Abstract
8-Hydroxy-2-deoxyguanosine (8OHdG) excreted into the urine is considered a marker of oxidative stress effect on DNA, and it is reported to be mainly produced by the DNA repair system. In previous works, we showed that autophagy was also involved in 8OHdG disposal through the degradation of oxidatively altered mitochondria. Here, we show that aging in Sprague–Dawley male rats is associated with a decline in the in vitro function of liver autophagy and a slight and not significant decrease in the urinary excretion of 8OHdG. In addition, we demonstrate that anti-aging caloric restriction maintains levels of both liver autophagy and urinary excretion of 8OHdG at very high levels throughout life. Finally, we show the in vivo stimulation of autophagy by the administration of an antilipolytic agent or everolimus, which rescues rats from the accumulation of 8OHdG in the liver mtDNA, also causes a dramatic increase in the urinary excretion of 8OHdG. The intensification of autophagy by the administration of the antilipolytic drugs to fasting rats faded progressively with increasing age, together with a reduced increase in 8OHdG output into the urine. It is concluded that the process of autophagy may play a major role in the disposal of 8OHdG with urine, and that the assay of 8OHdG levels in the urine before and after the stimulation of autophagy may provide a novel, non-invasive and safe procedure to monitor the in vivo functioning of the process.
Keywords: Caloric restriction, Autophagy, Antilipolytic drugs, 8OHdG, Urine
Introduction
Damage by endogenously produced oxygen radicals has been proposed as a major contributor to the aging process (Gruber et al. 2008). Biological aging is associated with a progressive accumulation of oxidative damage in protein, lipid, and mitochondrial (mt) and nuclear (n) DNA, which may account for age-associated dysfunction in many biological processes (Sohal and Weindruch 1996). In principle, any accumulation of altered components in tissues may be secondary to increased production, decreased repair, or both. The accumulation of oxidized nucleoside 8-hydroxy-2-deoxyguanosine (8OHdG) in older tissues is considered to result from the age-related increase in oxidative stress and its effects on mt and nDNA (Hamilton et al. 2001). The proposed results include decreased ability to repair DNA (Bohr 2002) or to a slow loss of DNA nuclease activity (Fraga et al. 1990). Despite the very high turnover rate of mitochondria (Pfeifer 1978), mtDNA is ten times more abundant in 8OHdG than nDNA (Nakamoto et al. 2007) due to its vicinity to the site of potential ROS generation and lack of protection by histones (Jin 2006).
Caloric restriction (CR) is the most powerful means of slowing aging and increasing longevity so far established. As compared to ad libitum (AL) feeding, CR attenuates the accumulation of oxidatively damaged molecules including oxidized DNA (Sohal et al. 1994). The responsible factor is the reduced intake of energy (Masoro 2009) which makes animals undergo a pattern of intermittent feeding (Bergamini et al. 2007). According to several authors, the mechanism(s) of the age-associated accumulation of the damaged molecules and its attenuation by CR have not been fully elucidated yet (Masoro 2009). It was proposed that aging either increases the rate of generation of reactive oxygen molecules, or it decreases the effectiveness of protective and repair processes, or it does both, and that CR may prevent or slow these changes (Sohal and Weindruch 1996). However, it is known that autophagy, a regulatable cell repair mechanism, which is active during fasting, is one of the mechanisms involved in biological aging and the anti-aging effects of caloric restriction (Bergamini and Gori 1995; Bergamini et al. 2004, 2007; see also Terman et al. 2010).
Autophagy is the intracellular degradation system that delivers aggresomes and cell organelles including altered 8OHdG-rich mitochondria (mitophagy) and damaged peroxysomes (pexophagy) to the lysosome for degradation and nutrient recycling (Donati et al. 2006; Cavallini et al. 2007; van Zutphen et al. 2011). Autophagy is regulated by amino acids, insulin, glucagon, and IGF-1 plasma levels and is induced in almost all organs by nutrient starvation (Mortimore and Pösö 1987; Mizushima 2009 and unpublished). Autophagy can be intensified or induced by drugs. The injection of an antilipolytic agent like 3.5 dimethylpyrazole (DMP) or (for human use) Acipimox during fasting leads to a shortage plasma FFA and causes hypoglycaemia, hypoinsulinemia and hyperglucagonemia and intensification of autophagy in minutes (Bergamini et al. 1993). The effects on autophagy can be blocked simply by a timed injection of glucose (Donati et al. 2008). Autophagy can also be induced by the injection of rapamycin or everolimus (Hartford and Ratain 2007). These drugs are known to pharmacologically remove the mTOR-mediated inhibition of autophagy in response to increased amino acid and insulin levels (Blommaart et al. 1995).
Oxidative DNA damage can be evaluated by measuring the abundance of 8OHdG in nDNA and mtDNA (Richter et al. 1988). 8OHdG released upon DNA repair or degradation is excreted into urine (Cooke et al. 2008). Diet does not appear to contribute to the urinary levels of 8OHdG, and the involvement of the degradation of dead cells is considered to be minimal (Cooke et al. 2008). Taken together, these results rule out various confounding factors and thus suggest that DNA repair pathways is the principal source of urinary purine, if not DNA lesions, enabling such measurements to be used as indicators of repair. In conclusion, the urinary output of 8OHdG may be both a marker of general oxidative stress (Wu et al. 2004) and of DNA degradation (Cooke et al. 2008).
In the current study, we explored the effects of aging and anti-aging CR both on liver autophagy and on the excretion of 8OHdG into urine. Furthermore, we explored the effects of the in vivo induction of autophagy on 8OHdG urinary output. The obtained results are in line with the hypothesis that the deleterious effects of aging are secondary to the suppression of the autophagic degradation of mitochondria (as signaled from the release and the excretion of 8OHdG in AL rats) which slows down mitochondrial turnover rate and promotes the secondary accumulation of malfunctioning, 8OHdG-rich organelles that lead to the ensuing increase in oxidative stress. The induction of autophagy by CR or drugs may stimulate degradation, promote replacement of older mitochondria with new organelles, lead to an increase in 8OHdG excretion and a decrease in oxidative stress and eventually to partial rejuvenation. Data also show that the urinary output of 8OHdG mainly depends on mtDNA oxidative damage but reflects the moment-to-moment rate of the autophagic degradation of damaged mitochondria.
