Abstract
The limbal niche in the corneoscleral junction of the eye, habitat of the limbal epithelial stem cells (LESC), facilitates corneal epithelial regeneration by providing physical support and chemical signalling. Anatomical structures within the limbus, namely, limbal epithelial crypts and focal stromal projections, are believed to function as a putative niche for LESCs. In this study, the impact of age on the topography of this niche was investigated. Also, the relationship between niche topography and limbal epithelial cell phenotype was assessed. Ex vivo imaging of the limbus in cadaveric tissue of donors aged from infancy to 90 years was carried out using electron and confocal microscopy. The data suggested that the area occupied by the crypts was sharply reduced after the age of 60 years. The niche microstructures also became smoother with donor age. The phenotypic assessment of cultured limbal epithelial cells harvested from donors of different ages showed that the levels of putative stem cell markers as well as telomerase activity and telomere length remained unchanged, regardless of niche topography. However, the colony forming efficiency of the cultures was significantly reduced with age (p < 0.05). This is the first comprehensive study of the effect of age on the structural and phenotypic characteristics of the human limbal niche. The results have a significant biological value as they suggest a correlation of limbal architecture with decline of re-epithelialisation rate in older patients. Overall, the data also suggest that LESCs harvested from younger donors may be more suitable for cultured LESC therapy production.
Keywords: Stem cells, Cornea, Stem cell markers, Telomerase activity, Telomere length, Imaging
Introduction
The cornea is the foremost transparent part of the eye that covers the pupil, the iris and the anterior chamber. Along with the lens, the cornea refracts light and facilitates focus. The outermost layer of the cornea is a regenerating squamous epithelium. During normal epithelial turnover, as well as following injury, the maintenance of the corneal epithelial cell mass is achieved by the activity of a population of unipotent limbal epithelial stem cells (LESC) believed to exist in the basal epithelium of the vascularised natural border between cornea and conjunctiva, namely, the limbus (Stepp and Zieske 2005; Dua et al. 2005; Daniels et al. 2001; Tseng 1996). Putative stem cells have been observed at the bottom of the epithelial papillae in the region of the limbal palisades of Vogt in histological tissue cross-sections (Schlotzer-Schrehardt and Kruse 2005). Our group (Shortt et al. 2007b) has studied the 3D LESC niche in detail and identified two candidate LESC niche structures, namely, limbal epithelial crypts (LECs) which extend from the palisades of Vogt and the focal stromal projections (FSPs) at the open ends of LECs (Shortt et al. 2007b). The non-uniform junction between the limbal epithelium and stroma probably protects the cells from shear forces, and the nearby blood vessels may provide a source of nutrition for the resident cells (Boulton and Albon 2004).
Loss of LESC function, due to disease or injury, can lead to conjunctivalisation of the corneal surface causing opacification, severe pain, and ultimately blindness. These cases can be treated with transplantation of cultured LESCs either derived by a biopsy from the patient’s healthy eye (in the case of unilateral injury) or harvested from donor tissue (LESC allograft) (Baylis et al. 2011; Shortt et al. 2010). There has been a clinical observation that pterygium, a condition where fibrotic tissue from the conjunctiva invades the cornea in a characteristic triangular shape, usually occurs in the nasal limbus (Kanski 2003). Interestingly, the nasal region does not contain LECs and FSPs and thus may suggest that these LESC-rich LECs and FSPs may prevent pterygium from occurring in these areas in the normal eye.
At present, a specific marker that defines LESC phenotype remains elusive. Putative LESC markers include the ABCG2 ATP binding cassette transporter protein ABCG2 (Watanabe et al. 2004), the alpha isoform of the transcription factor p63 (ΔNp63α) (Di Iorio et al. 2005), cytokeratin 15 (Yoshida et al. 2006). For that reason, a battery of markers and assays including colony forming efficiency (CFE) are used to investigate the progenitor-like character of LESCs.
In addition to marker expression, activity of the telomerase enzyme and the telomere length can also function as indicators of stemness; it was thought that telomerase expression is induced on differentiation of stem cells into transient-amplifying or progenitor cells and that this telomerase activity decreases once these cells have become fully differentiated (Chiu et al. 1996). Telomerase activity and telomere length are believed to be able to differentiate LESCs with progenitor-like characteristics. Specifically, putative LESCs are believed to have no telomerase activity and exist in a quiescent state, i.e. in the G0/G1 phase of the cell cycle (Umemoto et al. 2006).
Clinical evidence indicates that in elderly patients, which are also affected by additional ocular and systemic morbidities, corneal re-epithelialisation after injury, after infections such as microbial keratitis or following surgery such as corneal keratoplasty is often accompanied by persistent epithelial breakdown and delayed wound healing (Constantinou et al. 2009; Parmar et al. 2006; Ibrahim et al. 2009; van der Meulen et al. 2008). Possible underlying correlations, related to the LESC niche anatomy and limbal epithelial cell phenotype, have not as far been investigated.
In this study, the effect of age on niche topography and in vitro characteristics of limbal epithelial cells including putative stem cell marker expression, colony forming efficiency, telomerase activity and telomere length was investigated. The results presented here demonstrate a clear change in niche topography after the age of 60 years when a significant reduction in the surface area occupied by putative LESC niche structures occurs as well as flattening of the LECs and the palisades of Vogt (p < 0.05). These changes were associated with a significant reduction in colony forming efficiency (p < 0.05) of the epithelial cells isolated from these structures. Telomerase activity and telomere length of the cultured cells remained unchanged.
These findings may be clinically relevant as they demonstrate age-related changes in the LESC niche that coincide with reduced capacity for corneal re-epithelialisation following insult in the elderly.
Materials and methods
Collection of samples
Research-consented cadaveric human limbal rims from different age donors were obtained from Moorfields Eye Hospital Eye Bank. These samples were surplus from cases wherein the central corneal button was removed from the whole cornea for use in transplantation. The limbal rims were orientated under a dissecting microscope (Nikon SMZ1500). A least ten samples from each age group (0–30, 30–60, 60–90) were collected for each assay.
Confocal imaging of whole-mount tissue
Imaging of whole-mount donor corneas and fixation and staining of the specimens using phalloidin FITC and propidium iodide (both at a dilution of 1/500, Sigma/Dorset) were carried out as previously described (Shortt et al. 2007b). The specimens were imaged using a Zeiss LSM510UV confocal microscope. Montages were prepared using Adobe Photoshop.
