Abstract
Chronic inflammation has been linked to cancer. Prostaglandin E2 (PGE2) is the most pro-inflammatory lipid and one of the downstream products of 2 isoforms of cyclooxygenase (COX) enzymes: COX-1 and COX-2. Ovarian cancer is the most lethal gynecological malignancy and mainly occurs in older women. The factors that contribute to the correlation of age and ovarian cancer are unknown. The purpose of this study was to examine the expression of COX enzymes and PGE2 levels in ovaries and correlate them to ovarian cancer and aging. White Leghorn hens aged 1, 1.5, 2, 2.5, 3 and 3.5 years were used. The incidence of ovarian cancer was determined by gross pathology and histology. COX-1 and COX-2 protein and mRNA expression and PGE2 concentrations in ovaries were measured using Western blot, quantitative real-time PCR and ELISA, respectively. Our results indicated an increase in ovarian cancer incidence and expression of both COX enzymes in ovaries of older hens. In correlation with ovarian cancer incidence and COX enzymes expression, PGE2 concentrations were elevated with age. Ovaries with tumor had elevated COX-1 expression and PGE2 concentration compared to normal ovaries. Our findings suggest that the up-regulation of COX enzymes with age is the main contributing factor in the age associated increase in PGE2. Furthermore, elevated PGE2 in ovaries of hens concomitant with age suggests its important role in early stages of ovarian carcinogenesis. These finding may provide the basis for clinical trials utilizing COX specific inhibitors or dietary intervention targeting prostaglandin biosynthesis for the prevention and treatment of ovarian cancer.
Keywords: Ovarian cancer incidence, Age, Laying hen, Inflammation, Cyclooxygenase, Prostaglandin E2
1. Introduction
The link between chronic inflammation and cancer has been recognized for many years [1]. Originally, inflammation was believed to be primarily a beneficial host response, representing the body’s fight against invading tumor cells [2]. More recent data, however, suggest just the opposite; Inflammation may be the cause of some cancers and a powerful stimulus for tumor growth and invasion [1,3,4]. Conversely, anti-inflammatory drugs such as non-steroidal anti-inflammatory drugs (NSAIDs) decrease the risk of developing of many cancers.
Eicosanoids, including prostaglandins and leukotrienes, are products of local cell type specific arachidonic acid (AA) metabolism and can be potent mediators of inflammation [5,6]. Prostaglandins (PGs) are downstream products of cyclooxygenase enzymes. PGs are biologically active lipids that are associated with inflammation, fever, pain and tissue injury [7]. The most proinflammatory lipid and major prostaglandin is PGE2 which plays a predominant role in cancer initiation and development [8]. Furthermore, PGE2 is the most common prostaglandin found in different human cancers including colon, lung, breast, and head and neck cancers [9]. It has been proven that prostaglandins and their metabolites play a crucial role in regulating the cellular processes such as enhancement of proliferation, inhibition of apoptosis and stimulation of cell adhesion.
Cyclooxygenase (COX) is the rate limiting enzyme in catalyzing the conversion of arachidonic acid to prostaglandin H2. Specific prostaglandin synthases act on PGH2 to produce prostaglandins and thromboxanes. Two isoforms of cyclooxygenase has been identified, COX-1 and COX-2. Although both COX isoforms have similar structure and function, they are encoded with different genes (PTGS1 and PTGS2) and show distinct expression patterns. COX-1 is expressed in most cells and tissues and remains constant under most physiologic conditions to play a housekeeping role whereas the COX-2 form is inducible and usually only expressed in response to various inflammatory stimuli [10]. It has been shown that COX enzymes may be involved in both tumor establishment [11] and maintenance of existing tumors [12]. Up-regulation of COX-2 has been reported in many malignancies including breast [13], lung [14] and ovarian cancer [15]. However, we and others have shown that COX-1 is over-expressed in ovarian cancer [12,16,17].
Ovarian cancer is the fifth leading cause of cancer death among women and the most lethal gynecological malignancy. There are at least 3 well established risk factors for ovarian cancer: age, family history and environmental factors. The incidence of inclusion cysts increases with advancing age and are common in post-menopausal women [18] which is highly correlated with increased ovarian cancer risk. Ovarian cancer is mainly seen in older women when their ovaries are not reproductively functional. Close to half of the women with ovarian cancer (48%) are in the age group of 65 or older [19]. Several hypotheses about the etiology of ovarian cancer have been proposed. “Gonadotropin hypothesis” states that increased exposure to the gonadotropins, follicle stimulating hormone (FSH) and lutenizing hormone (LH), leads to ovarian surface epithelium (OSE) transformation [20] which is supported by evidence that as women enter menopause, their levels of FSH and LH are very high, which corresponds with the age that most women develop ovarian cancer [21]. “Tubal hypothesis” has emerged which proposes that ovarian cancer may not originate from the ovary at all, but rather arises from the oviduct [22,23] in a way that Fallopian tube exposure to the same microenvironment as the ovary which contains many inflammatory factors may potentially damage OSE and induce mutation and transformation. This hypothesis classifies ovarian cancer into two types: type I ovarian cancer where there are low grade, relatively stable cancers that arise in the ovary and type II which are very aggressive high grade serous carcinomas that may arise from damaged epithelium of the Fallopian tubes [24–26]. A third theory, the “incessant ovulation hypothesis” was first proposed by Fathalla in 1971 [27]. He hypothesized that continuous ovulation, with successive rounds of surface rupture and OSE cell mitosis to repair the wound, renders the cells susceptible to malignant transformation. In support of this hypothesis, multiparity, duration of lactation and use of birth control pills all decrease the risk of epithelial ovarian cancer [28]. The hen model strongly supports the incessant ovulation hypothesis because domestic hens ovulate almost daily and frequently develop ovarian cancer [29]. By the time a hen has completed 2 years of egg laying, she has ovulated about as many times as a woman approaching menopause. After each ovulation, the rapid repair of the ovarian surface epithelium may cause the formation of inclusion cysts where the surface cells become trapped and cause ovarian cancer.
Hens spontaneously develop ovarian adenocarcinomas that are similar in histological appearance to human ovarian carcinomas and share similar symptoms of the disease, such as perfuse ascitic fluid and peritoneal metastatic dissemination [30,31]. The laying hen is the only accessible animal model that recapitulates human ovarian cancer. Many hypotheses about etiology of ovarian cancer have been proposed but the factors that contribute to correlation of age and ovarian cancer is unknown. The purpose of this study was to examine the expression of COX enzymes and PGE2 levels in ovaries and correlate them to ovarian cancer and aging. The results of this study indicate that COX enzymes and PGE2 increase with age coinciding with onset of ovarian cancer which makes them suitable targets to prevent this highly lethal malignancy.
