Abstract
This study aims to examine the phase advance of sleep–wake rhythm, napping habit, nocturnal sleep duration, prolonged sleep latency and insomnia and their relationship with cognitive function. This is a cross-sectional study. Participants in this study are 2,947 community-dwelling adults older than 65 years old. Measurements of mini-mental examination (MMSE) score, go-to-bed time, wake-up time, nocturnal sleep duration, prolonged sleep latency, napping, and insomnia were done. The mean (standard deviation) nocturnal sleep hours was 7.96 (1.39) h. Twenty-one percent and 16.2% of the participants complained of prolonged sleep latency longer than 1 h and insomnia, respectively. Fifty-six percent of the participants napped once or more than once weekly. With advancing age, the participants reported longer sleep duration (p < 0.001), went to bed earlier, and woke up earlier, which were significant both before and after adjustment. The participants who had lower MMSE score went to bed earlier and woke up earlier, which were statistically significant both before and after adjustment. An inverted U-shaped relationship was observed between MMSE score and napping frequency, p for tend 0.026.The MMSE score decreased when the sleep duration prolonged from 7 h to ≧10 h (p for trend 0.006). No trend was observed from the sleep duration <4 up to 7.9 h (p for trend 0.500). Modest age-independent phase advance of the sleep–wake rhythm is associated with lower cognitive function. Whether this is a manifestation of early pre-clinical dementia and whether its recognition with early stabilization can slow cognitive decline remain elusive.
Keywords: Phase advance, Sleep/wake rhythm, Sleep duration, Dementia, Cognitive decline, Napping, Insomnia, Prolonged sleep latency
Introduction
Disturbed sleep–wake rhythm has consistently been recognized in dementia (Vitiello et al. 1990, 2002a; Bliwise 1993; Bootzin et al. 2001; Vitiello and Borson 2001; Moe et al. 1995; Ancoli-Israel et al. 1997; Pat-Horenczyk et al. 1998; Gehrman et al. 2005) while phase advance of sleep–wake cycle is regarded an age-associated variation.(Miles and Dement 1980). The significance of daytime sleepiness or napping has also been examined in patients suffering from dementia, which has indicated a high prevalence of day-time sleeping in patients suffering from Alzheimer's disease (Tractenberg et al. 2006); and Foley et al. (2001) have reported that daytime sleepiness was associated with 3-year incident dementia. This unfavorable association was further supported by (Ohayon and Vecchierini 2005) who demonstrated that prolonged daytime sleep of 1 h or more was associated with cognitive impairment. We, however, postulated that napping or excessive daytime sleepiness might be the early disturbance in sleep–wake cycle of dementia and perhaps, the extreme manifestation of the phase advance phenomenon; or phase advance, by itself, was the early presentation of sleep–wake rhythm disturbance in cognitive decline. To the best of our knowledge, the association between phase advance of sleep–wake rhythm and cognitive deficit has been scarcely examined.
Epidemiological studies of the relationship between sleep duration and cognitive function in older adults have revealed inconsistent findings. Some reported poor cognition in older persons sleeping for longer duration (Faubel et al. 2009; Benito-Leon et al. 2009; Schmutte et al. 2007) while others demonstrated the opposite (Ohayon and Vecchierini 2005; Tworoger et al. 2006). Kronholm et al. (2009), furthermore, has revealed an inverted U-shaped relationship between sleep duration and cognitive function in the general population, while Xu et al. (2011) have recently reported a similar relationship in older adults. While the duration or quantity of sleep is significant, the quality of sleep may be related to cognitive decline too. Several studies have reported that it was the self-reported quality but not the quantity of sleep that affected cognition (Blackwell et al. 2006; Nebes et al. 2009; Cricco et al. 2001). Therefore, the relationship between sleep quantity, quality, and cognitive deficit or dementia has remained elusive.
In the present cohort of community-dwelling older persons, who were free from clinical dementia, we therefore, examined their cognitive function and its relationship to phase advance of sleep–wake rhythm (go-to-bed-time and wake-up-time), napping habit, nocturnal sleep duration, and poor sleep quality of prolonged sleep latency, and subjective insomnia complaint.