Scanning electron microscopy
Preparation of tissue for scanning electron microscopy (SEM) examination was carried out as described before (Notara and Daniels 2010). Specifically, whole human limbal rims were decellularised using 100 mM EDTA solution and fixed overnight in 3% glutaraldehyde and 1% paraformaldehyde buffered to pH 7.4 with 0.07 M sodium cacodylate–HCl. The specimens were washed three times with cacodylate buffer (pH 7.4), treated for 2 h with 1% aqueous solution of osmium tetroxide, rinsed in deionised water and dehydrated through ascending grades of alcohol (50%, 70%, 90% and 100% at 10 min per step). After dehydration, they were critical point-dried, sputter-coated with silver and examined in a scanning electron microscope (6100SEM; JEOL) operating at 15 kV. Montages were prepared by using Adobe Photoshop.
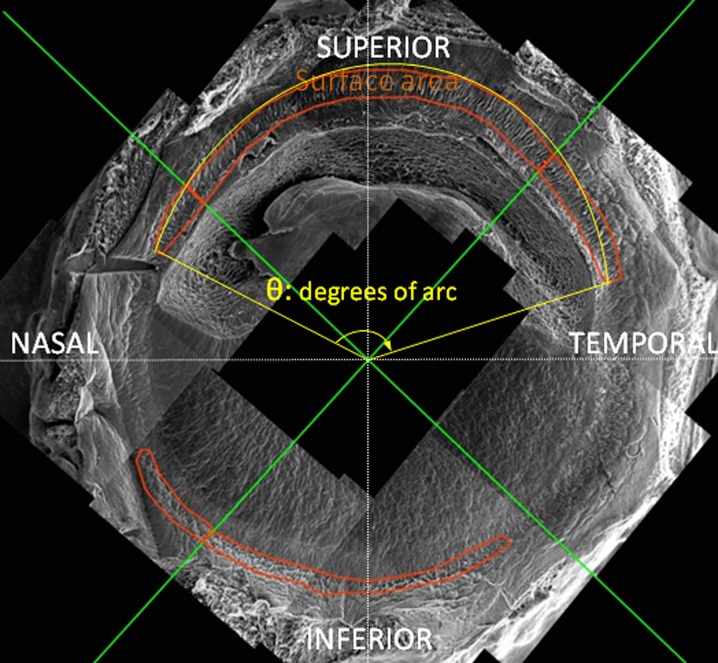
Measurement of surface area and degrees of arc occupied by limbal niche structures
SEM montages of donor limbal rims were prepared as outlined in the previous section. Image J was used to carry out measurements of surface area and degrees of a circle (degrees of arc) occupied up by the LECs and FSPs as shown in Fig. 1. The green crosses define the geometrical quarters of the limbal rims, namely, inferior (6 o’clock), superior (12 o’clock), nasal and temporal (3 and 9 o’clock; Fig. 1). The areas within the red line are occupied by putative niche structures and were measured by Image J by surrounding the area with the pensile tool and using the calculation of area pluggin. The degrees of arc, also calculated by image J, can be described as the angle corresponding to the ‘clock hours’ occupied by the LECs and FSPs. They are defined as the two points of beginning and end of the niche features as well as the geometrical centre of the cornea (Fig. 1, angle θ in yellow). These points were used to calculate the value of degrees of arc by using the appropriate image J pluggin.
Fig. 1.
Schematic description of the methods used in the study for the quantification of the physical extent of the limbal niche features. The pictured SEM microphotograph montage is separated by the green lines in four quadrants, namely, superior, inferior, nasal and temporal. The surface area of the niche features is bordered by the red line and was calculated using image J. The degrees of arc (also measured by image J) are defined by the beginning and the end of the LECs and FSPs as well as the geometrical centre of the cornea (yellow angle, θ)
The data, arranged in the age groups 0–30, 30–60 and 60–90 years, were presented in the following two ways: first, the surface area covered by the structures was expressed either as a percentage of the quadrant area or as a percentage of the total arbitrary circle; second, the degrees of arc occupied by the structures were expressed as a percentage of the quadrant (90°) or the total cornea (360°).
Primary human limbal epithelial cell isolation and culture
Research-consented cadaveric donor human limbal rims were obtained from Moorfields Eye Hospital Eye Bank. Human limbal epithelial (HLE) cells were isolated and cultured on growth-arrested 3T3/J2 mouse fibroblasts (a gift from Prof. Fiona Watt, Cancer Research UK) in corneal epithelial culture medium consisting of DMEM F12 (1:1) (Invitrogen/Paisley) with 10% foetal bovine serum, 1% penicillin/streptomycin (Invitrogen/Paisley), 0.1 nM cholera toxin (Sigma/Dorset), 5 μg/ml human recombinant insulin (Sigma/Dorset), 0.05 mM hydrocortisone (Sigma/Dorset) and 10 ng/ml epidermal growth factor (Invitrogen/Paisley) as previously described (Shortt et al. 2007c; Notara et al. 2007). To prepare feeder layers, the 3T3s were treated with culture medium containing 4 μg/ml mitomycin C (MMC; Sigma/Dorset) for 2 h. Cell morphology was monitored using a Nikon Eclipse TS100 inverted phase contrast microscope.
Quantitative RT-PCR
Cell cultures were trypsinised and centrifuged as described above. Total cellular RNA was isolated from cell pellets using the RNeasy system (Qiagen/USA). Reverse transcription (RT) was carried out as described previously (Shortt et al. 2007a) using Roche First Strand cDNA Synthesis Kit for RT-PCR-AMV. For QPCR, the SYBR Green JumpStart Taq ReadyMix kit for quantitative PCR mastermix (Sigma-Aldrich, UK) was used. Each 25-μl reaction consisted of 12.5 μl of the master mix, 0.25 μl of the indicator dye, 2 μl of the cDNA, 4 μl of the primers (sequences presented in Table 1) and water to make up the remaining volume. Three replicates of RNA from each donor sample were prepared.
Table 1.
Primer sequences used for quantitative PCR
| Gene | Sense primer | Antisense primer |
|---|---|---|
| Cytokeratin 15 | GGCAGAGATCGAGGGTGTC | GTCATCCTTCGCCTGCTGTAG |
| ABCG2 | TGCAACATGTACTGGCGAAGA | TCTTCCACAAGCCCCAGG |
| ΔΝp63α | GGAAAACAATGCCCAGACTC (ΔΝ) | ATGATGAACAGCCCAACCTC (α- termini) |
| β-Actin | GATGTACGTTGCTAT | CTCCTTAATGTCACGCACGAT |
The reactions were run in an applied biosystems ABI 7900HT cycler. The reaction steps included initial denaturation at 94°C for 2 min followed by 40 cycles of 94°C for 15 s, 60°C for 30 s, 72°C for 1 min and finally a stage of dissociation. Data were collected between the 60°C and 72°C stage of each cycle as well as during dissociation. Ramp rates were set to 100% for all of the stages except for the last step of dissociation, which was at 2%.
