Abstract
Midlife elevated blood pressure and hypertension contribute to the development of Alzheimer's disease (AD) and overall dementia. We sought to estimate whether angiotensin-converting enzyme inhibitors (ACE-Is) reduced the risk of developing mild cognitive impairment (MCI) in cognitively normal individuals. In the Italian Longitudinal Study on Aging, we evaluated 1,445 cognitively normal individuals treated for hypertension but without congestive heart failure from a population-based sample from eight Italian municipalities with a 3.5-year follow-up. MCI was diagnosed with current clinical criteria. Dementia, AD, and vascular dementia were diagnosed based on DSM-IIIR criteria, NINCDS–ADRDA criteria, and ICD-10 codes. Among 873 hypertension-treated cognitively normal subjects, there was no significant association between continuous exposure to all ACE-Is and risk of incident MCI compared with other antihypertensive drugs [hazard ratio (HR), 0.45, 95% confidence interval (CI), 0.16–1.28]. Captopril exposure alone did not significantly modify the risk of incident MCI (HR, 1.80, 95% CI, 0.39–8.37). However, the enalapril sub-group alone (HR, 0.17, 95% CI, 0.04 –0.84) or combined with the lisinopril sub-group (HR, 0.27, 95% CI, 0.08–0.96), another ACE-I structurally related to enalapril and with similar potency, were associated with a reduced risk of incident MCI. Study duration exposure to ACE-Is as a “class” was not associated with incident MCI in older hypertensive adults. However, within-class differences linked to different chemical structures and/or drug potencies may exist, with a possible effect of the enalapril and lisinopril sub-groups in reducing the risk of incident MCI.
Keywords: Angiotensin-converting enzyme inhibitors, Mild cognitive impairment, Dementia, Antihypertensive drugs
Introduction
Epidemiological evidence links hypertension to cognitive decline (Qiu et al. 2005; Peters and Beckett 2009). Both longitudinal and cross-sectional studies suggested that elevated midlife blood pressure or hypertension contribute to the development of Alzheimer's disease (AD) and overall dementia, although this effect is weaker in the years preceding dementia onset (Panza et al. 2010). In particular, hypertension at baseline was related to greater cognitive decline or mild cognitive impairment (MCI) (Panza et al. 2010). However, blood pressure reduction in randomized controlled trials (RCTs) demonstrated a complex association with reduced cognitive function, supporting the hypothesis that mechanisms beyond the blood-pressure-lowering effect of antihypertensive medications may be involved. In fact, several RCTs examined the impact of different antihypertensive drug classes, also including ACE-Is, upon cognitive function, incident dementia, or both, although not as a primary outcome, with conflicting results (Forette et al. 2002; Tzourio et al. 2003; Lithell et al. 2003; McGuinness et al. 2006; Birns et al. 2006; Peters et al. 2008; McGuinness et al. 2009; Staessen et al. 2011).
The nature of the involvement of the renin–angiotensin system (RAS) in AD remains controversial; a combination of preclinical and clinical evidence demonstrates abnormalities in this system that have the potential to exacerbate the disease (Kehoe and Wilcock 2007; Kehoe et al. 2009). Clinical studies investigating whether angiotensin-converting enzyme inhibitors (ACE-Is) and/or angiotensin receptor blockers (ARBs), which inhibit the production and action of angiotensin II, respectively, merit consideration as a treatment for cognitive decline have yielded contrasting findings (Forette et al. 2002; Tzourio et al. 2003; Ohrui et al. 2004a; Khachaturian et al. 2006; Peters et al. 2008; Sink et al. 2009; Li et al. 2010). Currently, large-scale clinical trials of ACE-Is in AD and cognitive decline are lacking (Weiner et al. 1992; Sudilovsky et al. 1993; Louis et al. 1999; Ohrui et al. 2004b). In other small observational studies of individuals with MCI, ACE-Is slowed cognitive decline and reduced progression to AD (Hajjar et al. 2005; He et al. 2006; Rozzini et al. 2006, 2008).
ACE-Is are described collectively as a “class” of drugs, but in reality, this classification is one of convenience based solely on their biological function which is the inhibition of ACE. Indeed, in some cases, these drugs are not only structurally similar but have similar potency (e.g., IC50 on ACE = enalapril 1.9 nM and lisinopril 1.5 nM) (Brown and Vaughan 1998). However, some studies have sub-grouped these drugs for analytical purposes according to their penetrance of the blood brain barrier (BBB) which would be expected to be important for diseases such as AD, but there are conflicting reports on the penetration of some ACE-Is (Jackson et al. 1987; Gohlke et al. 1989; Cushman et al. 1989; Tan et al. 2005), questioning the validity of this manner of sub-grouping (Miners et al. 2009). Instead, in the present study, we opted to sub-group drugs according to their chemical structures, which offers less ambiguity in some respects, although the potential for differential pharmacological profiles remains (Thind 1990; Ranadive et al. 1992). Our rationale is supported by evidence that the sulfhydryl containing molecules (e.g., captopril) have beneficial antioxidant properties (Westlin and Mullane 1988), but that sulfhydryl containing drugs tend to have similar clinical adverse reactions (Jaffe 1986) which bias prescription rates in some groups. Using a large, population-based cohort, we investigated whether ACE-Is collectively as a “class” and as sub-groups according to their chemical structures and drug potencies, compared with other antihypertensive agents and beyond the natural course in time of the hypertension, reduced the incidence of MCI in cognitively normal individuals.
Methods
Setting
The data of the present study were derived from the Italian Longitudinal Study on Aging (ILSA), the methods of which, including the first and second survey data collection, have been described elsewhere (Solfrizzi et al. 2004). Briefly, 5,632 subjects aged 65–84 years, free-living or institutionalized, were randomly selected from the electoral rolls of eight Italian municipalities after stratification for age and gender. In each of the eight centers, stratification was carried out by equal allocation strategy: four age groups of 88 subjects (65 to 69, 70 to 74, 75 to 79, and 80 to 84 years old) stratified by gender were randomly identified. The data presented have been derived from the first (March 1992 to June 1993, prevalence day: March 1st, 1992) and second (September 1995 to October 1996, prevalence day: September 1st, 1995) prevalence survey studies (Solfrizzi et al. 2004). The study project was approved by the Institutional Review Board of the eight municipalities. Informed consent was obtained from each subject before enrollment.
Clinical examination
Cases of coronary artery disease (CAD) (myocardial infarction or angina pectoris), type 2 diabetes mellitus, hypertension, stroke, and congestive heart failure (CHF) were identified by a two-phase procedure, utilizing clinical criteria described in detail elsewhere (Solfrizzi et al. 2004). Phase 1 saw subjects undergo clinical evaluation and a series of brief screening questionnaires and tests to identify cases for further investigation. In Phase 2, identified cases were clinically confirmed by standardized clinical examination by a certified neurologist or geriatrician. In particular, the diagnosis of hypertension fulfilled the criteria of the 1988 Report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure (no authors listed 1988) and was based on a screening phase with either a self-reported diagnosis or medical treatment or a recorded mean diastolic value ≥ 90 mm Hg or a systolic value ≥ 140 mm Hg. The values used in the analyses were the mean of the last two of the three sitting blood pressure measurements performed. Phase 2 consisted of a review of clinical records and a further blood pressure measurement with confirmation of the diagnosis. The main screening criteria used for cognitive impairment or dementia were the Mini Mental State Examination (MMSE) (cutoff score < 24) (Folstein et al. 1975) or a previous diagnosis reported by the respondent proxy. The MMSE has been previously validated in each of the eight study centers against the Diagnostic and Statistical Manual of Mental Disorders (DSM)–III-R (American Psychiatric Association 1987) clinical diagnosis of dementia; the cutoff score < 24 has a sensitivity of 95% and a specificity of 90% (Solfrizzi et al. 2004). Diagnosis was based on DSM-III-R criteria for dementia syndrome, NINCDS–ADRDA criteria for possible and probable AD (McKhann et al. 1984), and ICD-10 criteria for vascular dementia (VaD) and other dementing diseases (World Health Organization 1992). Smoking habits were self-reported on the number of cigarettes smoked and the ages when they started and stopped smoking, from which variable “pack–years” were derived [years smoked*usual number of cigarettes smoked/20 cigarettes per pack]. Abdominal circumference was measured with flexible steel tape to the nearest centimeter, in standing subjects at the level of the umbilicus (Sergi et al. 2005).
