Abstract
Carbon (C) allocation strongly influences plant and soil processes. Short-term C allocation dynamics in ecosystems and their responses to environmental changes are still poorly understood.
Using in situ 13CO2 pulse labeling, we studied the effects of 1 wk of shading on the transfer of recent photoassimilates between sugars and starch of above- and belowground plant organs and to soil microbial communities of a mountain meadow.
C allocation to roots and microbial communities was rapid. Shading strongly reduced sucrose and starch concentrations in shoots, but not roots, and affected tracer dynamics in sucrose and starch of shoots, but not roots: recent C was slowly incorporated into root starch irrespective of the shading treatment. Shading reduced leaf respiration more strongly than root respiration. It caused no reduction in the amount of 13C incorporated into fungi and Gram-negative bacteria, but increased its residence time.
These findings suggest that, under interrupted C supply, belowground C allocation (as reflected by the amount of tracer allocated to root starch, soil microbial communities and belowground respiration) was maintained at the expense of aboveground C status, and that C source strength may affect the turnover of recent plant-derived C in soil microbial communities.
Keywords: 13C tracer experiment, carbohydrate pools, carbon allocation, microbial phospholipid fatty acid (PLFA), respiration, starch, sugar
Introduction
Plants allocate photoassimilated carbon (C) to above- and belowground organs to fuel their metabolism, to provide C skeletons for growth and to build up storage pools (Larcher, 2003). A considerable amount of plant-assimilated C is also transferred to mycorrhizal symbionts, fungal endophytes and the rhizosphere, where exuded energy-rich compounds can stimulate nutrient mineralization in the soil and facilitate plant nutrient uptake (Lambers et al., 2008; Jones et al., 2009).
Plant C allocation has been suggested to be strongly sink driven, photosynthates being preferentially transferred to tissues with the highest demand (Lambers et al., 2008). Thus, under light limitation, plants tend to allocate a higher proportion of assimilated C to aboveground organs, whereas, under reduced nutrient and/or water supply, they invest more C to the root system (‘functional equilibrium hypothesis’, e.g. Bloom et al., 1985; Kobe et al., 2010; Poorter et al., 2012). However, it has been argued that the observed C allocation patterns could also be the result of a more complex suite of processes: in addition to sink strength, the C source could have a shared effect (Farrar & Jones, 2000), and C storage need not be purely passive, but could also be an active process, operating at the expense of growth (Chapin et al., 1990; Sala et al., 2012). In addition, with regard to C allocation to the rhizosphere, it is not clear to what degree it is driven by the plant (source) or mycorrhizal communities (sink) (Wright et al., 1998; Grimoldi et al., 2006; Jones et al., 2009; Lendenmann et al., 2011).
C allocation has been well studied with regard to carbohydrate metabolism (e.g. Smith & Stitt, 2007; Zeeman et al., 2007; Gibon et al., 2009; Graf & Smith, 2011; Werner & Gessler, 2011) and biomass partitioning (Poorter et al., 2012) of (often young) individual plants grown under controlled conditions, and as broader long-term patterns in ecosystems (Litton et al., 2007). Much less is known about the short-term dynamics of C allocation in ecosystems and how it responds to changing environmental conditions (Brüggemann et al., 2011; Epron et al., 2012). An analysis of the dynamic response of C transfer between different carbohydrate pools in above- and belowground plant organs, and between plants and microbial communities (Paterson et al., 2009), would improve our understanding of the processes underlying C allocation patterns in ecosystems.
Isotopic tracer studies have revealed a rapid and close coupling between photosynthesis and belowground C allocation to roots, soil organisms and respiratory processes in forests and grasslands (e.g. Ostle et al., 2000; Johnson et al., 2002; Leake et al., 2006; Denef et al., 2007; Högberg et al., 2008, 2010; Bahn et al., 2009; Epron et al., 2011). Only few studies have analyzed how such allocation dynamics are affected by changing environmental conditions. Reductions in light, nutrient or water supply have been shown to generally delay and reduce the release of recently assimilated C in soil or root respiration (Bahn et al., 2009; Rühr et al., 2009; Lehmeier et al., 2010; Barthel et al., 2011). It is largely unclear how such effects are related to changes in plant carbohydrate pools, although there is evidence in model plants that the respiratory substrate supply system may be very flexible, involving adjustments in the proportion of storage and turnover of current assimilates (Smith & Stitt, 2007; Lehmeier et al., 2010). Allocation to (and mobilization from) C storage pools, in particular, is poorly understood. In addition, the transfer of photosynthetic C to the rhizosphere may be affected by changing resource supply, and has been shown to decrease with fertilization in grassland (Denef et al., 2009). Direct effects of altered supply of photoassimilates (as, for example, induced by shading) on the dynamics of plant–microbe C allocation have so far not been studied in an ecosystem.
Here, we assess the importance of C storage in above- and belowground plant organs in grassland, and address the question of whether assimilate supply affects the dynamics of C allocation in the plant–soil system. Specifically, we test the hypothesis that shading (i.e. reduced C source strength) affects the dynamics of recently assimilated C in nonstructural carbohydrates and diminishes its transfer to roots and their storage pools, and to soil microbial communities. For this, canopy sections of a mountain grassland were pulse labeled with highly enriched 13CO2, and the fate of assimilated tracer was chased over a 1-month period in unshaded and shaded plots.
Materials and Methods
Site
The study was carried out on a mountain meadow at Kaserstattalm, Neustift, Austrian Central Alps, as described by Bahn et al. (2009). In brief, the meadow is fertilized with manure in spring every 2–4 yr, cut once in late July or early August and is lightly grazed in September. The dominating plant species are perennial and include the grasses Anthoxanthum odoratum L. and Festuca rubra L., and the forbs Alchemilla vulgaris L., Leontodon helveticus L., Leontodon hispidus L. and Trifolium repens L. The soil is a dystric cambisol on siliceous bedrock with a topsoil pH of 5.5. The meadow is characterized by a comparatively high productivity and high soil respiration rates (Bahn et al., 2010; Schmitt et al., 2010). In the study year 2007, the meadow reached its peak biomass towards the end of July and was mowed in early August, immediately after the completion of the experiments. Peak above- and belowground biomasses of the site are in the range 240–440 and 420–980 g m−2, respectively (Bahn et al., 2006; Schmitt et al., 2010; M. Bahn et al., unpublished).
Experimental set-up and treatments
In each of three consecutive campaigns during the period of peak biomass, we studied one experimental block containing a control and a shaded plot, as well as two plots which were pulse labeled and subsequently shaded or left unshaded. Labeling was performed during the late morning hours (starting between 08:45 and 11:20 h CET) on 15 and 26 July and on 1 August 2007. Microclimatic conditions during the experiments are shown in Supporting Information Fig. S1. Pulse labeling was accomplished by covering canopy sections with a transparent (95% light transmission) Perspex chamber of 1 × 1 × 0.7 m3 and continuously adding a small amount of 99.9 atom% 13C-CO2 to the chamber air, as described in Bahn et al. (2009). The chamber air temperature was stabilized by ice packs mounted on the back side of the chamber in the air flow. The frozen ice packs also prevented the condensation of water vapor on the chamber walls during measurements. During labeling, the temperature was maintained at 2°C near ambient, except for the last 20 min during the third labeling experiment, when the chamber air temperature reached values of up to 29°C, whereas ambient air temperature was near 22°C. The amount of 13CO2 added was manually regulated with a mass flow controller at average rates of c. 40 ml min−1 to keep the chamber CO2 mixing ratio at 600–800 ppmv throughout the labeling. This range was chosen to maximize photosynthetic uptake of the label, assuming that the short exposure of plants to elevated CO2 would not affect the allocation and respiratory use of C in the plant–soil system (Bahn et al., 2009).
