Abstract
Iron overload is the primary cause of mortality and morbidity in thalassemia major despite advances in chelation therapy. We performed a pilot clinical trial to evaluate the safety and efficacy of combined therapy with deferasirox (DFX, 20-30 mg/kg daily) and deferoxamine (DFO, 35-50 mg/kg on 3-7 days/week) in 22 patients with persistent iron overload or organ damage. In the 18 subjects completing 12 months of therapy, median liver iron concentration decreased by 31% from 17.4 mg/g (range 3.9-38.2 mg/g) to 12.0 mg/g (range 0.96-26.7 mg/g, P<0.001). Median ferritin decreased by 24% from 2,465 ng/mL (range 1,110-10,700 ng/mL) to 1,875 ng/mL (range 421-5,800 ng/mL, p=0.002). All 6 subjects with elevated myocardial iron showed improvement in MRI T2* (p=0.031). The mean ± S.E. plasma non-transferrin-bound iron (NTBI) declined from 3.10 ± 0.25 μM to 2.15 ± 0.29 μM (p=0.028). The administration of DFX during infusion of DFO further lowered NTBI (-0.28 ±0.08 μM, p=0.004) and labile plasma iron (LPI, -0.03 ± 0.01 μM, p=0.006). The simultaneous administration of DFO and DFX rapidly reduced systemic and myocardial iron, and provided an excellent control of the toxic labile plasma iron species without an increase in toxicity.
Keywords: Thalassemia Major, Iron overload, Deferoxamine, Deferasirox
Introduction
Individuals with thalassemia major, a severe inherited anemia arising from the failure of hemoglobin synthesis, are dependent upon regular blood transfusions for survival [1]. However, excess iron acquired from the transfused blood can cause irreversible organ damage and death. Despite advances in chelation therapy, initially with deferoxamine (DFO) [2], and subsequently with the oral iron chelators deferiprone (DFP) [3] and deferasirox (DFX) [4], iron overload continues to be the main determinant of mortality and morbidity in thalassemia [5; 6].
In patients who fail to respond adequately to a single drug, the intensity of chelation can be augmented by increasing the duration of exposure to the chelator [7], raising the dose to the maximum tolerated level [8], or adding a second chelator [9]. The simultaneous use of DFO and DFP can lead to synergistic ‘shuttling’ of iron by DFP onto DFO [10]. However, the rationale for combining DFO and DFX is derived from non-overlapping toxicity profiles and access to different intracellular iron pools. Whereas the low molecular weight, orally-absorbed DFP and DFX rapidly access intracellular iron in cytosol and organelles [11], the larger, parenterally-administered DFO accesses these intracellular iron pools relatively slowly [11; 12; 13], but interacts with lysosomal ferritin iron more effectively [14].
We reasoned that the combination of DFX with DFO could provide greater potency with a simpler dosing regimen than DFP with DFO, owing to the longer plasma half-life and easier monitoring requirements for DFX.
Materials and Methods
Study Design
This was a phase 2 pilot clinical trial designed to evaluate the safety and efficacy of the combination of DFX and DFO in transfusion-dependent thalassemia with a range of systemic iron burden. A secondary objective of the trial was to assess the rates of dose adjustment or discontinuation, effect on cardiac iron burden, and the control of labile plasma iron species. The trial was approved by the Institutional Review Board and conducted between December 2007 and May 2010 under Investigational New Drug application with an independent Data Safety and Monitoring Board. Informed consent was obtained from all participants prior to enrollment.
Subjects
Individuals over 8 years of age with transfusion-dependent (>8 transfusions per year) thalassemia were eligible if they had elevated systemic iron, iron-induced endocrine dysfunction or myocardial iron overload on the current chelation regimen. We enrolled 22 consecutive subjects who were assigned to two groups based upon the liver iron concentration (LIC) measured by Ferritometer [15]. Subjects in group A (moderate iron overload, n=7) had LIC ≤14 mg/g dry liver-weight and either endocrine dysfunction or cardiac siderosis, while those in group B (severe iron overload, n=15) had LIC >14 mg/g irrespective of end-organ injury. Two subjects in group A had low systemic iron burden (LIC <7 mg/g), but were included because of elevated myocardial iron. Eighteen subjects completed the study and were evaluated for efficacy, while data from all 22 subjects were analyzed for adverse effects. Response was evaluated by change in LIC, serum ferritin and myocardial MRI T2*.
Safety evaluation was conducted by monitoring serum creatinine, serum transaminases and urine protein excretion at each transfusion visit. Audiology and ophthalmology assessments were performed at baseline and the end of study. Additional monitoring for adverse events was accomplished through subject interviews and hospital records.
