Abstract
This study focuses on the analysis of polysaccharide residues from the cell walls of fruits and vegetables: tomato, potato, pumpkin, carrot and celery root. An alcohol-insoluble residue was prepared from plant material by extraction using the hot ethyl alcohol method and then cell wall fractions soluble in trans-1,2-diaminocyclohexane-N,N,N′,N′-tetraacetate, sodium carbonate and alkaline solution were sequentially extracted. Infrared spectroscopy combined with Fourier transform (FT-IR) was used to evaluate differences among cell wall residues and among species after each step of sequential extraction of pectins and hemicelluloses. Additionally, pectic substances were identified using an Automated Wet Chemistry Analyser. Principal component analysis (PCA) was applied to FT-IR spectra in two regions: 1,800–1,200 cm−1 and 1,200–800 cm−1 in order to distinguish different components of cell wall polysaccharides. This method also allowed us the possibility of highlighting the most important wavenumbers for each type of polysaccharide: 1,740, 1,610 and 1,240 cm−1 denoting pectins or 1,370 and 1,317 cm−1 denoting hemicelluloses and cellulose, respectively.
Keywords: FT-IR spectroscopy, Vegetable and fruit cell walls, Principal component analysis, Pectin, Hemicellulose
Introduction
The primary cell wall of fruits and vegetables mainly consists of various polysaccharides: pectins, hemicelulloses and cellulose, and comparably smaller amounts of structural glycoproteins, phenolic esters, minerals and enzymes [1, 2]. In the commonly accepted model of a plant cell wall, cellulose fibrils are embedded in matrix polysaccharides, which are pectins and hemicelluloses. Concentrations of each constituent vary according to the origin of the plant, the organs and the tissue [3]. Plant cell walls and their constitutive polysaccharide networks are vital with regard to the mechanical properties of the plant organ, such as stiffness or strength. In addition, the amount and structure of cell wall non-cellulosic polysaccharides changes during development and ripening, and again during senescence of fruit. During development and ripening, changes of cell wall properties are the result of compounds’ synthesis, whereas during post-harvest rather dominates degradation. Modifications of non-cellulosic polysaccharides also have a tremendous effect on structure and arrangement of cellulose microfibrils and hence on the mechanical properties of cell wall and plant tissue [3].
Infrared spectroscopy is one of the most frequently used spectroscopic techniques for studying plant cell wall material. The main advantage of this method is high sensitivity. Additionally, combination with Fourier transform (FT-IR) has provided a very rapid, versatile and relatively inexpensive tool for compounds’ identification. Transmission mode, attenuated total reflectance (ATR), diffusive reflectance and microspectroscopy are the most common among infrared spectroscopy methods [4, 5]. Among these techniques, ATR is a very convenient sampling method, which doesn’t require sample heating, pressing or grinding [6]. This method enables saving time and avoiding treatment that could lead to undesired structural changes of the sample.
The middle region (wavenumbers from 4,000 cm−1 to 400 cm−1) of the infrared radiation is most widely used in the study of biological objects [5–7]. The infrared absorption spectrum of each compound has its own unique pattern in the range from 1,400 cm−1 to 400 cm−1, often called the fingerprint region [8]. Additionally, the region from 1,800 cm−1 to 1,400 cm−1 could deliver information about functional groups occurring in investigated molecules. Infrared spectroscopy, as a very fast technique, has found application in carbohydrates’ investigation, especially for cell wall polysaccharides with the large diversity of pectins, hemicelluloses and cellulose. The polysaccharides show specific positioning and intensity of bands on the FT-IR spectra, therefore analysis of the 1,800–400 cm−1 region provides the possibility of specific carbohydrate recognition [5, 8].
Cellulose, which is an unbranched β(1,4)-linked glucan chain, is one of the most widely investigated polysaccharides. Pure cellulose exists in several crystalline polymorphs with different packing arrangements, which are known as cellulose I, II, III and IV. Cellulose I, which is the most abundant in nature, is a mixture composed of two distinct crystalline phases, cellulose Iα and Iβ, which have the same conformations, but differ in crystal structure—having triclinic (Iα) and monoclinic unit cells (Iβ). By means of infrared spectroscopy, both conformation changes accompanying the transition from cellulose I to cellulose III polymorph and identification of allomorphic type cellulose Iα or Iβ [9–12] have been observed. The hemicelluloses and pectins are a large family of cell wall compounds. The role of pectin and hemicelluloses is to bind to cellulose microfibrils in cell walls. Among the large variety of hemicelluloses, plant primary cell walls consist of xyloglucans and glucomannans, which are replaced by arabinoxylans in grass and cereal cell walls [13]. Xyloglucan coats the cellulose microfibrils, attaching to their surface as well as between microfibrils, limiting their aggregation. Absorption of xyloglucan on cellulose microfibrils is practically the same in the presence of low concentrations of pectin as in their absence. On the other hand, in the case of the presence of pectin, an increase of xyloglucan concentration causes a decrease of pectin absorption onto cellulose. The cellulose-pectin interaction seems to be weaker than the cellulose-xyloglucan interaction [14]. Pectin is believed to form an independent network, which works as a plasticizer and water binding agent [14, 15]. Pectins consist of compounds made up of a backbone of linear (1,4)-linked α-D-galacturonan and side chains mainly of α-D-galactopyranose, α-L-arabinofuranose and α -(1,2)-linked rhamnopyranosyl residue [5, 16–18].
The infrared investigation of pectic derivatives allows for the possibility of determining some functional groups which occur in pectic compounds [19]. Exploration of region 1,800–1,600 cm−1 offers the possibility of esterification degree estimation [20, 21]. Wellner et al. observed infrared band shift when investigating the interaction of divalent cations with potassium pectate, indicating a metal coordination by pectate [22]. Infrared spectroscopy of main hemicellulosic compounds covers identification of their specific bands and assignment of the monosaccharides constituting these compounds [5].
FT-IR has found application in monitoring of cell wall polysaccharides’ extraction, as well as cell wall changes during processing and quality control of fruits and vegetables [23, 24]. FT-IR spectroscopy combined with principal component analysis (PCA) has proven to be very useful for the fast evaluation of cell wall polysaccharides’ composition of pectic and hemicellulosic components derived from plant material when applied in the wavenumber region of 1,200–800 cm−1 [23–27]. It was also proven to be useful for determining structural and compositional changes of cell walls during the habituation of bean callus cultures treated by dichlobenil [8] or as result of thermal processing of pears [24]. Moreover, FT-IR with PCA was applied to determine the degree of amidation and methylesterification of pectic polysaccharides in plant cell wall extracts, and developed models were shown to be very consistent [19–21, 27].
FT-IR most frequently analysis was performed on supernatant containing pectic and hemicellulosic extracts, for example, from olive pulp and sun-dried pear cell walls [23–25, 28]. However, the method has not been applied yet for the analysis of cell wall residues directly obtained after the extraction. Therefore, this paper will try to identify cell wall polysaccharides according to their occurrence in each residue of extraction by FT-IR spectroscopy. The aim of this study is the analysis of differences in composition among cell wall residues and among plant species (fruits and vegetables) using FT-IR spectroscopy in association with PCA. This paper proposes the identification of specific wavenumbers that could discriminate the different polysaccharide constituents of cell walls according to their FT-IR spectra within two specific regions: 1,800–1,200 cm−1 and 1,200–800 cm−1.
