Abstract
It is well known that attenuated insulin/insulin-like growth factor signaling (IIS) has a positive effect on longevity in several animal species, including mice. Here, we demonstrate that a population of murine pluripotent very small embryonic-like stem cells (VSELs) that reside in bone marrow (BM) is protected from premature depletion during aging by intrinsic parental gene imprinting mechanisms and the level of circulating insulin-like growth factor-I (IGF-I). Accordingly, an increase in the circulating level of IGF-I, as seen in short-lived bovine growth hormone (bGH)-expressing transgenic mice, which age prematurely, as well as in wild-type animals injected for 2 months with bGH, leads to accelerated depletion of VSELs from bone marrow (BM). In contrast, long-living GHR-null or Ames dwarf mice, which have very low levels of circulating IGF-I, exhibit a significantly higher number of VSELs in BM than their littermates at the same age. However, the number of VSELs in these animals decreases after GH or IGF-I treatment. These changes in the level of plasma-circulating IGF-I corroborate with changes in the genomic imprinting status of crucial genes involved in IIS, such as Igf-2-H19, RasGRF1, and Ig2R. Thus, we propose that a chronic increase in IIS contributes to aging by premature depletion of pluripotent VSELs in adult tissues.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-011-9364-8) contains supplementary material, which is available to authorized users.
Keywords: VSELs, IGF-1, GH, Aging
Introduction
In all living species, senescence is an inevitable consequence of life. Several well-known risk factors, such as obesity, diabetes, high calorie consumption, and lack of physical activity, lead to atherosclerosis of the cardio-vascular system and cancer, impair the function of vital organs, and limit overall life span (Fontana et al. 2008; Ikeno et al. 2009; Piper and Bartke 2008). It is obvious that all of these risk factors are directly or indirectly related to prolonged insulin/insulin-like growth factor signaling (IIS) and must ultimately have an impact on the basic units of tissue rejuvenation, which are stem cells (SCs). These risk factors can directly affect SCs or damage the niches in which these cells reside and thereby impair SC self renewal and differentiation (Sharpless and DePinho 2007).
As a result of exposure to several intrinsic, as well as extrinsic, aging factors, cellular aging is triggered in SCs by gradually accumulating DNA damage due to the action of reactive oxygen species (ROS) and the accumulating epigenetic changes in gene expression that result (Oberdoerffer and Sinclair 2007; Sharpless and DePinho 2007; Shin et al. 2010a). Thus, aging can be envisioned, at the SC level, as the result of altered cell function in response to changes in DNA structure that directly affects proper gene expression. In addition, SCs also age by an intrinsic mechanism through the phenomenon of telomere shortening. It is well known that the length of telomeres decreases with SC age (Flores and Blasco 2010).
It has been reported that an increase in caloric intake and insulin and/or insulin-like growth factor (Ins/IGF) levels in peripheral blood (PB) have a significant impact on aging (Avogaro et al. 2010; Russell and Kahn 2007; Tatar et al. 2003). On the other hand, caloric restriction (CR) and decrease in IIS increase lifespan in worms, flies, and mammals (Bartke and Brown-Borg 2004; Clancy et al. 2001; Fontana et al. 2010; Masoro 2005). Consistent with this general observation, mice with low circulating insulin-like growth factor-1 (IGF-I) levels (e.g., Laron [GHR–/–], Ames, and Snell dwarfs) live much longer than their normal littermates. By contrast, mice with high levels of circulating IGF-I (e.g., transgenic mice that overexpress bovine growth hormone [bGH]) have significantly reduced life span (Bartke and Brown-Borg 2004; Bartke et al. 2002; Bonkowski et al. 2006; Coschigano et al. 2000; Zhou et al. 1997). Furthermore, as recently demonstrated, mice with attenuated expression of RasGRF1, a small GTP exchange factor (GEF) for Ras, also live longer (Borras et al. 2011). This latter finding can be explained by the involvement of RasGRF1 in signaling through insulin and insulin-like growth factor-1 receptors (InsR and IGF-IR, respectively), as we have recently proposed (Ratajczak et al. 2011d).
Interestingly, the expression of insulin-like growth factor-2 (Igf2), Igf2 receptor (Igf-2R), and RasGRF1 is regulated by changes in somatic imprinting (Reik and Walter 2001). These paternally imprinted genes play a crucial role in embryogenesis, fetal growth, the totipotential state of the zygote, and the pluripotency of developmentally early stem cells (Reik and Walter 2001). The expression of imprinted genes is regulated by DNA methylation within differentially methylated regions (DMRs), which are CpG-rich cis-elements within gene loci (Lopes et al. 2003).
Recently, our group demonstrated that adult tissues, including bone marrow (BM), harbor a population of pluripotent Oct4+ SSEA-1+Sca-1+Lin–CD45– very small embryonic-like stem cells (VSELs) (Kucia et al. 2006). In murine BM, these pluripotent stem cells (PSCs) are deposited during early embryogenesis and serve as a backup for long-term repopulating hematopoietic stem cells (LT-HSC) (Ratajczak et al. 2011b). Furthermore, molecular analysis of VSELs has revealed that their quiescence in adult BM and potentially premature depletion is controlled by epigenetic changes of imprinted genes that regulate signaling of Ins/Igf (e.g., Igf2-H19, Igf2R, and RasGrf1 loci) (Shin et al. 2010b; Shin et al. 2009). Accordingly, we observed that murine BM-sorted VSELs erase the paternally methylated imprints (e.g., DMRs at Igf2-H19 and RasGrf1 loci); however, they hypermethylate the maternally methylated imprints (e.g., DMRs at Igf2R). These changes in expression of imprinted genes in VSELs lead to downregulation of RasGRF1 (GEF for signaling from InsR and IGF-IR) and IGF-2 (autocrine factor involved in proliferation of VSELs) and overexpression of Igf-2R (which serves as a decoy receptor that prevents IGF-2 from binding to IGF-IR or InsR) (Shin et al. 2009). Overall, this epigenetic reprogramming of genomic imprinting negatively affects IIS signaling, maintains quiescence of VSELs, and protects them from premature aging and tumor formation (Ratajczak et al. 2010). Based on these findings, we proposed a novel hypothesis that relates aging, longevity, and IIS to the abundance and function of pluripotent VSELs deposited in adult tissues. A decrease in the number of these cells will affect pools of tissue-committed stem cells in various organs (e.g., HSCs in BM), and it has an impact on tissue rejuvenation and life span (Ratajczak et al. 2010). In the current paper, we provide additional evidence, based on several murine models of altered longevity, that lends support to this novel hypothesis to explain the aging process.
Material and methods
Animals
This study was performed in accordance with the guidelines of the Animal Care and Use Committee of the University of the Southern Illinois University Laboratory Animal Care Committee and University of Louisville School of Medicine and with the Guide for the Care and Use of Laboratory Animals (Department of Health and Human Services, publication no. NIH 86-23).
Ames dwarfs (Prop df/df)
Homozygous Ames dwarf (Prop1df/df) male mice were produced by mating heterozygous females and homozygous mutant males in our breeding colony at Southern Illinois University (SIU). In this colony, the Prop1df mutation is maintained on a heterogeneous genetic background. Animals were maintained under temperature- and light-controlled conditions (20–23°C, 12-h light/12-h dark cycle).
Laron dwarf (GHR–/–) mice
Control and GHR–/– (also termed Laron) male mice used in this study were produced in our breeding colony, developed by crossing 129Ola/BALB/c GHR+/– animals (generously provided by Dr. J. J. Kopchick) with mice derived from crosses of C57BL/6 J and C3H/J strains and maintained as a closed colony with inbreeding minimized by avoiding brother × sister matings. The animals were housed under temperature- and light-controlled conditions (20–23°C, 12-h light/12-h dark cycle). GHR–/– males were mated with heterozygous (GHR+/–) females to produce GHR–/– mice (Zhou et al. 1997).
Bovine GH transgenic (bGH Tg) mice
Male phosphoenolpyruvate carboxykinase (PEPCK)-bGH Tg male mice and their normal male siblings were originally produced by microinjecting the bGH structural gene fused with the promoter of the rat PEPCK gene into the pronuclei of fertilized mouse eggs (McGrane et al. 1988). The hemizygous Tg mice used in this study were produced by mating GH-Tg males with normal C57BL/6 × C3H F1 hybrid females. The animals were housed in temperature- and light-controlled conditions (20–23°C, 12-h light/12-h dark cycle) until the age of 12 months, when the animals were sacrificed and the tissues and cells collected.
Treatment of mice with porcine GH or human IGF-I
Young Ames dwarf mice (Prop df/df)
Groups of ten male Ames dwarf and WT males were subjected to treatment with porcine GH (pGH) via subcutaneous (sc) injection (6 μg/g/day), given twice daily starting at the age of 2 weeks and continuing for 6 weeks. On Saturdays and Sundays, animals were injected only once with a full dosage following a previous protocol (Masternak et al. 2010). Control Ames dwarfs (Prop1df/df) and wt littermates of the same age were treated with saline.