Chelation therapy
The duration of combined therapy was 12 months. DFX (20-30 mg/kg) was administered daily and DFO (35-50 mg/kg over 8-12 hours as subcutaneous or intravenous infusion) was given on 3 days/week (Group A) or 4-7 days/week (Group B). The total weekly dose of DFO was divided by 7 to obtain the average daily dose. In subjects who lacked prior exposure to deferasirox, the initial dose was lower than the intended doses (achieved after 4-8 weeks) in order to establish tolerability. Subjects in group B who had no decrease in serum ferritin at 3 or 6 months were asked to add one extra day of DFO per week or increase the dose of DFX to 30 mg/Kg. One or both chelators were withheld if serum ferritin fell below 500 ng/mL. The first two children in group B (ages 11 and 12 years) received DFO on 3 days per week, after which the protocol was amended to infuse DFO on 4-7 days per week in all subsequent subjects in group B. The average compliance with prescribed therapy, monitored by interviews at transfusions visits, was 89% for DFO and 94% for DFX.
Evaluation of plasma iron species
Blood samples were collected approximately 3 weeks after the last blood transfusion. All chelation was stopped 72 hours prior to the baseline visit. After the first blood sample (pre-dose), DFX was administered and the second blood sample (post-dose) was drawn 2 hours later. At subsequent time-points the pre-dose sample was obtained 24 hours from the last dose of DFX while DFO was being infused, and the post-dose sample was 2 hours after DFX.
Non-transferrin-bound iron (NTBI) is the summation of heterogeneous plasma iron species that are not bound to transferrin, consisting of monomeric, oligomeric and polymeric iron citrate as well as iron species bound to albumin or other plasma proteins [16; 17]. The NTBI assay [10; 16] involves a capture phase for Iron (III) using Nitrilo Triacetic Acid (NTA) followed with filtration and detection by high performance liquid chromatography (HLPC) using on-column derivatization with deferiprone in a metal-free Waters 625 LC system (Waters Ltd, Milford, MA) [18]. The labile plasma iron (LPI) assay measures a redox active low molecular subcomponent of NTBI, and is thought not to detect iron bound to DFO or DFX [19] as these chelate complexes are not redox active. The LPI assay was performed as previous described [20], but using standards prepared in plasma-like medium containing 20 mg/ml human serum albumin. Aliquots of serum were treated with buffered 2,3-dihydrorhodamine which is oxidized by pro-oxidants (including any redox active iron species) present in the serum to produce a fluorophore. The signal is made iron-specific by the inclusion of deferiprone in one of the aliquots which chelates the iron.
Statistical Methods
Data on safety are reported for all subjects, while analysis for efficacy is presented for those completing 12 months of combined chelation therapy. Data are presented as median and range, and the p values for change in LIC, ferritin and myocardial MRI T2* were calculated using the Wilcoxon matched-pairs signed rank test. The data on NTBI and LPI were analyzed using SAS (version 9.3, Cary, NC). Descriptive statistics were computed for each of the measures by time and pre-post analysis and presented as least square means and standard error. Employing mixed linear models with repeated measures, the pre-time points (i.e. before administration of DFX) were first compared for change over time. A model with both time and pre-post factors was used to assess the effects of administering DFX during infusion of DFO. A significance level of 0.05 was used for all statistical tests.
Results
Subject Characteristics
The diagnosis was beta thalassemia major (17), E beta0 thalassemia (4) or alpha thalassemia major (1). Four subjects (3 with beta thalassemia major and 1 with E beta0 thalassemia) exited the study before the 6 month evaluation. The reasons (duration on study) for discontinuing the trial were: death (2 months), relocation abroad (4 months), non-compliance (5 months) and recurrent abdominal pain (6 months). All 4 subjects had baseline LIC >15 mg/g and three also had elevated cardiac iron. While the change in LIC was not measured at study exit, the final serum ferritin was lower than baseline value in the three subjects who completed >3 months of therapy.
The iron burden and chelation therapy for 18 subjects completing 12 months of therapy is shown in table 1. The median LIC was 7.1 mg/g (3.9-10.8 mg/g) in group A and 21.7 mg/g (14.7-38.2 mg/g) in group B. The median serum ferritin in group A was 1,510 ng/mL (1,050-2,050 ng/mL) compared with 4,750 ng/mL (1,000-10,700 mg/g) in group B. Six subjects (3 from each group) had elevated myocardial iron with T2* ranging from 3.6 to 19.5 milliseconds. The chelation therapy prior to enrollment was variable (Table 1). Nine subjects were receiving DFO and three were receiving DFX as a single chelator. Five subjects had started receiving both DFO and DFX for 1-6 months before entering the study, while a sixth subject had been on combined therapy for 16 months.
Table 1.