Colony forming efficiency
3T3 fibroblasts were treated with MMC as previously described and plated at a cell density of 4.8 x 105 cells per well of a six-well plate. HLE cells, isolated from the different treatment groups, were seeded at a density of 1,000 cells per well. All cultures were fixed at the same time point and before 12 days (Barrandon and Green 1987) using cold methanol for 30 min at −20°C. Subsequently, the cells were stained with a solution of 1% rhodamine B (Sigma/Dorset) and 1% Toluidine Blue (Gurr) for 30 min at 37°C. The plates were photographed and Image J software was used to count the number of colonies founded by a single cell. Experiments were repeated in triplicate. Colony diameter was measured using Image J. Total colony forming efficiency, as well as the percentage of colonies with smooth, regular perimeters, containing small cells with a diameter greater than 3.5mm (Barrandon and Green 1987) was calculated using the equation:
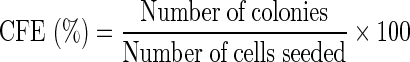 |
Telomerase activity assay
Telomerase activity was determined using the TeloTAGGG Telomerase PCR ELISAPLUS kit (Roche Applied Science) according to the manufacturer’s instructions. Per sample, 2 × 105 cells were analysed.
Telomere length assay
Telomere length was determined using the TeloTAGGG Telomere Length Assay kit (Roche Applied Science) according to the manufacturer’s instructions. DNA was extracted from 3.3 × 105 cells per sample using the QIAamp Mini Kit (Qiagen). This resulted in DNA being suspended in too large a volume for the telomere length assay, so DNA was isolated from the cells using the QIAamp Mini Kit. To precipitate DNA from this solution, the following were added to each sample: two to three volumes of ethanol and one tenth volume 3 M sodium acetate, pH 5.2. The resulting solution was mixed and stored overnight at −20°C. The solution was then spun at full speed in a microcentrifuge for 15–30 min at 4°C. The supernatant was removed, the pellet was washed with 70% ethanol and centrifuged for 5 min and the supernatant was removed. The resulting pellet was then air-dried for 15 min before adding 50 μl of water to re-suspend.
Statistical analysis
Data were analysed using Prism 4.0 (GraphPad/USA) software. One-way analysis of variance using Bonferroni correction for multiple comparisons was carried out on all data unless otherwise stated. Error bars represent standard error of the mean.
Results
Confocal microscopy
Confocal microscopy montages of limbal rim whole mounts from different age donors were used in order to assess the effect of ageing on the morphological features of the limbus.
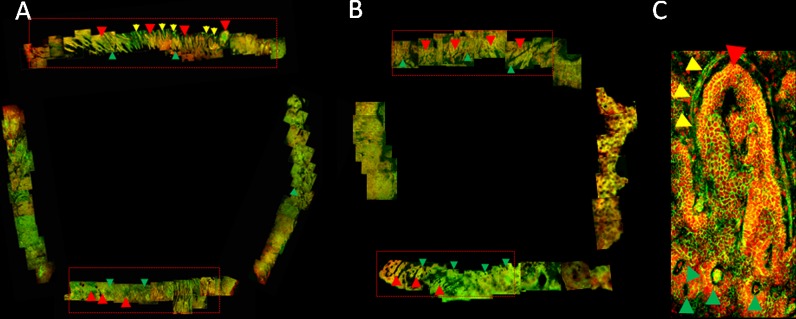
Figure 2a, b shows representative SEM montages from donors aged 22 and 70 years, respectively. The montages illustrate the complex network of invaginations and stromal projections that compose the limbal niche previously defined by our group as limbal epithelial crypts and focal stromal projections (Shortt et al. 2007b). LECs and FSP are noted in Fig. 2a, b with red and green arrows, respectively. Confocal imaging of whole-mount tissue allowed the visualisation of LECs, FSPs as well as parts of the surrounding vasculature (Fig. 2a, yellow arrows) of the LECs. A high power image (Fig. 2c) depicts the LEC (red arrows), FSP (green arrows) and surrounding blood vessel (yellow arrows) in more detail.
Fig. 2.
Confocal microscopy montages of limbal rim wholemounts harvested from a a 48-year-old donor and b a 70-year-old donor. c High-power image depicting a LEC (red arrows) surrounded by a blood vessel (yellow arrows). FSPs are noted with green arrows
Montages of limbal rims from 60–90-year-old donors indicated a reduced surface area and definition of the niche features compared to younger donors (Fig. 2, b—dotted areas). For a quantitative analysis of the changes of the morphology of these features with age, SEM imaging was used as a more precise and higher resolution method of topography analysis.
Quantitative effect of time on niche structures: SEM analysis
In order to get a comprehensive impression of the changes in the limbal niche with age, decellularised whole limbal rims from different age donors were examined using SEM. This technique was used to visualise the palisades of Vogt as well as the LECs and projections (FSPs) formed in the superior and inferior limbus. The SEM analysis demonstrated qualitative and quantitative differences in the structures that occur with age.
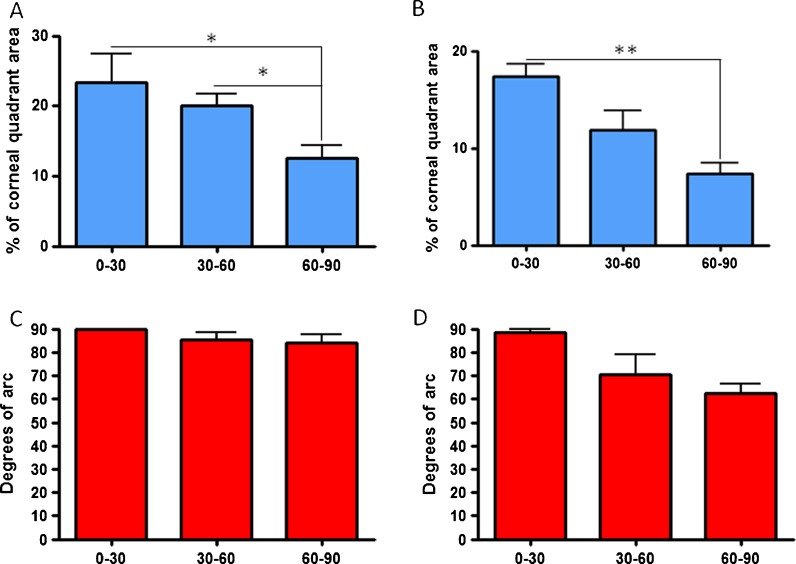
In the superior and inferior portions of the limbus, the surface area of the niche structures was significantly lower for the 60–90-year-old age group compared to the youngest age group of 0–30 years old (Fig. 3a, b—p < 0.05 and p < 0.001, respectively). Notably, in the superior segment, the difference between the grouping of 30–60-year-olds and 60–90-year-olds was also significant (Fig. 3a, p < 0.05). For the superior and inferior segments, there was no statistically significant difference in the degrees of arc covered by the putative niche, although a declining trend with age was observed in the inferior segment (Fig. 3c, d, respectively).