After 6 weeks of pGH treatment, half of the animals were sacrificed, and the tissues and cells were collected for immediate analysis. The other half of the animals had pGH treatment discontinued, and the animals were kept untreated for 12 weeks before final analysis.
Adult Ames dwarf mice (Prop df/df)
Groups of 9–10 male Ames dwarf and wt males were subjected to treatment with pGH via subcutaneous (sc) injection (6 μg/g/day), given twice daily starting at the age of 6 months and continuing for 6–8 weeks. On Saturdays and Sundays, animals were injected only once with a full dosage, following a previous protocol. Control male Ames dwarfs (Prop1df/df) and wt male littermates of the same age were treated with saline.
Laron dwarf (GHR–/–) mice
Young male GHR KO mice were subjected to human IGF-I (Biovision Inc.) treatment via subcutaneous (sc) injection (4 μg/g/day), given twice daily starting at the age of 6 weeks and continuing for 3 weeks. Control male GHR KO and wt male littermates of the same age were treated with saline.
Peripheral blood counts
Fifty microliters of PB was taken from the retro-orbital plexus of the mice and collected into microvette EDTA-coated tubes (Sarstedt Inc., Newton, NC). Samples were run within 2 h of collection on a Hemavet 950 (Drew Scientific Inc., Oxford, CT).
Clonogenic assays
Clonogenic assays were performed as described by us elsewhere (Ratajczak et al. 2011b). Briefly, BM-derived cells were resuspended in methylcellulose base media provided by the manufacturer (R&D Systems) and supplemented with granulocyte macrophage colony-stimulating factor (GM-CSF, 25 ng/ml) and interleukin-3 (IL-3, 10 ng/ml) for CFU-GM, with erythropoietin (EPO, 5 units/ml, Stem cell Tech.) and stem cell factor (SCF, 5 ng/ml) for burst-forming units (BFU-E), and with thrombopoietin (TPO, 100 ng/ml) for CFU-megakaryocyte (Meg). Cultures were incubated for 7–10 days, at which time they were scored under an inverted microscope for the number of each type of colony.
Staining and sorting of BM-derived stem cells
To determine the numbers of Sca-1+Lin−CD45− (VSEL) and Sca-1+Lin–CD45+ (HSC) cells, flow cytometry analysis was performed. In brief, a single-cell suspension was stained for lineage markers (CD45R/B220 clone RA3-6B2, Gr-1 clone RB6-8 C5, TCRαβ clone H57-597, TCRγζ clone GL3, CD11b clone M1/70, and Ter-119 clone TER-119) conjugated with phycoerythrin (PE), CD45 (clone 30-F11) conjugated with APC-Cy7, and Sca-1 (clone D7) conjugated with PE-Cy5 for 30 min on ice. After washing, samples were analyzed by fluorescence-activated cell sorting (LSRII flow cytometer, BD Biosciences). At least 106 events were acquired and analyzed by using BD FACSDiva™ software. Samples stained with appropriate isotype controls (BD Pharmingen, San Diego, CA) were examined in parallel.
Isolation and fluorescence-activated cell sorting (FACS) of VSELs and HSCs from murine BM
VSELs and HSCs were isolated from BM of adult female or male mice. Briefly, BM was flushed from tibias and femurs, and the population of total nucleated cells (TNCs) was obtained after lysis of red blood cells using 1× BD Pharm Lyse Buffer (BD Pharmingen, San Jose, CA, USA). TNCs were subsequently stained for CD45, hematopoietic lineage markers (Lineage [Lin]), and Sca-1 antigen for 30 min in medium containing 2% fetal bovine serum. The following anti-mouse antibodies (BD Pharmingen) were used for staining: rat anti-CD45 (allophycocyanin-Cy7, clone 30-F11), anti-CD45R/B220 (PE, clone RA3-6B2), anti-Gr-1 (PE, clone RB6-8 C5), anti-T-cell receptor-αβ (PE, clone H57-597), anti-T-cell receptor-γδ (PE, clone GL3), anti-CD11b (PE, clone M1/70), anti-Ter119 (PE, clone TER-119), and anti-Ly-6A/E (also known as Sca-1, biotin, clone E13-161.7, with streptavidin conjugated to PE-Cy5). Cells were then washed, resuspended in RPMI 1640 medium with 2% fetal bovine serum, and sorted with a MoFlo cell sorter (Dako, Carpinteria, CA, USA). The Sca-1+Lin−CD45− cells (VSELs) and control Sca-1+Lin−CD45+ cells (HSCs) were isolated.
Bisulfite-sequencing (BSS) and combined bisulfite-restriction analysis (COBRA)
The DNA methylation status of the DMRs of Igf2-H19 and RasGRF1 loci was investigated using bisulfite DNA modification followed by sequencing, as well as by the COBRA assay. In brief, genomic DNA for VSELs, HSCs, and BMMNCs isolated from the indicated mouse strains were prepared using the DNeasy Blood & Tissue Kit (Qiagen Inc, Valencia, CA, USA). Next, 100 ng of gDNA was used for bisulfite modification, performed using the EpiTect Bisulfite Kit (Qiagen Inc) according to the manufacturer's instructions. The BSS and COBRA analysis were performed as previously described (Shin et al. 2009).
Statistical analysis
All data were analyzed using one-factor analysis of variance with Bonferroni's multiple comparison test. We used the Instat1.14 program (GraphPad Software, La Jolla, CA, USA), and statistical significance was defined as P < 0.05 or P < 0.01.
Results
An increase in plasma circulating insulin-like growth factor-1 (IGF-I) level leads to premature depletion of VSELs in murine BM
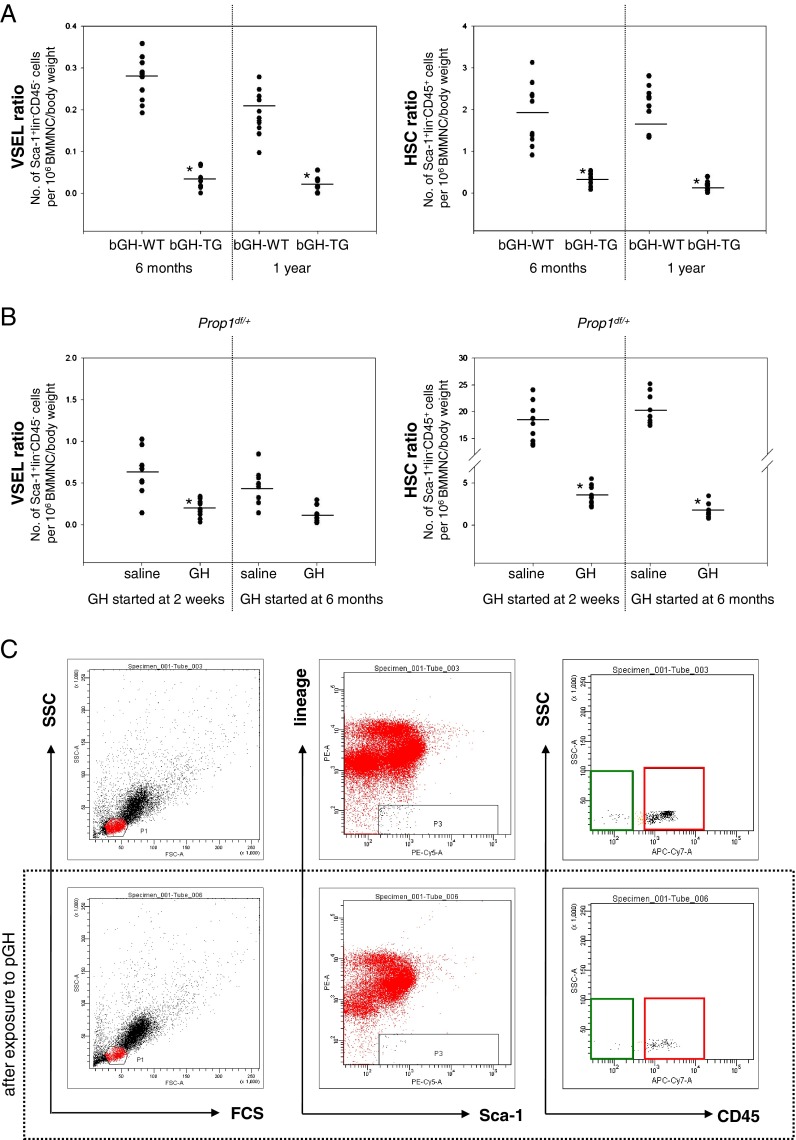
We initially became interested in the number of VSELs in BM of transgenic mice that overexpress bovine growth hormone (bGH) under control of the phosphoenolpyruvate carboxykinase (PEPCK) promoter. These mice live ~1 year, which is ~50% shorter than control littermates that lack this transgene. In our previous short report, we demonstrated that 6-month-old bGH-overexpressing transgenic mice have significantly reduced numbers of VSELs in BM (Kucia et al. 2011). In the current study, we have followed bGH transgenic mice up to 1 year of age. Figure 1a shows that these animals at 12 months of age have even more severely reduced numbers of Sca-1+lin–CD45– VSELs and Sca-1+lin–CD45+ HSCs in BM. In contrast, control mice maintain the number of HSCs in BM; however, they have lower numbers of VSELs at the age of 12 months (Fig. 1a).