Mild cognitive impairment diagnosis
For the diagnosis of MCI, we generally adhered to the diagnostic criteria as defined by Petersen and colleagues (Petersen et al. 1999), with some modifications (Solfrizzi et al. 2004, 2007), and we did not require subjective memory complaints (SMC), allowing also for the presence of non-cognitive disabilities and comorbid illnesses. We retroactively applied these criteria to the data collected in this study between 1992 and 1995: (1) no dementia; (2) normal general cognitive functioning as assessed by MMSE using age- and education-based norms (this cutoff was calculated by subtracting 1.5 SD from the mean age- and education-adjusted MMSE scores after excluding subjects with prevalent dementia). Elderly subjects with MMSE-adjusted scores greater than this cutoff were considered normal in terms of general cognitive functioning; (3) objective evidence of memory impairment as assessed by a total Babcock Story Recall Test score (immediate plus delayed recall) (Spinnler and Tognoni 1987) in the lowest tenth percentile of the distribution of age- and education-adjusted scores after exclusion of prevalent dementia at entry; and (4) independence in the basic activities of daily living, as measured by Activities of Daily Living scale (ADL) (Lawton and Brody 1969). In summary, our inclusion criteria to assess the functional status of MCI subjects included (1) subjects with no functional impairment (ADL = 6); (2) subjects who were slightly impaired (ADL = 7 or 8) but with no Instrumental Activities of Daily Living scale (Katz and Akpom 1976) impairment; (3) subjects with visual, auditory, or skeletal muscle (i.e., stroke) disabilities compromising ADL, but not cognitive skills; and 4) subjects with ADL impaired by comorbid illnesses (presence of two or more diseases).
Exposure
Many ACE-Is exist and, despite their shared function, can be further sub-divided into three groups based on chemical structures. These include (1) sulfhydryl-containing ACE-Is structurally related to captopril (i.e., fentiapril, pivalopril, zofenopril, and alacepril), (2) dicarboxyl-containing ACE-Is structurally related to enalapril (i.e., lisinopril, benazepril, quinapril, moexipril, ramipril, spirapril, perindopril, pentopril, and cilazapril), and (3) phosphorus-containing ACE-Is structurally related to fosinopril (Jackson 2001). It was taken into account that these drugs are not only structurally similar, but some of them have similar potency as for enalapril and lisinopril. We examined subject exposure to the drugs following the collection of a detailed pharmacological history as well as examination of drug boxes following request. Participants determined to already have dementia at the first evaluation were excluded, and analyses were restricted to participants who had treated or untreated self-reported hypertension, participants who were taking antihypertensive medication, or people unaware they had hypertension. Hypertension diagnosis was confirmed in each individual by medical examination. Patients with CHF at baseline were also excluded because of common use of ACE-Is and possible interaction with cognitive performance. A total of 873 non-cognitively impaired individuals were available for the present study (Fig. 1).
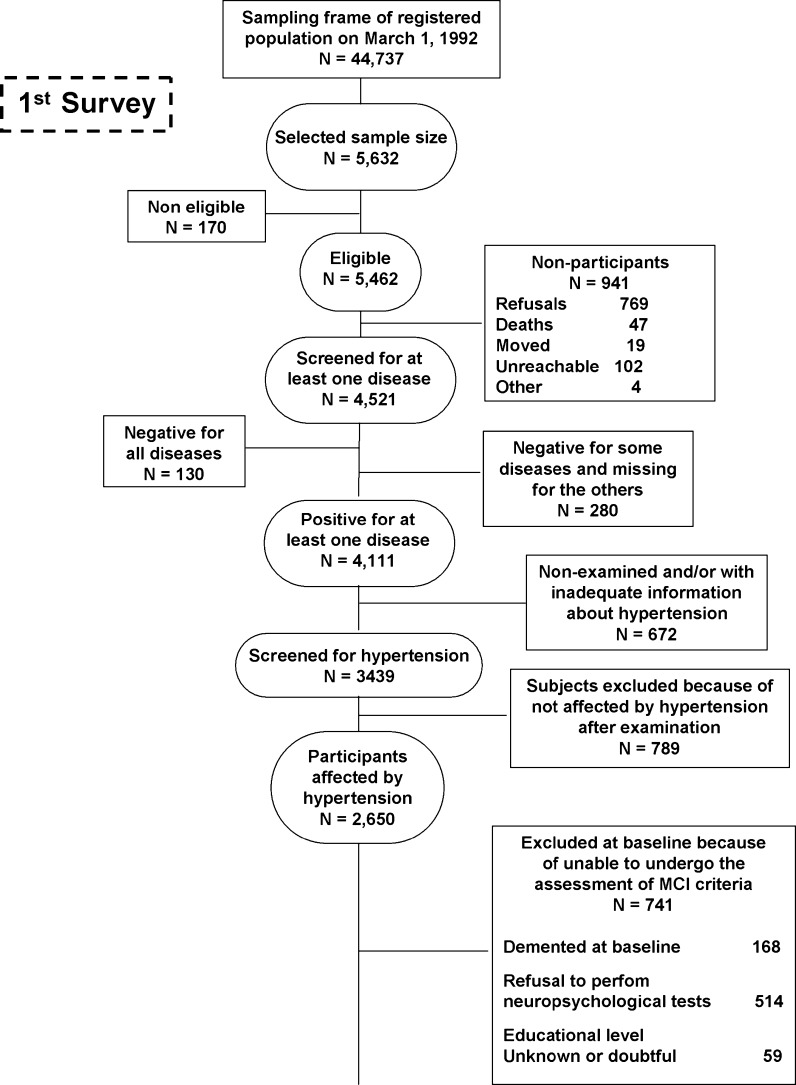
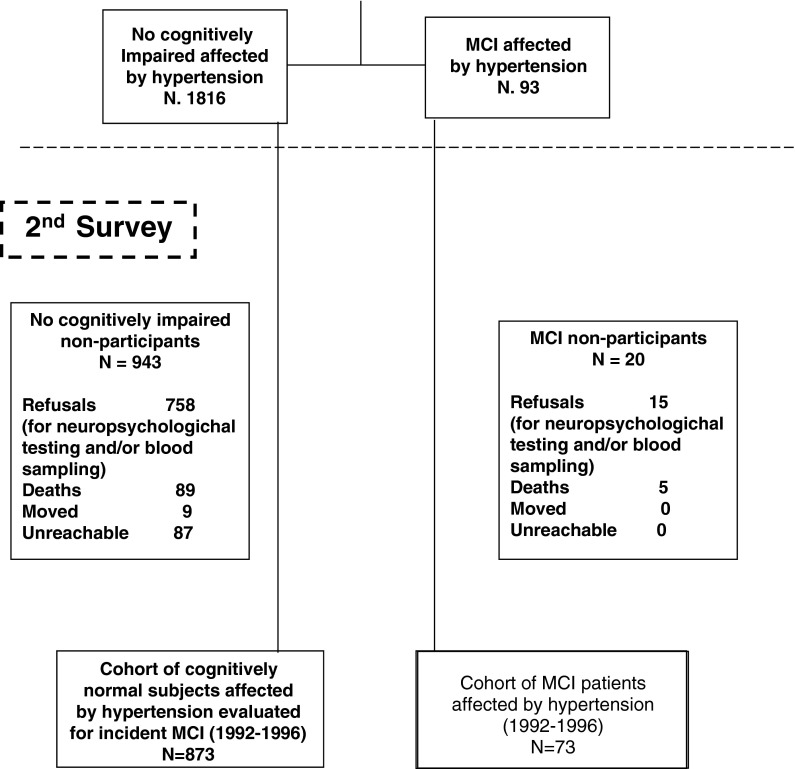
Fig. 1.