Shading of unlabeled and labeled plots was achieved with tents of 3 × 3 m2 ground area and 2 m height. The tents were covered with nontransparent plastic sheets. Small slots at the bottom of the tent and the four corners facilitated an exchange of air. In the centre of the tents, where the plots were located, 5–8% of the incoming photosynthetically active radiation (PAR) was incident on the vegetation, as measured with a PAR sensor (SunScan SS1, Delta-T, Cambridge, UK) during the course of a sunny day. Shading treatments were started 1 h after the pulse labeling had been completed and lasted for 6–8 d.
Sampling
Within each labeled and unlabeled plot, above- and belowground plant biomass and soil were sampled 2 (around noon; immediately before shading), 6, 12 and 34 h, 3.5 d, 1 wk (at the end of the shading treatment) and 1 month after the labeling had started. At each sampling, two 5 × 7-cm2 blocks of aboveground biomass and soil (10 cm depth, i.e. the main rooting horizon), located at the opposite ends of each plot, were harvested and mixed to obtain a pooled sample. Roots were separated from soil using tweezers, and root-free soil was immediately frozen using dry ice and stored in the laboratory at −20°C. Above- and belowground plant parts were killed and pre-dried by exposure to 1–2 min in a microwave, and oven dried to weight constancy on return to the laboratory. Aboveground biomass (subsequently termed ‘shoots’) included both leaves and stems, and belowground biomass (subsequently termed ‘roots’) comprised mostly fine roots and a minor amount of coarse roots, rhizomes and stolons.
Analysis of plant and microbial compounds and their C isotope composition
Isotopic analysis of above- and belowground plant material
After drying, the collected samples from above- and belowground plant components were ground to a fine powder with a steel ball mill (MM 200, Retsch, Haan, Germany). Aliquots of the powdered samples of 0.5–0.8 mg were weighed into tin capsules (Säntis Analytical AG, Teufen, Switzerland) and placed into an autosampler AS-128 and injected into an elemental analyzer (EA-1110 CHN, both Carlo Erba, Milan, Italy). Under excess oxygen, the samples were combusted. The resulting CO2 was transferred in helium carrier gas via a variable open split interface (Conflo II) to a sector mass spectrometer (Delta S, both Finnigan MAT, Bremen, Germany) for the determination of the isotope ratio.
Analysis of carbohydrates
Plant material was dried and ground in a ball mill before further processing. For the analysis of sucrose, 30 mg of plant material was extracted with 1.5 ml of deionized water at 85°C for 30 min. Samples were centrifuged and the supernatant was transferred to ion-exchange cartridges (OnGuard II H and A 1-cm3 cartridges; Dionex, Thermo Scientific, Vienna, Austria) to remove ionic components. The neutral fraction was analyzed by high-performance liquid chromatography-isotope ratio mass spectrometry (HPLC-IRMS) (for a description of the system, see Wild et al., 2010) on a HyperREZ XP Ca2+ column (Thermo Scientific) at 85°C with 0.5 ml min−1 of deionized water as eluent. Standards of glucose and sucrose at a range of concentrations were measured, interspersed with the samples, to calculate sucrose concentrations and to correct for offsets in sucrose 13C values during analysis (Wild et al., 2010). For the analysis of starch, 100 mg of plant material was digested with α-amylase (Göttlicher et al., 2006; Richter et al., 2009), and the resulting glucose was measured by elemental analysis-isotope ratio mass spectrometry (EA-IRMS) (EA 1110, CE Instruments, and Delta Plus IRMS, Thermo Scientific). In the following, we limit our presentation of sugars to that of sucrose for the following reasons: the concentration of glucose was clearly lower than that of sucrose (c. 10–20% of sucrose); glucose was only slightly labeled (< 10% of the label in sucrose); and changes in the concentrations of glucose followed broadly those of sucrose. A representative number of above- and belowground samples were also analyzed for fructan (high-molecular-mass but water-soluble carbohydrate) by high-performance liquid chromatography-pulsed amperometric detection (HPLC-PAD) on a CarboPack PA-100 column (Dionex Corporation) to exclude the possibility that a substantial amount of carbohydrate remains undetected, but no significant fructan concentrations could be found.
Analysis of phospholipid fatty acids (PLFAs)
PLFAs were extracted from frozen soil samples by a mixture of methanol, chloroform and citrate buffer (2 : 1 : 0.8, v/v/v), and then separated from neutral lipids on silica columns, and finally subjected to alkaline methanolysis, as described in detail by Koranda et al. (2011). Dried fatty acid methyl esters (FAMEs) were re-dissolved in isooctane, and the concentrations and C isotope ratios of PLFAs were determined by a Trace Ultra GC (Thermo Scientific) interfaced with an IRMS (Delta V Advantage, Thermo Scientific) via a combustion unit (GC combustion II/TC, Thermo Scientific). A mixture of FAMEs (Supelco; Sigma-Aldrich) was used as a qualitative standard. An internal standard (19 : 0) was used for the calculation of FAME concentrations, as well as for the correction of δ13C values. δ13C values of PLFAs were corrected for the δ13C values of C added during methanolysis. We used the sum of fatty acids i15:0, a15:0, i16:0, i17:0, a17:0 as an indicator of Gram-positive bacteria and the sum of fatty acids 16:1ω9, 16:1ω7, 18:1ω7, 18:1ω5, cy17:0, cy19:0, cy18:0 as an indicator of Gram-negative bacteria. PLFAs 16:1ω5 and 20:4ω6 were used as markers for arbuscular mycorrhizal fungi (AMF); 18:1ω9 and 18:2ω6 were used as general fungal markers (Denef et al., 2009; Kaiser et al., 2010a,b).
Calculation and expression of C isotope composition
For all plant samples, we expressed the results as the 13C atom% excess, which corresponds to the increase in 13C atoms caused by pulse labeling expressed as the percentage of total C atoms present in each sample, and was calculated as the difference between the 13C atom% of the labeled and unlabeled samples. For PLFA samples, we expressed the results as the amount of 13C incorporated, which was calculated by multiplying the 13C atom% excess by the amount of PLFA in each sample.
Measurements of leaf and root respiration
Leaf respiration was measured during early night-time (i.e. between 21:00 and 00:00 h) on five fully developed leaves of the dominant forb Leontodon helveticus, using a portable photosynthesis system (Li-6400, Li-Cor, Lincoln, NE, USA). Measurements were taken at the prevailing temperature of 15°C. Root respiration was measured during the daytime, as described by Bahn et al. (2006). At each sampling, six soil cores were extracted for each treatment, roots were carefully washed and fine roots (0–2 mm) were immediately measured in the field using a battery-operated gas exchange system (PLC-C connected to CIRAS-1, PPSystems, Hitchin, Hertfordshire, UK) (Bahn et al., 2006). The target temperature of 10°C, which corresponded to the soil temperature prevailing at the onset of the experiments, was achieved and stabilized using cooling boxes, in which the cuvette was placed.
