Abstract
The aim of the present study was to investigate resting measures of dehydroepiandrosterone (DHEA), dehydroepiandrosterone sulphate (DHEA-S) and cortisol, and the response and recovery of these hormones to acute exercise, in male and female older adults of different exercise training status. Participants were 49 community-dwelling older adults (23 females) aged between 60 and 77 years who were either sedentary (n = 14), moderately active (n = 14) or endurance trained (n = 21). Participants undertook an acute bout of exercise in the form of an incremental submaximal treadmill test. The exercise lasted on average 23 min 49 s (SD = 2 min 8 s) and participants reached 76.5% (SD = 5.44) of the predicted maximal heart rate. Blood samples were collected prior to exercise, immediately, and 1 h post-exercise. DHEA levels significantly increased immediately post-exercise; however, DHEA-S levels only significantly increased in females. Cortisol significantly decreased immediately post-exercise and 1 h post-exercise compared to pre-exercise. There were no significant differences in resting hormone levels or hormonal responses to exercise between training status groups. The findings suggest that exercise can stimulate DHEA production in older adults and that hormonal responses to exercise differ between male and female older adults.
Keywords: DHEA, DHEA-S, Cortisol, Acute exercise, Training status, Sex, Older adults
Introduction
Dehydroepiandrosterone (DHEA) and its sulphated metabolite, dehydroepiandrosterone sulphate (DHEA-S), are androgens produced by the adrenal cortex. DHEA/S has been proposed to affect various systems of the body and to be anti-ageing (Chahal and Drake 2007). It has been established that DHEA/S is immune enhancing, where cortisol, also produced by the adrenal cortex, is immunosuppressive if chronically elevated (Buford and Willoughby 2005). DHEA and DHEA-S production peaks at age 20–30 and then declines progressively with age (Belanger et al. 1994; Labrie et al. 1997; Orentreich et al. 1992). In contrast, cortisol has been reported to increase with age (Deuschle et al. 1997; VanCauter et al. 1996), although counter-evidence exists (Orentreich et al. 1992). Reductions in DHEA/S have been implicated in the disturbance of other physiological systems, such as the musculoskeletal system (Walston et al. 2006). Further, over-representation of cortisol compared to DHEA, and the consequent increase in the cortisol/DHEA ratio with ageing (Phillips et al. 2007), is associated with immune impairments and infection risk in older adults (Butcher et al. 2005). Exercise has been proposed as an intervention to protect against changes in the neuroendocrine system with ageing and improve immunity in older adults (Phillips et al. 2007).
DHEA-S has been found to be significantly higher in older men who are endurance trained (Tissandier et al. 2001) and who regularly cycled at moderate intensity (Ravaglia et al. 2001). In contrast, DHEA-S levels have been found to be similar between older male runners and sedentary controls (Arai et al. 2006). These studies did not include women; however, other studies investigating VO2max and energy expenditure rather than exercise training status have. DHEA-S correlated positively with VO2max (Bonnefoy et al. 1998, 2002) and estimated energy expenditure in older women, but not men (Bonnefoy et al. 1998; Kostka et al. 2002). In contrast, Abbasi et al. (1998) reported an association between VO2max and DHEA-S in men but not women, although this finding did not withstand adjustment for age. However, in these studies, participants were of average fitness levels and not endurance trained. In addition, those who take part in moderate activities have not been compared to those who are endurance trained within the same study nor has DHEA-S been examined in parallel with DHEA. Thus, it seems important to examine whether or not higher levels of habitual physical activity might have a greater effect on DHEA and DHEA-S levels in both men and women.
DHEA (Aldred et al. 2009; Cumming et al. 1986) and DHEA-S (Tremblay et al. 2004) have been shown to increase in response to acute exercise in younger adults. endurance trained young males showed attenuated increases in hormone concentrations in response to exercise compared to resistance-trained individuals (Tremblay et al. 2004). One study has compared hormonal responses to resistance exercise in middle-aged strength-trained and untrained men (Cadore et al. 2008). There were no differences in hormones between trained and untrained men at rest; however, untrained men demonstrated a significant increase in DHEA and cortisol in response to acute resistance exercise, where trained men did not. This study suggests that trained and untrained middle-aged individuals may elicit different hormonal responses to exercise; however, less is known regarding responses in elderly individuals. As older adults of different training status and fitness may vary in hormone levels at rest, they may also display different hormonal responses to acute exercise.
A small number of studies have investigated these hormones in older adults, but have mainly been restricted to postmenopausal females; less is known about males and older individuals. For example, DHEA significantly increased with exercise in females aged up to 69. This was restricted to resistance exercise and was not observed with endurance exercise (Copeland et al. 2002). Early postmenopausal females demonstrated an increase in DHEA-S immediately and 2 h after a combined endurance and strength training session (Kemmler et al. 2003). Another study in postmenopausal females found an increase in DHEA, but not DHEA-S, in response to submaximal exercise (Giannopoulou et al. 2003). A more recent study of older men and women reported that neither DHEA nor DHEA-S increased immediately after acute submaximal exercise (Aldred et al. 2009), although this study only tested seven participants and no samples were taken during the recovery period. The lack of clear consensus may be due to the different exercise protocols employed and participants studied. With regard to cortisol, one study examined older fit and unfit females’ baseline levels of cortisol and response and recovery to acute submaximal exercise and failed to observe any significant differences between groups (Traustadottir et al. 2004), although DHEA/S were not measured in this study. To our knowledge, responses of DHEA, DHEA-S and cortisol to acute exercise in older males and females in relation to different levels of training status has yet to be examined.