Materials and methods
Animals and treatments
Male Sprague–Dawley rats were aged on standard laboratory food (Harlan autoclavable Teklad diet, Harlan Italy S.r.l., containing 12.0% water, 18.4% crude protein, 5.5% crude fat, 4.4% crude fiber, 5.6% crude ash). Animals were housed in a controlled environment (22°C; 12/12-h light/dark cycle) and had free access to water. Animals were fed ad libitum or submitted to an alternate day fasting diet (every other day, EOD, ad libitum feeding, AL) beginning at age 2 months.
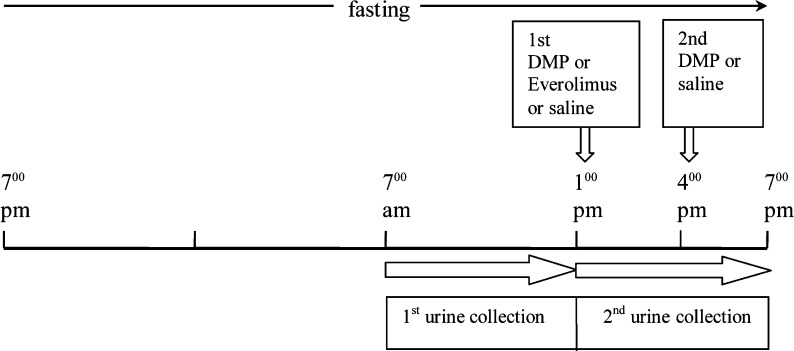
In the case of autophagy intensification by shortage of lipids, 3.5 dimethylpyrazole (DMP, Sigma Aldrich, 12 mg/kg body weight in 0.3 ml of saline) was given intraperitoneally after 18 and 21 h of fasting (Donati et al. 2008). As a control, a glucose load (1 g/kg body weight in 2 ml of water) was given intraperitoneally after the first DMP administration to prevent hypoglycaemia and the secondary decrease in insulin and increase in glucagon plasma levels. As an alternative, autophagy was induced by the administration of everolimus (Certican®, Novartis, 1 mg/kg body weight dissolved in 10% ethanol, 40% propylene glycol, 5% benzylic alcool) by the gastric tube after 18 h of fasting (see Fig. 1 for details). In order to label long-lived (resident) liver proteins, groups of rats were injected 6 mCi/rat 14C uniformly labelled valine (288.5 mCi/mmole) intraperitoneally 24 h prior to sacrifice. The official Italian regulation N.116/92 for the care and use of laboratory animals was followed.
Fig. 1.
Timetable of treatments and urine collection in the fasted rats
Urine biochemical analysis
Rat urine was collected in a refrigerated (−20°C) tube using metabolic cages, during a 6-h period (see timetable in Fig. 1, from 9.00 a.m. to 3.00 p.m. in the case of feeding state) and stored at −80°C until analysed. The urine of EOD rats was collected on the day of feeding. On the day of the assay, urine was thawed and centrifuged 2,000 × g in the cold (4°C) for 10 min. Urinary 8OHdG was measured using an ELISA kit (New 8-OHdG Check, ELISA kit, JaICA, Japan). Calibration, curve fitting and data analysis were conducted according to the manufacturers instructions. Urinary creatinine was assayed by the spectrophotometric method described by (Burtis et al. 2009). Results are expressed as nanogramme 8OHdG per milligramme of creatinine.
Rate of in vitro and ex vivo autophagic proteolysis
The methods used were published in detail in a previous paper (Donati et al. 2009). Briefly, the in vitro assay for autophagic proteolysis involves the following steps: (1) liver parenchymal cells were isolated by the collagenase perfusion of Seglen; (2) viability was assessed by Trypan Blue exclusion and was always better than 90%; (3) liver cells were suspended (3 ml, 1.5 106/ml) in Krebs–Ringer bicarbonate buffer and incubated at 37°C (gas was humidified 95% O2–5% CO2); (4) plasma amino acids were added as fraction multiples of a standard (valine-free) reference mixture reflecting levels of amino acids in rat plasma; (5) reuptake of valine into protein was blocked by adding cycloheximide (CHX; 10 μM); (6) the rate of proteolysis was assessed by measuring the autophagy-related increase in the concentration of valine in the medium normalized to 106 cells/ml (values were corrected by the subtraction of the valine released in the presence of the inhibitor of lysosomal proteolysis, 5 mM 3-methyladenine).
The ex vivo assay for autophagic proteolysis involved the following steps: (1) livers were perfused in situ as described by Mortimore and Mondon (1970); (2) surgery was performed in less than 3 min under anaesthesia (5–10 min after the intraperitoneal injection of 50 mg/kg body weight nembutal, in order to collect the samples of perfusate at the given times 0 and 150 min after the injection of DMP); (3) perfusion was performed in the single-pass mode with a medium free from amino acids (Krebs–Ringer bicarbonate buffer and bovine serum albumin (fraction V, Sigma Chemical, St. Louis, MO), 10 mM glucose, 18 mM cycloheximide and freshly washed no longer usable human erythrocyte, 27% v/v); (4) after a 7-min washout, two adjoining 1.5 min fractions of the outflow of the liver (approximately 12 ml each) were taken and centrifuged, and the surnatant was used for measurement of 14C6 and of amino acid release; (5) at the end of the perfusion, samples of liver tissue were taken to purify proteins and assess specific activity; (6) the plasma perfusate was deproteinized by the addition of 0.25 vol of ice-cold 25% TCA; (7) radioactivity in the supernatant was counted in a Beckman LS TD 500 liquid scintillation counter to an error less than 3%; (8) protein extracted by approximately 0.5 g of liver tissue was counted as described to an error less than 1%; (9) total values of liver protein degradation were computed from the released acid-soluble radioactivity in the perfusate divided by the specific activity of liver proteins. In the present paper, we reported the percent increase in liver protein degradation between 0 and 150 min after DMP administration.
