Abstract
It is well established that immunologic memory generated early in life can be maintained into old age and mediate robust anamnestic antibody responses. Little is known, however, about the initiation of memory B cells in the elderly. We have conducted a prospective analysis of the quantities and functionalities of antigen-specific B cell responses and its association with the functional helper CD4+T cell responses. The ability of naïve B cells from old (60–80 years) and young (20–31 years) humans to establish functional memory was examined following primary and booster vaccination with an inactivated-virus vaccine against tick-borne encephalitis. Our data show that the number of antigen-specific memory B cells generated during primary vaccination was ∼3-fold lower in old than in young individuals. The maintenance and booster responsiveness of these memory B cells were not compromised, as evidenced by similar increases in specific memory B cell frequencies upon revaccination in old and young adults. In contrast, the Ab response mediated per memory B cell after revaccination was dramatically diminished in the elderly. Also, antigen-specific IL-2-positive CD4+T cell responses were strongly reduced in the elderly and displayed an excellent correlation with Ab titres. The data suggest that the dramatically lower antibody response in the elderly could only partially be accounted for by the reduced B cell numbers and was strongly correlated with profound functional defects in CD4 help.
Keywords: Immunosenescence, B memory cells, Aging, TBE vaccination, CD4+T cells, CD154
Introduction
B cell memory induced by vaccination is crucial for the prevention of morbidity and mortality caused by infectious agents. The two cellular components of B cell memory are long-lived plasma cells (PCs) and memory B cells. Antibody (Ab)-secreting PCs produce large quantities of Ig that provide long-term immunity while remaining as non-dividing cells in the bone marrow (Manz et al. 1997; Slifka et al. 1998). Memory B cells reside in the blood circulation and secondary lymphoid organs and are capable of rapidly responding to antigen re-exposure, thereby expanding and differentiating into PCs (Blanchard-Rohner et al. 2009). The ability of the immune system to respond to antigenic stimulation, however, decreases with advanced age, which leads to reduced protective effects of vaccination and increased morbidity due to infectious diseases (Aw et al. 2007; Cancro et al. 2009). The decreased ability of aged individuals to produce high-affinity protective Ab responses to vaccination is well documented (McElhaney and Effros 2009) and has been supposed to originate from intrinsic changes in B cells and from additional defects in T cell signalling to B cells, which is required for efficient Ab production (Nicoletti et al. 1993; Pawelec et al. 2002; Eaton et al. 2004; Johnson and Cambier 2004; Haynes and Eaton 2005; Signer et al. 2007; Frasca et al. 2008; Cancro et al. 2009; Frasca and Blomberg 2011). Previous studies in mice and humans have provided substantial insights into age-related changes that impact the number as well as the functionality of naïve CD4+T cells, including reduced cognate helper activity (Eaton et al. 2004), impaired proliferation and dysregulations in cytokine production (Weksler and Hutteroth 1974; Bernstein and Murasko 1998; Haynes and Eaton 2005). Less is known, however, about the effect of age on the functional integrity of naïve and memory human B cells. In particular, studies on the number and maintenance of antigen (Ag)-specific IgG+ memory B cells when primary vaccination first occurs during old age and on the functional properties of these cells to respond in a secondary immune response have not yet been performed in humans. Vaccination against tick-borne encephalitis virus (TBEV) is a particularly appropriate model for addressing these issues because vaccine-induced protection is mediated by neutralizing Abs (Kreil et al. 1998), and both young and old TBE-naïve subjects are available for primary immunization. This allows a direct quantitative comparison of primary antigen-specific B cell responses and subsequent memory B cell generation and function.
TBEV is a human-pathogenic virus that is endemic in large parts of Europe and Northern and Eastern Asia. It is a member of the genus Flavivirus in the family Flaviviridae, which includes a number of other important human pathogens, such as dengue virus, yellow fever virus, Japanese encephalitis virus and West Nile virus. Due to their emergence in new geographic areas and the recent rise in the incidence of human infections, flaviviruses represent a significant public health problem (Gubler 2007; Kyle and Harris 2008). Effective flavivirus vaccines are available against yellow fever virus (strain 17D; live attenuated virus vaccines), Japanese encephalitis virus (inactivated whole-virus vaccines and live attenuated virus vaccines) and against TBEV (inactivated whole-virus vaccines; Barrett et al. 2008; Halstead and Jacobson 2008; Monath and Teuwen 2008). Older people exhibit significantly increased morbidity and mortality due to encephalitogenic flavivirus infections (Kaiser 2002; Mickiene et al. 2002; Haglund and Gunther 2003) and are therefore important targets for vaccination. In this work, we have conducted a prospective analysis of the quantities and functionalities of specific memory B cells after primary and booster TBEV vaccination and its relation to functional Ag-specific helper CD4+T cell responses. The results obtained indicate that the strongly reduced anamnestic Ab response in the elderly cannot be explained by the lower numbers of Ag-specific memory B cells alone. Our data rather suggest a reduced capacity of these cells to mediate the boosting of Ab titres which is strongly correlated to the dramatically lower numbers of Ag-specific CD4+T cells generated during primary vaccination in the elderly.
Methods
Study design
Twenty-one elderly (60–80 years, 9 female, and 12 male) and 12 young individuals (20–31 years, 6 female, 6 male) were included in the longitudinal study. The volunteers had no clinically significant diseases, acute infections or health conditions known to affect immune responses such as cancer and were not undergoing any treatment with immunosuppressive drugs. At the time of recruitment, they had no previous history of vaccination against TBE, yellow fever, Japanese encephalitis or any flavivirus diseases. The study was performed in compliance with the provisions of the Declaration of Helsinki and its amendments and was approved by the ethical committee of the Medical University of Vienna, Austria (approval no. 590/2007; EudraCT-no 2007-005645-38). Written informed consent for participation was obtained from all study participants. Volunteers received three doses of a licensed TBEV vaccine (FSME-IMMUN®), containing 2.4 μg of inactivated highly purified TBEV adsorbed to aluminium hydroxide. Immunization was conducted according to the standard vaccination protocol, consisting of two doses given 1 month apart for primary immunization and a third dose given at 9 ± 2.2 months after the first.
Peripheral blood samples collected in sodium citrate vials were obtained at the following time points: Before vaccination (day 0), 7 days, 1 and 9 months after the two-dose primary vaccination as well as 1 and 9 months after the booster vaccination. Initial experiments were conducted on PBMC samples from a pilot study of ten young healthy volunteers under the same consent conditions. The study was carried out from September 2007 to December 2010.