Fig. 1.
Reduced numbers of VSELs and HSPCs in BM of mice with elevated plasma GH and IGF-I levels. a Decreases in the number of VSELs (left) and HSCs (right) in 6-month-old and 1-year-old bGH transgenic mice. The numbers of Sca-1+Lin–CD45– (VSELs) and Sca-1+Lin–CD45+ (HSCs) were evaluated as the number of events per 1 × 106 BMMNC/body weight using the LSR II BD FACS analyzer with FACSDiva™ software. Analysis of variance (ANOVA) with Bonferroni's multiple comparison test (*p < 0.00001) with comparison to normal counterparts was performed. b Reduced numbers of VSELs (left) and HSCs (right) after injections of wild-type mice with porcine GH (pGH). The numbers of Sca-1+LinCD45– (VSELs) and Sca-1+LinCD45+ (HSCs) were evaluated as in a. ANOVA with Bonferroni's multiple comparison test (*p < 0.00001) with comparison to normal counterparts was performed. c Analysis of Sca-1+Lin–CD45– (VSELs) and Sca-1+Lin–CD45+ (HSCs) from mBM-derived MNCs using the LSR II BD FACS analyzer with FACSDiva™ software. BMMNCs from region P1 were analyzed for Sca-1 and Lin expression. The Sca-1+Lin– population, which is included in region P3, was subsequently analyzed based on CD45 antigen expression, and the CD45– and CD45+ subpopulations were visualized by dot plot: Sca-1+Lin–CD45– (VSELs, green area) and Sca-1+Lin–CD45+ (HSCs, red area). Lower panel decrease in the numbers of VSELs and HSCs after GH administration (6 μg/g/day) compared to animals treated with saline (upper panel, control group)
Next, to see whether a prolonged increase in plasma GH level by twice-daily injections of porcine GH (pGH) has a similar effect, 2-week- and 6-month-old wild-type (wt) mice were divided into two groups and treated for 8 weeks with pGH (6 μg/g/day) or saline (control group). Figure 1b shows that, like bGH transgenic mice, both 2-week- and 6-month-old wt animals injected with pGH for 8 weeks exhibited a reduction in the number of VSELs and HSCs in BM. Figure 1c shows an example of FACS analysis of BM VSELs in wt mice treated with saline (upper panel) and pGH (lower panel).
These results can be explained by the fact that GH stimulates secretion of IGF-I (somatomedin C) from liver (Brown-Borg 2009; Ohlsson et al. 2009), and this leads to high levels of circulating IGF-I in bGH transgenic and wt mice injected with pGH.
Long-living Laron (GHR–/–) and Ames (Prop1df/df) dwarf mice with low circulating levels of IGF-I retain high numbers of VSELs in BM during aging
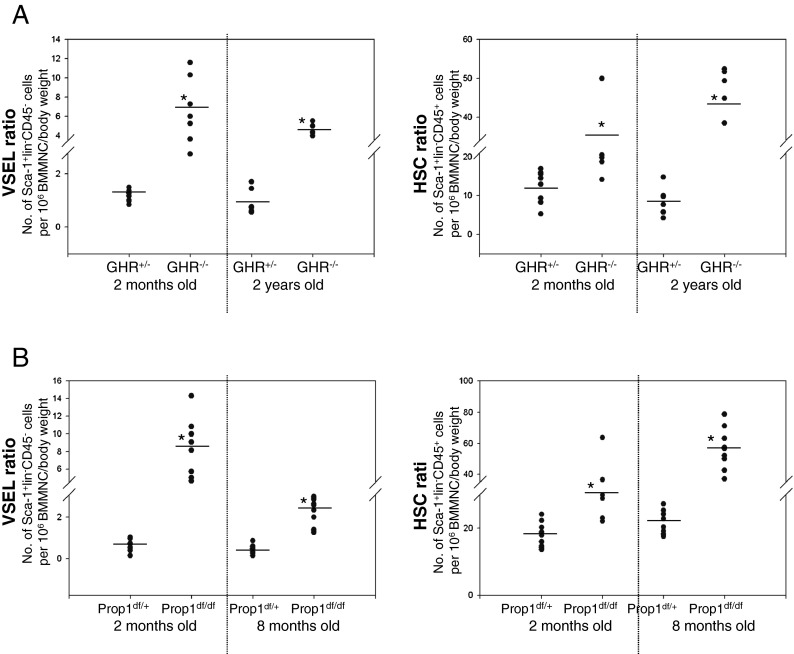
We recently demonstrated that long-living Laron dwarf mice (GHR–/–), with low circulating plasma levels of IGF-1, have a significantly higher number of VSELs and HSCs in BM at the age of 18 months than their normal littermates (Ratajczak et al. 2011a). Here, we have investigated the number of VSELs and HSCs in 2-year-old Laron dwarf mice (GHR–/–) and in a different type of long-lived mutant mice with low levels of IGF-1 in circulating plasma, 8-month-old Ames dwarfs (Prop1df/df), compared to normal control GHR–/+ and Prop1df/+ littermates.
Although both murine mutants exhibit extended longevity and have low levels of circulating IGF-I, the basis for these phenotypic characteristics is different (Andersen et al. 1995; Coschigano et al. 2003; Zhou et al. 1997). While Laron dwarf mice (GHR–/–) have a deletion of the GH-receptor (GHR) that makes them insensitive to GH signaling and thus do not secrete IGF-I from liver, the Ames dwarfs (Prop1df/df) have a mutation of the prophet of pituitary factor 1 (Prop1) gene involved in regulation of pituitary-specific transcription factor 1, which leads to a combined defect in expression of GH, thyroid-stimulating hormone (TSH), and prolactin (PRL) (Andersen et al. 1995; Brown-Borg et al. 1996). We now show that both Laron dwarfs and Ames dwarfs have a higher number of VSELs and HSCs in BM than age-matched normal littermates (GHR–/+ and Prop1df/+; Fig. 2a, b, respectively).
Fig. 2.
