Abstract
During mitosis, the Golgi membranes in mammalian cells undergo a continuous disassembly process and generate mitotic fragments that are distributed into the daughter cells and reassembled into new Golgi after mitosis. This disassembly and reassembly process is critical for Golgi biogenesis during cell division, but the underlying molecular mechanism is poorly understood. In this study, we have recapitulated this process using an in vitro assay and analyzed the proteins that are associated with interphase and mitotic Golgi membranes using quantitative proteomics that combines the isobaric tags for relative and absolute quantification approach with OFFGEL isoelectric focusing separation and LC-MALDI-MS/MS. A total of 1,193 Golgi-associated proteins were identified and quantified. These included broad functional categories: Golgi structural proteins, Golgi resident enzymes, SNAREs, Rab GTPases, and secretory and cytoskeletal proteins. More importantly, the combination of the quantitative proteomic approach with Western blot analysis allowed us to unveil 86 proteins with significant changes in abundance under the mitotic condition compared to the interphase condition. Altogether, this systematic quantitative proteomic study revealed candidate proteins of the molecular machinery that controls the Golgi disassembly and reassembly processes in the cell cycle.
Keywords: Liver Golgi, Cell cycle, Cell-free assay, Quantitative proteomics, iTRAQ, LC-MALDI-MS/MS
1. Introduction
The Golgi is the central organelle in the secretory pathway, essential for posttranslational modifications, sorting, and trafficking of secretory and membrane proteins and lipids in all eukaryotic cells. The unique feature of the Golgi in almost all eukaryotic cells, with only a few rare exceptions (1), is the densely packed stacks of flattened cisternal membranes. Processing enzymes in the Golgi, including those involved in modifying bound oligosaccharides, are arranged across the stack in the cis to trans order in which they function, which ensures the accuracy of glycosylation. Despite its complicated morphology and function, the Golgi is dynamic, capable of rapid disassembly and reassembly during mitosis or upon drug treatment. At the onset of mitosis, the characteristic stacked organization of the Golgi undergoes extensive disassembly. The resulting mitotic Golgi fragments are subsequently distributed to the daughter cells, where they are reassembled into new Golgi stacks during cytokinesis (2, 3).
The unique morphology of the Golgi apparatus and its high dynamic have intrigued a large number of cell biologists, and many studies have aimed to understand how the stacked Golgi structure is formed and how the Golgi disassembly and reassembly process during the cell cycle is regulated (2, 3). It is generally believed that Golgi structure formation is regulated by cytosolic and membrane proteins, and many studies have been performed in efforts to identify these proteins using biochemical and cell biology approaches. Early morphological studies showed that there are proteinaceous cross-bridges linking adjacent Golgi cisternae (4). Later on, a protein complex that was resistant to detergent and salt was isolated and named as the “Golgi matrix” proteins (5), which were identified in further studies as a group of coiled-coil proteins (i.e., golgins) and Golgi Reassembly Stacking Proteins (GRASPs). These proteins play essential roles in vesicle tethering, cisternal stacking, and ribbon formation as well as cell cycle regulation (6, 7). The function of these structural proteins is regulated by cytosolic factors, which results in the disassembly and reassembly of the Golgi during the cell cycle (7–9). However, the identities of these cytosolic proteins are still far from clear. Recently, several labs have attempted to identify Golgi membrane proteins using organellar proteomics, a fundamental and fast-expanding research technology in proteomics and cell biology that combines biochemical fractionation and comprehensive protein identification (10–15). However, quantitative studies documenting the comprehensive protein changes on the Golgi membranes during the cell cycle have not been reported thus far. Analysis of the dynamics of proteins in the Golgi membrane during disassembly has the advantage of identifying peripheral membrane proteins that exhibit changes, which are more likely to be specific regulators for the morphological change rather than contaminating components.
Biochemical reconstitution experiments have provided powerful tools with which we can dissect biological processes. One widely used method to study the Golgi disassembly and reassembly processes during the cell cycle involves purified Golgi membranes to which mitotic or interphase cytosol is added (16–20). After incubation, the membranes are separated from the cytosol by centrifugation through a sucrose cushion and then processed for biochemical and morphological analyses. When combined with modern quantitative proteomics approaches, this in vitro reconstitution system is expected to provide a powerful tool to further dissect the molecular mechanism that regulates Golgi membrane dynamics during the cell cycle (for strategy see Fig. 1a). In this study, we applied isobaric tags for relative and absolute quantification (iTRAQ) and LC-MALDI-MS/MS analysis to quantify protein changes on the Golgi membranes of different phases of the cell cycle (21). iTRAQ is a chemical labeling reagent that is widely used in proteomics studies in the recent years. Its advantage is to simultaneously identify and quantify proteins in up to eight samples in the same experiment. The ability to run multiplex samples at the same time minimizes experimental variations, resulting in the ability to detect relatively small changes in protein levels. This provides us an unbiased approach to quantitatively analyze proteins that are associated with the Golgi when the membranes are intact during interphase and when they are disassembled during mitosis, and thus to understand the global changes in the protein composition of the Golgi when its morphology is changed during cell division (Fig. 1b). This study revealed candidate proteins involved in the regulation of Golgi morphological changes during the cell cycle.
