to serum, pro-inflammatory cytokine mRNA was not increased in the brain during the stress or post-stress periods. Collectively, these data indicate that chronic RST decreases peripheral and central IL-10.
Figure 6. RST decreased expression of IL-10 in the brain.
mRNA expression of pro- and anti-inflammatory cytokines were measured in the cortex and hippocampus. For each data point n = 6–10 individuals. Data shown is mean +SEM. *p<0.05.
Recombinant IL-10 Reduced Stress-induced Behavioral Deficits
Because RST decreased IL-10, RST mice were treated with recombinant murine IL-10 or vehicle during the final seven days (days 14–20) of the 21-day restraint stress protocol and depressive-like behavior was determined. As expected, RST decreased time to first immobility (p<0.01) and increased total immobility (p<0.01) compared to controls (Fig. 7). The RST-induced depressive-like behavior was blocked by recombinant IL-10 treatment. For instance, IL-10 treatment extended time to first immobility and decreased total time spent immobile compared to vehicle-treated control mice (p<0.01 and p<0.01, respectively) and RST mice (p<0.01 and p<0.01, respectively).
Figure 7. IL-10 treatment exhibits a rescuing effect on restraint stress-induced behavioral deficits.
As part of a 21-day RST protocol, mice were treated with vehicle (control and RST mice) or murine IL-10 (IL-10 mice) for seven days immediately preceding FST. For each data point n = 5–15 individuals. Data shown is mean +SEM. *p<0.05.
Discussion
While a great deal of work has been done to characterize the events immediately following bouts of acute psychological stress, the vast majority of research utilizing RST is conducted over short experimental windows and frequently convey results obtained only at single time points upon completion of stress events [37]–[41]. Consequently, few long-term projects examine the ongoing biological and behavioral effects during extended periods of restraint stress and still fewer studies evaluate responses following stress cessation. For these reasons we extended the experimental timeframe, collecting data throughout both stress and post-stress periods. In proceeding with this investigation, we first characterized markers of stress response. We observed elevated and sustained stress responses throughout the stress period that returned to control levels following stress cessation. While corticosterone levels rise before rapidly returning to pre-stress baseline following acute psychological stress exposure, the model of chronic restraint stress employed here produced baseline AM corticosterone that were elevated even 18 hours following conclusion of the previous stress cycle (Fig. 2; RST - AM). This observation was seen throughout the stress period. These measurements were recorded at a time when corticosterone levels are typically at their lowest point during the diurnal cycle [61], [62] and convey a sustained elevation in minimum daily corticosterone levels throughout the 28-day stress period. Similarly, while PM corticosterone levels showed a maximum response on day 7, corticosterone levels measured immediately upon completion of stress exposure reflected an exaggerated and sustained stress response throughout the 28-day stress period (Fig. 2; RST - PM). Previous work using a 16-hour model of nocturnal restraint demonstrated disruption of diurnal corticosterone patterns following 8, but not 15 days of RST when combined with viral infection [63]. Here we found disruption of daily corticosterone patterns and elevated corticosterone levels that perdured throughout the 28-day stress period, perhaps owing to the sampling of naïve animals rather than repeated sampling of individuals as in other studies. This disruption of regular diurnal rhythm coupled with the inability to recover between RST exposures is indicative of chronic stress. Gross morphological changes have been described as part of an active response to restraint stress and observations of enlarged adrenal glands as well as decreased spleen and thymus proportions throughout the 28-day stress period are in line with previous studies [64]–[66] (Fig. 3). Together this indicates that chronic RST produces sustained stress responses throughout a 28-day RST protocol.
A key finding in this study was that RST caused depressive behavior that extended long after stress cessation. Forced swim tests are used in identifying depressive rodent phenotypes in which diminished total mobility and decreased time to first immobility are viewed as reflecting depressive-like behavior [32]–[34], [48], [54], [57]. Earlier reports demonstrate inconsistent behavioral responses to murine restraint stress: mice exposed to acute bouts of restraint (single exposure, >60 minutes) show, alternately, diminished latency to immobility and increased immobility [67] or no difference in behavior [68]. Additionally, murine restraint studies extending for up to 10 days report results ranging from no difference in depressive-like behavior to species-dependent variability [54]. In this study, application of uniform daily restraint exposure showed that time to first immobility was diminished at the earliest time point (Day 7) and reduced overall during the 28-day RST period (Fig. 4). Further, when the timeframe of examination was extended, mice showed decreased latency to immobility during the stress period which then continued into the period following stress cessation. To our knowledge this is the first report of depressive behavior extending into the weeks following restraint stress exposure and argues for further exploration of the mechanism linking psychological stress and depressive behavior.