Materials and Methods
Cell Wall Polysaccharide Extraction
The parenchyma samples of five species: pumpkin (Curcurbita L. cv. Bambino), celery (Apium graveolens L. var. rapaceum Mill. cv. Edward), carrot (Daucus carota L. cv. Finezja), tomato (Lycopersicon esculentum Mill. cv. Plomien) and potato (Solanum tuberosum cv. Irga), which were purchased at a local grocery market, were used for preparation of cell wall material. Cell wall material (CWM) from the species was isolated using the modified hot alcohol insoluble solids method as proposed by Renard (2005) [29]. Briefly, the fresh slices of material were ground by several bursts of a kitchen processor (Zauberstab, ESGE, Hockenheim, Switzerland), then dropped in hot alcohol (ethanol 70 %, temperature approx 82–85 °C) and left for 20 min of boiling. Then material was filtered through 65 G paper filter and then washed successively in 70 % and 96 % ethanol until the test for sugar presence using the phenol-sulphuric method of Dubois et al. 1956 [30] was negative, and finally placed in acetone. The sample was vacuum dried.
The obtained cell walls were further subjected to fractionation using the method proposed by Redgwell et al. (2008) [31] with some modifications. The CWM (450 mg) was stirred in 0.1 M trans-1,2-diaminocyclohexane-N,N,N′,N′-tetraacetic acid, (CDTA, pH 6.5, 25 mL) at 25 °C for 6 h. Then it was filtered and the residue disolved in 0.05 M CDTA (pH 6.5, 25 mL), stirred at 25 °C for 2 h and again filtered. The residue of this step has been called CDTA residue.
Then, the CDTA residue was stirred in 0.05 M sodium carbonate (Na2CO3) (30 mL) with the addition of 20 mM sodium borohydrite (NaBH4) for approximately 20 h at 1 °C, filtered, and again stirred for 2 h at 20 °C. The residue of this step has been called Na2CO3 residue.
Then, the Na2CO3 residue (depectinated cell walls) was stirred firstly in 12 mL of 0.5 M potassium hydroxide (KOH) and then in 12 mL of 1 M KOH, both with an addition of 20 mM sodium borohydride (NaBH4) each for 2 h at 20 °C every time. The residue of this step has been called KOH residue.
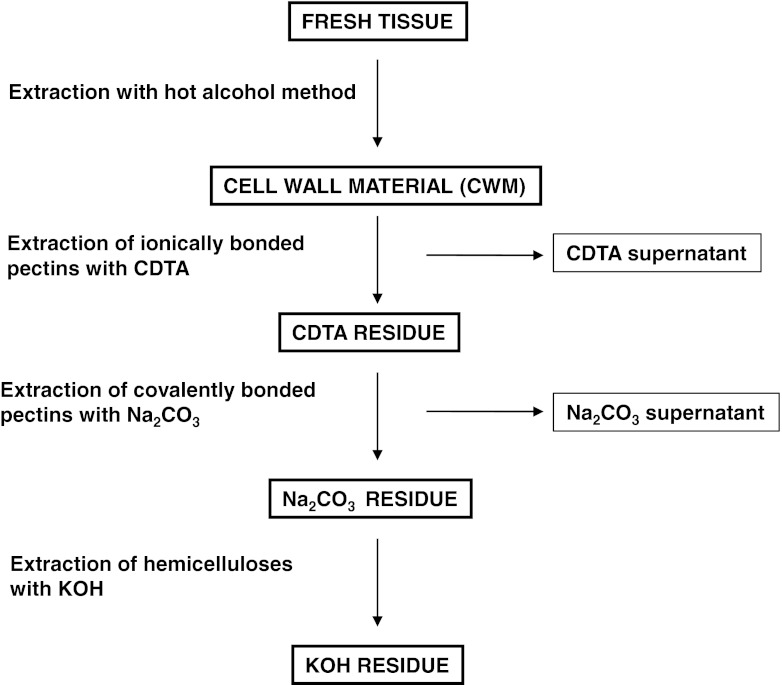
All KOH residues from tissue were rinsed several times in deionised water and finally stirred in deionised water all night. At all stages of filtration a nylon filter (Millipore, pore size 11 μm) was used. At every step of extraction lyophilization of each residue was performed and lyophilized material was weighted. The steps of extraction are presented in Fig. 1.
Fig. 1.
Steps of plant material extraction
All chemicals used for extraction were purchased from Sigma Aldrich and were used without further purification.
Additionally, to obtain exemplary infrared spectra of pectins, hemicelluloses and cellulose the following commercially available compounds were utilized: high methylated (degree of methylation 80 %) and low methylated (degree of methylation 30 %) pectins (Herbstreit and Fox, Neuenbürg, Germany), microcrystalline cellulose (powder, particle size ~ 20 μm, Sigma Aldrich) and xyloglucan (tamarind, purity >95 %, Megazyme, Bray, Ireland) as the one of the most common hemicelluloses occurring in fruits and vegetables.
FT-IR Spectroscopy
FT-IR spectra were collected with the use of a Nicolet 6700 FT-IR spectrometer (Thermo Scientific, Waltham, MA, USA) with the Smart iTR ATR sampling accessory. Each cell wall polysaccharide residue was applied on ATR as powder. The spectra were collected over the range 4,000–650 cm−1. For each material, five samples under the same conditions were examined. For each sample, 200 scans were averaged with a spectral resolution of 4 cm−1. Then for a given material, a final average spectrum was calculated. Baseline corrections were obtained using Omnic Software (Thermo Scientific).
Pectin Content
The chemical analysis of pectin content in the CDTA and Na2CO3 supernatant was performed with Automated Wet Chemistry Analyser also known as a Continuous Flow Analyser (San ++, Skalar Analytical, The Netherlands). This is an automated procedure for colorimetric determination of galacturonic acid based on total decomposition of pectin (hydrolysis with sulfuric acid) sample in acidic medium. The obtained products are transformed to furfuric derivatives, which when reacting with 3-phenyl phenol form a coloured dye, which is measured at 530 nm. The pectin content is expressed as galcturonic acid content in the dry mass of cell wall material (mg/g).
Principal Component Analysis
Principal component analysis (PCA) is one of the most commonly used chemometric methods for data reduction and exploratory analysis of high-dimensional data sets. The main goal of PCA is to obtain a small set of principal components (PC) that explain the most variability on these data sets. PCA creates a new sub-space defined by PCs, easier to interpret than original data. It usually allows the recognition and highlighting of characteristics and their correlation to physico-chemical properties of the sample. This method is especially useful in the interpretation of FT-IR spectra, which show band diversity and complication depending on the source of the sample. The Unscrambler 10.1 (Camo Software AS., Norway) was used for performing PCA. Each wavelength of FT-IR was treated as variable and they were weighted by dividing by standard deviation.