Long-lived dwarf mice with low circulating plasma IGF-I levels retain higher numbers of VSELs in BM during aging. a Increases in the number of VSELs (left) and HSCs (right) in 2-month-old and 2-year-old Laron dwarf mice (GHR–/–) compared to their normal heterozygote littermates (GHR+/–). The numbers of Sca-1+Lin–CD45– (VSELs) and Sca-1+Lin–CD45+ (HSCs) were evaluated as the number of events per 1 × 106 BMMNC/body weight using the LSR II BD FACS analyzer with FACSDiva™ software. Analysis of variance (ANOVA) with Bonferroni's multiple comparison test (*p < 0.00001) with comparison to normal counterparts was performed. b Increase in the numbers of VSELs (left) and HSCs (right) in 2- and 8-month-old Ames dwarf mice (Prop1df/df) compared to their normal heterozygote littermates (Prop1df/+). The numbers of Sca-1+LinCD45– (VSELs) and Sca-1+LinCD45+ (HSCs) were evaluated as in a. ANOVA with Bonferroni's multiple comparison test (*p < 0.00001) with comparison to normal counterparts was performed
Short-term GH or IGF-I treatment reduces the number of VSELs and HSCs in long-living, low-circulating-IGF-I-level Ames (Prop1df/df ) and Laron (GHR–/– ) dwarf mice
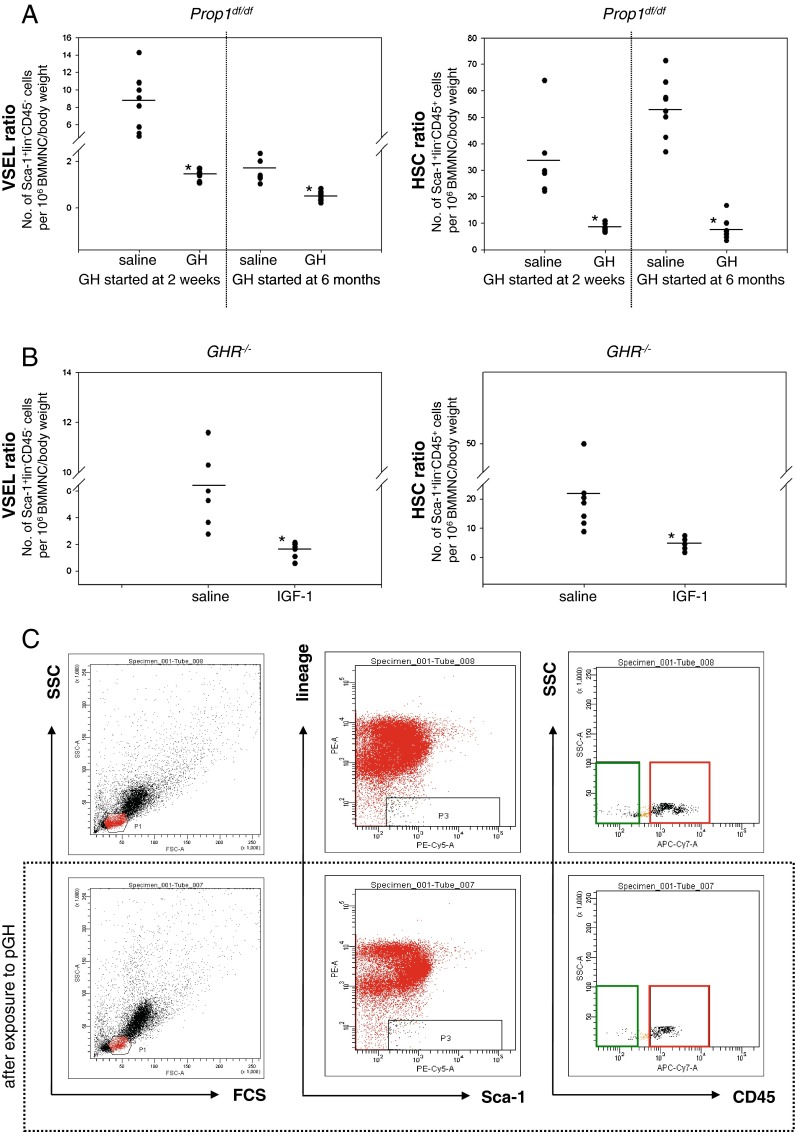
Data presented in Figs. 1 and 2 indicate that circulating plasma levels of bGH and IGF-I affect the number of VSELs in murine BM: High levels of these factors are associated with a decrease and low levels with an increase in the number of these cells. To address the underlying mechanism more directly, we treated Ames and Laron dwarf mice with pGH or recombinant human IGF-I injections, respectively.
As shown in Fig. 3a, Ames dwarfs (Prop1df/df) treated for 6 weeks starting at the ages of 14 days or 6 months by twice-daily injection of pGH (6 μg/g/day) exhibit significantly reduced numbers of VSELs and HSCs in BM compared to Ames dwarfs injected with saline vehicle (controls). Since these mice have low circulating levels of IGF-I due to GH deficiency, injections of GH elevated their circulating IGF-I levels.
Fig. 3.
GH or IGF-I treatment reduces the number of VSELs and HSCs in long-lived dwarf mice. a Reduced numbers of VSELs (left) and HSCs (right) in pGH- treated Ames dwarf mice (Prop1df/df). Ames dwarf mice were treated for 6 weeks starting at the age of 2 weeks or 6 months by twice-daily injection of GH (6 μg/g/day). The numbers of Sca-1+Lin–CD45– (VSELs) and Sca-1+Lin–CD45+ (HSCs) were evaluated as the number of events per 1 × 106 BMMNC/body weight using the LSR II BD FACS analyzer with FACSDiva™ software. Analysis of variance (ANOVA) with Bonferroni's multiple comparison test (*p < 0.00001) in comparison to normal counterparts was performed. b Reduced numbers of VSELs (left panel) and HSCs (right panel) in IGF-I treated Laron dwarf mice (GHR–/–). Laron dwarf mice were injected twice a day for 3 weeks with recombinant IGF-I. The numbers of Sca-1+LinCD45– ( VSELs) and Sca-1+LinCD45+ (HSCs) were evaluated as in a. ANOVA with Bonferroni's multiple comparison test (*p < 0.00001) with comparison to normal counterparts was performed. c Analysis of Sca-1+LinCD45– (VSELs) and Sca-1+LinCD45+ (HSCs) from mBM-derived MNCs using the LSR II BD FACS analyzer with FACSDiva™ software. BMMNCs from region P1 were analyzed for Sca-1 and Lin expression. The Sca-1+Lin– population, which is included in region P3, was subsequently analyzed based on CD45 antigen expression, and CD45– and CD45+ subpopulations were visualized by dot plot: Sca-1+Lin–CD45– (VSELs, green area) and Sca-1+Lin–CD45+ (HSCs, red area). Lower panel decrease in the number of VSELs and HSCs in Ames dwarf mice after GH administration (6 μg/g/day) compared to animals treated with saline (upper panel)
Finally, to confirm a direct role of IGF-I in the observed phenomena, we injected 6-week-old Laron dwarf mice, with low circulating plasma levels of IGF-I, twice daily for 3 weeks with human recombinant IGF-I (hIGF-I). As shown in Fig. 3b, twice-daily injection of hIGF-I (2 μg/g/day) leads to a significant decrease in the number of VSELs and HSCs in these animals compared to control Laron dwarf mice injected with saline.
Figure 3c shows an example of FACS analysis of BM VSELs in Ames dwarf mice treated with saline (upper panel) and GH (lower panel).
Changes in the number of hematopoietic clonogenic progenitors in BM and in peripheral blood parameters
Murine hematopoiesis during the aging process is affected by several deficiencies that, in C57Bl/6 mice, are characterized by an expansion of the hematopoietic stem cell phenotype and myeloid lineage in BM, impaired engraftment potential of HSPCs, mild-to-moderate normocytic anemia, and a decrease in acquired immunity (Liang et al. 2005; Rossi et al. 2005). Since we have previously demonstrated that VSELs in BM serve as precursors for LT-HSCs (Ratajczak et al. 2011b), we evaluated changes in clonogenic progenitors in BM and peripheral blood counts in animals investigated in our study.
Table 1 shows the relative change in the number of colony-forming units of granulocyte–monocyte (CFU-GM) cells, burst-forming units of erythrocytes (BFU-E), and colony-forming units of megakaryocytes (CFU-Meg) in BM of mice investigated in this study. These data correspond to changes in the number of Sca-1+Lin–CD45+ cells in BM and collectively indicate that prolonged IIS has a negative effect on the number of clonogenic progenitors in BM. In contrast, 2-year-old GHR–/– mice with low circulating IGF-I levels have higher numbers of clonogenic progenitors in BM than normal littermates (Table 1).
Table 1.
The number of clonogeneic progenitors in BM. The data are shown as number of colonies (Σ+/-SD)/105 BMMNC plateda
| Mice | CFU-GM | BFU-E | CFU-Megs |
|---|---|---|---|
| bGH-WT (6 M) | 156 ± 12 | 108 ± 20 | 156 ± 15 |
| bGH-TG (6 M) | 143 ± 14 | 75 ± 9 | 152 ± 17 |
| bGH-WT (12 M) | 125 ± 38 | 61 ± 16 | 60 ± 18 |
| bGH-TG (12 M) | 89 ± 35 | 38 ± 14 | 25 ± 7 |
| Prop1df/+ saline (2 M) | 75 ± 11 | 78 ± 14 | 64 ± 14 |
| Prop1df/+ GH (2 M) | 47 ± 9 | 35 ± 10 | 25 ± 9 |
| Prop1df/+ saline (8 M) | 58 ± 10 | 20 ± 4 | 38 ± 4 |
| Prop1df/+ GH (8 M) | 15 ± 3 | 8 ± 2 | 20 ± 3 |
| Prop1df/df saline (2 M) | 118 ± 20 | 99 ± 13 | 93 ± 11 |
| Prop1df/df GH (2 M) | 86 ± 29 | 55 ± 19 | 43 ± 13 |
| Prop1df/df saline (8 M) | 81 ± 8 | 36 ± 5 | 58 ± 6 |
| Prop1df/df GH (8 M) | 64 ± 7 | 20 ± 3 | 24 ± 6 |
| GHR+/- (24 M) | 124 ± 11 | 71 ± 11 | 67 ± 13 |
| GHR-/- (24 M) | 200 ± 17 | 113 ± 14 | 146 ± 13 |
aThe data are average from 6 mice/group plated in duplicates
These changes in the number of HSPCs in BM lead to changes in peripheral blood parameters. In particular, bGH at 6 months and 1 year of age develops microcytic anemia and exhibits elevated peripheral blood platelet counts (Supplementary Table I). However, we did not observe significant changes in peripheral blood parameters in wild-type mice or Ames dwarfs injected beginning at age 2 weeks or 6 months for 6 weeks with recombinant porcine GH (data not shown). Thus, under steady-state conditions, these animals are able to maintain normal peripheral blood counts despite a decrease in HSPCs in BM (Fig. 1b, Fig. 3a, and Table 1).