Subject characteristics.
|
|
Previous Chelation
|
Starting Doses on Study
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| UPN | Age (years) | Ferritin (ng/ml) | LIC (mg/g) | T2* (ms) | DFO1(mg/kg) | Days/week | DFX (mg/kg) | DFO1(mg/kg) | Days/week | DFX (mg/kg) |
| Group A | ||||||||||
|
| ||||||||||
| A1 | 28 | 1253 | 3.9 | 19.5 | 41 | 7 | -- | 41 | 3 | 21 |
| A2 | 14 | 1050 | 4.1 | 6.5 | 37 | 7 | 9 | 43 | 3 | 19 |
| A3 | 29 | 1510 | 7.1 | 19.4 | 44 | 6 | -- | 44 | 3 | 22 |
| A4 | 36 | 1970 | 7.1 | 26.2 | 43 | 3 | -- | 43 | 3 | 21 |
| A5 | 14 | 2050 | 7.3 | 35.2 | 41 | 6 | -- | 41 | 3 | 20 |
| A6 | 26 | 1579 | 8.2 | 26.4 | 32 | 3 | -- | 32 | 3 | 20 |
| A7 | 27 | 1370 | 10.8 | 32.3 | 29 | 3 | 10 | 29 | 3 | 21 |
| Median | 27 | 1510 | 7.1 | 26.2 | ||||||
| Range | 14-36 | 1050-2050 | 3.9-10.8 | 6.5-35.2 | ||||||
|
| ||||||||||
| Group B | ||||||||||
|
| ||||||||||
| B8 | 12 | 5230 | 14.7 | 32.7 | 31 | 3 | 27 | 46 | 3 | 27 |
| B9 | 9 | 1000 | 17.3 | 28.7 | 33 | 7 | -- | 33 | 5 | 19 |
| B10 | 11 | 2879 | 17.4 | 22.0 | 35 | 3 | 26 | 35 | 3 | 26 |
| B11 | 24 | 5055 | 19.3 | 45.4 | 59 | 6 | -- | 49 | 6 | 20 |
| B12 | 9 | 1620 | 20.3 | 32.7 | 35 | 2 | 35 | 35 | 5 | 29 |
| B13 | 29 | 4680 | 21.7 | 6.7 | 45 | 3 | 31 | 45 | 6 | 31 |
| B14 | 20 | 4050 | 33.5 | 32.6 | -- | -- | 23 | 31 | 3 | 23 |
| B15 | 9 | 7660 | 34.4 | 3.6 | 36 | 6 | -- | 36 | 7 | 18 |
| B16 | 19 | 4750 | 34.8 | 36.8 | -- | -- | 30 | 45 | 5 | 25 |
| B17 | 18 | 10700 | 38.0 | 33.5 | 48 | 3 | -- | 48 | 5 | 21 |
| B18 | 12 | 6927 | 38.2 | 8.0 | -- | -- | 40 | 35 | 3 | 23 |
| Median | 12 | 4750 | 21.7 | 32.6 | ||||||
| Range | 9-29 | 1000-10700 | 14.7-38.2 | 3.6-45.4 | ||||||
Six subjects were using both chelators prior to starting the study as described in the text
DFO mg/Kg per infusion; three subjects (A4, A6 and B17) were infusing DFO on 3 days per week instead of the prescribed 5 days per week
The median daily doses in groups A and B at the start of the study were 18 and 25 mg/kg for DFO, and 21 and 23 mg/kg for DFX, respectively (Figure 1). The intensity of chelation was greater in group B at later time points once the intended doses were achieved for both groups. At 6 months, the median dose of DFO was 18 mg/kg (14-19 mg/kg) in group A and 35 mg/kg (14-42 mg/kg) in group B, while the median dose of DFX was 21 mg/kg (18-22 mg/kg) in group A and 27 mg/kg (19-30 mg/kg) in group B. One or both chelators were stopped for periods ranging from 6-15 weeks in 3 subjects from group A when the serum ferritin fell below 500 ng/mL. Both DFO and DFX were continued throughout the duration of the study in all other subjects.
Figure 1. Doses of deferoxamine and deferasirox.

The median and range of doses of DFO (a) and DFX (b) at baseline, 6 months and end of therapy for group A (open symbols, n=7) and B (solid symbols, n=11). The average daily dose of DFO is shown in Figure 1a.
LIC and Ferritin
There was improvement in the systemic iron burden over the course of the study (Figure 2). The median LIC declined by 31% from 17.4 mg/g (3.9-38.2 mg/g) to 12.0 mg/g (0.96-26.7 mg/g, p<0.001, Figure 2a). The median change in LIC was -3.2 mg/g (-0.1 to -8.0 mg/g) in group A and -10.8 mg/g (2.7 to -23.1 mg/g) in group B. LIC increased in the two subjects from group B (by 0.7 and 2.7 mg/g) who received DFO on 3 days/week throughout the study. In contrast, all other 9 subjects in group B who infused DFO on 4-7 days per week achieved negative iron balance with median change of -11.3 mg/g (-3.9 to -23.1 mg/g) in LIC. The median serum ferritin declined by 24% from 2,465 ng/mL (1,000-10,700 ng/mL) to 1,863 ng/mL (421-5,570 ng/mL, p=0.002). Serum ferritin was observed to increase in two patients from group B despite an improvement in LIC (Figure 2b).
Figure 2. Improvement in systemic iron burden with combined chelation therapy.