Result and Discussion
FT-IR Spectra
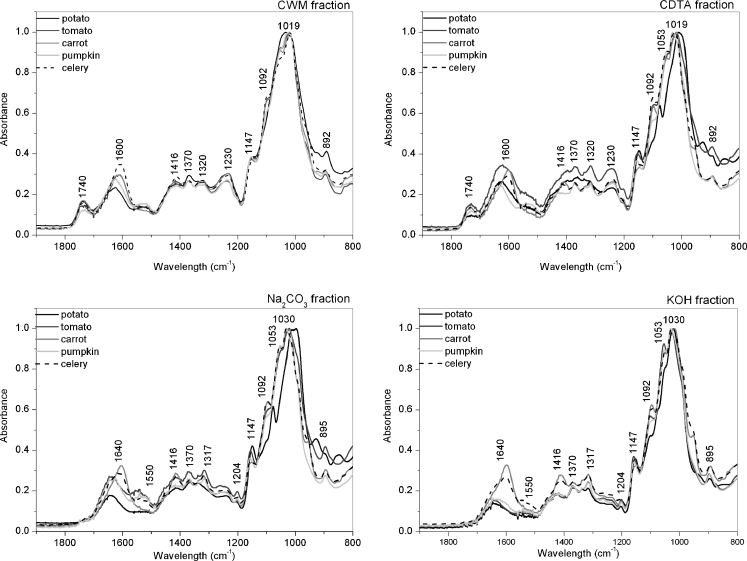
Figure 2 presents the spectra of investigated residues of fruits and vegetables after each step of extraction in the range of 1,800–650 cm−1. These spectra were normalized to 1.0 at 1,020 cm−1 (C-O stretching vibration). The analysis of FT-IR spectra showed that each particular polysaccharide has a maximum of specific bands in the region 1,800–800 cm−1 that are presented in Table 1. Additionally, there is in Table 1 comparison of infrared band assignments for cell wall polysaccharides found in literature as well as obtained in this experiment from commercially available polysacchrides. In the case of plant cell wall, polysaccharides are bonded with each other, therefore the shift of bands can be observed. The spectra in this region are characterized by strong absorption bands for specific wavenumbers, which could be assigned to pectic, hemicellulosic and cellulosic polysaccharides (Fig. 2) [32, 33]. Figure 3 depicts FT-IR spectra of pure plant cell wall polysaccharides.
Fig. 2.
FT-IR spectra in 2,000–650 cm−1 region of four residues after each step of extraction
Table 1.
Comparison of FT-IR absorption bands for assignment of cellulose, pectin and xyloglucan obtained in present experiment (column a) with previous results from refs [6, 20, 32–35] (column b). In column e, the residues of which spectra contains given frequency are denoted
| a | b | c | d | e |
|---|---|---|---|---|
| Frequency range (measured) | Frequency range (literature) | Assignment | Origin | Comment |
| 1740-1730 (P) | 1745-1740 | C = O stretching vibration of alkyl ester | Pectin | CWM and CDTA residue |
| – | 1650 | Amid I C-N stretching | Proteins | Mainly in Na2CO3 residue |
| – | 1640 | H-O-H bending vibration | Absorbed water | Mainly in Na2CO3 residue |
| 1600 (P) | 1630–1600 | COO- antisymmetric stretching | Polygalacturonic acid, carboxylate (pectin ester group) | Mainly CWM and CDTA residue |
| – | 1550 | Amid II N-H deformation | Proteins | |
| 1428 (C) | 1426 | CH2 symmetric bending | Cellulose | All residue, most intensive KOH residue |
| 1410 (P) | 1400 | COO- symmetric stretching | Carboxylate (pectin ester group) | CWM and CDTA residue |
| 1362 (XG, C) | 1370 | CH2 bending | Xyloglucan, Cellulose | All residues |
| 1330 (P) | 1320 | Ring vibration | Pectin | CWM and CDTA residue |
| 1313 (C) | 1317 | CH2 symmetric bending | Cellulose | KOH residue |
| 1268-1230 (P) | 1243 | C–O stretching | Pectin | CWM and CDTA residue |
| 1160 (C) | 1160 | O–C–O asymmetric stretching | Cellulose (glycosidic link) | All residues |
| 1143 (P) | 1146 | O–C–O asymmetric stretching | Pectin (glycosidic link) | All residues |
| 1147 (XG) | 1130 | O–C–O asymmetric stretching | Xyloglucan (glycosidic link) | None |
| 1103 (C) | 1115 | C–O stretching, C–C stretching | Cellulose (C2-O2) | KOH residue |
| 1093 (P) | 1100 | C–O stretching, C–C stretching | Pectin (ring) | None |
| 1071 (XG) | 1075 | C–O stretching, C–C stretching | Xyloglucan (ring) | None |
| Absence | 1042 | C–O stretching, C–C stretching | Xyloglucan (ring) | None |
| 1030 (C) | 1030 | C–O stretching, C–C stretching | Cellulose (C6-H2-O6) | |
| 1014 (P) | 1019 | C–O stretching, C–C stretching | Pectin (C2-C3, C2-O2, C1-O1) | KOH fraction |
| 1000 (C) | 1000 | C–O stretching, C–C stretching | Cellulose (C6-H2-O6) | Na2CO3 residue (potato) |
| 954 (P) | 960 | CO bending | Pectin | None |
| 941 (XG) | 944 | Ring vibration | Xyloglucan | None |
| 899 (C), 893 (XG) | 895 | C1-H bending | Xyloglucan, Cellulose (β-anomeric link) | All residues |
| 832 (P) | 833 | Ring vibration | Pectin | CDTA and NA2CO3 residue (potato) |
| Bands not assigned | ||||
| 1416 | XG | |||
| 1204 | C, XG | |||
| 1053 | C | |||
| 1019 | XG | |||
| 888 | P | |||
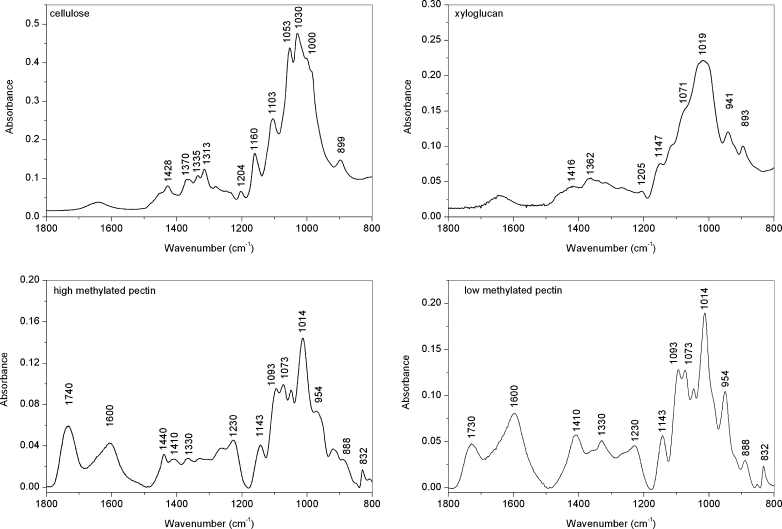
Fig. 3.