Preparation of blood samples
For limiting-dilution analysis, B cells were enriched, using the RosetteSep™ B cell enrichment cocktail by negative selection of CD2, CD3, CD16, CD36, CD56, CD66b and red blood cell glycophorin A, according to the manufacturer’s instructions (STEMCELL Technologies, Grenoble, France). For T cell assays, mononuclear cells were isolated from peripheral blood using Ficoll-Paque® PLUS (GE Healthcare) and stored in liquid nitrogen until tested. Plasma was obtained by centrifugation of the sodium-citrate-treated blood at 1,600×g for 10 min and stored at −20°C.
Limiting-dilution analysis of memory B cells
An ELISA-based limiting-dilution assay (LDA) was used for enumeration of TBEV-specific and total IgG memory B cells, essentially as described previously (Amanna and Slifka 2006). Briefly, purified B cells were resuspended in RPMI-1640 culture medium supplemented with 10% fetal calf serum, 2 mM l-glutamine and 100 U/ml of penicillin, 100 μg/ml streptomycin (Invitrogen), 20 mM HEPES (Invitrogen), 1 mM sodium pyruvate (Sigma), 50 μM β-mercaptoethanol and 0.1 mM non-essential amino acids (Sigma). Twofold dilutions of purified B cells (3 to 5 wells per dilution) were cultured, starting with 50,000 to 200,000 cells per well in 96-well tissue culture plates together with 5,000 mitomycin C-treated NIH 3T3 cells in a final volume of 200 μl per well and incubated with CpG oligonucleotides ODN 2006-G5 (1 μg/ml; InvivoGen, San Diego, CA), IL-2 (16 ng/ml), IL-6 (10 ng/ml) and IL-10 (17 ng/ml; all from Peprotech, USA), pokeweed mitogen extract (1/1,000,000; a generous gift from Shane Crotty, La Jolla Institute for Allergy and Immunology), Staphylococcus aureus Cowan strain (SAC, 1/10,000; Sigma-Aldrich) and lipopolysacharide (10 μg/ml; LPS, Escherichia coli, 0111:B4, Sigma-Aldrich). Background values were obtained from 16 wells containing NIH 3T3 feeder cells only. Plates were incubated at 37°C and 5% CO2. The cell supernatants were harvested on day 14, added to 96-well flat-bottom ELISA plates (Nunc) and frozen at −20°C until tested by ELISA. Total memory B cell precursor frequencies were calculated after IgG-ELISA-based LDA using a semi-logarithmic plot of the percentage of negative cultures per cell number, as described previously (Amanna and Slifka 2006). Frequencies were defined as the reciprocal of the number of cells that resulted in 37% of the wells being negative for TBEV-IgG or total IgG. TBEV-specific memory B cell frequencies were estimated from the ratio of cells secreting TBEV-specific IgG vs. total IgG-secreting cells. To analyse the reproducibility of memory B cell detection, PBMCs from three donors were cultured with polyclonal stimuli, and the supernatants were tested for IgG by ELISA assay. For each donor, three samples were analysed in parallel and the coefficient of variation (%) for each donor was determined to be less than 30% for ELISA-based LDA.
Detection of IgG in plasma and cell culture supernatants
Microtitre plates were coated with 0.5 μg/ml purified formalin-inactivated TBEV in carbonate buffer, pH 9.6. Plasma samples were tested at a dilution of 1:100, and peroxidase-labelled goat anti-human IgG (Nordic Immunological Laboratories, NL) was used as detecting antibody. TBEV-specific IgG was quantified in Vienna units (VIE U)/ml using a standard human anti-TBEV serum arbitrarily set at 1,000 VIE U/ml (Holzmann et al. 1996). TBEV-IgG values above 155 VIE U/ml were considered positive. The supernatants of polyclonally stimulated B cells were tested undiluted using the TBEV-IgG ELISA described above. Individual wells were scored positive if the absorbance read spectrophotometrically at 490 nm was greater than the mean of the unstimulated control wells +3 SD. When no TBEV-specific IgG was detectable with the lowest dilution (50,000–200,000 cells per well), it was assumed that 63% would have been positive at the next-lower dilution (100,000–400,000 cells per well). In addition, total IgG production was measured using a Human IgG ELISA Quantitation Set (Bethyl Laboratories, Montgomery, Texas) according to the manufacturer’s instructions.
Neutralization assay
Neutralization assays were carried out in duplicates in microtitre plates using baby hamster kidney cells (ATCC BHK-21) as described previously (Stiasny et al. 2009). Twofold serial dilutions of plasma samples were mixed with 25 pfu virus (strain Neudoerfl) and incubated for 1 h at 37°C (starting dilution of the plasma sample in the mixture, 1:10). BHK-21 cells were added, and incubation was continued for 3 days. The presence of virus in the supernatant was determined by four-layer ELISA. The virus neutralization titre (mean of duplicates) was defined as the reciprocal of the plasma sample dilution that gave a 90% reduction in the absorbance readout in the assay compared to the control without antibody. NT titres >10 were considered positive and NT titres <10 negative.
Detection of CMV-IgG in plasma
Plasma samples obtained from all study participants before primary vaccination were tested for the presence of human cytomegalovirus (CMV)-specific IgG, using a CMV-IgG-ELISA Kit (medac, Wedel, Germany) according to the manufacturer’s instructions. CMV-seroprevalence was found to be 42% in young and 52% in older study participants and was within the range what may have been expected from a previous, larger study in 100 healthy Austrian subjects (Steininger et al. 2009).
Peptides for identification of TBEV-specific CD4+T cell responses
Overlapping peptides derived from TBEV strain Neudoerfl covering the major TBEV envelope (E) protein were used. The complete protein-spanning peptide pools of 15-mers overlapping by 11 amino acids were obtained from JPT Peptide Technologies GmbH, Germany, dissolved in DMSO to a stock concentration of 1.25 mg/ml. Peptides were used at a final concentration of 8 μg/ml.