Data analysis
The effects of the shading treatment on the time courses of carbohydrate concentrations and plant and microbial 13C atom% excess and incorporated 13C were tested using repeated-measures ANOVA with sampling time as repeat, treatment as fixed factor and plot as random factor. To meet uncertainties concerning the normal distribution of the data caused by low replication, treatment effects per sampling time were analyzed using nonparametric tests. For carbohydrate concentrations with four to six replicates per sampling time and treatment, we applied Mann–Whitney U-tests; on isotope data with two to three replicates, we performed exact permutation tests.
Results
Plant tracer dynamics in unshaded plots
At the first sampling, 2 h after the start of labeling, shoots and roots were both highly enriched in 13C, which reflects a rapid photosynthetic uptake and belowground translocation of labeled C (Fig. 1). This tracer signal was particularly pronounced for shoot sucrose and starch, and for root sucrose (Fig. 2a–c), which all showed similar tracer dynamics: following a rapid initial increase in 13C to values of 10–12.5 and 4.7 13C atom% excess in shoots and roots, respectively, the tracer content declined over time, amounting to < 0.4 13C atom% excess 1 wk after the pulse (Fig. 2a–c). By contrast, root starch continuously accumulated tracer at low rates, exhibiting the highest values (0.14 13C atom% excess) 1 month after the labeling pulse (Fig. 2d).
Fig. 1.
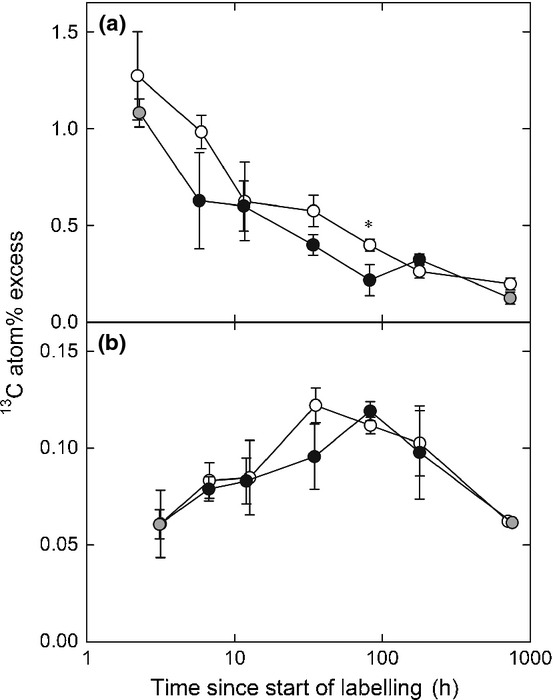
Time course of 13C atom% excess in (a) aboveground and (b) belowground plant biomass in unshaded (white circles) and shaded (black circles; gray circles indicate pre- and post-shading values) plots of a mountain meadow. *, Significant (P < 0.05) treatment effects for individual sampling dates.
Fig. 2.
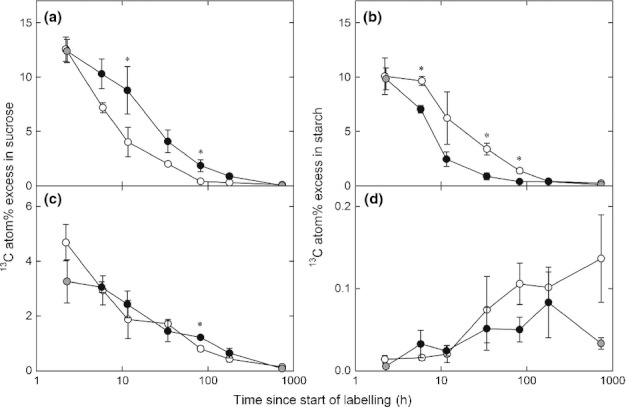
13C atom% excess in (a, c) sucrose and (b, d) starch of (a, b) aboveground and (c, d) belowground plant biomass in unshaded (white circles) and shaded (black circles; gray circles indicate pre- and post-shading values) plots of a mountain meadow. Error bars signify ± SE. *, Significant (P < 0.05) treatment effects for individual sampling dates.
Shading effects on plant carbohydrate concentrations, tracer dynamics and allocation
At the beginning of the experiments, mean shoot and root sucrose concentrations pooled across all plots were 16.2 and 6.3 mg g−1, respectively, and shoot and root starch concentrations were 8.9 and 24.5 mg g−1, respectively. Shoot sucrose and starch concentrations were reduced significantly by shading within half a day and, after 4–8 d of shading, amounted to only 31–39% (sucrose) and 37–45% (starch) of the values in control plots (Fig. 3, Table 1). By contrast, root sucrose and starch concentrations were not reduced significantly by shading (Fig. 3, Table 1). Three weeks after the shading experiment had been completed, shoot and root sucrose and starch concentrations showed no difference between previously shaded plots and control plots, and were generally lower than the values at the start of the experiment.
Fig. 3.

(a, c) Sucrose and (b, d) starch concentrations in (a, b) aboveground and (c, d) belowground plant biomass in unshaded (white circles) and shaded (black circles; gray circles indicate pre- and post-shading values) plots of a mountain meadow. Error bars signify ± SE. **, Significant (P < 0.01) treatment effects for individual sampling dates. Note that the first sampling was made around noon, the second in the afternoon, and the subsequent samples were taken at night.
Table 1.
Results of repeated-measures ANOVA to test for overall effects of treatment, time and time–treatment interactions in plant carbohydrate concentrations, 13C atom% excess of plant biomass and carbohydrates and incorporated 13C of soil microbial phospholipid fatty acids (PLFAs) in the mountain meadow
| Treatment | Time | Time × treatment | ||||
|---|---|---|---|---|---|---|
| F value | P value | F value | P value | F value | P value | |
| Sucrose concentration in shoots | 103.83 | < 0.001 | 0.30 | 0.874 | 6.13 | 0.001 |
| Sucrose concentration in roots | 1.14 | 0.313 | 1.16 | 0.345 | 1.06 | 0.391 |
| Starch concentration in shoots | 64.03 | < 0.001a | 5.20 | 0.002 | 10.13 | < 0.001 |
| Starch concentration in roots | 0.05 | 0.834a | 1.08 | 0.383 | 0.141 | 0.966 |
| 13C atom% excess in shoot biomass | 1.05 | 0.382 | 0.11 | 0.873b | 0.68 | 0.526b |
| 13C atom% excess in root biomass | 0.03 | 0.865 | 2.98 | 0.105b | 0.61 | 0.605b |
| 13C atom% excess in shoot sucrose | 6.90 | 0.079 | 7.97 | 0.041b | 1.53 | 0.299b |
| 13C atom% excess in root sucrose | 0.23 | 0.668 | 2.64 | 0.182b | 0.61 | 0.527b |
| 13C atom% excess in shoot starch | 7.08 | 0.076a | 3.97 | 0.135b | 1.41 | 0.321b |
| 13C atom% excess in root starch | 0.42 | 0.563a | 0.53 | 0.623b | 0.69 | 0.543b |
| 13C atom% excess in AMF PLFAsc | 31.23 | 0.113 | 0.97 | 0.506 | 1.31 | 0.457 |
| 13C atom% excess in fungal PLFAsc | 11.32 | 0.184- | 2.35 | 0.368b | 1.96 | 0.395b |
| 13C atom% excess in Gram-positive bacterial PLFAs | 0.70 | 0.557 | 10.28 | 0.193b | 1.49 | 0.438b |
| 13C atom% excess in Gram-negative bacterial PLFAs | 2947.86 | 0.012 | 0.64 | 0.571b | 1.19 | 0.473b |
| 13C atom% excess in all measured PLFAsc | 36.96 | 0.104 | 2.22 | 0.376b | 1.30 | 0.458b |
| Leaf respiration rate | 2.58 | 0.129 | 6.69 | 0.001 | 8.07 | < 0.001 |
| Root respiration rate | 4.67 | 0.059a | 3.91 | 0.01 | 1.11 | 0.368 |
Pre- and post-treatment data were excluded from the analysis. Bold values highlight significant effects at P < 0.05.