FT IR spectra of high- and low-methylated pectins, cellulose and xyloglucan in the range of 1,800–650 cm−1
The spectra of CWM and CDTA residues have very similar bands denoting pectins at: 1740, 1600, 1230, 1019, xyloglucan: 1370, 1147 and cellulose 895 cm−1. However, some bands are sharper and more intensive if CDTA is compared to CWM. For the Na2CO3 residue, the spectra bands, denoting xyloglucan and cellulose, become more visible at 1147 (XG) and 1030 (C) cm−1 (Table 1). These bands were probably masked in CDTA and CWM by bands corresponding to pectins. The KOH residue consists of mainly cellulose, but also branched pectins and hemicelluloses, which are usually difficult to remove [34]. The infrared spectrum bands characteristic for cellulose are at 1,370, 1,317 cm−1 (which are the sharpest for KOH spectra) and also at 1,097, 1,030 and 1,000 cm−1. The band visible in Na2CO3 and KOH spectra at 1,640 cm−1 probably correspond to water in the samples [35, 36]. Additionally, this band is affected by an amid I band centred at 1,650 cm−1, which indicates the presence of plant proteins. The presence of proteins is also confirmed by the existence of a band at 1,550 cm−1, which corresponds to amid II N-H vibration [20].
The main difference between spectra of CWM and CDTA residues, and Na2CO3 and KOH residues is revealed in the range of ester bands, which do not exist in the latter case. Significant differences can be noticed in the content of each polysaccharide and pectin esterification degree, as well as the intensity, shape and ratio of infrared bands situated around 1,740 cm−1 (C = O, ester carbonyl groups stretching) and 1,630–1,600 cm−1 (COO− carboxylate ion stretching band). Examples of FT-IR spectra of pectins with different esterification degree are shown in Fig. 3, where additionally cellulose and xyloglucan spectra are presented. The higher degree of esterification is reflected as the higher peak intensity and a peak shift to larger wavenumber band around 1,740 cm−1, and simultaneously, a decrease of the band situated at 1,600 cm−1 (Fig. 3). These spectra features have been previously used for evaluation of pectins’ degree of esterification [6, 20, 21], even though the consistency of the determination would be affected by water band (around 1,650 cm−1).
PCA
The most preferable region of FT-IR spectra for carbohydrates’ analysis is 1,200–850 cm−1, which is not practically influenced by bands derived from proteins and water molecules, but the region of 1,800–1,200 cm−1 was also previously used for PCA [23–26, 28]. The wavenumbers in the narrow region of 1,800–1,500 cm−1 are related to the carbonyl esters’ and carboxylates’ vibration, which reflects the pectic substances’ content [20, 21]. The region at 1,200–850 cm−1 is dominated by stretching vibrations of C – O, C – C, ring structures and deformation of CH2 groups’ vibration characteristic for polysaccharides and is therefore useful in their identification [17]. However, the bands characteristic for each polysaccharide are located closely to each other, and in the case of a polisaccharides’ mixture, this would cause problems with detection due to absorbance overlapping [5]. The common methods of polysaccharide extraction are imperfect hence in the very last residue, besides the cellulose, branched pectins and hemicelluloses are also present [34]. It can clearly be seen from a comparison of Figs. 2 and 3 that in the spectra of KOH residue, where only bands for pure cellulose should be present, in fact bands characteristic for pectic and hemicellulosic polysaccharides could also be found. This suggests implementing the PCA to both regions 1,800–1,200 cm−1 and 1,200–850 cm−1 to obtain the most complete information about investigated samples.
The PCA was carried out for 1) distinction cell wall residues within the studied species in terms of polysaccharide content and 2) revealing differences between species for the studied cell wall residues. The analysis were carried out within the two spectral ranges of FT-IR spectra. In each variant, three principal components were enough to explain differences among samples. For each residue/species sample, five FT-IR spectra were recorded which are replicates shown in PCA graphs.
Discrimination of Cell Wall Residues
1800–1200 cm−1 Region
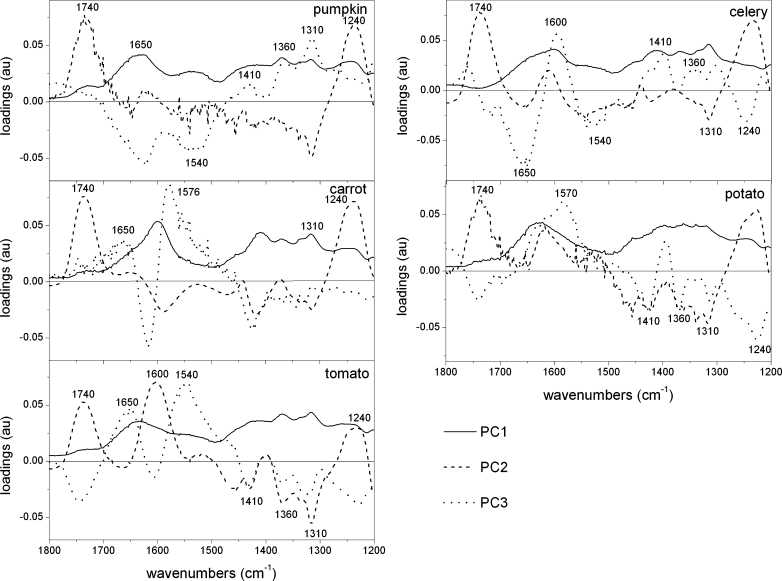
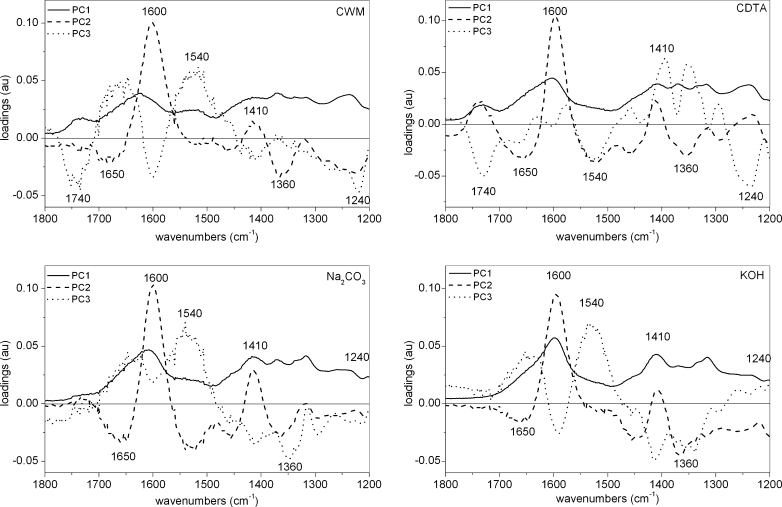
The scores scatter plots PC1 (explained above 90 % of variability)×PC2 (explained around 2–8 % of variability) were used to obtain separation of each group in the 1,800–1,200 cm−1 region (Fig. 4). Although the major of variability was explained by PC1, it seems that PC1 only varies scores within each group, indicating that PC1 could reflect the standard deviation of recorded spectra. The scores scatter plot PC1 vs. PC2 (Fig. 4) indicates that the cell wall residues are grouped along the PC2 axis and scattered along the PC1 axis. The scores of residues abundant in pectic substances (CWM and CDTA) lay on the positive side of PC2 scores, while residues Na2CO3 and KOH, which are rich in hemicelluloses and celluloses, were on the negative side of PC2 (Fig. 4). In Fig. 4 the tendency for the location of scores in four groups in a pattern going from positive to negative values of PC2:CWM, CDTA, Na2CO3 and KOH residue, which could reflect the subsequent steps of extraction, can be seen. Variable loadings for PC2 presented in Fig. 5 show peaks at 1,740, 1,610 and 1,230 cm−1 that are characteristic for the wavenumbers corresponding to pectic polysaccharides (Fig. 5). Whereas negative PC2 loading appeared for wavenumbers at 1,370 and 1,317 cm−1 characteristic for hemicellulosic and cellulosic polysaccharides, respectively.
