Abstract
Objective
Determine the effects on the vaccine cold chain of making different types of World Health Organization (WHO) Expanded Program on Immunizations (EPI) vaccines thermostable.
Methods
Utilizing a detailed computational, discrete-event simulation model of the Niger vaccine supply chain, we simulated the impact of making different combinations of the six current EPI vaccines thermostable.
Findings
Making any EPI vaccine thermostable relieved existing supply chain bottlenecks (especially at the lowest levels), increased vaccine availability of all EPI vaccines, and decreased cold storage and transport capacity utilization. By far, the most substantial impact came from making the pentavalent vaccine thermostable, increasing its own vaccine availability from 87% to 97% and the vaccine availabilities of all other remaining non-thermostable EPI vaccines to over 93%. By contrast, making each of the other vaccines thermostable had considerably less effect on the remaining vaccines, failing to increase the vaccine availabilities of other vaccines to more than 89%. Making tetanus toxoid vaccine along with the pentavalent thermostable further increased the vaccine availability of all EPI vaccines by at least 1–2%.
Conclusion
Our study shows the potential benefits of making any of Niger’s EPI vaccines thermostable and therefore supports further development of thermostable vaccines. Eliminating the need for refrigerators and freezers should not necessarily be the only benefit and goal of vaccine thermostability. Rather, making even a single vaccine (or some subset of the vaccines) thermostable could free up significant cold storage space for other vaccines, and thereby help alleviate supply chain bottlenecks that occur throughout the world.
Keywords: thermostable, vaccines, supply chain
INTRODUCTION
The dearth of reliable methods of keeping vaccines adequately cool or cold in many parts of the world has motivated calls for the development of thermostable vaccines, i.e., vaccines that do not degrade under heat or excessive cold exposure. To reach its destination, a vaccine travels through a country's vaccine supply chain (i.e., the series of storage locations and transport vehicles needed to get vaccines from manufacturers to vaccine recipients), and can traverse great distances and different climates. Many locations do not have reliable refrigerators or freezers (i.e., cold storage) or reliable means of monitoring temperatures in these devices [1–2]. Even when cold storage is present, space within cold storage may be limited or power availability may be intermittent and capricious[3]. One study in particular conducted in Indonesia found that 75% of vaccine lots shipped from the central level to peripheral levels were exposed to adverse temperatures[4]. Currently, thermostable formulations of a number of vaccines are under development including hepatitis B; diphtheria, tetanus and pertussis (DTP); and pentavalent (DTP, hepatitis B, Haemophilus influenza type b) vaccines[3].
However, some have raised concerns that unless all vaccines in a country's immunization program become thermostable, the benefit of making individual vaccines thermostable will be limited since the country will still have to maintain a cold chain for the non-thermostable vaccines[5]. In other words, unless technological advances can make all vaccines thermostable, developing individual thermostable vaccines may not be worth the effort. This is based on the belief that the greatest value of thermostable vaccines is the potential to eliminate the need for the cold chain, which is very costly and difficult to maintain.
Nevertheless, making individual vaccines thermostable could still have benefits if their removal from the cold chain could relieve bottlenecks in the vaccine supply chain. Therefore, our Vaccine Logistics Modeling team developed a computational model of Niger to simulate the effects of making different World Health Organization (WHO) Expanded Program on Immunizations (EPI) vaccines thermostable[6]. Many health centers in Niger have unreliable power or lack cold chain infrastructure, and therefore face difficulties in maintaining vaccines within 2–8°C[7].