2. Material and methods
2.1. Animals
Six hundred single comb white Leghorn hens, aged 12–45 months, were used. Hens were maintained 3 per cage and were provided with feed and water ad libitum and exposed to a photoperiod of 17 h light: 7 h dark, with lights on at 05:00 h and lights off at 22:00 h. Ovulation frequency was determined by counting the total number of eggs laid each week by the whole group, then dividing by the total number of hens in each group. Animal management and procedures were reviewed and approved by the Institutional Animal Care and Use Committees at the University of Illinois at Urbana-Champaign, University of Illinois at Chicago and Southern Illinois University at Carbondale.
2.2. Collection of tissue
At the age of 12, 19, 24, 31, 36 and 45 months, 20 hens were randomly selected, euthanized using CO2 asphyxiation and necropsied. Samples from chickens aged 19, 31 and 45 months were designated as 1.5, 2.5 and 3.5 years old, respectively. Ovaries were removed from hens and small yellow follicles (6–8 mm) and pre-ovulatory follicles (9–35 mm) were removed. Ovaries with suspected abnormalities were noted and confirmed as ovarian tissue with or without tumor by histology. Tumors were classified by stage as previously shown [32]. Tumors were characterized based on the size of the ovarian tumor, oviductal involvement, and if there was any ascites fluid and peritoneal metastaseis present by gross observation [31]. Basic histology was performed on early stage tumors to determine the grade of cancer [33]. The ovaries were dissected into several pieces. The first portion was frozen in liquid nitrogen and later stored at −80 °C; the second portion was put into RNAlater solution and stored at 4 °C before processing; the third and fourth portions were used for histological analysis and fixed in NBF fixative solution.
2.3. Reagents
Prostaglandin E2 EIA monoclonal kit and anti-human COX-1 and anti-human COX-2 antibodies were obtained from Cayman Chemical (Ann Arbor, MI, USA). High capacity cDNA archive kit, RNAlater and SYBR Green were obtained from Applied Biosystems (Foster city, CA, USA). Neutral buffered formalin (NBF) was obtained from Sigma-Aldrich (St Louis, MO, USA); Experion RNA StdSens Analysis and CFX384 Real-Time System were obtained from Bio-Rad (Hercules, CA, USA). Halt protease inhibitor cocktail EDTA-Free and BCA protein assay were obtained from Pierce Biotechnology (Rockford, IL, USA). The homogenizer Ultra-Turrax was from Jenke and Kunkel (Staufenh, Germany). Synergy MxMonochromator-Based Multi-Mode Microplate reader was from BioTek (Winoosk, VT, USA). SNAP i.d. was obtained from Millipore (Billerica, MA, USA). Odyssey blocking buffer, goat anti-mouse IgG antibody (H&L)-DyLight™680 conjugated and goat anti-rabbit IgG antibody (H&L)-DyLight™800 conjugated were obtained from LI-COR Bioscience (Lincoln, NE, USA). β-Actin was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Solid phase extraction columns were purchased from Waters Corporation (Milford, MA, USA).
2.4. Histology
Ovarian tissues fixed in NBF were processed and paraffin embedded as previously described [16,29,30]. Four micrometer sections were cut and mounted on SuperFrost Plus microscope slides. Slides were deparaffinized and rehydrated through xylene and graded ethanol solutions. Hematoxylin and eosin staining were performed as described [34].
2.5. RNA extraction and analysis
Total RNA was extracted from ovarian tissue using Trizol reagent. Quantification was performed by determination of absorbance at A260, and qualified by Experion RNA StdSens Analysis. All RNA samples used in this study had a 260:280 ratio range between 1.97 and 2.15, and had 3 bands: 5 S, 18 S and 28 S for electrophoresis results. RNA samples were then treated with RQ1 RNase-free DNase prior to reverse transcription reaction. cDNA was synthesized from DNase treated RNA with the high capacity cDNA archive kit.
2.6. Quantitative real-time PCR
The chicken-specific primers and plasmid standards used for each gene were designed. Primer sequences are as follows:
| COX-1 | Forward | 5′ TCAGGTGGTTCTGGGACATCA 3′ |
| Reverse | 5′ TGTAGCCGTACTGGGAGTTGAA 3′ | |
| COX-2 | Forward | 5′ CTGCTCCCTCCCATGTCAGA 3′ |
| Reverse | 5′ CACGTGAAGAATTCCGGTGTT 3′ | |
| GAPDH | Forward | 5′ GATGGGTGTCAACCATGAGAAA 3′ |
| Reverse | 5′ CAATGCCAAAGTTGTCATGGA 3′ |
qRT-PCR was conducted by amplifying cDNA with SYBR Green on CFX384 Real-Time System and analyzed with Bio-Rad CFX Manager software. Control reactions lacking template were run for each gene. Reactions were 10 μl in total volume and 200 nM of each primer. The plasmid standards and cDNA were simultaneously assayed in duplicate reactions. The amplification conditions were as follows: 50 °C 5 S, 95 °C 10 min, 40 cycles for 95 °C 15 S, 60 °C 1 min.
2.7. Western blot
Snap frozen ovarian tissue samples were pulverized on dry ice, re-suspended in ice-cold lysis buffer (PBS/0.1% sodium dodecyl sulfate (SDS), supplemented with Halt protease inhibitor cocktail EDTA-Free), and homogenized using Ultra-Turrax. Protein concentrations were determined by BCA protein assay and Synergy MxMonochromator-Based Multi-Mode Microplate reader. Twenty milligrams of total protein were separated by SDS-PAGE using 12.5% acrylamide/SDS separating gels and transferred to nitrocellulose membranes. Using SNAP i.d. the nitrocellulose membranes were incubated for 10 min with Odyssey blocking buffer and monoclonal anti-human antibodies for detection of COX-1 or COX-2. Data were normalized to β-Actin. Detection of bound antibody on the blot was assessed with a goat anti-mouse IgG antibody (H&L), DyLight™680 conjugated and goat anti-rabbit IgG antibody (H&L), DyLight™800 conjugated scanned for infrared signal using Odyssey imaging system.