The combined role of IL-6 and IL-10 in depression have garnered recent attention with the recognition of human populations suffering from major depression displaying commensurate increases in IL-6 and decreases in IL-10 [15], [16], providing direct biological correlation with this animal model. While elevated IL-6 is itself reported as a biomarker of depression [7], [8], [69], animal studies show the behavioral effects of IL-6 alone to be inconsistent. IL-6 stimulates the murine HPA axis [70] and peripheral administration increases brain tryptophan levels and elevates serotonin metabolism [55], together providing a rationale for depressive behavioral modifications. However, reports have also shown that peripheral IL-6 treatment increases exploratory and locomotive behavior in mice [71] without affecting feeding [72] or reward response [73], and intracerebroventricular IL-6 has alternately resulted in decreased locomotion and reward response [74] or no change in locomotive and social investigatory behavior in rodents [13]. Further, IL-6 KO mice show no difference in depressive-like behavior as measured by tail suspension or forced swim tests [55]. Taken together, this indicates that while increased circulating IL-6 as described here may affect behavior (Fig. 5), altered IL-6 expression cannot alone account for depressive symptoms. The inflammatory status of the cortex and hippocampus are directly implicated in mood disorders and major depression [75]–[77] and while no intracranial elevation in IL-6 was observed here, IL-10 expression was reduced in the cortex and hippocampus of stressed animals both throughout and following the stress period (Fig. 6). Despite suppression of anti-inflammatory IL-10, no corresponding broad increase in pro-inflammatory markers was recorded. This is in line with previous work demonstrating decreased IL-10 expression and increased IL-6/IL-10 ratios in the cortex of rats exposed to an extended unpredictable chronic mild restrain protocol and [78]. This also provides context for the restraint stress model when considering studies showing the inability of 28 days of psychosocial stress to elicit changes in IL-6 or IL-10 mRNA expression in the mouse brain [79].
Whereas acute psychological stress has shown either no effect on circulating IL-10 levels in mice [80] or increased IL-10 levels in rats [81], in line with clinical studies of major depression [15], [16] here chronic restraint stress resulted in diminished circulating IL-10 throughout stress period and for two weeks afterward. These findings describe a systemic peripheral and central suppression of IL-10 both during and following the restraint period. There has been little work describing a role for IL-4 in depressive disorders and decreases in circulating IL-4 observed here may correspond to a more generalized suppression of anti-inflammatory cytokines in response to sustained restraint stress rather than specific features of depressive response. Together, this suggests that depressive behavior may be influenced not only by elevations in pro-inflammatory cytokines, but also independently affected by suppression of anti-inflammatory cytokines. Following from the observations of decreased IL-10 expression and increased measures of depressive-like behavior induced by RST, we found that depressive-like behavior did not develop in mice treated with recombinant murine IL-10 (fig. 7) though further work is require to elucidate the full mechanism. Traditional cytokine-based theories of depression place great emphasis on the role of inflammatory cytokine elevations in depressive illness, though important work suggests that IL-10 itself plays a direct role in affecting depressive behavior. Numerous clinical studies show that multiple classes of effective antidepressants elevate circulating IL-10 from pre-treatment levels upon successful therapy, though no direct mechanism is described [26]–[29], [82]. IL-10 ameliorates LPS-induced sickness behavior in rodents [83] while IL-10-deficient mice show increased fatigue and motor deficits following LPS exposure [84]. Transgenic studies using IL-10 knockout mice show that an absence of IL-10 expression results in constitutively depressive behavior (as measured by FST) and that treatment with recombinant IL-10 can ameliorate these behaviors [24], [25]. Further, IL-10 overexpression decreases measures of depressive behavior in mice in FST assessments and exogenous IL-10 treatment amplifies physical activity and measures of exploratory behavior in wild-type mice [23]–[25]. In this work we observed that chronic restraint stress induced a measure of depressive-like behavior (Fig. 4) and decreased expression of IL-10 in the brain and in the periphery, both during and in the weeks following restraint (Fig. 5 & 5) and further work will include additional examinations of depressive-like behavior such as sucrose-preference tests. FST has been frequently used in assessing antidepressant efficacy, though many recent studies have included it as an independent assessment of depressive behavior [85]–[87]. Whereas IL-6 levels normalized following stress cessation, this pattern of sustained IL-10 suppression corresponded with sustained measures of depressive-like behavior. Moreover, while IL-6 shows inconsistent ability to modify depressive behavior, behavioral deficits brought about by RST were reversible by treatment with recombinant IL-10, thereby highlighting the role of IL-10 in directly affecting behavior. This demonstrates that while depressive disorders may frequently display comorbidity with elevations in pro-inflammatory markers, depressive behavior itself may be attributable to suppression of anti-inflammatory cytokines rather than increases in pro-inflammatory cytokines.