Fig. 4.
PCA scores scatter plots of tomato, potato, carrot, celery and pumpkin cell wall FT-IR spectra in the 1,800–1,200 cm−1 region after sequential extraction of pectins and hemicelluloses
Fig. 5.
PCA loadings plots (PC1, PC2 and PC3) of tomato, potato, carrot, celery and pumpkin cell wall FT-IR spectra in the 1,800–1,200 cm−1 region after sequential extraction of pectins and hemicelluloses
The plot of PC2×PC3 (PC3 explains about 1 % of variability), which is not shown in this paper, revealed less clear groupings and interpretation of the loadings was not as straightforward as it was in the case of the first two components (loading along PC3 are shown in Fig. 5). The significant bands of positive loading in PC3 were at 1,650 (carrot, tomato) and 1,550 (tomato) cm−1, which corresponds to amid I and amid II vibrations, respectively, and denotes the presence of proteins in plant cell wall material. However, in the case of pumpkin and celery, these wavelengths had negative impact on the scores. Also confusingly, positive values of loading were observed for wavenumbers at 1,410, 1,370 and 1,317 cm−1 (cellulose bands) for pumpkin and celery samples, whereas for potato, carrot and tomato samples, loadings for these wavelengths were negative (Fig. 5).
1200–850 cm−1 Region
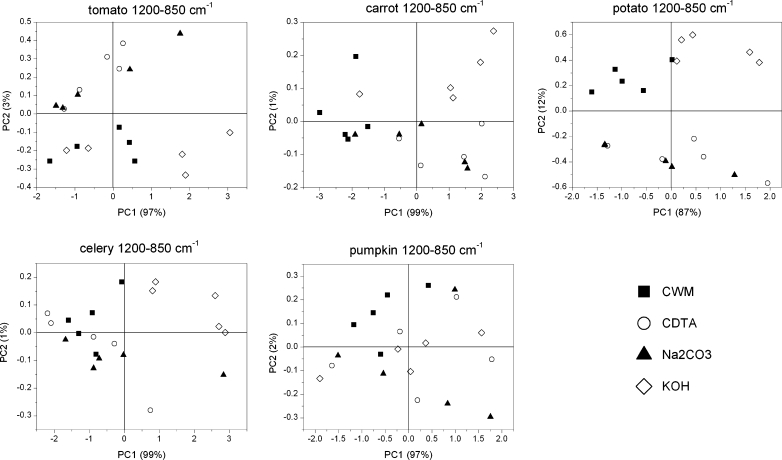
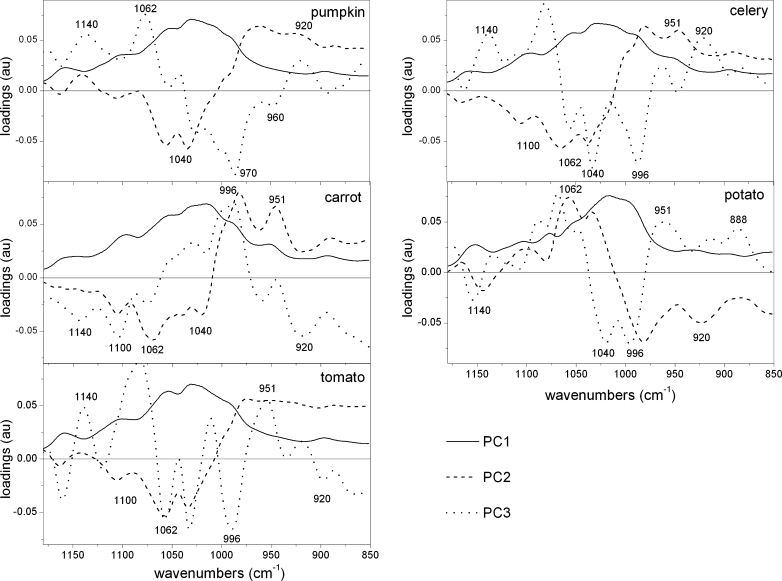
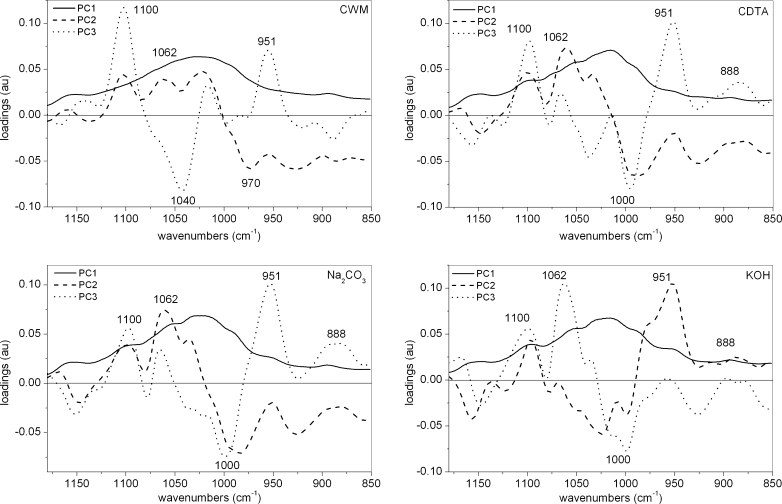
Considering the region of 1,200–850 cm−1, the scores scatter plots PC1 (explained above 87 % of variability)×PC2 (explained up to 12 % of variability), showed clear cell wall residue grouping only in the case of potato and tomato samples (Fig. 6). PC3 explains around 1 % of variability (not schown). The PC1 explained the majority of variability and its loadings had only positive values (Fig. 7), however, scores within groups are randomly scattered along PC1 axis. Common among species, significant bands on PC2 loading have positive influence (apart from potato samples) on scores around 970 cm−1 (pectins) and negative around 1,035 cm−1 (cellulose) (Fig. 7). While for PC3 loadings, the wavenumbers which positively influence the scores are around 1,070 cm−1, denoting xyloglucan, and around 1,140 and 960 cm−1 denoting pectins (apart from carrot samples). Loadings with negative values usually occur at 1,030 and 996 cm−1, which are close to wavenumber characteristics for cellulose vibration bands.
Fig. 6.
PCA scores scatter plots of tomato, potato, carrot, celery and pumpkin cell wall FT-IR spectra in the 1,200–850 cm−1 region after sequential extraction of pectins and hemicelluloses
Fig. 7.
PCA loadings plots (PC1, PC2 and PC3) of tomato, potato, carrot, celery and pumpkin cell wall FT-IR spectra in the 1,200–850 cm−1 region after sequential extraction of pectins and hemicelluloses
To summarize this part, the two bands analysed above suggest that for the each sample the region of 1,800–1,200 cm−1 discriminates cell wall residues. Each residue was well separated and scores varied the most significantly along the PC2. The loadings indicate that positive influence on scores have wavenumbers denoting pectins (1,740, 1,610 and 1,240 cm−1) and negative on scores have wavenumbers denoting hemicelluloses and cellulose (1,370 and 1,317 cm−1, respectively). Therefore, residues with high content of pectins (CWM and CDTA) lie on the positive side of the PC2 axis and residues with only hemicellulose and cellulose content (Na2CO3 and KOH) lie on the negative side.