Violation of homoscedasticity in at least one dataset (P value of Levene test < 0.05).
Glasshouse-Geisser correction was applied because of violation of sphericity (P value of Mauchly test < 0.05).
Dataset of last sampling time was excluded to obtain consistent time series across all samplings.
Shading reduced the initial rate of decline in tracer content in shoot biomass (Fig. 1) and in shoot and root sucrose (Fig. 2a,c). It accelerated the initial decline in tracer content in shoot starch, causing consistently lower values of 13C excess during the first 3 d of shading (Fig. 2b). This was clearly reflected in the allocation dynamics of shoot carbohydrate pools (Fig. 4), indicating that the 13C pool in starch decreased by 15% within 3–4 h after shading, whereas the 13C pool in sucrose increased by 11% (Fig. 4b). The difference in tracer contents and pools in shoot sucrose and starch between shaded and unshaded plots disappeared largely after 1 wk of shading (Figs 2a,b, 4). In root starch, tracer contents increased more slowly in shaded relative to control plots (Fig. 2d), and accumulated a somewhat smaller fraction of assimilated tracer relative to controls. The relative amount of 13C allocated to the root starch pool was generally small (0.8–1.8% of the initial amount of 13C recovered in the measured carbohydrates; Fig. 4). Three weeks after the end of the shading experiment, tracer concentrations did not differ significantly between previously shaded and unshaded plots, except for root starch, which showed lower tracer concentrations in previously shaded plots (Fig. 2a–d), although the differences were not significant.
Fig. 4.
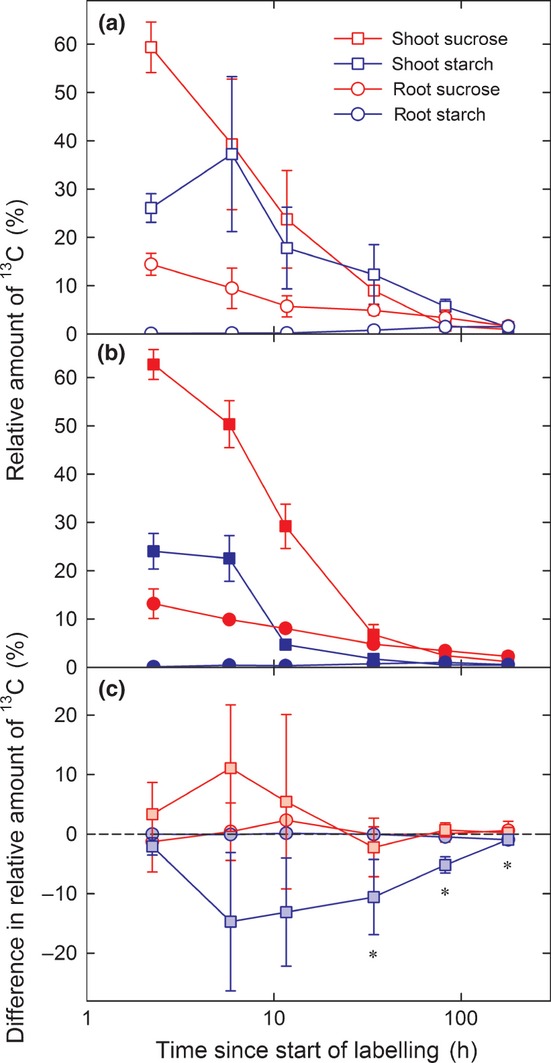
Relative amount of tracer 13C pools in shoot and root sucrose and starch in (a) unshaded (open symbols) and (b) shaded (closed symbols) plots of a mountain meadow, and (c) the difference in the respective relative pool sizes of shaded minus unshaded plots. The amounts of 13C in the respective pools are expressed as a fraction (%) of the total amount of 13C recovered in all of these carbohydrate pools at the first sampling (2 h after the start of labeling and immediately before the start of the shading experiment). Error bars signify ± SE. *, Values for shoot starch are significantly (P < 0.05) different from zero. The partitioning of pools is based on the assumption that biomass and root/shoot ratios remained constant during the week of shading.
Shading effects on leaf and root respiration
Shading caused a significant reduction in night-time leaf respiration rates at the reference temperature: by the end of the shading experiment, leaf respiration of Leontodon helveticus amounted to < 50% in shaded plots relative to control plots (Fig. 5a), when expressed on a dry weight basis. As a result of a significant (P < 0.001) decrease in leaf mass per area (LMA) from 33.2 to 26.7 g m−2 under shading, leaf respiration per unit leaf area was reduced to < 30% of the values measured in control plots. Shading had a less pronounced effect on specific root respiration at the reference temperature: after 1 wk of shading, it was reduced to 77% of the values observed in control plots (Fig. 5b).
Fig. 5.

(a) Specific (mass-based) night-time leaf respiration (at 15°C) of the dominant forb Leontodon helveticus and (b) specific fine root respiration (at 10°C) in unshaded (white circles) and shaded (black circles; gray circles indicate pre-shading values) plots of a mountain meadow. Error bars signify ± SE. * and **, Significant treatment effects for individual sampling dates at the levels of P < 0.05 and P < 0.001, respectively.
C transfer to microbial communities in unshaded and shaded plots
Four hours after the labeling had been completed, a first distinct tracer signal was detected in the PLFAs of fungal communities and Gram-negative bacteria (Fig. 6). For these microbial groups, the amount of 13C incorporated in the PLFAs of control plots peaked 2 d after labeling. One month after labeling, tracer content was reduced significantly in the fungal PLFAs, but had increased further in the PLFAs of Gram-negative bacteria (Fig. 6). During the first days after labeling, Gram-positive bacteria incorporated only negligible amounts of 13C into their PLFAs, and showed a significant tracer signal after 1 month.
Fig. 6.

Time course of the amount of 13C incorporated into phospholipid fatty acids (PLFAs) of microbial groups in unshaded (white bars) and shaded (black bars; gray bars indicate pre- and post-shading values) plots of a mountain meadow. (a) Arbuscular mycorrhizal fungal (AMF) PLFAs (16:1ω5, 20:4ω6), (b) general fungal PLFAs (18:1ω9c, 18:2ω6,9c), (c) Gram-positive bacterial PLFAs (i15:0, a15:0, i16:0, i17:0, a17:0), (d) Gram-negative bacterial PLFAs (18:1ω7, cy18:0(11/12), 16:1ω9, cyc17:0), (e) all PLFAs (including also general bacterial biomarkers). Error bars signify + SE. For individual sampling dates, treatment effects were not significant at P < 0.05.
Shading had no significant effect on 13C tracer content or dynamics (except for Gram-negative bacteria, Table 1); however, the peak values observed for PLFAs of AMF and Gram-negative bacteria tended to occur somewhat later, so that, at the end of the shading treatment (Fig. 6, 1 wk), slightly more 13C was incorporated relative to PLFAs in control plots, but the differences were not significant. Three weeks after the shading experiment ended, the amount of tracer in PLFAs of all microbial groups was similar for unshaded and previously shaded plots (Fig. 6). In general, no relationship between 13C in root sucrose and in PLFAs was found in unshaded and shaded plots (not shown).