Attrition of the study population at the different phases of the survey, Italian Longitudinal Study on Aging, 1992–1996. MCI mild cognitive impairment
Statistical analysis
Analyses were performed using SAS statistical software (SAS/STAT user's guide, version 9.1 Cary, NC: SAS Institute, 2004). Continuous variables were examined with t-tests or Mann–Whitney test, and categorical variables were examined using the χ2 test. We used time-dependent Cox regression analyses to model the relationship of time to the development of MCI in cognitively normal individuals. In fact, the analyses did not meet proportionality of hazards assumptions for ACE-I use, checked by plotting log-minus-log curves. ACE-Is exposure was recorded longitudinally. Cumulative exposure to ACE-Is was defined as the total number of years, within the study follow-up, in which the participant had been continually taking ACE-Is. Time covariate was coded as 0 to indicate no exposure at first and/or second survey, 0 exposure at first survey, and 3.5 for cumulative exposure to ACE-I second survey. Drug use was determined on the basis of no exposure to the other antihypertensive study drugs on entry and over the course of the study. Cohorts who shared similar health profiles were selected to avoid bias due to misclassification. In order to assess the possible mechanism for the beneficial effects of these ACE-Is, we used two different reference ACE-I naive groups: Those who were (1) exposed to other hypertensive medications (calcium channel blockers, beta blockers, and diuretics) and (2) who had untreated hypertension. We adjusted for the use of other antihypertensive medications at each visit to assess the impact of ACE-Is exposure on our outcome measures independent of exposure to other antihypertensive agents. We included people with untreated hypertension because hypertension management contributes to the global risk of incident MCI. Finally, to avoid confounding by multiple indication for ACE-Is use, we restricted our study population to patients treated with these medications, excluding those who, at baseline and during the study period (i.e., new cases), were affected by other diseases such as CHF. In this cognitively normal cohort, we performed secondary analyses considering the enalapril sub-group alone (and the enalapril plus lisinopril combined sub-groups) vs. the captopril sub-group. Exposure to ACE-Is of less than 5 % at baseline was considered in the reference group with other antihypertensive medications (calcium channel blockers, beta blockers, and diuretics). Those who, over the course of the study, changed from ACE-Is to other antihypertensive drugs or were exposed at second evaluation to a new antihypertensive therapy were also included in the reference group of other antihypertensive medications.
Independently of reference groups used, potential confounders of the relationship between ACE-I use and cognition were considered in models partially adjusted for age and gender (Model 1) and in models fully adjusted for age, gender, education, pack–years (0 for smokers and 1 for ever smokers), type 2 diabetes, coronary artery disease, serum creatinine level, and apolipoprotein B to apolipoprotein A1 ratio at baseline as well as history of stroke, history of hypertension, and other hypertension drug use at each wave evaluation (Model 2). Patients with congestive heart failure exposed to ACE-Is were excluded from statistical evaluation
Results
Out of 3,439 participants screened for hypertension, 2,650 (77.1%) were affected, and 38.7% were pharmacologically untreated. Among the different classes of antihypertensive medications, 680 patients (41.9%) took ACE-Is broken down as: 277 exposed to captopril, 252 to enalapril, 71 to lisinopril, 29 to quinapril, 19 to ramipril and fosinopril, 7 to cilazapril, and 3 to perindopril and benazapril. Of 1,445 subjects longitudinally evaluated for incident MCI, 204 took ACE-Is (Table 1). Beyond these groups of individuals, 460 persons of the study population were hypertensive and treated with ACE-Is (mean age ± SD: 74.81 ± 5.55, 58.6% women), 799 took other antihypertensive medications (mean age 75.5 ± 5.56, 57.8% women), and 395 patients had untreated hypertension (mean age ± SD: 74.25 ± 5.74, women 43.5%). Participants determined to already have dementia at the first evaluation were excluded, and analyses were restricted to participants who had treated or untreated self-reported hypertension, participants who were taking antihypertensive medication, or people unaware they had hypertension. Hypertension diagnosis was confirmed in each individual by medical examination. Patients with CHF at baseline were also excluded because of common use of ACE-Is and possible interaction with cognitive performance. A total of 873 non-cognitively impaired individuals were available for this study, while we diagnosed MCI in 73 patients also with hypertension (Fig. 1).
Table 1.