Methods
Four thousand community-dwelling men and women aged 65 years or over were invited to attend a health check carried out in the School of Public Health of The Chinese University of Hong Kong between August 2001 and December 2003 by placing recruitment notices in community centers for the elderly and housing estates. This project was primarily examining the bone mineral density of older Chinese adults. Written informed consents were obtained. Only ethnical Chinese subjects were recruited. We excluded those who (1) were unable to walk without assistance of another person; (2) had had a bilateral hip replacement because that would have affected the bone mineral density measurement; (3) were cognitively incompetent to give informed consent; (4) had medical conditions, in the judgment of the study physicians, which made it unlikely that they would survive the duration of the study. The sample was stratified so that approximately 33% were in each of the age groups: 65–69, 70–74, and 75 and over. This study was approved by the Clinical Research Ethics Committee of The Chinese University of Hong Kong. The recruitment has been described in more details in the parent osteoporosis study (Lau et al. 2006).
The present sleep study was conducted during the second year follow-up of the parent osteoporosis study. Two thousand nine hundred and forty five subjects who turned up for the second year follow-up assessment of their bone mineral density were invited to participate. Each participant was tested by the mini-mental status examination (MMSE) (Folstein et al. 1975) according to the original osteoporosis study protocol (Lau et al. 2006). A sleep questionnaire (Li et al. 2002; Wing et al. 2002) was additionally administered to record the subjects' go-to-bed time, wake-up time, self-report nocturnal sleep duration, prolonged sleep latency (more than 1 h), subjective insomnia complaint, napping habit, and its frequency per week. Information of demographics, years of education, smoking habit, regular alcohol, tea and coffee consumption, habitual snoring, Geriatric Depression Scale score (Yesavage et al. 1982), psychotropic medication usage, and the diagnosis of diabetes mellitus, hypertension, stroke, chronic obstructive pulmonary disease, and coronary heart disease were collected. The specific questions asked in the Sleep Questionnaires were included in the Appendix.
Statistical methods
Nocturnal sleep duration was categorized arbitrarily into eight categories: <4, 4–4.9, 5–5.9, 6–6.9, 7–7.9, 8–8.9, 9–9.9, and ≧10 h; and the napping habit frequency into 5 categories: 0 per week, <1 per week, 1–2 per week, 3–5 weeks and daily. The adjusted mean MMSE score was separately plotted against each nocturnal sleep duration and napping frequency category to observe any U- or linear relationship. The relationship was examined by p-for-trend if either an incremental or decrescendo trend was observed.
The relationship between MMSE score and each of the sleep variables: nocturnal sleep duration, go-to-bed time, and wake-up time was tested by linear regression singly and then repeated with adjustment for the covariates: age, gender, years of education, smoking habit, regular alcohol, tea and coffee consumption, habitual snoring, depression (Geriatric Depression Scale score ≥ 8), use of psychotropic medications, and the diagnosis of diabetes mellitus, hypertension, stroke, chronic obstructive pulmonary disease, and coronary heart disease. The relationship with the remaining three binary sleep variables: napping habit, prolonged sleep latency (>1 h), and subjective complaint of insomnia was likewise tested by logistic regression singly and then repeated with adjustment for the same set of covariates.
The statistical tests were undertaken using SPSS 10.0. All tests were two-sided and any p value less than 0.05 was regarded as statistically significant.
Results
Two thousand nine hundred and forty-five participants were recruited. The mean (standard deviation) age was 73.8 (4.9) years and 1,203 (40.8%) were women. Their mean (standard deviation) MMSE score was 26.1 (3.3). The average go-to-bed time was 22:27 and the wake-up-time was 06:07. The mean (standard deviation) nocturnal sleep hours was 7.96 (1.39) h. Twenty-one percent and 16.2% of the participants complained of prolonged sleep latency longer than 1 h and insomnia, respectively. Fifty-six percent of the participants napped once or more weekly. All these results were listed in Table 1.
Table 1.