If exercise is to be used as a possible intervention to buffer against age-induced changes in the neuroendocrine system, such as the reduction in DHEA/DHEA-S, then it is important to establish, first, whether exercise training influences levels of these hormones in older age and, second, whether training or sex affects hormonal responses to exercise. Therefore, the aim of the present study was to investigate resting measures of DHEA, DHEA-S and cortisol, and their response to and recovery from acute exercise, in male and female older adults of different exercise training status. First, it was hypothesised that older adults who were exercise trained would present with a more favourable hormonal profile: higher levels of DHEA/S and a lower cortisol/DHEA ratio. Second, it was hypothesised that sedentary individuals would have a greater hormonal response to acute exercise.
Methods
Participants
Participants were 49 community-dwelling older adults (23 females) recruited from the West Midlands area aged between 60 and 77 years. Inclusion criteria were no endocrine or immune disorder, no psychiatric illness, no eating disorder and not taking glucocorticoid medication. Twenty percent of participants reported suffering from a chronic illness, which were hypertension and asthma, and 43% reported taking medication, such as antihypertensives, non-corticosteroid inhalers, statins and gastrointestinal medications. All participants described themselves as ‘White’ ethnicity, and in terms of socio-economic status, 86% of participants classified themselves as non-manual based on their previous or current occupation using the Registrar General’s Classification of Occupations 1980. Participants were either sedentary (n = 14), moderately active (n = 14) or endurance trained (n = 21). The sedentary participants were recruited from the local community and were not currently involved in any regular exercise nor had they been for 5 years prior to the study. Moderately active participants were recruited from local rambling groups, keep fit classes, aqua fit classes and gymnasia. endurance trained older athletes were recruited from local running clubs and at races. Details of the exercise behaviour of participants are described in relation to the exercise diary below. There was no significant difference in socio-economic status, chronic illness or medication use between the exercise groups.
Study design
This study was a cross-sectional investigation of the DHEA, DHEA-S and cortisol response and recovery to acute exercise in untrained, moderately trained and endurance trained older adults. It comprised an acute exercise bout and the completion of a 14-day exercise diary. All participants gave written informed consent prior to the study, which was approved by the University Research Ethics Committee.
Exercise diary
To confirm the allocation at recruitment to sedentary, moderately active and endurance trained groups, participants completed a consecutive 14-day exercise diary where they recorded what activity they did, the duration of the activity and the intensity of the exercise. The intensity of the exercise was determined using a 0–10 Rating of Perceived Exertion (RPE) Scale (Borg 1998) where 0 was rest and 10 was maximal effort; participants were briefed on the use of the RPE Scale and instructions and examples were also provided in the diary. The diary was analysed to determine how long was spent in moderate and vigorous activity based on metabolic equivalent values for a given activity (Ainsworth et al. 2000) and RPE (Nelson et al. 2007). The activities of the moderate group were mainly rambling, golf, yoga, badminton, swimming and keep fit classes. The endurance trained group were runners, and cycling, circuit training and karate were also reported among this group. Minutes spent in moderate and vigorous exercise over the 14-day period were averaged per week. From this, an exercise score was created using the criteria from the Whitehall study (Marmot et al. 1991). This uses a 0–5 categorical scoring system, e.g. if they spent 1–2 h performing an activity, they were awarded a score of 1, 3–5 h a score of 2 and so on. A combined exercise score was calculated by multiplying the category score by a weighting of 2 for moderate activity and 3 for vigorous activity. As shown in Table 1, the exercise groups had significantly different exercise scores and significantly varied in moderate and vigorous exercise.
Table 1.
Exercise score and time participating in moderate and vigorous exercise per week for sedentary, moderately active and endurance trained older adults
| Sedentary (n = 14) | Moderate (n = 14) | Trained (n = 21) | F (df) | p value | η 2 | |
|---|---|---|---|---|---|---|
| Exercise score diary | 0.00 (0.00) | 4.85 (2.31) | 7.38 (3.02) | 41.8 (2,46) | <0.001 | 0.645 |
| Time spent in moderate activity per week (min) | 281.6 (183.26) | 94.4 (102.14) | 20.7 (2,46) | <0.001 | 0.474 | |
| Time spent in vigorous activity per week (min) | 197.8 (121.94) | 36.3 (2,46) | <0.001 | 0.612 |
Blood samples and hormone analysis
Three blood samples were collected: prior to exercise, immediately post-exercise and 1 h post-exercise. The first blood sample was taken between 8.30 and 9.30 a.m., this was on average 3 h after participants had woken up. For each blood sample, a 6-ml venous blood was collected from an antecubital vein into plain tubes (BD Vacutainer, Plymouth, UK). Blood was allowed to clot at room temperature for 1 h and then centrifuged at 4,000 rpm for 5 min, and the separated serum was stored at −20°C until analysis. DHEA, DHEA-S and cortisol were analysed in duplicate using ELISAs based on the principle of competitive binding (IBL International, Hamburg, Germany). The microtiter wells are coated with a polyclonal antibody directed towards DHEA/DHEA-S or cortisol. The hormone in the sample competes with horseradish peroxidase conjugate for binding to the coated antibody. After 60 min incubation, the unbound conjugate is washed off. After addition of a substrate solution and further 15 min incubation, the enzymatic reaction is stopped and the concentration of these hormones is inversely proportional to the optical density measured at 450 nm. Intra-assay coefficients were <10%.
Procedure
Pre-study screening
Prior to entry to the study, participants completed pre-study questionnaires. Participants were asked if they suffered from any chronic illness or any acute illness and if they were taking any medication. Participants completed the Physical Activity Readiness Questionnaire (Tharrett and Peterson 1997), the Hospital Anxiety and Depression Scale (Zigmond and Snaith 1983) and the Life Events Survey from the West of Scotland Twenty-07 Study (Ford et al. 1994) to assess stressful life events exposure over the past year. No participants met the criteria for high probability of anxiety or depression, and there was no significant difference in stressful life events exposure between exercise groups. Participants were given a 14-day exercise diary and instructions on how to complete it. They were then given an appointment for the acute exercise trial.