Supporting Information
Sample size examined for each data point.
(TIF)
Funding Statement
This work was supported by National Institutes of Health (NIH) T32 HL07946, NIH R01 HL067176, NIH R01 HL102464, and startup funds from TDE. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Mathers CD, Loncar D (2006) Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 3: e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, et al. (2007) Depression, chronic diseases, and decrements in health: Results from the world health surveys. Lancet 370: 851–858. [DOI] [PubMed] [Google Scholar]
- 3. Dantzer R, O’Connor JC, Lawson MA, Kelley KW (2011) Inflammation-associated depression: From serotonin to kynurenine. Psychoneuroendocrinology 36: 426–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Miller AH (2009) Norman cousins lecture. mechanisms of cytokine-induced behavioral changes: Psychoneuroimmunology at the translational interface. Brain Behav Immun 23: 149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Raedler TJ (2011) Inflammatory mechanisms in major depressive disorder. Curr Opin Psychiatry 24: 519–525. [DOI] [PubMed] [Google Scholar]
- 6. Raison CL, Capuron L, Miller AH (2006) Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends Immunol 27: 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, et al. (2010) A meta-analysis of cytokines in major depression. Biol Psychiatry 67: 446–457. [DOI] [PubMed] [Google Scholar]
- 8. Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, et al. (2001) The relationship of depression and stressors to immunological assays: A meta-analytic review. Brain Behav Immun 15: 199–226. [DOI] [PubMed] [Google Scholar]
- 9. Lanquillon S, Krieg JC, Bening-Abu-Shach U, Vedder H (2000) Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology 22: 370–379. [DOI] [PubMed] [Google Scholar]
- 10. Maes M, Bosmans E, De Jongh R, Kenis G, Vandoolaeghe E, et al. (1997) Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine 9: 853–858. [DOI] [PubMed] [Google Scholar]
- 11. Sakic B, Szechtman H, Braciak T, Richards C, Gauldie J, et al. (1997) Reduced preference for sucrose in autoimmune mice: A possible role of interleukin-6. Brain Res Bull 44: 155–165. [DOI] [PubMed] [Google Scholar]
- 12. Sakic B, Gauldie J, Denburg JA, Szechtman H (2001) Behavioral effects of infection with IL-6 adenovector. Brain Behav Immun 15: 25–42. [DOI] [PubMed] [Google Scholar]
- 13. Lenczowski MJ, Bluthe RM, Roth J, Rees GS, Rushforth DA, et al. (1999) Central administration of rat IL-6 induces HPA activation and fever but not sickness behavior in rats. Am J Physiol 276: R652–8. [DOI] [PubMed] [Google Scholar]
- 14. Zalcman S, Murray L, Dyck DG, Greenberg AH, Nance DM (1998) Interleukin-2 and -6 induce behavioral-activating effects in mice. Brain Res 811: 111–121. [DOI] [PubMed] [Google Scholar]
- 15. Blume J, Douglas SD, Evans DL (2011) Immune suppression and immune activation in depression. Brain Behav Immun 25: 221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dhabhar FS, Burke HM, Epel ES, Mellon SH, Rosser R, et al. (2009) Low serum IL-10 concentrations and loss of regulatory association between IL-6 and IL-10 in adults with major depression. J Psychiatr Res 43: 962–969. [DOI] [PubMed] [Google Scholar]
- 17. Kiecolt-Glaser JK, Glaser R (2002) Depression and immune function: Central pathways to morbidity and mortality. J Psychosom Res 53: 873–876. [DOI] [PubMed] [Google Scholar]
- 18. O’Brien SM, Scully P, Fitzgerald P, Scott LV, Dinan TG (2007) Plasma cytokine profiles in depressed patients who fail to respond to selective serotonin reuptake inhibitor therapy. J Psychiatr Res 41: 326–331. [DOI] [PubMed] [Google Scholar]
- 19. Koldzic-Zivanovic N, Tu H, Juelich TL, Rady PL, Tyring SK, et al. (2006) Regulation of adrenal glucocorticoid synthesis by interleukin-10: A preponderance of IL-10 receptor in the adrenal zona fasciculata. Brain Behav Immun 20: 460–468. [DOI] [PubMed] [Google Scholar]