Baseline characteristics and comparison between male and female subjects
| All (N = 2,945) | Male (N = 1,742) | Female (N = 1,203) | p value | |
|---|---|---|---|---|
| Age | 73.89 (4.99) | 73.92 (4.90) | 73.86 (5.12) | 0.760 |
| Go-to-bed-time | 22:27 (1:15) | 22:26 (1:18) | 22:29 (1:11) | 0.320 |
| Wake-up-time | 06:07 (1:21) | 06:11 (1:26) | 06:01 (1:13) | 0.001 |
| Sleep duration (hour) | 7.96 (1.39) | 8.10 (1.46) | 7.76 (1.26) | <0.001 |
| Prolonged sleep latency (%) | 640 (21.8%) | 322 (18.5%) | 318 (26.5%) | <0.001 |
| Insomnia (%) | 477 (16.2%) | 187 (10.7%) | 290 (24.2%) | <0.001 |
| Napping (%) | 1,602 (56.0%) | 1,058 (61.3%) | 544 (47.8%) | <0.001 |
| Depression GDS ≧ 8 (%) | 144 (4.9%) | 65 (3.7%) | 79 (6.6%) | <0.001 |
| MMSE | 26.12 (3.37) | 27.13 (2.60) | 24.66 (3.80) | <0.001 |
| Psychotropic medications (%) | 71 (2.4%) | 32 (1.8%) | 39 (3.2%) | 0.015 |
With advancing age, the participants reported longer sleep duration (p < 0.001), went to bed earlier, and woke up earlier, which were significant both before and after adjustment for the covariates (Tables 2 and 3). Furthermore, the participants who had lower MMSE score went to bed earlier and woke up earlier, which were statistically significant both before and after adjustment (Tables 2 and 3). Women went to bed and woke up at the hour similar to men (Table 3) but slept 0.34 h less (p < 0.001) before and 0.36 h less (p < 0.001) than men after adjustment for the same set of covariates (Tables 2 and 3).
Table 2.
Relationship between sleep–wake rhythm, sleep duration, age, gender, and cognitive function (univariate analysis)
| Go-to-bed time (hour) | Wake-up-time (hour) | Sleep duration (hour) | Prolonged sleep latency | Insomnia | Napping | ||
|---|---|---|---|---|---|---|---|
| Mean (SD) | OR (95% CI) | ||||||
| Age | SD (5 years) | −0.24 (0.02)* | −0.09 (0.02)* | 0.16 (0.03)* | 1.09 (0.998, 1.19) | 0.99 (0.90, 1.10) | 1.04 (0.96, 1.12) |
| Female gender | Female/ male | 0.05 (0.05) | −0.16 (0.05)** | −0.34 (0.05)* | 1.59 (1.33, 1.90)* | 2.65 (2.17, 3.25)* | 0.58 (0.50, 0.67)* |
| MMSE | SD(3 units) | 0.14 (0.02)* | 0.09 (0.02)* | −0.03 (0.02) | 0.82 (0.76, 0.88)* | 0.86 (0.80, 0.94)** | 1.13 (1.06, 1.21)* |
Values are change in time duration (hours) or odds ratio (OR) per unit change of independent variable; CI = confidence interval; SD = standard deviation
*p < 0.001, **p < 0.01
Table 3.