Materials
The following materials were purchased from the following companies: 3,5-dimethyl pyrazole, from Fluka AG; amino acid standards, glutamine, cycloheximide, bovine serum albumin and methionine sulphonate from Sigma Italy, Milano. 14C6-valine was purchased from Amersham International, Amersham, UK.
Statistical analysis
One- or two-way analysis of variance (ANOVA) test was applied where appropriate to evaluate differences among multiple conditions. If main effects were noted, the Tukey test was used to test for their statistical significance. Student’s t test was used to evaluate differences between two conditions. Values of P < 0.05 were considered to be statistically significant.
Results
Age-related changes in the urinary excretion of 8OHdG and in cell liver autophagy in rats fed ad libitum or submitted to EOD diet
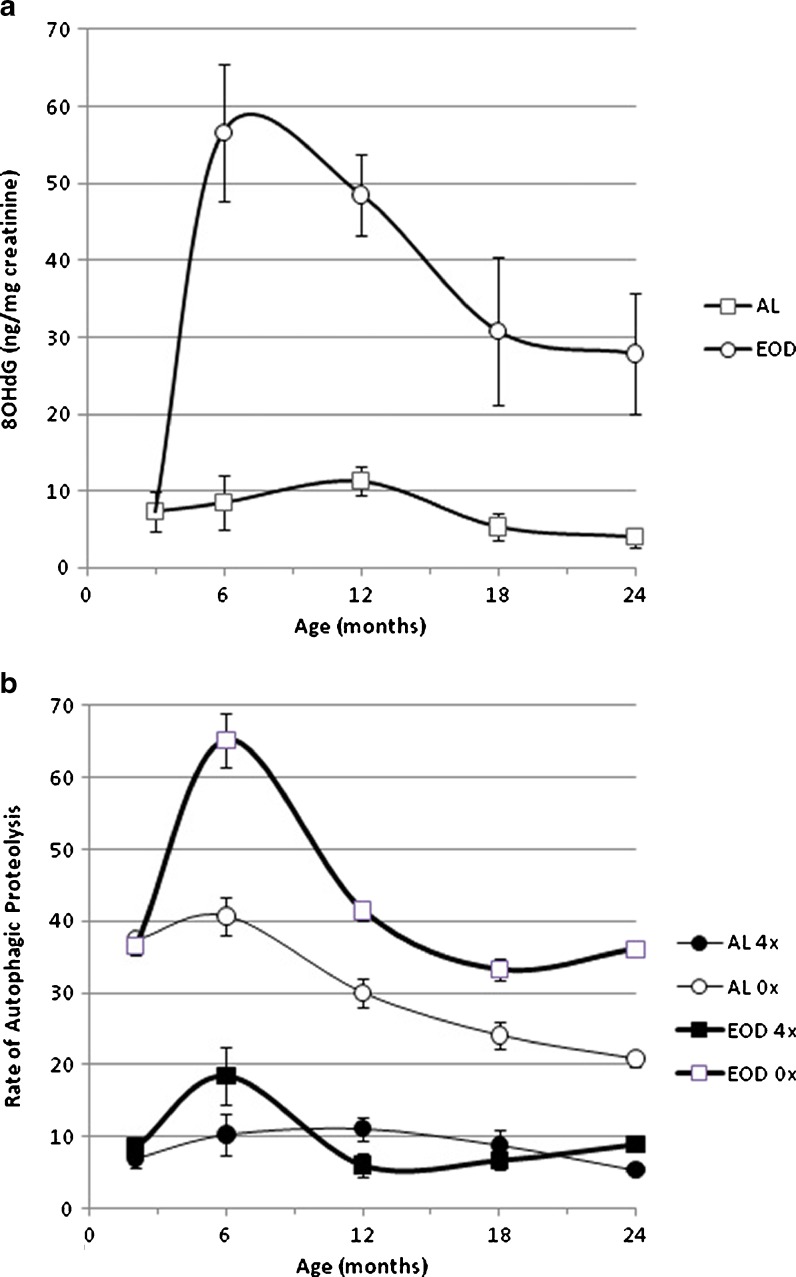
The daily excretion of 8OHdG in the urine of AL and food restricted rats is presented in Fig. 2. In AL animals, excretion slightly declined after age 12 months; in the EOD rats, excretion of 8OHdG increased dramatically after the start of the diet, peaked by age 6 months, stayed high at least until age 12 months and declined thereafter but always remained three- to fourfold higher than that of AL rats.
Fig. 2.
