Abstract
It is well known that genotypic differences can account for the subject-specific responses to opiate administration. In this regard, the basal activity of the endogenous system (either at the receptor or ligand level) can modulate the effects of exogenous agonists as morphine and vice versa. The μ opioid receptor from zebrafish, dre-oprm1, binds endogenous peptides and morphine with similar affinities. Morphine administration during development altered the expression of the endogenous opioid propeptides proenkephalins and proopiomelanocortin. Treatment with opioid peptides (Met-enkephalin [Met-ENK], Met-enkephalin-Gly-Tyr [MEGY] and β-endorphin [β-END]) modulated dre-oprm1 expression during development. Knocking down the dre-oprm1 gene significantly modified the mRNA expression of the penk and pomc genes, thus indicating that oprm1 is involved in shaping penk and pomc expression. In addition, the absence of a functional oprm1 clearly disrupted the embryonic development, since proliferation was disorganized in the central nervous system of oprm1-morphant embryos: mitotic cells were found widespread through the optic tectum and were not restricted to the proliferative areas of the mid- and hindbrain. Transferase-mediated dUTP nick-end labeling (TUNEL) staining revealed that the number of apoptotic cells in the central nervous system (CNS) of morphants was clearly increased at 24-h postfertilization. These findings clarify the role of the endogenous opioid system in CNS development. Our results will also help unravel the complex feedback loops that modulate opioid activity and that may be involved in establishing a coordinated expression of both receptors and endogenous ligands. Further knowledge of the complex interactions between the opioid system and analgesic drugs will provide insights that may be relevant for analgesic therapy.
INTRODUCTION
Opiates elicit their pharmacological actions by activating the endogenous opioid receptors oprm1 (μ), oprd1 (δ) and oprk1 (κ). The endogenous ligands of these receptors are of peptidic nature and are derived from three different precursors (Table 1), namely proenkephalin (PENK) (1,2), proopiomelanocortin (POMC) (3) and prodynorphin (PDYN). When the larger propeptides are cleaved, they give rise to the active ligands, which are either the pentapeptides, Met-enkephalin (Met-ENK: YGGFM) and Leu-enkephalin (Leu-ENK: YGGFL), or longer peptides that contain an N-terminal Met-ENK, such as β-endorphin (β-END), or an N-terminal Leu-ENK sequence, as for example dynorphin A (DYN A) (for a complete review of the endogenous opioid system, please refer to the study by Corbett et al. [4]). The endogenous opioid ligands do not show high selectivity toward one of the three receptor types, although Met-ENK–derived peptides and β-END display higher affinities toward μ and δ receptors and dynorphin-derived peptides toward κ receptor (5).
Table 1.
Proenkephalin and proopiomelanocortin opioid precursors.
| Precursor | Opioid peptides | |
|---|---|---|
| Proenkephalin (hsa-penk) (1,2) | 4 Met-ENK (ME) | YGGFM |
| 1 Leu-ENK (LE) | YGGFL | |
| 1 Met-ENK-Arg6-Phe7 (MERF) | YGGFMRF | |
| 1 Met-ENK-Arg6-Gly7-Leu8 | (MERGL)YGGFMRGL | |
| Proenkephalin b (dre-penk b) (18) | 4 Met-ENK (ME) | YGGFM |
| 1 Leu-ENK (LE) | YGGFL | |
| 1 Met-ENK-Gly6-Tyr7 (MEGY) | YGGFMGY | |
| Proenkephalin a (dre-penk a) (18) | 4 Met-ENK (ME) | YGGFM |
| 1 Met-ENK-Ile6 (MEI) | YGGFMI | |
| 1 Met-ENK-Asp6 (MED) | YGGFMD | |
| Proopiomelanocortin (hsa-pomc) (3) | β-END (1–31) | YGGFMTSEKSQTPLVTLFKNAIIKNAYKKGE |
| Proopiomelanocortin a (dre-pomc a) (19) | β-END (1–32) | YGGFMKSWDERAQKPLLTLFKVMHKDQPRKDE |
| Proopiomelanocortin b (dre-pomc b) (19) | β-END (1–32) | YGGLMRPYLDESHKPLITLIRNAIGNGQQFTD |
Different opioid peptides that are encoded by the PENK and POMC propeptides in human and zebrafish are shown.
The opiate morphine still remains the drug of choice to treat moderate to severe pain, despite its adverse side effects, such as tolerance and dependence. Morphine elicits its analgesic actions mainly by acting on the μ opioid receptor (6,7), although the δ receptor can play a role in the instatement of morphine tolerance (8). However, the effects of opiates are not restricted to eliciting analgesia or to causing addiction, since they can have broader effects during development. It was previously shown that opioid antagonists such as naltrexone impair central nervous system (CNS) development, since they affect dendritic spine formation, possibly by disrupting trophic signals (9). Met-ENK treatment can inhibit cell proliferation of the cerebellar external germinal cells, and this effect can be blocked by the opioid antagonist naloxone (10). In addition, in utero opioid exposure can cause neonatal abstinence syndrome (11) in the newborn and in the infant (12), which indicates that opiates may be responsible for long-term CNS dysfunction during development.
There is also evidence that opiates may affect cell proliferation and differentiation into neural or glial cell types in the CNS. For example, the presence of functional μ and κ receptors in mouse embryonic stem cells and neural progenitors was recently reported (13,14). The selective agonists [D-Ala2, NMe-Phe4, Gly-ol5]-enkephalin (DAMGO) (μ-selective) and U69,593 (κ-selective) promote cell proliferation of stem cells as well as induce stem cells and neural progenitors to differentiate via ERK activation (13). DAMGO and U69,593 can also modulate the terminal differentiation of neural precursors, since they inhibited the differentiation of neural precursors to neurons and astrocytes, but stimulated the differentiation to oligodendrocytes (14). δ Receptors are related to neurogenesis and neuroprotection, and these effects can be mediated by tyrosine kinase (TRK) receptors (15).
Our group has characterized the opioid system in the zebrafish (for a broader revision, please refer to the study by Gonzalez-Nunez and Rodriguez [16]). The μ opioid receptor from zebrafish (dre-μ) displays a similar pharmacological profile to mammalian μ receptors (17). Also, we found six opioid precursors: two of them are proenkephalins (penk) and another two are proopiome-lanocortins (pomc) (Table 1) (16). dre-penk a (formerly zfPENK-like) codes for four Met-ENKs, one Met-enkephalin-Ile (MEI) and one Met-enkephalin-Asp (MED), although the fourth core Met-ENK does not conserve one of the proteolytic cleavage motifs (18). dre-penk b (formerly zf-PENK) contains six peptides: four Met-ENKs, one Leu-ENK and one Met-enkephalin-Gly-Tyr (MEGY). dre-pomc a (formerly zfPOMC) contains the consensus sequences for adenocorti-cotropin (ACTH), γ-lipotropin (γ-LPH), β-melanotropin (β-MSH) and β-END. In dre-pomc b (formerly zfPOMC-like), only the α-MSH and β-END are considered end products, since some of the prote-olytic cleavage sites for ACTH, β-LPH and γ-LPH are not conserved. In addition, β-END displays a rather degenerate sequence (19). The pharmacological profile of these endogenous peptides was studied for both δ receptors (dre-oprd1a and dre-oprd1b) (20) and for the κ receptor (21), but the biological effects of the endogenous ligands on dre-μ have not yet been assessed.