Fig. 3.
Percentage of niche surface area and degrees of arc in a, c superior and b, d inferior quadrants, respectively, in different age groups (*p < 0.05, **p < 0.001)
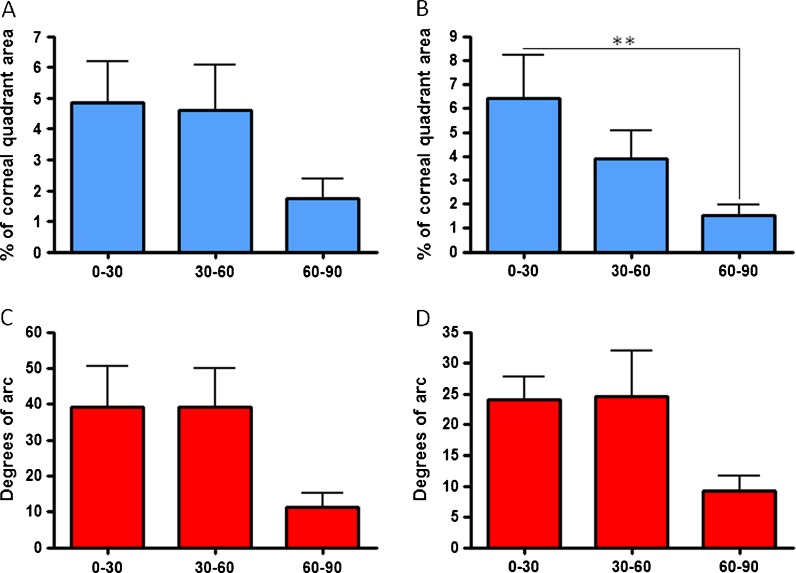
In the temporal and nasal segments, on the other hand (Fig. 4a–d), no significant differences in the niche area and degrees of arc were observed between age groups. The only exception was in the nasal segment where the surface area covered by niche features in the 60–90-year-olds was significantly lower than in the 0–30-year-olds (Fig. 4b, p < 0.001). Notably though, in both segments, both surface areas and degrees of arc of the 60–90-year-old group were smaller; however, these were not statistically different compared to the other groups, possibly due to biological variability in the structures between individuals (Fig. 4a–d).
Fig. 4.
Percentage of niche surface area and degrees of arc in a, c nasal and b, d temporal quadrants, respectively, in different age groups (**p < 0.001)
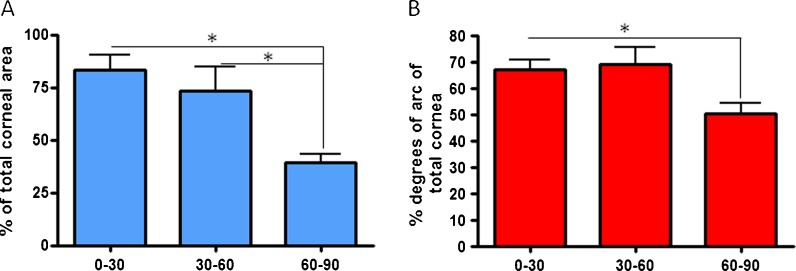
The total surface area of the niche structures expressed as a percentage of the total arbitrary cycle area was significantly lower in 60–90-year-old donors compared to 0–30- and 30–60-year-old donors (Fig. 5a, p < 0.05). The total degrees of arc occupied by niche structures were significantly lower for the 60–90-year-olds compared to the youngest donors aged 0–30 years (Fig. 5b, p < 0.05).
Fig. 5.
Percentage of a total niche surface area and b total degrees of arc compared to the total cornea in different age groups (*p < 0.05)
Qualitative age impact on niche structures
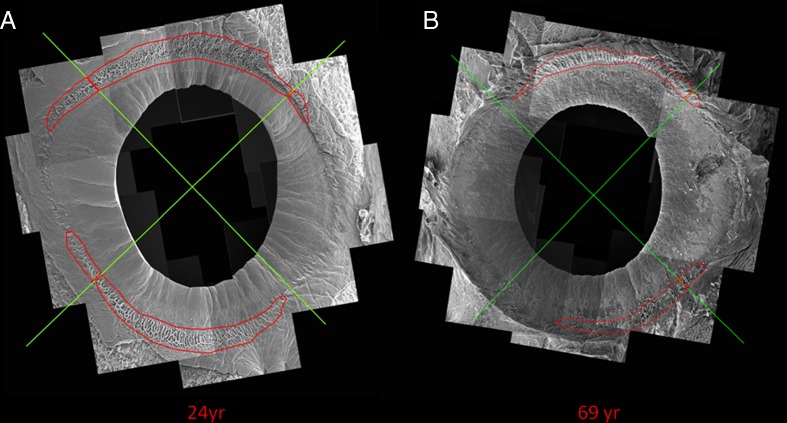
A closer examination of the putative LESC niche structures revealed a change in topography as well as surface area. A clear difference in the surface area coverage was observed between a 24-year-old and a 69-year-old donor (Fig. 6a, b, respectively).
Fig. 6.