Age-related changes in the urinary levels of 8OHdG (a) and in the rate of autophagic proteolysis of liver cells isolated from rats fed ad libitum or submitted to EOD diet, incubated in the absence (0×) and in the presence of added aminoacid (4×) (b). Results represent the mean ± SEM of at least seven cases. Two-way ANOVA statistical analysis (age × diet): 8OHdG: age main effect, p < 0.0001; diet main effect, p < 0.0001; age by diet interaction, p < 0.0001. Post-ANOVA Tuckey test (p < 0.05), 2 vs 6,12, 18 months; 6 vs 24 months. Autophagic proteolysis: (0×) age main effect, p < 0.0001; diet main effect, p < 0.0001; age by diet interaction, p < 0.0001. Post-ANOVA Tuckey test (p < 0.05), 2 vs 6,18, 24 months; 6 vs 12, 18, 24 months; 12 vs 18, 24 months (4×) age main effect, p = 0.0004; diet main effect, n.s.; age by diet interaction, p = 0.0054. Post-ANOVA Tuckey test (p < 0.05), 2 vs 6 months; 6 vs 12, 18, 24 months
In view of the essential role of autophagy in mitochondrial degradation and in the antiaging mechanism of diet/caloric restriction, here the effects are reported for aging and anti-aging dietary restriction on the minimum and the maximum rate of autophagic proteolysis in in vitro incubated isolated rat liver cells (Fig. 2). The minimum rate of autophagic proteolysis was measured in the presence of an amino acid concentration in the incubation medium similar to the postprandial amino acid concentration in the portal vein and may reflect the postprandial rate of liver autophagic proteolysis, while the maximum rate of proteolysis was measured in the absence of any added amino acid and may reflect the maximum attainable rate of autophagic proteolysis during fasting. The minimum rate of autophagic proteolysis was not significantly affected by increasing age in AL animals and slightly but significantly increased in food-restricted rats by age 6 months; on the other hand, the age-related changes in the maximum rate of autophagic proteolysis were affected significantly by CR. In EOD rats, the increase between 3 and 6 months of age was dramatic, and levels declined thereafter, always remaining higher than AL control for the remainder of life. As a very interesting observation, we noted in AL rats the urinary 8OHdG output appeared to follow the age-related changes in autophagic proteolysis in the “fed” condition; whereas, with EOD rats the urinary 8OHdG pattern resembled age-related changes in the maximum autophagic proteolytic rate. In conclusion, data with fully fed and food-restricted rats suggest that age-related changes in the main pattern of autophagic proteolysis and the 8OHdG urinary output run parallel.
Effects of the intensification of autophagy on the urinary 8OHdG in young rats
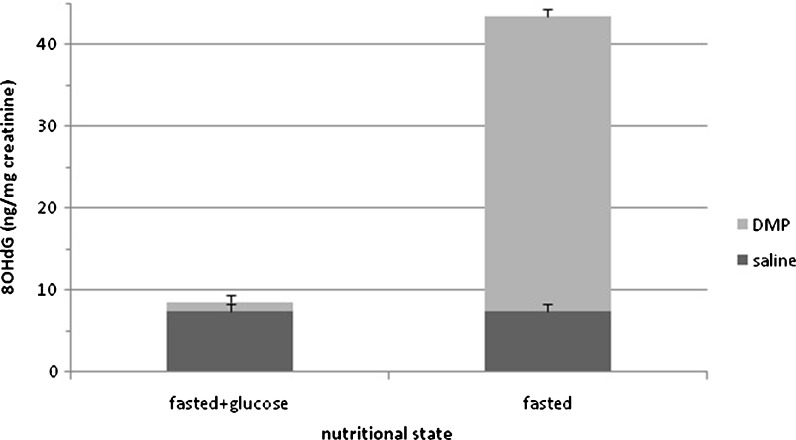
In view of the obtained data, it was decided to further explore the relationship between higher autophagic activity and levels of urinary 8OHdG by examining effects of intensified autophagy induced by the administration of an antilipolytic drug during fasting similar to past reports (Bergamini et al. 1993). As observed in Fig. 3, fasting by itself did not cause any significant increase in the urinary excretion of 8OHdG. The intensification of autophagy by the antilipolytic drug caused a dramatic increase in the urinary output of 8OHdG which was fully suppressed by the administration of glucose (1 g/kg) used to suppress DMP-induced autophagy (Donati et al. 2008).
Fig. 3.
Effects of the induction of autophagy by DMP administration and of suppression by glucose on urinary 8OHdG. Glucose (1 g/kg body weight) was administered after first DMP injection. Results represent the mean ± SEM of five cases. Two-way ANOVA statistical analysis (nutritional state × DMP): nutritional state main effect, p < 0.0001; DMP main effect, p < 0.0001; nutritional state by DMP interaction, p < 0.0001
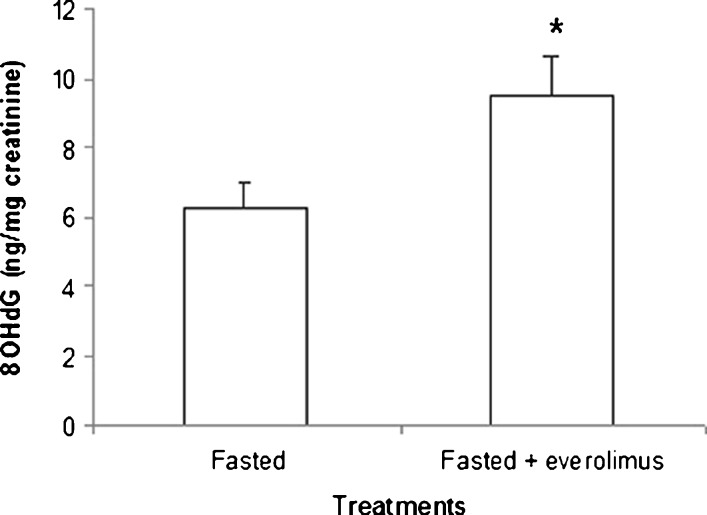
The stimulation of autophagy by blocking inhibitory signaling of mTOR following treatment with everolimus also increased the output of 8OHdG significantly. However, although dosage of everolimus (1 mg/kg body weight) is fully effective in inhibiting the mTOR pathway (Boulay et al. 2004), the increase in 8OHdG output was much smaller than that observed after DMP injection (Fig. 4).
Fig. 4.