2.8. PGE2 EIA
The amount of PGE2 in ovarian tissue was measured using a specific enzyme immunoassay according to manufacturer’s instructions. Snap frozen ovarian tissues maintained at −80 °C were pulverized on dry ice and re-suspended in 5 ml of homogenizing buffer (0.1 M phosphate, pH 7.4, containing 1 mM EDTA and 10 μM indomethacin) per 1 g of tissue. Samples were homogenized, acidified to ~pH 4, centrifuged at 3000 g for 5 min and supernatants were transferred to clean tubes. Solid phase extraction columns (C-18) were prepared by rinsing with 5 ml methanol followed by 5 ml deionized water. Samples were applied to the columns and washed with 5 ml of deionized water followed by 5 ml of HPLC grade hexane. Cartridges were allowed to dry. PGE2 was eluted from the columns by ethylacetate containing 1% methanol. Ethyl acetate was evaporated under steam of nitrogen to complete dryness. To re-suspend the samples, 500 μl EIA buffer (provided with the kit) were added and vortexed. The contents of PGE2 EIA standard vial (provided with the kit) were reconstituted with 1.0 ml of EIA buffer and serially diluted. 96-well goat polyclonal anti-mouse IgG covered plate was set up and EIA buffer was added to appropriate wells. Fifty micro-liter of standards and 50 μl of samples, 50 μl of PGE2–acetylcholinesterase tracer and PGE2 monoclonal antibody were added to appropriate wells, respectively. The plate was covered and incubated for 18 h at 4 °C. Wells were emptied and rinsed with wash buffer (provided with kit) 5 times. Ellman’s reagent was reconstituted and 200 μl of it were added to each well. Five micro-liter of tracer were added to appropriate wells. The plate was covered and allowed to develop on an orbital shaker for 75 min. The plate was read at wavelength of 405 nm and Cayman chemical computer spreadsheet was used to calculate the concentration of PGE2.
2.9. Statistical analysis
All experiments were performed in duplicate at each time-point (n=20) and differences in data from groups were analyzed with Graph Pad InStat by using One-way ANOVA with Student–Newman–Keuls. A value of P<0.05 was considered significant whereas a value of P<0.01 was considered highly significant.
3. Results
3.1. Ovarian cancer incidence increases with age
Upon necropsy of 20 hens at each time-point, ovaries were classified as normal, cancerous or suspected abnormalities. Ovaries with suspected abnormalities were analyzed by histology to confirm the cancerous ovarian tissue. Fig. 1A and D shows a normal ovary of hen consists of a hierarchy of 4–5 pre-ovulatory follicles. The ovary shown in Fig. 1B was classified as a suspected ovary. The ovary was active and there were no metastasis and abdominal ascites but it had less ovulatory follicles than normal ovary and was stiff, highly vascular and bigger than normal. Microscopic examination of the suspected ovary after H&E staining (Fig. 1E) showed a disassociated stroma with unusually high numbers of blood vessels. Furthermore, formation of focal lesions in the stroma below the ovarian surface was apparent. Fig. 1C was classified as cancer due to stiffness and huge size of the ovary, lack of any follicle indicating inactivity of the ovary, peritoneal ascites and metastasis. Under the microscope, the cancerous ovary had solid areas composed of slit-like sheets containing cells with high-grade nuclear atypia and a few tiny glands were also seen without any papillae (Fig. 1F). No ovarian malignancy was detected in hens aged 1, 1.5 and 2 years; however 6 month later,10% of 2.5 year old chickens (n=2) had ovarian cancer (Fig. 2). There was an increase in ovarian cancer incidence in 3 year old hens (n=13) compared to younger hens with 65% of 3 year old hens having ovarian cancer.
Fig. 1.
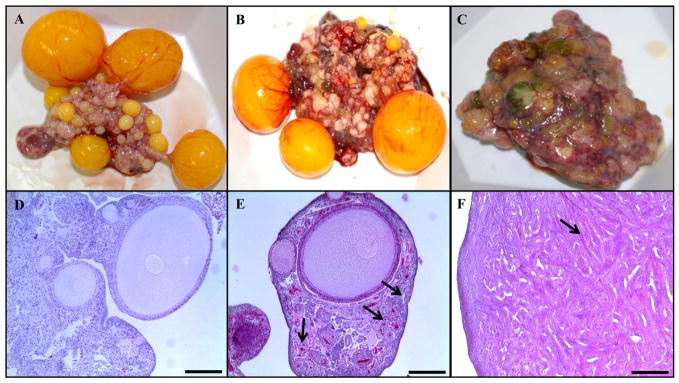
Normal and cancerous chicken ovary. (A) Gross anatomy of normal ovary with a hierarchy of developing follicles; (B) ovary classified as suspected; (C) ovary taken from a hen with metastatic late stage ovarian cancer; (D) H&E stain of normal ovary with developing small follicles; (E) H&E stain of suspected ovary showed a disassociated stoma with unusually high numbers of blood vessels and formation of focal lesions in the stroma below the ovarian surface was apparent (arrow); (F) H&E stain of metastatic late stage ovarian cancer with solid areas composed of slit-like sheets containing cells with high-grade nuclear atypia and a few tiny glands were also seen without any papillae. Calibration Bar, 200 μm.
Fig. 2.
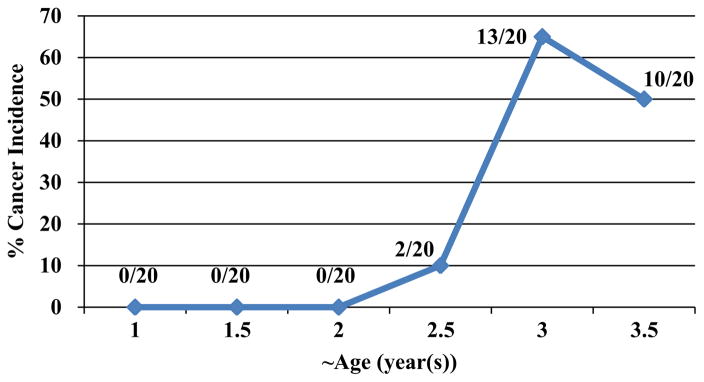
Percentage of early and late stages ovarian cancer incidence at different ages. Necropsy of hens was performed at different time-points (n=20) and ovaries were classified as normal, cancerous or questionable. Questionable ovarian tissues were analyzed by histology to confirm the cancerous ovarian tissue. No ovarian malignancy was detected in 1 (n=0/20), 1.5 (n=0/20) and 2 year old hens (n=0/20); however, 10% of 2.5 year old (n=2/20) hens had ovarian cancer. There was a significant increase in ovarian cancer incidence in 3 year old hens (n=13/20) (65%) compared to younger hens. Fifty percent of 3.5 year old birds (n=10/20) had ovarian cancer.