Relationship between sleep–wake rhythm, sleep duration, age, gender, and cognitive function (multivariate analysis)
| Go-to-bed time (hour)a | Wake-up-time (hour) a | Sleep duration (hour) | Prolonged sleep latency | Insomnia | Napping | ||
|---|---|---|---|---|---|---|---|
| Unit | Mean (SD) | OR (95% CI) | |||||
| Age | SD (5 years) | −0.15 (0.02)* | −0.14 (0.02)* | 0.14 (0.03)* | 1.03 (0.93, 1.14) | 0.97 (0.87, 1.08) | 1.04 (0.96, 1.13) |
| Female gender | Female/male | −0.02 (0.06) | −0.001 (0.06) | −0.36 (0.07)* | 1.13 (0.86, 1.47) | 2.61 (1.96, 3.48)* | 0.64 (0.52, 0.80)* |
| MMSE | SD(3 units) | 0.08 (0.02)* | 0.09 (0.02)* | −0.05 (0.03)*** | 0.86 (0.79, 0.94)** | 1.03 (0.94, 1.14) | 1.05 (0.98, 1.14) |
Values are change in time duration (hours) or odds ratio (OR) per unit change of independent variable; CI = confidence interval; SD = standard deviation
Adjusted for age, gender, MMSE score, years of education, smoking habit, regular alcohol, tea and coffee consumption, habitual snoring, depression (Geriatric depression scale score ≥ 8), use of psychotropic medications, and the diagnosis of diabetes mellitus, hypertension, stroke, chronic obstructive pulmonary disease, and coronary heart disease by multiple linear regression for continuous dependent variables and logistic regression for binary dependent variables
*p < 0.001, **p < 0.01, ***p < 0.05
aFurther adjusted for sleep duration, prolonged sleep latency, insomnia, and napping
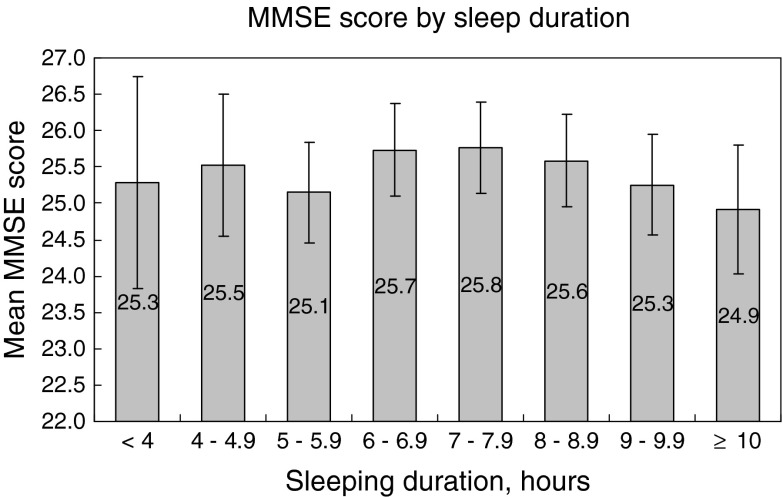
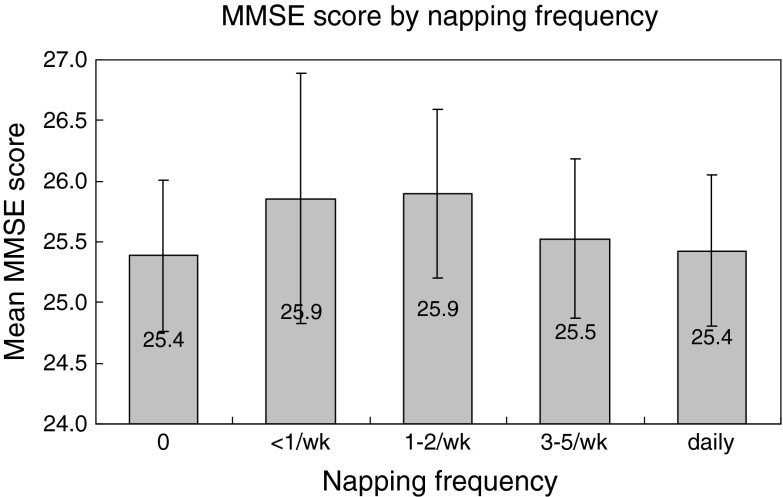
There was a decrease in mean MMSE score when the sleep duration prolonged from 7 h to ≧10 h (p for trend 0.006). No trend was observed from the sleep duration <4 up to 7.9 h (p for trend 0.500). A lower MMSE score was significantly associated with longer nocturnal sleep duration after adjustment for the covariates (p < 0.05, Table 3). A higher MMSE score was associated with fewer reports of prolonged sleep latency (OR, 0.82; p < 0.001), less insomnia complaint (OR, 0.86; p < 0.01) and napping (OR, 1.13; p < 0.001) before adjustment (Table 2). Only prolonged sleep latency (OR 0.86; p < 0.01) persisted to be statistically significant after adjustment (Table 3). An inverted U-shaped relationship was observed between MMSE score and napping frequency, p for tend was 0.026 for both decrescendo and incremental trend from ≥1–2/week to either ends with adjustment for the covariates (Fig. 1).
Fig. 1.