Baseline demographic and clinical characteristics of cognitively normal individuals (n = 873) with untreated hypertension or exposed to angiotensin-converting enzyme inhibitors (ACE-Is) or to other antihypertensive medication. Values are expressed as mean (SD) unless otherwise indicated. The Italian Longitudinal Study on Aging (1st prevalence Survey, 1992–1993)
| Variable | Entire cohort (n = 873) | Untreated Hypertension (n = 377) | Exposed to ACE-Is (n = 204) | Exposed to other antihypertensive drugs (n = 292) |
|---|---|---|---|---|
| Women (%) | 410 (46.96) | 162 (42.97) | 100 (49.02) | 148 (50.68) |
| Age (years) | 72.04 ± 4.93 | 71.88 ± 4.92 | 72.17 ± 4.96 | 72.00 ± 4.93 |
| Education (years) | 6.85 ± 4.61 | 6.99 ± 4.75 | 6.81 ± 4.78 | 6.78 ± 4.30 |
| Pack–years | 15.96 ± 24.53 | 17.39 ± 25.94 | 13.20 ± 20.52 | 16.04 ± 23.97 |
| 0 (0–27) | 0.9 (0–29) | 0(0–22.40) | 0 (0–26.65) | |
| Type 2 diabetes (%) | 101 (11.57) | 43 (11.41) | 22 (10.78) | 36 (12.33) |
| Coronary artery disease (%) | 148 (16.95) | 48 (12.73) | 28 (13.73) | 72 (24.66)a |
| Stroke (%) | 58 (6.64) | 20 (5.31) | 17 (8.33) | 21 (7.19) |
| Systolic blood pressure | 156.52 ± 19.50 | 158.37 ± 16.89 | 155.28 ± 19.98 | 155.01 ± 20.36 |
| Mini-mental state examination | 26.96 ± 3.07 | 26.87 ± 3.31 | 27.12 ± 2.95 | 26.96 ± 2.83 |
| Creatinine (mg/dL) | 0.99 ± 0.25 | 0.96 ± 0.20b | 1.01 ± 0.23 | 1.01 ± 0.31 |
| Abdominal circumference | 98.27 ± 11.31 | 96.67 ± 10.97b | 99.31 ± 11.66 | 99.62 ± 11.27 |
| Apolipoprotein B/apolipoprotein A-1 | 0.90 ± 0.43 | 0.87 ± 0.27 | 0.92 ± 0.59 | 0.92 ± 0.47 |
aPearson's chi-squared: untreated hypertension and exposed to ACE-Is vs. exposed to other antihypertensive medications (Bonferroni p value < 0.01)
bStudent's t-test for unpaired data: exposed to ACE-Is and other antihypertensive medications vs. untreated hypertension (Bonferroni p value < 0.05)
Incidence of mild cognitive impairment
The average age at baseline for the 1,445 participants longitudinally evaluated for MCI was 71.9 years, and 43.6% were women. Significant differences in CAD were found between those who were already exposed to ACE-Is (n = 204), those untreated for hypertension (n = 377, 43.2% of entire cohort), and those who took other antihypertensive medications (n = 292) (Bonferroni p-value < 0.01) (Table 1). Differences were also observed in abdominal circumference and serum creatinine between those exposed to ACE-Is and the other two groups excluding CHF patients (Bonferroni p-value < 0.05) (Table 1). Of the 204 participants previously exposed to ACE-Is at baseline, 36% took enalapril, 42% took captopril, 10% took lisinopril, and other ACE-Is were each less than 5%. The characteristics of participants who took the most commonly used drugs (i.e., enalapril vs. captopril) were similar (data not shown). Over a median follow-up of 3.5 years, the exposure to all ACE-Is was 282 person–years, 125 person–years for the enalapril sub-group alone (for the enalapril plus lisinopril combined sub-group, it was 149 person–years) and 100 person–years for captopril. Approximately, 40% of ACE-I users (81 individuals) were continuous users with the same therapy throughout the study period, with no difference in the length of continuous use between those taking captopril and those taking enalapril (χ2: 1.41, P = 0.24). In contrast, exposure to other antihypertensive medications was estimated at 1,803 person–years, and exposure to hypertensive untreated was estimated at 958 person–years. Of 68 incident MCI cases, 50 were exposed to other antihypertensive medications, and 18 were exposed to ACE-Is.
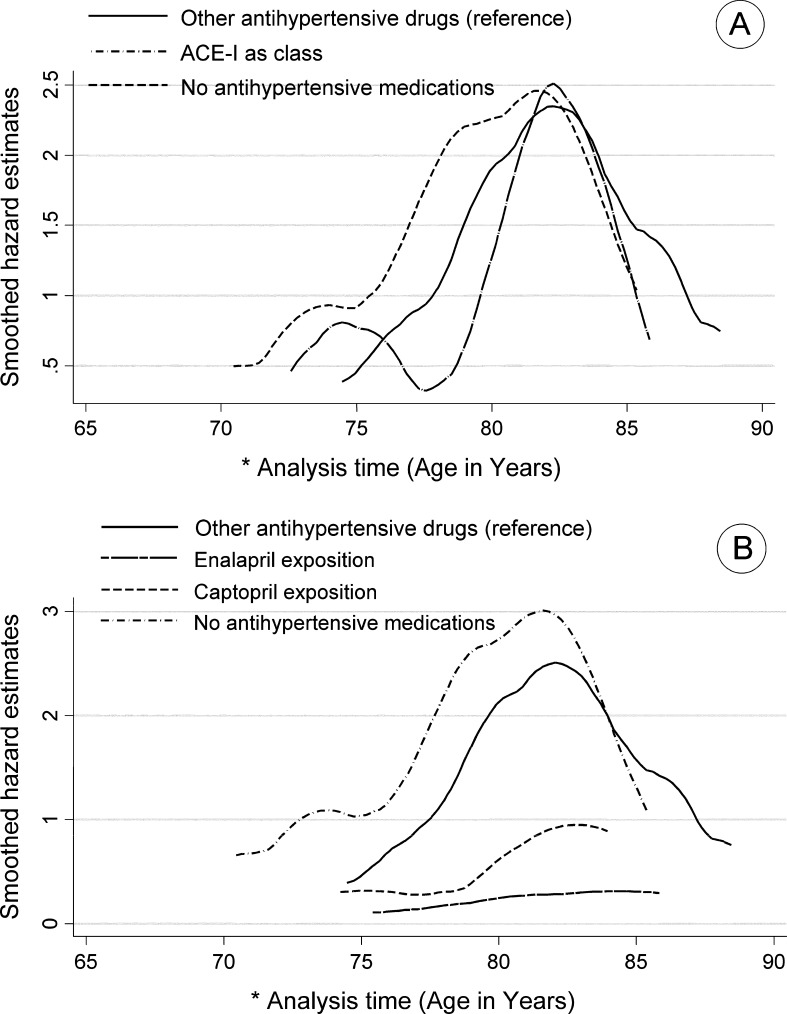
Among hypertensive older adults receiving drug therapy, no difference in risk for MCI was found for continuous exposure to ACE-Is (as a class) compared to other antihypertensive medication users (Model 2: HR, 0.45; 95% CI, 0.16–1.28) or no antihypertensive medication users (Model 2: HR 0.39 95% CI 0.12–1.24). When examined separately by type of ACE-I, continuous exposure to captopril was not significantly associated with incident MCI (Model 2: HR 1.80 95% CI 0.39–8.37) compared to other antihypertensive medication users or to no antihypertensive medication users (Model 2: HR 1.32 95% CI 0.25–6.87). In contrast, enalapril was associated with significantly reduced risk of MCI compared to other antihypertensive medication users (Model 2: HR 0.17, 95% CI 0.04–0.84) or to no antihypertensive medication users (Model 2: HR 0.13, 95% CI 0.02–0.69) (Table 2 and Fig. 2). Combining the group of patients exposed to lisinopril with that exposed to enalapril (because of their high comparability chemically and functionally), a reduced risk of incident MCI was found compared to other antihypertensive medication users (Model 2: HR 0.27, 95% CI 0.08–0.96), as well as to untreated hypertension (Model 2: HR 0.23, 95% CI 0.06–0.92) (Table 2 and Fig. 2).
Table 2.