3.2. COX-1 is up-regulated in ovarian cancer with age
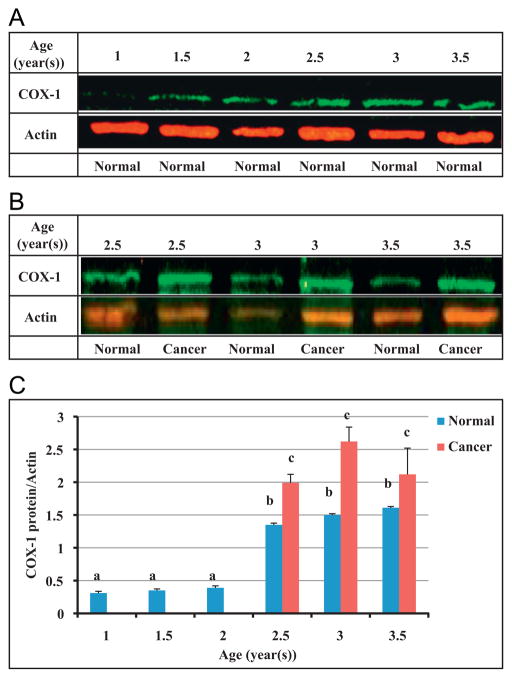
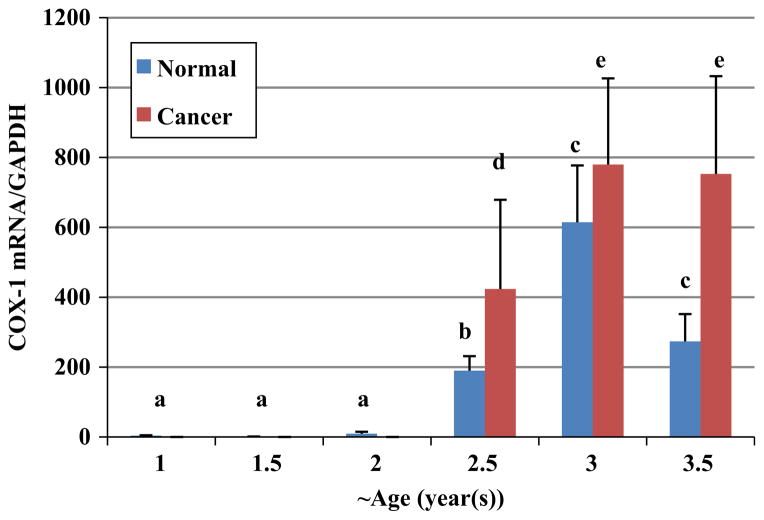
Expression of COX-1 protein was measured using western blot (Figs. 3A–C). There was no significant difference in expression of COX-1 among 1 (n=20), 1.5 (n=20) and 2 (n=20) year old hens. Comparing COX-1 protein expression in normal ovaries of 2.5 year old hens (n=18) to the first three age groups (ages 1, 1.5 and 2) revealed a significant increase in 2.5 year old hens (P<0.001). It is important to note that in ovaries of 1, 1.5 and 2 year old hens, no ovarian cancer was seen and the first ovarian cancer was observed in 2.5 year old hens. Using qPCR, COX-1 mRNA was quantified (Fig. 4). Similar to the protein expression, no significant change in COX-1 mRNA expression was observed among the three youngest ages (1, 1.5 and 2 year old). Normal ovarian tissue of 2.5 year old chickens expressed greater amounts of COX-1 mRNA than three younger groups (P<0.01). A significant elevation in COX-1 mRNA expression was seen in 3 year old normal hens compared to 2.5 year old normal hens (P<0.01); However a decrease in normal ovarian tissue’s COX-1 mRNA and protein expression from 3 to 3.5 year old hens was found which was not statistically significant (P>0.05).
Fig. 3.
Expression of COX-1 protein was measured in ovaries of hens at different ages. (A and C) A significant increase in COX-1 expression was observed in normal ovaries of 2.5 year old hens (n=18) compared to 1 (n=20), 1.5 (n=20) and 2 (n=20) year old hens. (B and C) There was a significant difference in COX-1 protein expression between normal and cancerous ovaries of hens at 2.5, 3 and 3.5 year old. a versus b, P<0.001; b versus c, P<0.01; Bars indicate standard error.
Fig. 4.
COX-1 mRNA expression in ovaries of hens at approximately 6 month intervals. Normal ovaries of 2.5 year old hens (n=18) had significantly greater expression of COX-1 mRNA than 1 (n=20), 1.5 (n=20) and 2 (n=20) year old hens. A significant elevation in COX-1 mRNA expression occurred in ovaries of 3 year old normal hens (n=7) compared to ovaries of 2.5 year old normal hens. There was a significant difference in COX-1 mRNA expression between normal and cancerous ovaries of hens at 2.5, 3 and 3.5 year old. a versus b, b versus c, b versus d, c versus e, e versus d, P<0.01; c versus d, P>0.05. Bars indicate standard error.
A significant (P<0.01) elevation of COX-1 protein and mRNA expression occurred when comparing normal ovaries to cancerous ovaries of 2.5, 3 and 3.5 year old hens, respectively (Fig. 3B and C; and Fig. 4).
3.3. COX-2 is over-expressed in ovaries with age
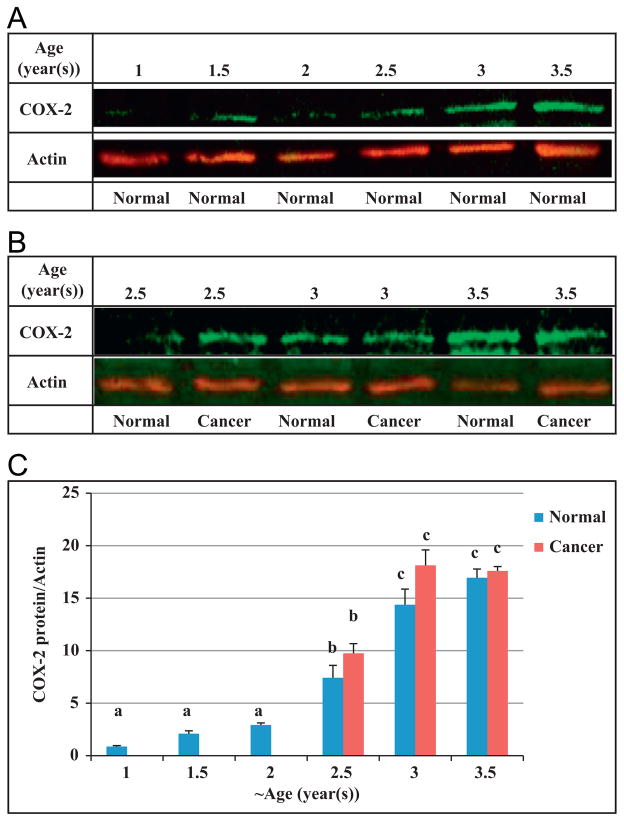
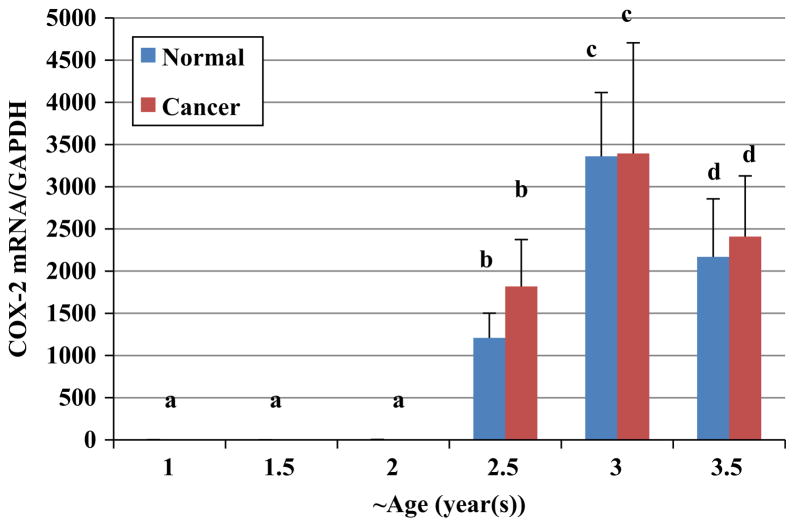
There were no significant changes in COX-2 protein expression in ovaries of hens aged 1 (n=20), 1.5 (n=20) and 2 (n=20) years. Birds aged 2.5 years, had significantly higher expression of COX-2 protein in their normal ovaries (n=18) compared to normal ovarian tissue of 2 year old hens (n=20) (Fig. 5). A further significant increase in expression of COX-2 protein was seen in normal ovaries of 3 year old hens (n=7) compared to normal ovaries of 2.5 year old hens. The COX-2 mRNA expression in all groups was measured using qPCR and compared to each other (Fig. 6). There were no significant differences in COX-2 mRNA expression among 1, 1.5 and 2 year old hens (n=20). In contrast, there was a significant increase in COX-2 mRNA expression in normal ovaries of 2.5 year old chickens (n=18) in comparison to younger hens (P<0.01). Normal ovarian tissue from 3 year old hens (n=7) had higher expression of COX-2 mRNA than 2.5 year old normal hens; and 3.5 year old chickens with normal ovaries (n=10) had significantly less COX-2 expression than normal ovaries of 3 year old hens (P<0.05).
