Abstract
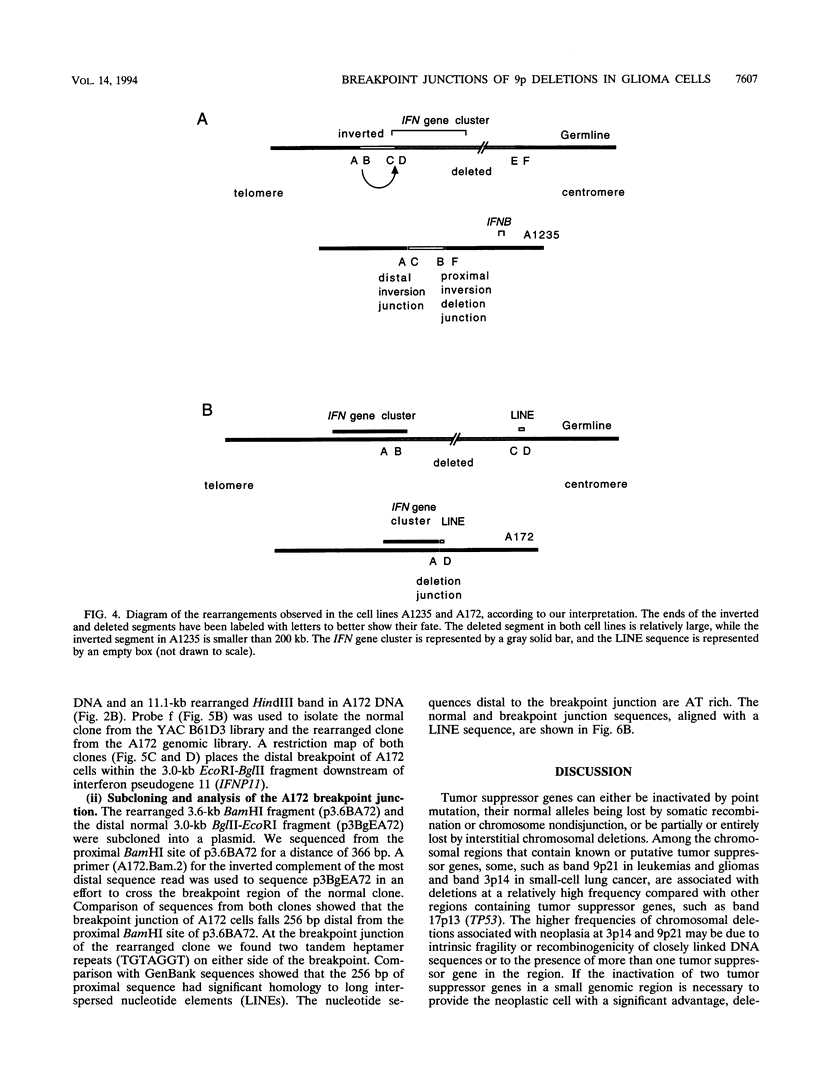
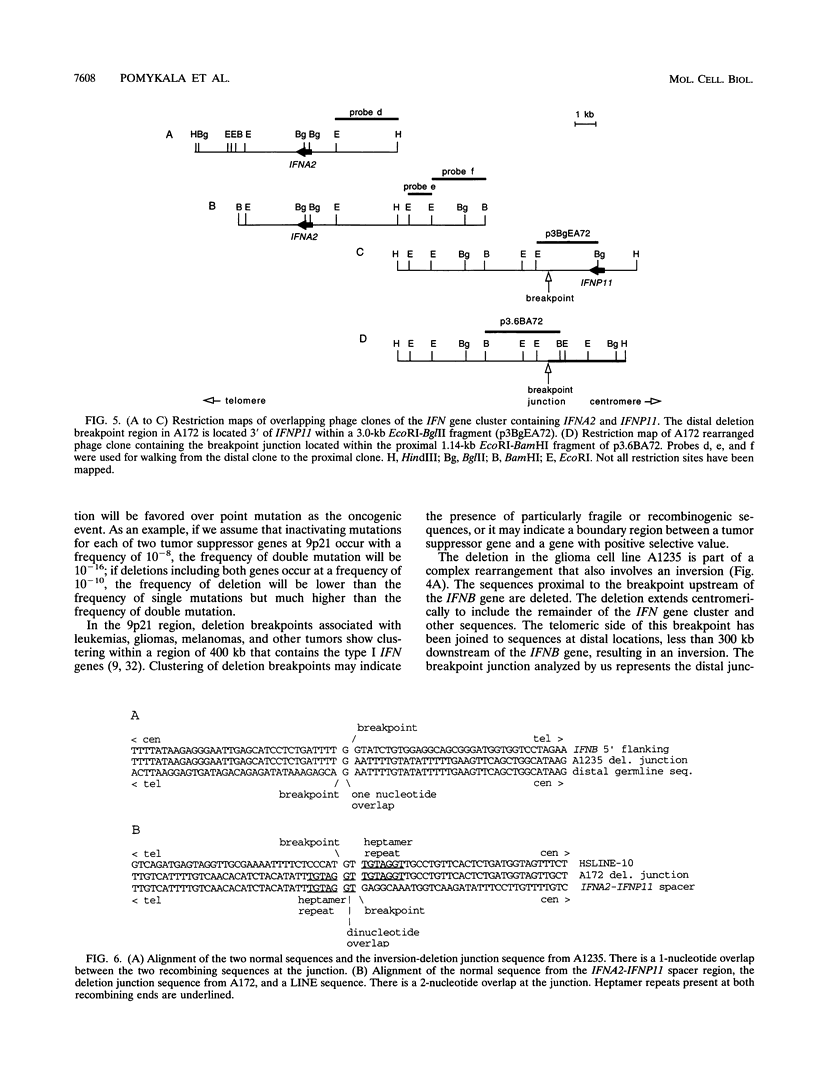
Interstitial deletions of the short arm of chromosome 9 are associated with glioma, acute lymphoblastic leukemia, melanoma, mesothelioma, lung cancer, and bladder cancer. The distal breakpoints of the deletions (in relation to the centromere) in 14 glioma and leukemia cell lines have been mapped within the 400 kb IFN gene cluster located at band 9p21. To obtain information about the mechanism of these deletions, we have isolated and analyzed the nucleotide sequences at the breakpoint junctions in two glioma-derived cell lines. The A1235 cell line has a complex rearrangement of chromosome 9, including a deletion and an inversion that results in two breakpoint junctions. Both breakpoints of the distal inversion junction occurred within AT-rich regions. In the A172 cell line, a tandem heptamer repeat was found on either side of the deletion breakpoint junction. The distal breakpoint occurred 5' of IFNA2; the 256 bp sequenced from the proximal side of the breakpoint revealed 95% homology to long interspersed nuclear elements. One- and two-base-pair overlaps were observed at these junctions. The possible role of sequence overlaps, and repetitive sequences, in the rearrangement is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bello M. J., Moreno S., Rey J. A. Involvement of 9p in metastatic ovarian adenocarcinomas. Cancer Genet Cytogenet. 1990 Apr;45(2):223–229. doi: 10.1016/0165-4608(90)90086-p. [DOI] [PubMed] [Google Scholar]
- Bigner S. H., Mark J., Bullard D. E., Mahaley M. S., Jr, Bigner D. D. Chromosomal evolution in malignant human gliomas starts with specific and usually numerical deviations. Cancer Genet Cytogenet. 1986 Jun;22(2):121–135. doi: 10.1016/0165-4608(86)90172-x. [DOI] [PubMed] [Google Scholar]
- Bode J., Maass K. Chromatin domain surrounding the human interferon-beta gene as defined by scaffold-attached regions. Biochemistry. 1988 Jun 28;27(13):4706–4711. doi: 10.1021/bi00413a019. [DOI] [PubMed] [Google Scholar]