Effects of everolimus administration on urinary levels of 8OHdG. *p < 0.05, Student’s t test
Effects of increasing age on the DMP-induced intensification of autophagy and the associated increase in the urinary 8OHdG excretion
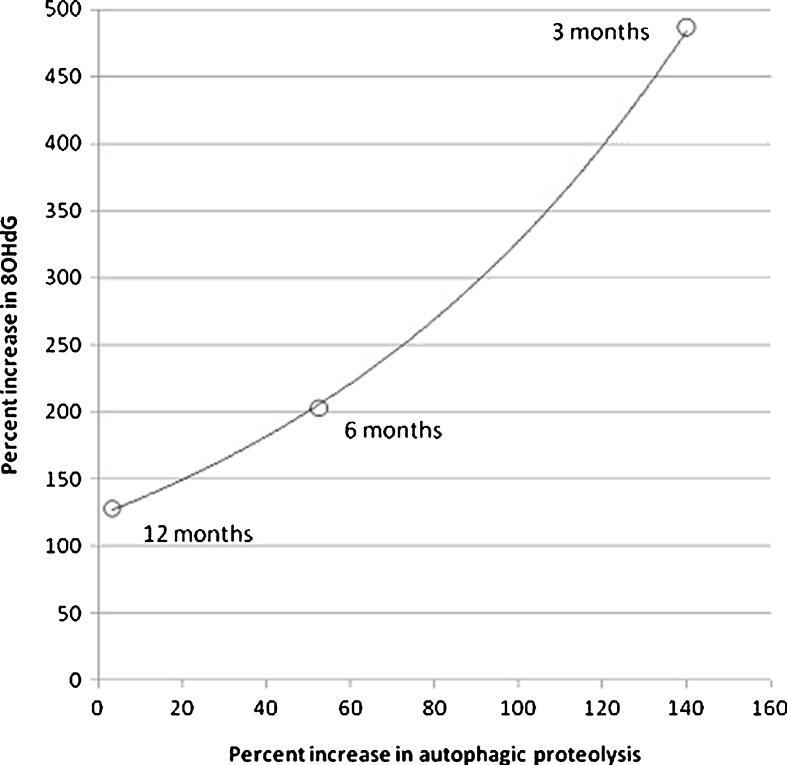
The effects of an in vivo induction of autophagy on the urinary excretion of 8OHdG were tested at different ages. Figure 5 shows that the effects of the administration of DMP to fasted rats on the liver ex vivo autophagy declined progressively with increasing age until 12 months of age and are exponentially correlated with the urinary excretion of 8OHdG. Previous studies showed that the age-associated decline in induced autophagy was not secondary to impediments in the autophagic machinery but rather to the decline in metabolic and endocrine responses to DMP injection (Del Roso et al. 2003).
Fig. 5.
The age-dependent decreases in the effects of DMP administration on autophagic proteolysis and urinary excretion of 8OHdG correlate each other in an exponential fashion. Results are expressed as percent increase in urinary 8OHdG and in ex vivo liver autophagic proteolysis associated with administration of DMP to fasted rats (see “Material and methods” section for details). Control values of liver autophagic proteolysis were 2 months, 22 ± 5; 6 months, 19 ± 2.1; 12 months, 30 ± 3.3 (milligrammes of labelled protein degraded/100 g body weight per hour). Control values of urinary 8OHdG were 2 months, 7.4 ± 0.33; 6 months, 13.9 ± 0.61; 12 months, 6.86 ± 0.85 (nanogrammes 8OHdG/mg creatinine)
Discussion
Our data clearly reveal that aging, CR and autophagy, all have remarkable effects on the 8-OHdG output into urine, and that the effects of aging and CR are likely to be secondary to their effects on autophagy. An age-dependent decrease in urinary excretion of 8OHdG was reported by Fraga et al. (1990) in the Fischer 344 rat. Studies of effects of CR on this marker have produced conflicting results with some reports of no effects (Hofer et al. 2008): increased 8OHdG under CR (Loft et al. 1995), and decreased levels (Simic and Bergtold 1991) in urine 8OHdG in CR animals. Reports of effect of increased autophagy on the urinary excretion of 8OHdG have not been established previously.
Our discovery that increased autophagy is associated with an increased urinary output of 8OHdG appears to be the key finding. Results could be expected from our previous observation that an acute pharmacological stimulation of autophagy can rescue rats from the twofold increase in mtDNA 8OHdG content observed between ages 3 and 16 months in less than 6 h. Specifically we observed that levels of 8OHdG in liver mtDNA returned to juvenile values without any appreciable change in mitochondrial mass, suggesting a selective removal of few severely injured mitochondria (Donati et al. 2006; Cavallini et al. 2007). In view of the observed correlations (Figs. 2 and 5), it is conceivable that the age-related decline in autophagy function might impair the degradation of altered mitochondria and lower levels of 8OHdG output into urine, and that CR may increase 8OHdG urinary levels by preventing the age-related decline of autophagy.
To better appreciate the role of the autophagic degradation of mitochondria (mitophagy) in the urinary excretion of 8OHdG, this process was intensified in vivo by the administration of an antilipolytic agent (DMP) and also by everolimus. Treatment with DMP intensifies the physiological response to fasting and causes a decrease in blood glucose and insulin and an increase in plasma glucagon and corticosteroids. These metabolic and endocrine changes induce liver autophagy and autophagic proteolysis, and increase the expression of LC3, a protein involved in autophagosome formation (Donati et al. 2008). The suppression of hypoglycaemia and endocrine changes by an injection of glucose can attenuate autophagy induction (Donati et al. 2008). In the present study, we show that in the 6-h time period required for the autophagic removal of the oxidatively altered mitochondria, levels of 8OHdG in the urine exhibited a sevenfold increase and that the increase was not observed if autophagy was blocked by glucose administration (Fig. 3). The induction of autophagy by blocking the inhibitory signal of mTOR caused a much smaller increase of urinary 8OHdG. It is known that rapamycin is unable to completely remove the inhibitory effect of an amino acid mixture on autophagy function of liver cells (Blommaart et al. 1995). Furthermore, recent results show that mTOR may not be the unique pathway which controls autophagy in response to starvation. Lysosome positioning may be a major cofactor (Korolchuk et al. 2011). In addition, mTOR may be an inhibitor of the process during feeding state opposed to a stimulatory signal, which could activate autophagy during fasting (Kim et al. 2011).