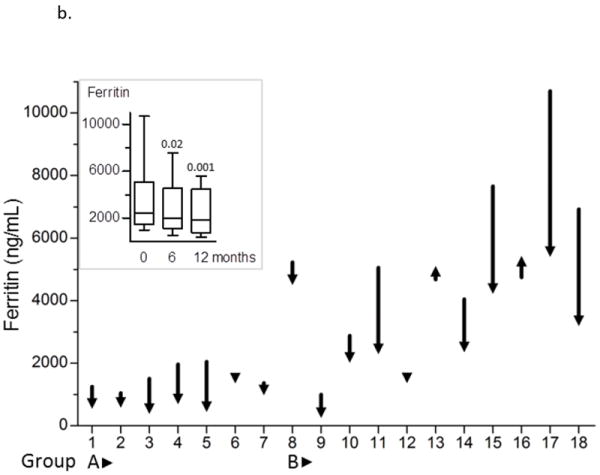
The magnitude of change in LIC (a) and ferritin (b) over 12 months is shown as decrease or increase in the measured value compared with baseline for each subject. The inset graph shows group medians and range. P values evaluate the significance of improvement in LIC at 6 months and 12 months.
Myocardial Iron
Six subjects, 3 each from groups A and B, had elevated cardiac iron at baseline with T2* values ranging from 3.6 to 19.5 milliseconds. An improvement in myocardial T2* occurred in all 6 subjects, with a median change of 2.7 milliseconds (range, 1.5-11.7 milliseconds, p=0.031). No subject with a baseline T2* value >20 ms developed detectable myocardial iron during the study. Cardiac function was abnormal in only one subject at baseline who had left ventricular ejection fraction 47.5% and T2* 19.5 milliseconds. At the end of the study, the T2* increased to 21.3 milliseconds and LVEF had improved to the normal range (62.8%).
Plasma NTBI and LPI
The mean ± S.E. NTBI and LPI at baseline, after the 72-hour washout period, were 3.10 ± 0.25 μM and 0.92 ±0.21 μM, respectively. NTBI showed a progressive decline during the study and the mean level was significantly lower at 6 and 12 months compared with baseline (Figure 3a). The level of LPI at 1, 6 and 12 months was <0.3 μM at each visit. While this effect on plasma iron species may be exaggerated by the washout interval at baseline and by the concurrent infusion of DFO at later time points, the declining trend in both parameters indicates progressive improvement during combined therapy.
Figure 3. Control of plasma NTBI and LPI with combined chelation therapy.
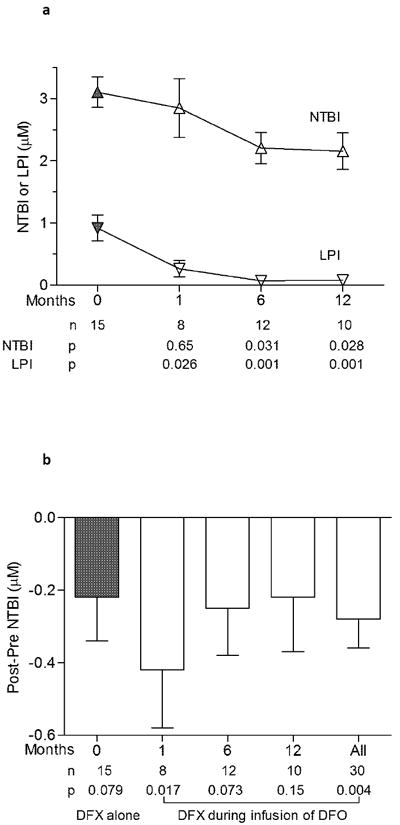
Trend in NTBI and LPI over 12 months is shown in figure 3a. Symbols represent means and standard error at 4 different visits. The baseline sample (solid symbols) was obtained 72 hours after stopping all chelation. Later samples (open symbols) were obtained 24 hours from the previous dose of DFX while infusing DFO. P values evaluate the significance of difference from baseline. The effect of DFX on NTBI in presence of DFO is shown in figure 3b. A decrease in NTBI was observed when DFX was administered alone (time 0) or during infusion of DFO (time 1, 6 and 12 months). Samples were drawn before and 2 hours following administration of DFX. P values evaluate the difference (post-pre) at each time point.
The effect of DFX administration on plasma iron species was examined with or without concurrent DFO infusion. At the baseline visit, DFX was administered alone and reduced NTBI by -0.22 ± 0.12 μM (p=0.08). At later visits, DFX administration during concurrent infusion of DFO led to a similar decrease in NTBI with a mean difference of -0.28 ± 0.08 μM between post- and pre-dose samples (p=0.004, Figure 3b). The reduction in NTBI was comparable whether DFX was given alone at baseline or during the infusion of DFO at later time points (p=0.71). Administration of DFX alone at baseline lowered LPI by -0.80 ± 0.18 μM (p<0.001). A small further change in LPI (-0.03 ± 0.01 μM) was observed when DFX was administered during DFO infusion at later visits (p = 0.006).