Flow cytometric analysis
Enumeration of B cells was done with whole-blood samples, using a combination of anti-CD19-APC (BD Pharmingen) and anti-CD45-PE Cy7 (BD Pharmingen) and BD TruCOUNT tubes as recommended by the manufacturer (BD). In addition, naïve and switched IgG+ B cell subpopulations were also enumerated in enriched B cell preparations using BD TruCOUNT tubes and the following monoclonal Abs: anti-IgG-FITC, streptavidin-PE, anti-CD45-PE-Cy™7, anti-CD19-APC, anti-CD20-APC-H7 (all from BD Pharmingen) and anti-CD27-Biotin (BioLegend, San Diego); 7-AAD was used to allow the exclusion of dead cells. The purity of enriched B cell samples was assessed using anti-CD16-PE, anti-CD45-PE-Cy™7, anti-CD19-APC, anti-CD3-APC and anti-CD4-Pacific Blue™ (all from BD Pharmingen), and these tests always revealed <0.2% CD3+ T cells.
Intracellular cytokine staining
To determine Ag-specific CD4+T cell responses, cells were thawed and incubated overnight at 37°C in a 5% CO2 atmosphere. Aliquots of 500 μl containing 2 × 106 PBMCs were placed in 15-ml Falcon tubes (BD) and stimulated in the presence of 1 μg/ml anti-CD28/49d antibody (BD Pharmingen, USA) with either a TBEV peptide pool at 8 μg/ml or without peptides for 6 h at 37°C, 5% CO2, in the presence of 2 μM monensin (Sigma) and 40 μl PE-labelled anti-CD154 (BD Pharmingen) according to a protocol described previously (Chattopadhyay et al. 2006). After stimulation, cells were washed and stained using a LIVE/DEAD® Cell Viability Assay Kit (Invitrogen) according to the manufacturer’s instructions to allow the exclusion of dead cells. The cells were washed and surface-stained, using APC-H7-labeled anti-CD3 and Pacific Blue-labeled anti-CD4. After surface staining, cells were fixed and permeabilized using a Caltag Laboratories Fix&Perm® cell permeabilization kit (Invitrogen) as recommended by the manufacturer. Following permeabilization, cells were stained with anti-IL-2-APC, anti-IFN-γ-FITC and anti-TNF-PE-Cy7. The number of CD4+T lymphocyte-gated events ranged from 80,000 to 300,000 in all experiments. Cells were analysed on a FACS Canto™ II (BD, USA) cytometer. Data were further analysed using BD Diva software version 6.1.2 or FlowJo software version 7.2.5 (Tree Star, USA). The percentage of CD4+T cells staining for each cytokine or CD154 was determined after TBEV peptide stimulation. Because the TBEV peptide stimulations include costimulation with anti-CD28/anti-CD49d, to quantify the responses, the frequency of cells responding to costimulation alone was subtracted from the frequency of cells responding to TBEV peptides.
Statistical analysis
Ab concentrations obtained by ELISA and Ab titres from the neutralization test were log-transformed and results are expressed as geometric mean concentrations or geometric mean titres, respectively. Likewise, cell count data were log-transformed to obtain homogenous variances and symmetric distributions of residuals. Percentages of responsive cells were arcsine transformed. Statistical analyses were performed on these transformed data by repeated measures mixed-model analysis of variance (if data from more than one time point were present) or Student’s t tests (for data analysed at only one time point). In the case of ANOVA, age groups were compared by linear contrasts. Univariate regression analysis was used to test the relationship between TBEV-specific cells producing IL-2, TNF, IFN-γ or CD154 and Ab titres. For all statistical tests, a p value below 0.05 was considered significant. No correction for multiple endpoints was applied but comparisons of age groups for each parameter was alpha protected.
Results
Memory B cell responses after primary vaccination
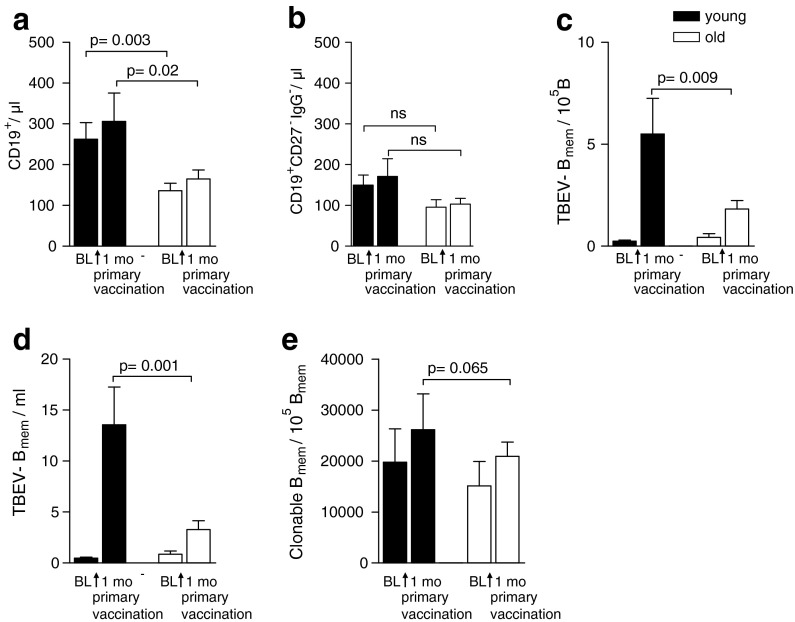
To examine the impact of age on the generation of memory B cells in a primary immune response, the frequencies of antigen-specific memory B cells were assessed in 21 old (60–80 years) and 12 young (20–31 years) individuals before, and 1 month after, primary TBE vaccination. Previous studies have demonstrated that elderly individuals have significantly lower numbers of peripheral blood B cells in comparison to young adults (Franceschi et al. 1995; Shi et al. 2005; Frasca et al. 2008; Frasca and Blomberg 2011). To confirm these findings in our study population, we first measured the absolute numbers of total CD19+B cells in whole blood samples obtained from all study participants. Consistent with published data, our results show that these numbers are about 50% lower in older adults than in young adults (Fig. 1a), whereas the number of naïve CD27−IgG−CD19+B cells did not differ between young and old subjects (Fig. 1b). For determining frequencies of TBEV-specific memory B cells, identical numbers of purified CD19+B cells were subjected to limiting-dilution analysis. The use of highly purified B cells in these experiments also allowed us to eliminate a possible contribution of helper CD4+T cells to in vitro B cell responses, which may display age-related differences in their number and functionality (Pawelec et al. 2002; Eaton et al. 2004; Haynes and Eaton 2005). Figure 1c relates the number of TBEV-specific memory B cells to the total number of CD19+B cells, revealing a significant reduction in the elderly. A significant age-related difference was also found when we calculated the absolute numbers of newly generated TBEV-specific memory B cells in all study participants (Fig. 1d).