METHODS
Niger Vaccine Supply Chain Model
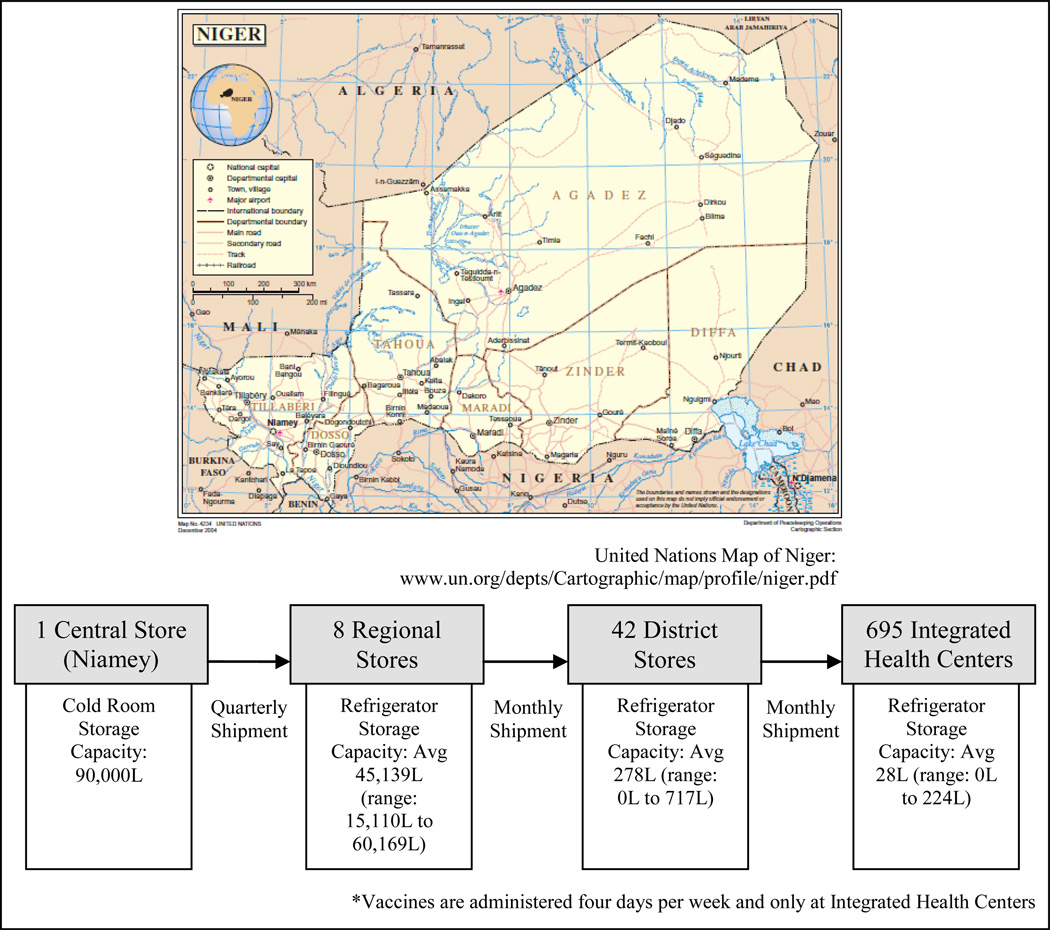
Our team constructed a detailed discrete-event simulation computational model of the Niger vaccine supply chain utilizing our Highly Extensible Resource for Modeling Supply Chains (HERMES) framework. HERMES is programmed in Python and uses features provided by the SimPy package. Previously published studies detail the structure, data, and assumptions of our Niger supply chain model, which represents every storage location, refrigerator, freezer and transport device in the Niger supply chain as well as shipping schedules and policies and every EPI vaccine vial flowing through the supply chain (Figure 1)[6, 8]. This includes Bacille Calmette-Guerin (BCG), pentavalent, yellow fever (YF), oral polio virus (OPV), tetanus toxoid (TT), and measles (M). The model includes the type, make, model, age, and the specific capacity of every single cold room, refrigerator, and freezer at each location in the Niger supply chain with data collected by the 2008 WHO Cold Chain Inventory, verified and augmented by 2009 team visits. Each vaccine vial has a specified lifetime during which the vaccine remains effective. Exposure to temperatures beyond a vial’s recommended range shortens this lifetime. Each immunization day, virtual patients arrive to be vaccinated based on census and birth data from Niger. Personnel at each location open and reconstitute (if necessary) a vaccine vial to immunize a child. Any reconstituted but unused doses are discarded at the end of the session.
Figure 1.
The Structure of Niger’s Vaccine Cold Chain
Converting Vaccines into Thermostable Formulations
The packaged volumes and temperature profile for each vaccine (Table 1) govern where (freezer versus refrigerator versus room temperature) each vaccine can and should be kept while in storage or transport[9–10]. It also determines the shelf-life of a vaccine. Converting a vaccine into a thermostable formulation means that the vaccine no longer requires cold storage or could be kept out of cold storage long enough to traverse locations and routes that may experience cold storage constraints resulting in bottlenecks and would have a shelf-life equivalent to established upper limits. Our experiments involved making various combinations of the EPI vaccines thermostable.
Table 1.
Niger’s EPI Vaccine Characteristics
| Expanded Program on Immunization (EPI) Vaccine |
Immunization Schedule |
Doses per person |
Doses per vial |
Packaged volume per dose (cm3) |
Packaged volume per diluent (cm3) |
Storage | Source |
|---|---|---|---|---|---|---|---|
|
Bacille Calmette- Guerin (BCG) |
birth | 1 | 20 | 1.2 | 0.7 | 2–8°C | a, b |
|
Diphtheria- tetanus- pertussis- hepatitis B- haemophilus influenza type B (Pentavalent) |
6, 10, 14 weeks | 3 | 1 | 16.8 | none | 2–8°C | a, b |
|
Yellow Fever (YF) |
9 months | 1 | 10 | 2.5 | 6 | 2–8°C | a, b |
|
Oral Polio Vaccine (OPV) |
0, 6, 10, 14 weeks |
4 | 20 | 1 | none | −20°C* | a, b |
|
Tetanus Toxoid (TT) |
0, 4 weeks, 6, 12 months |
5 | 10 | 3 | none | 2–8°C | a, b |
| Measles (M) | 9 months | 1 | 10 | 2.6 | 0.5 | 2–8°C | a, b |
OPV is stored in the refrigerator (2–8°C) in integrated health centers.