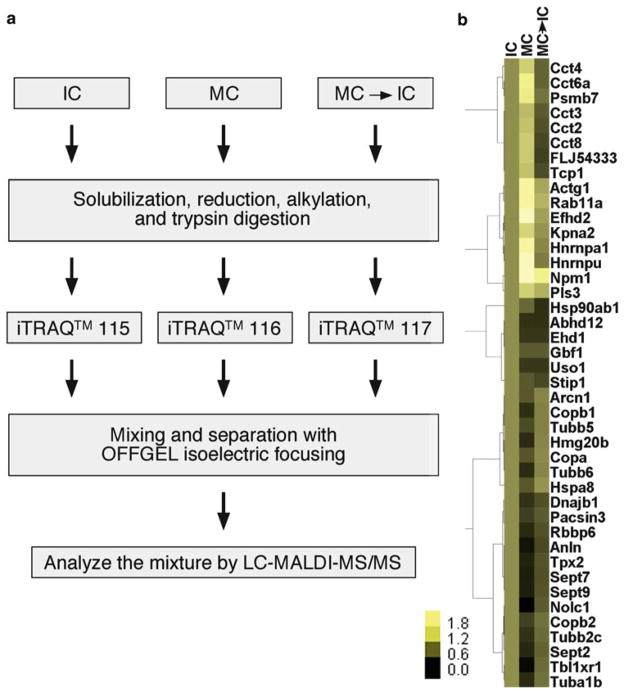
Fig. 1.
Flowchart of the experimental procedures and hierarchical clustering of identified Golgi proteins whose binding to the membranes undergoes cell cycle regulation. (a) Experiment flowchart. Purified rat liver Golgi membranes were incubated with indicated cytosols and subjected to the subsequent treatments to identify and quantify Golgi-associated proteins. (b) Hierarchical clustering of Golgi proteins under indicated conditions. The protein amount after treatment with interphase cytosol was normalized to 1 and the relative amounts under other conditions were shown.
2. Materials
2.1. Purification of Golgi Membranes from Rat Liver
All reagents were purchased from Sigma (St. Louis, MO, USA), Roche(Roche, Indianapolis, IN, USA), or Calbiochem (Gibbstown, NJ, USA), unless otherwise stated.
Typically, livers are obtained from six Sprague Dawley rats with body weight between 150 and 200 g (22).
Homogenization buffer: 0.5 M potassium phosphate buffer, pH 6.7. To prepare the buffer, make up 500 ml solutions of 0.5 M anhydrous K2HPO4 and 0.5 M anhydrous KH2PO4. To 400 ml of the latter, gradually add the former until the pH reaches 6.7.
Gradient buffers: Make up sucrose buffers A–E in 100 mM phosphate buffer, pH 6.7, and 5 mM MgCl2 from the indicated stock solutions and ice-cold water as shown in Table 1. 50 ml of each buffer A, B, and E and 100 ml of buffers C and D are needed. EDTA-free protease inhibitor tablet (Roche, Indianapolis, IN, USA) and pepstatin A are added to buffers C and D. Water should be precooled to 4°C overnight to ensure that all the buffers are ice cold. It is important to be as accurate as possible when mixing various components and to check the refractive index of each buffer using a refractometer (Reichert, Depew, NY, USA). The final refractive index should be adjusted using water or 2 M sucrose to within ±0.5% sucrose (about 0.001 in refractive index) for buffers C and D in particular. Leave the buffers on ice all the time during the experiment.
Beckman L8-70M ultracentrifuge, SW-41 rotor, and centrifuge tubes (Beckman, Cat. #: 344059, Brea, CA, USA), or equivalents.
Table 1.
Sucrose solutions for Golgi purification
| Buffer | A | B | C | D | E |
|---|---|---|---|---|---|
| Sucrose concentration (M) | 0 | 0.25 | 0.5 | 0.86 | 1.3 |
| 0.5 M Phosphate buffer, pH 6.7 (ml) | 10 | 10 | 20 | 20 | 10 |
| 2 M Sucrose (ml) | 0 | 6.25 | 25 | 43 | 32.5 |
| 2 M MgCl2 (ml) | 0.125 | 0.125 | 0.25 | 0.25 | 0.125 |
| Water (ml) | 39.9 | 33.6 | 54.8 | 36.8 | 7.4 |
| Total volume (ml) | 50 | 50 | 100 | 100 | 50 |
| Sucrose % (w/v) | 0 | 8.6 | 16.0 | 26.4 | 38.6 |
| Refractive index | 1.3330 | 1.3456 | 1.3574 | 1.3747 | 1.3973 |
2.2. In Vitro Reconstitution of Mitotic Golgi Disassembly and Postmitotic Golgi Reassembly Using a Cell-Free System
2.2.1. Preparation of Cytosol
The interphase (IC) and mitotic (MC) cytosols are prepared from HeLa S3 cells (16, 18, 20).
EBS buffer: 0. 1 M sucrose, 80 mM β-glycerophosphate, 20 mM EGTA, 15 mM MgCl2, 2 mM ATP, 1 mM glutathione, EDTA-free protease inhibitor cocktail (Roche, 1 in 50 ml), 5 mM pepstatin A. Adjust to pH 7.2 with KOH. Freshly made before the experiment and leave on ice.