Fig. 1.
Analysis of the frequency and number of TBEV-specific memory B cells after primary TBE vaccination. Absolute numbers of CD19+B cells (a) and naïve (CD27−IgG−) CD19+ B cells (b) determined in peripheral blood samples using the TrueCount® kit; c frequency of TBEV-IgG+ memory B cells per 105 B cells, determined after polyclonal stimulation in limiting-dilution analysis; d absolute numbers of TBEV-specific memory B cells/ml, calculated as the product of the absolute CD19+B cell count determined in whole blood and the percentage of TBEV-specific memory B cells obtained by limiting-dilution assay; e numbers of total IgG-producing B cells per 105 IgG+ B cells in young and older adults. BL baseline, B mem memory B cell. Results are expressed as mean + SEM
The lower Ag-specific memory B cell numbers in older adults as determined by LDA may indeed be due to the presence of lower numbers of newly generated memory B cells but could also be the result of weaker Ab production by these cells under the stimulation conditions employed in our assay. To address this question, we determined the total number of IgG-secreting cells after polyclonal stimulation from young and old adults by LDA. As can be seen in Fig. 1e, there was no significant difference in the number of IgG-producing B cells relative to the total number of input IgG+ memory B cells between young and old adults, indicating that memory B cells from older subjects have no general impairment of IgG Ab production after polyclonal stimulation of these cells. Together, these results demonstrate a significant age-related decrease in the generation of Ag-specific memory B cells upon primary immunization.
Kinetics of B cell responses during primary and booster vaccination
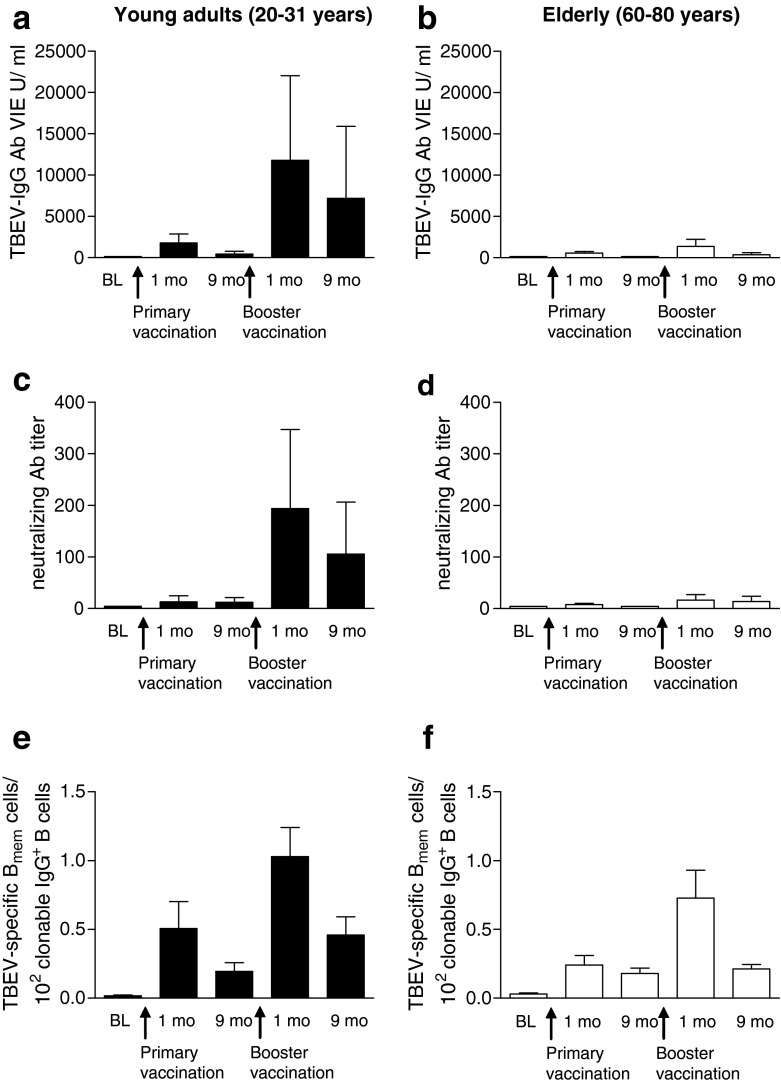
The major objective of this study was to compare the generation, maintenance and function of memory B cells along with Ab responses elicited during a primary and secondary immune response in old compared to young adults. For this purpose, we analysed Ab titres in ELISA and neutralization tests and memory B cell frequencies at the time points 0, 1 and 9 months after primary vaccination, as well as 1 and 9 months after booster vaccination. Consistent with a previous study (Loew-Baselli et al. 2009), ELISA TBEV-specific Ab concentrations were significantly lower in older than in young adults at 1 and 9 months after primary vaccination (p = 0.03; p < 0.0001, respectively) and at 1 and 9 months after booster vaccination (p < 0.0001; p < 0.0001, respectively; Fig. 2 a, b). Older adults also exhibited significantly lower TBEV-neutralizing Ab titres at 9 months, but not 1 month after primary vaccination (p < 0.0001; p = 0.8, respectively) and at 1 and 9 months after booster vaccination (p < 0.0001; p < 0.0001, respectively; Fig. 2 c, d). After booster vaccination, Ab titres were significantly higher than they were after primary vaccination in both age groups, but the extent of this increase was much lower in the elderly (5-fold vs 3-fold for ELISA, 11-fold vs 3-fold for NT). The data confirm that age strongly affects the formation of Ab responses during both the primary and secondary immune responses.
Fig. 2.