Hazard ratios (HR) and 95% confidence interval (CI) of incident mild cognitive impairment (MCI) in individuals exposed to enalapril, captopril, and other angiotensin-converting enzyme inhibitors (ACE-Is) as a class. The Italian Longitudinal Study on Aging (1st and 2nd Surveys, 1992–1996)1
| Number of subjects exposed to ACE-Is at baseline and those continuatively exposed to ACE-Is/new events | HR unadjusted (95% CI) | HR in Model 1a (95% CI) | HR in Model 2b (95% CI) | |
|---|---|---|---|---|
| 1. (A) ACE-Is versus other antihypertensive medications | 204 and 81/68 | 1.21 (0.54–2.71) | 0.50 (0.20–1.24) | 0.39 (0.12–1.24) |
| (B) ACE-Is versus no antihypertensive medications | 204 and 81/68 | 1.43 (0.66–3.08) | 0.72 (0.31–1.70) | 0.45 (0.16–1.28) |
| 2. (A) Enalapril versus other antihypertensive medications | 72 and 37/68 | 0.97 (0.29–3.22) | 0.17 (0.05–0.66) | 0.13 (0.02–0.69) |
| Captopril versus other antihypertensive medications | 86 and 29/68 | 1.01 (0.30–3.37) | 1.56 (0.44–5.46) | 1.32 (0.25–6.87) |
| (B) Enalapril versus no antihypertensive medications | 72 and 37/68 | 0.14 (0.35–3.69) | 0.24 (0.06–0.88) | 0.17 (0.04–0.84) |
| Captopril versus no antihypertensive medications | 86 and 29/68 | 1.19 (0.36–3.86) | 2.15 (0.63–7.32) | 1.80 (0.39–8.37) |
| 3. (A) Enalapril + Lisinopril versus other antihypertensive medications | 92 and 44/68 | 1.51 (0.57–3.98) | 0.34 (0.11–1.02) | 0.23 (0.06–0.92) |
| (B) Enalapril + Lisinopril versus no antihypertensive medications | 92 and 44/68 | 1.76 (0.69–4.49) | 0.47 (0.16–1.36) | 0.27 (0.08–0.96) |
1The reference groups consisted of those (A) who were exposed to other antihypertensive drugs (considering those with untreated hypertension as confounder) or (B) had untreated hypertension (considering those who were exposed to other antihypertensive drugs as confounder)
The statistical analyses were performed in separate models:
1. Cumulative exposure to ACE-Is as a class compared with (A) those who were exposed to other antihypertensive drugs or (B) those with untreated hypertension
2. Cumulative exposure to enalapril and captopril compared with (A) those who were exposed to other antihypertensive drugs or (B) those with untreated hypertension
3. Cumulative exposure of enalapril plus lisinopril and captopril (results not showed) compared with (A) those who were exposed to other antihypertensive drugs or (B) those with untreated hypertension
aModel 1: partially adjusted models for age and gender
bModel 2: fully adjusted models for age, gender, education, pack–years (0 for smokers and 1 for ever smokers), type 2 diabetes, coronary artery disease, serum creatinine level, and apolipoprotein B to apolipoprotein A1 ratio at baseline as well as history of stroke, history of hypertension, and other hypertension drug use at each wave evaluation. Patients with congestive heart failure exposed to ACE-Is were excluded from statistical evaluation
Fig. 2.
a Smoothed hazard estimates of mild cognitive impairment (MCI) according to cumulative exposure to angiotensin-converting enzyme inhibitors (ACE-Is) as a class and b cumulative exposure to enalapril and captopril compared with those who were exposed to other antihypertensive drugs. Italian Longitudinal Study on Aging, 1992–1996. The asterisk means that the analysis time (time unit: years) indicates the time at risk of the study population. The beginning of time at risk has been stated at the age of 65 years old, respecting the lower limit in age range of participants to the study. The analysis of time exceeds 85 years, indicating that some individuals who remained at risk have passed the age of 85 years. The figure is derived from a model that is adjusted for several parameters (Model 2 in Table 2)
Discussion
In this study, exposure to ACE-Is as a class was not independently associated with incident MCI in hypertensive elderly people in a median 3.5-year follow-up. Secondary analysis of within-class differences revealed that the sub-group of the dicarboxyl-containing ACE-Is enalapril alone or the enalapril and lisinopril sub-groups combined were associated with reduced risks of 83% and 73%, respectively, of developing MCI, in comparison with other antihypertensive medications. A similar pattern of reduced risk of incident MCI was observed in individuals with untreated hypertension.
A few small observational and case–control studies of individuals with MCI have suggested that ACE-Is slowed cognitive decline and reduced progression to AD (Hajjar et al. 2005; He et al. 2006; Rozzini et al. 2006, 2008). The present findings also support some suggestions from secondary analyses in two large stroke-prevention trials Syst-Eur (Forette et al. 2002) and PROGRESS (Tzourio et al. 2003), where a significant reduction in the incidence of dementia was found with antihypertensive therapies also including ACE-Is. However, the Systolic Hypertension in Europe (Syst-Eur) trial was nitrendipine based, with enalapril as an add-on therapy (Forette et al. 2002), while in the Perindopril Protection against Recurrent Stroke Study (PROGRESS), dementia incidence was only reduced in the combined perindopril and indapamide sub-group and not for perindopril alone (Tzourio et al. 2003). On the other hand, the perindopril findings were not replicated in another large trial (Hypertension in the Very Elderly Trial cognitive function assessment. HYVET-COG) in very old subjects with hypertension (Peters et al. 2008), and two systematic reviews by the Cochrane collaboration found “no convincing” evidence that blood pressure lowering in late-life prevented the development of dementia or cognitive impairment in hypertensive patients with no apparent prior cerebrovascular disease (McGuinness et al. 2006, 2009). However, in a smaller Cardiovascular Health Study (CHS) Cognition sub-study that followed up 1,054 elderly people with treated hypertension and no diagnosis of CHF after six years, exposure to all ACE-Is was not implicated with the risk of dementia or difference in MMSE scores (Sink et al. 2009). Similar findings were made in the CACHE county cohort on both incident AD (Khachaturian et al. 2006) and rate of functional decline in AD (Rosenberg et al. 2008). However, further analysis within the CHS cohort suggested that the so-called centrally active ACE-Is (e.g., lisinopril, perindopril, and ramipril) were associated with a 65% lower decline in MMSE score per year of exposure, but the so-called peripherally acting compounds such as enalapril may contribute to increased AD risk in contrast to ACE-Is that cross the BBB (Sink et al. 2009).
The present findings and those from both the CHS (Sink et al. 2009) and the CACHE county cohort (Khachaturian et al. 2006; Rosenberg et al. 2008) do not support the existence of a class effect of ACE-Is in protecting against cognitive decline. Other studies suggested that ACE-Is as a class had an elevated HR (Khachaturian et al. 2006), which to some extent agrees with the trends that we observed with the widely used captopril. Our finding that dicarboxyl-containing enalapril sub-group alone and enalapril plus lisinopril sub-groups combined may reduced the risk of incident MCI contradicts conclusions drawn from studies where ACE-I classification was based on BBB permeability (Sink et al. 2009). However, the cognitive outcomes were defined differently in the CHS (incident dementia, cognitive decline, or disability) (Sink et al. 2009) and in the ILSA (incident MCI). Furthermore, conflicting evidence exists around the central action of lisinopril (Jackson et al. 1987; Cushman et al. 1989; Furberg and Pitt 2001) and ramipril (Furberg and Pitt 2001; Jouquey et al. 1995). Clearly, the classification by either chemical structure or BBB penetrability has some limitations. One possible advantage of chemical classification is that structure determines to some extent pharmacological parameters, and the grouping of drugs based on key chemical groups that can have shared functions such as antioxidative properties (Westlin and Mullane 1988) or potential for adverse events (Jaffe 1986) could help identify other drug-molecule-based mechanisms that might be important. These could be properties independent of blood-pressure-lowering effects and inhibition of ACE which already have credible mechanistic links with AD. Such structure-based classifications may be the only means to examine effects, with increasing evidence that viewing ACE-Is as a class is somewhat convenient and based mainly on their primary role, i.e., inhibition of ACE, yet there is a wide variation in the structures involved and lack of interchangeability of ACE-Is in some contexts (Furberg and Pitt 2001). We are not discounting the principle of classifying ACE-Is according to their ability to cross the BBB; indeed, we would advocate the need for further work to clarify the status of these drugs and perhaps the need for consensus on what constitutes the criteria under which they should be tested and judged. However, in the interim and in the absence of strong consistent evidence for some ACE-Is, we suggest that chemical classification is more robust because the chemical structures are immutable, and indeed, the chemical properties will partly influence the BBB permeability of these drugs as the chemical structure influences a number of factors, including molecule size, charge, and lipophilicity among others; all of which will also need to be assessed and further altered by variable BBB integrity which is common in AD. This is particularly relevant for MCI which is recognized to be a pathology-based condition with a high rate of progression to AD (Petersen et al. 1999), and thus, the BBB may have already been compromised in our MCI patients. The inclusion in the analyses of untreated hypertensives was important to identify the risk contribution of hypertension to incident MCI (Panza et al. 2010). The exclusion of this sub-sample would have reduced the risk of incident MCI.