Discussion
C allocation is a major process underlying the patterns of plant growth and biomass partitioning, rhizosphere processes and the coupling of photosynthesis and respiration in plants and terrestrial ecosystems (Brüggemann et al., 2011; Poorter et al., 2012). Unfortunately, our understanding of the multiple pathways and controls underlying C allocation is still rather limited, although progress has been made in model plants with regard to the role and controls of carbohydrate metabolism and its links to respiration and growth (Smith & Stitt, 2007; Zeeman et al., 2007; Graf & Smith, 2011). At the ecosystem scale, the assessment of pathways and responses of C allocation to environmental changes is particularly challenging, because of the large numbers of species and their interactions that typically constitute natural and semi-natural ecosystems, the fluctuating environmental conditions and spatial variability, and the technical limitations which complicate frequent in situ monitoring of the complete set of C fluxes between different ecosystem pools and compartments.
We assessed belowground C allocation in grassland and its response to extended shading by pulse labeling canopy sections with highly enriched 13CO2 and measuring the temporal dynamics of five complementary sets of parameters: (1) the 13C content in plant biomass and root and shoot sucrose and starch pools; (2) root and shoot sucrose and starch concentrations; (3) leaf and root respiration at the respective reference temperatures; (4) the 13C content in PLFAs of microbial groups; and (5) the 13C content and amount of soil-respired CO2 (published in Bahn et al., 2009). Isotopic pulse labeling permits an assessment of the allocation dynamics of C of a well-defined age (as assimilated during the short labeling period). However, it does not indicate the total amount of C being allocated in the system. Thus, temporal changes in sucrose and starch concentrations provide important complementary information on the allocation dynamics of the total nonstructural C pool. In addition, leaf and root respiration rates at reference temperatures indicate, to what extent, reductions in respiratory substrate pools under shading limit metabolic processes.
Tracer dynamics in plant carbohydrate pools
Tracer dynamics showed that the C assimilated during labeling was rapidly incorporated into shoot sucrose and starch pools and transferred below ground, a very distinct tracer signal appearing in root sucrose and, to a lesser extent, in starch within 2 h after labeling had started (Fig. 2). The tracer uptake and translocation were so rapid and substantial that they were also immediately evident in the total plant biomass (Fig. 1) and in belowground respiration (Bahn et al., 2009). Tracer dynamics in shoot starch were consistent with the notion that, during the daytime, a significant amount of newly assimilated C is deposited in starch and mobilized in the subsequent night (Zeeman et al., 2007; Graf & Smith, 2011): from the first (noon) to the second (afternoon) sampling, the amount of 13C in starch pools increased, but decreased sharply at night (Fig. 4a). C mobilized from starch at night has been suggested to be an important substrate for respiration and growth both above- and belowground (Smith & Stitt, 2007; Lehmeier et al., 2008; Barthel et al., 2011). Accordingly, the diurnal patterns in C isotope composition of soil-respired CO2 observed after labeling (Bahn et al., 2009) could be related to such diurnal patterns of transitory starch accumulation and remobilization. In contrast with shoot starch, root starch did not exhibit a reduction in tracer concentration during the week of the experiment, but continuously accumulated a small amount of 13C (Fig. 4a). This could indicate that root starch acts as a seasonal store, which is also supported by a higher starch-to-sucrose ratio in roots relative to shoots.
Shading triggered a rapid and extensive mobilization of C stores in shoot starch: shoot starch rapidly lost 13C, whereas shoot sucrose transiently increased its 13C pool (Fig. 4). By contrast, shading did not alter the pattern of tracer accumulation in root starch (Fig. 4). The implications are discussed further below (section ‘Effects of extended shading on C allocation’).
C transfer to microbial communities of unshaded and shaded plots
Our study has demonstrated a rapid transfer of fresh photoassimilates from leaves to microbial communities and a significant incorporation in PLFAs within a few hours, with peak values occurring after 1–2 d. This is in support of earlier grassland studies (Treonis et al., 2004; Leake et al., 2006; Denef et al., 2007; De Deyn et al., 2011), whereas, for forests, peak values of tracer recovery in microbial biomass have been reported after 3–6 d (Högberg et al., 2010; Epron et al., 2011).
The patterns of 13C incorporation by different microbial groups observed in our study are consistent with observations in earlier grassland studies: fungi (including AMF) and Gram-negative bacteria incorporate the tracer rapidly, whereas Gram-positive bacteria are characterized by a delayed uptake of labeled C. The rapid uptake of plant-derived C by fungi has been documented previously (Johnson et al., 2002; Treonis et al., 2004; Olsson & Johnson, 2005; Denef et al., 2007; Drigo et al., 2010; De Deyn et al., 2011) and is in line with the notion that AMF and other fungi have direct access to plant carbohydrates. Amongst nonmycorrhizal fungal groups, dark septate endophytes could have contributed to the tracer dynamics at our site, where they have been observed abundantly (M. Van der Heijden, pers. comm.). For Gram-negative bacteria, our study suggests a slightly faster 13C uptake than reported by Denef et al. (2007), demonstrating that, within < 12 h from the start of labeling, significant amounts of tracer were incorporated in their PLFAs. This suggests that Gram-negative bacteria were probably able to feed directly on root or fungal exudates, but they were also able to recycle C which had previously been incorporated in roots and/or other microbial groups, as indicated by peak values of 13C occurring 1 month after labeling.
Our results confirm previous observations that Gram-positive bacteria take up tracer more slowly than do fungi and Gram-negative bacteria (Olsson & Johnson, 2005; Denef et al., 2007), which has been attributed to an assimilation of C from fungal necromass or dead root material (Denef et al., 2007). This indicates that root exudates are only a minor source of C for the growth of Gram-positive bacteria, although they may potentially play an important role for an enhanced turnover of older soil organic matter by this microbial group through priming effects (Bird et al., 2011).
While the results for unshaded plots thus largely confirm and refine earlier findings on the close linkage between plants and microbial communities, our study provides novel insights as to how the supply of photoassimilates affects this linkage. Surprisingly, shading did not reduce the transfer of freshly assimilated C to soil microbial communities: neither the speed of transfer nor the amount of 13C incorporated in PLFAs was reduced by shading (Fig. 6). By contrast, the amount of recovered tracer tended to be higher in shaded plots. In unshaded plots, the amount of 13C incorporated in PLFAs of fungi and Gram-negative bacteria decreased after 2 d, whereas it remained constant (or increased slightly) under shading. It has been discussed previously that a decrease in tracer concentration in PLFAs could potentially be caused by a dilution of 13C by unlabeled fresh C via plant roots after pulse labeling (Denef et al., 2007; De Deyn et al., 2011; Balasooriya et al., 2012). To analyze tracer dynamics independently of such a possible dilution effect, we calculated the absolute amount of 13C contained in PLFAs (Fig. 6). The observed reductions in the amounts of 13C thus reflect solely the fact that the tracer previously incorporated in PLFAs was turned over (and subsequently respired, taken up by saprophytic organisms or remained in the soil solution). We conclude that the residence time of C in microbial groups characterized by a rapid incorporation of recent C (i.e. fungi and Gram-negative bacteria) is affected by assimilate supply. Possible mechanisms could be an increased lifetime (reduced turnover) under conditions of decreased C input and increased recycling of C contained in these microbial groups (i.e. a shift in predominant C source), as well as a change in C use efficiency (Manzoni et al., 2012).