By contrast, 6-week-old Laron dwarf mice injected for 3 weeks with human recombinant IGF-I exhibited slight polycythemia (Supplementary Table II), which supports a role for IGF-I in augmenting proliferation and maturation of pro-erythroblasts, as previously reported (Ratajczak et al. 1998).
Changes in methylation pattern of imprinted genes involved in Ins/Igf signaling
We have reported that VSELs deposited in adult BM are in a quiescent state protected from proliferation by erasure of differentially methylated regions (DMRs) in paternally imprinted genes that encode Igf2-H19 and RasGRF1 (Shin et al. 2009). We also proposed that the number of these cells is prematurely depleted by chronic IIS signaling (Ratajczak et al. 2010). Thus, changes in expression of imprinted genes in VSELs lead to downregulation of (1) IGF-2, which is an autocrine factor involved in proliferation of VSELs, and (2) RasGRF1, a GEF for signaling from InsR and IGF-IR. Both of these changes play a major role in attenuating IIS in these cells (Ratajczak et al. 2011d).
To address the status of paternal imprints in VSELs, we evaluated the imprinting (methylation) of DMRs at the Igf2-H19 and RasGRF1 loci by employing bisulfide modification of DNA followed by sequencing (Fig. 4) or by employing restriction analysis of PCR products employing primers designed for DMRs of interest (COBRA assay, Supplementary Fig. 1). In young wild-type mice, the DNA of these paternally imprinted regions in VSELs remains unmethylated, in contrast to HSCs and BMMNCs (Fig. 4 and Supplementary Fig. 1). Furthermore, a decrease in the pool of VSELs after chronic exposure to GH/IGF-I (Fig. 1) corresponded with enhanced methylation of the DMR for the Igf2-H19 locus in VSELs, as seen in both bGH transgenic mice (Fig. 4a) and wt mice injected with pGH (Fig. 4b).
Fig. 4.
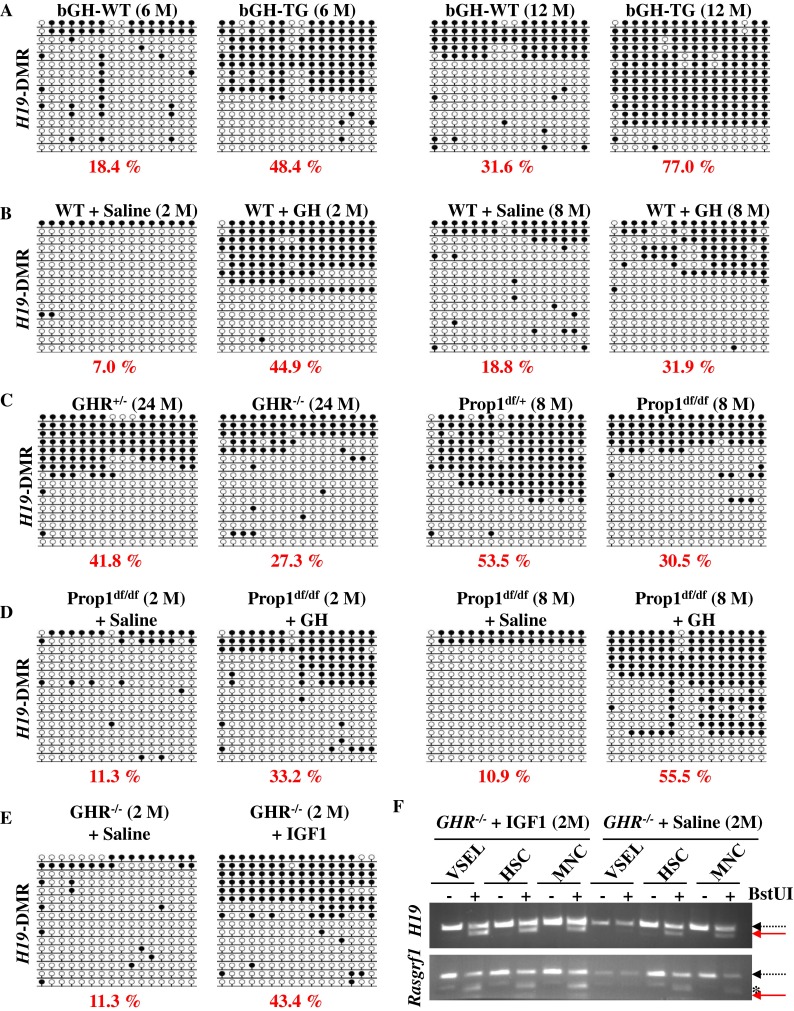
The effect of IIS on changes in DNA methylation within the Igf2-H19 DMR in VSELs. Bisulfite sequencing profiles of DNA methylation of DMRs for Igf2-H19 in VSELs isolated from the indicated mice. Methylated and unmethylated CpG sites are shown as filled and open circles, respectively. The numbers under the bisulfite sequencing profiles indicate the percentage of methylated CpG sites. a VSELs were FACS-isolated from 6-month- ( left ) and 1-year-old ( right ) bGH transgenic (bGH-TG) mice and their control (bGH-wt) littermates. b Wild-type mice were treated with porcine GH (pGH) at the age of 2 weeks ( left ) or 6 months ( right ) for 6 weeks. As a control, same-age mice were treated with saline. c VSELs were isolated from 2-year-old Laron dwarf (GHR–/–, left) and Ames dwarfs (Prop1df/df, right) mice and their control heterozygote (GHR+– and Prop1df/+) littermates. d Two-month- and 8-month-old Ames dwarfs (Prop1df/df) and e 2-month-old Laron dwarfs (GHR–/–) were treated with pGH or recombinant IGF-I, respectively, at the age of 2 weeks or at the age of 6 months, for 6 weeks. The control same-age mice were treated with saline. f COBRA assay of the Igf2-H19 DMR1 ( upper panel ) and RasGRF1 DMR ( lower panel ) by BstUI restriction enzyme cleavage. The unmethylated DNA (dashed arrow) was not cleaved, in contrast to methylated DNA (solid arrow), because of a sequence change at the site recognized by the restriction enzyme after bisulfite reaction. *Non-specific PCR product
We previously reported that erasure of genomic imprints at the Igf2-H19 locus in VSELs turns into the normal somatic pattern during aging (Ratajczak et al. 2011a), which is why VSELs from 2-year-old wild-type or Laron dwarf heterozygote mice (GHR–/+) and 8-month-old control mice for Ames dwarfs (Prop1df/+) show the somatic type of methylation (~50% methylation) in the DMR for the Igf2-H19 locus (Fig. 4c). Interestingly, at the same time, we observed that VSELs from both Laron dwarf (GHR–/–) and Ames (Prop1df/df) mice exhibit a delay in aging, which is related to DNA methylation of the Igf2-H19 locus (~30% methylation, Fig. 4c). However, this locus may become prematurely methylated if young 2-month-old Ames or 8-month-old Laron dwarf mice are exposed to daily pGH and IGF-I injections (Fig. 4d–f).
Taken together, our bisulfite modification of DNA followed by sequencing (Fig. 4) and COBRA assay (Fig. 4f and Supplementary Fig. 1) results suggest that prolonged IIS signaling induces reprogramming of the DNA methylation of imprinted loci (Igf2-H19 and RasGRF1) and increases sensitivity of these cells to IIS, which may lead to premature depletion of primitive VSELs from adult BM.
Discussion
Several animal models were employed in our studies to evaluate the correlation between the number of VSELs and longevity. Results of these studies demonstrate that the circulating plasma level of IGF-I directly affects the number of these cells deposited in adult murine BM. Thus, a novel paradigm emerges that directly links the number of PSCs deposited in adult tissues with IIS and longevity.
It has been proposed that VSELs are a backup population of PSCs that supply stem cells committed to particular tissues and thus play a role in regeneration and rejuvenation of adult tissues (Kucia et al. 2006; Ratajczak et al. 2011b). This would explain why VSELs are precursor cells for hematopoietic stem cells (HSCs) (Ratajczak et al. 2011b) and mesenchymal stem cells (MSCs) (Taichman et al. 2010) in murine BM. Accumulating evidence indicates that this is also the case for human VSELs (Ratajczak et al. 2011c). Further studies in other mammalian species are needed to see whether this is a common mechanism that controls tissue rejuvenation and impacts life span in all mammals.