Irrespective of the means of ACE-I classification, there are some studies demonstrating a possible role of ACE-Is in preventing dementia or AD (Fournier et al. 2009). There are actually several biological explanations to possibly explain this. The brain possesses an intrinsic RAS, and ACE is overexpressed in the hippocampi of patients with AD (Kehoe and Wilcock 2007; Kehoe et al. 2009). Stimulation of the RAS also drives the activation of inflammatory cytokines implicated in AD (Tuppo and Arias 2005; Duron and Hanon 2010), while angiotensin II, a product of ACE function, has been shown to inhibit acetylcholine release, suggesting therefore that ACE-Is could increase acetylcholine concentration and thus be beneficial for cognition (Barnes et al. 1990). There is also decreased cerebral blood flow in AD (Ruitenberg et al. 2005), and angiotensin II, a vasoconstrictor likely to be involved, also highlights the intervening potential of ACE-Is (Duron and Hanon 2010). Finally, ACE-Is could mitigate against oxidative stress that is mediated through AT1 receptor activation and which leads to release of glutamate and induced synaptic plasticity (Fournier et al. 2009).
Some limitations in the present study should be described. In particular, misclassification bias of the exposure was possible because we do not know whether some subjects were exposed to ACE-Is before baseline. Unmanaged vascular risk factors in middle-age may be decisive for the onset of cognitive impairment in late-life (Panza et al. 2010). Although our study covered a 3.5-year period, a longer time frame starting from the middle age would have been more ideal. Moreover, the distribution of comorbidity (such as diabetes and stroke where ACE-Is are also indicated) is very heterogeneous among elderly people, and as such, drug biasing towards the null hypothesis on the rates of these diseases is possible. Differences in blood pressure lowering within any class of antihypertensive drugs could contribute to differences in reducing risk of MCI of these medications. We controlled these as much as possible in the analyses, although we faced the complex problem of controlling risks of interest for confounding due to multiple indications. Indeed, we excluded patients with the other main indication for ACE-Is, particularly CHF, and censored them at follow-up. In the ILSA, among factors that are potential risk factors for dementia and might be associated with the response to antihypertensive treatment, thus acting as potential confounders, we did not have information on the apolipoprotein E (APOE) ε4 allele status. Finally, we did not have information on length and severity of hypertension in the ILSA sample. The present promising findings on reduced risk of developing MCI associated with dicarboxyl-containing ACE-Is enalapril sub-group alone or enalapril and lisinopril sub-groups combined in comparison with other antihypertensive medications reinforce previous calls for the need for further larger RCTs on ACE-Is in predementia and dementia syndromes. Nonetheless, these results need to be replicated in independent studies with a greater number of events before taking them into account.
Footnotes
The ILSA Working Group
E. Scafato, MD (Scientific Coordinator), G. Farchi, MSc, L. Galluzzo, MA, C. Gandin, MD, Istituto Superiore di Sanità, Roma; A. Capurso, MD, F. Panza, MD, PhD, V. Solfrizzi, MD, PhD, V. Lepore, MD, P. Livrea, MD, University of Bari; L. Motta, MD, G. Carnazzo, MD, M. Motta, MD, P. Bentivegna, MD, University of Catania; S. Bonaiuto, MD, G. Cruciani, MD, D. Postacchini, MD, Italian National Research Centre on Aging (INRCA), Fermo; D. Inzitari, MD, L. Amaducci, MD, University of Firenze; A. Di Carlo, MD, M. Baldereschi, MD, Italian National Research Council (CNR), Firenze; C. Gandolfo, MD, M. Conti, MD, University of Genova; N. Canal, MD, M. Franceschi, MD, San Raffaele Institute, Milano; G. Scarlato, MD, L. Candelise, MD, E. Scapini, MD, University of Milano; F. Rengo, MD, P. Abete, MD, F. Cacciatore, MD, University of Napoli; G. Enzi, MD, L. Battistin, MD, G. Sergi, MD, G. Crepaldi, MD, University of Padova; S. Maggi, MD, N. Minicucci, MD, M. Noale, MD, Italian National Research Council (CNR), Aging Section, Padova; F. Grigoletto, ScD, E. Perissinotto, ScD, Institute of Hygiene, University of Padova; P. Carbonin, MD, Università Cattolica del Sacro Cuore, Roma.
Contributor Information
Vincenzo Solfrizzi, Phone: +39-80-5473685, FAX: +39-80-5478860, Email: v.solfrizzi@geriatria.uniba.it.
Francesco Panza, Email: geriat.dot@geriatria.uniba.it, Email: f.panza@operapadrepio.it.
References
- Diagnostic and Statistical Manual of Mental Disorders. 3rd ed. revised (DSM-III-R) Washington: American Psychiatric Association; 1987. pp. 103–107. [Google Scholar]
- Barnes JM, Barnes NM, Costall B, Horovitz ZP, Ironside JW, Naylor RJ, Williams TJ. Angiotensin II inhibits acetylcholine release from human temporal cortex: implications for cognition. Brain Res. 1990;507:341–343. doi: 10.1016/0006-8993(90)90294-L. [DOI] [PubMed] [Google Scholar]
- Birns J, Morris R, Donaldson N, Kalra L. The effects of blood pressure reduction on cognitive function: a review of effects based on pooled data from clinical trials. J Hypertens. 2006;24:1907–1914. doi: 10.1097/01.hjh.0000244934.81180.16. [DOI] [PubMed] [Google Scholar]
- Brown NJ, Vaughan DE. Angiotensin-converting enzyme inhibitors. Circulation. 1998;97:1411–1420. doi: 10.1161/01.CIR.97.14.1411. [DOI] [PubMed] [Google Scholar]
- Cushman DW, Wang FL, Fung WC, Harvey CM, DeForrest JM. Differentiation of angiotensin-converting enzyme (ACE) inhibitors by their selective inhibition of ACE in physiologically important target organs. Am J Hypertens. 1989;2:294–306. doi: 10.1093/ajh/2.4.294. [DOI] [PubMed] [Google Scholar]
- Duron E, Hanon O. Antihypertensive treatments, cognitive decline, and dementia. J Alzheimers Dis. 2010;20:903–914. doi: 10.3233/JAD-2010-091552. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. «Mini-Mental State»: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Forette F, Seux ML, Staessen JA, Thijs L, Babarskiene MR, Babeanu S, Bossini A, Fagard R, Gil-Extremera B, Laks T, Kobalava Z, Sarti C, Tuomilehto J, Vanhanen H, Webster J, Yodfat Y, Birkenhäger WH, Systolic Hypertension in Europe Investigators The prevention of dementia with antihypertensive treatment: new evidence from the Systolic Hypertension in Europe (Syst-Eur) study. Arch Intern Med. 2002;162:2046–2052. doi: 10.1001/archinte.162.18.2046. [DOI] [PubMed] [Google Scholar]