WHO. Immunization Profile: Niger. 2010 [cited 2010 27 October]; Available from: http://apps.who.int/immunization_monitoring/en/globalsummary/countryprofileresult.cfm?C='ner'
WHO. WHO Prequalified Vaccines. 2010 [cited 2010; Available from: http://www.who.int/immunization_standards/vaccine_quality/PQ_vaccine_list_en/en/index.html
Measured Outcomes
Each simulation experiment generated the following measures of supply chain performance:
-
Vaccine Availability (for each vaccine type at each immunization location)
This measures the proportion of patients arriving at each immunization session for which vaccines were available for administration. This is calculated by the following:- Vaccine Availability = Number of patients receiving vaccine/Number of patients presenting at immunization locations to be vaccinated
-
Transport Capacity Utilization
This measures the amount of cold storage space used by vaccines during transportation for each transport device (e.g., truck, cold box, or vaccine carrier). This is calculated by the following:- Transport Capacity Utilization = Transport space consumed in vehicle per shipment/Total transport space available in vehicle per shipment
-
Storage Capacity Utilization
This measures the proportion of available storage space used by vaccines in cold rooms, refrigerators, and freezers at each level in the supply chain. This is calculated by the following:- Storage Capacity Utilization = Storage space consumed in cold chain equipment per storage period/Total storage space available in cold chain equipment
For transport, thermostable vaccines could then be placed in one of the following alternative locations: (1) the remaining space in the bed of a 4×4 truck, (2) the backseat of a double cab, or (3) a transport vehicle/mode that is not currently part of the cold chain but may be easier to utilize.
Sensitivity Analyses
Sensitivity analyses explored the impact of varying storage capacity utilization and transport capacity utilization (range: 50% to 100%). Previously published studies have tested the model on additional parameters, including inventory loss, shipping demand, population demand and percent of target population arriving to be vaccinated [6, 8].
Computational Details
Each simulation run represented vaccine supply chain operations for one year. Reported results are means over ten runs.
RESULTS
Making any of the EPI vaccines thermostable had a positive impact on the supply chain, relieving existing bottlenecks (especially at the district and integrated health center levels), increasing the vaccine availabilities of all EPI vaccines and decreasing the cold capacity utilization during both storage and transport. Reducing the available capacity to 50% or 85% did not significantly affect the pattern of results (i.e., which vaccine removals have the greatest or least effects). However, reducing the capacity to 50% available space resulted in a greater number of bottlenecks at storage locations and along transport segments, considerably lowering vaccine availabilities. Due to the fact that as available storage capacity decreases, the benefit of a thermostable vaccine increases, we take a conservative approach and report scenarios with 100% available storage capacity. We also assume a 1% vaccine inventory loss rate and a 1% vaccine transport loss rate from expiration or mishandling of vaccines. All results reported in the manuscript have standard deviations less than 1%, while cold capacity utilization (presented in Tables 2 and 3) report standard deviations greater than 1%.
Table 2.
The Effect of Thermostable Vaccines on Vaccine Storage Capacity Utilization
| EPI Vaccines that are Thermostable |
Mean Central Cold Storage Capacity* |
Mean Regional Cold Storage Capacity (range) |
SD‡ | Mean District Cold Storage Capacity (range) |
SD‡ | Mean IHC Cold Storage Capacity (range) |
SD‡ |
|---|---|---|---|---|---|---|---|
| None | 62% | 12% (1 – 28) | 10% | 63% (3 – 100) | 32% | 42% (2 – 99) | 25% |
| Pentavalent | 25% | 4% (1 – 11) | 4% | 34% (1 – 100) | 26% | 29% (0 – 79) | 18% |
| TT | 55% | 10% (1 – 25) | 9% | 59% (2 – 100) | 32% | 39% (1 – 99) | 24% |
| YF | 56% | 10% (1 – 24) | 9% | 59% (2 – 100) | 31% | 32% (2 – 97) | 21% |
| BCG | 56% | 10% (1 – 24) | 9% | 59% (2 – 100) | 32% | 39% (0 – 99) | 24% |
| M | 56% | 10% (1 – 24) | 9% | 60% (2 – 100) | 31% | 40% (3 – 99) | 25% |
| OPV | 60% | 11% (1 – 27) | 10% | 62% (2 – 100) | 32% | 40% (2 – 99) | 25% |
| Pentavalent, TT | 19% | 3% (0 – 8) | 3% | 27% (1 – 100) | 22% | 24% (0 – 58) | 15% |
| Pentavalent, TT, YF | 12% | 2% (0 – 6) | 2% | 18% (1 – 72) | 15% | 12% (0 – 29) | 7% |
| Pentavalent, TT, YF, BCG | 7% | 1% (0 – 3) | 1% | 10% (0 – 40) | 9% | 7% (0 – 16) | 4% |
| Pentavalent, TT, YF, BCG, M | 2% | 0% (0 – 1) | 0% | 3% (0 – 11) | 2% | 3% (0 – 7) | 2% |
| All | 0% | 0%(0 – 0) | 0% | 0%(0 – 0) | 0% | 0%(0 – 0) | 0% |
The central store is in a single location; therefore, it does not have a range or standard deviation.