Relationship between MMSE score (95% confidence interval) and sleep duration. Adjusted for age, gender, years of education, smoking habit, regular alcohol, tea and coffee consumption, habitual snoring, depression (Geriatric depression scale score ≥ 8), use of psychotropic medications, and the diagnosis of diabetes mellitus, hypertension, stroke, chronic obstructive pulmonary disease, and coronary heart disease. p for quadratic regression = 0.179; <4–7.9 h: p for trend = 0.500; 7–≥10 h: p for trend = 0.006
Discussion
The reported mean nocturnal duration of sleep in this cohort of older adults was 7.96 (1.39) h, which was not particularly shorter than the general adult population (Ohayon and Vecchierini 2005; National Sleep Foundation 2003). Furthermore, we have observed that with every 5 years increase in age, the adjusted sleep duration was lengthened modestly by 0.14 h (p < 0.001), in contrary to the belief that sleep duration will shorten with aging. In our participants with an age range of 65 years and above, we did not reveal any increase in the frequency of subjective insomnia complaints and prolonged sleep latency (Tables 2 and 3), supporting the notion that sleep complaints in old age are usually secondary to other co-morbidities instead of aging singly (Foley et al. 1999, 2004; Vitiello et al. 2002b).
We have reconfirmed the phase advance of sleep–wake cycle in old age (Miles and Dement 1980). With every 5 years older, our participants went to bed 0.15 h earlier and woke up 0.14 h earlier (Table 3). The magnitude of this phase advance may appear modest, but it was demonstrable even in the age range from 65 years and onwards. It is plausible that the phase advance is more prominent from middle age to old age. The clinical significance of this phase advance of sleep cycle and its relationship to any neurodegenerative diseases in old age warrants further studies.
Similar to the effect of age, cognitive deficit was associated with the phase advance of sleep–wake rhythm. With every three decrements of MMSE score, the go-to-bed time was 0.08 h and the wake-up time 0.09 h earlier, which were independent of age and other co-variates. This phase advance, though modest, may be the manifestation of the pre-clinical stage of cognitive decline in this cohort of community-dwelling older adults, who are yet free from clinical dementia. Since the go-to-bed-time and wake-up-time might be confounded by other sleep-related complaints and sleep duration, we have additionally included them as covariates in analyzing the two phase advance dependent variables in the regression models. It is observed that both go-to-bed time and wake-up time were advanced with lower MMSE score, independent of sleep duration, and other sleep-related complaints (Table 3). The sleep–wake rhythm is controlled by the suprachiasmal nuclei in the anterior hypothalamus, which receive light and dark information and entrain biological rhythms to environmental time (Wulff et al. 2010). Exaggerated degeneration of this nucleus, more than accounted for by aging, has been reported in patients suffering from Alzheimer's disease (Hofman and Swaab 1994). Whether this can account for the phase advance in sleep cycle associated with cognitive deficit is uncertain and warrants further research. It has been postulated that sleep stabilization in neurodegenerative diseases with associated sleep cycle disturbance, may retard cognitive decline (Wulff et al. 2010). The clinical advantage of early recognition and stabilization of the phase advance in sleep–wake rhythm remains elusive.
If that napping represents the extreme manifestation of phase advance as we have postulated had been true, it would have been associated with a lower MMSE score. Napping, in contrary to the previous reports (Tractenberg et al. 2006; Foley et al. 2001; Ohayon and Vecchierini 2005) about the association between excessive daytime sleepiness and dementia, appeared to be protective against cognitive deficit (Table 2) though the effect did not persist after adjustment (Table 3). On further analysis of napping frequency instead of napping habit alone, we have demonstrated an inverted U-shaped relationship with cognitive function (Fig. 1). Optimal napping less than once to twice a week appeared to be protective to cognitive function, comparing to those never napped and those who napped more frequently. We have not inquired about the age at which the subjects began to acquire their napping habit. It is plausible that habitual napping acquired from middle or younger age is benign and is different from the irresistible daytime sleepiness that appears in old age. Only the latter, perhaps, represents the phase advance or the disturbance in the sleep–wake rhythm of cognitive decline.