In this work, we have established the binding affinities of opioid peptides on dre-μ as a prior step to study the complex interactions of dre-μ and its endogenous ligands during development. We have determined that opioid peptides modulate dre-oprm1 expression during development, and likewise, dre-oprm1 is involved in shaping opioid propeptide expression. In addition, morphine exposure alters the expression of the endogenous opioid propeptides. The in vivo results that we present here might help in understanding the influence of the endogenous opioid system in the modulation of CNS development.
MATERIALS AND METHODS
Drugs and Radioligands
Morphine hydrochloride was provided by the Spanish Ministry of Health. Naloxone was purchased from Sigma-Aldrich (St. Louis, MO, USA); Met-ENK, Leu-ENK and β-END from Bachem (Weil am Rhein, Germany); and [3H]-diprenorphine (50 Ci/mmol) from PerkinElmer (Boston, MA, USA). Unlabelled MEGY and its two analogs (d-Ala2)-MEGY (Tyr-d-Ala-GlyPhe-Met-Gly-Tyr) and (d-Ala2, Val5)-MEGY (Tyr-d-Ala-Gly-Phe-Val-Gly-Tyr) were synthesized as trifluoroacetic derivatives by G Arsequell and G Valencia at the Consejo Superior de Investigaciones Científicas (Barcelona, Spain). All other reagents used were from analytical grade.
Zebrafish Maintenance, Breeding and Drug Treatments
The experiments were performed using the wild-type AB zebrafish line. General maintenance and care of fish were carried out according to standard protocols in our own zebrafish facility. Animals were maintained at a constant temperature of 28°C in a 14-h light cycle and fed three times a day. Embryos were obtained by natural mating and cultured in E3 medium, with or without the addition of 0.003% 1-phenyl-2-thiourea (PTU) (Sigma-Aldrich) to inhibit pigmentation. Embryos were staged according to hours postfertilization (hpf) (22). In all experiments, adequate measures were taken to minimize pain or discomfort, and animals were handled according to the guidelines of the European Communities Council Directive 2010/63/UE (23), to the current Spanish legislation for the use and care of animals RD 1201/2005 (24) and to the Guide for the Care and Use of Laboratory Animals, as adopted and promulgated by the U.S. National Institutes of Health (25).
Zebrafish embryos were exposed to 10 nmol/L morphine or 10 μmol/L opioid peptides (Met-ENK, MEGY and β-END) in E3 embryo buffer with protease inhibitors (0.1 mg/mL bacitracin, 3.3 μmol/L captopril and protease inhibitor cocktail; from Sigma-Aldrich) from 5 to 24 hpf and to 48 hpf. In the latter case, medium was changed and fresh peptide solution was added at 24 hpf. Two control groups (E3 and E3 only with protease inhibitors) were performed in parallel. We have chosen these time points (24 and 48 hpf) because they stand for two key stages in embryonic development: at 24 hpf, the development on the CNS is finished, and the organogenesis concludes at 48 hpf.
Cell Culture and Membrane Preparation
Stably transfected HEK293 cells expressing the μ receptor from zebrafish were maintained in Dulbecco’s modified Eagle medium supplemented with 10% (v/v) fetal calf serum, 2 mmol/L glutamine, 100 U/mL penicillin, 0.1 mg/mL streptomycin and 250 μg/mL Geneticin (G-418) (all from Gibco; Life Technologies, Carlsbad, CA, USA), at 37ºC in a humidified atmosphere containing 5% (v/v) CO2 in a Forma incubator.
Cells were grown to 80% confluence, harvested in phosphate-buffered saline (pH 7.4) containing 2 mmol/L EDTA and collected by centrifugation at 500g. The cell pellets were frozen at −80ºC for at least 1 h and resuspended in 50 mmol/L Tris HCl buffer (pH 7.4) (assay buffer) with protease inhibitors (0.1 mg/mL bacitracin, 3.3 μmol/L captopril and protease inhibitor cocktail; from Sigma-Aldrich). Cell suspensions were homogenized with a Potter-Elvehjem tissue grinder in assay buffer, and the homogenates were centrifuged at 500g for 10 min at 4ºC. The nuclear pellet was homogenized again, centrifuged and discarded. The two supernatants were combined and homogenized again with the tissue grinder, and the membrane pellet was collected upon centrifugation at 18,000g for 30 min at 4ºC. The crude membrane fraction was resuspended in ice-cold assay buffer with protease inhibitors, and protein concentration was determined by Bradford (Bio-Rad Laboratories, Alcobendas, Madrid, Spain).
Competition Binding Assays and Data Analysis
The following unlabeled ligands were used: Met-ENK, Leu-ENK, β-END, MEGY, (d-Ala2)-MEGY, (d-Ala2, Val5)-MEGY, naloxone and morphine. Radioligand binding was performed as previously described (20). Briefly, 10 μg protein were incubated with different concentrations of unlabeled ligand ranging from 0.3 nmol/L to 10 μmol/L and using [3H]-diprenorphine as radioligand (the working concentration was similar to the affinity constant, KD = 1 nmol/L). Reactions were incubated for 1 h at 25ºC in a final volume of 250 μL assay buffer, and 10 μmol/L naloxone was used to determine nonspecific binding. After incubation, the reaction was stopped by adding 4 mL ice-cold 50 mmol/L Tris HCl buffer (pH 7.4); the mixture was rapidly filtrated by using a Brandel Cell Harvester and washed two times onto GF/B glass-fiber filters that were presoaked with 0.2% (v/v) polyethylenimine for at least 1 h. The filters were placed in scintillation vials and incubated overnight at room temperature in EcoScint A scintillation liquid (EcoScint A, London, UK). Radioactivity was counted by using a Beckman Coulter 6500 scintillation counter (Pasadena, CA, USA). All experiments were performed in triplicate and repeated three times.
Specific binding was defined as the difference between total binding and nonspecific binding, as measured in the presence of 10 μmol/L naloxone. Radioligand binding data were analyzed by computer-assisted nonlinear regression analysis by using GraphPad Prism software (San Diego, CA, USA), and inhibition constants (Ki) were obtained for each ligand using the Cheng–Prusoff equation, which corrects for the concentration of radioligand used in each experiment as well as for the affinity of the radioligand for its binding site (KD) (26).
Morpholino Antinucleotide Injections: Knockdown Assays at One-Cell Stage
The translation blocking antisense morpholino AATGTTGCCAGTG TTTTCCATCATG complementary to the region from −1 to +24 of the dre-oprm1 open reading frame (ORF) was purchased from GeneTools LLC and was microinjected at the one-cell stage at a concentration of 0.33 mmol/L. As control, the standard control morpholino (also available from GeneTools) was injected at the same concentration.
In Situ Hybridization
The open reading frames of dre-penk a, dre-penk b, dre-pomc a and dre-oprm1 were amplified by reverse transcriptase (RT)–polymerase chain reaction (PCR) and subcloned in pCR®4-TOPO® vector (Invitrogen; Life Technologies) by using standard methodology. To confirm the sequences of the cloned inserts, DNA sequencing was performed by the Sequencing Facility (Servicio de Secuenciación, Universidad de Salamanca). These clones were used to generate the corresponding in situ riboprobes, which were transcribed from their templates by using the DIG RNA Labeling Kit (SP6/T7) (Roche Applied Science, Sant Cugat del Vallès, Spain), following the manufacturer’s instructions. In situ hybridizations were performed on whole-mounts as previously described (27), and probes were detected by using BM purple or NBT/BCIP reagents (Roche Applied Science). Control experiments were always performed in parallel. Images were taken with a Leica M165FC Stereomicroscope.