Acute exercise bout
Participants were asked to refrain from exercise and alcohol 24 h prior and food and caffeine 12 h prior to arriving at the laboratory. Participants arrived between 8 and 9 a.m. and, on arrival, had the timeline for the visit described and asked if they had any questions. Their height and weight were measured. They were then fitted with a heart rate (HR) monitor (Polar, Electro Kempele, Finland). The procedure for Douglas bag gas analysis was explained and they watched a researcher demonstrate how to position the nose clips and insert the mouthpiece. For familiarisation, participants practiced using the nose clip and mouthpiece and breathed for 1 min in a seated position for familiarisation. They then sat quietly and rested for 15 min before the first blood sample was taken; on average, this was taken 2 h 45 min after waking (SD = 33 min), and timing of this sample did not differ significantly between groups.
Participants then undertook an acute bout of exercise in the form of an incremental submaximal treadmill test. A researcher demonstrated how to walk on the treadmill for those who had not used one before. Participants then began walking on the treadmill and speed was gradually increased until the participant reached a pace they considered ‘brisk walking’. Once this pace was reached, the test commenced comprising 4-min stages. Every 4 min, the gradient increased between 2% and 3.5%, depending on the participants HR. During the final minute of each 4-min stage, expired air samples were collected into Douglas bags (Cranlea, Birmingham, UK) for the determination of oxygen consumption. HR was monitored continuously throughout the exercise and recorded every 15 s during the final minute of each stage, and RPE was obtained at the end of each stage and prior to the termination of the exercise. The test was terminated once the participant had reached 75% of their predicted maximum HR, as determined by the formula: 208 − (age × 0.7) (Tanka et al. 2001). In a few cases, participant’s HR did not increase proportionally with the exercise; in which case, the exercise was terminated once they reached ‘hard’ on the RPE Scale. Once the exercise was finished, participants were seated immediately for another blood sample, and a final blood sample was taken 1 h post-exercise.
Carbon dioxide production and oxygen consumption were determined from Douglas bag samples using an infrared carbon dioxide analyser and a paramagnetic oxygen analyser (Analyser Series 1440, Servomex, Crowborough, East Sussex, UK). Expired air volumes were measured using a dry gas meter (Harvard Apparatus, Edenbridge, Kent, UK) and corrected to standard temperature and pressure. Maximal oxygen consumption (VO2max) was predicted using a regression equation created from plotting the relationship between HR and oxygen consumption during the final three stages of exercise.
Statistical analyses
Univariate ANOVA was used to examine the differences between exercise groups for age, BMI, VO2max, HR, RPE and exercise duration. Repeated-measures ANOVA was used to examine the DHEA, DHEA-S and cortisol response to exercise, to test any main effects of group and, finally, to examine any group × time interactions. Repeated-measures ANOVA was also used to investigate any sex differences in hormone responses. Where significant effects emerged, subsequent ANCOVA was performed to adjust for any potential confounding variables, such as age. Greenhouse–Geisser corrections were applied with the repeated-measures analyses and partial η2, a measure of effect size, is reported throughout.
Results
Group characteristics
Table 2 displays the age, BMI and predicted VO2max for the exercise groups. Age [F(2,46) = 5.06, p = 0.01, η2 = 0.180] and BMI [F(2,46) = 8.94, p < 0.001, η2 = 0.280] differed significantly between exercise groups. VO2max also differed significantly between groups [F(2,39) = 22.14, p < 0.001, η2 = 0.532], and this group effect withstood adjustment for age. In comparison to the VO2max criteria for sex and age group (McArdle et al. 2001), males in the untrained, moderate and trained groups were classified as having average, good and excellent aerobic fitness for their age group, respectively. Females in the untrained and moderate groups were classed as average for their age group, and trained females were classed as excellent for their age group.
Table 2.
Mean (SD) age, BMI and VO2max for exercise training status and sex
| Sedentary | Moderate | Trained | ||||
|---|---|---|---|---|---|---|
| Males | Females | Males | Females | Males | Females | |
| Age | 66.8 (3.48) | 70.6 (4.63) | 67.5 (5.36) | 67.6 (3.25) | 65.6 (4.82) | 62.3 (4.23) |
| BMI | 26.3 (3.40) | 25.4 (3.61) | 27.9 (2.75) | 25.4 (3.07) | 23.5 (1.93) | 21.3 (1.71) |
| VO2max (ml kg−1 min−1) | 35.4 (3.53) | 27.0 (5.89) | 40.1 (7.69) | 29.5 (3.53) | 48.1 (6.30) | 44.4 (6.35) |
Acute exercise
The mean duration of the incremental exercise test was 23 min 49 s (SD = 2 min 8 s) and the mean final RPE obtained at the end of exercise was 5.1 (SD = 1.08), which was equivalent to ‘hard’. The final HR achieved at the end of the exercise was 132.7 bpm (SD = 9.91), this was equivalent to 76.5% (SD = 5.44) of the predicted maximal HR. There were no significant differences between exercise training status groups or sex for exercise duration, final RPE, final HR or percentage HR.
DHEA, DHEA-S and cortisol
Training status
There were no significant group differences in any of the hormone parameters at any of the time points. Accordingly, all subsequent results are reported for participants as a whole. VO2max was positively associated with DHEA-S levels in women, although this was not statistically significant [r(18) = 0.42, p = 0.07]. There were no other trends for VO2max and hormone levels.