Standard deviation
Table 3.
The Effect of Thermostable Vaccines on Vaccine Transport Capacity Utilization
| EPI Vaccines that are Thermostable |
Mean Cold Truck Capacity (range) |
SD‡ | Mean 4×4 Truck Capacity (range) |
SD‡ | Mean Vaccine Carrier Capacity (range) |
SD‡ |
|---|---|---|---|---|---|---|
| None | 63% (57 – 70) | 7% | 70% (4 – 592) | 86% | 158% (79 – 303) | 44% |
| Pentavalent | 27% (24 – 30) | 3% | 32% (2 – 249) | 36% | 71% (51 – 91) | 11% |
| TT | 57% (51 – 63) | 6% | 64% (3 – 531) | 77% | 139% (71 – 257) | 35% |
| YF | 57% (51 – 623) | 6% | 64% (3 – 532) | 77% | 141% (57 – 283) | 41% |
| BCG | 57% (51 – 63) | 6% | 65% (3 – 535) | 78% | 142% (59 – 284) | 42% |
| M | 58% (52 – 64) | 6% | 65% (3 – 540) | 79% | 144% (60 – 287) | 42% |
| OPV | 61% (55 – 68) | 7% | 68% (4 – 571) | 83% | 149% (75 – 287) | 41% |
| Pentavalent, TT | 20% (10 – 22) | 2% | 24% (1 – 188) | 27% | 54% (44 – 68) | 8% |
| Pentavalent, TT, YF | 14% (12 – 15) | 1% | 16% (1 – 129) | 19% | 37% (30 – 49) | 6% |
| Pentavalent, TT, YF, BCG | 8% (7 – 9) | 1% | 9% (0 – 72) | 10% | 21% (15 – 31) | 4% |
| Pentavalent, TT, YF, BCG, M | 2%(2 – 2) | 0% | 3% (0 – 20) | 3% | 9% (3 – 15) | 4% |
| All | 0%(0 – 0) | 0% | 0%(0 – 0) | 0% | 0%(0 – 0) | 0% |
Standard Deviation
Making a Single Vaccine Thermostable
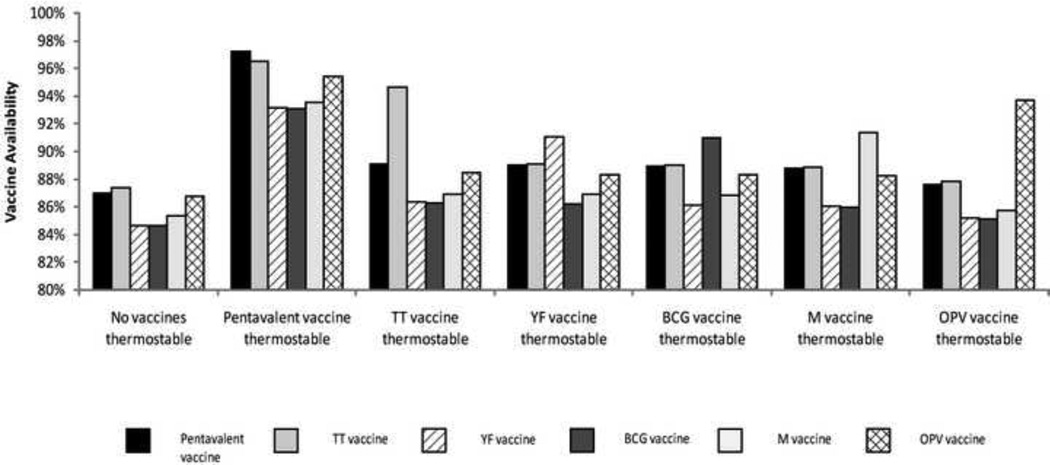
By far the most substantial impact came from making the pentavalent thermostable. Figure 2 shows how making each EPI vaccine thermostable affects the vaccine availabilities of all the other EPI vaccines. As seen in Figure 2, a thermostable pentavalent increased its own vaccine availability from 87% to 97% and the vaccine availabilities of all other remaining non-thermostable EPI vaccines to over 93%. While each thermostable vaccine increased its own vaccine availability significantly more than it increased the remaining vaccines, a thermostable pentavalent increased all remaining vaccines more than their respective thermostable forms could increase their vaccine availability. Removing the pentavalent had the largest impact on relieving bottlenecks in storage at the district level. Without these storage bottlenecks, more vaccine could be held at district stores. While transport bottlenecks exist between the district and IHC levels, shipments are collection-based, which means that even though the first shipments are overfilled, they can return to collect additional vaccines in subsequent shipments. The thermostable pentavalent increased the TT availability from 87% to 97% (versus a thermostable TT which increased the TT availability to only 95%), the YF availability from 85% to 93% (versus a thermostable YF which increased the YF availability to only 91%), the BCG availability from 85% to 93% (versus a thermostable BCG which increased the BCG availability to only 91%), the M availability from 85% to 94% (versus a thermostable M which increased the M availability to only 92%), and the OPV availability from 87% to 95% (versus a thermostable OPV which increased the OPV availability to only 94%). By contrast, making each of the other vaccines thermostable had considerably less effect on the remaining EPI vaccines, failing to increase the vaccine availabilities of other vaccines to more than 89%.