Immunohistochemistry
Immunohistochemical studies were performed on whole-mounts and analyzed with an Olympus AX70 fluorescence microscope or with a Leica M165FC Stere-omicroscope. The rabbit anti-histone H3 (phospho Ser10) (Abcam, Cambridge, UK) was used as primary antibody and Alexa Fluor 594–conjugated anti-rabbit (Molecular Probes; Life Technologies) as secondary antibody. Cell nuclei were counterstained with 4,6-diamidino-2-phenylindole (DAPI) (Roche Applied Science). Transferase-mediated dUTP nick-end labeling (TUNEL) assays to determine apoptosis were performed in toto by using the ApopTag® Peroxidase In Situ Apoptosis Detection Kit (Millipore, Madrid, Spain) following the manufacturer’s instructions.
Quantitative Real-Time PCR Analysis
Total RNA from zebrafish embryos was isolated by using the Trizol reagent (In-vitrogen; Life Technologies), treated with DNase I (New England Biolabs, Ipswich, MA, USA), and 1 μg total RNA was reverse-transcribed with the ImProm-II™ Reverse Transcription System (Promega, Alcobendas, Spain). To specifically amplify the DNA fragments, primers were designed with the Universal ProbeLibrary Assay Design Center web tool from Roche Applied Science (https://www.roche-applied-science.com/sis/rtpcr/upl/acenter.jsp?id=030000). The sequence of the quantitative PCR (qPCR) primers is given in Supplementary Table S1. The amplification reactions were carried out in a 25-μL final volume by using Power SYBR Green PCR Master Mix (Applied Biosystems; Life Technologies), and the real-time PCR was performed using the ABI PRISM 7300 (Applied Biosystems; Life Technologies). Cycle threshold (Ct) values were calculated with the SDS v1.3.1 software (Applied Biosystems; Life Technologies) for each gene, and the cDNA abundance of each transcript was calculated relative to the expression of the housekeeping gene ee1f1a1l1 by using the REST-384 v2 software (28). Also, standard curves for each gene and negative controls (NTC [no template control] and RNA, which was not reverse transcribed) were included in each PCR.
All supplementary materials are available online at www.molmed.org.
RESULTS
Binding Affinities of Opioid Peptides on the μ Opioid Receptor from Zebrafish
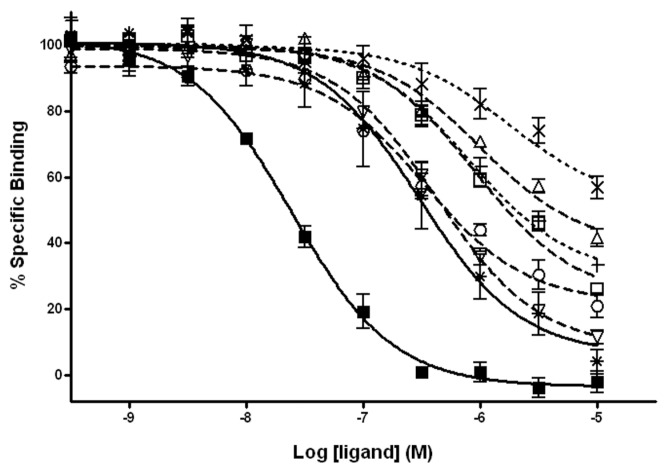
Competition binding experiments with [3H]-diprenorphine were carried out to establish the binding affinities of opioid ligands on dre-μ, and displacements with morphine and naloxone were performed in parallel as positive controls. All tested ligands displaced [3H]-diprenorphine binding, yet with different affinities (Figure 1), and in all cases, the experimental data fit better in the one-site displacement model. Naloxone showed the highest affinity toward dre-μ, with a Ki value on the nanomolar range. The rank order of affinity of the tested ligands was as follows: naloxone >> β-END = morphine >> MEGY >> Met-ENK > (d-Ala2)-MEGY >> Leu-ENK >> (d-Ala2, Val5)-MEGY (Table 2; statistical analysis in enclosed in Supplementary Table S2). Interestingly, while both naloxone and morphine were able to displace up to 100% of the specific binding, all of the peptidic agents left some residual [3H]-diprenorphine binding that could not be effectively displaced, even at a concentration of 10 μmol/L.
Figure 1.
Pharmacological properties of endogenous opioid peptides on dre-μ. Competition binding curves of endogenous opioid peptides on dre-μ membrane homogenates are shown. Data were fit to the one-site competition model, and each point represents the mean ± standard error of the mean (SEM) (capped bars) of three independent experiments performed in triplicate. Morphine, a μ agonist, and naloxone, a non-specific antagonist, were added for comparison. □, Met-ENK; △, Leu-ENK; ▽, β-END; ○, Met-ENK-Gly-Tyr (MEGY); +, (d-Ala2)-MEGY; ×, (d -Ala2, Val5)-MEGY; *, morphine; ■, naloxone.
Table 2.
Competition affinities for different opioid receptors and ligands.
| Receptor | Ligand | Ki (mean ± SEM) (nmol/L) | % Displacement | |
|---|---|---|---|---|
| dre-μ | Met-ENK | 684 ± 15 | 73.97 ± 1.29 | |
| Leu-ENK | 1,317 ± 166 | 58.51 ± 2.68 | ||
| β-END | 186 ± 25 | 88.45 ± 2.03 | ||
| MEGY | 204 ± 53 | 78.98 ± 3.73 | ||
| (d-Ala2)-MEGY | 744 ± 27 | 66.72 ± 0.81 | ||
| (d-Ala2, Val5)-MEGY | 3,645 ± 621 | 43.16 ± 3.39 | ||
| Morphine | 187 ± 94 | 100 | ||
| Naloxone | 10.65 ± 1.53 | 100 | ||
|
| ||||
| Ki (nmol/L) | Reference | |||
|
| ||||
| dre-δ1 | β-END | 36.6 | 29 | |
| MEGY | 427 | 21 | ||
| Morphine | 22 | 29 | ||
| dre-δ2 | Met-ENK | 45 |
|
30 |
| Leu-ENK | 175 | |||
| MEGY | 146 | 21 | ||
| Morphine | 1,400 | 30 | ||
| rn-μa | Met-ENK | 1.80 |
|
5 |
| Leu-ENK | 6.19 | |||
| MERF | 0.37 | |||
| Morphine | 7.48 | |||
| rn-δb | Met-ENK | 0.45 | ||
| Leu-ENK | 0.37 | |||
| MERF | 0.57 | |||
| Morphine | 302 | |||
| tg-μc | Met-ENK | 70.7 |
|
32 |
| Leu-ENK | 117 | |||
| β-END | 55.9 | |||
| tg-δc | Met-ENK | 24.9 | ||
| Leu-ENK | 198 | |||
| β-END | 284 | |||
This table summarizes the results obtained in competition binding assays (Ki and % displacement) using [3H]-diprenorphine on membrane homogenates from HEK293 cells that stably express the μ opioid receptor from zebrafish. The results of other competition binding studies for the zebrafish δ opioid receptors dre-δ1 and dre-δ2, prototypical mammalian δ and μ receptors rn-oprm1 (rn, Rattus norvegicus [rat]) and rn-oprm1 and the amphibian μ and δ receptors tg-oprm1 and tg-oprd1 (tg, Taricha granulosa [newt]) have also been included for comparison.
In this case, the radioligand used was the μ-selective peptidic analog [3H]-DAMGO and not [3H]-diprenorphine.
In this case, the radioligand used was the δ-selective peptidic analog [3H]-DPDPE and not [3H]-diprenorphine.