DHEA
There was a significant main effect of time for DHEA, where DHEA levels increased immediately post-exercise [F(2,92) = 6.62, p = 0.004, η2 = 0.126]. This effect is displayed in Fig. 1a.
Fig. 1.
a Serum DHEA response to acute exercise in older adults. DHEA values immediately post-exercise increased significantly from pre-exercise values, *p = 0.004. b Serum cortisol response to acute exercise in older adults. Significantly different from pre-exercise values, *p < 0.001
DHEA-S
There was a trend for DHEA-S to increase immediately post-exercise, but this failed to reach statistical significance (p = 0.07). However, the effect was significant for females [F(2,42) = 4.37, p = 0.02, η2 = 0.172]. Compared to pre-exercise, females had a significant increase in DHEA-S immediately post-exercise. Although they did not exhibit a response to exercise, males had significantly higher overall DHEA-S levels than females [F(1,43) = 4.48, p = 0.04, η2 = 0.094]. Descriptive statistics of hormone levels for males and females are displayed in Table 3.
Table 3.
Mean (SD) hormone values (in nanomoles per litre) for males and females: overall, pre-exercise, immediately post-exercise and 1 h post-exercise
| Males (n = 26) | Females (n = 23) | |
|---|---|---|
| DHEA (nmol/l) | 18.9 (12.23) | 20.8 (12.70) |
| Pre-exercise | 18.9 (8.61) | 19.5 (12.72) |
| Immediately post-exercise | 20.2 (9.66) | 23.6 (22.44) |
| 1 h post-exercise | 17.6 (8.38) | 19.2 (14.24) |
| DHEA-S (nmol/l) | 2,649.8 (971.25) | 2,036.9 (971.24) |
| Pre-exercise | 2,650.3 (1,028.99) | 2,002.4 (923.70) |
| Immediately post-exercise | 2,659.4 (1,028.99) | 2,096.8 (935.97) |
| 1 h post-exercise | 2,639.7 (1,007.09) | 2,011.5 (939.47) |
| Cortisol (nmol/l) | 362.6 (107.22) | 300.8 (108.24) |
| Pre-exercise | 402.6 (132.77) | 355.3 (110.46) |
| Immediately post-exercise | 375.6 (130.80) | 308.4 (118.40) |
| 1 h post-exercise | 309.6 (141.10) | 238.6 (112.47) |
Cortisol
As shown in Fig. 1b, there was a significant main effect of time for cortisol, which decreased immediately post-exercise and 1 h post-exercise compared to pre-exercise [F(2,92) = 19.58, p < 0.001, η2 = 0.299]. Males had significantly higher cortisol levels than females [F(1,47) = 4.06, p = 0.05, η2 = 0.079] (Table 3).
Cortisol/DHEA or DHEA-S ratio
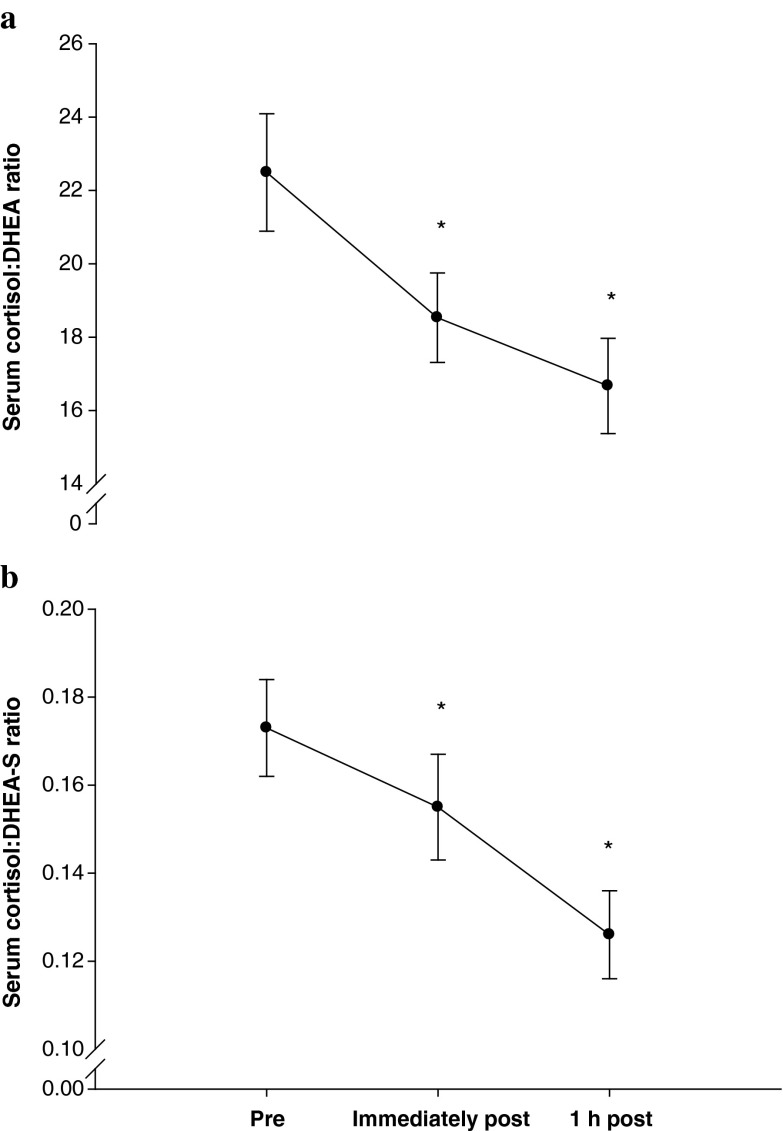
There was a significant main effect of time for the cortisol/DHEA ratio [F(2,90) = 20.04, p < 0.001, η2 = 0.308], where the ratio decreased immediately post-exercise and 1 h post-exercise compared to pre-exercise (Fig. 2a). The cortisol/DHEA-S ratio also decreased significantly immediately post-exercise and 1 h post-exercise compared to pre-exercise [F(2,78) = 19.08, p < 0.001, η2 = 0.329] (Fig. 2b).