Representative SEM montages of limbal rims from a a 24-year-old donor and b a 69-year-old donor. It is clearly shown that the surface area and degrees of arc coverage of the niche features (surrounded with a red line) is reduced in the older donor
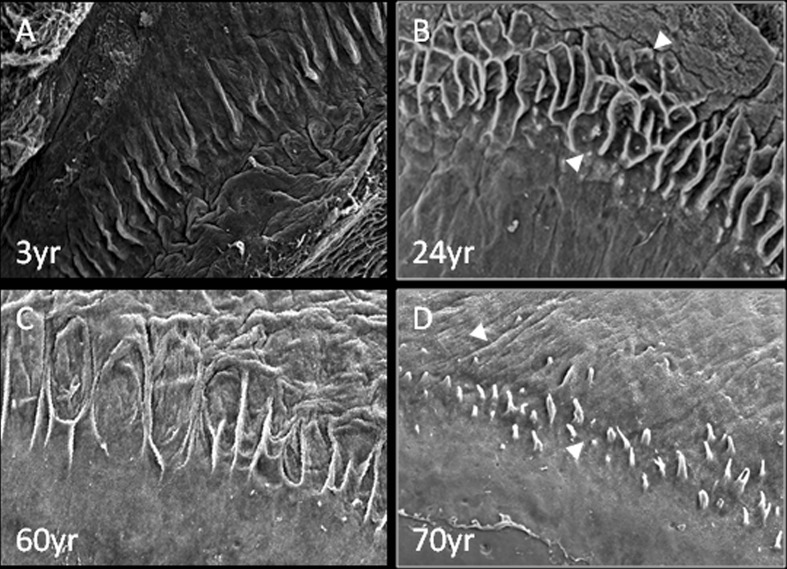
SEM analysis showed that the stromal invaginations forming the palisades and LECs became less defined with age (Fig. 7). Specifically, the niche structures appear to be well defined in younger age, as shown in representative examples of donors aged 3 and 24 years (Fig. 7a, b, respectively). However, the LECs and FSPs in ages of 60 and 70 years are less well demarcated and appear to deteriorate with time (Fig. 7d, e).
Fig. 7.
Representative SEM images of LEC and FSP structures in a 3-, b 24-, c 60- and d 70-year-old donors: the FSP features (white arrows) gradually become less defined with progression of age
Characterisation of cultured HLE cells harvested from donors of different ages
Telomerase activity and telomere length assays
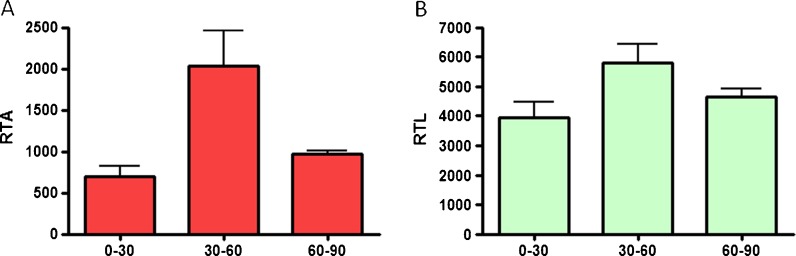
The relative telomerase activity (RTA) and relative telomere length (RTL) of in vitro expanded HLE cells harvested from a range of donors of different ages were assessed. Specifically, there was no significant difference in the RTA levels for donors in all age groups (Fig. 8a). No significant difference was observed for the RTL levels either (Fig. 8b).
Fig. 8.
a Relative telomerase activity and b relative telomere length of cultured limbal epithelial cells harvested from cadaveric donor tissue. No significant difference was observed between the different age groups
Putative stem cell marker expression as assessed by Q-PCR
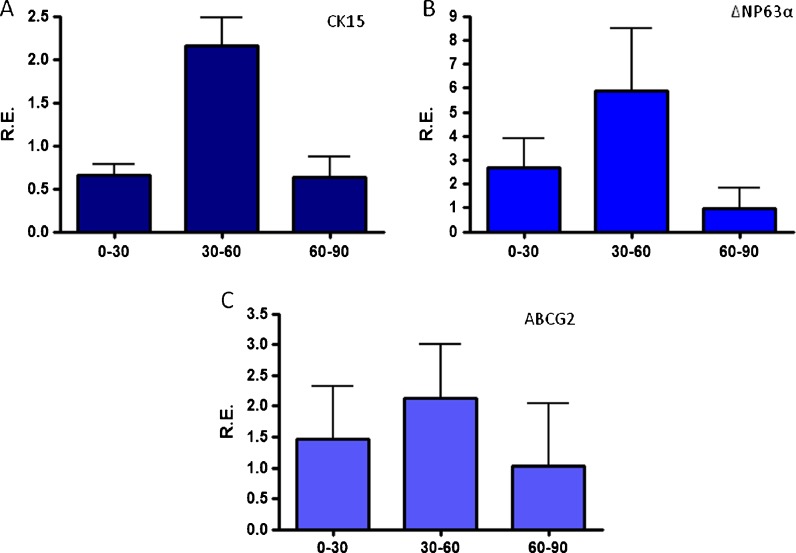
The relative expression (RE) of putative stem cell markers in cultured HLE cells harvested from donors of different ages was measured with Q-PCR. No significant difference was found in the RE levels of cytokeratin 15, ΔΝp63α and ABCG2 for donors in all age groups (Fig. 9a–c, respectively).
Fig. 9.
a Cytokeratin 15, b ΔΝp63α and c ABCG2 expression levels of cultured limbal epithelial cells harvested from cadaveric donor tissue as measured by quantitative PCR. No significant difference was observed between the different age groups
Colony forming efficiency assay
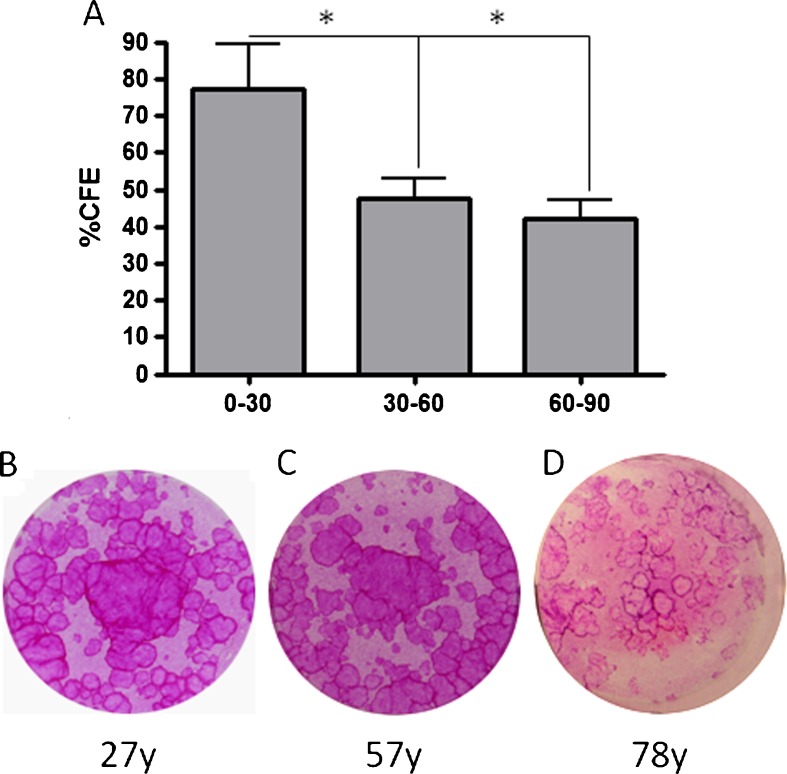
The proliferative capacity of cultured primary HLE cells was assessed by measuring their colony forming efficiency. CFE results (Fig. 10a) showed that indeed cells derived from younger donors (0–30 years old) have a significantly higher colony forming efficiency compared to cells harvested from donors aged 30–60 years (p < 0.05) and older donors (over 60–90 years) (p < 0.05). As a general observation, the colonies from HLEs harvested from younger and middle-aged donors (Fig. 10b, c) had more tightly packed cells and smooth periphery compared to those found in cells isolated from donors of 60 years and over (Fig. 10c).