- 20. Raison CL, Miller AH (2003) When not enough is too much: The role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry 160: 1554–1565. [DOI] [PubMed] [Google Scholar]
- 21. Smith EM, Cadet P, Stefano GB, Opp MR, Hughes TK (1999) Jr (1999) IL-10 as a mediator in the HPA axis and brain. J Neuroimmunol 100: 140–148. [DOI] [PubMed] [Google Scholar]
- 22. Tu H, Rady PL, Juelich T, Tyring SK, Koldzic-Zivanovic N, et al. (2007) Interleukin-10 regulated gene expression in cells of hypothalamic-pituitary-adrenal axis origin. Cell Mol Neurobiol 27: 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harvey D, Smith R, English K, Mahon B, Commins S (2006) Interleukin-10 (IL-10) but not lipopolysaccharide (LPS) produces increased motor activity and abnormal exploratory patterns while impairing spatial learning in Balb/c mice. Physiol Behav 87: 842–847. [DOI] [PubMed] [Google Scholar]
- 24. Mesquita AR, Correia-Neves M, Roque S, Castro AG, Vieira P, et al. (2008) IL-10 modulates depressive-like behavior. J Psychiatr Res 43: 89–97. [DOI] [PubMed] [Google Scholar]
- 25. Roque S, Correia-Neves M, Mesquita AR, Palha JA, Sousa N (2009) Interleukin-10: A key cytokine in depression? Cardiovasc Psychiatry Neurol 2009: 187894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kubera M, Maes M, Holan V, Basta-Kaim A, Roman A, et al. (2001) Prolonged desipramine treatment increases the production of interleukin-10, an anti-inflammatory cytokine, in C57BL/6 mice subjected to the chronic mild stress model of depression. J Affect Disord 63: 171–178. [DOI] [PubMed] [Google Scholar]
- 27. Kubera M, Lin AH, Kenis G, Bosmans E, van Bockstaele D, et al. (2001) Anti-inflammatory effects of antidepressants through suppression of the interferon-gamma/interleukin-10 production ratio. J Clin Psychopharmacol 21: 199–206. [DOI] [PubMed] [Google Scholar]
- 28. Kenis G, Maes M (2002) Effects of antidepressants on the production of cytokines. Int J Neuropsychopharmacol 5: 401–412. [DOI] [PubMed] [Google Scholar]
- 29. Maes M, Song C, Lin AH, Bonaccorso S, Kenis G, et al. (1999) Negative immunoregulatory effects of antidepressants: Inhibition of interferon-gamma and stimulation of interleukin-10 secretion. Neuropsychopharmacology 20: 370–379. [DOI] [PubMed] [Google Scholar]
- 30. Selye H (1936) The alarm reaction. Can Med Assoc J 34: 704. [Google Scholar]
- 31. Keim KL, Sigg EB (1977) Plasma corticosterone and brain catecholamines in stress: Effect of psychotropic drugs. Pharmacol Biochem Behav 6: 79–85. [DOI] [PubMed] [Google Scholar]
- 32. Boyle MP, Brewer JA, Funatsu M, Wozniak DF, Tsien JZ, et al. (2005) Acquired deficit of forebrain glucocorticoid receptor produces depression-like changes in adrenal axis regulation and behavior. Proc Natl Acad Sci U S A 102: 473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW (2008) From inflammation to sickness and depression: When the immune system subjugates the brain. Nat Rev Neurosci 9: 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lawson MA, Kelley KW, Dantzer R (2011) Intracerebroventricular administration of HIV-1 tat induces brain cytokine and indoleamine 2,3-dioxygenase expression: A possible mechanism for AIDS comorbid depression. Brain Behav Immun 25: 1569–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Maccari S, Morley-Fletcher S (2007) Effects of prenatal restraint stress on the hypothalamus-pituitary-adrenal axis and related behavioural and neurobiological alterations. Psychoneuroendocrinology 32 Suppl 1S10–5. [DOI] [PubMed] [Google Scholar]
- 36.Solomon MB, Furay AR, Jones K, Packard AE, Packard BA, et al.. (2011) Deletion of forebrain glucocorticoid receptors impairs neuroendocrine stress responses and induces depression-like behavior in males but not females. Neuroscience. [DOI] [PMC free article] [PubMed]