Cytosols are changed into related buffers using Bio-spin 6-Tris columns (Bio-Rad, Hercules, CA, USA) following the manufacturer’s instructions.
Beckman Max-XP ultracentrifuge, TLA 100.3 rotor, and centrifuge tubes (Beckman, Brea, CA, USA), or equivalents.
2.2.2. The Golgi Disassembly and Reassembly Assay
MEB buffer: 50 mM Tris–HCl, pH 7.4 (adjusted with HCl), 0.2 M sucrose, 50 mM KCl, 20 mM β-glycerophosphate, 15 mM EGTA, 10 mM MgCl2, 2 mM ATP, 1 mM GTP, 1 mM glutathione, and EDTA-free protease inhibitors. Freshly made before the experiment and leave on ice.
ATP regeneration system (10×): 100 mM creatine phosphate (200 mM stock, in distilled water), 1 mM ATP (10 mM stock, in 250 mM Hepes-KOH, pH 7.3), 0.2 mg/ml cytochalasin B (2 mg/ml stock, in DMSO), 0.2 mg/ml creatine kinase (2 mg/ml stock, in 50% glycerol). Freshly mix the stock solutions with distilled water before use. Dilute 1:10 into the reactions.
Beckman Max-XP ultracentrifuge, TLA55 rotor, and centrifuge tubes, or equivalents.
Eppendorf 5417R microcentrifuge (Eppendorf, Hauppauge, NY, USA, or a similar centrifuge without cooling in a 4°C cold room) with a swing bucket rotor.
KHM buffer: 20 mM HEPES, pH 7.4, 0.2 M sucrose, 60 mM KCl, 5 mM Mg(OAc)2, 2 mM ATP, 1 mM GTP, 1 mM glutathione, protease inhibitors (1 EDTA-free tablet for 50 ml buffer).
Electron microscopy (EM) fixation buffer: 2% glutaraldehyde in KHM buffer. Dilute glutaraldehyde from a 25% or 50% stock into KHM buffer freshly, warm to room temperature (18–22°C) before use.
EM post-fixative: 1% (w/v) OsO4, 1.5% K3(Fe(CN)6), 0.1 M sodium cacodylate, pH 7.4.
Epon-mix: Before use, mix 14.5 g Embed 812, 10.5 g NMA, and 5.0 g DDSA (Electron Microscopy Sciences, Hatfield, PA, USA). Mix well until no streaks are left; add 0.54 g DMP-30 and mix. Prepare freshly before use.
Uranyl acetate solution: Dissolve 2% uranyl acetate in H2O. Push the solution through a 0.22 μm syringe filter before use.
Lead citrate solution: Weigh 1.33 g lead nitrate and 1.76 g sodium citrate, add 30 ml H2O, and shake for 2 min at room temperature. Add 8 ml 1 M NaOH, adjust volume to 50 ml with H2O. Push through a 0.22 μm syringe filter before use.
2.3. In-Solution Digestion and iTRAQ™ Labeling
Sequencing grade modified trypsin (Promega, Madison, WI, USA).
iTRAQ™ reagents (Applied Biosystems, Foster City, CA, USA).
Resuspension buffer: 0.5 M triethyl ammonium bicarbonate (TEAB), 8 M urea, and 0.4% SDS.
Bradford assay kit (Bio-Rad, Hercules, CA, USA).
2.4. OFFGEL Isoelectric Focusing and LC-MALDI-MS/MS
A 3100 OFFGEL Fractionator (Agilent Technologies, Santa Clara, CA, USA).
IPG strip pH 3–10 (GE healthcare, Pittsburgh, PA, USA).
C18 spin columns (Thermo Scientific, Rockford, IL, USA).
Reversed-phase C18 trap columns (Zorbax C18; 5 mm by 0.3 mm i.d.; 5 μm particles) (Agilent Technologies, Santa Clara, CA, USA).
Reversed-phase C18 analytical columns (Zorbax 300 SB C18 column, 75 μm × 150 mm, 3.5 μm particles) (Agilent Technologies, Santa Clara, CA, USA).
Agilent 1100 HPLC system with micro-collection/spotting system.
Solvent A: 0.1% TFA in Optima™ H2O (LC/MS grade) (Fisher Scientific, Pittsburgh, PA, USA) and solvent B: 90% acetonitrile and 0.1% TFA in Optima™ H2O.
MALDI matrix: α-cyano-4-hydroxycinnamic acid (2 mg/ml) (Agilent Technologies, Santa Clara, CA, USA).
Infusion pump (Harvard Apparatus, Holliston, MA, USA).
MALDI-TOF/TOF instrument: Applied Biosystems 4800 Proteomics Analyzer (AB Sciex, Foster City, CA, USA).
Peptide Mass Standards Kit (AB Sciex, Foster City, CA, USA).
2.5. Database Searching, Quantification, and Categorization of the Identified Proteins
ProteinPilot™ v3.0 (AB Sciex, Foster City, CA, USA).
IPI rat (version 3.49) and IPI human databases (version 3.53).
Hierarchical clustering using Cluster 3.0 and viewed with the TreeView program.
“Compute pI/Mw” tool accessible on the ExPASY Web site.