- Fournier A, Oprisiu-Fournier R, Serot JM, Godefroy O, Achard JM, Faure S, Mazouz H, Temmar M, Albu A, Bordet R, Hanon O, Gueyffier F, Wang J, Black S, Sato N. Prevention of dementia by antihypertensive drugs: how AT1-receptor-blockers and dihydropyridines better prevent dementia in hypertensive patients than thiazides and ACE-inhibitors. Expert Rev Neurother. 2009;9:1413–1431. doi: 10.1586/ern.09.89. [DOI] [PubMed] [Google Scholar]
- Furberg CD, Pitt B. Are all angiotensin-converting enzyme inhibitors interchangeable? J Am Coll Cardiol. 2001;37:1456–1460. doi: 10.1016/S0735-1097(01)01161-5. [DOI] [PubMed] [Google Scholar]
- Gohlke P, Schölkens B, Henning R, Urbach H, Unger T. Inhibition of converting enzyme in brain tissue and cerebrospinal fluid of rats following chronic oral treatment with the converting enzyme inhibitors ramipril and Hoe 288. J Cardiovasc Pharmacol. 1989;14(Suppl 4):S32–S36. [PubMed] [Google Scholar]
- Hajjar I, Catoe H, Sixta S, Boland R, Johnson D, Hirth V, Wieland D, Eleazer P. Cross-sectional and longitudinal association between antihypertensive medications and cognitive impairment in an elderly population. J Gerontol A Biol Sci Med Sci. 2005;60:67–73. doi: 10.1093/gerona/60.1.67. [DOI] [PubMed] [Google Scholar]
- He M, Ohrui T, Maruyama M, Tomita N, Nakayama K, Higuchi M, Furukawa K, Arai H. ACE activity in CSF of patients with mild cognitive impairment and Alzheimer disease. Neurology. 2006;67:1309–1310. doi: 10.1212/01.wnl.0000238102.04582.ec. [DOI] [PubMed] [Google Scholar]
- Jackson EK (2001) Renin and angiotensin. In: Hardman JG, Limbird LE, Gilman AG (eds) Goodman & Gilman's the pharmacological basis of therapeutics. Chapter 31, 10th Edition, McGraw-Hill Professional: 809–842
- Jackson B, Cubela R, Sakaguchi K, Johnston CI. Blockade of angiotensin converting enzyme in circumventricular organs of the brain after oral lisinopril administration demonstrated by quantitative in vitro autoradiography. Clin Exp Pharmacol Physiol. 1987;14:155–158. doi: 10.1111/j.1440-1681.1987.tb00367.x. [DOI] [PubMed] [Google Scholar]
- Jaffe IA. Adverse effects profile of sulfhydryl compounds in man. Am J Med. 1986;80:471–476. doi: 10.1016/0002-9343(86)90722-9. [DOI] [PubMed] [Google Scholar]
- Jouquey S, Mathieu MN, Hamon G, Chevillard C. Effect of chronic treatment with trandolapril or enalapril on brain ACE activity in spontaneously hypertensive rats. Neuropharmacology. 1995;34:1689–1692. doi: 10.1016/0028-3908(95)00146-8. [DOI] [PubMed] [Google Scholar]
- Katz S, Akpom CA. A measure of primary sociobiological functions. Int J Health Serv. 1976;6:493–507. doi: 10.2190/UURL-2RYU-WRYD-EY3K. [DOI] [PubMed] [Google Scholar]
- Kehoe PG, Wilcock GK. Is inhibition of the renin–angiotensin system a new treatment option for Alzheimer's disease? Lancet Neurol. 2007;6:373–378. doi: 10.1016/S1474-4422(07)70077-7. [DOI] [PubMed] [Google Scholar]
- Kehoe PG, Miners S, Love S. Angiotensins in Alzheimer's disease—friend or foe? Trends Neurosci. 2009;32:619–628. doi: 10.1016/j.tins.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Khachaturian AS, Zandi PP, Lyketsos CG, Hayden KM, Skoog I, Norton MC, Tschanz JT, Mayer LS, Welsh-Bohmer KA, Breitner JC. Antihypertensive medication use and incident Alzheimer disease: the CACHE County Study. Arch Neurol. 2006;63:686–692. doi: 10.1001/archneur.63.5.noc60013. [DOI] [PubMed] [Google Scholar]
- Lawton MP, Brody EM. Assessment of older people: self maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. doi: 10.1093/geront/9.3_Part_1.179. [DOI] [PubMed] [Google Scholar]
- Li NC, Lee A, Whitmer RA, Kivipelto M, Lawler E, Kazis LE, Wolozin B. Use of angiotensin receptor blockers and risk of dementia in a predominantly male population: prospective cohort analysis. BMJ. 2010;340:b5465. doi: 10.1136/bmj.b5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lithell H, Hansson L, Skoog I, Elmfeldt D, Hofman A, Olofsson B, Trenkwalder P, Zanchetti A, SCOPE Study Group The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double blind intervention trial. J Hypertens. 2003;21:875–886. doi: 10.1097/00004872-200305000-00011. [DOI] [PubMed] [Google Scholar]
- Louis WJ, Mander AG, Dawson M, O'Callaghan C, Conway EL. Use of computerized neuropsychological tests (CANTAB) to assess cognitive effects of antihypertensive drugs in the elderly. Cambridge Neuropsychological Test Automated Battery. J Hypertens. 1999;17:1813–1819. doi: 10.1097/00004872-199917121-00005. [DOI] [PubMed] [Google Scholar]
- McGuinness B, Todd S, Passmore P, Bullock R. The effects of blood pressure lowering on development of cognitive impairment and dementia in patients without apparent prior cerebrovascular disease. Cochrane Database Syst Rev. 2006;2:CD004034. doi: 10.1002/14651858.CD004034.pub2. [DOI] [PubMed] [Google Scholar]
- McGuinness B, Todd S, Passmore P, Bullock R. Blood pressure lowering in patients without prior cerebrovascular disease for prevention of cognitive impairment and dementia. Cochrane Database Syst Rev. 2009;4:CD004034. doi: 10.1002/14651858.CD004034.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS–ADRDA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34:939–944. doi: 10.1212/WNL.34.7.939. [DOI] [PubMed] [Google Scholar]
- Miners S, Ashby E, Baig S, Harrison R, Tayler H, Speedy E, Prince JA, Love S, Kehoe PG. Angiotensin-converting enzyme levels and activity in Alzheimer's disease: differences in brain and CSF ACE and association with ACE1 genotypes. Am J Transl Res. 2009;1:163–177. [PMC free article] [PubMed] [Google Scholar]
- No authors listed (1988) The 1988 Report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure. Arch Intern Med 148: 1023–1038 [PubMed]
- Ohrui T, Matsui T, Yamaya M, Arai H, Ebihara S, Maruyama M, Sasaki H. Angiotensin-converting enzyme inhibitors and incidence of Alzheimer's disease in Japan. J Am Geriatr Soc. 2004;52:649–650. doi: 10.1111/j.1532-5415.2004.52178_7.x. [DOI] [PubMed] [Google Scholar]
- Ohrui T, Tomita N, Sato-Nakagawa T, Matsui T, Maruyama M, Niwa K, Arai H, Sasaki H. Effects of brain-penetrating ACE inhibitors on Alzheimer disease progression. Neurology. 2004;63:1324–1325. doi: 10.1212/01.WNL.0000140705.23869.E9. [DOI] [PubMed] [Google Scholar]