- Cairns P., Shaw M. E., Knowles M. A. Preliminary mapping of the deleted region of chromosome 9 in bladder cancer. Cancer Res. 1993 Mar 15;53(6):1230–1232. [PubMed] [Google Scholar]
- Call K. M., Glaser T., Ito C. Y., Buckler A. J., Pelletier J., Haber D. A., Rose E. A., Kral A., Yeger H., Lewis W. H. Isolation and characterization of a zinc finger polypeptide gene at the human chromosome 11 Wilms' tumor locus. Cell. 1990 Feb 9;60(3):509–520. doi: 10.1016/0092-8674(90)90601-a. [DOI] [PubMed] [Google Scholar]
- Carrera C. J., Eddy R. L., Shows T. B., Carson D. A. Assignment of the gene for methylthioadenosine phosphorylase to human chromosome 9 by mouse-human somatic cell hybridization. Proc Natl Acad Sci U S A. 1984 May;81(9):2665–2668. doi: 10.1073/pnas.81.9.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll A. J., Castleberry R. P., Crist W. M. Lack of association between abnormalities of the chromosome 9 short arm and either "lymphomatous" features or T cell phenotype in childhood acute lymphocytic leukemia. Blood. 1987 Mar;69(3):735–738. [PubMed] [Google Scholar]
- Cowan J. M., Halaban R., Francke U. Cytogenetic analysis of melanocytes from premalignant nevi and melanomas. J Natl Cancer Inst. 1988 Sep 21;80(14):1159–1164. doi: 10.1093/jnci/80.14.1159. [DOI] [PubMed] [Google Scholar]
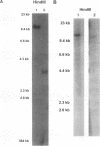
- Diaz M. O., Rubin C. M., Harden A., Ziemin S., Larson R. A., Le Beau M. M., Rowley J. D. Deletions of interferon genes in acute lymphoblastic leukemia. N Engl J Med. 1990 Jan 11;322(2):77–82. doi: 10.1056/NEJM199001113220202. [DOI] [PubMed] [Google Scholar]
- Diaz M. O., Ziemin S., Le Beau M. M., Pitha P., Smith S. D., Chilcote R. R., Rowley J. D. Homozygous deletion of the alpha- and beta 1-interferon genes in human leukemia and derived cell lines. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5259–5263. doi: 10.1073/pnas.85.14.5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
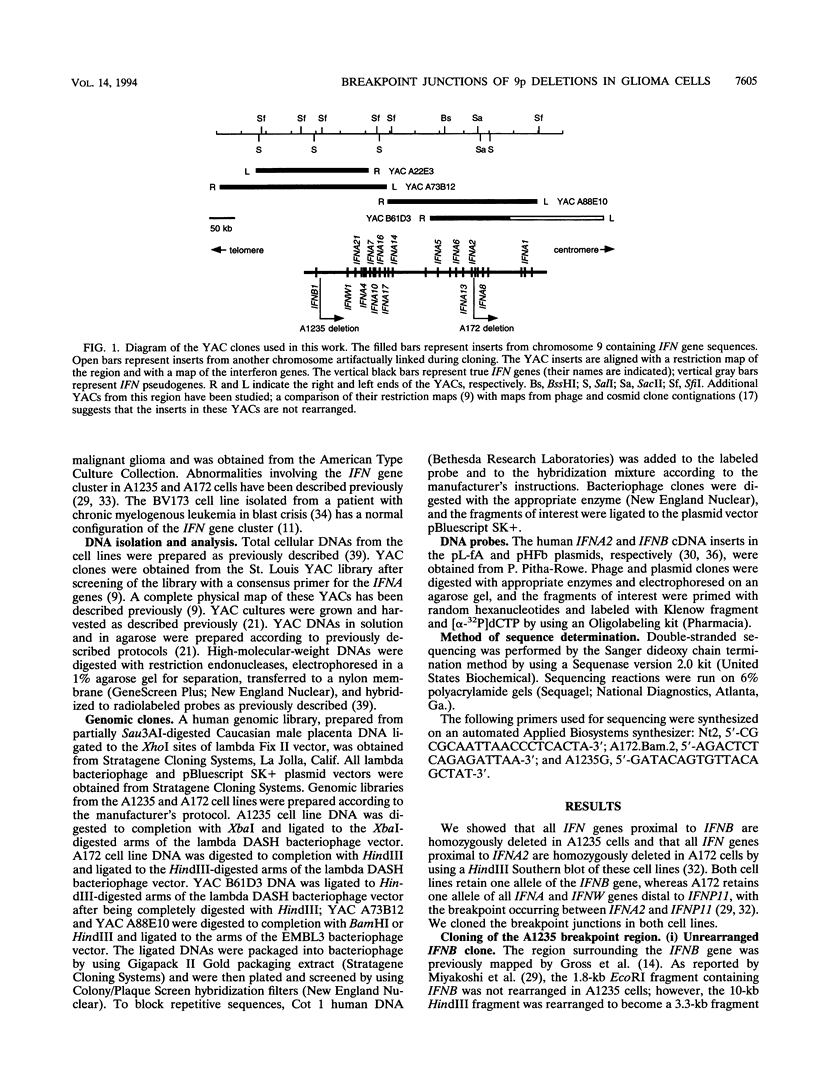
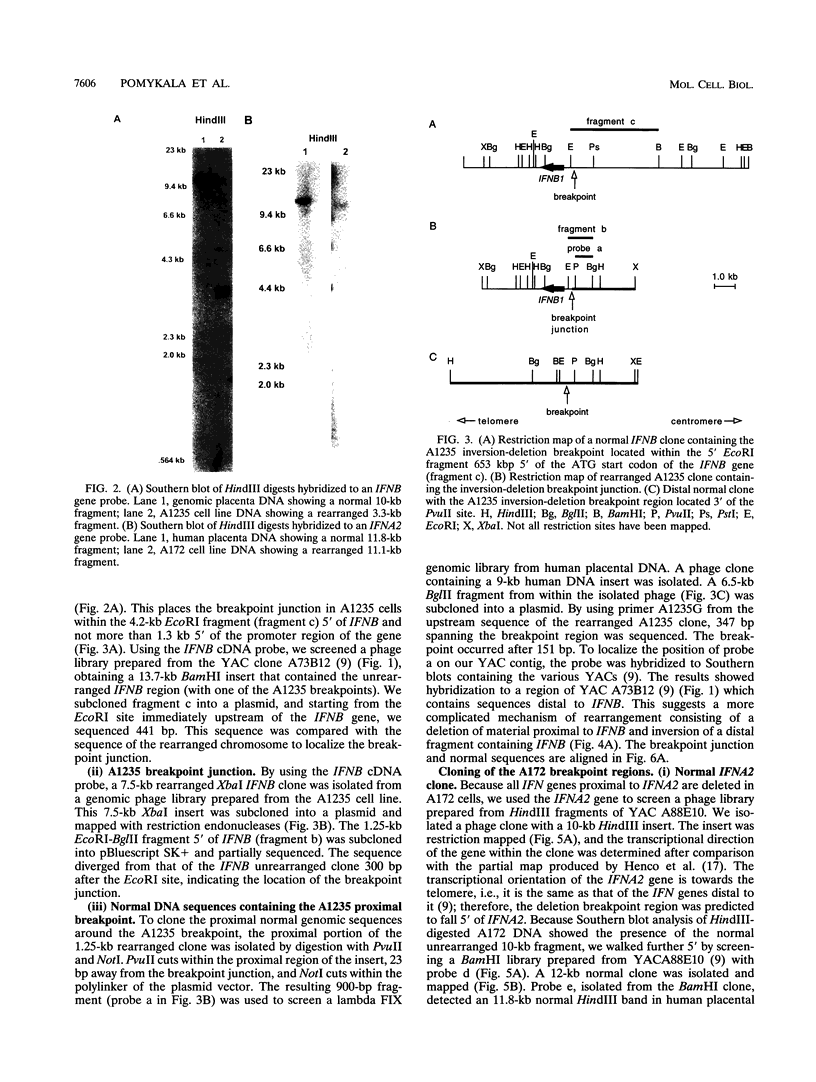