We envision that VSELs, like other more committed SCs in the adult body, are exposed to several factors that affect their number and function, such as reactive oxygen species (ROS) that promote gradual accumulation of DNA damage (Ito et al. 2004; Rossi et al. 2005). However, in comparison to other SCs, VSELs are more resistant to DNA-damaging agents (e.g., irradiation) (Ratajczak et al. 2011b) and possess higher telomerase activity (Kucia et al. 2006). Because caloric intake and Ins/IGF level in PB have a significant impact on aging in numerous species (Bartke and Brown-Borg 2004; Clancy et al. 2001; Fontana et al. 2008; Longo and Finch 2003), we became interested in whether changes in plasma Ins/IGF level affect the number of VSELs deposited in adult organs and correlate with life span in various mouse models with altered longevity.
The rationale for these studies was the discovery of a crucial genomic imprinting-related epigenetic mechanism that governs the quiescent state of VSELs, preventing their proliferation and premature depletion from the tissues (Shin et al. 2009). This mechanism is based on the epigenetic changes in selected somatic-imprinted genes (i.e., Igf-2-H19, RasGRF1, and IGF-2R) that are involved in IIS (Drake et al. 2009; Font de Mora et al. 2003; Shin et al. 2009; Yoon et al. 2005). By erasing paternal imprints on Igf2-H19 and RasGRF1, VSELs downregulate expression of IGF-2, which seems to be an autocrine growth factor for these cells, and repress RasGRF1 gene expression, which appears to serve as a downstream signaling protein for activated insulin and IGF-IR (Font de Mora et al. 2003). Furthermore, in addition to erasure of imprinting on paternally imprinted genes in VSELs, some of the maternally imprinted genes become, by contrast, hypermethylated. One of these genes is Igf-2R, which serves as a molecular sink for IGF-2 and prevents its interaction with IGF-IR (Braulke 1999). Thus, impaired signaling from IGF-IR and InsR is responsible for maintaining VSELs in a quiescent state in adult tissues.
Interestingly, the molecular mechanisms that keep VSELs in adult tissues in a quiescent state and prevent their premature depletion are similar to the molecular mechanisms that are the basis for extension of life span in mice (Ratajczak et al. 2010). Long-living Laron and Ames dwarf mice have low levels of circulating IGF-I (Zhou et al. 1997) (Andersen et al. 1995), while RasGRF1 is repressed in long-living bi-maternal and RasGRF1–/– mice (Borras et al. 2011; de Magalhaes 2011; Kawahara and Kono 2010). It is also well known that changes in IIS have important implications for aging, and comparable effects on the extension of longevity can be produced by CR that attenuates IIS (Fontana et al. 2010; Masoro 2005).
Thus, attenuated signaling from IGF-IR and InsR is responsible for keeping VSELs quiescent in adult tissues. A decrease in the number of these cells would directly impact the regenerative capacity of their progeny, the pool of tissue-committed stem cells that are more restricted in their ability to differentiate. In support of a role for VSELs in organ/tissue rejuvenation, our previous studies performed on normal young (4-week-old) and old (2-year-old) wild-type mice revealed that the number of VSELs and their pluripotency decrease during aging (Ratajczak et al. 2011a). Consistent with our proposed mechanism, VSELs from young mice show erasure of DMRs for the Igf-2-H19 and RasGrf1 loci and thus do not express IGF-2 and RasGRF1. In contrast, VSELs from old mice show the somatic type of methylation at both Igf2-H19 and RasGrf1 loci, which increases expression of Igf-2 and RasGRF1 and thus their sensitivity to insulin-factor signaling. This suggests that chronic IIS via RasGRF1 may contribute to age-related depletion of VSELs and the senescence process and corresponds with a parallel reduction in expression of the pluripotency master regulators, such as Oct4, Nanog, Sox2, Klf4, and cMyc. As we have demonstrated, at the molecular level, the Oct4 promoter in VSELs becomes gradually hypermethylated with age and shows a closed chromatin structure (Ratajczak et al. 2011a).
In the current study, we provide more direct evidence that the number of VSELs deposited during ontogenesis in BM is related to plasma IGF-I level. In particular, mice with an elevated IGF-I level in plasma due to the expression of the bGH transgene and wt mice injected for a prolonged time with pGH exhibit decreased numbers of VSELs in BM compared to control animals. This was paralleled by epigenetic changes in the Igf2-H19 and RasGrf1 loci in which DMRs become hypermethylated over time, leading to increases in Igf2 and RasGRF1 expression. As mentioned previously, bGH transgenic mice have a significantly reduced life span. In contrast, Laron and Ames dwarf mice have an extended life span, with low circulating plasma IGF-I levels and high numbers of VSELs in BM that, in contrast to aged-matched normal littermates, are maintained at high levels into advanced age. The molecular signature of VSELs in these animals revealed prolonged retention of hypomethylation in DMRs at Igf2-H19 and RasGrf1 loci, which attenuates IIS in these cells. The numbers of VSELs, however, decrease in these animals after prolonged treatment with pGH or IGF-I, while in Ames dwarfs, GH treatment started early in life was recently shown to shorten their survival (Panici et al. 2010). Further studies are needed to determine whether CR has a similar effect on the number of VSELs residing in adult tissues.
Therefore, our data indicate a detrimental effect of elevated GH and/or IGF-I on the number of VSELs in adult murine tissues. We have demonstrated that, in both mice and humans, VSELs are precursors for LT-HSCs (Ratajczak et al. 2011b, c), and reduction of these cells in BM may lead to impaired hematopoiesis. Furthermore, our recent studies show a correlation between VSEL number and the number of HSCs and clonogenic progenitors in BM, which may be reflected by peripheral blood parameters in advanced age.
Interestingly, the role of IGF-I in hematopoiesis is controversial. IGF-I was initially described as stimulating proliferation of hematopoietic progenitors (Doepfner et al. 2007; Tazzari et al. 2007), but more recently, this effect has been explained by the presence of kit ligand (KL) in the culture conditions employed (Ratajczak et al. 1998, 1994). Similarly, a report describing the beneficial effects of short ex vivo exposure to IGF-I in rejuvenation of HSCs (Mayack et al. 2010) has been withdrawn, and further studies are needed to reevaluate these data. IGF-I, however, may play an important role in the final stages of erythropoiesis by promoting the expansion of erythroid precursors and their hemoglobinization (Ratajczak et al. 1998, 1994). In fact, we observed that healthy Laron dwarf mice injected with IGF-I for 2 months display slight polycythemia.
We have also proposed that since regeneration and tumor formation are closely related processes, the same mechanism that controls proliferation of VSELs may play a role in neoplastic transformation of these cells (Ratajczak et al. 2009). Accordingly, prolonged IIS may lead to uncontrolled neoplastic transformation of these cells. In support of this notion, loss of imprinting at the Igf2-H19 locus is a common feature in Beckwith–Wiedemann syndrome, which is associated with fetal overgrowth and pediatric sarcomas (Koufos et al. 1985; Prawitt et al. 2005). In addition, there is considerable evidence that an increase in IIS can lead to tumorogenesis (Pollak 2008; Steuerman et al. 2011). An increase in tumor formation also correlates positively with the level of circulating plasma IGF-I level in mice employed in our studies (e.g., bGH transgenic animals vs. Ames and Laron dwarfs). Thus, the potential involvement of VSELs in tumor formation in these animals requires further study.
In conclusion, we propose a novel paradigm for aging in which epiblast-derived VSELs, which are deposited in adult tissues as the most primitive population of stem cells involved in tissue/organ rejuvenation, are depleted by chronic IIS. Furthermore, since Igf2-H19 and RasGrf1 genes are paternally imprinted genes and thus expressed from paternal (sperm-derived) chromosomes (Reik and Walter 2001), our data demonstrate indirectly that some paternally imprinted genes play a crucial role in the control of longevity. These studies imply that the sperm genome has a detrimental effect on longevity in mammals. To summarize, longevity studies have increased in sophistication to the point where explanations can now be sought at the level of imprinted genes and their epigenetic regulation.