Figure 2.
The Effect of Making a Single Vaccine Thermostable on the Vaccine Availabilities of all WHO EPI Vaccines
The effects of making TT, YF, BCG or M thermostable were fairly comparable. Making the OPV thermostable had the least effect. Of these latter vaccines, TT had the greatest effect due to its relatively large size and number of doses per fully immunized child (FIC). The vaccine availability never reaches 100% because some vaccines are lost due to breakage along the way or open vial waste, with the highest rates for those vaccines requiring reconstitution; BCG, M, and YF at 84%, 68%, and 68%, respectively. Additionally, a 25% buffer vaccine stock ordering policy is implemented throughout the supply chain, which over-supplies some locations and under-supplies others. Finally, as all vaccines are transported together in the same vehicle from location to location, regardless of their temperature profile, transport bottlenecks still exist, preventing all vaccines from reaching their target patients.
Table 2 delineates the impact on the mean (as well as the minimum and maximum) cold storage capacity utilization, at all levels when different vaccines are made thermostable. With vaccine availabilities, the greatest benefit occurred when the pentavalent became thermostable and the least benefit occurred when the OPV became thermostable.
As seen in Table 3, the most significant bottleneck is cold transport capacity. At baseline, transport capacity utilization for many shipping routes exceeds 100% with each percentage above 100% representing transport overfill, i.e., vaccines that cannot be shipped due to lack of space. Making pentavalent thermostable reduced transport capacity utilization in most locations below 100%, meaning that occasions when vaccines cannot be shipped from being common to being rare. Making other vaccines thermostable did alleviate transport bottlenecks but not to the level of the pentavalent.
The substantial impact of making the pentavalent thermostable stems from its large size per dose (i.e., the only single dose vial in the EPI) and frequency of administration, i.e., three doses per person (Table 1). Therefore, the pentavalent occupies a disproportionately large amount of space in the cold chain. The TT, YF, BCG and M are more similar in size (range of packaged volume per dose: 1.0–3.0cm3) and regimen and therefore had similar effects across all levels. The least effect came from making the OPV thermostable. OPV is stored in freezers for the majority of the supply chain until the IHC level where it is stored in the refrigerator and therefore competes for space with other vaccines.
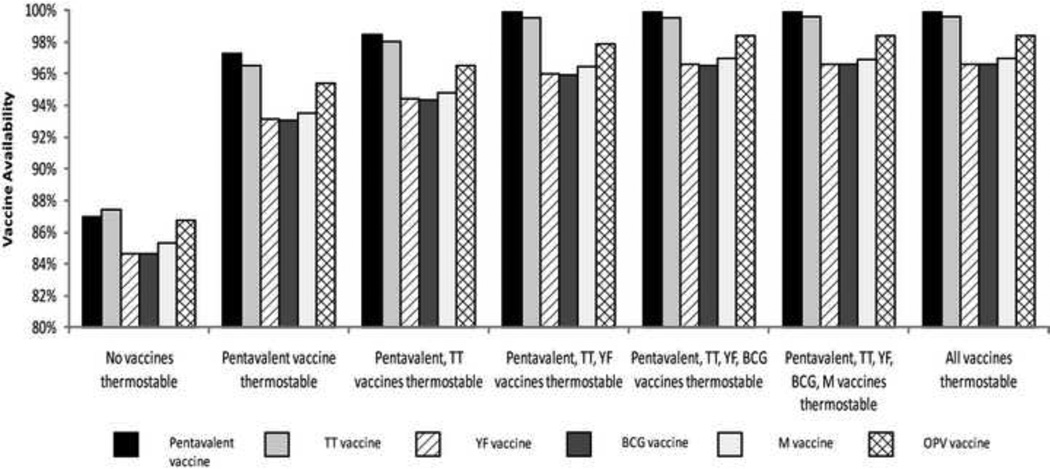
Making More than One Vaccine Thermostable
If making the pentavalent thermostable has by far the greatest impact, what gains are possible from making other vaccines thermostable in addition to the pentavalent? As Figure 2 shows, making TT along with the pentavalent thermostable further increased the vaccine availability of all EPI vaccines by at least 1–2%. Additional gains occurred when YF is added to the thermostable group. However, after these three vaccines (i.e., pentavalent, TT and YF) became thermostable, benefits were minimal by making the BCG, M or OPV thermostable (unless of course the need for the cold chain is completely eliminated, or if there are regular campaigns which are relatively common with M and OPV). This is because making the pentavalent, TT, and YF thermostable pushed the vaccine availabilities of all vaccines close to 96% with the remaining 4% due to factors unrelated to thermostability such as breakage and open vial waste, with the highest waste estimates being for those vaccines requiring reconstitution; e.g., BCG, M, and YF at 85%, 68%, and 68%, respectively. The order in which vaccines were removed from the cold chain was determined based on which vaccines had the greatest impact on alleviating bottlenecks and increasing vaccine availability, which largely depends on a vaccines packaged volume per dose and number of doses per FIC.