2.6. Confirmation of the Proteomic Analysis by Western Blotting
The antibodies used are monoclonal antibodies against Bet1, Gos28, GM130, and HSP90 from BD Transduction Laboratories (Rockville, MD, USA); monoclonal antibodies against α-actin (St. Louis, MO, USA), ARF1 (1D9, Abcam, Cambridge, MA, USA), β-COP (M3A5, gift of Dr. I Mellman, Genentech, South San Francisco, CA, USA), GRASP65 (23), PP2A C subunit (Millipore, Billerica, MA, USA), and β-tubulin (Dr. K. Gull, University of Oxford, UK); polyclonal antibodies against ARF1 (Dr. D. Sheff, University of Iowa, IA, USA), cdc2 (Millipore, Billerica, MA, USA), β-COP (EAGE, Dr. I Mellman, Genentech, South San Francisco, CA, USA), ERK2 (Millipore, Billerica, MA, USA), GM130 (MLO7, M. Lowe, University of Manchester, UK), GRASP55 (Dr. J. Seemann, University of Texas, Dallas, USA) (24), GRASP65 (23, 25), HSP70 (Synaptic Systems, Goettingen, Germany), α-Mannosidase (Man) I (Dr. G. Warren, Max F. Perutz Laboratories, Vienna, Austria) and II (Dr. K. Moremen, University of Georgia, GA, USA), golgin-84 (Dr. A. Satoh, Okayama University, Okayama, Japan), NSF (Dr. G. Warren), p115 (Dr. G. Warren), Rab1A (Santa Cruz, Santa Cruz, CA, USA), Rab6 (Santa Cruz), Rab11 (Santa Cruz), rat serum albumin (Dr. G. Warren), sec31 (Dr. F. Gorelick, Yale University, New Haven, CT, USA), syntaxin 5 (Dr. G. Warren), SNAP29 (Synaptic Systems), and TGN38 (Dr. G. Warren). Antibodies to β′-, γ-, δ-, and ε-COPs were kindly provided by Dr. D. Sheff. The monoclonal antibody for phosphorylated GRASP65 was raised in mouse with recombinant GST-GRASP65 (aa 202–446) treated with mitotic HeLa cell cytosol. Secondary antibodies for immunofluorescence and for Western blotting were from Molecular Probes (Carlsbad, CA, USA) and Jackson Immunoresearch Laboratories (West Grove, PA, USA), respectively.
3. Methods
3.1. Purification of Golgi Membranes from Rat Liver
The Golgi membranes are purified from rat liver homogenate by two sequential sucrose gradient centrifugations as previously described (22). This method uses rat liver as the source since liver cells have abundant Golgi membranes due to the high activity in protein and lipid secretion. Liver tissue is homogenized by passing through a 150 μm mesh sieve. This method is relatively mild and is essential for the preservation of the stacked structure of the Golgi membranes. The first gradient separates the Golgi membranes from unbroken cells, other cellular organelles (e.g., nuclei and mitochondria), and the majority of cytosol and free lipids. The second sucrose gradient further separates the Golgi membranes from cytosolic contaminants and concentrates the Golgi membranes. A typical preparation can yield 5–6 mg Golgi membranes that are purified about 100-fold over the homogenate, and 60–70% in stacks analyzed by electron microscopy.
Starve six female Sprague–Dawley rats (body weight 150–200 g) for 24 h.
Sacrifice the rats using high doses of CO2 followed by cervical dislocation. Rapidly remove the livers into a large volume of ice-cold buffer C (protease inhibitors can be omitted in the wash buffer) to wash off the blood. Transfer the livers into ice-cold buffer C with EDTA-free protease inhibitors and pepstatin A. Mince the liver into small pieces with a pair of scissors.
Weigh livers, which are normally 40–45 g from six rats.
Homogenize the tissue by pressing through a 150 mm mesh stainless-steel sieve with the bottom of a 200 ml conical flask in a rolling action. Adding a small amount of buffer C (with protease inhibitor) to the sieve will help the liver homogenate press through more easily. The final volume of the homogenate should be about 50 ml.
Make the gradients: Place 6 ml of buffer D (Table 1) in each of the 12 SW-41 Ultraclear tubes, overlay 4.5 ml of homogenates, and 1.5 ml of buffer B on the top of the gradient, and balance the tubes.
Centrifuge at 103,800 × g (29,000 rpm) in an SW-41 rotor for 60 min at 4°C.
Aspirate the lipid at the top and the cytosol indicated by red color of hemoglobin, and collect Golgi membranes that accumulate at the 0.5/0.86 M interfaces with a plastic Pasteur pipette.
Pool the collected fractions. Measure the sucrose concentration of the sample by a refractometer, and adjust the sucrose concentration to 0.25 M (refractive index 1.3456) using buffer A (no sucrose).
Pool the second gradient with 1 ml 1.3 M sucrose (buffer E) and 2 ml 0.5 M sucrose (buffer C) in each tube, and overlay 9 ml diluted Golgi sample.
Centrifuge at 7,900 × g (8,000 rpm) in an SW-41 rotor for 30 min at 4°C.
Discard the supernatant and collect the membranes at the 0.5 M/1.3 M sucrose interface. Adjust the sucrose concentration to 0.5 M (refractive index 1.3574) using buffer A.
Check protein concentration using Bio-Rad Bradford assay. Check morphology and purity of the purified Golgi membranes by EM.