We have observed a plausible inverted U-shaped relationship between cognitive function and sleep duration by eye-balling (Fig. 2), which, however, was not supported by further statistical test (p = 0.179 by quadratic regression). The MMSE score decreased with increasing sleeping duration from 7 h onwards (p for trend 0.007), with no similar relationship demonstrable from 7 h and below (p for trend 0.500). This relationship has been adjusted for age, gender, years of education, smoking habit, regular alcohol, tea and coffee consumption, insomnia, depression (GDS ≧ 8), psychotropic medications, diabetes mellitus, hypertension, stroke, chronic obstructive pulmonary disease, coronary heart disease, and habitual snoring. Our observations were consistent with the work of others who have revealed that cognitive deficit was associated with longer sleep duration (Faubel et al. 2009; Benito-Leon et al. 2009; Schmutte et al. 2007). It is uncertain whether it is the co-existing morbidities, for which we have adjusted as far as possible, or the poor sleep quality associated with cognitive decline that prolonged sleep duration.
Fig. 2.
Relationship between MMSE score (95% confidence interval) and napping frequency. Adjusted for age, gender, years of education, smoking habit, regular alcohol, tea and coffee consumption, habitual snoring, depression (Geriatric depression scale score ≥ 8), use of psychotropic medications, and the diagnosis of diabetes mellitus, hypertension, stroke, chronic obstructive pulmonary disease and coronary heart disease. p for quadratic regression = 0.017. 0 to ≤1–2/week: p for trend = 0.026; ≥1–2/week to daily: p for trend = 0.026
Regarding sleep quality, we have observed that cognitive deficit was associated with prolonged sleep latency but not subjective complaint of insomnia (Table 3). Disturbed sleep cycle and poor sleep quality has been consistently reported in demented patients (Vitiello et al. 1990, 2002a; Bliwise 1993; Bootzin et al. 2001; Vitiello and Borson 2001; Moe et al. 1995; Ancoli-Israel et al. 1997; Pat-Horenczyk et al. 1998; Gehrman et al. 2005). Our participants having lower MMSE score might still be in the early stage of cognitive decline and poor sleep quality was therefore not yet evident. Our results, however, suggest that one of the poor sleep complaints, prolonged sleep latency, may already be present in the pre-clinical stage of cognitive deficit.
Our study has several limitations. Being cross-sectional, the causal relationship between cognitive deficit and sleep–wake rhythm and sleep duration cannot be inferred. All our data were generated from self-report, and we have not used actigraphy or sleep EEG to measure the sleep duration, sleep-onset time, and wake-up time. Self report in old age may be subjected to error, particularly for those having cognitive deficit. However, recall error should occur in both directions and may not be biased towards either end. All participants were independent in daily functioning, community dwelling, and free from clinical dementia though some of them having low MMSE score. By including all participants regardless of MMSE scores, we were able to examine the phase advance in the preclinical stage of cognitive deficit before the onset of dementia.
The association between cognitive function and sleep–wake rhythm, sleep duration, and sleep quality could have been confounded by many co-existing sleep or cognition related medical conditions. We have attempted to adjust for two prominent confounding factors, mood (GDS) and the psychotropic medication usage and several common medical co-morbidities. Obstructive sleep apnea syndrome, which is associated with cognitive decline, is common in old age (Fulda and Schulz 2001; Ancoli-Israel et al. 1991). Habitual snoring has been adjusted for, hoping to minimize the confounding effect. Given all these efforts, our findings, however, may still be alternatively accounted for by other confounders, which were unknown and had not been adjusted for. The participants were community-dwelling, healthy volunteers, and free from any clinical dementia. As such, our findings cannot be extrapolated to the frail and demented patients.
Conclusions
Modest age-independent phase advance of the sleep–wake rhythm is associated with lower cognitive function. Whether this is a manifestation of early pre-clinical dementia and whether its recognition with early stabilization can slow cognitive decline remain elusive.
Appendix
Footnotes
This work was done at the S. H. Ho Centre for Gerontology and Geriatrics, the Chinese University of Hong Kong.