In this case, the radioligand used was the nonspecific antagonist [3H]-naloxone and not [3H]-diprenorphine.
Transcriptional Regulation of dre-oprm1 by Opioid Peptides
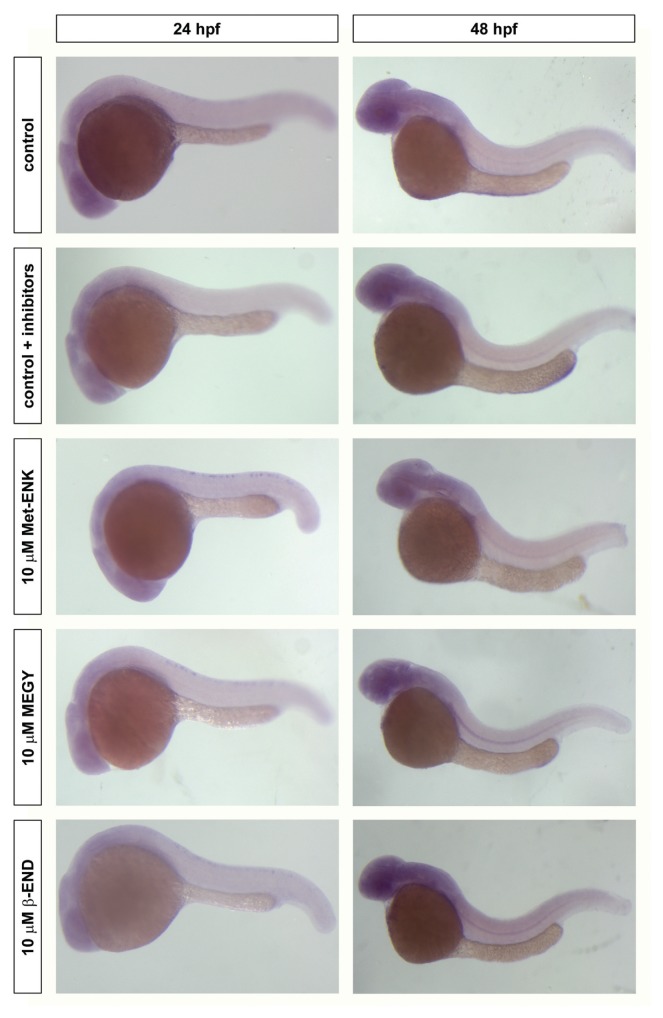
To determine whether dre-oprm1 expression can be regulated by chronic exposure to opioid peptides, zebrafish embryos were exposed to 10 μmol/L Met-ENK, MEGY and β-END, respectively, from 5 to 24 hpf or 48 hpf. It is noteworthy to mention that 10% of the embryos were found dead at 24 hpf when incubated with 10 μmol/L Met-ENK, and 10% were delayed in their development when incubated with 10 μmol/L of either Met-ENK or MEGY. Also, Met-ENK–treated embryos were smaller than controls and showed a certain degree of developmental delay, which could affect the result of the subsequent analysis. These effects were not seen when the embryos were exposed to 10 μmol/L β-END or in both controls (incubated with and without protease inhibitors). The quantitative changes were analyzed by qPCR (Table 3), and changes in the expression pattern were assayed by in situ hybridization (Figure 2). Met-ENK, the smallest peptide, did not seem to greatly alter dre-oprm1 expression at 24 hpf, and a slight increase was found at 48 hpf. Treatment with the heptapeptide MEGY resulted in a decrease in dre-oprm1 levels at 24 and 48 hpf, showing a cumulative effect. β-END displayed a biphasic effect, since dre-oprm1 expression dropped by onefold at 24 hpf, but it almost recovered control levels at 48 hpf. As shown in Figure 2, chronic exposure to opioid peptides did not induce significant changes on the expression pattern of dre-oprm1 during development, maybe because dre-oprm1 shows a diffuse expression pattern in the developing brain.
Table 3.
Effect of the endogenous opioid peptides in dre-oprm1 expression.
| 10 μmol/L Met-ENK | 10 μmol/L MEGY | 10 μmol/L β-END | |
|---|---|---|---|
| 24 hpf | 1.03 ± 0.11 | 0.78 ± 0.17 | 0.18 ± 0.01a |
| 48 hpf | 1.11 ± 0.20 | 0.47 ± 0.07a | 0.98 ± 0.09 |
Relative dre-oprm1 expression fold-changes in embryos that were exposed to a dose of 10 μmol/L opioid peptides (Met-ENK, MEGY and β-END) for 24 and 48 hpf are shown. Data are normalized to dre-eef1a1l1. A total of 50 embryos were used for each treatment and for each time point; the experiment was performed in triplicate and repeated three times.
p < 0.05 (unpaired Student t test, n = 3).
Figure 2.
Effect of opioid peptide treatment of the developmental pattern of dre-oprm1. dre-oprm1 expression was assayed by in situ hybridization on whole-mount embryos that were exposed to 10 μmol/L Met-ENK, MEGY or β-END from 5 to 24 hpf or 48 hpf. Embryos are oriented anterior toward the left and posterior toward the right. A total of 25 embryos were used for each treatment and for each time point, and the experiment was repeated three times.
Morphine Disrupts the Expression of the Endogenous Opioid Propeptides
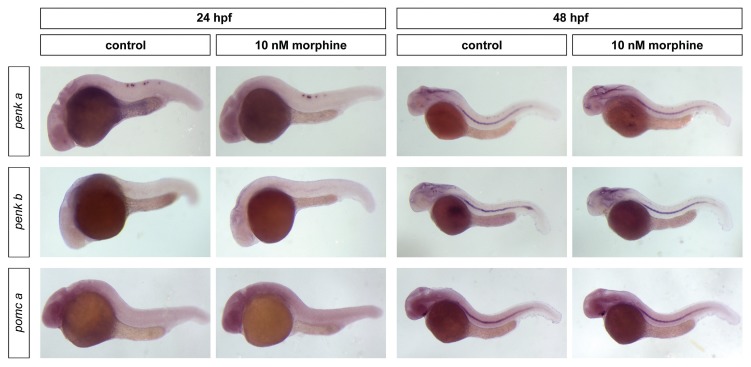
Zebrafish embryos were exposed to 10 nmol/L morphine to assess whether this ligand modulates the expression of the propeptides penk a, penk b and pomc a, which code for the endogenous ligands for μ, namely enkephalins and β-END. At 24 hpf, morphine downregulated penk a and upregulated penk b expression, while transcriptional activity of pomc a remained mostly unaffected (Table 4). At 48 hpf, morphine increased the expression of both enkephalin-containing propeptides and decreased the transcriptional rate of the β-END–containing precursor. At 48 hpf, morphine-treated embryos displayed penk a expression in periventricular areas of the forebrain, which did not seem to be stained in control embryos. penk b expression pattern seemed to not be affected by morphine treatment (Figure 3), although a more intense staining was found in areas located at the rim of the fourth ventricle.
Table 4.
Changes of expression of zebrafish proenkephalins and proopiomelanocortin in morphine-treated embryos.
| dre-penk a | dre-penk b | dre-pomc a | |
|---|---|---|---|
| 24 hpf | 0.61 ± 0.12 | 1.32 ± 0.05a | 0.90 ± 0.01 |
| 48 hpf | 1.45 ± 0.06b | 1.31 ± 0.10a | 0.48 ± 0.15b |
Results represent the relative gene expression fold-changes of dre-penk a, dre-penk b and dre-pomc a in morphine-treated embryos of 24 and 48 hpf. Data shown are normalized to dr-eef1a1l1. A total of 50 embryos were used for each treatment and for each time point; the experiment was performed in triplicate and repeated four times.
p < 0.01 and
p < 0.05 (unpaired Student t test, n = 4).