Electronic supplementary material
COBRA assay for Igf2-H19 and RasGRF1 DMRs in the response to prolonged GH treatment. Panel A. COBRA assay of Igf2-H19 DMR1 (upper panel) and RasGRF1 DMR (lower panel) by BstUI restriction enzyme cleavage in the indicated cells isolated from six-month- (left) and one-year-old (right) bGH transgenic (bGH-TG) mice and their control wt littermates (bGH-wt). Panels B and C. COBRA assay of Igf2-H19 DMR1 by BstUI restriction enzyme cleavage of the indicated cells isolated from (Panel B) two-year-old Ames dwarf (Prop1df/df, left panel) and Laron dwarf (GHR––, right panel) mice and their control heterozygote (Prop1df/+ or GHR+/–) littermates. Panel C Ames dwarf mice were injected with porcine GH (pGH) at the age of 2 weeks (left) or 6 months (right) for 6 weeks. As a control, same-age mice were treated with saline. The unmethylated DNA (dashed arrow) was not cleaved, in contrast to methylated DNA (solid arrow), because of a sequence change at the site recognized by a restriction enzyme after bisulfite reaction. *non-specific PCR product. (PPT 452 kb)
Peripheral blood parameters in male 6-month and 1-year-old normal and bGH transgenic mice (n=10) (DOC 37 kb)
Peripheral blood parameters in male 2-month old GHR-/- and GHR-/- after exposure to IGF-1 (n=10). (DOC 31 kb)
Acknowledgements
This work was supported by NIH R01 DK074720, EU structural funds, Innovative Economy Operational Program POIG.01.01.01-00-109/09-01, and the Henry M. and Stella M. Hoenig Endowment to MZR; by NIH P20RR018733 from the National Center for Research Resources to MK; NIH P01 AG031736 to AB; and NIH AG032290, KBN grant N N401 042638, and U19 AG023122 to MM.
References
- Andersen B, Pearse RV, 2nd, Jenne K, Sornson M, Lin SC, Bartke A, Rosenfeld MG. The Ames dwarf gene is required for Pit-1 gene activation. Dev Biol. 1995;172:495–503. doi: 10.1006/dbio.1995.8040. [DOI] [PubMed] [Google Scholar]
- Avogaro A, de Kreutzenberg SV, Fadini GP. Insulin signaling and life span. Pflugers Arch. 2010;459:301–314. doi: 10.1007/s00424-009-0721-8. [DOI] [PubMed] [Google Scholar]
- Bartke A, Brown-Borg H. Life extension in the dwarf mouse. Curr Top Dev Biol. 2004;63:189–225. doi: 10.1016/S0070-2153(04)63006-7. [DOI] [PubMed] [Google Scholar]
- Bartke A, Chandrashekar V, Bailey B, Zaczek D, Turyn D. Consequences of growth hormone (GH) overexpression and GH resistance. Neuropeptides. 2002;36:201–208. doi: 10.1054/npep.2002.0889. [DOI] [PubMed] [Google Scholar]
- Bonkowski MS, Rocha JS, Masternak MM, Al Regaiey KA, Bartke A. Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc Natl Acad Sci U S A. 2006;103:7901–7905. doi: 10.1073/pnas.0600161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borras C, Monleon D, Lopez-Grueso R, Gambini J, Orlando L, Pallardo FV, Santos E, Vina J, Font de Mora J. RasGrf1 deficiency delays aging in mice. Aging (Albany NY) 2011;3:262–276. doi: 10.18632/aging.100279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braulke T. Type-2 IGF receptor: a multi-ligand binding protein. Horm Metab Res. 1999;31:242–246. doi: 10.1055/s-2007-978725. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM. Hormonal control of aging in rodents: the somatotropic axis. Mol Cell Endocrinol. 2009;299:64–71. doi: 10.1016/j.mce.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384:33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, Hafen E, Leevers SJ, Partridge L. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001;292:104–106. doi: 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- Coschigano KT, Clemmons D, Bellush LL, Kopchick JJ. Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology. 2000;141:2608–2613. doi: 10.1210/en.141.7.2608. [DOI] [PubMed] [Google Scholar]
- Coschigano KT, Holland AN, Riders ME, List EO, Flyvbjerg A, Kopchick JJ. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology. 2003;144:3799–3810. doi: 10.1210/en.2003-0374. [DOI] [PubMed] [Google Scholar]
- de Magalhaes JP. Paternal genome effects on aging: evidence for a role of Rasgrf1 in longevity determination? Mech Ageing Dev. 2011;132:72–73. doi: 10.1016/j.mad.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Doepfner KT, Spertini O, Arcaro A. Autocrine insulin-like growth factor-I signaling promotes growth and survival of human acute myeloid leukemia cells via the phosphoinositide 3-kinase/Akt pathway. Leukemia. 2007;21:1921–1930. doi: 10.1038/sj.leu.2404813. [DOI] [PubMed] [Google Scholar]
- Drake NM, Park YJ, Shirali AS, Cleland TA, Soloway PD. Imprint switch mutations at Rasgrf1 support conflict hypothesis of imprinting and define a growth control mechanism upstream of IGF1. Mamm Genome. 2009;20:654–663. doi: 10.1007/s00335-009-9192-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores I, Blasco MA. The role of telomeres and telomerase in stem cell aging. FEBS Lett. 2010;584:3826–3830. doi: 10.1016/j.febslet.2010.07.042. [DOI] [PubMed] [Google Scholar]
- Font de Mora J, Esteban LM, Burks DJ, Nunez A, Garces C, Garcia-Barrado MJ, Iglesias-Osma MC, Moratinos J, Ward JM, Santos E. Ras-GRF1 signaling is required for normal beta-cell development and glucose homeostasis. EMBO J. 2003;22:3039–3049. doi: 10.1093/emboj/cdg280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Weiss EP, Villareal DT, Klein S, Holloszy JO. Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell. 2008;7:681–687. doi: 10.1111/j.1474-9726.2008.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Partridge L, Longo VD. Extending healthy life span—from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeno Y, Hubbard GB, Lee S, Cortez LA, Lew CM, Webb CR, Berryman DE, List EO, Kopchick JJ, Bartke A. Reduced incidence and delayed occurrence of fatal neoplastic diseases in growth hormone receptor/binding protein knockout mice. J Gerontol A Biol Sci Med Sci. 2009;64:522–529. doi: 10.1093/gerona/glp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Hirao A, Arai F, Matsuoka S, Takubo K, Hamaguchi I, Nomiyama K, Hosokawa K, Sakurada K, Nakagata N, et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- Kawahara M, Kono T. Longevity in mice without a father. Hum Reprod. 2010;25:457–461. doi: 10.1093/humrep/dep400. [DOI] [PubMed] [Google Scholar]
- Koufos A, Hansen MF, Copeland NG, Jenkins NA, Lampkin BC, Cavenee WK. Loss of heterozygosity in three embryonal tumours suggests a common pathogenetic mechanism. Nature. 1985;316:330–334. doi: 10.1038/316330a0. [DOI] [PubMed] [Google Scholar]
- Kucia M, Reca R, Campbell FR, Zuba-Surma E, Majka M, Ratajczak J, Ratajczak MZ. A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells identified in adult bone marrow. Leukemia. 2006;20:857–869. doi: 10.1038/sj.leu.2404171. [DOI] [PubMed] [Google Scholar]
- Kucia M, Shin DM, Liu R, Ratajczak J, Bryndza E, Masternak MM, Bartke A, Ratajczak MZ. Reduced number of VSELs in the bone marrow of growth hormone transgenic mice indicates that chronically elevated Igf1 level accelerates age-dependent exhaustion of pluripotent stem cell pool: a novel view on aging. Leukemia. 2011;25:1370–1374. doi: 10.1038/leu.2011.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Van Zant G, Szilvassy SJ. Effects of aging on the homing and engraftment of murine hematopoietic stem and progenitor cells. Blood. 2005;106:1479–1487. doi: 10.1182/blood-2004-11-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD, Finch CE. Evolutionary medicine: from dwarf model systems to healthy centenarians? Science. 2003;299:1342–1346. doi: 10.1126/science.1077991. [DOI] [PubMed] [Google Scholar]
- Lopes S, Lewis A, Hajkova P, Dean W, Oswald J, Forne T, Murrell A, Constancia M, Bartolomei M, Walter J, et al. Epigenetic modifications in an imprinting cluster are controlled by a hierarchy of DMRs suggesting long-range chromatin interactions. Hum Mol Genet. 2003;12:295–305. doi: 10.1093/hmg/ddg022. [DOI] [PubMed] [Google Scholar]
- Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Masternak MM, Panici JA, Wang F, Wang Z, Spong A. The effects of growth hormone (GH) treatment on GH and insulin/IGF-1 signaling in long-lived Ames dwarf mice. J Gerontol A Biol Sci Med Sci. 2010;65:24–30. doi: 10.1093/gerona/glp172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayack SR, Shadrach JL, Kim FS, Wagers AJ. Systemic signals regulate ageing and rejuvenation of blood stem cell niches. Nature. 2010;463:495–500. doi: 10.1038/nature08749. [DOI] [PubMed] [Google Scholar]