Toxicity
No significant toxicity or unexpected adverse events were observed with combined chelation therapy in this group of high-risk subjects with thalassemia. In particular, measures of liver function and plasma creatinine showed no adverse trends (Figure 4). Improvement in ALT level was observed in all five subjects with elevated values (>65 IU/mL) at baseline. The average serum creatinine during the study was 0.09 mg/dL (95% confidence interval 0.05 to 0.12 mg/dL) higher than baseline, but no value >33% above baseline was recorded. Interruption of treatment or modification of dose was not required in any subject secondary to elevated serum creatinine or transaminases. There was no change in the urine protein:creatinine ratio during the study period. No new abnormalities were detected upon audiology or ophthalmology assessments.
Figure 4. Monitoring for hepatic and renal toxicity.
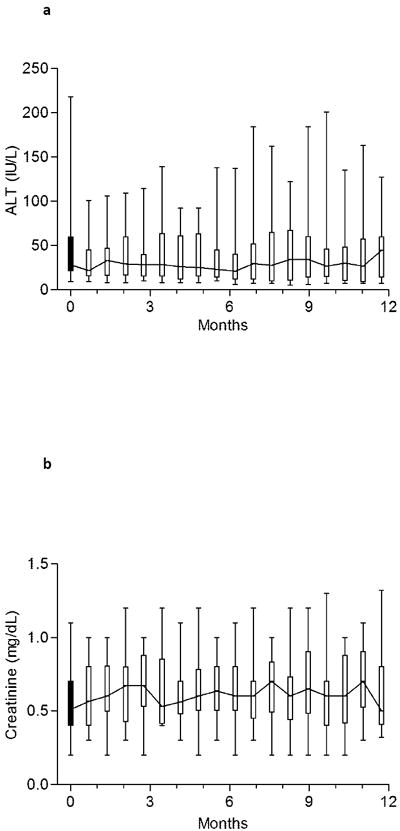
Median and range of alanine aminotransferase (a) and creatinine (b) at baseline and during study. The trend line connects the medians. Each time-point represents between 13-22 observations.
Serious adverse events
Two subjects from group B had serious adverse events. One subject with recurrent abdominal pain underwent cholecystectomy and treatment for H. pylori, and developed infection of an implanted port with Trichosporon asahii. This subject was excluded from the study after 6 months for inability to maintain a consistent dose of DFX. One adult subject from group B died at 2 months from the start of the study. This subject was splenectomized and had insulin-dependent diabetes mellitus. At baseline evaluation the LIC was 24.6 mg/g, while the cardiac MRI showed T2* 4.8 milliseconds and a normal LVEF value of 67%. The subject presented to the emergency room with abdominal pain, ascites, diarrhea and leukocytosis, and died during an emergency colectomy (for colitis) from cardiac arrhythmias two days later. No infectious agent was identified, and cardiac disease was the likely immediate cause of mortality.
Discussion
Treatment with either DFO or DFX as a single agent is capable of inducing negative iron balance in the majority of patients with transfusion-dependent thalassemia [21]. However, the occurrence of adverse effects, non-compliance or high transfusion requirement may prevent lowering of iron burden below the threshold for organ damage [21; 22]. Moreover, patients with extremely high iron burden are at increased risk of failing therapy with the oral chelators [23; 24]. The combination of DFO and DFX may offer a distinct advantage in this setting as long as the adverse effects are not higher than expected with a single agent.
In this trial, combined therapy with DFO/DFX reduced LIC by 11.3 mg/g in the high-iron group when DFO was infused ≥4 days per week. In previous studies on patients with comparable iron burden, DFX (30 mg/Kg) and DFO (average daily dose 51 mg/Kg) as single chelators lowered LIC by 8.9 mg/g and 6.4 mg/g, respectively [4]. However, 25% of patients who continued receiving DFX at doses between 15-35 mg/Kg still had LIC >14 mg/g after 4 years [25]. More recent trials using higher dose of DFX (30-45 mg/Kg) in patients with heavy iron burden demonstrated a mean reduction in LIC of 3.9 mg/g after 1 year[26] and 13.9 mg/g after 3 years [27]. In the latter study, only half of the high-risk patients achieved LIC <14 mg/g after 3 years [27]. The results from our study suggest that greater excretion of iron is likely with DFO and DFX are given simultaneously. This is supported by a short-term iron-balance study comparing single agent (DFO or DFX) with combined therapy that demonstrated at least an additive effect when the two chelators are used simultaneously [28]. A direct comparison between DFO/DFX and DFX alone will be necessary to establish if the proportion of patients achieving a safe level of LIC is improved with combination therapy.
The combined chelation therapy improved myocardial iron by a magnitude similar to the combination of DFO and DFP over a 1-year period [9]. The DFO/DFX combination may have greater activity against myocardial iron than DFX alone [27] since we found no evidence that the improvement in T2* was prevented by extremely elevated LIC [23]. While myocardial iron improved in all subjects on this study, there was one death from cardiac complications at 2 months from the start of the study. Consequently, the role of DFO/DFX in patients at very high risk of cardiac failure or arrhythmia requires careful further evaluation.