Figure 3.
Morphine effect on the expression of the opioid propeptides. Zebrafish embryos were exposed to 10 nmol/L morphine, and penk a, penk b and pomc a expression was established at 24 and 48 hpf and compared with controls. Embryos are oriented anterior toward the left and posterior toward the right. A total of 25 embryos were used for each treatment and for each time point, and the experiment was repeated three times.
Effect of dre-oprm1 Knocking Down on the Expression of the Opioid Precursors
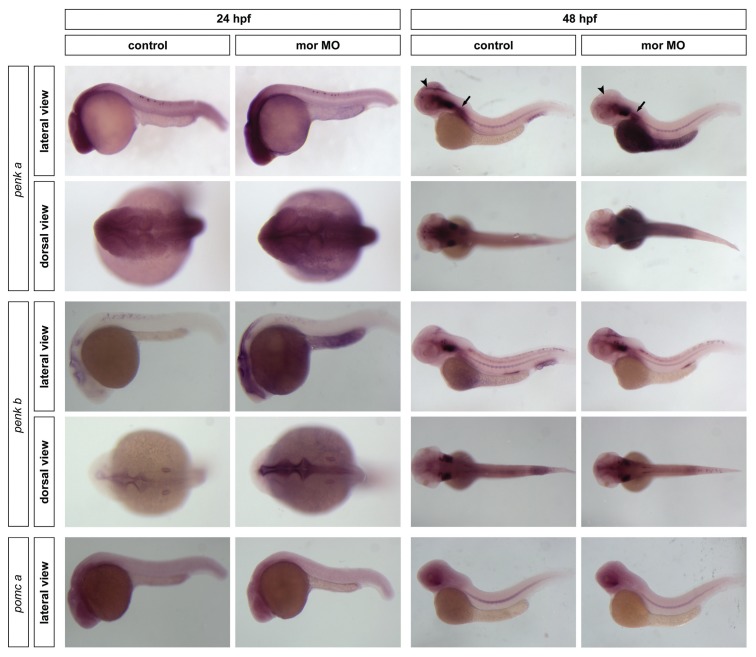
To establish whether dre-oprm1 plays a role in the transcriptional regulation of the opioid precursors, an ATG morpholino was microinjected to knockdown dre-oprm1 mRNA expression. Embryos were harvested at 24 and 48 hpf, and changes in the expression of penk a, penk b, pomc a and pomc b were assayed by qPCR (Table 5) and in situ hybridization (Figure 4). The absence of a functional μ receptor affected the expression of Met-ENK– and β-END–containing precursors, yet in a different fashion: while penk genes were up-regulated at 24 hpf, such an increase was not observed for pomc a. At 48 hpf, penk a expression was reduced in morphants, while this change was not observed for penk b, and a slight decrease was seen for pomc a. At 24 hpf, penk a showed diffuse and widespread labeling in the CNS of control embryos, whereas a more intense staining was found in morphants. At 48 hpf, penk a expression in control embryos was found at the border between tegmentum and optic tectum and at the most posterior area of the medulla oblongata. This expression pattern was lost in the morphant phenotype. penk b showed a very constrained expression pattern at 24 hpf, being localized at the periventricular areas of the brain and in few cells at the spinal cord. In the morphant embryos, the staining was more intense and spread to more anterior and posterior areas, being found at the rim of the forebrain ventricle. No significant changes in the expression pattern were observed for pomc a.
Table 5.
Changes in the expression of zebrafish proenkephalins and proopiomelanocortin in dre-oprm1 morphants.
| dre-penk a | dre-penk b | dre-pomc a | |
|---|---|---|---|
| 24 hpf | 1.37 ± 0.04a | 1.54 ± 0.14a | 1.03 ± 0.29 |
| 48 hpf | 0.54 ± 0.10a | 0.98 ± 0.04 | 0.88 ± 0.20 |
Results represent the relative gene expression fold-changes of dre-penk a, dre-penk b and dre-pomc a in morpholino-injected embryos of 24 and 48 hpf. Data shown are normalized to dre-eef1a1l1. Note the differential regulation of enkephalin- and β-END–containing genes in the morphant embryos. A total of 50 embryos were used for each treatment and for each time point; the experiment was performed in triplicate and repeated four times.
p < 0.05 (unpaired Student t test, n = 4).
Figure 4.
Effect of knocking down dre-oprm1 on the expression pattern of the opioid precursors. The expression of proenkephalins (dre-penk a and dre-penk b) and proopiomelanocortin (dre-pomc a) was assayed in dre-oprm1 morphants of 24 and 48 hpf. Embryos are oriented anterior toward the left and posterior toward the right. Arrowheads indicate the border between tegmentum and optic tectum; and arrows indicate the most posterior area of the medulla oblongata. A total of 25 embryos were used for each treatment and for each time point, and the experiment was repeated three times. mor MO, morpholino designed against dre-oprm1 gene.
Effect of dre-oprm1 Morpholino Microinjections on the CNS Development in the Zebrafish
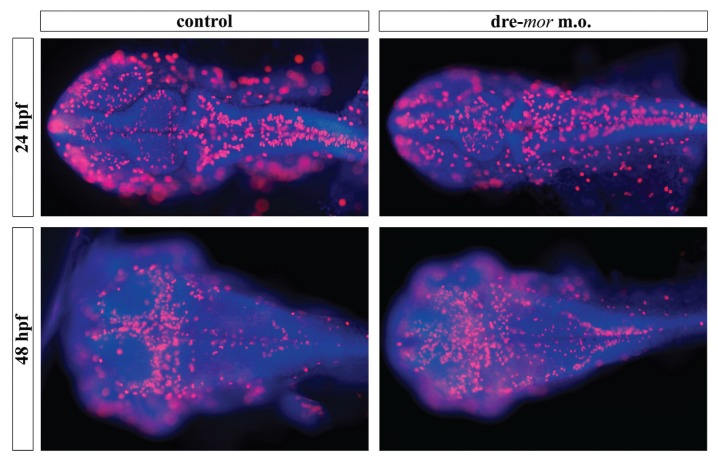
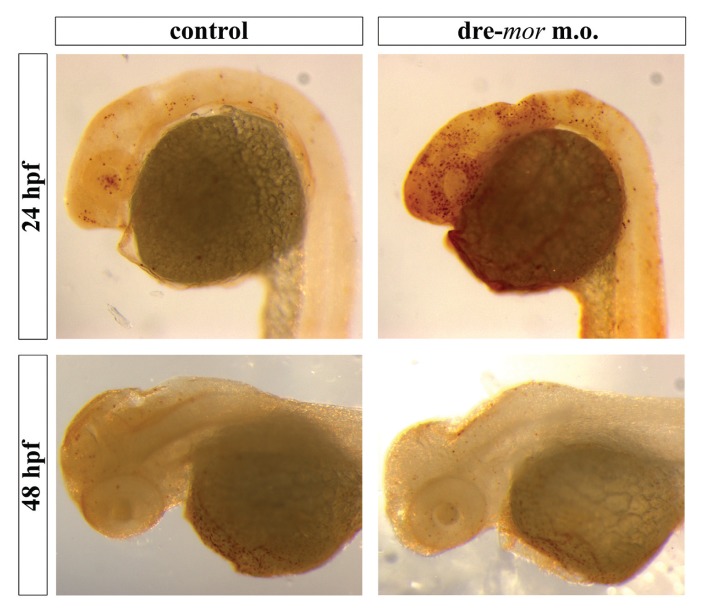
To determine whether the absence of a functional μ opioid receptor affects CNS development, proliferation (Figure 5) and apoptosis (Figure 6) assays were conducted on morpholino-injected embryos. Both at 24 and 48 hpf, the expression of the mitotic marker phospho-histone 3 (Ser10) was disorganized in dre-oprm1 morphants, since it was not restricted to the periventricular areas of the brain. Phospho-histone 3–positive cells were found at the midline of the optic tectum at 24 hpf. At 48 hpf, control embryos displayed the proliferative cells in the optic tectum in a characteristic ωshape. This pattern was not seen in morphants, since more mitotic cells were found scattered in the optic tectum of these embryos. In addition, the rim of the fourth ventricle was clearly labeled with phospho-histone 3 staining in the morphant phenotype. TUNEL staining assays revealed several apoptotic cell nuclei widespread in the forebrain, eyes, cerebellum and the most anterior part of the hindbrain at 24 hpf. Interestingly, these areas, where apoptosis was clearly evident, overlap with the ones that were labeled with the dre-oprm1 in situ hybridization probe in control embryos. At 48 hpf, no major differences were seen between controls and dre-oprm1 morphants.