Aliquot and freeze samples in liquid nitrogen and store at −80°C (see Note 1).
3.2. In Vitro Reconstitution of Mitotic Golgi Disassembly and Postmitotic Golgi Reassembly Using a Cell-Free System
The interphase and mitotic cytosols were prepared from HeLa S3 cells as previously described (16, 18, 20). Cytosols are centrifuged for 30 min at full speed in a tabletop centrifuge to remove protein precipitates and changed into indicated buffers using Bio-Spin 6 columns before used to treat Golgi membranes.
The Golgi disassembly assay is performed as described previously (19, 20). In brief, purified Golgi membranes (200 μg) are mixed with 10 mg of mitotic cytosol (see Note 2), 1 mM GTP, and an ATP-regenerating system in MEB buffer with a final volume of 1 ml.
After incubation for 60 min at 37°C, mitotic Golgi fragments (MGFs) are isolated and soluble proteins are removed by centrifugation (55,000 rpm or 136,000 × g, for 30 min in a TLA55 rotor) through a 0.4 M sucrose cushion in KHM buffer onto a 6 μl 2 M sucrose cushion (see Note 3).
The membranes are resuspended in KHM buffer, and aliquots are succeeded to fixation and processing for EM, to reassembly reactions described below, or kept frozen at −80°C until further use for the proteomics analysis.
Incubation of Golgi membrane with interphase cytosol in KHM buffer is used as control.
For Golgi reassembly, 200 μg of MGFs are resuspended in 4 mg IC in KHM buffer in the presence of an ATP regeneration system in a final volume of 300 μl and incubated at 37°C for 60 min. Gently mix the samples every 20 min by tapping the Eppendorf tube.
Membranes are pelleted by centrifugation as described above, processed for EM, or kept at −80°C for the proteomic analysis or Western blotting. All results are confirmed by three or more independent experiments.
For EM analysis, the percentage of membranes in cisternae or in vesicles is determined by the intersection method (16, 18, 19, 23, 26) (see Note 4).
3.3. In-Solution Digestion and iTRAQ™ Labeling
For quantitative proteomics analysis, membranes are collected under three experimental conditions: (1) incubation of membranes with interphase cytosol alone; (2) with mitotic cytosol alone; and (3) sequential incubation with mitotic cytosol and interphase cytosol (21).
After incubation, Golgi membrane is separated from cytosol by centrifugation through a 0.4 M sucrose cushion and the Golgi membrane pellets are stored at −80°C until use.
For in-solution digestion, Golgi membrane pellets are solubilized on ice in 50 μl resuspension buffer for 30 min.
Protein concentrations are determined with the Bio-Rad Bradford assay kit.
Golgi membrane proteins (30 μg) from each of the previously mentioned three groups are reduced with 5 mM tris(2-carboxyethyl)phosphine (TCEP) for 1 h at 37°C. Cysteines are then blocked with 10 mM methyl methanethiosulfonate (MMTS) for 20 min at room temperature.
The protein solution is diluted four times with 0.5 M TEAB containing 3 μg trypsin (1:10 w/w) and incubated at 37°C overnight.
To quantitatively compare Golgi membrane treated with mitotic cytosol to that treated with interphase cytosol, multiplexed isobaric tags (iTRAQ™ reagents) are used to label tryptic peptides from different conditions: 115 for interphase cytosol treatment, 116 for mitotic cytosol treatment, and 117 for sequential mitotic and interphase cytosol treatments. The labeling procedure is performed according to the manufacturer’s instructions, with more details described below.
Dissolve the iTRAQ™ reagents by adding 70 μl of ethanol, vortex thoroughly for a minute, and then spin down briefly in a minicentrifuge.
The tryptic peptides from three different treatments are then mixed with the 115, 116, and 117 iTRAQ™ reagents, respectively, at room temperature for an hour.
The labeling reactions are terminated by diluting the mixture with 10 volumes of H2O.
3.4. OFFGEL Isoelectric Focusing and LC-MALDI-MS/MS
The combined peptide mixture is first fractionated based on isoelectric points (pIs) on a 3100 OFFGEL Fractionator (Agilent Technologies) using pH 3–10 IPG strip with a 12-well manifold according to the manufacturer’s instruction.
The iTRAQ-labeled peptides are diluted in the peptide-focusing buffer to a final volume of 1.8 ml, 150 μl of which is loaded into each well.
The samples are focused with a maximum current of 50 μA until 50 kV h is achieved.
Peptide fractions are harvested and desalted using Pierce C18 spin columns.
The resulting elute is dried using a vacuum centrifuge and resuspended in 40 μl 0.1% trifluoroacetic acid (TFA) and 5% acetonitrile.
The resuspended peptide sample from each OFFGEL fraction is concentrated on a reversed-phase C18 cartridge (Agilent Technologies) and then separated by reversed-phase C18 analytical column (Agilent Technologies) using an Agilent 1100 HPLC system with the following binary gradient with a flow rate of 300 nl/min: 0 min, 6.5% B; 9 min, 6.5% B; 12 min, 15% B; 92 min, 45% B; 97 min, 60% B; 102 min, 100% B; 104 min, 100% B; 105 min, 6.5% B; and 115 min, 6.5% B. Solvent A is 0.1% TFA and solvent B is 90% acetonitrile and 0.1% TFA.