Storage and transport capacity utilization followed a similar trend as seen in Tables 2 and 3 respectively. Thermostable pentavalent, TT, and YF essentially brought storage and transport cold capacity utilization across all levels well below 100%. Of course, making all vaccines thermostable brought cold capacity utilization down to 0% everywhere.
DISCUSSION
Our results support the further development of thermostable vaccines by demonstrating that even making only one vaccine thermostable could help alleviate bottlenecks. Eliminating the need for refrigerators and freezers should not necessarily be the only benefit and goal of vaccine thermostability. Even countries with currently well-functioning supply chains could experience bottlenecks when new vaccines (rotavirus, pneumococcal, dengue) are introduced over the next decade[11].
While making vaccines truly thermostable for extensive durations is very challenging, promising steps have occurred. In recent years, researchers have explored both liquid and freeze dried possibilities[3]. So far, the hepatitis B formulation has shown the most promise[12]. While Cambridge Biostability's work towards stabilizing the pentavalent was terminated, others are now taking on the challenge[13–14]. Trials of a needle-free and thermostable aerosol formulation of the measles vaccine have yielded encouraging immunogenic responses[15]. Nova Laboratories continues research on spray-dried powder vaccines that would remain viable at even elevated temperatures[16]. International interest and funding (e.g., Bill and Melinda Gates Foundation Grand Challenges in Global Health and the Wellcome Trust) has grown over the past several years[17–18]. Our previous studies show that introductions of new vaccines (e.g., pneumococcal and rotavirus) could disrupt the cold chain. Making these new vaccines thermostable could prevent these disruptions. Our study also suggests that there is value even when vaccines can be kept out of the cold chain just long enough to traverse locations and routes that have cold storage constraints (e.g., a few days for transport routes and at most a month for most storage locations).
A key finding from this study is the dynamic benefits that making vaccines thermostable would have not only on delivery of the thermostable vaccine but also all other vaccines in the supply chain. While one may expect that making larger vaccines (e.g., pentavalent) thermostable would have greater benefits on vaccine distribution, it can be difficult quantifying the full scope of these effects without using a detailed supply chain simulation model of the supply chain. Simpler "back-of-the-envelope" calculations likely will miss and underestimate many of these benefits. Of course, making vaccines thermostable is certainly not the only or necessarily the best way of relieving supply chain bottlenecks (e.g., changing vaccine vial sizes or ordering policies)[19–20]. Making vaccines thermostable in combination with other measures could further improve supply chain performance. Our HERMES platform could serve as virtual laboratory to determine these potentially additive (or even multiplicative) benefits in future studies.
Our study also may help funders, policy makers, scientists, and manufacturers prioritize which vaccines to make thermostable, delineating the potential differential benefits of each on the vaccine supply chain. It favors prioritizing development of a thermostable pentavalent over that of other EPI vaccines. Of course, many other factors affect the prioritization of vaccine thermostability research. Certainly, technical feasibility is paramount. Disease burden also can help guide prioritization. Efforts to reduce vaccine wastage and increase vaccine accessibility also influence decisions in regards to changing the way the supply chain works. An economic study conducted in Cambodia, Ghana and Bangladesh of single dose thermostable vaccines proved to be cost-effective by both WHO standards and the World Bank[21]. However, while this evidence is helpful, it is difficult to accurately estimate pricing of such vaccines. In addition, clinical trials will also affect the overall program costs[3].
While technical feasibility and disease burden should be the primary drivers, ultimately, effects on the vaccine supply chain should also play a role on guiding vaccine thermostability research agendas. Neglecting supply chain effects can lead to unpleasant surprises, as evidenced by the rotavirus vaccine introduction in Latin America which unexpectedly caused supply chain bottlenecks[22]. After all, vaccines must be able to reach the population to be effective. Since retrospective and prospective supply chain studies can be prohibitively costly, impractical and difficult to perform, computational models of vaccine supply chains can play an important role in predicting the potential impact of new vaccine technology introduction[11].