Figure 5.
Proliferation studies on dre-oprm1 morphants. Phospho-histone 3 (Ser10) immunostaining (red) was assayed on whole-mounts of control and dre-oprm1 morphants of 24 and 48 hpf. The cell nuclei are counterstained with DAPI (blue) to give a better overview of the embryo. Dorsal views of the flat-mounts are shown, and embryos are oriented anterior toward the left and posterior toward the right. A total of 25 embryos were used for each treatment and for each time point, and the experiment was repeated three times. dre-mor MOR, morpholino designed against dre-oprm1 gene.
Figure 6.
Apoptosis assays on dre-oprm1 morphants. TUNEL staining on control and dre-oprm1 morphants of 24 and 48 hpf. The apoptotic nuclei are positive for peroxidase labeling. Lateral views are shown, and embryos are oriented anterior toward the left and posterior toward the right. A total of 25 embryos were used for each treatment and for each time point, and the experiment was repeated three times.
DISCUSSION
Pharmacological analysis was performed to establish whether the endogenous peptides (Met-ENK, MEGY and β-END) bind the μ opioid receptor from zebrafish; results are shown in Table 2 and are compared with previous results for both μ and δ receptors from different species. In zebrafish, Met-ENK–derived peptides and β-END bind both receptor types (20,29,30). The peptidic analogs (d-Ala2)-MEGY and (d-Ala2, Val5)-MEGY showed reduced affinities for dre-μ, and Leu-ENK showed a Ki value on the micromolar range, thus indicating that the presence of a Gly in position 2 and a Met in position 5 are decisive for a proper peptide-receptor interaction. In addition, longer peptides such as MEGY and β-END displayed higher affinities than Met-ENK on dre-μ. Given that Met-ENK, MEGY and β-END have the same “opioid message” at their N-terminus, the differences in amino acid composition and the length of the C-terminus might be responsible for establishing a high-affinity binding of these peptides upon dre-μ. A similar result was reported for the different Met-ENK–derived heptapeptides that are encoded by the penk gene (31).
Morphine displayed a similar affinity for dre-μ than β-END and MEGY; in contrast, its Ki value is one order lower for dre-δ1 (29) and lies on the micromolar range for dre-δ2 (30). These results indicate that morphine might preferentially bind dre-δ1 > dre-μ >> dre-δ2 and that some of the morphine effects on the zebrafish might be mediated by δ rather than by μ receptors.
The results obtained for other species are difficult to compare to those from zebrafish, since a different radioligand was used in the competition binding assays. The highly selective ligands [H3]-DAMGO and [H3]-DPDPE ([H3]-[D-Pen2,5]Enkephalin, [D-Pen2,D-Pen5]Enkephalin) were used in the experiments with rodent receptors, and this is the reason why the endogenous peptides displayed much higher affinities (below the nanomolar range) for both δ and μ receptors (5). In the case of amphibian receptors, competition binding experiments were performed with [H3]-naloxone (32), and the obtained results are similar to those seen in zebrafish. These results indicate that the ability of endogenous opioid peptides to both δ and μ receptors is conserved among vertebrate evolution, and thus, the results found in zebrafish may be extrapolated to mammalian systems.
Once it was established that both morphine and endogenous opioid peptides bind to the μ receptor, we investigated the complex interactions among the μ receptor, exogenous ligands as morphine and the endogenous peptides. The cross-talk between the endogenous opioid system and exogenous agents is of great interest to understand the secondary effects of analgesic agents, such as morphine. For example, it is well known that Met-ENK levels are decreased during the state of physical dependence and that an increase in Met-ENK reversed chronic morphine-induced physical dependence. Also, a Met-ENK increase can help to alleviate the symptoms of naloxone-precipitated withdrawal (33). The analgesic effect of opiates cannot be split from their side effects as tolerance and dependence (which can lead to opiate addiction and abuse). In addition, the developmental effects of several drugs of abuse could also explain the different predisposition to develop addictive disorders during adult life. Also, the heterogeneity in the individual response to morphine is also closely related to changes during the embryonic development.
Peptide treatments modulated the transcriptional activity of oprm1, yet in a different fashion. While Met-ENK did not seem to alter oprm1 expression, MEGY downregulated the expression levels at both stages (24 and 48 hpf), and β-END had a transient effect at 24 hpf. No changes in the oprm1 expression pattern were seen, maybe because the mRNA for this receptor displays widespread and diffuse expression areas throughout the CNS. Met-ENK and MEGY show different binding affinities for δ and μ opioid receptors (20,30), and the enlarged forms of Met-ENK display a shift from δ- to μ-receptor selectivity compared with the parent peptide. Therefore, the effect of MEGY on dre-oprm1 expression could be related to this change in selectivity. It was previously shown that MERF peptide modulated the expression of the three opioid receptors after chronic treatment, although these changes in the expression levels had no effect on MERF antinociceptive properties (34).
Morphine treatment disrupts the expression of the propeptide genes, yet in a different fashion. Both penk genes are up-regulated at 48 hpf, and penk b is upregulated at 24 hpf as well; there is a decrease in the expression of penk a at 24 hpf and pomc a at 48 hpf. Former studies about the effect of chronic morphine administration on the levels of opioid peptides or of their precursors seem to be controversial. It was reported that chronic morphine administration caused a decrease of pomc expression in the arcuate nucleus of the basal hypothalamus of female guinea pigs (35). On the other hand, radioimmunoassays have shown a large increase in Met-ENK in the periaqueductal gray matter in morphine-dependent rats (36). Other authors have reported that morphine administration decreases penk expression in the nucleus accumbens and olfactory tuberculum, whereas no changes were seen in the striatum(37,38).
These opposing results seem hard to reconcile, unless we take into consideration that the genetic background and the genotype might influence the modulation of the opioid system by exogenous agents. In this regard, it was shown that different mouse inbred strains, which display distinct sensitivity to the rewarding effects of morphine, presented marked differences in the basal expression of both penk and pdyn genes in the nucleus accumbens(39). In addition, the different expression levels of the opioid peptides can explain the morphine self-administration behavior in inbred rat strains. In fact, Lewis rats, which display a high rate of morphine self-administration, exhibit a lower basal penk mRNA expression in the caudate-putamen and nucleus accumbens compared with Fischer rats. penk expression levels were decreased after morphine self-administration in Lewis rats, but significantly increased in the Fischer strain (40).