Kinetics of B cell responses before and at 1 and 9 months after two-dose primary vaccination and at 1 and 9 months after booster. a, b Geometric means with 95% CI of plasma TBEV-specific IgG concentrations (ELISA) and c, d neutralizing Ab titres; e, f TBEV-specific memory B cell frequencies expressed relative to clonable IgG+ memory B cells, mean + SEM. BL baseline, B mem memory B cell
In addition to Abs, we measured the frequencies of TBEV-specific memory B cells at the same time points (Fig. 2e, f). For displaying the data in this figure, we took into account that the cloning efficiency under the culture conditions used was only about 20%, i.e., only ∼20% of IgG+ memory B cells were capable of IgG production (Fig. 1e). Therefore, to provide a closer estimate of the actual proportion of newly generated memory B cell populations, we expressed all results as a percentage of clonable IgG+ memory B cells, in accordance with Pinna et al. (2009). The overall pattern obtained was quite similar in old and young vaccinees, i.e., (a) increase of TBEV-specific memory B cell frequencies after primary vaccination, (b) a strong booster response in both age groups and (c) maintenance of these memory cells, albeit at a lower level.
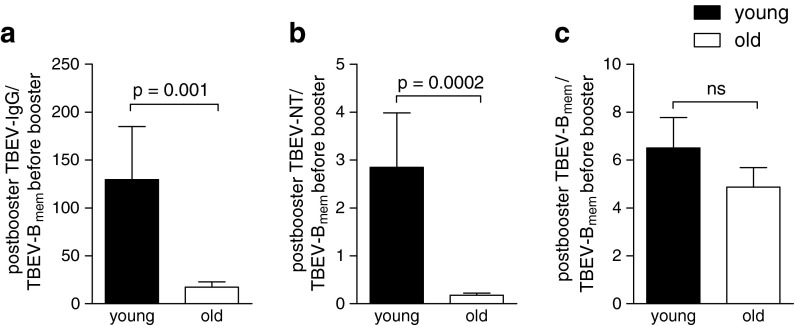
Inspection of the data in Fig. 2 reveals that the difference between old and young subjects was much more pronounced with respect to the Ab titres (Fig. 2a–d) than with respect to the numbers of specific memory B cells (Fig. 2e, f). This discrepancy is quantitatively displayed in Fig. 3, which relates the post-booster Ab (Fig. 3a, b) and specific memory B cell numbers (Fig. 3c) to the number of specific memory B cells present at the time of booster vaccination. As illustrated in the figure, the ratios of post-booster Ab titres to memory B cell numbers before booster were strikingly different between the old and young adults. In contrast, there was only a minor, not statistically significant age-related difference in the relative increase of memory B cell frequencies after booster vaccination (Fig. 3c). Together, these data indicate that the poor Ab responses in older individuals could not be explained by reduced numbers of Ag-specific memory B cells or the reduced responsiveness of these cells to booster vaccination but suggest the existence of additional deficiency factors that impair Ab production in the elderly.
Fig. 3.
Comparison of the proportions of specific memory B cell and Ab responses at 1 month after booster vaccination. Mean of individual ratios of a post-booster TBEV-specific IgG concentrations, b post-booster neutralizing Ab titres and c post-booster TBEV-specific memory B cell numbers and the number of TBEV-specific memory B cells present before booster. Data are mean + SEM
TBEV-specific CD4+T cell response
The observed impairment of the Ab response in the elderly may be related to the well-documented age-related reduction of T helper function (Weksler and Hutteroth 1974; Pawelec et al. 2002; Haynes et al. 2003; Eaton et al. 2004; Haynes and Eaton 2005). We therefore determined TBEV-specific CD4+T cells 1 week after primary TBE vaccination in 17 old and 12 young individuals. CD4+T cells were analysed for the expression of IFN-γ, TNF, IL-2 and CD154 after stimulation for 6 h with a library of peptides spanning the major TBEV E protein. We determined the frequencies of TBEV-specific CD4+T cells as a percentage of the total CD4+T cells (Fig. 4). As can be seen in Fig. 4a, b, the frequencies of TBEV-specific IL-2- as well as TNF-positive CD4+T cells were significantly lower in older than in young adults (mean frequency 0.15%, young vs 0.01%, old for IL-2, 0.23% vs 0.04% for TNF), whereas differences observed with IFN-γ or CD154 did not reach statistical significance (mean frequency 0.02% vs 0.01% for IFN-γ; 0.05% vs 0.03% for CD154). Frequencies of TBEV-specific CD4+T cells producing IL-2, TNF, IFN-γ or CD154 positively correlated with plasma TBEV-IgG Ab concentrations and neutralizing Ab titres 4 weeks after primary vaccination, although significance was obtained only for IL-2 and TNF positive CD4+T cell frequencies (Fig. 4c, Table 1). In contrast, no significant correlation was found between IFN-γ+ or CD154+CD4+T cell frequencies and plasma TBEV-specific Ab titres (Table 1). These experiments demonstrate that age affects the quantity and functionality of primary CD4+T cell responses and that the reduced Ag-specific CD4+T cell frequencies strongly correlate with the low Ab titres achieved in the elderly.
Fig. 4.
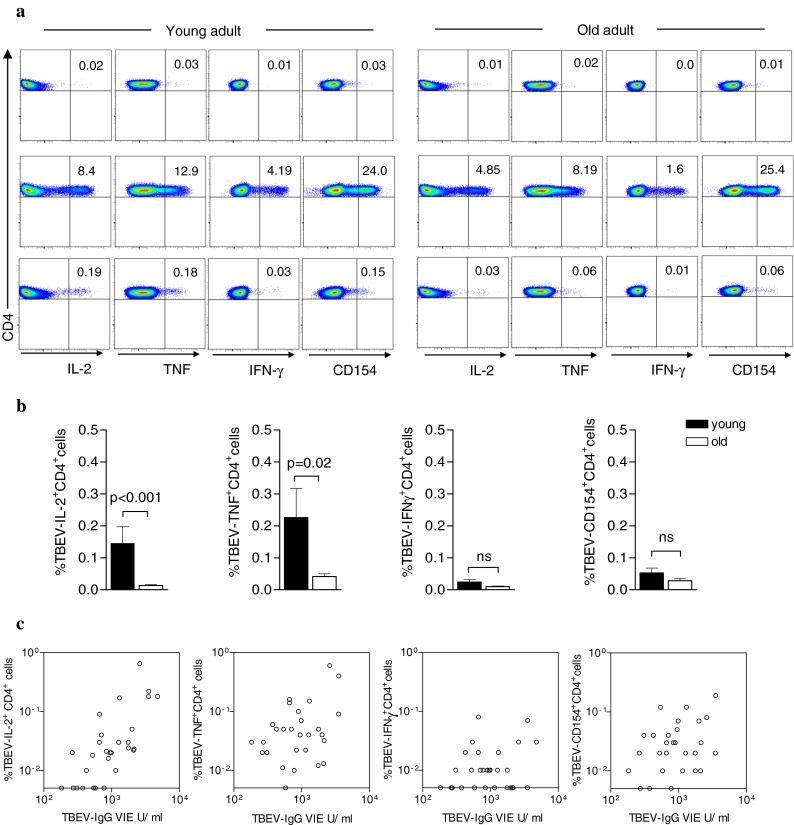
Flow cytometric analysis of Ag-specific CD4+T cells induced after primary TBE vaccination. Blood samples were collected 1 week after primary TBE vaccination. a Representative examples of one young and one old vaccinee showing the frequencies of IL-2-, TNF-, IFN-γ- or CD154-expressing CD4+T cells after in vitro stimulation of PBMC with a library of peptides spanning the TBEV major envelope protein E (bottom panel), SEB (middle panel) or costimulation only as a negative control (top panel). Numbers in the upper right quadrant of each FACS plot represent percentages of total CD4+T cells. b Frequencies of TBEV-activated CD4+T cells that expressed IL-2, TNF, IFN-γ or upregulated CD154 in 12 young and 17 old adults. Data are mean + SEM. c Correlation between plasma TBEV-IgG concentrations (ELISA) 1 month after primary vaccination and the frequency of TBEV-specific CD4+T cells expressing IL-2, TNF, IFN-γ or CD154 at 1 week after primary vaccination
Table 1.