Effects of extended shading on C allocation
It is commonly held that C allocation in plants is driven by the ‘demand’ and the ‘sink strength’ of different tissues/organs, although it is extremely difficult to quantify such parameters or to assess the relative role of source strength and of different sinks (Farrar & Jones, 2000; Poorter et al., 2012). We tested the response of C allocation dynamics to manipulations of the C source, and observed that sustained shading decreased carbohydrate pools above- but not belowground, and reduced leaf respiration more strongly than root respiration.
Notably, the response of leaf respiration to shading was delayed relative to that of carbohydrate pools, which is in line with an earlier study on alpine species, where, after a single day of shading, carbohydrates but not leaf respiration rates were consistently diminished (McCutchan & Monson, 2001). It has been suggested that, in response to extended shading, the pools of respiratory intermediates could be replenished by a switch to catabolism of proteins, cell walls and lipids (Brouquisse et al., 1998; Smith & Stitt, 2007). This might explain the observed partial decoupling of leaf carbohydrates and respiration rates. Root respiration was less affected by shading, which largely supports conclusions from a clipping experiment in the same grassland indicating that belowground C pools can buffer root metabolic activity over periods of at least 1–2 wk (Bahn et al., 2006). The observed reductions in root respiration might be explained by reduced C allocation to growth (and thus growth respiration) under extended darkness (Robson & Parsons, 1981; Smith & Stitt, 2007).
C allocation to storage has often been considered to be largely ‘passive’, resulting from an overflow of carbohydrates not used for respiration and growth (see review by Chapin et al., 1990). Interestingly, our results indicate that, even under severe C limitation, when aboveground carbohydrate pools were strongly depleted, leaf respiration was down-regulated and leaf mass per leaf area declined, recent C continued to be incorporated into belowground storage pools (i.e. starch). Such ‘active’ storage (Chapin et al., 1990) may play an underestimated role for the survival and growth under potentially C-limiting situations, as has been argued for trees only very recently (Sala et al., 2012; Wiley & Helliker, 2012). In mountain grassland, it could reflect an evolutionary adaptation to grazing, as well as the short growing season, which both require the presence of belowground storage pools for regrowth (Chapin et al., 1990; Donaghy & Fulkerson, 1998). By buffering against asynchrony between supply and C requirement of respiration and growth, ‘active’ storage is driven by a ‘demand’ extending beyond the immediate C requirements for maintenance and growth. Thus, even though the functional equilibrium hypothesis predicts a preferential allocation of C to aboveground organs in situations of severe light limitation, our results suggest that no major change in allocation occurred. More generally, it should be noted that C allocation to a storage pool is probably always the result of an active process, as, for example, reflected in the highly complex and dynamic relationships between sugar and starch pools between day and night (Smith & Stitt, 2007; Zeeman et al., 2007), and that therefore the expression ‘passive storage’ may need to be reconsidered altogether.
C allocation to soil microbial communities can be substantial, amounting to c. 5–20% of photosynthesis for AMF alone (Jakobsen & Rosendahl, 1990; Johnson et al., 2002; Grimoldi et al., 2006). It is unclear to what extent it is driven by the plant (source) or fungal/mycorrhizal communities (sink) (Grimoldi et al., 2006; Jones et al., 2009). There is evidence that C sink strength by AMF can stimulate rates of photosynthesis and thus C source strength (Wright et al., 1998; Kaschuk et al., 2009; Lendenmann et al., 2011). Our study showed that the amount of recent C incorporated into PLFAs of fungal (including AMF) and bacterial communities was not reduced by shading, that is, their C sink strength was not affected by reduced C source strength. This suggests that C allocation to mycorrhizae and the rhizosphere is strongly sink driven. However, it should also be noted that we did not quantify mycorrhizal (and rhizosphere) respiration, which may consume a large percentage of C allocated to AMF (Johnson et al., 2002; Grimoldi et al., 2006). Combining the findings that shading had only a minor effect on total soil respiration (Bahn et al., 2009) or root respiration (Fig. 5), and did not reduce the total amount of tracer recovered from soil-respired CO2 (Bahn et al., 2009; Fig. S2), we can nevertheless assume that the respiration (and its use of recent C) of AMF and other microbial communities was not reduced, and that therefore no reduction in overall rhizosphere sink strength occurred under reduced source strength.
Conclusions
In the grassland studied, shoot and root starch played very different roles for C storage. Shoot starch accumulated recent C rapidly, and shading triggered an immediate mobilization of this C store. By contrast, root starch, which received comparatively little C, acted as a long-term storage, whose behavior remained unaffected by shading.
Notably, belowground C allocation was insensitive to a distinct reduction in C source, as the amount of tracer allocated to root starch, incorporated into soil microbial communities and lost in belowground respiration was similar in shaded and unshaded plots. This supports the hypothesis of a preferential C flow to belowground plant functions (respiration and storage), and to fungal communities and rhizosphere microbes, at the expense of the C status of aboveground organs. However, the altered patterns of tracer residence times under shading also suggest an influence of C source strength on the utilization and turnover of recent plant-derived C in soil microbial communities.
Acknowledgments
We thank Sebastian Waldhuber, Zofia Garajova, Thomas Ladreiter-Knauss, Karin Bianchi, Vincent Saxl and Dominik Steigner for assistance with the experimental set-up and for help during sampling and analysis, and Karolien Denef for helpful discussions concerning PLFAs. This study was financially supported by the Austrian Science Fund (FWF) projects P18756-B16 and P22214-B17 and the Tiroler Wissenschaftsfonds (TWF). F.A.L. received funding from Deutsche Forschungsgemeinschaft (DFG/BMZ) (LA2390/1-1).
Supporting Information
Additional supporting information may be found in the online version of this article.
Fig. S1 Photosynthetically active radiation, air and soil temperature, and soil water content during the study period.