The column effluent is mixed with MALDI matrix (2 mg/ml α-cyano-4-hydroxycinnamic acid) (Agilent Technologies) through a 25 nl mixing tee and spotted on 1536 well OptiTOF™ MALDI target plates which are later analyzed by tandem mass spectrometry (see Note 5).
The MS and MS/MS spectra are acquired on an Applied Biosystems 4800 Proteomics Analyzer (TOF/TOF) in positive ion reflection mode with a 200 Hz Nd:YAG laser operating at 355 nm. The accelerating voltage is 20 kV with a 400-ns delay.
For MS/MS spectra, the collision energy is 2 keV and the collision gas is air.
Each 1536 well OptiTOF™ MALDI plate is first calibrated on nine calibration wells using standards from Applied Biosystems with a 20 ppm mass accuracy in the MS mode.
Both MS and MS/MS data are then acquired in the sample wells using the instrument default calibration. Typical MS spectra are obtained with the minimum possible laser energy in order to maintain the best resolution. Single-stage MS spectra for the entire samples are first collected and in each sample well MS/MS spectra are acquired from the 12 most intense peaks above the signal-to-noise ratio threshold of 30.
3.5. Database Searching, Quantification, and Categorizing the Identified Proteins
Database searching is performed using ABI’s ProteinPilot™ v3.0 using the Paragon algorithm (27). This software interacts directly with the Oracle database in which the mass spectrometer stores its data, and submits monoisotopic peak lists in batch to a local instance of the search engine for protein identities. No additional peak list filtering is specified.
Peak lists are generated by the mass spectrometer during data acquisition based on a specified signal-to-noise threshold (30 in this case).
ProteinPilot™ also performs protein grouping to remove redundant hits and comparative quantifications using iTRAQ ratios.
In an effort to comprehensively identify both rat (originated from the Golgi membranes) and human proteins (recruited from HeLa cell cytosol), a combined human and rat database is created by manually concatenating IPI rat (version 3.49, with 40,131 of Rattus norvegicus proteins) and IPI human databases (version 3.53, with 73,748 of Homo sapiens proteins). All data sets are then searched against this database using ProteinPilot™.
All reported proteins are identified with 90% or greater confidence as determined by ProteinPilot™ Unused scores (≥1.0) with the corresponding false positive discovery rate below 1%. The iTRAQ peak area data were normalized for loading error by biased corrections calculated using ProteinPilot™. The default ion intensity threshold in ProteinPilot™ for calculating the peptide ratios is 40 counts.
Hierarchical clustering is performed based on Pearson correlation using Cluster 3.0 program and clusters are viewed with the TreeView program.
The theoretical pIs of the peptides in each OFFGEL fraction are calculated using “Compute pI/Mw” tool accessible on the ExPASY Web site. The identified peptides with a confidence greater than 90% are included in the peptide list for the calculation.
3.6. Confirmation of the Proteomic Analysis by Western Blotting
To validate the iTRAQ-based results, Western blot analyses are conducted on a large number of proteins, including many in the major clusters.
The protein levels in the interphase and mitotic cytosols are first compared to confirm that the change of Golgi-associated proteins is not due to the availabilities of the proteins in either cytosol.
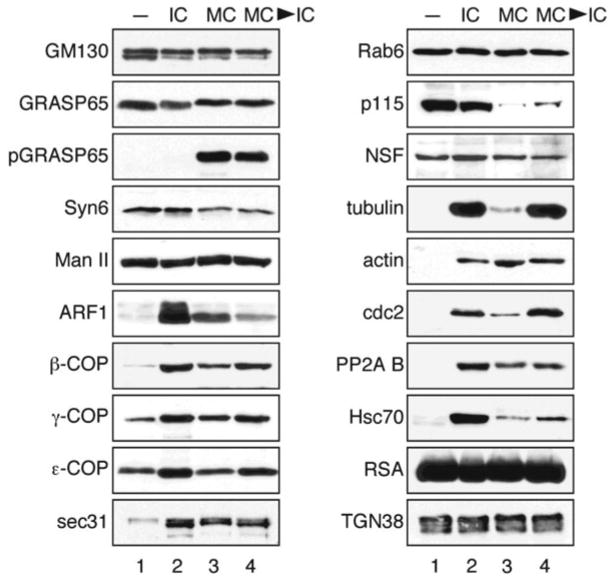
Then the proteins associated with either interphase or mitotic Golgi membranes are compared. As expected, Golgi resident proteins such as TGN38 and enzymes such as α-Mannosidase II have no change across all conditions. Consistent with our iTRAQ results, the amount of membrane proteins such as GM130 and GRASP65 does not change upon different treatments (Fig. 2, also see Note 6).
The unique patterns of changes of COPI coatomer subunits are confirmed by Western blot with antibodies against five out of all six subunits identified by iTRAQ. The different patterns of the heat-shock proteins and the cytoskeleton proteins actin and tubulin are also confirmed (Fig. 2, see Note 7).
Fig. 2.