Knocking down oprm1 expression altered the mRNA levels for penk genes, up-regulating their expression at 24 hpf and decreasing penk a levels at 48 hpf. In contrast, no changes in penk or pomc expression were seen in μ-deficient knockout mice (7). In addition, no gross morphological alterations and changes in proliferation were seen in these mice, albeit the studies were conducted on adult animals (41). In our case, we use two complementary techniques: in situ hybridization, a qualitative method to determine changes in the expression pattern, and real-time PCR, a quantitative approach. In the case of animals with uterine development, the maternal contribution is quite important, and it may be compensating for the absence of some gene and thus masking the real effect. In the case of zebrafish, where the development is external, this problem is easily avoided.
However, other studies revealed that there is a close relationship between opioid system activation and effects in proliferation. For example, Met-ENK, Leu-ENK and MERF bind the δ opioid receptor and provide cardioprotection in an ischemia model (42). It was also shown that agonist-dependent μ activation modulates Wntless activity, a protein that mediates WNT function by regulating its secretion (43). Besides, Met-ENK–derived peptides can act as tonic inhibitory peptides on the opioid growth factor receptor, thus inhibiting proliferation either during normal growth and development or even in cancer models from neural and non-neural cell lines (11,44).
This body of evidence is in agreement with our findings in the dre-oprm1 morphants, in which proliferation is markedly affected, since mitotic cells were disorganized in the brain at both 24 and 48 hpf. In addition, the number of apoptotic cells was significantly increased at 24 hpf. Interestingly, penk b expression at 24 hpf was found at the periventricular areas of the brain, which stand for those brain regions that will harbor the stem cell niches in the adult organism. These results clearly indicate that the endogenous opioid system, namely μ receptor and enkephalins, play a role during the development of the CNS and suggest a possible mechanism to explain the morphine effect in proliferation: chronic morphine treatment upregulates penk b expression, thus increasing the levels of Met-ENK and MEGY. These enkephalins bind the opioid growth factor receptor, thus eliciting an inhibitory signal for proliferation. In the morphant embryos, the absence of a functional μ receptor could deregulate the interaction of enkephalins with the opioid growth factor receptor and/or the δ opioid receptors, hence losing the characteristic pattern of proliferation in the zebrafish embryo.
CONCLUSION
The μ opioid receptor from zebrafish (dre-oprm1) binds endogenous peptides (Met-ENK–derived peptides and β-END) and morphine with similar affinity. We have shown that peptidic ligands can regulate the expression levels of oprm1 mRNA and that the absence of a functional μ receptor changes the expression of the propeptide genes. In addition, the activity of the endogenous opioid system can be modulated by exogenous agents such as morphine, either at the receptor or at the ligand level. Therefore, we can deduce that the basal activity of the endogenous opioid system can modulate the effects of exogenous agonists such as morphine and might partially explain the disruption of this homeostasis caused by opiates and that this disruption can play a role in the instatement of the Neonatal Abstinence Syndrome. These findings will contribute to a better understanding of the complex interactions between the opioid system and antinociceptive drugs and provide insights that may be relevant for analgesic therapy.
Supplemental Data
ACKNOWLEDGMENTS
This work was supported by grants from the Spanish Ministry of Science and Education (SAF2010-18597) and from Consejeria de Sanidad, Junta de Castilla y León (SAN673/SA25/08 and B1039/SA25/10). The authors would like to thank G Valencia and G Arsequell for synthesizing the zebrafish MEGY peptide and its two analogs: (d-Ala2)-MEGY and (d-Ala2, Val5)-MEGY.
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
- 1.Comb M, Seeburg PH, Adelman J, Eiden L, Herbert E. Primary structure of the human Met- and Leu-enkephalin precursor and its mRNA. Nature. 1982;295:663–6. doi: 10.1038/295663a0. [DOI] [PubMed] [Google Scholar]
- 2.Gubler U, Seeburg P, Hoffman BJ, Gage LP, Udenfriend S. Molecular cloning establishes proenkephalin as precursor of enkephalin-containing peptides. Nature. 1982;295:206–8. doi: 10.1038/295206a0. [DOI] [PubMed] [Google Scholar]
- 3.Chang AC, Cochet M, Cohen SN. Structural organization of human genomic DNA encoding the pro-opiomelanocortin peptide. Proc Natl Acad Sci U S A. 1980;77:4890–4. doi: 10.1073/pnas.77.8.4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corbett AD, Henderson G, McKnight AT, Paterson SJ. 75 years of opioid research: the exciting but vain quest for the Holy Grail. Br. J. Pharmacol. 2006;147(Suppl 1):S153–62. doi: 10.1038/sj.bjp.0706435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mansour A, Hoversten MT, Taylor LP, Watson SJ, Akil H. The cloned mu, delta and kappa receptors and their endogenous ligands: evidence for two opioid peptide recognition cores. Brain Res. 1995;700:89–98. doi: 10.1016/0006-8993(95)00928-j. [DOI] [PubMed] [Google Scholar]
- 6.Borgland SL, Connor M, Osborne PB, Furness JB, Christie MJ. Opioid agonists have different efficacy profiles for G protein activation, rapid desensitization, and endocytosis of mu-opioid receptors. J Biol Chem. 2003;278:18776–84. doi: 10.1074/jbc.M300525200. [DOI] [PubMed] [Google Scholar]
- 7.Matthes HW, et al. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383:819–23. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- 8.Zhu Y, et al. Retention of supraspinal delta-like analgesia and loss of morphine tolerance in delta opioid receptor knockout mice. Neuron. 1999;24:243–52. doi: 10.1016/s0896-6273(00)80836-3. [DOI] [PubMed] [Google Scholar]
- 9.Hauser KF, McLaughlin PJ, Zagon IS. Endogenous opioids regulate dendritic growth and spine formation in developing rat brain. Brain Res. 1987;416:157–61. doi: 10.1016/0006-8993(87)91509-5. [DOI] [PubMed] [Google Scholar]
- 10.Zagon IS, McLaughlin PJ. Endogenous opioid systems regulate cell proliferation in the developing rat brain. Brain Res. 1987;412:68–72. doi: 10.1016/0006-8993(87)91440-5. [DOI] [PubMed] [Google Scholar]
- 11.Zagon IS, Verderame MF, McLaughlin PJ. The biology of the opioid growth factor receptor (OGFr) Brain Res Brain Res Rev. 2002;38:351–76. doi: 10.1016/s0165-0173(01)00160-6. [DOI] [PubMed] [Google Scholar]
- 12.Jansson LM, Velez M, Harrow C. The opioid-exposed newborn: assessment and pharmacologic management. J Opioid Manag. 2009;5:47–55. [PMC free article] [PubMed] [Google Scholar]