Fig. 5.
Expression of COX-2 protein was measured in hen ovaries at different ages. Normal ovaries of 2.5 year old hens (n=18) showed significantly higher expression of COX-2 protein compared to ovaries of 1 (n=20), 1.5 (n=20) and 2 (n=20) year old hens. A significant increase in expression of COX-2 protein was seen in 3 year old chickens (n=7) compared to 2.5 year old. a versus b and b versus c, P<0.001; Bars indicate standard error.
Fig. 6.
COX-2 mRNA expression in ovaries of hens aged 1 (n=20), 1.5 (n=20) and 2 (n=20) year was similar followed by a significant increase in normal ovaries of hens aged 2.5 (n=18) and 3 (n=7) year. Normal ovaries of 3.5 year old hens (n=10) had less COX-2 mRNA expression than 2.5 year old normal hens. a versus b and b versus c, P<0.01; c versus d. P<0.05; Bars indicate standard error.
Interestingly, there were no statistically significant differences in expression of both COX-2 protein and mRNA between birds with ovarian cancer and birds with normal ovaries at each time-point.
3.4. PGE2 concentration in ovary increases with age
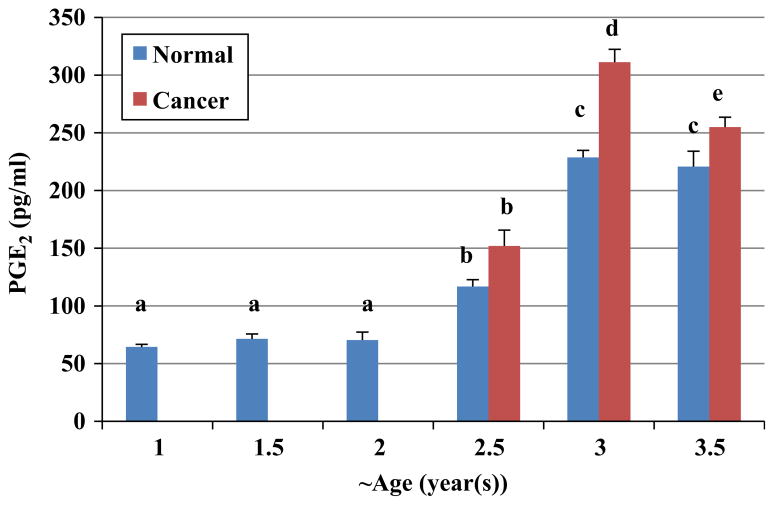
In parallel with the results that we obtained for COX enzymes expression, PGE2 was measured in ovaries of hens as a function of age. There were not any significant differences in concentration of PGE2 in normal ovaries of 1 (n=8), 1.5 (n=8) and 2 (n=8) year old hens. Significantly higher amounts of PGE2 were present in normal ovaries of 2.5 year old hens (n=8) compared to normal ovaries of 1 (n=8), 1.5 (n=8) and 2 year old (n=8) hens (P<0.01; Fig. 7). The concentration of PGE2 in normal ovaries of 3 year old hens (n=7) was higher than normal ovaries of 2.5 year old hens (P<0.001).
Fig. 7.
Comparison of prostaglandin E2 concentrations in ovaries of chicken at different ages. There was a significant increase in concentrations of PGE2 in normal ovaries of 2.5 year old hens (n=8) compared to ovaries of 1 (n=8) 1.5 (n=8) and 2 (n=8) year old birds. Normal ovaries of 3 year old hens (n=7) had the higher PGE2 concentrations than normal ovaries of 2.5 year old hens. Cancerous ovaries of 3 year old hens (n=8) had significantly more PGE2 concentrations compared to normal ovaries of hens aged the same (n=7). There was a significant difference between normal ovaries of 3.5 year old hens (n=8) and cancerous ovaries of hens at the same age (n=8). a versus b, c versus d, P<0.01; b versus c and b versus d, P<0.001; c versus e, P<0.05;Bars indicate standard error.
There was no significant difference in concentration of PGE2 between normal and cancerous ovaries of 2.5 year old birds; however cancerous ovaries of 3 year old hens (n=8) had significantly more PGE2 concentrations compared to normal ovaries of hens aged the same (n=7; P<0.01). Furthermore, cancerous ovaries of 3.5 year old hens (n=8) had significantly higher PGE2 concentration than normal ovaries of birds of the same age (n=8; P<0.05).