- Panza F, Frisardi V, Capurso C, Imbimbo BP, Vendemiale G, Santamato A, D'Onofrio G, Seripa D, Sancarlo D, Pilotto A, Solfrizzi V. Metabolic syndrome and cognitive impairment: current epidemiology and possible underlying mechanisms. J Alzheimers Dis. 2010;21:691–724. doi: 10.3233/JAD-2010-091669. [DOI] [PubMed] [Google Scholar]
- Peters R, Beckett N. Hypertension, dementia, and antihypertensive treatment: implications for the very elderly. Curr Hypertens Rep. 2009;11:277–282. doi: 10.1007/s11906-009-0047-0. [DOI] [PubMed] [Google Scholar]
- Peters R, Beckett N, Forette F, Tuomilehto J, Clarke R, Ritchie C, Waldman A, Walton I, Poulter R, Ma S, Comsa M, Burch L, Fletcher A, Bulpitt C, HYVET investigators Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG). A double-blind, placebo-controlled trial. Lancet Neurol. 2008;7:683–689. doi: 10.1016/S1474-4422(08)70143-1. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4:487–499. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- Ranadive SA, Chen AX, Serajuddin AT. Relative lipophilicities and structural-pharmacological considerations of various angiotensin-converting enzyme (ACE) inhibitors. Pharm Res. 1992;9:1480–1486. doi: 10.1023/A:1015823315983. [DOI] [PubMed] [Google Scholar]
- Rosenberg PB, Mielke MM, Tschanz J, Cook L, Corcoran C, Hayden KM, Norton M, Rabins PV, Green RC, Welsh-Bohmer KA, Breitner JC, Munger R, Lyketsos CG. Effects of cardiovascular medications on rate of functional decline in Alzheimer disease. Am J Geriatr Psychiatry. 2008;16:883–892. doi: 10.1097/JGP.0b013e318181276a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozzini L, Vicini Chilovi B, Bertoletti E, Conti M, Del Rio I, Trabucchi M, Padovani A. Angiotensin converting enzyme (ACE) inhibitors modulate the rate of progression of amnestic mild cognitive impairment. Int J Geriatr Psychiatry. 2006;21:550–555. doi: 10.1002/gps.1523. [DOI] [PubMed] [Google Scholar]
- Rozzini L, Vicini Chilovi B, Trabucchi M, Padovani A. Antihypertensive medications influence the rate of conversion from mild cognitive impairment to Alzheimer disease. Arch Neurol. 2008;65:993–994. doi: 10.1001/archneur.65.7.993. [DOI] [PubMed] [Google Scholar]
- Ruitenberg A, den Heijer T, Bakker SL, van Swieten JC, Koudstaal PJ, Hofman A, Breteler MM. Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam Study. Ann Neurol. 2005;57:789–794. doi: 10.1002/ana.20493. [DOI] [PubMed] [Google Scholar]
- Sergi G, Perissinotto E, Pisent C, Buja A, Maggi S, Coin A, Grigoletto F, Enzi G, ILSA Working Group An adequate threshold for body mass index to detect underweight condition in elderly persons: the Italian Longitudinal Study on Aging (ILSA) J Gerontol A Biol Sci Med Sci. 2005;60:866–871. doi: 10.1093/gerona/60.7.866. [DOI] [PubMed] [Google Scholar]
- Sink KM, Leng X, Williamson J, Kritchevsky SB, Yaffe K, Kuller L, Yasar S, Atkinson H, Robbins M, Psaty B, Goff DC., Jr Angiotensin-converting enzyme inhibitors and cognitive decline in older adults with hypertension: results from the Cardiovascular Health Study. Arch Intern Med. 2009;169:1195–1202. doi: 10.1001/archinternmed.2009.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solfrizzi V, Panza F, Colacicco AM, D'Introno A, Capurso C, Torres F, Grigoletto F, Maggi S, Del Parigi A, Reiman EM, Caselli RJ, Scafato E, Farchi G, Capurso A, Italian Longitudinal Study on Aging Working Group Vascular risk factors, incidence of MCI, and rates of progression to dementia. Neurology. 2004;63:1882–1891. doi: 10.1212/01.WNL.0000144281.38555.E3. [DOI] [PubMed] [Google Scholar]
- Solfrizzi V, D'Introno A, Colacicco AM, Capurso C, Del Parigi A, Caselli RJ, Scapicchio PL, Scafato E, Gandin C, Capurso A, Panza F, for the Italian Longitudinal Study on Aging Working Group Incident occurrence of depressive symptoms among patients with mild cognitive impairment. The Italian Longitudinal Study on Aging. Dement Geriatr Cogn Disord. 2007;24:55–64. doi: 10.1159/000103632. [DOI] [PubMed] [Google Scholar]
- Spinnler H, Tognoni G. Standardizzazione e taratura italiana di test neuropsicologici. Ital J Neurol Sci. 1987;6(supp. 8):12–120. [PubMed] [Google Scholar]
- Staessen JA, Thijs L, Richart T, Odili AN, Birkenhäger WH. Placebo-controlled trials of blood pressure-lowering therapies for primary prevention of dementia. Hypertension. 2011;57:e6–e7. doi: 10.1161/HYPERTENSIONAHA.110.165142. [DOI] [PubMed] [Google Scholar]
- Sudilovsky A, Cutler NR, Sramek JJ, Wardle T, Veroff AE, Mickelson W, Markowitz J, Repetti S. A pilot clinical trial of the angiotensin-converting enzyme inhibitor ceranapril in Alzheimer disease. Alzheimer Dis Assoc Disord. 1993;7:105–111. doi: 10.1097/00002093-199307020-00006. [DOI] [PubMed] [Google Scholar]
- Tan J, Wang JM, Leenen FH. Inhibition of brain angiotensin-converting enzyme by peripheral administration of trandolapril versus lisinopril in Wistar rats. Am J Hypertens. 2005;18:158–164. doi: 10.1016/j.amjhyper.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Thind GS. Angiotensin converting enzyme inhibitors: comparative structure, pharmacokinetics, and pharmacodynamics. Cardiovasc Drugs Ther. 1990;4:199–206. doi: 10.1007/BF01857634. [DOI] [PubMed] [Google Scholar]
- Tuppo EE, Arias HR. The role of inflammation in Alzheimer's disease. Int J Biochem Cell Biol. 2005;37:289–305. doi: 10.1016/j.biocel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Tzourio C, Anderson C, Chapman N, Woodward M, Neal B, MacMahon S, Chalmers J, PROGRESS Collaborative Group Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch Intern Med. 2003;163:1069–1075. doi: 10.1001/archinte.163.9.1069. [DOI] [PubMed] [Google Scholar]
- Weiner MF, Bonte FJ, Tintner R, Ford N, Svetlik D, Riall T. ACE inhibitor lacks acute effect on cognition or brain blood flow in Alzheimer's disease. Drug Dev Res. 1992;26:467–471. doi: 10.1002/ddr.430260410. [DOI] [Google Scholar]
- Westlin W, Mullane K. Does captopril attenuate reperfusion-induced myocardial dysfunction by scavenging free radicals? Circulation. 1988;77:I30–I139. [PubMed] [Google Scholar]
- International statistical classification of diseases and related health problems, 10th revision (ICD-10). Chapter V, categories F00–F99. Mental, behavioural, and developmental disorders, clinical description and diagnostic guidelines. Geneva: World Health Organization; 1992. [Google Scholar]