With regard to aging, past reports have shown a progressive increase in the DNA oxidative damage measured as tissue content of 8OHdG (Wu et al. 2004). It was already mentioned that mtDNA is ten times more abundant in 8OHdG than nDNA (Nakamoto et al. 2007) and may be the major source of urinary 8OHdG. Previous research from this laboratory (Donati et al. 2006; Cavallini et al. 2007) has shown that the age-related decline in efficiency of autophagy may be the main causative factor, since stimulation may rescue older cells from 8OHdG accumulation in mtDNA in less than 6 h. It was already emphasized that autophagy is the cellular repair mechanism that engulfs any altered mitochondria (“mitophagy”) and degrades their components including mtDNA. The observed correlation between the age-associated changes in autophagy and the urinary output of 8OHdG (Figs. 2 and 5) is in line with this hypothesis.
With regard to CR, this intervention has been known for years to extend the healthspan and lifespan of virtually all tested animal species by slowing aging processes (Masoro 2009) by keeping cells “cleaner” and improving cell repair function and quality of all cell components (Donati 2006). Over the last three decades, numerous laboratories examined the effects of CR on the integrity of the genome and the ability of cells to repair DNA and have shown that CR significantly reduces age-related accumulation of oxidative damage to DNA and enhances DNA repair, including major DNA repair pathways, such as, nucleotide excision repair (NER), BER and double-strand break repair (Unnikrishnan et al. 2007). However, our data show that the beneficial effects of CR on the age-related accumulation of oxidative damage to DNA may be secondary to the amelioration of the function of mitophagy and argue against the hypothesis that a major anti-aging mechanism of CR relates to decreased levels of oxidative stress inside the mitochondrial compartment, thus preventing mtDNA oxidation. Indeed, long-term CR was shown to be associated with a dramatic increase in the urinary output of 8OHdG. As pointed out by Feuers et al. (1993), most findings on decreased generation of reactive oxygen species are from in vitro studies, and little is known about the effects of CR on in vivo free radical production. If the in vivo free radical production (and mtDNA oxidative damage) correlates with oxygen consumption and energy production and consumption, perhaps papers should be recalled here showing that energy consumption per unit lean body mass is not be altered by long-term CR (McCarter et al. 1985). However, due to the autophagic removal of the altered (high free-radical generators) mitochondria that otherwise would accumulate in older cells, perhaps CR might have the secondary effect of preventing an age-related increase in the oxidative damage to extramitochondrial cell components, including nDNA. Since 8OHdG is remarkably stable in urine and is not degraded nor produced by artifactual oxidation of dG in the systemic circulation, measurement of the urinary levels of 8OHdG can be offered as a non-invasive means of oxidative stress assessment (Cooke et al. 2008). Support for this hypothesis emerges from demonstrations that high levels of urine 8OHdG are associated with smoking, air pollution and pathologies that generate reactive oxygen species (Cooke et al. 2008). However, in this context it is important to emphasize that the magnitude of observed changes in urinary excretion is rather small, for example, the effect of smoking is an increase of 15–50%, and the effect of cruciferous vegetable is a reduction of 30% in urinary 8OHdG (Loft et al. 1992; Prieme et al. 1998; Verhagen et al. 1995). In accord with Fraga et al. (1990), our data reveal that biological aging, which is characterized by the accumulation of 8OHdG in mt- and n-DNA of older cells (Hamilton et al. 2001) is not associated with any increase in the urinary output of 8OHdG and that CR, which prevents the accumulation, increases it. Furthermore, the stimulation of autophagy causes an almost immediate increase in the urinary output of 8OHdG, which can be attenuated simply by injecting glucose and cannot be attributed to changes in the level of oxidative stress. Hence, there is no support for the hypothesis that the age-related changes in the urine 8OHdG levels may be due to a change in oxidative damage or in the activity of whichever DNA repair enzymes, even if NER, transcription-coupled repair, nucleotide incision repair, mismatch repair and various exonuclease activities, can all feasibly generate initial products that could yield 8-oxodG after further metabolism (Evans et al. 2010). On the contrary, there is strong support for the hypothesis that the autophagic removal of mitochondria could be a major source of urinary 8OHdG. The role of autophagy in the disposal of 8OHdG into urine is confirmed by the previously mentioned association between the age-related changes in autophagy response in vivo to DMP and changes in urinary excretion of 8OHdG. The function of autophagy was evaluated ex vivo by measuring the autophagic proteolytic function of the perfused liver in the 150-min period following DMP administration. Results indicated that both autophagy and its associated increase in urine 8OHdG progressively decline between 3 and 12 months of age and correlate with each other.
In summary, we conclude that urinary excretion of 8OHdG mainly reflects the moment-to-moment activity of autophagy, and that the assay for urinary 8OHdG levels before and after the stimulation of autophagy by an antilipolytic drug may provide a novel, non-invasive and safe procedure to monitor the in vivo functioning of this process.