Fig. S2 Cumulative amount of tracer respired below ground in unshaded and shaded plots during the first and the second experiment.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
References
- Bahn M, Knapp M, Garajova Z, Pfahringer N, Cernusca A. Root respiration in temperate mountain grasslands differing in land use. Global Change Biology. 2006;12:995–1006. [Google Scholar]
- Bahn M, Reichstein M, Davidson EA, Grünzweig J, Jung M, Carbone MS, Epron D, Misson L, Nouvellon Y, Roupsard O, et al. Soil respiration at mean annual temperature predicts annual total across vegetation types and biomes. Biogeosciences. 2010;7:2147–2157. doi: 10.5194/bg-7-2147-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahn M, Schmitt M, Siegwolf R, Richter A, Brüggemann N. Does photosynthesis affect grassland soil-respired CO2 and its carbon isotope composition on a diurnal timescale? New Phytologist. 2009;182:451–460. doi: 10.1111/j.1469-8137.2008.02755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasooriya WK, Denef K, Huygens D, Boeckx P. Translocation and turnover of rhizodeposit carbon within soil microbial communities of an extensive grassland ecosystem. Plant and Soil. 2012 doi: 10.1007/s11104-012-1343-z [Author, if possible, please update the doi with vol and page range] [Google Scholar]
- Barthel M, Hammerle A, Sturm P, Baur T, Gentsch L, Knohl A. The diel imprint of leaf metabolism on the δ13C signal of soil respiration under control and drought conditions. New Phytologist. 2011;192:925–938. doi: 10.1111/j.1469-8137.2011.03848.x. [DOI] [PubMed] [Google Scholar]
- Bird JA, Herman D, Firestone MK. Rhizosphere priming of soil organic matter by bacterial groups in a grassland soil. Soil Biology and Biochemistry. 2011;43:718–725. [Google Scholar]
- Bloom A, Chapin F, Mooney H. Resource limitations in plants – an economic analogy. Annual Review of Ecology and Systematics. 1985;16:363–392. [Google Scholar]
- Brouquisse R, Gaudillere JP, Raymond P. Induction of a carbon-starvation-related proteolysis in whole maize plants submitted to light/dark cycles and to extended darkness. Plant Physiology. 1998;117:1281–1291. doi: 10.1104/pp.117.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüggemann N, Gessler A, Kayler Z, Keel SG, Badeck F, Barthel M, Boeckx P, Buchmann N, Brugnoli E, Esperschütz J, et al. Carbon allocation and carbon isotope fluxes in the plant–soil–atmosphere continuum: a review. Biogeosciences. 2011;8:3457–3489. [Google Scholar]
- Chapin F, Schulze ED, Mooney H. The ecology and economics of storage in plants. Annual Review of Ecology and Systematics. 1990;21:423–447. [Google Scholar]
- De Deyn GB, Quirk H, Oakley S, Ostle N, Bardgett RD. Rapid transfer of photosynthetic carbon through the plant–soil system in differently managed species-rich grasslands. Biogeosciences. 2011;8:1131–1139. [Google Scholar]
- Denef K, Bubenheim H, Lenhart K, Vermeulen J, Van Cleemput O, Boeckx P, Müller C. Community shifts and carbon translocation within metabolically-active rhizosphere microorganisms in grasslands under elevated CO2. Biogeosciences. 2007;4:769–779. [Google Scholar]
- Denef K, Roobroeck D, Wadu MCWM, Lootens P, Boeckx P. Microbial community composition and rhizodeposit-carbon assimilation in differently managed temperate grassland soils. Soil Biology and Biochemistry. 2009;41:144–153. [Google Scholar]
- Donaghy DJ, Fulkerson WJ. Priority for allocation of water-soluble carbohydrate reserves during regrowth of Lolium perenne. Grass and Forage Science. 1998;53:211–218. [Google Scholar]
- Drigo B, Pijl AS, Duyts H, Kielak A, Gamper HA, Houtekamer MJ, Boschker HTS, Bodelier PLE, Whiteley AS, van Veen JA, et al. Shifting carbon flow from roots into associated microbial communities in response to elevated atmospheric CO2. Proceedings of the National Academy of Sciences, USA. 2010;107:10938–10942. doi: 10.1073/pnas.0912421107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epron D, Bahn M, Derrien D, Lattanzi FA, Pumpanen J, Gessler A, Högberg P, Maillard P, Dannoura M, Gerant D, et al. Pulse-labelling trees to study carbon allocation dynamics: a review of methods, current knowledge and future prospects. Tree Physiology. 2012;32:776–798. doi: 10.1093/treephys/tps057. [DOI] [PubMed] [Google Scholar]
- Epron D, Ngao J, Dannoura M, Bakker MR, Zeller B, Bazot S, Bosc A, Plain C, Lata JC, Priault P, et al. Seasonal variations of belowground carbon transfer assessed by in situ13CO2 pulse labelling of trees. Biogeosciences. 2011;8:1153–1168. [Google Scholar]
- Farrar JF, Jones DL. The control of carbon acquisition by roots. New Phytologist. 2000;147:43–53. [Google Scholar]
- Gibon Y, Pyl E-T, Sulpice R, Lunn JE, Hihne M, Gunther M, Stitt M. Adjustment of growth, starch turnover, protein content and central metabolism to a decrease of the carbon supply when Arabidopsis is grown in very short photoperiods. Plant, Cell & Environment. 2009;32:859–874. doi: 10.1111/j.1365-3040.2009.01965.x. [DOI] [PubMed] [Google Scholar]
- Göttlicher S, Knohl A, Wanek W, Buchmann N, Richter A. Short-term changes in carbon isotope composition of soluble carbohydrates and starch: from canopy leaves to the root system. Rapid Communications in Mass Spectrometry. 2006;20:653–660. doi: 10.1002/rcm.2352. [DOI] [PubMed] [Google Scholar]
- Graf A, Smith AM. Starch and the clock: the dark side of plant productivity. Trends in Plant Science. 2011;16:169–175. doi: 10.1016/j.tplants.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Grimoldi AA, Kavanová M, Lattanzi FA, Schäufele R, Schnyder H. Arbuscular mycorrhizal colonization on carbon economy in perennial ryegrass: quantification by 13CO212CO2 steady-state labelling and gas exchange. New Phytologist. 2006;172:544–553. doi: 10.1111/j.1469-8137.2006.01853.x. [DOI] [PubMed] [Google Scholar]
- Högberg MN, Briones MJI, Keel SG, Metcalfe DB, Campbell C, Midwood AJ, Thornton B, Hurry V, Linder S, Näsholm T, et al. Quantification of effects of season and nitrogen supply on tree belowground carbon transfer to ectomycorrhizal fungi and other soil organisms in boreal pine forest. New Phytologist. 2010;187:485–493. doi: 10.1111/j.1469-8137.2010.03274.x. [DOI] [PubMed] [Google Scholar]
- Högberg P, Högberg MN, Göttlicher SG, Betson NR, Keel SG, Metcalfe DB, Campbell C, Schindlbacher A, Hurry V, Lundmark T, et al. High temporal resolution tracing of photosynthate carbon from the tree canopy to forest soil microorganisms. New Phytologist. 2008;177:220–228. doi: 10.1111/j.1469-8137.2007.02238.x. [DOI] [PubMed] [Google Scholar]
- Jakobsen I, Rosendahl L. Carbon flow into soil and external hyphae from roots of mycorrhizal cucumber plants. New Phytologist. 1990;115:77–83. [Google Scholar]
- Johnson D, Leake JR, Ostle N, Ineson P, Read DJ. In situ13CO2 pulse-labelling of upland grassland demonstrates a rapid pathway of carbon flux from arbuscular mycorrhizal mycelia to the soil. New Phytologist. 2002;153:327–334. [Google Scholar]
- Jones DL, Nguyen C, Finlay RD. Carbon flow in the rhizosphere: carbon trading at the soil–root interface. Plant and Soil. 2009;321:5–33. [Google Scholar]