Discrimination of Species
1800–1200 cm−1 Region
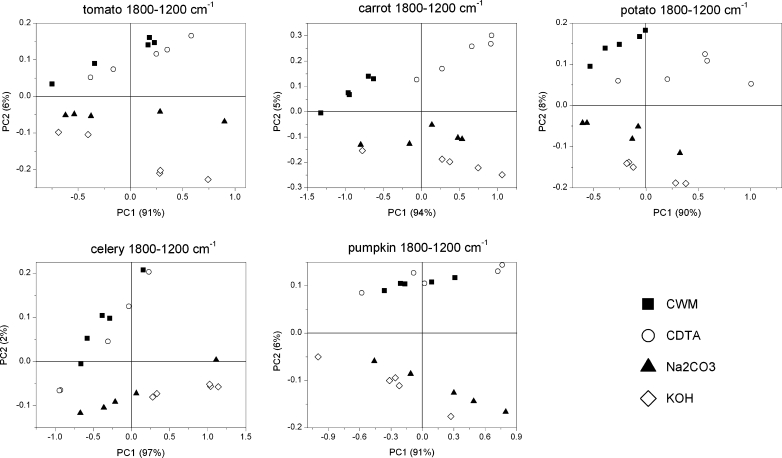
Similar to cell wall residue discrimination, the scores scatter plots PC1×PC2 and PC2×PC3 were used to separate the five investigated species.
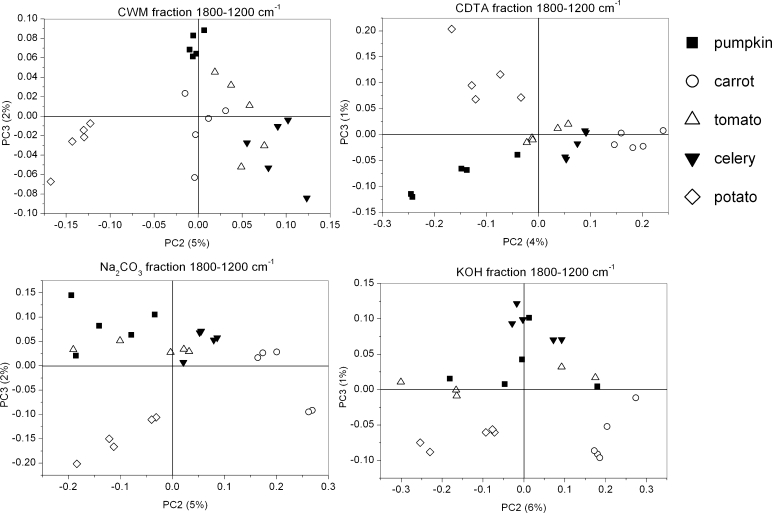
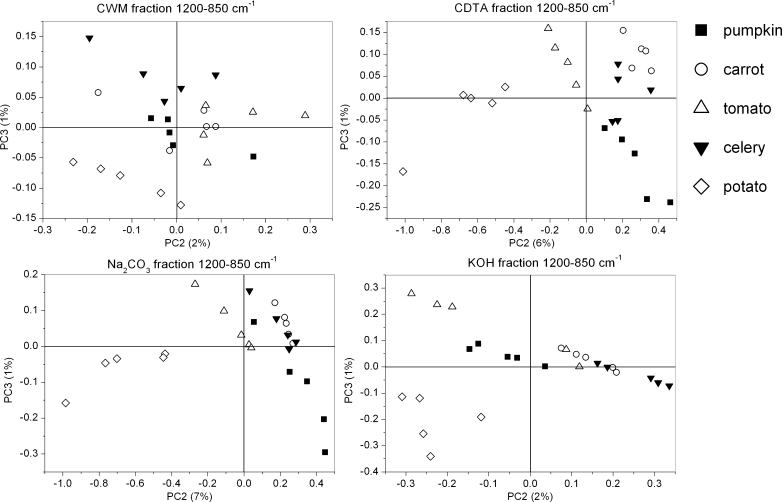
The scores scatter plots PC1 (explained above 91 % of variability, figure not shown)×PC2 (explained around 4–6 % of variability) and PC2×PC3 (explained around 2 % of variability) were used to obtain separation of each group, but only in the PC2×PC3 (3 % of explained variability) plot was the grouping visible (Fig. 8). The PCA of CWM residue showed only a small grouping effect for species (Fig. 8). Only pumpkin, potato and carrot scores are grouped separately as scores for tomato and celery samples are mixed together. The component PC1 explains 92 % of variability, however, its loadings took only positive values and scores were again scattered along this axis (figure not shown). Only the potato CWM sample lay on the negative side of the PC2 axis (5 % of explained variability), the remaining samples lay on its positive side or oscillated around zero (Fig. 8). PC2 loading had only one significant positive maximum corresponding to the 1,600 cm−1, which denotes COO− antisymmetric stretching of ester groups in pectins (Fig. 9). PC3 loadings took positive values around 1,650 and 1,550 cm−1, which corresponds to amid I and amid II infrared bands, respectively, and a negative value for 1,600 cm−1.
Fig. 8.
PCA scores scatter plots of the FT-IR spectra of CWM, CDTA, Na2CO3 and KOH residue obtained by sequential extraction of tomato, potato, carrot, celery and pumpkin cell wall in the 1,800–1,200 cm−1 region
Fig. 9.
PCA loadings plots (PC1, PC2 and PC3) of the FT-IR spectra of CWM, CDTA, Na2CO3 and KOH residues obtained by sequential extraction of tomato, potato, carrot, celery and pumpkin cell wall in the 1,800–1,200 cm−1 region
In the case of CDTA, the residue grouping effect of the studied species was more visible on both scatter plots PC1×PC2 (not shown) and PC2×PC3 (Fig. 8) with scores dispersed along the PC1 axis. The loadings in the case of PC2 obtained positive values at 1,740 and 1,600, 1,400 and 1,240 cm−1 corresponding to functional group vibrations in pectins (Fig. 9). The negative impact on the scores had wavelengths at 1,650, 1,550 (amid I and amid II), 1,430 and 1,360 cm−1 (cellulose vibration bands). In the case of PC3, a positive impact on scores corresponds to wavenumbers denoting vibration for cellulose: 1,360 cm−1 and pectins: 1,400 cm−1. Whereas negative impact on scores corresponds to wavenumbers: 1,740 and 1,240 cm−1 denoting vibration for pectins and wavenumber 1,550 cm−1 denoting vibration for amid II.