References
- Bergamini E, Gori Z. Towards an understanding of the biological mechanism of dietary restriction: a signal transduction theory of aging. Aging Clin Exp Res. 1995;7:473–475. doi: 10.1007/BF03324374. [DOI] [Google Scholar]
- Bergamini E, Del Roso A, Fierabracci V, Gori Z, Masiello P, Masini M, Pollera M. A new method for the investigation of endocrine-regulated autophagy and protein degradation in rat liver. Exp Mol Pathol. 1993;59:13–26. doi: 10.1006/exmp.1993.1023. [DOI] [PubMed] [Google Scholar]
- Bergamini E, Cavallini G, Donati A, Gori Z. The role of macroautophagy in the ageing process, anti-ageing intervention and age-associated diseases. Int J Biochem Cell Biol. 2004;36(12):2392–2404. doi: 10.1016/j.biocel.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Bergamini E, Cavallini G, Donati A, Gori Z. The role of autophagy in aging: its essential part in the anti-aging mechanism of caloric restriction. Ann N Y Acad Sci. 2007;1114:69–78. doi: 10.1196/annals.1396.020. [DOI] [PubMed] [Google Scholar]
- Blommaart EF, Luiken JJ, Blommaart PJ, van Woerkom GM, Meijer AJ. Phosphorylation of ribosomal protein S6 is inhibitory for autophagy in isolated rat hepatocytes. J Biol Chem. 1995;270(5):2320–2326. doi: 10.1074/jbc.270.5.2320. [DOI] [PubMed] [Google Scholar]
- Bohr VA. Repair of oxidative DNA damage in nuclear and mitochondrial DNA, and some changes with aging in mammalian cells. Free Radic Biol Med. 2002;32(9):804–812. doi: 10.1016/S0891-5849(02)00787-6. [DOI] [PubMed] [Google Scholar]
- Boulay A, Zumstein-Mecker S, Stephan C, Beuvink I, Zilbermann F, Haller R, Tobler S, Heusser C, O'Reilly T, Stolz B, Marti A, Thomas G, Lane HA. Antitumor efficacy of intermittent treatment schedules with the rapamycin derivative RAD001 correlates with prolonged inactivation of ribosomal protein S6 kinase 1 in peripheral blood mononuclear cells. Cancer Res. 2004;64(1):252–261. doi: 10.1158/0008-5472.CAN-3554-2. [DOI] [PubMed] [Google Scholar]
- Burtis CA, Ashwood ER, Bruns DE. Tietz textbook of clinical chemistry and molecular diagnostic. 4. St. Louis: Elsevier; 2009. [Google Scholar]
- Cavallini G, Donati A, Taddei M, Bergamini E. Evidence for selective mitochondrial autophagy and failure in aging. Autophagy. 2007;3(1):26–27. doi: 10.4161/auto.3268. [DOI] [PubMed] [Google Scholar]
- Cooke MS, Olinski R, Loft S, European Standards Committee on Urinary (DNA) Lesion Analysis Measurement and meaning of oxidatively modified DNA lesions in urine. Cancer Epidemiol Biomark Prev. 2008;17(1):3–14. doi: 10.1158/1055-9965.EPI-07-0751. [DOI] [PubMed] [Google Scholar]
- Del Roso A, Vittorini S, Cavallini G, Donati A, Gori Z, Masini M, Pollera M, Bergamini E. Ageing-related changes in the in vivo function of rat liver macroautophagy and proteolysis. Exp Gerontol. 2003;38(5):519–527. doi: 10.1016/S0531-5565(03)00002-0. [DOI] [PubMed] [Google Scholar]
- Donati A. The involvement of macroautophagy in aging and anti-aging interventions. Mol Aspects Med. 2006;27(5–6):455–470. doi: 10.1016/j.mam.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Donati A, Taddei M, Cavallini G, Bergamini E. Stimulation of macroautophagy can rescue older cells from 8-OHdG mtDNA accumulation: a safe and easy way to meet goals in the SENS agenda. Rejuvenation Res. 2006;9(3):408–412. doi: 10.1089/rej.2006.9.408. [DOI] [PubMed] [Google Scholar]
- Donati A, Ventruti A, Cavallini G, Masini M, Vittorini S, Chantret I, Codogno P, Bergamini E. In vivo effect of an antilipolytic drug (3,5'-dimethylpyrazole) on autophagic proteolysis and autophagy-related gene expression in rat liver. Biochem Biophys Res Commun. 2008;366(3):786–792. doi: 10.1016/j.bbrc.2007.12.023. [DOI] [PubMed] [Google Scholar]
- Donati A, Cavallini G, Bergamini E. Methods for inducing and monitoring liver autophagy relative to aging and antiaging caloric restriction in rats. Methods Enzymol. 2009;452:441–455. doi: 10.1016/S0076-6879(08)03626-4. [DOI] [PubMed] [Google Scholar]
- Evans MD, Saparbaev M, Cooke MS. DNA repair and the origins of urinary oxidized 2'-deoxyribonucleosides. Mutagenesis. 2010;25(5):433–442. doi: 10.1093/mutage/geq031. [DOI] [PubMed] [Google Scholar]
- Feuers RJ, Weindruch R, Hart RW. Caloric restriction, aging, and antioxidant enzymes. Mutat Res. 1993;295(4–6):191–200. doi: 10.1016/0921-8734(93)90020-4. [DOI] [PubMed] [Google Scholar]
- Fraga CG, Shigenaga MK, Park JW, Degan P, Ames BN. Oxidative damage to DNA during aging: 8-hydroxy-2-deoxyguanosine in rat organ DNA and urine. Proc Natl Acad Sci U S A. 1990;7:4533–4537. doi: 10.1073/pnas.87.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber J, Schaffer S, Halliwell B. The mitochondrial free radical theory of ageing—where do we stand? Front Biosci. 2008;13:6554–6579. doi: 10.2741/3174. [DOI] [PubMed] [Google Scholar]
- Hamilton ML, Van Remmen H, Drake JA, Yang H, Guo ZM, Kewitt K, Walter CA, Richardson A. Does oxidative damage to DNA increase with age? Proc Natl Acad Sci U S A. 2001;98(18):10469–10474. doi: 10.1073/pnas.171202698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartford CM, Ratain MJ. Rapamycin: something old, something new, sometimes borrowed and now renewed. Clin Pharmacol Ther. 2007;82(4):381–388. doi: 10.1038/sj.clpt.6100317. [DOI] [PubMed] [Google Scholar]