- Kaiser C, Frank A, Wild B, Koranda M, Richter A. Negligible contribution from roots to soil-borne phospholipid fatty acid fungal biomarkers 18:2ω6,9 and 18:1ω9. Soil Biology and Biochemistry. 2010a;42:1650–1652. doi: 10.1016/j.soilbio.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser C, Koranda M, Kitzler B, Fuchslueger L, Schnecker J, Schweiger P, Rasche F, Zechmeister-Boltenstern S, Sessitsch A, Richter A. Belowground carbon allocation by trees drives seasonal patterns of extracellular enzyme activities by altering microbial community composition in a beech forest soil. New Phytologist. 2010b;187:843–858. doi: 10.1111/j.1469-8137.2010.03321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaschuk G, Kuyper TW, Leffelaar PA, Hungria M, Giller KE. Are the rates of photosynthesis stimulated by the carbon sink strength of rhizobial and arbuscular mycorrhizal symbioses? Soil Biology and Biochemistry. 2009;41:1233–1244. [Google Scholar]
- Kobe RK, Iyer M, Walters MB. Optimal partitioning theory revisited: nonstructural carbohydrates dominate root mass responses to nitrogen. Ecology. 2010;91:166–179. doi: 10.1890/09-0027.1. [DOI] [PubMed] [Google Scholar]
- Koranda M, Schnecker J, Kaiser C, Fuchslueger L, Kitzler B, Stange CF, Sessitsch A, Zechmeister-Boltenstern S, Richter A. Microbial processes and community composition in the rhizosphere of European beech – the influence of plant C exudates. Soil Biology & Biochemistry. 2011;43:551–558. doi: 10.1016/j.soilbio.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambers H, Chapin FS, III, Pons TL. Plant physiological ecology. New York, USA: Springer; 2008. [Google Scholar]
- Larcher W. Physiological plant ecology. 4th edn. Berlin, Germany: Springer; 2003. [Google Scholar]
- Leake JR, Ostle NJ, Rangel-Castro JI, Johnson D. Carbon fluxes from plants through soil organisms determined by field 13CO2 pulse-labelling in an upland grassland. Applied Soil Ecology. 2006;33:152–175. [Google Scholar]
- Lehmeier CA, Lattanzi FA, Gamnitzer U, Schaufele R, Schnyder H. Day-length effects on carbon stores for respiration of perennial ryegrass. New Phytologist. 2010;188:719–725. doi: 10.1111/j.1469-8137.2010.03457.x. [DOI] [PubMed] [Google Scholar]
- Lehmeier CA, Lattanzi FA, Schaeufele R, Wild M, Schnyder H. Root and shoot respiration of perennial ryegrass are supplied by the same substrate pools: assessment by dynamic 13C labeling and compartmental analysis of tracer kinetics. Plant Physiology. 2008;148:1148–1158. doi: 10.1104/pp.108.127324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lendenmann M, Thonar C, Barnard RL, Salmon Y, Werner RA, Frossard E, Jansa J. Symbiont identity matters: carbon and phosphorus fluxes between Medicago truncatula and different arbuscular mycorrhizal fungi. Mycorrhiza. 2011;21:689–702. doi: 10.1007/s00572-011-0371-5. [DOI] [PubMed] [Google Scholar]
- Litton CM, Raich JW, Ryan MG. Carbon allocation in forest ecosystems. Global Change Biology. 2007;13:2089–2109. [Google Scholar]
- Manzoni S, Taylor P, Richter A, Porporato A, Ågren GI. Environmental and stoichiometric controls on microbial carbon-use efficiency in soils. New Phytologist. 2012;196:79–91. doi: 10.1111/j.1469-8137.2012.04225.x. [DOI] [PubMed] [Google Scholar]
- McCutchan CL, Monson RK. Night-time respiration rate and leaf carbohydrate concentrations are not coupled in two alpine perennial species. New Phytologist. 2001;149:419–430. doi: 10.1046/j.1469-8137.2001.00039.x. [DOI] [PubMed] [Google Scholar]
- Olsson PA, Johnson NC. Tracking carbon from the atmosphere to the rhizosphere. Ecology Letters. 2005;8:1264–1270. [Google Scholar]
- Ostle N, Ineson P, Benham D, Sleep D. Carbon assimilation and turnover in grassland vegetation using an in situ13CO2 pulse labelling system. Rapid Communications in Mass Spectrometry. 2000;14:1345–1350. doi: 10.1002/1097-0231(20000815)14:15<1345::AID-RCM22>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Paterson E, Midwood AJ, Millard P. Through the eye of the needle: a review of isotope approaches to quantify microbial processes mediating soil carbon balance. New Phytologist. 2009;184:19–33. doi: 10.1111/j.1469-8137.2009.03001.x. [DOI] [PubMed] [Google Scholar]
- Poorter H, Niklas KJ, Reich PB, Oleksyn J, Poot P, Mommer L. Biomass allocation to leaves, stems and roots: meta-analyses of interspecific variation and environmental control. New Phytologist. 2012;193:30–50. doi: 10.1111/j.1469-8137.2011.03952.x. [DOI] [PubMed] [Google Scholar]
- Richter A, Wanek W, Werner RA, Ghashghaie J, Jäggi M, Gessler A, Brugnoli E, Hettmann E, Göttlicher SG, Salmon Y, et al. Preparation of starch and soluble sugars of plant material for the analysis of carbon isotope composition: a comparison of methods. Rapid Communications in Mass Spectrometry. 2009;23:2476–2488. doi: 10.1002/rcm.4088. [DOI] [PubMed] [Google Scholar]
- Robson MJ, Parsons AJ. Respiratory efflux of carbon-dioxide from mature and meristematic tissue of Uniculm barley during 80 hours of continuous darkness. Annals of Botany. 1981;48:727–731. [Google Scholar]
- Rühr NK, Offermann CA, Gessler A, Winkler JB, Ferrio JP, Buchmann N, Barnard RL. Drought effects on allocation of recent carbon: from beech leaves to soil CO2 efflux. New Phytologist. 2009;184:950–961. doi: 10.1111/j.1469-8137.2009.03044.x. [DOI] [PubMed] [Google Scholar]
- Sala A, Woodruff DR, Meinzer FC. Carbon dynamics in trees: feast or famine? Tree Physiology. 2012;32:764–775. doi: 10.1093/treephys/tpr143. [DOI] [PubMed] [Google Scholar]
- Schmitt M, Bahn M, Wohlfahrt G, Tappeiner U, Cernusca A. Land use affects the net ecosystem CO2 exchange and its components in mountain grasslands. Biogeosciences. 2010;7:2297–2309. doi: 10.5194/bg-7-2297-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A, Stitt M. Coordination of carbon supply and plant growth. Plant, Cell & Environment. 2007;30:1126–1149. doi: 10.1111/j.1365-3040.2007.01708.x. [DOI] [PubMed] [Google Scholar]
- Treonis AM, Ostle NJ, Stott AW, Primrose R, Grayston SJ, Ineson P. Identification of groups of metabolically-active rhizosphere microorganisms by stable isotope probing of PLFAs. Soil Biology and Biochemistry. 2004;36:533–537. [Google Scholar]
- Werner C, Gessler A. Diel variations in the carbon isotope composition of respired CO2 and associated carbon sources: a review of dynamics and mechanisms. Biogeosciences. 2011;8:2437–2459. [Google Scholar]
- Wild B, Wanek W, Postl W, Richter A. Contribution of carbon fixed by Rubisco and PEPC to phloem export in the Crassulacean acid metabolism plant Kalanchoe daigremontiana. Journal of Experimental Botany. 2010;61:1375–1383. doi: 10.1093/jxb/erq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley E, Helliker B. A re-evaluation of carbon storage in trees lends greater support for carbon limitation to growth. New Phytologist. 2012;195:285–289. doi: 10.1111/j.1469-8137.2012.04180.x. [DOI] [PubMed] [Google Scholar]
- Wright DP, Read DJ, Scholes JD. Mycorrhizal sink strength influences whole plant carbon balance of Trifolium repens L. Plant, Cell & Environment. 1998;21:881–891. [Google Scholar]
- Zeeman SC, Smith SM, Smith AM. The diurnal metabolism of leaf starch. Biochemical Journal. 2007;401:13–28. doi: 10.1042/BJ20061393. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