In Na2CO3 and KOH residues, the grouping of species was very similar, as well as loadings for specific components (Figs. 8 and 9). Significant values for PC1 appeared at 1,600 and 1,400 cm−1 which were characteristic for pectins, and 1,317 cm−1, which was characteristic for cellulose and was positive. There were in fact two loadings having positive impact on PC2 scores: strong around 1,600 cm−1 and smaller around 1,410 cm−1, both denoting pectins. The negative impact had values around 1,430 and 1,360 cm−1 which are characteristic for cellulose. For PC3 there were two strong positive values around 1,650 and 1,550 cm−1 (amid I and amid II bands) and two negative, 1,630 and 1,410 cm−1, typical for pectins and cellulose, respectively.
1200–850 cm−1 Region
The scores scatter plots PC1 (explained above 93 % of variability)×PC2 (explained around 2–7 % of variability) and PC2×PC3 (explained 1 % of variability) were used to obtain separation of each group, but only in the PC2×PC3 plot was the grouping visible (Fig. 10). Similar to the previous analyses, scores were scattered along the PC1 axis and its loadings had only positive values. The smallest grouping effect was obtained in the case of CWM for this spectral region. The PC3 loading obtained for every four sets of variables (CWM, CDTA, Na2CO3 and KOH) had significant positive value corresponding to 1,100 cm-1 (cellulose) and to 951 cm−1 (pectins), but in the case of the second wavelength its value decreased to zero in the last residue (KOH) (Fig. 11). Negative influence had a wavenumber around 1,042 and 1,000 cm−1 (bands assigned to cellulose) for scores of CWM and CDTA residues. In the case of Na2CO3 and KOH residues, loadings for 1,042 cm−1 changed their values to positive, while for 1,000 cm−1, loadings remained negative.
Fig. 10.
PCA scores scatter plots of the FT-IR spectra of CWM, CDTA, Na2CO3 and KOH residue obtained by sequential extraction of tomato, potato, carrot, celery and pumpkin cell wall in the 1,200–850 cm−1 region
Fig. 11.
PCA loadings plots (PC1, PC2 and PC3) of the FT-IR spectra of CWM, CDTA, Na2CO3 and KOH residues obtained by sequential extraction of tomato, potato, carrot, celery and pumpkin cell wall in the 1,200–800 cm−1 region
PC2 loadings indicated that positive influence on CWM scores had values around 1,100 (cellulose), 1,070 and 1,020 cm−1 (xyloglucan), whereas negative at 970, 927 and 888 cm−1, which could be assigned to pectins. This pattern is similar for CDTA and Na2CO3 residues, but 1,020 is shifted to 1,030 cm−1 (band assigned to cellulose) and there is lack of a minimum around 888 cm−1 . The biggest differences in loadings maximums and minimums were obtained for KOH residue PC3 loadings: positive influence was at 1,100 cm−1 (cellulose) and 951 cm−1 (pectins), and negative at 1,015 cm−1 (cellulose). The presence of a wavenumber which can be assigned to pectins or hemicellulose in KOH residue confirms that a part of the branched non-cellulosic polysaccharides are hard to remove from between the cellulose microfibrils [34].
Although a similar grouping effect for both of the chosen regions was obtained, a more pronounced grouping effect was achieved for the region 1,200–850 cm−1 and with use of PC2 and PC3 components. The groups were probably created due to pectin and hemicellulose content. Generally, the primary cell wall of plants consists of 25 % cellulose, 25 % hemicellulose, 35 % pectins, 1–8 % structural proteins, 1–5 % minerals and 2 % phenolics compounds (% of dry weight), but it must be remembered that these values can vary between species [3, 37]. In Table 2 the results of pectin content in CDTA and Na2CO3 supernatants (as a galacturonic acid content mg/g of dry mass of CWM residue) are shown. These values of pectin content are in good agreement with values obtained previously [38–42]. The most separated groups have been obtained for the potato sample. By comparing this result with Table 2 it can be concluded that potato contains the lowest content of ionically and covalently-bonded pectins among the samples. In the CWM residues, scores for other species are mixed together (not separated), however, for tomato, which is characterized by one of the biggest values of pectin content in the CDTA supernatant, all scores lie on the positive side of PC2 axis. Again for CDTA residue, scores (PC2×PC3) for potato and pumpkin, with the lowest content of covalently-bonded pectins, lie on the negative side of the PC3 axis. Scores for the remaining samples are arranged into groups, which are close to each other.
Table 2.
Pectin content in cell wall material from chosen vegetables as content of galacturonic acid (Gal A mg/g of dry mass of CWM). In parenthesis standard deviation
| Sample | Gal A content (mg/g dry mass CWM) | |
|---|---|---|
| CDTA and Na2CO3 supernatant | Na2CO3 supernatant | |
| Potato | 46,64 (±2,18) | 28,49 (±0,24) |
| Pumpkin | 102,95 (±0,72) | 20,40 (±0,36) |
| Carrot | 141,95 (±1,80) | 35,12 (±0,49) |
| Tomato | 253,04 (±2,44) | 56,16 (±0,82) |
| Celery root | 282,78 (±10,18) | 114,64 (±7,32) |
Conclusion
This study showed that discrimination between the cell wall residues of fruits and vegetables is possible with the use of FT-IR spectra in the regions 1,800–1,200 cm−1 and 1,200–850 cm−1 analysed using the PCA method, however, better distinction was obtained for region 1,800–1,200 cm−1. The grouping is due to the pectin content and separation in PC2, and significant positive loadings of FT-IR absorbancies for residues distinction are: 1,740, 1,610 and 1,240 cm−1. The residues abundant in hemicelluloses and cellulose are separated in PC2 by the wavenumbers of 1,370 and 1,317 cm−1, which influence the scores negatively. These results suggest that analysis of polysaccharides from cell walls can be successfully performed directly from subsequent residues.
The differences among species when all polysaccharide residues are considered are most highlighted when the PCA is implemented on the FT-IR region 1,200–850 cm−1. The species mainly vary due to the content of ionically or covalently-bonded pectins (CWM and CDTA residues). However, species distinction by PCA was not as clear as in the case of discrimination of cell wall residues.
Acknowledgments
The research was co-founded by National Centre of Science (research grant nr 2011/01/D/NZ9/02494).