Univariate regression analysis to estimate the relationship between TBEV-specific CD4+T cell populations, age and plasma TBEV-neutralizing Ab titres and TBEV-IgG
| Predictor | TBEV-neutralizing Ab | TBEV-IgG (ELISA) | ||
|---|---|---|---|---|
| Coefficient | p value | Coefficient | p value | |
| Age | −0.777 | <0.001 | −0.593 | <0.001 |
| IL-2 | 0.638 | 0.005 | 0.719 | <0.001 |
| TNF | 0.416 | 0.014 | 0.451 | 0.014 |
| IFN-γ | 0.261 | 0.234 | 0.228 | 0.235 |
| CD154 | 0.379 | 0.059 | 0.362 | 0.058 |
Standardized coefficients and p-value of log TBEV-specific CD4+T cells expressing IL-2, TNF, IFN-γ or CD154 on log TBEV-neutralizing Ab titres and log TBEV-IgG (VIE U/ml) after TBE primary vaccination
Discussion
The decreased ability of elderly people to produce high Ab titres in response to vaccination has been well documented (Wolters et al. 2003; Kaml et al. 2006; Frasca and Blomberg 2011), specifically also for TBE vaccination (Hainz et al. 2005; Rendi-Wagner et al. 2006; Loew-Baselli et al. 2009; Paulke-Korinek et al. 2009; Weinberger et al. 2010). This defect has been attributed in part to intrinsic changes in B cells, most notably to an age-related decline in the production of naïve B cells from their precursors (Johnson and Cambier 2004; Signer et al. 2007) and changes in the pre-immune B cell receptor repertoire diversity (Nicoletti et al. 1993; Cancro et al. 2009). Similarly, defects in molecular events that lead to a decline in Ig class-switch recombination impact Ab production in the elderly (Frasca et al. 2008; Frasca and Blomberg 2011). The effect of age on the generation of IgG+ memory B cells in a primary immune response and their capacity to respond in a secondary immune response has not yet been studied in humans. In our work we have addressed this question and demonstrate that memory B cells generated in older persons are neither impaired in maintenance nor in their capacity to expand upon booster vaccination. However, we show that the Ag-specific memory B cell numbers generated after primary vaccination are lower in elderly subjects, consistent with previous studies on age-related changes in numbers of B cells and their precursors (Franceschi et al. 1995; Shi et al. 2005; Frasca et al. 2008; Frasca and Blomberg 2011). This relatively slight reduction in memory B cell numbers could not explain the much stronger impairment of Ab production in the elderly, which is consistent with in vitro data which showed a profound impairment of Ab production even though B cell activation and proliferation were equal to or greater in old as compared to young subjects (Ennist et al. 1986).
An analysis of the age-specific CD4+T cell responses 1 week after primary vaccination revealed that CD4+T cell help was strongly impaired in older subjects. We demonstrate that the lower amount of circulating neutralizing Ab correlated with the reduced IL-2 and TNF-positive CD4+T cell response. Our data suggest that the reduced Ab response was not entirely due to an age-related reduction in B cell numbers, but that impaired CD4+T cell responses play a decisive role in the decline of Ab responses. The strongest correlation of IgG concentrations and neutralizing Ab titres was observed with IL-2 producing CD4+T cells, consistent with a previous study (Litjens et al. 2008). IL-2 appears to be especially important for the induction of IgG-producing PCs. It acts on Ag-activated B cells, thereby inducing transcripts of the Blimp-1 gene, which is considered the crucial regulator for the differentiation of B cells into Ig-producing PCs (Turner et al. 1994; Kallies and Nutt 2007). Another molecule expressed by activated T helper cells that has been shown to be important for Ab production is CD154 (CD40L). Previous work demonstrated that the expression of CD154 on CD4+T cells is reduced in aged mice (Eaton et al. 2004). This leads to reduced CD40L:CD40 interactions between B and T cells, which are required for germinal centre formation as well as Ig class switching (Kawabe et al. 1994; Xu et al. 1994). The present study revealed only minor differences in the frequencies of Ag-specific CD154+CD4+T cells in older compared to young adults. However, it is also known from studies in mice (Eaton et al. 2004) that age-related changes in CD154 upregulation are evident only after 40–60 h. The same study found no difference in CD154 expression early (4–6 h) after TCR stimulation (Eaton et al. 2004; Swain et al. 2005) which is in agreement with our results. For future studies, larger cohorts will be required to robustly determine the possible age dependence of the kinetics of CD154 expression in CD4+T cells.
Recent evidence suggests that there may be a significant participation of naïve in addition to memory B cells in secondary Ab responses (Goins et al. 2010). It was shown that these cells are activated by immuncomplexes formed between the pre-existing Ab from a primary immune response and the incoming Ag, and participate in rapid and robust Ab production upon secondary Ag exposure. On the basis of our data, this means that if a significant portion of Ab responses after booster vaccination actually came from naïve B cells, the lower Ab titres could also partly be explained by the reduced numbers of naïve B cells and/or by very low or undetectable pre-existing Ab from primary responses observed in the elderly. To which extent the involvement of naïve B cells also contributes to the impaired Ab response upon booster vaccination in the elderly remains to be determined.