Fig. 2.
a Serum cortisol/DHEA ratio response to acute exercise in older adults. Significantly different from pre-exercise values, *p < 0.001. b Serum cortisol/DHEA-S ratio response to acute exercise in older adults. Significantly different from pre-exercise values, *p < 0.001
Discussion
Older adults demonstrated a significant increase in DHEA immediately post-exercise. DHEA has been previously shown to increase in response to submaximal aerobic (Giannopoulou et al. 2003) and resistance exercise (Copeland et al. 2002) in postmenopausal women, and this study extends previous findings to older males. Despite a similar exercise protocol, the present findings contrast with those from a recent study which failed to find an increase in DHEA with exercise in individuals aged between 65 and 75 years (Aldred et al. 2009). However, this could reflect the small sample size employed in this previous investigation.
Cortisol decreased immediately post and 1 h post-exercise compared to pre-exercise. Independent activation of the zona fasciculata and zona reticularis has been noted previously (Velardo et al. 1991). A decrease in cortisol post-exercise has also been noted in postmenopausal females (Kemmler et al. 2003); this decrease continued throughout the 2-h recovery period. As with the present study, Kemmler et al. (2003) exercised their participants in the morning; however, the authors suggested that their observed decreases were above that of normal diurnal decline. It is difficult to separate the effects of exercise from the effects of diurnal variation. However, the first blood sample taken in the present study was nearly 3 h after waking; therefore, participants would have already experienced the large decrease in cortisol that occurs following the cortisol awakening response. This is suggested by cortisol diurnal rhythm data collected on average a week before acute exercise testing in the present participants (unpublished data available on request). Further, the present finding is in contrast to Traustadottir et al. (2004) who reported an increase in cortisol in older females after 15 min of cycling; this exercise bout also took place in the morning period, suggesting that time of day is not responsible for this difference.
As a result of the decrease in cortisol, which was not apparent with DHEA/S, the cortisol/DHEA/S ratio significantly decreased immediately and 1 h post-exercise. This represents a more favourable endocrine profile, although it may be that this only occurs if exercise takes place in the morning period. Identical exercise in the afternoon would be required to determine whether this is the case. If the effect is limited to morning exercise, it could be that this is because it is the optimal time to alter the balance between cortisol and DHEA/S and thus promote greater protection against decrements in immunity linked to higher cortisol levels. However, this is highly speculative, and exercise of a longer duration or a higher intensity in the morning may still produce an increase in cortisol. It is known that only high-intensity or exhaustive exercise results in increases in cortisol, with a threshold of 60% VO2max required for its release (Bishop 2006; Pedersen and Hoffman-Goetz 2000). Therefore, although participants were exercised to 75% of their predicted maximum capacity, as the exercise was graded and not at a predetermined intensity, it is possible that they did not exercise for a sufficient period at 60% VO2max or above to elicit an increase in cortisol.
To our knowledge, this is the first study to examine the responses of DHEA/S alongside cortisol to acute exercise in relation to exercise training status in elderly participants. The present findings suggest that sedentary, moderately trained and endurance trained older adults do not vary in their hormonal responses to exercise. This implies that older adults, regardless of training status, are able to produce a DHEA response to exercise. This finding is in contrast to those of Cadore et al. (2008) who found that trained middle-aged men required a greater exercise stimulus to produce a hormonal response. However, this study used strength-trained individuals and investigated acute resistance exercise in middle-aged, not elderly, individuals. Although no effects of training status were found, the response of DHEA-S to exercise did differ between sexes. Females showed a significant increase in DHEA-S immediately post-exercise, whereas males did not. This increase in DHEA-S is consistent with prior research in early postmenopausal females (Kemmler et al. 2003), although males were not tested in this previous study.
Possible mechanisms for exercise-induced increases in DHEA/S have been outlined previously, with increased secretion rate by the adrenal cortex as a result of ACTH stimulation (Johnson et al. 1997; Keizer et al. 1987, 1989) and decreased metabolic clearance due to a reduction in hepatic blood flow during exercise (Ponjee et al. 1994) being the most commonly cited. The mechanism responsible for the observed increase in DHEA-S in females, but not in males, is not clear. DHEA-S is found in higher and more stable concentrations due to its longer half-life; it has been suggested that larger increases in DHEA-S are required to observe significant changes (Johnson et al. 1997). Therefore, as women have significantly lower levels of DHEA-S at baseline, they may have greater potential to exhibit a significant increase in response to exercise. Resting DHEA-S levels have been shown to be associated with physical activity in females, but not males, within the same study (Bonnefoy et al. 1998; Kostka et al. 2002); consequently, it could be speculated that females may be more sensitive to exercise-induced changes. The ability of an acute exercise bout to increase DHEA/S in older adults may afford a non-pharmacological method for increasing anabolic hormones. However, as levels returned back to baseline within an hour, it is debatable how such a short-term increase could be beneficial. Exercise of a longer duration and higher intensity may be required in order to elevate and maintain DHEA/S levels for a significant period post-exercise, although this needs to be balanced against what exercise protocols older adults can realistically perform.