4. Discussion
Our goal was to examine the expression of COX-1, COX-2 and PGE2 in normal and cancerous hen ovaries and compare expression to age and ovarian cancer incidence. Here, we report that the incidence of ovarian cancer significantly increases with age in the laying hen. Also the expression of both COX-1 protein and mRNA was remarkably increased in normal ovaries of older hens compared to normal ovaries of younger hens. A significant increase was found in COX-1 protein and mRNA expression in cancerous ovaries compared to normal ovaries of old hens (aged 2.5, 3 and 3.5 years). Furthermore, normal ovaries of older hens had significantly higher COX-2 protein and mRNA expression compared to normal ovaries of younger hens indicating a positive correlation between age and COX-2 expression. There was no difference in COX-2 protein and mRNA expression between normal and cancerous ovaries of hens (P>0.05). In correlation with increased incidence of ovarian cancer and expression of both COX enzymes, PGE2 was elevated significantly with age. Similar to COX-1, cancerous ovaries of 3 and 3.5 year old hens had higher PGE2 concentrations than normal ovaries of hens at the same age.
We detected an increase in incidence of ovarian cancer in hens specifically at 3–3.5 years of age. The average age of menopause among women is 51 years [35], which closely precedes the average age of ovarian cancer diagnosis, which is 54 years [36]. The incidence of ovarian cancer continues to increase after menopause, and age—even more so than a family history of ovarian cancer—is the best prediction of ovarian cancer risk [37]. Ovarian cancer is mainly seen in older women and our results correlated with age related ovarian cancer incidence in humans. Many years of ovulation, longer exposure to environmental factors, ovarian aging and accumulated DNA damage might be associated with increased rate of ovarian cancer with age. Our finding was in agreement with others that have shown an increase in ovarian cancer incidence in hens after 2 years of age [38]. This finding further validates the chicken as a suitable model to study human ovarian cancer.
We detected an up-regulation of COX-1 protein and mRNA in ovarian cancer in the laying hen which is in correlation with our previous findings [16]. Elevation of COX-1 expression with age might be in respond to the high frequency of ovulation during the reproductive age which may help to establish a pro-carcinogenic microenvironment where malignant transformation of OSE originates. The age-associated increase in COX-derived products of arachidonic acid in humans has been previously reported [39–41]. Martinez et al. found that in stomach, small bowel and large bowel tissue COX-1 mRNA levels in the adult are significantly higher than in the newborn [42]. Kino et al. indicated that PGE2 synthesis in ovarian cancer is regulated by COX-1 enzyme [43]. Urick and Johnson reported that increased PGE2 levels in hen ovaries with tumors might be due to increased COX-1expression [17].
Our data indicated that the expression of COX-2 protein and mRNA were significantly increased with age in the hen ovaries. Similar to COX-1, COX-2 was up-regulated at the same age cancer was noted for the first time in hen ovaries; However, none of hens with ovarian cancer had greater COX-2 expression compared to age matched normal hens. COX-2 mediates the growth hormone regulated proliferation of granulosa cells in chickens [44]. Because chickens have a high ovulation frequency, high expression and accumulation of COX-2 in hen ovaries are possible. There are many reports on the role of COX-2 enzyme and its over-expression in cancer. Enhanced expression of COX-2 with age in murine spleen [45] and human papillary thyroid cancer [46] previously has been reported. Casolini et al. showed that inhibition of COX-2 reduces the age dependent increase of hippocampal inflammatory markers in rats [47] suggesting a natural tendency to offset the age-dependent increase in brain inflammatory process. Our findings are in agreement with previous reports regarding increase in expression of COX-2 mRNA in old mice compared to younger mice [39].
The normal ovaries of older chickens, which are highly susceptible to ovarian cancer, had greater PGE2 concentrations compared to younger hens. Also, comparing the older (3 and 3.5 year old) hens with ovarian cancer to normal ones at the same age revealed a significant elevation in PGE2 concentration. This finding was positively correlated to significantly increased expression of COX enzymes with age. PGE2 as a key pro-inflammatory lipid and the major prostaglandin has a predominant role in cancer initiation and development. Elevation of prostaglandin E2 in ovarian cancer [48] and an increase in incidence of, and mortality from neoplastic diseases with age have been previously reported [39]. Prostaglandins are potent mediators of intercellular communication, and high concentrations of PGE2 are believed to be immunosuppressive for T cell-mediated immunity [49], increase angiogenesis [50] and stimulate cell proliferation and inhibit apoptosis in ovarian cancer cell lines [51]. PGE2 mediated immune suppression may lead to increased susceptibility to tumor formation and impaired defense against previously formed tumors [17,52]. The increase in PGE2 at the age when cancer begins to increase implies that PGE2 is an important factor in early stages of ovarian carcinogenesis in the aging hen. This finding further establishes the link between PGE2 derived inflammation and consequent ovarian diseases with age.
Taken together, our results are consistent with the notion that the up-regulation of COX protein and mRNA levels with age is the main contributing factor in the age associated increase in prostaglandin E2. Our results demonstrate that the age-associated increases in PGE2 may correspond to the increased incidence of ovarian cancer suggesting that cumulative inflammation contributes to initiation and/or progression of ovarian cancer. The mechanism of an increase in expression of COX enzymes is not clear. However, several studies have reported increased production of ROS during aging indicating that enhanced expression of COX-2 mRNA with age might be due to increased production of ROS [53–55]. To our knowledge the present study provides the first insight into the age-related changes in the expression pattern of COX-1, COX-2, prostaglandin E2 levels and onset of ovarian cancer. These finding may provide the basis for clinical trials utilizing COX specific inhibitors or dietary intervention targeting prostaglandin biosynthesis for the prevention and treatment of ovarian cancer.
Acknowledgments
This work was funded by NIH/National Center for Complementary and Alternative Medicine Grant AT004085 (DBH) and American Institute for Cancer Research Grant 06-A043 (DBH). We are grateful for the lab management by Carrie Small and poultry farm management by Chet Utterback, Douglas Hilgendorf and Pam Utterback. We also thank Dr. Karen Hales for her suggestions and review of this manuscript.