In conclusion, our study shows that B cell function as measured by Ab production is strongly impaired in older people, not so much due to a reduction in B cell numbers, but due to an impairment of CD4+ helper cell responses, which appears to be a major determinant of strongly reduced Ab titres in the elderly. The present findings may provide leads for the specific design of vaccines for the elderly, e.g., by using adjuvants that compensate the CD4 defects shown to prevent an efficient Ab response.
Acknowledgements
We thank Ursula Sinzinger, Jutta Hutecek and Cornelia Stöckl for expert technical assistance. Franz X. Heinz is an inventor of patents on flavivirus vaccines and has consulted Baxter for developing tick-borne encephalitis vaccines. Michael Kundi has served as a consultant to Baxter.
References
- Amanna IJ, Slifka MK. Quantitation of rare memory B cell populations by two independent and complementary approaches. J Immunol Methods. 2006;317(1–2):175–185. doi: 10.1016/j.jim.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aw D, Silva AB, et al. Immunosenescence: emerging challenges for an ageing population. Immunology. 2007;120(4):435–446. doi: 10.1111/j.1365-2567.2007.02555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett PN, Plotkin SA, Ehrlich HJ (2008) Tick-borne encephalitits virus vaccines. Vaccine. O. W. Plotkin SA, Offit PA, Saunders Elsevier, pp 841–856
- Bernstein ED, Murasko DM. Effect of age on cytokine production in humans. AGE. 1998;21:137–151. doi: 10.1007/s11357-998-0024-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard-Rohner G, Pulickal AS, et al. Appearance of peripheral blood plasma cells and memory B cells in a primary and secondary immune response in humans. Blood. 2009;114(24):4998–5002. doi: 10.1182/blood-2009-03-211052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancro MP, Hao Y, et al. B cells and aging: molecules and mechanisms. Trends Immunol. 2009;30(7):313–318. doi: 10.1016/j.it.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay PK, Yu J, et al. Live-cell assay to detect antigen-specific CD4+ T-cell responses by CD154 expression. Nat Protoc. 2006;1(1):1–6. doi: 10.1038/nprot.2006.1. [DOI] [PubMed] [Google Scholar]
- Eaton SM, Burns EM, et al. Age-related defects in CD4 T cell cognate helper function lead to reductions in humoral responses. J Exp Med. 2004;200(12):1613–1622. doi: 10.1084/jem.20041395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennist DL, Jones KH, et al. Functional analysis of the immunosenescence of the human B cell system: dissociation of normal activation and proliferation from impaired terminal differentiation into IgM immunoglobulin-secreting cells. J Immunol. 1986;136(1):99–105. [PubMed] [Google Scholar]
- Franceschi C, Monti D, et al. The immunology of exceptional individuals: the lesson of centenarians. Immunol Today. 1995;16(1):12–16. doi: 10.1016/0167-5699(95)80064-6. [DOI] [PubMed] [Google Scholar]
- Frasca D, Blomberg BB. Aging affects human B cell responses. J Clin Immunol. 2011;31(3):430–435. doi: 10.1007/s10875-010-9501-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasca D, Landin AM, et al. Aging down-regulates the transcription factor E2A, activation-induced cytidine deaminase, and Ig class switch in human B cells. J Immunol. 2008;180(8):5283–5290. doi: 10.4049/jimmunol.180.8.5283. [DOI] [PubMed] [Google Scholar]
- Goins CL, Chappell CP, et al. Immune complex-mediated enhancement of secondary antibody responses. J Immunol. 2010;184(11):6293–6298. doi: 10.4049/jimmunol.0902530. [DOI] [PubMed] [Google Scholar]
- Gubler DJ. The continuing spread of West Nile virus in the western hemisphere. Clin Infect Dis. 2007;45(8):1039–1046. doi: 10.1086/521911. [DOI] [PubMed] [Google Scholar]
- Haglund M, Gunther G. Tick-borne encephalitis—pathogenesis, clinical course and long-term follow-up. Vaccine. 2003;21(Suppl 1):S11–S18. doi: 10.1016/S0264-410X(02)00811-3. [DOI] [PubMed] [Google Scholar]
- Hainz U, Jenewein B, et al. Insufficient protection for healthy elderly adults by tetanus and TBE vaccines. Vaccine. 2005;23(25):3232–3235. doi: 10.1016/j.vaccine.2005.01.085. [DOI] [PubMed] [Google Scholar]
- Halstead SB, Jacobson J (2008) Japanese encephalitis vaccines. Vaccine. O. W. Plotkin SA, Offit PA, Saunders Elsevier, pp 311–352
- Haynes L, Eaton SM. The effect of age on the cognate function of CD4+ T cells. Immunol Rev. 2005;205:220–228. doi: 10.1111/j.0105-2896.2005.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes L, Eaton SM, et al. CD4 T cell memory derived from young naive cells functions well into old age, but memory generated from aged naive cells functions poorly. Proc Natl Acad Sci U S A. 2003;100(25):15053–15058. doi: 10.1073/pnas.2433717100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzmann H, Kundi M, et al. Correlation between ELISA, hemagglutination inhibition, and neutralization tests after vaccination against tick-borne encephalitis. J Med Virol. 1996;48(1):102–107. doi: 10.1002/(SICI)1096-9071(199601)48:1<102::AID-JMV16>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Johnson SA, Cambier JC. Ageing, autoimmunity and arthritis: senescence of the B cell compartment—implications for humoral immunity. Arthritis Res Ther. 2004;6(4):131–139. doi: 10.1186/ar1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser R. Tick-borne encephalitis (TBE) in Germany and clinical course of the disease. Int J Med Microbiol. 2002;291(Suppl 33):58–61. doi: 10.1016/S1438-4221(02)80012-1. [DOI] [PubMed] [Google Scholar]