Co-administration of DFX and DFO had a favorable and progressive effect of decreasing both plasma NTBI and LPI levels (Figure 3a). This is the first study where LPI and NTBI have been measured simultaneously during chelation therapy over 1 year. Although transient decrements in NTBI during DFO infusions are well described [13; 29], progressive fall in NTBI was not seen previously with either DFO or DFX as single agents [30]. Decrease in NTBI following administration of DFX in patients concurrently infused with DFO (Figure 3b) suggests that DFX accesses NTBI pools unavailable to DFO alone. This provides further evidence for additive effects of DFO and DFX. These results are of mechanistic interest as synergistic removal of NTBI by combined DFP with DFO has been demonstrated in vitro[10].
The NTBI assay detects the iron complexes of DFP, which can therefore obscure change in NTBI with DFP therapy [31]. Although iron complexes of DFO are too stable to be detected in this assay [10], high levels of DFX complexes may be partially detected (Evans, personal communication). The LPI assay, which detects only redox active iron, overcomes this limitation, and the concentration of LPI is low while any iron-free chelator remains in plasma [19; 32]. Longer-term progressive decline in LPI is observed with DFX [33; 34] or DFP [35], but LPI decrease at 1 year is less impressive than at initial time points with DFX [33; 34]. The consistent long term LPI response seen with our study could in principle be a benefit of combined DFO and DFX.
Although we enrolled a consecutive sample on this study, several subjects were already on combination of DFO and DFX at study entry. Still, selection bias cannot be excluded and this is a limitation of our study since it could have lowered the observed incidence of adverse events. In previous studies, reduction in the dose of DFX was necessary in 10% of the patients secondary to elevated serum creatinine [8; 36; 37] and in 18% of the patients secondary to any adverse event [8]. No subject in our study required a reduction in dose or interruption of treatment for abnormalities in renal or hepatic function. However, this study lacks the ability to detect a 10% rate of adverse events, since the binomial probability of finding zero adverse events (if P=0.10) with 22 subjects extends to 13%. Therefore, close monitoring and adjustment of the doses of the chelators as the iron burden improves are essential.
Combined therapy with DFO/DFX provides another option besides DFO/DFP to tailor therapy in patients with inadequate response to a single chelator. The manner of delivering simultaneous chelation therapy is similar in the two regimens; the oral chelator is given daily while DFO is infused on 2-7 days per week [38]. However, the longer half-life of DFX allows for once daily dosing compared with three doses per day for DFP. Although both regimens increase total iron excretion beyond that seen with single agents [28; 38], our results suggest that the control of labile plasma iron species may be superior with DFO/DFX [19]. These two regimens are also distinguished by the difference in toxicities. Concern over neutropenia and agranulocytosis associated with DFP necessitates monitoring of patients using weekly blood tests [3]. The experience from our study suggests that the frequency of laboratory monitoring of DFO/DFX can be the same as DFX used as a single agent (i.e. monthly). The greater convenience of DFO/DFX may improve adherence to prescribed therapy, but the relative benefits of these two regimens should be evaluated in future clinical trials.
Conclusions
This pilot trial suggests that simultaneous use of DFO and DFX has the potential to offer higher potency without a concomitant increase in side effects. Combination therapy could be considered as an option for patients who require a swift and predictable reduction in iron overload, or are unable to maintain negative iron balance with a single chelator due to toxicity or high iron-loading rate.
Acknowledgments
This work was supported by ICL670AUS24T from Novartis (E.V.) and UL1RR024131-01 from the National Center for Research Resources. We are grateful for the bio-statistical review provided by Mark Hudes, Ph.D. and Ginny Gildengorin, Ph.D.
Footnotes
Authorship Contributions
A.L. and E.V. designed research, collected, analyzed and interpreted the data, and wrote the manuscript. J.P. designed research, contributed data, interpreted the results, and wrote the manuscript. N.S. and V.N. collected subject data and reviewed results. P.E. analyzed the plasma iron species and interpreted results. L.N. assisted in statistical analysis and writing of manuscript. G.K. performed and interpreted cardiac iron analysis. P.H. analyzed liver iron data and reviewed the manuscript.