- Díaz M. O., Pomykala H. M., Bohlander S. K., Maltepe E., Malik K., Brownstein B., Olopade O. I. Structure of the human type-I interferon gene cluster determined from a YAC clone contig. Genomics. 1994 Aug;22(3):540–552. doi: 10.1006/geno.1994.1427. [DOI] [PubMed] [Google Scholar]
- Fountain J. W., Karayiorgou M., Ernstoff M. S., Kirkwood J. M., Vlock D. R., Titus-Ernstoff L., Bouchard B., Vijayasaradhi S., Houghton A. N., Lahti J. Homozygous deletions within human chromosome band 9p21 in melanoma. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10557–10561. doi: 10.1073/pnas.89.21.10557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend S. H., Bernards R., Rogelj S., Weinberg R. A., Rapaport J. M., Albert D. M., Dryja T. P. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986 Oct 16;323(6089):643–646. doi: 10.1038/323643a0. [DOI] [PubMed] [Google Scholar]
- Gross G., Mayr U., Bruns W., Grosveld F., Dahl H. M., Collins J. The structure of a thirty-six kilobase region of the human chromosome including the fibroblast interferon gene IFN-beta. Nucleic Acids Res. 1981 Jun 11;9(11):2495–2507. doi: 10.1093/nar/9.11.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H., Förster I., Rajewsky K. Sequence homologies, N sequence insertion and JH gene utilization in VHDJH joining: implications for the joining mechanism and the ontogenetic timing of Ly1 B cell and B-CLL progenitor generation. EMBO J. 1990 Jul;9(7):2133–2140. doi: 10.1002/j.1460-2075.1990.tb07382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henco K., Brosius J., Fujisawa A., Fujisawa J. I., Haynes J. R., Hochstadt J., Kovacic T., Pasek M., Schamböck A., Schmid J. Structural relationship of human interferon alpha genes and pseudogenes. J Mol Biol. 1985 Sep 20;185(2):227–260. doi: 10.1016/0022-2836(85)90401-2. [DOI] [PubMed] [Google Scholar]
- Henthorn P. S., Smithies O., Mager D. L. Molecular analysis of deletions in the human beta-globin gene cluster: deletion junctions and locations of breakpoints. Genomics. 1990 Feb;6(2):226–237. doi: 10.1016/0888-7543(90)90561-8. [DOI] [PubMed] [Google Scholar]