- Hofer T, Fontana L, Anton SD, Weiss EP, Villareal D, Malayappan B, Leeuwenburgh C. Long-term effects of caloric restriction or exercise on DNA and RNA oxidation levels in white blood cells and urine in humans. Rejuvenation Res. 2008;11(4):793–799. doi: 10.1089/rej.2008.0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S. Autophagy, mitochondrial quality control, and oncogenesis. Autophagy. 2006;2(2):80–84. doi: 10.4161/auto.2.2.2460. [DOI] [PubMed] [Google Scholar]
- Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13(2):132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korolchuk VI, Saiki S, Lichtenberg M, Siddiqi FH, Roberts EA, Imarisio S, Jahreiss L, Sarkar S, Futter M, Menzies FM, O'Kane CJ, Deretic V, Rubinsztein DC. Lysosomal positioning coordinates cellular nutrient responses. Nat Cell Biol. 2011;13(4):453–460. doi: 10.1038/ncb2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loft S, Vistisen K, Ewertz M, Tjonneland A, Overvad K, Poulsen HE. Oxidative DNA damage estimated by 8-hydroxydeoxyguanosine excretion in humans: influence of smoking, gender and body mass index. Carcinogenesis. 1992;13:2241–2247. doi: 10.1093/carcin/13.12.2241. [DOI] [PubMed] [Google Scholar]
- Loft S, Velthuis-te Wierik EJ, van den Berg H, Poulsen HE. Energy restriction and oxidative DNA damage in humans. Cancer Epidemiol Biomark Prev. 1995;4(5):515–519. [PubMed] [Google Scholar]
- Masoro EJ. Caloric restriction-induced life extension of rats and mice: a critique of proposed mechanisms. Biochim Biophys Acta. 2009;1790(10):1040–1048. doi: 10.1016/j.bbagen.2009.02.011. [DOI] [PubMed] [Google Scholar]
- McCarter R, Masoro EJ, Yu BP. Does food restriction retard aging by reducing the metabolic rate? Am J Physiol. 1985;248:E488–E490. doi: 10.1152/ajpendo.1985.248.4.E488. [DOI] [PubMed] [Google Scholar]
- Mizushima N. Physiological functions of autophagy. Curr Top Microbiol Immunol. 2009;335:71–84. doi: 10.1007/978-3-642-00302-8_3. [DOI] [PubMed] [Google Scholar]
- Mortimore GE, Mondon CE. Inhibition by insulin of valine turnover in liver. Evidence for a general control of proteolysis. J Biol Chem. 1970;245(9):2375–2383. [PubMed] [Google Scholar]
- Mortimore GE, Pösö AR. Intracellular protein catabolism and its control during nutrient deprivation and supply. Annu Rev Nutr. 1987;7:539–564. doi: 10.1146/annurev.nu.07.070187.002543. [DOI] [PubMed] [Google Scholar]
- Nakamoto H, Kaneko T, Tahara S, Hayashi E, Naito H, Radak Z, Goto S. Regular exercise reduces 8-oxodG in the nuclear and mitochondrial DNA and modulates the DNA repair activity in the liver of old rats. Exp Gerontol. 2007;42(4):287–295. doi: 10.1016/j.exger.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Pfeifer U. Inhibition by insulin of the formation of autophagic vacuoles in rat liver. A morphometric approach to the kinetics of intracellular degradation by autophagy. J Cell Biol. 1978;78:152–167. doi: 10.1083/jcb.78.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieme H, Loft S, Klarlund M, Gronbaek K, Tonnesen P, Poulsen HE. Effect of smoking cessation on oxidative DNA modification estimated by 8-oxo-7,8-dihydro-2- deoxyguanosine excretion. Carcinogenesis. 1998;19:347–351. doi: 10.1093/carcin/19.2.347. [DOI] [PubMed] [Google Scholar]
- Richter C, Park JW, Ames BN (1988) Normal oxidative damage to mitochondrial and nuclear DNA is extensive. Proc Natl Acad Sci U S A 85(17):6465–7 [DOI] [PMC free article] [PubMed]
- Simic MG, Bergtold DS. Dietary modulation of DNA damage in human. Mutat Res. 1991;250(1–2):17–24. doi: 10.1016/0027-5107(91)90158-K. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273(5271):59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal RS, Agarwal S, Candas M, Forster MJ, Lal H. Effect of age and caloric restriction on DNA oxidative damage in different tissues of C57BL/6 mice. Mech Ageing Dev. 1994;76(2–3):215–224. doi: 10.1016/0047-6374(94)91595-4. [DOI] [PubMed] [Google Scholar]
- Terman A, Kurz T, Navratil M, Arriaga EA, Brunk UT. Mitochondrial turnover and aging of long-lived postmitotic cells: the mitochondrial–lysosomal axis theory of aging. Antioxid Redox Signal. 2010;12:503–535. doi: 10.1089/ars.2009.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unnikrishnan A, Lucente LV, Richardson A. Caloric restriction and genomic stability. Nucleic Acids Res. 2007;35(22):7485–7496. doi: 10.1093/nar/gkm860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zutphen T, Veenhuis M, van der Klei IJ. Damaged peroxisomes are subject to rapid autophagic degradation in the yeast Hansenula polymorpha. Autophagy. 2011;7(8):863–872. doi: 10.4161/auto.7.8.15697. [DOI] [PubMed] [Google Scholar]
- Verhagen H, Poulsen HE, Loft S, van Poppel G, Willems MI, van Bladeren PJ. Reduction of oxidative DNA-damage in humans by Brussels sprouts. Carcinogenesis. 1995;16:969–970. doi: 10.1093/carcin/16.4.969. [DOI] [PubMed] [Google Scholar]
- Wu LL, Chiou CC, Chang PY, Wu JT. Urinary 8-OHdG: a marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clin Chim Acta. 2004;339(1–2):1–9. doi: 10.1016/j.cccn.2003.09.010. [DOI] [PubMed] [Google Scholar]