Fig. 10.
a %CFE of cultured limbal epithelial cells harvested from cadaveric donor tissue was significantly higher for 0–30-year-olds compared to both 30–60 and 60–90-year-olds (*p < 0.05). Representative CFE cultures of limbal epithelial cells from b 27-, c 57- and a 78-year-old donors are shown
Discussion
In almost every country of the developed world, the ratio of people aged over 60 years is growing faster than any other age group. Average life expectancy is currently at 75.7–80 years in the US and Europe and is projected to increase to over 90 years by 2050 (source: World Health Organisation and CIA World Factbook). For that reason, research focussing on the effect of ageing on tissues and their implications for health span is timely.
In this study, the effect of age on the anatomy and phenotype of the epithelial cells of the limbal niche was investigated for the first time. More than 100 human donor limbal rims were used to investigate the topography of the limbal niche using scanning and confocal microscopy. These data were complemented by characterisation of the phenotypic changes in cultured limbal epithelial cells from a spectrum of ages in terms of CFE, putative LESC marker expression, telomerase activity and telomere length.
This study demonstrated age-related changes in the limbal niche. The surface coverage as well as the total degrees of arc occupied by the niche features was significantly reduced in the age group of 60–90-year-olds (p < 0.05). Although the reduction in the occurrence of the palisades of Vogt with age has been previously noted (Zheng and Xu 2008), ours is the first report to examine the effects of age on the physical properties of the niche as well as the limbal epithelial cell phenotype. These physical changes were accompanied with a reduction in CFE of cultured limbal epithelial cells while the marker expression, telomerase activity and telomere length remained unchanged.
The effect of age on stem cell niches has been demonstrated in humans and animal models in a variety of tissues including the hippocampus (Knoth et al. 2010), haematopoietic stem cell niche (Oakley and Van Zant 2010; Wagner et al. 2008; Geiger et al. 2007; Pearce et al. 2007), skin (Gago et al. 2009), dental pulp stem cell niche (Zheng et al. 2009), muscle satellite stem cell niche (Carlson et al. 2009) and germline stem cell niches (Zhao et al. 2008; Zahidov et al. 2010; Cheng et al. 2008; Boyle et al. 2007; Pan et al. 2007).
The deteriorating ageing niche microenvironment has been shown to have a critical effect on the stem cell number, phenotype and regenerative capacity. In fact, the niche habitat is so critical for the preservation of the stem cell potency with age that exposure of stem cells from older individuals to a ‘young’ stem cell niche can rejuvenate hematopoietic stem cell function which normally declines with age (Zheng et al. 2009; Oakley and Van Zant). The physical wearing of the niche areas has been shown to affect stem cell properties, too: in mice, thinning of the subventricular zone, a neurogenic stem cell niche, has been associated with decrease of cell proliferation and neuroblast numbers (Luo et al. 2006). Moreover, declining mouse testis weight with age has also been linked to reduction in levels of spermatogenesis (Ryu et al. 2006). In the same manner, the physical reduction and wear of the limbal niche after the age of 60 could be correlated to the decelerated wound healing clinically observed in that age group, including the delayed corneal epithelial wound healing after injury or infections such as microbial keratitis or following surgery such as corneal keratoplasty (Constantinou et al. 2009; Parmar et al. 2006; Ibrahim et al. 2009; van der Meulen et al. 2008).
In addition to the physical changes on the niche microenvironment, signalling mechanisms and gradual loss of progenitor cell properties such as marker expression and proliferative capacity can indicate ageing of the niche. Our data showed a significant drop of %CFE after the age of 60 years, indicating an overall drop in proliferative capacity of limbal epithelial cells. These data are in agreement with studies in other progenitor cell populations. In drosophila, changes of cell signalling from the ageing niche have been shown to be related to slowing down of germ line stem cells in females (Zhao et al. 2008). Other studies have also confirmed a deceleration of germline stem cell division in both drosophila ovaries (Pan et al. 2007) and testis (Boyle et al. 2007) that is associated to loss of E-cadherin expression in somatic niche cells.
In our study, although a clear decline of %CFE with age in cultured LESC was observed, no significant difference was found in the telomerase activity and telomere length, although it should be noted that the biological variability between different samples from the same age group may have affected these results. In other systems, ageing has been linked with reduced telomerase activity and consequent shortening of telomere length in some stem cell populations, for example, in hematopoietic stem cells (Rossi et al. 2007; Cawthon et al. 2003). However, findings in stem cells from muscle (O’Connor et al. 2009) and skin (Carlson et al. 2009; Krunic et al. 2009), a tissue with many functional and phenotypic similarities to the cornea, agree with the results of our study by showing that age had no significant effect in telomerase and telomere lengths.
In terms of marker expression, our data indicated that the key putative LESC markers ABCG2, cytokeratin 15 and ΔΝp63α remained unchanged with age. This has been previously observed in other studies in skin cells that were cultured for use in the clinic and were shown to sustain their phenotype with age (Carlson et al. 2009).
Overall, although the putative stem cell markers, telomerase activity and telomere length of cultured limbal epithelial cells remained unchanged with age, the CFE% data indicate that limbal epithelial cells from younger donors had higher proliferative capacity. This is of high clinical significance as allogeneic cultured limbal epithelial cells and limbal tissue grafting are therapeutic options in cases of bilateral LESC deficiency. This is important for both clinicians and patients as a more accurate selection of donor for therapy may help in reducing the number of failures in LESC grafting and thus save funds and valuable time of clinicians, patients and the health system.