Disclosure of Conflicts of Interest
E.V. has received consultancy fees and research funding from Novartis and reports membership of the Novartis Speakers’ Bureau. J.P. has received research funding from Novartis and reports membership of Novartis advisory boards and Speakers’ Bureau. P.H. has received research funding from Novartis.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rund D, Rachmilewitz E. Beta-thalassemia. New England Journal of Medicine. 2005;353:1135–46. doi: 10.1056/NEJMra050436. [DOI] [PubMed] [Google Scholar]
- 2.Brittenham GM, Griffith PM, Nienhuis AW, et al. Efficacy of deferoxamine in preventing complications of iron overload in patients with thalassemia major. New England Journal of Medicine. 1994;331:567–73. doi: 10.1056/NEJM199409013310902. [DOI] [PubMed] [Google Scholar]
- 3.Cohen AR, Galanello R, Piga A, De Sanctis V, Tricta F. Safety and effectiveness of long-term therapy with the oral iron chelator deferiprone. Blood. 2003;102:1583–7. doi: 10.1182/blood-2002-10-3280. [DOI] [PubMed] [Google Scholar]
- 4.Cappellini MD, Cohen A, Piga A, et al. A phase 3 study of deferasirox (ICL670), a once-daily oral iron chelator, in patients with beta-thalassemia. Blood. 2006;107:3455–3462. doi: 10.1182/blood-2005-08-3430. [DOI] [PubMed] [Google Scholar]
- 5.Borgna-Pignatti C, Rugolotto S, De Stefano P, et al. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica. 2004;89:1187–1193. [PubMed] [Google Scholar]
- 6.Kirk P, Roughton M, Porter JB, et al. Cardiac T2* Magnetic Resonance for Prediction of Cardiac Complications in Thalassemia Major. Circulation. 2009;120:1961–1968. doi: 10.1161/CIRCULATIONAHA.109.874487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis BA, Porter JB. Long-term outcome of continuous 24-hour deferoxamine infusion via indwelling intravenous catheters in high-risk β-thalassemia. Blood. 2000;95:1229–1236. [PubMed] [Google Scholar]
- 8.Taher A, Cappellini MD, Vichinsky E, et al. Efficacy and safety of deferasirox doses of >30 mg/kg per d in patients with transfusion-dependent anaemia and iron overload. British Journal of Haematology. 2009;147:752–759. doi: 10.1111/j.1365-2141.2009.07908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanner MA, Galanello R, Dessi C, et al. A Randomized, Placebo-Controlled, Double-Blind Trial of the Effect of Combined Therapy With Deferoxamine and Deferiprone on Myocardial Iron in Thalassemia Major Using Cardiovascular Magnetic Resonance. Circulation. 2007;115:1876–1884. doi: 10.1161/CIRCULATIONAHA.106.648790. [DOI] [PubMed] [Google Scholar]
- 10.Evans P, Kayyali R, Hider RC, Eccleston J, Porter JB. Mechanisms for the shuttling of plasma non-transferrin-bound iron (NTBI) onto deferoxamine by deferiprone. Translational Research. 2010;156:55–67. doi: 10.1016/j.trsl.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glickstein H, El RB, Link G, et al. Action of chelators in iron-loaded cardiac cells: Accessibility to intracellular labile iron and functional consequences. Blood. 2006;108:3195–203. doi: 10.1182/blood-2006-05-020867. [DOI] [PubMed] [Google Scholar]
- 12.Hoyes KP, Porter JB. Subcellular distribution of desferrioxamine and hydroxypyridin-4-one chelators in K562 cells affects chelation of intracellular iron pools. British Journal of Haematology. 1993;85:393–400. doi: 10.1111/j.1365-2141.1993.tb03184.x. [DOI] [PubMed] [Google Scholar]
- 13.Porter JB, Rafique R, Srichairatanakool S, et al. Recent insights into interactions of deferoxamine with cellular and plasma iron pools: Implications for clinical use. Annals of the New York Academy of Sciences. 2005;1054:155–68. doi: 10.1196/annals.1345.018. [DOI] [PubMed] [Google Scholar]
- 14.De Domenico I, Ward DM, Kaplan J. Specific iron chelators determine the route of ferritin degradation. Blood. 2009;114:4546–4551. doi: 10.1182/blood-2009-05-224188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer R, Piga A, Harmatz P, Nielsen P. Monitoring long-term efficacy of iron chelation treatment with biomagnetic liver susceptometry. Annals of the New York Academy of Sciences. 2005;1054:350–7. doi: 10.1196/annals.1345.043. [DOI] [PubMed] [Google Scholar]
- 16.Evans RW, Rafique R, Zarea A, et al. Nature of non-transferrin-bound iron: studies on iron citrate complexes and thalassemic sera. Journal of biological inorganic chemistry : JBIC : a publication of the Society of Biological Inorganic Chemistry. 2008;13:57–74. doi: 10.1007/s00775-007-0297-8. [DOI] [PubMed] [Google Scholar]
- 17.Silva AM, Hider RC. Influence of non-enzymatic post-translation modifications on the ability of human serum albumin to bind iron. Implications for non-transferrin-bound iron speciation. Biochimica et Biophysica Acta. 2009;1794:1449–58. doi: 10.1016/j.bbapap.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Singh S, Hider RC, Porter JB. A direct method for quantification of non-transferrin-bound iron. Analytical biochemistry. 1990;186:320–3. doi: 10.1016/0003-2697(90)90088-q. [DOI] [PubMed] [Google Scholar]