References
- 1.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 2.Erdman SE, Poutahidis T. Cancer inflammation and regulatory T cells. Int J Cancer. 2010;127:768–779. doi: 10.1002/ijc.25430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balkwill F, Coussens LM. Cancer: an inflammatory link. Nature. 2004;431:405–406. doi: 10.1038/431405a. [DOI] [PubMed] [Google Scholar]
- 4.Allavena P, Garlanda C, Borrello MG, Sica A, Mantovani A. Pathways connecting inflammation and cancer. Curr Opin Genet Dev. 2008;18:3–10. doi: 10.1016/j.gde.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Zeldin DC. Epoxygenase pathways of arachidonic acid metabolism. J Biol Chem. 2001;276:36059–36062. doi: 10.1074/jbc.R100030200. [DOI] [PubMed] [Google Scholar]
- 6.Imig JD, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat Rev Drug Discovery. 2009;8:794–805. doi: 10.1038/nrd2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reese J, Zhao X, Ma WG, Brown N, Maziasz TJ, Dey SK. Comparative analysis of pharmacologic and/or genetic disruption of cyclooxygenase-1 and cyclooxygenase-2 function in female reproduction in mice. Endocrinology. 2001;142:3198–3206. doi: 10.1210/endo.142.7.8307. [DOI] [PubMed] [Google Scholar]
- 8.Dempke W, Rie C, Grothey A, Schmoll HJ. Cyclooxygenase-2: a novel target for cancer chemotherapy? J Cancer Res Clin Oncol. 2001;127:411–417. doi: 10.1007/s004320000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greene ER, Huang S, Serhan CN, Panigrahy D. Regulation of inflammation in cancer by eicosanoids. Prostaglandins Other Lipid Mediators. 2011;96:27–36. doi: 10.1016/j.prostaglandins.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams CS, Mann M, DuBois RN. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene. 1999;18:7908–7916. doi: 10.1038/sj.onc.1203286. [DOI] [PubMed] [Google Scholar]
- 11.Gupta RA, Tejada LV, Tong BJ, Das SK, Morrow JD, Dey SK, DuBois RN. Cyclooxygenase-1 is overexpressed and promotes angiogenic growth factor production in ovarian cancer. Cancer Res. 2003;63:906–911. [PubMed] [Google Scholar]
- 12.Daikoku T, Wang D, Tranguch S, Morrow JD, Orsulic S, DuBois RN, Dey SK. Cyclooxygenase-1 is a potential target for prevention and treatment of ovarian epithelial cancer. Cancer Res. 2005;65:3735–3744. doi: 10.1158/0008-5472.CAN-04-3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takeshita E, Osanai T, Higuchi T, Soumaoro LT, Sugihara K. Elevated cyclooxygenase-2 expression is associated with histological grade in invasive ductal breast carcinoma. J Med Dent Sci. 2005;52:189–193. [PubMed] [Google Scholar]
- 14.Wolff H, Saukkonen K, Anttila S, Karjalainen A, Vainio H, Ristimaki A. Expression of cyclooxygenase-2 in human lung carcinoma. Cancer Res. 1998;58:4997–5001. [PubMed] [Google Scholar]
- 15.Denkert C, Kobel M, Pest S, Koch I, Berger S, Schwabe M, Siegert A, Reles A, Klosterhalfen B, Hauptmann S. Expression of cyclooxygenase 2 is an independent prognostic factor in human ovarian carcinoma. Am J Pathol. 2002;160:893–903. doi: 10.1016/S0002-9440(10)64912-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hales DB, Zhuge Y, Lagman JA, Ansenberger K, Mahon C, Barua A, Luborsky JL, Bahr JM. Cyclooxygenases expression and distribution in the normal ovary and their role in ovarian cancer in the domestic hen (Gallus domesticus) Endocrine. 2008;33:235–244. doi: 10.1007/s12020-008-9080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Urick ME, Johnson PA. Cyclooxygenase 1 and 2 mRNA and protein expression in the Gallus domesticus model of ovarian cancer. Gynecol Oncol. 2006;103:673–678. doi: 10.1016/j.ygyno.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 18.Vanderhyden BC, Shaw TJ, Ethier JF. Animal models of ovarian cancer. Reprod Biol Endocrinol. 2003;1:67. doi: 10.1186/1477-7827-1-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yancik R, Ries LG. Cancer in the aged. An epidemiologic perspective on treatment issues. Cancer. 1991;68:2502–2510. doi: 10.1002/1097-0142(19911201)68:11+<2502::aid-cncr2820681504>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 20.Cramer DW, Hutchison GB, Welch WR, Scully RE, Ryan KJ. Determinants of ovarian cancer risk. I. Reproductive experiences and family history. J Natl Cancer Inst. 1983;71:711–716. [PubMed] [Google Scholar]
- 21.Beltsos AN, Odem RR. Ovulation induction and ovarian malignancy. Semin Reprod Endocrinol. 1996;14:367–374. doi: 10.1055/s-2008-1067981. [DOI] [PubMed] [Google Scholar]
- 22.Crum CP, Drapkin R, Miron A, Ince TA, Muto M, Kindelberger DW, Lee Y. The distal fallopian tube: a new model for pelvic serous carcinogenesis. Curr Opin Obstet Gynecol. 2007;19:3–9. doi: 10.1097/GCO.0b013e328011a21f. [DOI] [PubMed] [Google Scholar]
- 23.Kim J, Coffey DM, Creighton CJ, Yu Z, Hawkins SM, Matzuk MM. High-grade serous ovarian cancer arises from fallopian tube in a mouse model. Proc Natl Acad Sci USA. 2012;109:3921–3926. doi: 10.1073/pnas.1117135109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho KR, Shih I-M. Ovarian cancer. Ann Rev Pathol: Mech Dis. 2009;4:287–313. doi: 10.1146/annurev.pathol.4.110807.092246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurman RJ, Shih IeM. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34:433–443. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salvador S, Gilks B, Kobel M, Huntsman D, Rosen B, Miller D. The fallopian tube: primary site of most pelvic high-grade serous carcinomas. Int J Gynecol Cancer. 2009;19:58–64. doi: 10.1111/IGC.0b013e318199009c. [DOI] [PubMed] [Google Scholar]
- 27.Fathalla MF. Incessant ovulation—a factor in ovarian neoplasia? Lancet. 1971;2:163. doi: 10.1016/s0140-6736(71)92335-x. [DOI] [PubMed] [Google Scholar]
- 28.Landen CN, Jr, Birrer MJ, Sood AK. Early events in the pathogenesis of epithelial ovarian cancer. J Clin Oncol. 2008;26:995–1005. doi: 10.1200/JCO.2006.07.9970. [DOI] [PubMed] [Google Scholar]