- Kallies A, Nutt SL. Terminal differentiation of lymphocytes depends on Blimp-1. Curr Opin Immunol. 2007;19(2):156–162. doi: 10.1016/j.coi.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Kaml M, Weiskirchner I, et al. Booster vaccination in the elderly: their success depends on the vaccine type applied earlier in life as well as on pre-vaccination antibody titers. Vaccine. 2006;24(47–48):6808–6811. doi: 10.1016/j.vaccine.2006.06.037. [DOI] [PubMed] [Google Scholar]
- Kawabe T, Naka T, et al. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1994;1(3):167–178. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Kreil TR, Maier E, et al. Neutralizing antibodies protect against lethal flavivirus challenge but allow for the development of active humoral immunity to a nonstructural virus protein. J Virol. 1998;72(4):3076–3081. doi: 10.1128/jvi.72.4.3076-3081.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle JL, Harris E. Global spread and persistence of dengue. Annu Rev Microbiol. 2008;62:71–92. doi: 10.1146/annurev.micro.62.081307.163005. [DOI] [PubMed] [Google Scholar]
- Litjens NH, Huisman M, et al. IL-2 producing memory CD4+ T lymphocytes are closely associated with the generation of IgG-secreting plasma cells. J Immunol. 2008;181(5):3665–3673. doi: 10.4049/jimmunol.181.5.3665. [DOI] [PubMed] [Google Scholar]
- Loew-Baselli A, Poellabauer EM, et al. Seropersistence of tick-borne encephalitis antibodies, safety and booster response to FSME-IMMUN 0.5 ml in adults aged 18–67 years. Hum Vaccin. 2009;5(8):551–556. doi: 10.4161/hv.5.8.8571. [DOI] [PubMed] [Google Scholar]
- Manz RA, Thiel A, et al. Lifetime of plasma cells in the bone marrow. Nature. 1997;388(6638):133–134. doi: 10.1038/40540. [DOI] [PubMed] [Google Scholar]
- McElhaney JE, Effros RB. Immunosenescence: what does it mean to health outcomes in older adults? Curr Opin Immunol. 2009;21(4):418–424. doi: 10.1016/j.coi.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickiene A, Laiskonis A, et al. Tick-borne encephalitis in an area of high endemicity in lithuania: disease severity and long-term prognosis. Clin Infect Dis. 2002;35(6):650–658. doi: 10.1086/342059. [DOI] [PubMed] [Google Scholar]
- Monath TP, Cetron M, Teuwen DE (2008). Yellow fever vaccine. Vaccine. O. W. Plotkin SA, Offit PA, Saunders Elsevier, pp 959–1055
- Nicoletti C, Yang X, et al. Repertoire diversity of antibody response to bacterial antigens in aged mice. III. Phosphorylcholine antibody from young and aged mice differ in structure and protective activity against infection with Streptococcus pneumoniae. J Immunol. 1993;150(2):543–549. [PubMed] [Google Scholar]
- Paulke-Korinek M, Rendi-Wagner P, et al. Booster vaccinations against tick-borne encephalitis: 6 years follow-up indicates long-term protection. Vaccine. 2009;27(50):7027–7030. doi: 10.1016/j.vaccine.2009.09.068. [DOI] [PubMed] [Google Scholar]
- Pawelec G, Barnett Y, et al. T cells and aging, January 2002 update. Front Biosci. 2002;7:d1056–d1183. doi: 10.2741/a831. [DOI] [PubMed] [Google Scholar]
- Pinna D, Corti D, et al. Clonal dissection of the human memory B-cell repertoire following infection and vaccination. Eur J Immunol. 2009;39(5):1260–1270. doi: 10.1002/eji.200839129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendi-Wagner P, Zent O, et al. Persistence of antibodies after vaccination against tick-borne encephalitis. Int J Med Microbiol. 2006;296(Suppl 40):202–207. doi: 10.1016/j.ijmm.2006.01.030. [DOI] [PubMed] [Google Scholar]
- Shi Y, Yamazaki T, et al. Regulation of aged humoral immune defense against pneumococcal bacteria by IgM memory B cell. J Immunol. 2005;175(5):3262–3267. doi: 10.4049/jimmunol.175.5.3262. [DOI] [PubMed] [Google Scholar]
- Signer RA, Montecino-Rodriguez E, et al. Aging, B lymphopoiesis, and patterns of leukemogenesis. Exp Gerontol. 2007;42(5):391–395. doi: 10.1016/j.exger.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifka MK, Antia R, et al. Humoral immunity due to long-lived plasma cells. Immunity. 1998;8(3):363–372. doi: 10.1016/S1074-7613(00)80541-5. [DOI] [PubMed] [Google Scholar]
- Steininger C, Rassenti LZ, et al. Relative seroprevalence of human herpes viruses in patients with chronic lymphocytic leukaemia. Eur J Clin Invest. 2009;39:497–506. doi: 10.1111/j.1365-2362.2009.02131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiasny K, Holzmann H, et al. Characteristics of antibody responses in tick-borne encephalitis vaccination breakthroughs. Vaccine. 2009;27(50):7021–7026. doi: 10.1016/j.vaccine.2009.09.069. [DOI] [PubMed] [Google Scholar]
- Swain S, Clise-Dwyer K, et al. Homeostasis and the age-associated defect of CD4 T cells. Semin Immunol. 2005;17(5):370–377. doi: 10.1016/j.smim.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CA, Jr, Mack DH, et al. Blimp-1, a novel zinc finger-containing protein that can drive the maturation of B lymphocytes into immunoglobulin-secreting cells. Cell. 1994;77(2):297–306. doi: 10.1016/0092-8674(94)90321-2. [DOI] [PubMed] [Google Scholar]
- Weinberger B, Keller M, et al. Decreased antibody titers and booster responses in tick-borne encephalitis vaccinees aged 50–90 years. Vaccine. 2010;28(20):3511–3515. doi: 10.1016/j.vaccine.2010.03.024. [DOI] [PubMed] [Google Scholar]
- Weksler ME, Hutteroth TH. Impaired lymphocyte function in aged humans. J Clin Invest. 1974;53(1):99–104. doi: 10.1172/JCI107565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters B, Junge U, et al. Immunogenicity of combined hepatitis A and B vaccine in elderly persons. Vaccine. 2003;21(25–26):3623–3628. doi: 10.1016/S0264-410X(03)00399-2. [DOI] [PubMed] [Google Scholar]
- Xu J, Foy TM, et al. Mice deficient for the CD40 ligand. Immunity. 1994;1(5):423–431. doi: 10.1016/1074-7613(94)90073-6. [DOI] [PubMed] [Google Scholar]