- 37. Buynitsky T, Mostofsky DI (2009) Restraint stress in biobehavioral research: Recent developments. Neurosci Biobehav Rev 33: 1089–1098. [DOI] [PubMed] [Google Scholar]
- 38. Harris RB, Mitchell TD, Simpson J, Redmann SM, Jr, Youngblood BD, et al. (2002) Weight loss in rats exposed to repeated acute restraint stress is independent of energy or leptin status. Am J Physiol Regul Integr Comp Physiol 282: R77–88. [DOI] [PubMed] [Google Scholar]
- 39. Houshyar H, Cooper ZD, Woods JH (2001) Paradoxical effects of chronic morphine treatment on the temperature and pituitary-adrenal responses to acute restraint stress: A chronic stress paradigm. J Neuroendocrinol 13: 862–874. [DOI] [PubMed] [Google Scholar]
- 40. Jankord R, Herman JP (2008) Limbic regulation of hypothalamo-pituitary-adrenocortical function during acute and chronic stress. Ann N Y Acad Sci 1148: 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mohawk JA, Lee TM (2005) Restraint stress delays reentrainment in male and female diurnal and nocturnal rodents. J Biol Rhythms 20: 245–256. [DOI] [PubMed] [Google Scholar]
- 42. Delgado-Morales R, del Rio E, Gomez-Roman A, Bisagno V, Nadal R, et al. (2012) Adrenocortical and behavioural response to chronic restraint stress in neurokinin-1 receptor knockout mice. Physiol Behav 105: 669–675. [DOI] [PubMed] [Google Scholar]
- 43. Lamkin DM, Sloan EK, Patel AJ, Chiang BS, Pimentel MA, et al. (2012) Chronic stress enhances progression of acute lymphoblastic leukemia via beta-adrenergic signaling. Brain Behav Immun 26: 635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yu H, Wang DD, Wang Y, Liu T, Lee FS, et al. (2012) Variant brain-derived neurotrophic factor Val66Met polymorphism alters vulnerability to stress and response to antidepressants. J Neurosci 32: 4092–4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhao TJ, Liang G, Li RL, Xie X, Sleeman MW, et al. (2010) Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc Natl Acad Sci U S A 107: 7467–7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. El Bouhassani M, Gilibert S, Moreau M, Saint-Charles F, Treguier M, et al. (2011) Cholesteryl ester transfer protein expression partially attenuates the adverse effects of SR-BI receptor deficiency on cholesterol metabolism and atherosclerosis. J Biol Chem 286: 17227–17238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bowers SL, Bilbo SD, Dhabhar FS, Nelson RJ (2008) Stressor-specific alterations in corticosterone and immune responses in mice. Brain Behav Immun 22: 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Godbout JP, Moreau M, Lestage J, Chen J, Sparkman NL, et al. (2008) Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharmacology 33: 2341–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kinsey SG, Bailey MT, Sheridan JF, Padgett DA, Avitsur R (2007) Repeated social defeat causes increased anxiety-like behavior and alters splenocyte function in C57BL/6 and CD-1 mice. Brain Behav Immun 21: 458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Porsolt RD, Le Pichon M, Jalfre M (1977) Depression: A new animal model sensitive to antidepressant treatments. Nature 266: 730–732. [DOI] [PubMed] [Google Scholar]
- 51. Porsolt RD (2000) Animal models of depression: Utility for transgenic research. Rev Neurosci 11: 53–58. [DOI] [PubMed] [Google Scholar]
- 52. Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, et al. (2011) Beta-adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci 31: 6277–6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Holm S (1979) A simple sequentially rejective multiple ATest procedure. Scandinavian Journal of Statistics 6: 65–70. [Google Scholar]