References
- Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Sleep-disordered breathing in community-dwelling elderly. Sleep. 1991;14:486–495. doi: 10.1093/sleep/14.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancoli-Israel S, Klauber MR, Jones DW, et al. Variations in circadian rhythms of activity, sleep and light exposure related to dementia in nursing home patients. Sleep. 1997;20:18–23. [PubMed] [Google Scholar]
- Benito-Leon J, Bermego-Pareja F, Vega S, Louis ED (2009) Total daily sleep duration and the risk of dementia: a prospective population-based study. Eur J Neurol 16:990–997 [DOI] [PubMed]
- Blackwell T, Yaffe K, Ancoli-Israel S, et al. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2006;61:405–410. doi: 10.1093/gerona/61.4.405. [DOI] [PubMed] [Google Scholar]
- Bliwise D. Sleep in normal aging and dementia. Sleep. 1993;16:40–81. doi: 10.1093/sleep/16.1.40. [DOI] [PubMed] [Google Scholar]
- Bootzin RR, Manber R, Lowey DH, Kuo TF, Franzen PL. Sleep disorders. In: Adams HE, Sutker PB, editors. Comprehensive handbook of psychopathology. 3. New York: Plenum Press; 2001. [Google Scholar]
- Cricco M, Simonsick EM, Foley DJ. The impact of insomnia on cognitive functioning in older adults. J Am Geriatr Soc. 2001;49:1185–1189. doi: 10.1046/j.1532-5415.2001.49235.x. [DOI] [PubMed] [Google Scholar]
- Faubel R, Lopez-Garcia E, Guallar-Castillon P, Graciani A, Banegas JR, Rodriguez-Artalejo F. Usual sleep duration and cognitive function in older adults in Spain. J Sleep Res. 2009;18:427–435. doi: 10.1111/j.1365-2869.2009.00759.x. [DOI] [PubMed] [Google Scholar]
- Foley DJ, Monjan A, Simonsick EM, Wallace RB, Blazer DG. Incidence and remission of insomnia among elderly adults: an epidemiologic study of 6,800 persons over three years. Sleep. 1999;22:S366–S372. [PubMed] [Google Scholar]
- Foley D, Monjan A, Masaki K, et al. Daytime sleepiness is associated with 3-year incident dementia and cognitive decline in older Japanese–American men. J Am Geriatr Soc. 2001;49:1628–1632. doi: 10.1111/j.1532-5415.2001.49271.x. [DOI] [PubMed] [Google Scholar]
- Foley D, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56:497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fulda S, Schulz H. Sleep cognitive dysfunction in sleep disorders. Medicine Reviews. 2001;5:423–445. doi: 10.1053/smrv.2001.0157. [DOI] [PubMed] [Google Scholar]
- Gehrman PR, Marler M, Martin JL, Shochat T, Corey-Bloom J, Ancoli-Israel S. The relationship between dementia severity and rest/activity circadian rhythms. Neuropsychiatr Dis Treat. 2005;1:155–163. doi: 10.2147/nedt.1.2.155.61043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofman MA, Swaab DF. Alterations in circadian rhythmicity of the vasopressin-producing neurons of the human suprachiasmatic nucleus (SCN) with aging. Brain Res. 1994;651:134–142. doi: 10.1016/0006-8993(94)90689-0. [DOI] [PubMed] [Google Scholar]
- Kronholm E, Sallinen M, Suutama T, Sulkava R, Era P, Partonen T. Self-reported sleep duration and cognitive functioning in the general population. J Sleep Res. 2009;18:436–446. doi: 10.1111/j.1365-2869.2009.00765.x. [DOI] [PubMed] [Google Scholar]
- Lau EMC, Leung PC, Kwok T et al (2006) The determinants of bone mineral density in Chinese men-results from Mr Os (Hong Kong), the first osteoporosis study on Asian men. Osteoporos Int 17:297–303 [DOI] [PubMed]
- Li RHY, Wing YK, Ho SC, Fong SYY. Gender differences in insomnia—a study in the Hong Kong Chinese population. J Psychosom Res. 2002;53:601–609. doi: 10.1016/S0022-3999(02)00437-3. [DOI] [PubMed] [Google Scholar]