Acute exercise performed on a regular basis over a period of years does not appear to influence resting hormone levels, as interestingly, there were no differences among older adults in relation to their exercise training status. This is consistent with Arai et al. (2006) who found no significant difference in DHEA-S between older male runners and those who were sedentary, and Traustadottir et al. (2004) who reported no differences in cortisol levels between fit older women and those who were of average fitness. Although this present finding is in contrast to other studies which suggest that DHEA/S is higher in endurance trained (de Gonzalo-Calvo et al. 2011; Tissandier et al. 2001) and moderately trained (Ravaglia et al. 2001) older men. In these previous and current investigations, blood samples were collected at similar times under the same conditions (fasted, rested, etc.). However, differences could be due to variation in the participants studied in terms of fitness, training load and how long they have been exercising for.
Our current findings suggest that long-term exercise training, be it at a vigorous or moderate intensity, does not lead to a more favourable hormonal profile among older adults compared to those who do not exercise. All participants in the present study were healthy older adults with no endocrine or immune disorders; it is possible that the sedentary group were healthier and fitter than the average older adult and this is why no differences emerged between exercisers and non-exercisers. They were of relatively high socio-economic status, as is characteristic of study volunteers and is associated with better health (Anderson and Armstead 1995). It may be that long-term exercise training does not impact upon hormone levels of healthy older adults, but it could have the potential to have a positive effect on more vulnerable older adults, for example, those who are frail, who suffer from an illness or depression or who are experiencing chronic stress (Phillips et al. 2007).
In healthy older adults, 6 months of resistance (Häkkinen et al. 2000) or endurance training (Hersey et al. 1994) did not elicit an increase in DHEA/S. However, exercise intervention studies in this area are currently lacking, especially long-duration studies and studies of unhealthy populations. Even if long-term exercise turns out not to be beneficial from a hormonal perspective, it should still be encouraged as evidence suggests that it has the potential to decelerate immunosenescence (Arai et al. 2006; Nieman et al. 1993; Shinkai et al. 1995), as well as to maintain aerobic capacity and muscular strength, thereby preserving physical function with ageing.
The present study suffers from several limitations. Firstly, as a VO2max test was not performed and aerobic fitness was estimated from a submaximal test. However, maximal exercise testing in older adults raises a number of issues (Hugget et al. 2005), not least the ethics of such testing. As a result of not obtaining a VO2max, exercise was graded in intensity and was not steady state, which may have produced a different response. Investigating hormonal responses to exercise of different durations and intensities in this population would be valuable, although the feasibility of multiple trials and what demands can be placed on participants may be limiting factors.
In conclusion, older adults demonstrated a significant increase in DHEA in response to acute submaximal aerobic exercise, whereas only older females showed a significant increase in DHEA-S. Further, neither resting hormone levels nor the response to exercise was influenced by exercise training status. Although these findings suggest that long-term exercise may not affect the hormonal status of older adults, future research involving longitudinal studies and exercise interventions are required to definitively determine whether exercise can be used to maintain or improve the ageing endocrine system.
Acknowledgments
The authors would like to thank the participants and Siobhan Forrest, Deborah Shearring, Alison Wood, Patrick Dawkes, Rosa James-Watling, Ruth Mackay, Emma Dodd and Lucy Dowsett for their help with data collection.
References
- Abbasi A, Duthie EH, Sheldahl L, Wilson C, Sasse E, Rudman I, Mattson DE (1998) Association of dehydroepiandrosterone sulphate, body composition and physical fitness in independent community-dwelling older men and women. J Am Geriatr Soc 46:263–273 [DOI] [PubMed]
- Ainsworth BE, Haskell WL, Whitt CM, Irwin ML, Swartz AN, Strath SJ, O'Brien WL. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- Aldred S, Rohalu M, Edwards K, Burns V. Altered DHEA and DHEAS response to exercise in healthy older adults. J Ageing Phys Act. 2009;17:77–88. doi: 10.1123/japa.17.1.77. [DOI] [PubMed] [Google Scholar]
- Anderson NB, Armstead CA. Toward understanding the association of socio-economic status and health—a new challenge for the biopsychosocial approach. Psychosom Med. 1995;57:213–225. doi: 10.1097/00006842-199505000-00003. [DOI] [PubMed] [Google Scholar]