Limitations
All models are simplified representations of real life and cannot capture every detail and possibility associated with the vaccine cold chain[23–24]. Our experiments assumed that the physical characteristics (e.g., size and number of doses per vial) of a vaccine would remain the same for thermostable formulations. Another assumption is that transport vehicles would have adequate non-cold storage capacity to carry thermostable vaccines, based on calculations of the available storage space (e.g., the back of a 4 × 4 truck minus what would be occupied by cold boxes). However, in reality, the vehicles may be carrying other items (e.g., other medical devices, tools, dried foods, etc.) that occupy non-cold space. Our study in fact may underestimate the logistical benefits of thermostable vaccines. It assumed that refrigerators and freezers in the Niger supply chain were fully functioning and able to maintain their prescribed temperatures. Instead, many refrigerators and freezers experience temperature fluctuations above and below their indicated ranges[25]. Many refrigerators and freezers are older and can malfunction. Power sources can be unreliable.
CONCLUSION
Our study shows the potential benefits of making any vaccine thermostable and therefore supports further development of thermostable vaccines. Eliminating the entire cold chain should not necessarily be the only benefit and goal of vaccine thermostability. Indeed, making single vaccines thermostable could help alleviate supply chain bottlenecks throughout the world. Our study also may help funders, policy makers, scientists, and manufacturers prioritize which vaccines to make thermostable, delineating the potential differential benefits of each on the vaccine supply chain. It favors prioritizing development of a thermostable pentavalent vaccine over that of other EPI vaccines.
Figure 3.
The Effect of Making Multiple Vaccines Thermostable on the Vaccine Availabilities of all WHO EPI Vaccines
Highlights.
HERMES computational simulation model of Niger’s vaccine supply chain.
Simulated the impact of making different combinations of vaccines thermostable.
Making any EPI vaccine thermostable relieved existing supply chain bottlenecks.
Making any EPI vaccine thermostable increased vaccine availability of all vaccines.
The most substantial impact came from making the pentavalent vaccine thermostable.
ACKNOWLEDGEMENTS
The HERMES logistics modeling team consists of (in alphabetical order): Tina-Marie Assi, PhD, Shawn T. Brown, PhD, Brigid E. Cakouros, MPH, Sheng-I Chen, PhD, Diana L. Connor, MPH, Erin G. Claypool, PhD, Leila A. Haidari, BS, Veena Karir, PharmD, Bruce Y. Lee, MD, MBA, Jim Leonard, Leslie E. Mueller, BS, Bryan A. Norman, PhD, Proma Paul, MHS, Jayant Rajgopal, PhD, Michelle M. Schmitz, BA, Rachel B. Slayton, PhD, Angela R. Wateska, MPH, Joel S. Welling, PhD, and Yu-Ting Weng, MS. This study was supported by the Vaccine Modeling Initiative (VMI), funded by the Bill and Melinda Gates Foundation and the National Institute of General Medical Sciences Models of Infectious Disease Agent Study (MIDAS) grant 1U54GM088491-0109. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. No other financial disclosures were reported by the authors of this paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Brigid E. Cakouros, Email: bec16@pitt.edu.
Tina-Marie Assi, Email: tina-marie.assi@mail.mcgill.ca.
Diana L. Connor, Email: diana_connor@hotmail.com.
Joel Welling, Email: welling@psc.edu.
Souleymane Kone, Email: kones@who.int.
Ali Djibo, Email: ali_djibo@yahoo.fr.
Angela R. Wateska, Email: arw38@pitt.edu.
Lionel Pierre, Email: lionel@logisticsforhealth.com.
Shawn T. Brown, Email: stbrown@psc.edu.
REFERENCES
- 1.Kartoglu U, Nelaj E, Maire D. Improving temperature monitoring in the vaccine cold chain at the periphery: an intervention study using a 30-day electronic refrigerator temperature logger (Fridge-tag) Vaccine. 2010 May 28;28(24):4065–4072. doi: 10.1016/j.vaccine.2010.03.076. [DOI] [PubMed] [Google Scholar]
- 2.Ren Q, Xiong H, Li Y, Xu R, Zhu C. Evaluation of an outside-the-cold-chain vaccine delivery strategy in remote regions of western China. Public Health Rep. 2009 Sep-Oct;124(5):745–750. doi: 10.1177/003335490912400517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen D, Kristensen D. Opportunities and challenges of developing thermostable vaccines. Expert Rev Vaccines. 2009 May;8(5):547–557. doi: 10.1586/erv.09.20. [DOI] [PubMed] [Google Scholar]
- 4.Nelson CM, Wibisono H, Purwanto H, Mansyur I, Moniaga V, Widjaya A. Hepatitis B vaccine freezing in the Indonesian cold chain: evidence and solutions. Bull World Health Organ. 2004 Feb;82(2):99–105. [PMC free article] [PubMed] [Google Scholar]
- 5.McNeil DG. Gauging Impact of Gates Grants. The New York Times; 2010. Dec 20, Five Years. 2010. [Google Scholar]
- 6.Assi TM, Brown ST, Djibo A, Norman BA, Rajgopal J, Welling JS, et al. Impact of changing the measles vaccine vial size on Niger's vaccine supply chain: a computational model. BMC Public Health. 2011;11:425. doi: 10.1186/1471-2458-11-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaillard P. Vaccine du PEV: Le conditions de leur conservation permettent-elles de garantir leur qualite [Google Scholar]
- 8.Lee BY, Assi TM, Rookkapan K, Connor DL, Rajgopal J, Sornsrivichai V, et al. Replacing the measles ten-dose vaccine presentation with the single-dose presentation in Thailand. Vaccine. 2011 May 12;29(21):3811–3817. doi: 10.1016/j.vaccine.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO. [cited 2010 27 October];Immunization Profile: Niger. 2010 Available from: http://apps.who.int/immunization_monitoring/en/globalsummary/countryprofileresult.cfm?C='ner'.