Abbreviations
- C
Cellulose
- CDTA
Trans-1,2-Diaminocyclohexane-N,N,N′,N′-tetraacetate
- CWM
Cell wall material
- FT-IR
Fourier transform infrared
- P
Pectins
- PC
Principal component
- PCA
Principal component analysis
- XG
Xyloglucan
References
- 1.Brett C, Waldron K. Physiology and Biochemistry of Plant Cell Wall, Topics in Plant Physiology. London: Unwin Hyman Ltd; 1990. pp. 4–22. [Google Scholar]
- 2.Carpita NC, Gibeaut DM. Plant J. 1993;3:1–30. doi: 10.1111/j.1365-313X.1993.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 3.Taiz L, Zeiger E. Plant Physiology. 3. Sunderland: Sinauer Associates; 2002. pp. 314–337. [Google Scholar]
- 4.P.R. Griffiths, J.A. De Haseth, Fourier Transform Infrared Spectrometry, 2nd edn. (Wiley, 2007), pp 321–362
- 5.Kačurakova M, Wilson RH. Carbohydr. Pol. 2001;44:291–303. doi: 10.1016/S0144-8617(00)00245-9. [DOI] [Google Scholar]
- 6.Fellah A, Anjukandi P, Waterland MR, Williams MAK. Carbohydr. Pol. 2009;78:847–853. doi: 10.1016/j.carbpol.2009.07.003. [DOI] [Google Scholar]
- 7.Černa M, Barros AS, Nunes A, Rocha SM, Delgadillo I, Čopikova J, Coimbra MA. Carbohydr. Pol. 2003;51:383–389. doi: 10.1016/S0144-8617(02)00259-X. [DOI] [Google Scholar]
- 8.Alonso-Simon A, Encina AE, Garcia-Angulo P, Alvarez JM, Acebes JL. Plant Sci. 2004;167:1273–1281. doi: 10.1016/j.plantsci.2004.06.025. [DOI] [Google Scholar]
- 9.Gümüskaya E, Usta M, Kirci H. Polym. Degrad. Stab. 2003;81:559–564. doi: 10.1016/S0141-3910(03)00157-5. [DOI] [Google Scholar]
- 10.Rondeau-Mouro C, Bouchet B, Pontoire BP, Mazoyer RJ, Buléon A. Carbohydr. Pol. 2003;53:241–252. doi: 10.1016/S0144-8617(03)00069-9. [DOI] [Google Scholar]
- 11.Szymańska-Chargot M, Cybulska J, Zdunek A. Sensors. 2011;11:5543–5560. doi: 10.3390/s110605543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhbankov RG, Firsov SP, Buslov DK, Nikonenko NA, Marchewka MK, Ratajczak H. J. Mol. Struct. 2002;614:117–125. doi: 10.1016/S0022-2860(02)00252-1. [DOI] [Google Scholar]
- 13.Jarvis MC. Food Hydrocoll. 2011;25:257–262. doi: 10.1016/j.foodhyd.2009.09.010. [DOI] [Google Scholar]
- 14.Zykwinska A, Thibault J-F, Ralet M-C. Carbohydr. Pol. 2008;74:957–961. doi: 10.1016/j.carbpol.2008.05.004. [DOI] [Google Scholar]
- 15.Atalla RH, Hackney JM, Uhlin I, Thompson NS. Int. J. Biol. Macromol. 1993;15:109–112. doi: 10.1016/0141-8130(93)90007-9. [DOI] [PubMed] [Google Scholar]
- 16.Caffall KH, Mohnen D. Carbohydr. Res. 2009;344:1879–1900. doi: 10.1016/j.carres.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 17.Kačurakova M, Capek P, Sasinkova V, Wellner N, Ebringerova A. Carbohydr. Pol. 2000;43:195–203. doi: 10.1016/S0144-8617(00)00151-X. [DOI] [Google Scholar]
- 18.Mohnen D. Curr. Opin. Plant Biol. 2008;11:266–277. doi: 10.1016/j.pbi.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 19.Engelsen SB, Norgaard L. Carbohydr. Pol. 1996;30:9–24. doi: 10.1016/S0144-8617(96)00068-9. [DOI] [Google Scholar]
- 20.Chatjigakis AK, Pappas C, Proxenia N, Kalantzi O, Rodis P, Polissiou M. Carbohydr. Pol. 1998;37:395–408. doi: 10.1016/S0144-8617(98)00057-5. [DOI] [Google Scholar]
- 21.R. Gnansambandam, A. Proctor, Food Chem. 68(327–332) (2000)
- 22.Wellner N, Kačurakova M, Malovikova A, Wilson RH, Belton PS. Carbohydr. Res. 1998;308:123–131. doi: 10.1016/S0008-6215(98)00065-2. [DOI] [Google Scholar]
- 23.Barros AS, Mafra I, Ferreira D, Cardoso S, Reis A, Lopes da Silva JA, Delgadillo I, Rutledge DN, Coimbra MA. Carbohydr. Pol. 2002;50:85–94. doi: 10.1016/S0144-8617(02)00017-6. [DOI] [Google Scholar]
- 24.Ferreira D, Barros A, Coimbra MA, Delgadillo I. Carbohydr. Pol. 2001;45:175–182. doi: 10.1016/S0144-8617(00)00320-9. [DOI] [Google Scholar]
- 25.Coimbra MA, Barros A, Rutledge DN, Delgadillo I. Carbohydr. Res. 1999;317:145–154. doi: 10.1016/S0008-6215(99)00071-3. [DOI] [Google Scholar]
- 26.Hori R, Sugiyama J. Carbohydr. Pol. 2003;52:449–453. doi: 10.1016/S0144-8617(03)00013-4. [DOI] [Google Scholar]
- 27.Winning H, Viereck N, Salomonsen T, Larsen J, Engelsen SB. Carbohydr. Res. 2009;344:1833–1841. doi: 10.1016/j.carres.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 28.Coimbra MA, Barros A, Barros M, Rutledge DN, Delgadillo I. Carbohydr. Pol. 1998;37:241–248. doi: 10.1016/S0144-8617(98)00066-6. [DOI] [Google Scholar]
- 29.Renard CMGC. Carbohydr. Pol. 2005;60:512–522. doi: 10.1016/j.carbpol.2005.03.002. [DOI] [Google Scholar]
- 30.Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Anal. Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- 31.Redgwell RJ, Curti D, Gehin-Delval C. Eur. Food Res. Technol. 2008;227:607–618. doi: 10.1007/s00217-007-0762-1. [DOI] [Google Scholar]
- 32.Kačurakova M, Smith AC, Gidley MJ, Wilson RH. Carbohydr. Res. 2002;337:1145–1153. doi: 10.1016/S0008-6215(02)00102-7. [DOI] [PubMed] [Google Scholar]
- 33.Sene CFB, McCann MC, Wilson RH, Grinter R. Plant Physiol. 2004;106:1623–1631. doi: 10.1104/pp.106.4.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fry SC. The Growing Plant Cell Wall: Chemical and Metabolic Analysis. UK: The Blackburn Press. Longman Scientific & Technical; 1988. pp. 64–65. [Google Scholar]
- 35.Synytsya A, Čopikova J, Matĕjka P, Machovič V. Carbohydr. Pol. 2003;54:97–106. doi: 10.1016/S0144-8617(03)00158-9. [DOI] [Google Scholar]
- 36.Wilson RH, Smith AC, Kačurakova M, Saunders PK, Wellner N, Waldron KW. Plant Physiol. 2000;124:397–405. doi: 10.1104/pp.124.1.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cybulska J, Konstankiewicz K, Zdunek A, Skrzypiec K. Int. Agrophys. 2010;24:107–114. [Google Scholar]
- 38.Gross KC, Wallner SJ. Plant Physiol. 1979;63:117–120. doi: 10.1104/pp.63.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Houben K, Jolie RP, Fraye I, Van Loey AM, Henddrickx ME. Carbohydr. Res. 2011;346:1105–1111. doi: 10.1016/j.carres.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 40.Jarvis MC, Hall MA, Trelfall DR, Friend J. Planta. 1981;152:93–100. doi: 10.1007/BF00391179. [DOI] [PubMed] [Google Scholar]
- 41.Jarvis MC. Planta. 1982;154:344–346. doi: 10.1007/BF00393913. [DOI] [PubMed] [Google Scholar]
- 42.Kotcharian A, Kunzek H, Dongowski G. Food Chem. 2004;87:231–245. doi: 10.1016/j.foodchem.2003.11.015. [DOI] [Google Scholar]