Western blot analysis of major Golgi-associated proteins. Purified rat liver Golgi membranes were treated with buffer (lane 1 ), interphase cytosol (IC, lane 2), mitotic cytosol (MC, lane 3 ), or with subsequent treatments with interphase and mitotic cytosol (MC → IC, lane 4 ) followed by isolation of the total membranes by centrifugation. Equal fractions were analyzed by Western blot for indicated proteins; representative images from three independent experiments were shown. pGRASP65, phospho-specific GRASP65; NSF, N-ethylmaleimide-sensitive fusion protein; RSA, rat serum albumin. Modified from Chen et al. (21) with permission from the journal.
Acknowledgments
We gratefully acknowledge Drs. F. Gorelick, K. Gull, T. Kreis, M. Lowe, K. Moremen, A. Price, A. Satoh, J. Seemann, D. Sheff, D. Shields, and G. Warren for generously providing antibodies. We thank J. Williams and D. Tang for suggestions and reagents. We thank Sarah Volk for her assistance in OFFGEL electrophoresis. This work was supported by National Institute of Health grant P41 RR018627 to P. Andrews, and was partially supported by the National Institutes of Health (GM087364) and the American Cancer Society (RGS-09-278-01-CSM) to Y.W.
Footnotes
Golgi membrane pellets can be used immediately after preparation or stored in the centrifuge tubes at −80°C with the caps sealed with parafilm.
The protein ratio of mitotic cytosol:Golgi in the assay is based on the quantification results of these two components in rat liver cells. Prior to use, spin the mitotic cytosol in a benchtop microcentrifuge for 20 min at 18,000 × g (14,000 rpm) at 4°C to remove protein precipitates. Desalt the mitotic cytosol into MEB buffer using Bio-spin 6-Tris column following the manufacturer’s instruction.
This step is critical to reduce excess cytosolic proteins. If the reaction is in a smaller volume, increase the volume of the 0.4 M sucrose cushion so that the total volume in the tube is between 1.0 and 1.4 ml. The centrifuge tube may collapse after the high-speed spin if the volume is too small.
To analyze the efficiency of Golgi disassembly and reassembly, the percentage of membranes in Golgi stacks, single cisternae, vesicles, or tubular structures can be quantified. Cisternae are defined as long membrane profiles with a length greater than four times their width, the latter being not more than 60 nm. Normal cisterna ranges 20–30 nm in width and is longer than 200 nm. Stacks are defined as two or more cisternae that were separated by no more than 15 nm and overlapped in parallel by more than 50% of their length. Vesicles are spherical or nearly spherical membrane profiles with 50–200 nm diameter, often can be seen with the protein coat. The vesicles in our study are approximately 70 nm in diameter. Tubular network are single tubules with a length more than 1.5 times but less than four times their maximal width. The width is often uneven, sometimes more than 60 nm, and sometimes with branches.
The MALDI matrix is delivered to the mixing tee by external infusion pump at 800 nl/min. A reduced concentration of α-cyano-4-hydroxycinnamic acid is used to prevent matrix crystals from clogging the capillary in the nanoflow pump. In addition, the capillaries are washed with matrix solvent using the infusion pump after each experiment to prevent matrix crystals’ formation.
Western blot analysis for GRASP65 is performed using two antibodies, one recognizes all forms of GRASP65, which shows that the amount of GRASP65 is not changed upon different treatments of the membranes although the protein migrates slower on the SDS-PAGE when treated with mitotic cytosol; the other recognizes only the phosphorylated form, pGRASP65.
References
- 1.Mowbrey K, Dacks JB. Evolution and diversity of the Golgi body. FEBS Lett. 2009;583(23):3738–3745. doi: 10.1016/j.febslet.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y. Golgi apparatus inheritance. In: Mironov A, Pavelka M, Luini A, editors. The Golgi apparatus State of the art 110 years after Camillo Golgi’s discovery. Springer; New York: 2008. pp. 580–607. [Google Scholar]
- 3.Shorter J, Warren G. Golgi architecture and inheritance. Annu Rev Cell Dev Biol. 2002;18:379–420. doi: 10.1146/annurev.cellbio.18.030602.133733. [DOI] [PubMed] [Google Scholar]
- 4.Cluett EB, Brown WJ. Adhesion of Golgi cisternae by proteinaceous interactions: intercisternal bridges as putative adhesive structures. J Cell Sci. 1992;103:773–784. doi: 10.1242/jcs.103.3.773. [DOI] [PubMed] [Google Scholar]
- 5.Slusarewicz P, Nilsson T, Hui N, Watson R, Warren G. Isolation of a matrix that binds medial Golgi enzymes. J Cell Biol. 1994;124(4):405–413. doi: 10.1083/jcb.124.4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barr FA, Short B. Golgins in the structure and dynamics of the Golgi apparatus. Curr Opin Cell Biol. 2003;15(4):405–413. doi: 10.1016/s0955-0674(03)00054-1. [DOI] [PubMed] [Google Scholar]