- 54. Mozhui K, Karlsson RM, Kash TL, Ihne J, Norcross M, et al. (2010) Strain differences in stress responsivity are associated with divergent amygdala gene expression and glutamate-mediated neuronal excitability. J Neurosci 30: 5357–5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Swiergiel AH, Dunn AJ (2006) Feeding, exploratory, anxiety- and depression-related behaviors are not altered in interleukin-6-deficient male mice. Behav Brain Res 171: 94–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Carlezon WA, Pliakas AM, Parow AM, Detke MJ, Cohen BM, et al. (2002) Antidepressant-like effects of cytidine in the forced swim test in rats. Biol Psychiatry 51: 882–889. [DOI] [PubMed] [Google Scholar]
- 57. Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, et al. (2001) Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J Neurosci 21: 7397–7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Liu J, Halene S, Yang M, Iqbal J, Yang R, et al. (2012) Gaucher disease gene GBA functions in immune regulation. Proc Natl Acad Sci U S A 109: 10018–10023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhou Q, Leeman SE, Amar S (2011) Signaling mechanisms in the restoration of impaired immune function due to diet-induced obesity. Proc Natl Acad Sci U S A 108: 2867–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mendez-Ferrer S, Lucas D, Battista M, Frenette PS (2008) Haematopoietic stem cell release is regulated by circadian oscillations. Nature 452: 442–447. [DOI] [PubMed] [Google Scholar]
- 61. Leitch MM, Ingram CD, Young AH, McQuade R, Gartside SE (2003) Flattening the corticosterone rhythm attenuates 5-HT1A autoreceptor function in the rat: Relevance for depression. Neuropsychopharmacology 28: 119–125. [DOI] [PubMed] [Google Scholar]
- 62. Windle RJ, Wood SA, Shanks N, Lightman SL, Ingram CD (1998) Ultradian rhythm of basal corticosterone release in the female rat: Dynamic interaction with the response to acute stress. Endocrinology 139: 443–450. [DOI] [PubMed] [Google Scholar]
- 63. Sheridan JF, Feng NG, Bonneau RH, Allen CM, Huneycutt BS, et al. (1991) Restraint stress differentially affects anti-viral cellular and humoral immune responses in mice. J Neuroimmunol 31: 245–255. [DOI] [PubMed] [Google Scholar]
- 64. Campbell T, Meagher MW, Sieve A, Scott B, Storts R, et al. (2001) The effects of restraint stress on the neuropathogenesis of theiler’s virus infection: I. acute disease. Brain Behav Immun 15: 235–254. [DOI] [PubMed] [Google Scholar]
- 65. Wang Y, Lu Y, Yu D, Wang Y, Chen F, et al. (2008) Enhanced resistance of restraint-stressed mice to sepsis. J Immunol 181: 3441–3448. [DOI] [PubMed] [Google Scholar]
- 66. Zelena D, Mergl Z, Foldes A, Kovacs KJ, Toth Z, et al. (2003) Role of hypothalamic inputs in maintaining pituitary-adrenal responsiveness in repeated restraint. Am J Physiol Endocrinol Metab 285: E1110–7. [DOI] [PubMed] [Google Scholar]
- 67. Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA (2011) Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 476: 458–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Swiergiel AH, Leskov IL, Dunn AJ (2008) Effects of chronic and acute stressors and CRF on depression-like behavior in mice. Behav Brain Res 186: 32–40. [DOI] [PubMed] [Google Scholar]
- 69. Alesci S, Martinez PE, Kelkar S, Ilias I, Ronsaville DS, et al. (2005) Major depression is associated with significant diurnal elevations in plasma interleukin-6 levels, a shift of its circadian rhythm, and loss of physiological complexity in its secretion: Clinical implications. J Clin Endocrinol Metab 90: 2522–2530. [DOI] [PubMed] [Google Scholar]
- 70. Wang J, Dunn AJ (1998) Mouse interleukin-6 stimulates the HPA axis and increases brain tryptophan and serotonin metabolism. Neurochem Int 33: 143–154. [DOI] [PubMed] [Google Scholar]
- 71. Zalcman S, Green-Johnson JM, Murray L, Nance DM, Dyck D, et al. (1994) Cytokine-specific central monoamine alterations induced by interleukin-1, -2 and -6. Brain Res 643: 40–49. [DOI] [PubMed] [Google Scholar]