- Hollstein M., Sidransky D., Vogelstein B., Harris C. C. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- James C. D., He J., Collins V. P., Allalunis-Turner M. J., Day R. S., 3rd Localization of chromosome 9p homozygous deletions in glioma cell lines with markers constituting a continuous linkage group. Cancer Res. 1993 Aug 15;53(16):3674–3676. [PubMed] [Google Scholar]
- Jilk R. A., Makris J. C., Borchardt L., Reznikoff W. S. Implications of Tn5-associated adjacent deletions. J Bacteriol. 1993 Mar;175(5):1264–1271. doi: 10.1128/jb.175.5.1264-1271.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y., Mertens F., Mandahl N., Heim S., Olegård C., Wennerberg J., Biörklund A., Mitelman F. Chromosome abnormalities in eighty-three head and neck squamous cell carcinomas: influence of culture conditions on karyotypic pattern. Cancer Res. 1993 May 1;53(9):2140–2146. [PubMed] [Google Scholar]
- Kamb A., Gruis N. A., Weaver-Feldhaus J., Liu Q., Harshman K., Tavtigian S. V., Stockert E., Day R. S., 3rd, Johnson B. E., Skolnick M. H. A cell cycle regulator potentially involved in genesis of many tumor types. Science. 1994 Apr 15;264(5157):436–440. doi: 10.1126/science.8153634. [DOI] [PubMed] [Google Scholar]
- Kinzler K. W., Nilbert M. C., Su L. K., Vogelstein B., Bryan T. M., Levy D. B., Smith K. J., Preisinger A. C., Hedge P., McKechnie D. Identification of FAP locus genes from chromosome 5q21. Science. 1991 Aug 9;253(5020):661–665. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- Korenberg J. R., Rykowski M. C. Human genome organization: Alu, lines, and the molecular structure of metaphase chromosome bands. Cell. 1988 May 6;53(3):391–400. doi: 10.1016/0092-8674(88)90159-6. [DOI] [PubMed] [Google Scholar]
- Lehrman M. A., Russell D. W., Goldstein J. L., Brown M. S. Exon-Alu recombination deletes 5 kilobases from the low density lipoprotein receptor gene, producing a null phenotype in familial hypercholesterolemia. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3679–3683. doi: 10.1073/pnas.83.11.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukeis R., Irving L., Garson M., Hasthorpe S. Cytogenetics of non-small cell lung cancer: analysis of consistent non-random abnormalities. Genes Chromosomes Cancer. 1990 Jul;2(2):116–124. doi: 10.1002/gcc.2870020207. [DOI] [PubMed] [Google Scholar]