Overall, the limbus provides an accessible model for the study of the ageing niche. This study illustrates some of the changes occurring to this system with age and demonstrates the link between its physical characteristics and cell phenotype. These results can be related to other relevant systems and open the way for further studies associated to the mechanisms involved in the ageing process of the stem cell niche.
Acknowledgments
The authors would like to thank the Medical Research Council, NIHR BRC for Ophthalmology, Moorfields Eye Hospital & the UCL Institute of Ophthalmology as well as the Engineering and Physical Sciences Research Council for their support and funding.
References
- Barrandon Y, Green H. Three clonal types of keratinocyte with different capacities for multiplication. Proc Natl Acad Sci USA. 1987;84(8):2302–2306. doi: 10.1073/pnas.84.8.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylis O, Figueiredo F, Henein C, Lako M, Ahmad S (2011) 13 years of cultured limbal epithelial cell therapy: a review of the outcomes. J Cell Biochem 112 (4). doi:10.1002/jcb.23028 [DOI] [PubMed]
- Boulton M, Albon J. Stem cells in the eye. Int J Biochem Cell Biol. 2004;36(4):643–657. doi: 10.1016/j.biocel.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Boyle M, Wong C, Rocha M, Jones DL. Decline in self-renewal factors contributes to aging of the stem cell niche in the Drosophila testis. Cell Stem Cell. 2007;1:470–478. doi: 10.1016/j.stem.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Carlson ME, Conboy MJ, Hsu M, Barchas L, Jeong J, Agrawal A, Mikels AJ, Agrawal S, Schaffer DV, Conboy IM. Relative roles of TGF-beta 1 and Wnt in the systemic regulation and aging of satellite cell responses. Aging Cell. 2009;8(6):676–689. doi: 10.1111/j.1474-9726.2009.00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361(9355):393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- Cheng J, Turkel N, Hemati N, Fuller MT, Hunt AJ, Yamashita YM. Centrosome misorientation reduces stem cell division during ageing. Nature. 2008;456(7222):599–U540. doi: 10.1038/nature07386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CP, Dragowska W, Kim NW, Vaziri H, Yui J, Thomas TE, Harley CB, Lansdorp PM. Differential expression of telomerase activity in hematopoietic progenitors from adult human bone marrow. Stem Cells. 1996;14(2):239–248. doi: 10.1002/stem.140239. [DOI] [PubMed] [Google Scholar]
- Constantinou M, Jhanji V, Tao LW, Vajpayee RB. Clinical review of corneal ulcers resulting in evisceration and enucleation in elderly population. Graefes Arch Clin Exp Ophthalmol. 2009;247(10):1389–1393. doi: 10.1007/s00417-009-1111-9. [DOI] [PubMed] [Google Scholar]
- Daniels JT, Dart JKG, Tuft SJ, Khaw PT. Corneal stem cells in review. Wound Repair Regen. 2001;9(6):483–494. doi: 10.1046/j.1524-475x.2001.00483.x. [DOI] [PubMed] [Google Scholar]
- Di Iorio E, Barbaro V, Ruzza A, Ponzin D, Pellegrini G, De Luca M. Isoforms of {Delta}Np63 and the migration of ocular limbal cells in human corneal regeneration. Proc Natl Acad Sci USA. 2005;102(27):9523–9528. doi: 10.1073/pnas.0503437102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dua HS, Shanmuganathan VA, Powell-Richards AO, Tighe PJ, Joseph A. Limbal epithelial crypts: a novel anatomical structure and a putative limbal stem cell niche. Br J Ophthalmol. 2005;89(5):529–532. doi: 10.1136/bjo.2004.049742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gago N, Perez-Lopez V, Sanz-Jaka JP, Cormenzana P, Eizaguirre I, Bernad A, Izeta A. Age-dependent depletion of human skin-derived progenitor cells. Stem Cells. 2009;27(5):1164–1172. doi: 10.1002/stem.27. [DOI] [PubMed] [Google Scholar]
- Geiger H, Koehler A, Gunzer M. Stem cells, aging, niche, adhesion and Cdc42—a model for changes in cell–cell interactions and hematopoietic stem cell aging. Cell Cycle. 2007;6(8):884–887. doi: 10.4161/cc.6.8.4131. [DOI] [PubMed] [Google Scholar]
- Ibrahim YW, Boase DL, Cree IA. Epidemiological characteristics, predisposing factors and microbiological profiles of infectious corneal ulcers: the Portsmouth corneal ulcer study. Br J Ophthalmol. 2009;93(10):1319–1324. doi: 10.1136/bjo.2008.151167. [DOI] [PubMed] [Google Scholar]
- Kanski J. Clinical ophthalmology: a systematic approach. 5. Boston: Butterworth-Heinemann; 2003. [Google Scholar]
- Knoth R, Singec I, Ditter M, Pantazis G, Capetian P, Meyer RP, Horvat V, Volk B, Kempermann G (2010) Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. Plos One 5 (1). doi:10.1371/journal.pone.0008809 [DOI] [PMC free article] [PubMed]
- Krunic D, Moshir S, Greulich-Bode KM, Figueroa R, Cerezo A, Stammer H, Stark HJ, Gray SG, Nielsen KV, Hartschuh W, Boukamp P. Tissue context-activated telomerase in human epidermis correlates with little age-dependent telomere loss. Biochim Biophys Acta-Mol Basis Dis. 2009;1792(4):297–308. doi: 10.1016/j.bbadis.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Luo J, Daniels SB, Lennington JB, Notti RQ, Conover JC. The aging neurogenic subventricular zone. Aging Cell. 2006;5(2):139–152. doi: 10.1111/j.1474-9726.2006.00197.x. [DOI] [PubMed] [Google Scholar]
- Notara M, Daniels JT. Characterisation and functional features of a spontaneously immortalised human corneal epithelial cell line with progenitor-like characteristics. Brain Res Bull. 2010;81(2–3):279–286. doi: 10.1016/j.brainresbull.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Notara M, Haddow DB, MacNeilss S, Daniels JT. A xenobiotic-free culture system for human limbal epithelial stem cells. Regen Med. 2007;2:919–927. doi: 10.2217/17460751.2.6.919. [DOI] [PubMed] [Google Scholar]