- 72. Swiergiel AH, Dunn AJ (1999) The roles of IL-1, IL-6, and TNFalpha in the feeding responses to endotoxin and influenza virus infection in mice. Brain Behav Immun 13: 252–265. [DOI] [PubMed] [Google Scholar]
- 73. Anisman H, Kokkinidis L, Borowski T, Merali Z (1998) Differential effects of interleukin (IL)-1beta, IL-2 and IL-6 on responding for rewarding lateral hypothalamic stimulation. Brain Res 779: 177–187. [DOI] [PubMed] [Google Scholar]
- 74. Schobitz B, Pezeshki G, Pohl T, Hemmann U, Heinrich PC, et al. (1995) Soluble interleukin-6 (IL-6) receptor augments central effects of IL-6 in vivo. FASEB J 9: 659–664. [DOI] [PubMed] [Google Scholar]
- 75. Khairova RA, Machado-Vieira R, Du J, Manji HK (2009) A potential role for pro-inflammatory cytokines in regulating synaptic plasticity in major depressive disorder. Int J Neuropsychopharmacol 12: 561–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Loftis JM, Huckans M, Morasco BJ (2010) Neuroimmune mechanisms of cytokine-induced depression: Current theories and novel treatment strategies. Neurobiol Dis 37: 519–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Shelton RC, Claiborne J, Sidoryk-Wegrzynowicz M, Reddy R, Aschner M, et al. (2011) Altered expression of genes involved in inflammation and apoptosis in frontal cortex in major depression. Mol Psychiatry 16: 751–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. You Z, Luo C, Zhang W, Chen Y, He J, et al. (2011) Pro- and anti-inflammatory cytokines expression in rat’s brain and spleen exposed to chronic mild stress: Involvement in depression. Behav Brain Res 225: 135–141. [DOI] [PubMed] [Google Scholar]
- 79. Bartolomucci A, Palanza P, Parmigiani S, Pederzani T, Merlot E, et al. (2003) Chronic psychosocial stress down-regulates central cytokines mRNA. Brain Res Bull 62: 173–178. [DOI] [PubMed] [Google Scholar]
- 80. Curtin NM, Mills KH, Connor TJ (2009) Psychological stress increases expression of IL-10 and its homolog IL-19 via beta-adrenoceptor activation: Reversal by the anxiolytic chlordiazepoxide. Brain Behav Immun 23: 371–379. [DOI] [PubMed] [Google Scholar]
- 81. Connor TJ, Brewer C, Kelly JP, Harkin A (2005) Acute stress suppresses pro-inflammatory cytokines TNF-alpha and IL-1 beta independent of a catecholamine-driven increase in IL-10 production. J Neuroimmunol 159: 119–128. [DOI] [PubMed] [Google Scholar]
- 82. O’Brien SM, Scott LV, Dinan TG (2004) Cytokines: Abnormalities in major depression and implications for pharmacological treatment. Hum Psychopharmacol 19: 397–403. [DOI] [PubMed] [Google Scholar]
- 83. Bluthe RM, Castanon N, Pousset F, Bristow A, Ball C, et al. (1999) Central injection of IL-10 antagonizes the behavioural effects of lipopolysaccharide in rats. Psychoneuroendocrinology 24: 301–311. [DOI] [PubMed] [Google Scholar]
- 84. Krzyszton CP, Sparkman NL, Grant RW, Buchanan JB, Broussard SR, et al. (2008) Exacerbated fatigue and motor deficits in interleukin-10-deficient mice after peripheral immune stimulation. Am J Physiol Regul Integr Comp Physiol 295: R1109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kim SJ, Lee H, Lee G, Oh SJ, Shin MK, et al. (2012) CD4+CD25+ regulatory T cell depletion modulates anxiety and depression-like behaviors in mice. PLoS One 7: e42054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lundh SH, Soylu R, Petersen A (2012) Expression of mutant huntingtin in leptin receptor-expressing neurons does not control the metabolic and psychiatric phenotype of the BACHD mouse. PLoS One 7: e51168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Walker TL, Vukovic J, Koudijs MM, Blackmore DG, Mackay EW, et al. (2012) Prolactin stimulates precursor cells in the adult mouse hippocampus. PLoS One 7: e44371. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sample size examined for each data point.
(TIF)