- Arai MH, Deuarte AJ, Natale VM. The effects of long-term endurance training on the immune and endocrine systems of elderly men: the role of cytokines and anabolic hormones. Immun Ageing. 2006;25:3–9. doi: 10.1186/1742-4933-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanger A, Candas B, Dupont A, Cusan L, Diamond P, Gomez JL, Labrie F. Changes in serum concentrations of conjugated and unconjugated steriods in 40-year-old to 80-year-old men. J Clin Endocrinol Met. 1994;79:1086–1090. doi: 10.1210/jc.79.4.1086. [DOI] [PubMed] [Google Scholar]
- Bishop NC. Acute exercise and aquired immune function. In: Glesson M, editor. Immune function in sport and exercise. London: Churchill Livingstone Elsevier; 2006. [Google Scholar]
- Bonnefoy M, Kostka T, Patricot MC, Berthouze SE, Mathian B, Lacour JR. Physical activity and dehydroepiandrosterone sulphate, insulin-like growth factor and testosterone in healthy active elderly. Age Ageing. 1998;1998:745–751. doi: 10.1093/ageing/27.6.745. [DOI] [PubMed] [Google Scholar]
- Bonnefoy M, Patricot MC, Lacour JR, Rahmani A, Berthouze SE, Kostka T. Relation between physical activity, muscle function and IGF-1, testosterone and DHEAS concentrations in the elderly. Rev Med Interne. 2002;23:819–827. doi: 10.1016/S0248-8663(02)00689-6. [DOI] [PubMed] [Google Scholar]
- Borg GA. Borg's ratings of percieved exhertion and pain scales. Champaign: Human Kinetics; 1998. [Google Scholar]
- Buford TW, Willoughby DS. Impact of DHEA(S) and cortisol on immune function in ageing: a brief review. Appl Physiol Nutr Metab. 2005;33:429–433. doi: 10.1139/H08-013. [DOI] [PubMed] [Google Scholar]
- Butcher SK, Killampalli V, Lascelles D, Wang K, Alpar EK, Lord JM. Raised cortisol:DHEAS ratios in the elderly after injury: potential impact upon neutrophil function and immunity. Aging Cell. 2005;4:319–324. doi: 10.1111/j.1474-9726.2005.00178.x. [DOI] [PubMed] [Google Scholar]
- Cadore EL, Lhullier FL, Brentano MA, da Silva RF, Ambrosini MB, Spinelli R, Silva RF, Kruel LF. Hormonal responses to resistance exercise in long term trained and untrained middle-aged men. J Strength Cond Res. 2008;22:1617–1627. doi: 10.1519/JSC.0b013e31817bd45d. [DOI] [PubMed] [Google Scholar]
- Chahal HS, Drake WM. The endocrine system and ageing. J Pathol. 2007;211:173–180. doi: 10.1002/path.2110. [DOI] [PubMed] [Google Scholar]
- Copeland JL, Consitt LA, Tremblay MS. Hormal responses to endurance and resistance exercise in females aged 19–69 years. J Gerontol A Biol Sci Med Sci. 2002;57:158–165. doi: 10.1093/gerona/57.4.B158. [DOI] [PubMed] [Google Scholar]
- Cumming DC, Brunsting LA, Strich G, Reis AL, Rebar W. Reproductive hormone increases in response to acute exercise in men. Med Sci Sports Exerc. 1986;18:369–373. doi: 10.1249/00005768-198608000-00001. [DOI] [PubMed] [Google Scholar]
- de Gonzalo-Calvo D, Fernandez-Garcia B, de Luxan-Delago B, Rodriguez-Gonzalez S, Garcia-Marcia M, Suarez FM, Solano JJ, Rodriguez-Colunga MJ, Coto-Montes A (2011) Long-term training induces a healthy inflammatory and endocrine emergent biomarker profile in elderly men. Age (Dordr) (in press) [DOI] [PMC free article] [PubMed]
- Deuschle M, Gotthardt U, Schweiger U, Weber B, Körner A, Schmider J, Standhardt H, Lammers C-H, Heuser I. With aging in humans the activity of the hypothalamus–pituitary–adrenal system increases and its diurnal amplitude flattens. Life Sci. 1997;61:2239–2246. doi: 10.1016/S0024-3205(97)00926-0. [DOI] [PubMed] [Google Scholar]
- Ford G, Ecob R, Hunt K, Macintyre S, West P. Patterns of class inequality in health through the life span: class gradients at 15, 35 and 55 years in the west of Scotland. Soc Sci Med. 1994;39:1037–1050. doi: 10.1016/0277-9536(94)90375-1. [DOI] [PubMed] [Google Scholar]
- Giannopoulou L, Carhart R, Sauro LM, Kanaley JA. Adrenocortical responses to submaximal exercise in postmenopausal black and white women. Metabolism. 2003;52:1643–1647. doi: 10.1016/S0026-0495(03)00312-3. [DOI] [PubMed] [Google Scholar]
- Häkkinen K, Parkarinen A, Kraemer WJ, Newton RU, Alen M. Basal concentrations and acute responses of serum hormones and strength development during heavy resistance training in middle-aged and elderly men and woman. J Gerontol A Biol Sci Med Sci. 2000;55:B95–B105. doi: 10.1093/gerona/55.2.B95. [DOI] [PubMed] [Google Scholar]
- Hersey WCr, Graves JE, Pollock ML, Gingerich R, Shireman RB, Heath GW, Spierto F, McCole SD, Hagberg JM. Endurance exercise training improves body composition and plasma insulin responses in 70- to 79-year old men and women. Metabolism. 1994;43:847–854. doi: 10.1016/0026-0495(94)90265-8. [DOI] [PubMed] [Google Scholar]
- Hugget DL, Connelly DM, Overend TJ. Maximal aerobic capacity testing of older adults: a critical review. J Gerontol A Biol Sci Med Sci. 2005;60:57–66. doi: 10.1093/gerona/60.1.57. [DOI] [PubMed] [Google Scholar]
- Johnson LG, Kraemer RR, Haltom R, Kraemer GR, Gaines HE, Castracane VD. Effects of estrogen replacement therapy on dehydroepiandrosterone, dehydroepiandrosterone sulphate, and cortisol responses to exercise in postmenopausal women. Fertil Steril. 1997;68:836–843. doi: 10.1016/S0015-0282(97)00369-5. [DOI] [PubMed] [Google Scholar]
- Keizer HA, Kuipers H, de Haan J, Janssen GM, Beckers E, Habets L, van Kranenberg G, Geurten P. Effect of a 3 month endurance training program on metabolic and multiple hormonal responses to exercise. Int J Sports Med. 1987;8:154–160. doi: 10.1055/s-2008-1025722. [DOI] [PubMed] [Google Scholar]