- McGrane MM, de Vente J, Yun J, Bloom J, Park E, Wynshaw-Boris A, Wagner T, Rottman FM, Hanson RW. Tissue-specific expression and dietary regulation of a chimeric phosphoenolpyruvate carboxykinase/bovine growth hormone gene in transgenic mice. J Biol Chem. 1988;263:11443–11451. [PubMed] [Google Scholar]
- Oberdoerffer P, Sinclair DA. The role of nuclear architecture in genomic instability and ageing. Nat Rev Mol Cell Biol. 2007;8:692–702. doi: 10.1038/nrm2238. [DOI] [PubMed] [Google Scholar]
- Ohlsson C, Mohan S, Sjogren K, Tivesten A, Isgaard J, Isaksson O, Jansson JO, Svensson J. The role of liver-derived insulin-like growth factor-I. Endocr Rev. 2009;30:494–535. doi: 10.1210/er.2009-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panici JA, Harper JM, Miller RA, Bartke A, Spong A, Masternak MM. Early life growth hormone treatment shortens longevity and decreases cellular stress resistance in long-lived mutant mice. FASEB J. 2010;24:5073–5079. doi: 10.1096/fj.10-163253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper MD, Bartke A. Diet and aging. Cell Metab. 2008;8:99–104. doi: 10.1016/j.cmet.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- Prawitt D, Enklaar T, Gartner-Rupprecht B, Spangenberg C, Lausch E, Reutzel D, Fees S, Korzon M, Brozek I, Limon J, et al. Microdeletion and IGF2 loss of imprinting in a cascade causing Beckwith–Wiedemann syndrome with Wilms' tumor. Nat Genet. 2005;37:785–786. doi: 10.1038/ng0805-785. [DOI] [PubMed] [Google Scholar]
- Ratajczak MZ, Kuczynski WI, Onodera K, Moore J, Ratajczak J, Kregenow DA, DeRiel K, Gewirtz AM. A reappraisal of the role of insulin-like growth factor I in the regulation of human hematopoiesis. J Clin Invest. 1994;94:320–327. doi: 10.1172/JCI117324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak J, Zhang Q, Pertusini E, Wojczyk BS, Wasik MA, Ratajczak MZ. The role of insulin (INS) and insulin-like growth factor-I (IGF-I) in regulating human erythropoiesis. Studies in vitro under serum-free conditions—comparison to other cytokines and growth factors. Leukemia. 1998;12:371–381. doi: 10.1038/sj.leu.2400927. [DOI] [PubMed] [Google Scholar]
- Ratajczak MZ, Shin DM, Kucia M. Very small embryonic/epiblast-like stem cells: a missing link to support the germ line hypothesis of cancer development? Am J Pathol. 2009;174:1985–1992. doi: 10.2353/ajpath.2009.081143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak MZ, Shin DM, Ratajczak J, Kucia M, Bartke A. A novel insight into aging: are there pluripotent very small embryonic-like stem cells (VSELs) in adult tissues overtime depleted in an Igf-1-dependent manner? Aging (Albany NY) 2010;2:875–883. doi: 10.18632/aging.100231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak J, Shin DM, Wan W, Liu R, Masternak MM, Piotrowska K, Wiszniewska B, Kucia M, Bartke A, Ratajczak MZ. Higher number of stem cells in the bone marrow of circulating low Igf-1 level Laron dwarf mice—novel view on Igf-1, stem cells and aging. Leukemia. 2011;25:729–733. doi: 10.1038/leu.2010.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak J, Wysoczynski M, Zuba-Surma E, Wan W, Kucia M, Yoder MC, Ratajczak MZ. Adult murine bone marrow-derived very small embryonic-like stem cells differentiate into the hematopoietic lineage after coculture over OP9 stromal cells. Exp Hematol. 2011;39:225–237. doi: 10.1016/j.exphem.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak J, Zuba-Surma E, Klich I, Liu R, Wysoczynski M, Greco N, Kucia M, Laughlin MJ, Ratajczak MZ. Hematopoietic differentiation of umbilical cord blood-derived very small embryonic/epiblast-like stem cells. Leukemia. 2011;25:1278–1285. doi: 10.1038/leu.2011.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak MZ, Kucia M, Liu R, Shin DM, Bryndza E, Masternak MM, Tarnowski M, Ratajczak J, Bartke A. RasGrf1: genomic imprinting, VSELs, and aging. Aging (Albany NY) 2011;3:692–697. doi: 10.18632/aging.100354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reik W, Walter J. Genomic imprinting: parental influence on the genome. Nat Rev Genet. 2001;2:21–32. doi: 10.1038/35047554. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Bryder D, Zahn JM, Ahlenius H, Sonu R, Wagers AJ, Weissman IL. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci U S A. 2005;102:9194–9199. doi: 10.1073/pnas.0503280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell SJ, Kahn CR. Endocrine regulation of ageing. Nat Rev Mol Cell Biol. 2007;8:681–691. doi: 10.1038/nrm2234. [DOI] [PubMed] [Google Scholar]
- Sharpless NE, DePinho RA. How stem cells age and why this makes us grow old. Nat Rev Mol Cell Biol. 2007;8:703–713. doi: 10.1038/nrm2241. [DOI] [PubMed] [Google Scholar]
- Shin DM, Zuba-Surma EK, Wu W, Ratajczak J, Wysoczynski M, Ratajczak MZ, Kucia M. Novel epigenetic mechanisms that control pluripotency and quiescence of adult bone marrow-derived Oct4(+) very small embryonic-like stem cells. Leukemia. 2009;23:2042–2051. doi: 10.1038/leu.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin DM, Kucia M, Ratajczak MZ. Nuclear and chromatin reorganization during cell senescence and aging—a mini-review. Gerontology. 2010;57:76–84. doi: 10.1159/000281882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin DM, Liu R, Klich I, Wu W, Ratajczak J, Kucia M, Ratajczak MZ. Molecular signature of adult bone marrow-purified very small embryonic-like stem cells supports their developmental epiblast/germ line origin. Leukemia. 2010;24:1450–1461. doi: 10.1038/leu.2010.121. [DOI] [PubMed] [Google Scholar]
- Steuerman R, Shevah O, Laron Z. Congenital IGF1 deficiency tends to confer protection against post-natal development of malignancies. Eur J Endocrinol. 2011;164:485–489. doi: 10.1530/EJE-10-0859. [DOI] [PubMed] [Google Scholar]
- Taichman RS, Wang Z, Shiozawa Y, Jung Y, Song J, Balduino A, Wang J, Patel LR, Havens AM, Kucia M, et al. Prospective identification and skeletal localization of cells capable of multilineage differentiation in vivo. Stem Cells Dev. 2010;19:1557–1570. doi: 10.1089/scd.2009.0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299:1346–1351. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- Tazzari PL, Tabellini G, Bortul R, Papa V, Evangelisti C, Grafone T, Martinelli G, McCubrey JA, Martelli AM. The insulin-like growth factor-I receptor kinase inhibitor NVP-AEW541 induces apoptosis in acute myeloid leukemia cells exhibiting autocrine insulin-like growth factor-I secretion. Leukemia. 2007;21:886–896. doi: 10.1038/sj.leu.2404523. [DOI] [PubMed] [Google Scholar]
- Yoon B, Herman H, Hu B, Park YJ, Lindroth A, Bell A, West AG, Chang Y, Stablewski A, Piel JC, et al. Rasgrf1 imprinting is regulated by a CTCF-dependent methylation-sensitive enhancer blocker. Mol Cell Biol. 2005;25:11184–11190. doi: 10.1128/MCB.25.24.11184-11190.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Xu BC, Maheshwari HG, He L, Reed M, Lozykowski M, Okada S, Cataldo L, Coschigamo K, Wagner TE, et al. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse) Proc Natl Acad Sci U S A. 1997;94:13215–13220. doi: 10.1073/pnas.94.24.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
COBRA assay for Igf2-H19 and RasGRF1 DMRs in the response to prolonged GH treatment. Panel A. COBRA assay of Igf2-H19 DMR1 (upper panel) and RasGRF1 DMR (lower panel) by BstUI restriction enzyme cleavage in the indicated cells isolated from six-month- (left) and one-year-old (right) bGH transgenic (bGH-TG) mice and their control wt littermates (bGH-wt). Panels B and C. COBRA assay of Igf2-H19 DMR1 by BstUI restriction enzyme cleavage of the indicated cells isolated from (Panel B) two-year-old Ames dwarf (Prop1df/df, left panel) and Laron dwarf (GHR––, right panel) mice and their control heterozygote (Prop1df/+ or GHR+/–) littermates. Panel C Ames dwarf mice were injected with porcine GH (pGH) at the age of 2 weeks (left) or 6 months (right) for 6 weeks. As a control, same-age mice were treated with saline. The unmethylated DNA (dashed arrow) was not cleaved, in contrast to methylated DNA (solid arrow), because of a sequence change at the site recognized by a restriction enzyme after bisulfite reaction. *non-specific PCR product. (PPT 452 kb)
Peripheral blood parameters in male 6-month and 1-year-old normal and bGH transgenic mice (n=10) (DOC 37 kb)
Peripheral blood parameters in male 2-month old GHR-/- and GHR-/- after exposure to IGF-1 (n=10). (DOC 31 kb)