- Miles LE, Dement WC. Sleep and aging. Sleep. 1980;3:1–220. [PubMed] [Google Scholar]
- Moe KE, Vitiello MV, Larsen LH, Prinz PN. Symposium: cognitive processes and sleep disturbances: sleep/wake patterns in Alzheimer's disease: relationships with cognition and function. J Sleep Res. 1995;4:15–20. doi: 10.1111/j.1365-2869.1995.tb00145.x. [DOI] [PubMed] [Google Scholar]
- National Sleep Foundation. 2003 Sleep in America Poll. At http://www.sleepfoundation.org
- Nebes RD, Buysse DJ, Halligan EM, Houck PR, Monk TH. Self-reported sleep quality predicts poor cognitive performance in healthy older adults. J Gerontol B Psychol Sci Soc Sci. 2009;64:180–187. doi: 10.1093/geronb/gbn037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayon MM, Vecchierini MF. Normative sleep data, cognitive function and daily living activities in older adults in the community. Sleep. 2005;28(8):981–989. [PubMed] [Google Scholar]
- Pat-Horenczyk R, Klauber MR, Shochat T, Ancoli-Israel S. Hourly profiles of sleep and wakefulness in severely versus mild-moderately demented nursing home patients. Aging Clin Exp Res. 1998;10:308–315. doi: 10.1007/BF03339793. [DOI] [PubMed] [Google Scholar]
- Schmutte T, Harris S, Levin R, Zweig R, Katz M, Lipton R. The relation between cognitive functioning and self-reported sleep complaints in nondemented older adults: results from the Bronx aging study. Behav Sleep Med. 2007;5:39–56. doi: 10.1207/s15402010bsm0501_3. [DOI] [PubMed] [Google Scholar]
- Tractenberg RE, Singer CM, Kaye JA. Characterizing sleep problems in persons with Alzheimer's disease and normal elderly. J Sleep Res. 2006;15:97–103. doi: 10.1111/j.1365-2869.2006.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tworoger SS, Lee S, Schernhammer ES, Grodstein F. The association of self-reported sleep duration, difficulty sleeping, and snoring with cognitive function in older women. Alzheimer Dis Assoc Disord. 2006;20:41–48. doi: 10.1097/01.wad.0000201850.52707.80. [DOI] [PubMed] [Google Scholar]
- Vitiello M, Borson S. Sleep disturbances in patients with Alzheimer's disease: epidemiology, pathophysiology and treatment. CNS Drugs. 2001;15:777–796. doi: 10.2165/00023210-200115100-00004. [DOI] [PubMed] [Google Scholar]
- Vitiello MV, Prinz PN, Williams DE, Frommlet MS, Ries RK. Sleep disturbances in patients with mild-stage Alzheimer's disease. J Gerontol. 1990;45:M131–M138. doi: 10.1093/geronj/45.4.M131. [DOI] [PubMed] [Google Scholar]
- Vitiello MV, Moe KE, Prinz PN. Sleep complaints cosegregate with illness in older adults: clinical research informed by and informing epidemiological studies of sleep. J Psychosom Res. 2002;53:555–559. doi: 10.1016/S0022-3999(02)00435-X. [DOI] [PubMed] [Google Scholar]
- Vitiello MV, Moe KE, Prinz PN. Sleep complaints cosegregate with illness in older adults: clinical research informed by and informing epidemiological studies of sleep. J Psychosom Res. 2002;53:555–559. doi: 10.1016/S0022-3999(02)00435-X. [DOI] [PubMed] [Google Scholar]
- Wing YK, Li RHY, Lam CW, Ho CKW, Leung T. Prevalence of narcolepsy in Hong Kong Chinese. Ann Neurol. 2002;51:578–584. doi: 10.1002/ana.10162. [DOI] [PubMed] [Google Scholar]
- Wulff K, Gatti S, Wettstein JG, Foster RG. Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat Rev Neurosci. 2010;11:589–599. doi: 10.1038/nrn2868. [DOI] [PubMed] [Google Scholar]
- Xu L, Jiang CQ, Lam TH, Liu B, Jin YL, Zhu T, Zhang WS, Cheng KK, Thomas GN. Short or long sleep duration is associated with memory impairment in older Chinese: the Guangzhou Bioban Cohort Study. Sleep. 2011;34:575–580. doi: 10.1093/sleep/34.5.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]