- 19.Zanninelli G, Breuer W, Cabantchik ZI. Daily labile plasma iron as an indicator of chelator activity in Thalassaemia major patients. British Journal of Haematology. 2009;147:744–751. doi: 10.1111/j.1365-2141.2009.07907.x. [DOI] [PubMed] [Google Scholar]
- 20.Esposito BP, Breuer W, Sirankapracha P, et al. Labile plasma iron in iron overload: redox activity and susceptibility to chelation. Blood. 2003;102:2670–2677. doi: 10.1182/blood-2003-03-0807. [DOI] [PubMed] [Google Scholar]
- 21.Porter JB. Optimizing iron chelation strategies in beta-thalassaemia major. Blood Reviews. 2009;23:S3–S7. doi: 10.1016/S0268-960X(09)70003-7. [DOI] [PubMed] [Google Scholar]
- 22.Cohen AR, Glimm E, Porter JB. Effect of transfusional iron intake on response to chelation therapy in beta-thalassemia major. Blood. 2008;111:583–7. doi: 10.1182/blood-2007-08-109306. [DOI] [PubMed] [Google Scholar]
- 23.Wood JC, Kang BP, Thompson A, et al. The effect of deferasirox on cardiac iron in thalassemia major: impact of total body iron stores. Blood. 2010;116:537–543. doi: 10.1182/blood-2009-11-250308. [DOI] [PubMed] [Google Scholar]
- 24.Hoffbrand AV, AL-Refaie F, Davis B, et al. Long-term trial of deferiprone in 51 transfusion-dependent iron overloaded patients. Blood. 1998;91:295–300. [PubMed] [Google Scholar]
- 25.Cappellini MD, Bejaoui M, Agaoglu L, et al. Iron chelation with deferasirox in adult and pediatric patients with thalassemia major: efficacy and safety during 5 years’ follow-up. Blood. 2011;118:884–93. doi: 10.1182/blood-2010-11-316646. [DOI] [PubMed] [Google Scholar]
- 26.Taher A, Elalfy MS, Al Zir K, et al. Importance of optimal dosing >/= 30 mg/kg/d during deferasirox treatment: 2.7-yr follow-up from the ESCALATOR study in patients with beta-thalassaemia. European journal of haematology. 2011;87:355–65. doi: 10.1111/j.1600-0609.2011.01662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pennell DJ, Porter JB, Cappellini MD, et al. Deferasirox for up to 3 years leads to continued improvement of myocardial T2* in patients with beta-thalassemia major. Haematologica. 2012 doi: 10.3324/haematol.2011.049957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grady RW, Galanello R, Randolph RE, et al. Toward optimizing the use of deferasirox: potential benefits of combined use with deferoxamine. Haematologica. 2012 doi: 10.3324/haematol.2012.070607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Porter JB, Abeysinghe RD, Marshall L, Hider RC, Singh S. Kinetics of removal and reappearance of non-transferrin-bound plasma iron with deferoxamine therapy. Blood. 1996;88:705–713. [PubMed] [Google Scholar]
- 30.Walter PB, Macklin EA, Porter J, et al. Inflammation and oxidant-stress in beta-thalassemia patients treated with iron chelators deferasirox (ICL670) or deferoxamine: an ancillary study of the Novartis CICL670A0107 trial. Haematologica. 2008;93:817–25. doi: 10.3324/haematol.11755. [DOI] [PubMed] [Google Scholar]
- 31.Aydinok Y, Evans P, Manz CY, Porter JB. Timed non-transferrin bound iron determinations probe the origin of chelatable iron pools during deferiprone regimens and predict chelation response. Haematologica. 2012;97:835–41. doi: 10.3324/haematol.2011.056317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cabantchik ZI, Breuer W, Zanninelli G, Cianciulli P. LPI-labile plasma iron in iron overload. Best Practice & Research. Clinical Haematology. 2005;18:277–287. doi: 10.1016/j.beha.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Daar S, Pathare A, Nick H, et al. Reduction in labile plasma iron during treatment with deferasirox, a once-daily oral iron chelator, in heavily iron-overloaded patients with beta-thalassaemia. European Journal of Haematology. 2009;82:454–7. doi: 10.1111/j.1600-0609.2008.01204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Porter JB, Lin KH, Beris P, et al. Response of iron overload to deferasirox in rare transfusion-dependent anaemias: equivalent effects on serum ferritin and labile plasma iron for haemolytic or production anaemias. European Journal of Haematology. 2011;87:338–48. doi: 10.1111/j.1600-0609.2011.01660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pootrakul P, Breuer W, Sametband M, et al. Labile plasma iron (LPI) as an indicator of chelatable plasma redox activity in iron-overloaded {beta}-thalassemia/HbE patients treated with an oral chelator. Blood. 2004;104:1504–1510. doi: 10.1182/blood-2004-02-0630. [DOI] [PubMed] [Google Scholar]
- 36.Pennell DJ, Porter JB, Cappellini MD, et al. Efficacy of deferasirox in reducing and preventing cardiac iron overload in beta-thalassemia. Blood. 2010;115:2364–2371. doi: 10.1182/blood-2009-04-217455. [DOI] [PubMed] [Google Scholar]
- 37.Vichinsky E. Clinical application of deferasirox: Practical patient management. American Journal of Hematology. 2008;83:398–402. doi: 10.1002/ajh.21119. [DOI] [PubMed] [Google Scholar]
- 38.Galanello R, Agus A, Campus S, et al. Combined iron chelation therapy. Annals of the New York Academy of Sciences. 2010;1202:79–86. doi: 10.1111/j.1749-6632.2010.05591.x. [DOI] [PubMed] [Google Scholar]