- 7.Ramirez IB, Lowe M. Golgins and GRASPs: holding the Golgi together. Semin Cell Dev Biol. 2009;20(7):770–779. doi: 10.1016/j.semcdb.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 8.Lupashin V, Sztul E. Golgi tethering factors. Biochim Biophys Acta. 2005;1744(3):325–339. doi: 10.1016/j.bbamcr.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 9.Short B, Haas A, Barr FA. Golgins and GTPases, giving identity and structure to the Golgi apparatus. Biochim Biophys Acta. 2005;1744(3):383–395. doi: 10.1016/j.bbamcr.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Bell AW, Ward MA, Blackstock WP, Freeman HN, Choudhary JS, Lewis AP, Chotai D, Fazel A, Gushue JN, Paiement J, Palcy S, Chevet E, Lafreniere-Roula M, Solari R, Thomas DY, et al. Proteomics characterization of abundant Golgi membrane proteins. J Biol Chem. 2001;276(7):5152–5165. doi: 10.1074/jbc.M006143200. [DOI] [PubMed] [Google Scholar]
- 11.Wu CC, MacCoss MJ, Mardones G, Finnigan C, Mogelsvang S, Yates JR, 3rd, Howell KE. Organellar proteomics reveals Golgi arginine dimethylation. Mol Biol Cell. 2004;15(6):2907–2919. doi: 10.1091/mbc.E04-02-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu CC, Taylor RS, Lane DR, Ladinsky MS, Weisz JA, Howell KE. GMx33: a novel family of trans-Golgi proteins identified by proteomics. Traffic. 2000;1(12):963–975. [PubMed] [Google Scholar]
- 13.Wu CC, Yates JR, 3rd, Neville MC, Howell KE. Proteomic analysis of two functional states of the Golgi complex in mammary epithelial cells. Traffic. 2000;1(10):769–782. doi: 10.1034/j.1600-0854.2000.011004.x. [DOI] [PubMed] [Google Scholar]
- 14.Taylor RS, Wu CC, Hays LG, Eng JK, Yates JR, 3rd, Howell KE. Proteomics of rat liver Golgi complex: minor proteins are identified through sequential fractionation. Electrophoresis. 2000;21(16):3441–3459. doi: 10.1002/1522-2683(20001001)21:16<3441::AID-ELPS3441>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 15.Mogelsvang S, Howell KE. Global approaches to study Golgi function. Curr Opin Cell Biol. 2006;18(4):438–443. doi: 10.1016/j.ceb.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Misteli T, Warren G. COP-coated vesicles are involved in the mitotic fragmentation of Golgi stacks in a cell-free system. J Cell Biol. 1994;125(2):269–282. doi: 10.1083/jcb.125.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rabouille C, Kondo H, Newman R, Hui N, Freemont P, Warren G. Syntaxin 5 is a common component of the NSF- and p97-mediated reassembly pathways of Golgi cisternae from mitotic Golgi fragments in vitro. Cell. 1998;92(5):603–610. doi: 10.1016/s0092-8674(00)81128-9. [DOI] [PubMed] [Google Scholar]
- 18.Rabouille C, Misteli T, Watson R, Warren G. Reassembly of Golgi stacks from mitotic Golgi fragments in a cell-free system. J Cell Biol. 1995;129(3):605–618. doi: 10.1083/jcb.129.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang D, Mar K, Warren G, Wang Y. Molecular mechanism of mitotic Golgi disassembly and reassembly revealed by a defined reconstitution assay. J Biol Chem. 2008;283(10):6085–6094. doi: 10.1074/jbc.M707715200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang D, Xiang Y, Wang Y. Reconstitution of the cell cycle regulated Golgi disassembly and reassembly in a cell free system. Nat Protoc. 2010;5(4):758–772. doi: 10.1038/nprot.2010.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen X, Simon ES, Xiang Y, Kachman M, Andrews PC, Wang Y. Quantitative proteomics analysis of cell cycle regulated Golgi disassembly and reassembly. J Biol Chem. 2010;285(10):7197–7207. doi: 10.1074/jbc.M109.047084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Taguchi T, Warren G. Purification of rat liver golgi stacks. In: Celis J, editor. Cell biology: a laboratory handbook. 3. Elsevier Science; San Diego: 2006. pp. 33–39. [Google Scholar]
- 23.Wang Y, Seemann J, Pypaert M, Shorter J, Warren G. A direct role for GRASP65 as a mitotically regulated Golgi stacking factor. EMBO J. 2003;22(13):3279–3290. doi: 10.1093/emboj/cdg317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiang Y, Wang Y. GRASP55 and GRASP65 play complementary and essential roles in Golgi cisternal stacking. J Cell Biol. 2010;188(2):237–251. doi: 10.1083/jcb.200907132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Satoh A, Warren G. Mapping the functional domains of the Golgi stacking factor GRASP65. J Biol Chem. 2005;280(6):4921–4928. doi: 10.1074/jbc.M412407200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiang Y, Seemann J, Bisel B, Punthambaker S, Wang Y. Active ADP-ribosylation factor-1 (ARF1) is required for mitotic Golgi fragmentation. J Biol Chem. 2007;282(30):21829–21837. doi: 10.1074/jbc.M611716200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shilov IV, Seymour SL, Patel AA, Loboda A, Tang WH, Keating SP, Hunter CL, Nuwaysir LM, Schaeffer DA. The Paragon Algorithm, a next generation search engine that uses sequence temperature values and feature probabilities to identify peptides from tandem mass spectra. Mol Cell Proteomics. 2007;6(9):1638–1655. doi: 10.1074/mcp.T600050-MCP200. [DOI] [PubMed] [Google Scholar]