- Miyakoshi J., Dobler K. D., Allalunis-Turner J., McKean J. D., Petruk K., Allen P. B., Aronyk K. N., Weir B., Huyser-Wierenga D., Fulton D. Absence of IFNA and IFNB genes from human malignant glioma cell lines and lack of correlation with cellular sensitivity to interferons. Cancer Res. 1990 Jan 15;50(2):278–283. [PubMed] [Google Scholar]
- Mizoguchi J., Pitha P. M., Raj N. B. Efficient expression in Escherichia coli of two species of human interferon-alpha and their hybrid molecules. DNA. 1985 Jun;4(3):221–232. doi: 10.1089/dna.1985.4.221. [DOI] [PubMed] [Google Scholar]
- Nobori T., Miura K., Wu D. J., Lois A., Takabayashi K., Carson D. A. Deletions of the cyclin-dependent kinase-4 inhibitor gene in multiple human cancers. Nature. 1994 Apr 21;368(6473):753–756. doi: 10.1038/368753a0. [DOI] [PubMed] [Google Scholar]
- Olopade O. I., Bohlander S. K., Pomykala H., Maltepe E., Van Melle E., Le Beau M. M., Diaz M. O. Mapping of the shortest region of overlap of deletions of the short arm of chromosome 9 associated with human neoplasia. Genomics. 1992 Oct;14(2):437–443. doi: 10.1016/s0888-7543(05)80238-1. [DOI] [PubMed] [Google Scholar]
- Olopade O. I., Jenkins R. B., Ransom D. T., Malik K., Pomykala H., Nobori T., Cowan J. M., Rowley J. D., Diaz M. O. Molecular analysis of deletions of the short arm of chromosome 9 in human gliomas. Cancer Res. 1992 May 1;52(9):2523–2529. [PubMed] [Google Scholar]
- Pegoraro L., Matera L., Ritz J., Levis A., Palumbo A., Biagini G. Establishment of a Ph1-positive human cell line (BV173). J Natl Cancer Inst. 1983 Mar;70(3):447–453. [PubMed] [Google Scholar]
- Pollak C., Hagemeijer A. Abnormalities of the short arm of chromosome 9 with partial loss of material in hematological disorders. Leukemia. 1987 Jul;1(7):541–548. [PubMed] [Google Scholar]