- Keizer H, Janssen GM, Menheere P, Kranenberg G. Changes in basal plasma testosterone, cortisol, and dehydroepiandrosterone sulphate in previously untrained males and females preparing for a marathon. Int J Sports Med. 1989;10:S139–S145. doi: 10.1055/s-2007-1024962. [DOI] [PubMed] [Google Scholar]
- Kemmler W, Wildt L, Engelke K, Pintag R, Pavel M, Bracher B, Weineck J, Kalender W. Acute hormonal responses of a high impact physical exercise session in early postmenopausal women. Eur J Appl Physiol. 2003;90:199–200. doi: 10.1007/s00421-003-0874-7. [DOI] [PubMed] [Google Scholar]
- Kostka T, Pariante CM, Berthouze SE, Lacour JR, Bonnefoy M. Influence of 6 month changes in habitual physical activity on dehydroepiandrosterone sulphate in elderly subjects. Biol Sport. 2002;19:33–41. [Google Scholar]
- Labrie F, Belanger A, Cusan L, Gomez JL, Candas B. Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging. J Clin Endocrinol Met. 1997;82:2396–2402. doi: 10.1210/jc.82.8.2396. [DOI] [PubMed] [Google Scholar]
- Marmot MG, Smith GD, Stansfeld S, Patel C, North F, Head J, White I, Brunner E, Feeney A. Health inequalities amoung British civil servants: the Whitehall II study. Lancet. 1991;337:1387–1399. doi: 10.1016/0140-6736(91)93068-K. [DOI] [PubMed] [Google Scholar]
- McArdle WD, Katch FI, Katch VL. Exercise physiology: energy, nutrition and human performance. Baltimore: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC. Physical acivity and public health in older adults. Circulation. 2007;116:1094. doi: 10.1161/CIRCULATIONAHA.107.185650. [DOI] [PubMed] [Google Scholar]
- Nieman DC, Henson DA, Gusewitch G, Warren BJ, Dotson RC, Butterworth DE, Nehlsencannarella SL. Physical activity and immune function in elderly women. Med Sci Sports Exerc. 1993;25:823–831. doi: 10.1249/00005768-199307000-00011. [DOI] [PubMed] [Google Scholar]
- Orentreich N, Brind JL, Vogelman JH, Andres R, Baldwin H. Long term longitudinal measurements of plasma dehydroepiandrosterone sulphate in normal man. J Clin Endocrinol Met. 1992;75:1002–1004. doi: 10.1210/jc.75.4.1002. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Hoffman-Goetz L. Exercise and the immune system: regulation, integration, and adaptation. Physiol Rev. 2000;80:1055–1081. doi: 10.1152/physrev.2000.80.3.1055. [DOI] [PubMed] [Google Scholar]
- Phillips AC, Burns VE, Lord JM. Stress and exercise: getting the balance right for aging immunity. Exerc Sport Sci Rev. 2007;35:35–39. doi: 10.1097/jes.0b013e31802d7008. [DOI] [PubMed] [Google Scholar]
- Ponjee GA, De Rooy HA, Vader HL. Androgen turnover during marathon running. Med Sci Sports Exerc. 1994;26:1274–1277. doi: 10.1249/00005768-199410000-00015. [DOI] [PubMed] [Google Scholar]
- Ravaglia G, Forti P, Maioli F, Pratelli L, Vettori C, Bastagli L, Mariani E, Facchini A, Cucinotta D. Regular moderate intensity physical activity and blood concentrations of endogenous anabolic hormones and thyroid hormones in aging men. Mech Ageing Dev. 2001;122:191–203. doi: 10.1016/S0047-6374(00)00234-7. [DOI] [PubMed] [Google Scholar]
- Shinkai S, Kohno H, Kimura K, Komura T, Asai H, Inai R, Oka K, Kurokawa Y, Shephard RJ. Physical activity and immune senescence in men. Med Sci Sports Exerc. 1995;27:1516–1526. doi: 10.1249/00005768-199511000-00008. [DOI] [PubMed] [Google Scholar]
- Tanka H, Monahan KD, Seals DR. Age predicted maximal heart rate revisited. J Am Coll Cardiol. 2001;37:153–156. doi: 10.1016/S0735-1097(00)01054-8. [DOI] [PubMed] [Google Scholar]
- Tharrett SJ, Peterson JA. ACSM's health/fitness facility standards and guidelines. Champaign: Human Kinetics; 1997. [Google Scholar]
- Tissandier O, Peres G, Fiet J, Piette F. Testosterone, dehydroepiandrosterone, insulin-like growth factor 1, and insulin in sedentary and physically trained aged men. Eur J Appl Physiol. 2001;85:177–184. doi: 10.1007/s004210100420. [DOI] [PubMed] [Google Scholar]
- Traustadottir T, Bosch PR, Cantu T, Matt KS. Hypothalamic–pituitary–adrenal axis response and recovery from high-intensity exercise in women: effects of aging and fitness. J Clin Endocrinol Met. 2004;89:3248–3254. doi: 10.1210/jc.2003-031713. [DOI] [PubMed] [Google Scholar]
- Tremblay MS, Copeland JL, Van Helder W. Effect of training status and exercise mode on endogenous steroid hormones in men. J Appl Physiol. 2004;96:531–539. doi: 10.1152/japplphysiol.00656.2003. [DOI] [PubMed] [Google Scholar]
- VanCauter E, Leproult R, Kupfer DJ. Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. J Clin Endocrinol Met. 1996;81:2468–2473. doi: 10.1210/jc.81.7.2468. [DOI] [PubMed] [Google Scholar]
- Velardo A, Pantaleoni M, Valerio L, Barini A, Marrama P. Influence of exercise on dehydroepiandrosterone sulphate and delta 4-androsterone plasma levels in man. Exp Clin Endocrinol. 1991;97:99–101. doi: 10.1055/s-0029-1211046. [DOI] [PubMed] [Google Scholar]
- Walston J, Hadley EC, Ferrucci L, Guralnik JM, Newman AB, Studenski SA, Ershler WB, Harris T, Fried LP. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54:991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]