- 29.Zhuge Y, Lagman JA, Ansenberger K, Mahon CJ, Daikoku T, Dey SK, Bahr JM, Hales DB. CYP1B1 expression in ovarian cancer in the laying hen Gallus domesticus. Gynecol Oncol. 2009;112:171–178. doi: 10.1016/j.ygyno.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ansenberger K, Zhuge Y, Lagman JA, Richards C, Barua A, Bahr JM, Hales DB. E-cadherin expression in ovarian cancer in the laying hen, Gallus domesticus, compared to human ovarian cancer. Gynecol Oncol. 2009;113:362–369. doi: 10.1016/j.ygyno.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barua A, Bitterman P, Abramowicz JS, Dirks AL, Bahr JM, Hales DB, Bradaric MJ, Edassery SL, Rotmensch J, Luborsky JL. Histopathology of ovarian tumors in laying hens: a preclinical model of human ovarian cancer. Int J Gynecol Cancer. 2009;19:531–539. doi: 10.1111/IGC.0b013e3181a41613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ansenberger K, Richards C, Zhuge Y, Barua A, Bahr JM, Luborsky JL, Hales DB. Decreased severity of ovarian cancer and increased survival in hens fed a flaxseed-enriched diet for 1 year. Gynecol Oncol. 2010;117:341–347. doi: 10.1016/j.ygyno.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lynch MJ. Medical Laboratory Technology and Clinical Pathology. 2. Saunders (W.B.) Co Ltd; 1969. Revised edition edition. [Google Scholar]
- 34.Skeehan DC, Hrapchak BB. Theory and Practice of Histotechnology. Mosby; St. Louis: 1973. Theory and practice of histotechnology; pp. 111–112. [Google Scholar]
- 35.Gosden RG. The Biology of Menopause: The causes and consequences of ovarian aging. Academic Press; New York: 1985. [Google Scholar]
- 36.Lobo RA, Kelsey J, Marcus R. Menopause: Biology and pathobiology. 1. Academic press; Burlington, MA: 2000. [Google Scholar]
- 37.Smith ER, Xu XX. Ovarian ageing, follicle depletion, and cancer: a hypothesis for the aetiology of epithelial ovarian cancer involving follicle depletion. Lancet Oncol. 2008;9:1108–1111. doi: 10.1016/S1470-2045(08)70281-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fredrickson TN. Ovarian tumors of the hen. Environ Health Perspect. 1987;73:35–51. doi: 10.1289/ehp.877335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hayek MG, Mura C, Wu D, Beharka AA, Han SN, Paulson KE, Hwang D, Meydani SN. Enhanced expression of inducible cyclooxygenase with age in murine macrophages. J Immunol. 1997;159:2445–2451. [PubMed] [Google Scholar]
- 40.Wilson TW, McCaulay FA, Waslen TA. Effects of aging on responses to furosemide. Prostaglandins. 1989;38:675–687. doi: 10.1016/0090-6980(89)90049-x. [DOI] [PubMed] [Google Scholar]
- 41.Vericel E, Croset M, Sedivy P, Courpron P, Dechavanne M, Lagarde M. Platelets and aging. I—Aggregation, arachidonate metabolism and antioxidant status. Thromb Res. 1988;49:331–342. doi: 10.1016/0049-3848(88)90313-1. [DOI] [PubMed] [Google Scholar]
- 42.Martinez FE, Reno C, Trevenen CL, Hart DA, Belik J. Age-dependent changes in the regulation of cyclooxygenases in the gastrointestinal tract after gram-negative endotoxemia. J Pediatr Gastroenterol Nutr. 2001;33:165–170. doi: 10.1097/00005176-200108000-00013. [DOI] [PubMed] [Google Scholar]
- 43.Kino Y, Kojima F, Kiguchi K, Igarashi R, Ishizuka B, Kawai S. Prostaglandin E2 production in ovarian cancer cell lines is regulated by cyclooxygenase-1, not cyclooxygenase-2. Prostaglandins Leukotrienes Essent Fatty Acids. 2005;73:103–111. doi: 10.1016/j.plefa.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 44.Jin Y, Zhang C, Zeng W, Taya K, Tan TQ. Interactive actions of prostaglandin and epidermal growth factor to enhance proliferation of granulosa cells from chicken prehierarchical follicles. Prostaglandins Other Lipid Mediators. 2007;83:285–294. doi: 10.1016/j.prostaglandins.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 45.Rosenstein MM, Strausser HR. Macrophage-induced T cell mitogen suppression with age. J Reticuloendothel Soc. 1980;27:159–166. [PubMed] [Google Scholar]
- 46.Siironen P, Ristimaki A, Nordling S, Louhimo J, Haapiainen R, Haglund C. Expression of COX-2 is increased with age in papillary thyroid cancer. Histopathology. 2004;44:490–497. doi: 10.1111/j.1365-2559.2004.01880. [DOI] [PubMed] [Google Scholar]
- 47.Casolini P, Catalani A, Zuena AR, Angelucci L. Inhibition of COX-2 reduces the age-dependent increase of hippocampal inflammatory markers, corticosterone secretion, and behavioral impairments in the rat. J Neurosci Res. 2002;68:337–343. doi: 10.1002/jnr.10192. [DOI] [PubMed] [Google Scholar]
- 48.Kushlinskii NE, Podistov IuI, Laktionov KP, Karseladze AI, Babkina IV, Kerimova GI. Prostaglandins E in the primary tumor, metastases, and ascitic fluid of patients with ovarian cancer. Biull Eksp Biol Med. 1997;123:83–86. [PubMed] [Google Scholar]
- 49.Goodwin JS, Ceuppens J. Regulation of the immune response by prostaglandins. J Clin Immunol. 1983;3:295–315. doi: 10.1007/BF00915791. [DOI] [PubMed] [Google Scholar]
- 50.Brecht K, Weigert A, Hu J, Popp R, Fisslthaler B, Korff T, Fleming I, Geisslinger G, Brune B. Macrophages programmed by apoptotic cells promote angiogenesis via prostaglandin E2. FASEB J. 2011;25:2408–2417. doi: 10.1096/fj.10-179473. [DOI] [PubMed] [Google Scholar]
- 51.Munkarah AR, Morris R, Baumann P, Deppe G, Malone J, Diamond MP, Saed GM. Effects of prostaglandin E(2) on proliferation and apoptosis of epithelial ovarian cancer cells. J Soc Gynecol Invest. 2002;9:168–173. [PubMed] [Google Scholar]
- 52.Fischer SM. Prostaglandins and cancer. Front Biosci. 1997;2:d482–d500. doi: 10.2741/a207. [DOI] [PubMed] [Google Scholar]
- 53.Sohal RS, Orr WC. Role of oxidative stress in senescence. Aging (Milano) 1998;10:149–151. [PubMed] [Google Scholar]
- 54.Beharka AA, Wu D, Serafini M, Meydani SN. Mechanism of vitamin E inhibition of cyclooxygenase activity in macrophages from old mice: role of peroxynitrite. Free Radical Biol Med. 2002;32:503–511. doi: 10.1016/s0891-5849(01)00817-6. [DOI] [PubMed] [Google Scholar]
- 55.Feng L, Xia Y, Garcia GE, Hwang D, Wilson CB. Involvement of reactive oxygen intermediates in cyclooxygenase-2 expression induced by interleukin-1, tumor necrosis factor-alpha, and lipopolysaccharide. J Clin Invest. 1995;95:1669–1675. doi: 10.1172/JCI117842. [DOI] [PMC free article] [PubMed] [Google Scholar]