- Raj N. B., Pitha P. M. Analysis of interferon mRNA in human fibroblast cells induced to produce interferon. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7426–7430. doi: 10.1073/pnas.78.12.7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth D. B., Wilson J. H. Nonhomologous recombination in mammalian cells: role for short sequence homologies in the joining reaction. Mol Cell Biol. 1986 Dec;6(12):4295–4304. doi: 10.1128/mcb.6.12.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M., Hannon G. J., Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993 Dec 16;366(6456):704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- Shima E. A., Le Beau M. M., McKeithan T. W., Minowada J., Showe L. C., Mak T. W., Minden M. D., Rowley J. D., Diaz M. O. Gene encoding the alpha chain of the T-cell receptor is moved immediately downstream of c-myc in a chromosomal 8;14 translocation in a cell line from a human T-cell leukemia. Proc Natl Acad Sci U S A. 1986 May;83(10):3439–3443. doi: 10.1073/pnas.83.10.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets W., Pauwels R., Laarakkers L., Debruyne F., Geraedts J. Chromosomal analysis of bladder cancer. III. Nonrandom alterations. Cancer Genet Cytogenet. 1987 Nov;29(1):29–41. doi: 10.1016/0165-4608(87)90028-8. [DOI] [PubMed] [Google Scholar]
- Sperry A. O., Blasquez V. C., Garrard W. T. Dysfunction of chromosomal loop attachment sites: illegitimate recombination linked to matrix association regions and topoisomerase II. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5497–5501. doi: 10.1073/pnas.86.14.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi T., Jhanwar S. C., Siegfried J. M., Keller S. M., Testa J. R. Recurrent deletions of specific chromosomal sites in 1p, 3p, 6q, and 9p in human malignant mesothelioma. Cancer Res. 1993 Sep 15;53(18):4349–4355. [PubMed] [Google Scholar]
- Takahashi T., Nau M. M., Chiba I., Birrer M. J., Rosenberg R. K., Vinocour M., Levitt M., Pass H., Gazdar A. F., Minna J. D. p53: a frequent target for genetic abnormalities in lung cancer. Science. 1989 Oct 27;246(4929):491–494. doi: 10.1126/science.2554494. [DOI] [PubMed] [Google Scholar]