- 13.Kim E, et al. Mu- and kappa-opioids induce the differentiation of embryonic stem cells to neural progenitors. J Biol Chem. 2006;281:33749–60. doi: 10.1074/jbc.M603862200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hahn JW, et al. Mu and kappa opioids modulate mouse embryonic stem cell-derived neural progenitor differentiation via MAP kinases. J Neurochem. 2010;112:1431–41. doi: 10.1111/j.1471-4159.2009.06479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narita M, et al. Role of delta-opioid receptor function in neurogenesis and neuroprotection. J Neurochem. 2006;97:1494–505. doi: 10.1111/j.1471-4159.2006.03849.x. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Nunez V, Rodriguez RE. The zebrafish: a model to study the endogenous mechanisms of pain. ILAR J. 2009;50:373–86. doi: 10.1093/ilar.50.4.373. [DOI] [PubMed] [Google Scholar]
- 17.de Velasco EM, Law PY, Rodriguez RE. Mu opioid receptor from the zebrafish exhibits functional characteristics as those of mammalian mu opioid receptor. Zebrafish. 2009;6:259–68. doi: 10.1089/zeb.2009.0594. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez Nunez V, Gonzalez Sarmiento R, Rodriguez RE. Characterization of zebrafish proenkephalin reveals novel opioid sequences. Brain Res Mol Brain Res. 2003;114:31–9. doi: 10.1016/s0169-328x(03)00126-8. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez-Nunez V, Gonzalez-Sarmiento R, Rodriguez RE. Identification of two proopiomelanocortin genes in zebrafish (Danio rerio) Brain Res Mol Brain Res. 2003;120:1–8. doi: 10.1016/j.molbrainres.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez-Nunez V, Toth G, Rodriguez RE. Endogenous heptapeptide Met-enkephalin-Gly-Tyr binds differentially to duplicate delta opioid receptors from zebrafish. Peptides. 2007;28:2340–7. doi: 10.1016/j.peptides.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez-Nunez V, Marron Fernandez, de Velasco E, Arsequell G, Valencia G, Rodriguez RE. Identification of dynorphin a from zebrafish: a comparative study with mammalian dynorphin A. Neuroscience. 2007;144:675–84. doi: 10.1016/j.neuroscience.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 22.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 23.European Parliament; Council of the EU. Directive 2010/63/UE of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Off. J. European Union. 2010;53:L 276/33–79. [Google Scholar]
- 24.17344: REAL DECRETO 1201/2005, de 10 de octubre, sobre protección de los animales utilizados para experimentación y otros fines científicos. Boletin Oficial del Estado (BOE. 2005;252:34367–34391 252. [Google Scholar]
- 25.Committee for the Update of the Guide for the Care and Use of Laboratory Animals, Institute for Laboratory Animal Research, Division on Earth and Life Studies, National Research Council of the National Academies. Guide for the Care and Use of Laboratory Animals. 8th edition. Washington (DC): National Academies Press; 2011. [cited 2013 Jan 30]. Available from: http://oacu.od.nih.gov/regs/ [Google Scholar]
- 26.Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez-Nunez V, Nocco V, Budd A. Characterization of drCol 15a1b: a novel component of the stem cell niche in the zebrafish retina. Stem Cells. 2010;28:1399–411. doi: 10.1002/stem.461. [DOI] [PubMed] [Google Scholar]
- 28.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez RE, et al. Characterization of ZFOR1, a putative delta-opioid receptor from the teleost zebrafish (Danio rerio) Neurosci Lett. 2000;288:207–10. doi: 10.1016/s0304-3940(00)01239-8. [DOI] [PubMed] [Google Scholar]
- 30.Pinal-Seoane N, et al. Characterization of a new duplicate delta-opioid receptor from zebrafish. J Mol Endocrinol. 2006;37:391–403. doi: 10.1677/jme.1.02136. [DOI] [PubMed] [Google Scholar]
- 31.Bojnik E, et al. Phylogenetic diversity and functional efficacy of the C-terminally expressed heptapeptide unit in the opioid precursor polypep-tide proenkephalin A. Neuroscience. 2011;178:56–67. doi: 10.1016/j.neuroscience.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 32.Bradford CS, Walthers EA, Stanley DJ, Baugh MM, Moore FL. Delta and mu opioid receptors from the brain of a urodele amphibian, the rough-skinned newt Taricha granulosa: cloning, heterologous expression, and pharmacological characterization. Gen Comp Endocrinol. 2006;146:275–90. doi: 10.1016/j.ygcen.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 33.Hao S, Hu J, Fink DJ. Transgene-mediated enkephalin expression attenuates signs of nalox-one-precipitated morphine withdrawal in rats with neuropathic pain. Behav Brain Res. 2009;197:84–9. doi: 10.1016/j.bbr.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vats ID, et al. Endogenous peptide: Met-enkephalin-Arg-Phe, differently regulate expression of opioid receptors on chronic treatment. Neuropeptides. 2009;43:355–62. doi: 10.1016/j.npep.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Fang Y, Kelly MJ, Ronnekleiv OK. Proopiomelanocortin (POMC) mRNA expression: distribution and region-specific down-regulation by chronic morphine in female guinea pig hypothalamus. Brain Res Mol Brain Res. 1998;55:1–8. doi: 10.1016/s0169-328x(97)00348-3. [DOI] [PubMed] [Google Scholar]
- 36.Nieto MM, Wilson J, Cupo A, Roques BP, Noble F. Chronic morphine treatment modulates the extracellular levels of endogenous enkephalins in rat brain structures involved in opiate dependence: a microdialysis study. J Neurosci. 2002;22:1034–41. doi: 10.1523/JNEUROSCI.22-03-01034.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turchan J, Lason W, Budziszewska B, Przewlocka B. Effects of single and repeated morphine administration on the prodynorphin, proenkephalin and dopamine D2 receptor gene expression in the mouse brain. Neuropeptides. 1997;31:24–8. doi: 10.1016/s0143-4179(97)90015-9. [DOI] [PubMed] [Google Scholar]
- 38.Yukhananov RY, Handa RJ. Effect of morphine on proenkephalin gene expression in the rat brain. Brain Res Bull. 1997;43:349–56. doi: 10.1016/s0361-9230(97)00019-1. [DOI] [PubMed] [Google Scholar]
- 39.Gieryk A, Ziolkowska B, Solecki W, Kubik J, Przewlocki R. Forebrain PENK and PDYN gene expression levels in three inbred strains of mice and their relationship to genotype-dependent morphine reward sensitivity. Psychopharmacology (Berl) 2010;208:291–300. doi: 10.1007/s00213-009-1730-1. [DOI] [PubMed] [Google Scholar]
- 40.Sanchez-Cardoso P, et al. Modulation of the endogenous opioid system after morphine self-administration and during its extinction: a study in Lewis and Fischer 344 rats. Neuropharmacology. 2007;52:931–48. doi: 10.1016/j.neuropharm.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 41.Kieffer BL, Gaveriaux-Ruff C. Exploring the opioid system by gene knockout. Prog Neurobiol. 2002;66:285–306. doi: 10.1016/s0301-0082(02)00008-4. [DOI] [PubMed] [Google Scholar]
- 42.Takasaki Y, Wolff RA, Chien GL, van Winkle DM. Met5-enkephalin protects isolated adult rabbit cardiomyocytes via delta-opioid receptors. Am J Physiol. 1999;277:H2442–50. doi: 10.1152/ajpheart.1999.277.6.H2442. [DOI] [PubMed] [Google Scholar]
- 43.Reyes BA, et al. Opiate agonist-induced redistribution of Wntless, a mu-opioid receptor interacting protein, in rat striatal neurons. Exp Neurol. 2012;233:205–13. doi: 10.1016/j.expneurol.2011.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McLaughlin PJ, Verderame MF, Hankins JL, Zagon IS. Overexpression of the opioid growth factor receptor downregulates cell proliferation of human squamous carcinoma cells of the head and neck. Int J Mol Med. 2007;19:421–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.