- O’Connor MS, Carlson ME, Conboy IM. Differentiation rather than aging of muscle stem cells abolishes their telomerase activity. Biotechnol Prog. 2009;25(4):1130–1137. doi: 10.1002/btpr.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley EJ, Van Zant G. Age-related changes in niche cells influence hematopoietic stem cell function. Cell Stem Cell. 2010;6(2):93–94. doi: 10.1016/j.stem.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Pan L, Chen SY, Weng CJ, Call G, Zhu DX, Tang H, Zhang N, Xie T. Stem cell aging is controlled both intrinsically and extrinsically in the Drosophila ovary. Cell Stem Cell. 2007;1(4):458–469. doi: 10.1016/j.stem.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Parmar P, Salman A, Kalavathy CM, Kaliamurthy J, Thomas PA, Jesudasan CAN. Microbial keratitis at extremes of age. Cornea. 2006;25(2):153–158. doi: 10.1097/01.ico.0000167881.78513.d9. [DOI] [PubMed] [Google Scholar]
- Pearce DJ, Anjos-Afonso F, Ridler CM, Eddaoudi A, Bonnet D. Age-dependent increase in side population distribution within hematopoiesis: implications for our understanding of the mechanism of aging. Stem Cells. 2007;25(4):828–835. doi: 10.1634/stemcells.2006-0405. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447(7145):725–U715. doi: 10.1038/nature05862. [DOI] [PubMed] [Google Scholar]
- Ryu BY, Orwig KE, Oatley JM, Avarbock MR, Brinster RL. Effects of aging and niche microenvironment on spermatogonial stem cell self-renewal. Stem Cells. 2006;24(6):1505–1511. doi: 10.1634/stemcells.2005-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlotzer-Schrehardt U, Kruse FE. Identification and characterization of limbal stem cells. Exp Eye Res. 2005;81:247–264. doi: 10.1016/j.exer.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Shortt AJ, Secker GA, Munro PM, Khaw PT, Tuft SJ, Daniels JT. Characterization of the limbal epithelial stem cell niche: Novel imaging techniques permit in-vivo observation and targeted biopsy of limbal epithelial stem cells. Stem Cells. 2007;25:1402–1409. doi: 10.1634/stemcells.2006-0580. [DOI] [PubMed] [Google Scholar]
- Shortt AJ, Secker GA, Munro PM, Khaw PT, Tuft SJ, Daniels JT. Characterization of the limbal epithelial stem cell niche: novel imaging techniques permit in vivo observation and targeted biopsy of limbal epithelial stem cells. Stem Cells. 2007;25(6):1402–1409. doi: 10.1634/stemcells.2006-0580. [DOI] [PubMed] [Google Scholar]
- Shortt AJ, Secker GA, Notara MD, Limb GA, Khaw PT, Tuft SJ, Daniels JT. Transplantation of ex-vivo cultured limbal epithelial stem cells—a review of current techniques and clinical results. Surv Ophthalmol. 2007;52:483–502. doi: 10.1016/j.survophthal.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Shortt AJ, Tuft SJ, Daniels JT. Ex vivo cultured limbal epithelial transplantation. A clinical perspective. Ocul Surf. 2010;8(2):80–90. doi: 10.1016/S1542-0124(12)70072-1. [DOI] [PubMed] [Google Scholar]
- Stepp MA, Zieske JD. The corneal epithelial stem cell niche. Ocul Surf. 2005;3(1):15–26. doi: 10.1016/S1542-0124(12)70119-2. [DOI] [PubMed] [Google Scholar]
- Tseng SCG. Regulation and clinical implications of corneal epithelial stem cells. Mol Biol Rep. 1996;23(1):47–58. doi: 10.1007/BF00357072. [DOI] [PubMed] [Google Scholar]
- Umemoto T, Yamato M, Nishida K, Yang J, Tano Y, Okano T. Limbal epithelial side-population cells have stem cell-like properties, including quiescent state. Stem Cells. 2006;24(1):86–94. doi: 10.1634/stemcells.2005-0064. [DOI] [PubMed] [Google Scholar]
- van der Meulen IJ, van Rooij D, Nieuwendaal CP, Van Cleijnenbreugel H, Geerards AJ, Remeijer L. Age-related risk factors, culture outcomes, and prognosis in patients admitted with infectious keratitis to two Dutch tertiary referral centers. Cornea. 2008;27(5):539–544. doi: 10.1097/ICO.0b013e318165b200. [DOI] [PubMed] [Google Scholar]
- Wagner W, Horn P, Bork S, Ho AD. Aging of hematopoietic stem cells is regulated by the stem cell niche. Exp Gerontol. 2008;43(11):974–980. doi: 10.1016/j.exger.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Nishida K, Yamato M, Umemoto T, Sumide T, Yamamoto K, Maeda N, Watanabe H, Okano T, Tano Y. Human limbal epithelium contains side population cells expressing the ATP-binding cassette transporter ABCG2. FEBS Lett. 2004;565:6–20. doi: 10.1016/j.febslet.2004.03.064. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Shimmura S, Kawakita T, Miyashita H, Den S, Shimazaki J, Tsubota K. Cytokeratin 15 can be used to identify the limbal phenotype in normal and diseased ocular surfaces. Invest Ophthalmol Vis Sci. 2006;47(11):4780–4786. doi: 10.1167/iovs.06-0574. [DOI] [PubMed] [Google Scholar]
- Zahidov ST, Hohlov AN, Malolina EA, Kulibin AY, Marshak TL. Ageing of the spermatogenesis system. Biol Bull. 2010;37(1):10–17. doi: 10.1134/S1062359010010024. [DOI] [PubMed] [Google Scholar]
- Zhao R, Xuan Y, Li XH, Xi RW. Age-related changes of germline stem cell activity, niche signaling activity and egg production in Drosophila. Aging Cell. 2008;7(3):344–354. doi: 10.1111/j.1474-9726.2008.00379.x. [DOI] [PubMed] [Google Scholar]
- Zheng TY, Xu JJ. Age-related changes of human limbus on in vivo confocal microscopy. Cornea. 2008;27(7):782–786. doi: 10.1097/ICO.0b013e31816f5ec3. [DOI] [PubMed] [Google Scholar]
- Zheng W, Wang S, Ma DD, Tang L, Duan YZ, Jin Y. Loss of proliferation and differentiation capacity of aged human periodontal ligament stem cells and rejuvenation by exposure to the young extrinsic environment. Tissue Eng Part A. 2009;15(9):2363–2371. doi: 10.1089/ten.tea.2008.0562. [DOI] [PubMed] [Google Scholar]