- 10.WHO. WHO Prequalified Vaccines. 2010 cited 2010; Available from: http://www.who.int/immunization_standards/vaccine_quality/PQ_vaccine_list_en/en/index.html.
- 11.Kaufmann JR, Miller R, Cheyne J. Vaccine supply chains need to be better funded and strengthened, or lives will be at risk. Health Aff (Millwood) 2011 Jun;30(6):1113–1121. doi: 10.1377/hlthaff.2011.0368. [DOI] [PubMed] [Google Scholar]
- 12.Braun LJ, Jezek J, Peterson S, Tyagi A, Perkins S, Sylvester D, et al. Characterization of a thermostable hepatitis B vaccine formulation. Vaccine. 2009 Jul 23;27(34):4609–4614. doi: 10.1016/j.vaccine.2009.05.069. [DOI] [PubMed] [Google Scholar]
- 13.Nicholls H. Cash injection for thermostable vaccines. Drug Discovery Today. 2004;9(22):945. doi: 10.1016/S1359-6446(04)03281-7. [DOI] [PubMed] [Google Scholar]
- 14. [cited Accessed on March 5, 2012];Nova Laboratories Acquires Pioneering Vaccine Technology. 2012 Available from: http://www.novalabs.co.uk/news-events/?newsId=17.
- 15.Low N, Kraemer S, Schneider M, Restrepo AM. Immunogenicity and safety of aerosolized measles vaccine: systematic review and meta-analysis. Vaccine. 2008 Jan 17;26(3):383–398. doi: 10.1016/j.vaccine.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Alcock R, Cottingham MG, Rollier CS, Furze J, De Costa SD, Hanlon M, et al. Long-term thermostabilization of live poxviral and adenoviral vaccine vectors at supraphysiological temperatures in carbohydrate glass. Sci Transl Med. 2010 Feb 17;2(19):19ra2. doi: 10.1126/scitranslmed.3000490. [DOI] [PubMed] [Google Scholar]
- 17.Laboratories H. Thermostability. 2012 cited; Available from: http://www.hillemanlabs.org/science/thermostability.html.
- 18.Challenge 2: Prepare Vaccines that Do Not Require Refrigeration. 2012 cited; Available from: http://www.grandchallenges.org/ImproveVaccines/Challenges/HeatStable/Pages/default.aspx.
- 19.Rajgopal J, Connor DL, Assi TM, Norman BA, Chen SI, Bailey RR, et al. The optimal number of routine vaccines to order at health clinics in low or middle income countries. Vaccine. 2011 Jul 26;29(33):5512–5518. doi: 10.1016/j.vaccine.2011.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee BY, Norman BA, Assi TM, Chen SI, Bailey RR, Rajgopal J, et al. Single versus multi-dose vaccine vials: an economic computational model. Vaccine. 2010 Jul 19;28(32):5292–5300. doi: 10.1016/j.vaccine.2010.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levin A, Levin C, Kristensen D, Matthias D. An economic evaluation of thermostable vaccines in Cambodia, Ghana and Bangladesh. Vaccine. 2007 Sep 28;25(39–40):6945–6957. doi: 10.1016/j.vaccine.2007.06.065. [DOI] [PubMed] [Google Scholar]
- 22.Lee BY, Burke DS. Constructing target product profiles (TPPs) to help vaccines overcome post-approval obstacles. Vaccine. 2010 Apr 1;28(16):2806–2809. doi: 10.1016/j.vaccine.2009.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee BY, Biggerstaff BJ. Screening the United States blood supply for West Nile Virus: a question of blood, dollars, and sense. PLoS Med. 2006 Feb;3(2):e99. doi: 10.1371/journal.pmed.0030099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee BY. Digital decision making: computer models and antibiotic prescribing in the twenty-first century. Clin Infect Dis. 2008 Apr 15;46(8):1139–1141. doi: 10.1086/529441. [DOI] [PubMed] [Google Scholar]
- 25.Matthias DM, Robertson J, Garrison MM, Newland S, Nelson C. Freezing temperatures in the vaccine cold chain: a systematic literature review. Vaccine. 2007 May 16;25(20):3980–3986. doi: 10.1016/j.vaccine.2007.02.052. [DOI] [PubMed] [Google Scholar]