Abstract
Purpose
Congenital pseudarthrosis of the tibia (CPT) is a rare disease. Epidemiological data are limited, and treatment of the condition is challenging. The purpose of our study was to gain epidemiological data on the incidence of CPT in Norway and to evaluate the treatment outcome of the disease.
Methods
During the period 1987–2006 22 patients with CPT were born in Norway (11 boys, 11 girls; mean age 15 years, age range 8–24 years) and are included in this study. During the same time period 1,183,380 live-births were registered by the Norwegian Birth Register. Primary surgical treatment was the Ilizarov method in 15 patients, intramedullary nailing in three patients, and plate osteosynthesis in two patients; two patients never developed a fracture and were treated with an orthosis.
Results
The incidence of CPT based on this period was 1:53,000. The rate of primary healing was 66 % for the Ilizarov group. Primary healing occurred in three patients treated with intramedullary nailing and in none of the patients treated with plate osteosynthesis. However, almost all patients required additional surgery due to refracture or deformity correction. Currently, all 12 skeletally mature patients are considered to be healed, whereas two of the skeletally immature patients are still under treatment.
Conclusion
The incidence of CPT in Norway seems to be notably higher than that based on epidemiological data from other studies. Primary healing rates are satisfactory when treated either with an Ilizarov device or intramedullary nailing. Refractures must be avoided, and alignment of the leg must be maintained. Healing is usually achieved before skeletal maturity. However, residual deformities are common.
Keywords: Congenital pseudarthrosis of the tibia, Ilizarov method, Treatment, Epidemiology, Telescopic nail, External fixation
Introduction
Congenital pseudarthrosis of the tibia (CPT) is a rare disease with a variable history and appearance [1, 2] (Fig. 1). Pseudarthrosis in most cases of CPT are not present at birth. Therefore the term “pseudarthrosis” might be somewhat inaccurate, and dysplasia would be the preferred term [3]. The underlying disease process and anterolateral bowing are usually present at birth, while fracture with subsequent pseudarthrosis occurs during the first decade of life [1, 3]. Andersen [4] reported a CPT incidence of 1:190,000 live births in Denmark. The etiology of the disease is unknown, and epidemiological data on these patients are limited. However, up to 55 % of the cases of anterolateral bowing and pseudarthrosis are associated with neurofibromatosis [5, 6] and 5.7 % of patients with neurofibromatosis type 1 have the deformity [7] (Fig. 2). Neurofibromatosis is the most common single gene disorder found in humans and is considered to be an autosomal trait with variable penetrance and a very high rate of spontaneous mutation [7, 8]. Up to 15 % of the patients with anterolateral bowing have fibrous dysplasia [9]. Several radiographic classifications of CPT have been described [7, 10, 11]. These classification systems describe the untreated appearance of the diseased bone, but unfortunately, none of the classification systems provide guidance to the management or long-term outcome of the condition [3].
Fig. 1.
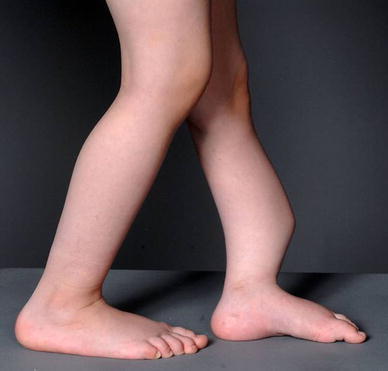
Girl at age 4 years (patient no. 20) before primary treatment
Fig. 2.
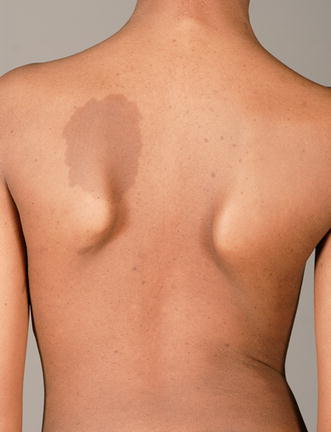
Nevrofibromatosis with a characteristic “cafe-au-lait spot”
The natural history of CPT is very unfavorable, and when fracture occurs spontaneous healing is highly unlikely [12]. The treatment of CPT remains challenging [13], but the rate of primary union appears to have improved through the use of treatment methods, such as the Ilizarov technique [14–17], microvascular fibula transfer [18], intramedullary fixation [19], or a combination of these procedures. Regardless of the surgical method used, some form of resection of the pseudarthrosis is commonly recommended as part of the initial treatment [20]. Residual challenges regarding the quality and longevity of any primary union are common. Most of the patients require multiple surgeries, and ultimate function is affected by residual deformities, joint stiffness and possible leg length discrepancy. The purpose of our study was to obtain epidemiological data on the incidence of CPT in Norway and to evaluate the treatment outcome of the disease.
Patients and methods
In Norway, the treatment of CPT is centralized to university hospitals. For the purposes of our study, we therefore requested all university hospitals in Norway to forward to us information on all cases of CPT in patients born in Norway during the period 1987–2006 (20-year period). Haukeland University Hospital in Bergen reported two cases, whereas 20 patients had been treated at our institution (Oslo University Hospital). Thus, 22 patients (11 boys, 11 girls; mean age at time of study 15 years; age range at time of study 8–24 years) with CPT were born and treated for CPT from 1987 to 2006 in Norway (Table 1) and could be included in this study. Twelve patients had reached skeletal maturity at the last follow-up. Mean follow-up was 6.7 (range 0.5–16) years. The number of live-births for the time period from 1987 to 2006 was obtained from the Norwegian Birth Register in order to calculate the incidence of the disease in Norway [21].
Table 1.
Clinical data of 22 patients with congenital pseudarthrosis of the tibia
| Patient no. | Age (years), gender | Early onset | Crawford type I–IV | Neurofibromatosis | Primary treatment | Age at first surgery (years) | No. of refractures at pseudarthrosis site | No. of refractures at lengthening site | Additional surgery lengthening, axis corr | Current status | Follow-up since healing of pseudarthrosis (years) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 24/m | + | – | + | Ilizarov | 8 | 1 | Healed | 15 | ||
| 2 | 24/f | + | – | + | Plate | 6 | 2 | 2 | Healed | 14 | |
| 3 | 22/f | + | – | + | Plate | 4 | 2 | 1 | Healed | 16 | |
| 4 | 21/f | + | IV | + | Ilizarov | 3 | 2 | 2 | 2 | Healed | 1.5 |
| 5 | 20/m | + | IV | + | Ilizarov | 3 | 1 | 1 | Healed (fibula graft) | 15 | |
| 6 | 19/m | + | IV | − | Ilizarov | 3 | 5 | 1 | Healed | 1 | |
| 7 | 19/m | + | IV | + | Ilizarov | 3 | 2 | 3 | Healed, rush-pin | 0.5 | |
| 8 | 18/f | + | IV | − | Ilizarov | 3 | 3 | 3 | 4 | Healed (fibula graft) | 12 |
| 9 | 18/f | + | IV | + | Ilizarov | 3 | 3 | 2 | Healed | 2.5 | |
| 10 | 18//f | − | I | − | Nail | 12 | Healed, telescopic nail | 3.5 | |||
| 11 | 17/m | + | – | + | Nail | 3 | 1 | Healed | 7 | ||
| 12 | 16/f | + | III | + | Ilizarov | 5 | 1 | 1 | Healed | 8 | |
| 13 | 14/m | − | I | + | Never fractured | ||||||
| 14 | 13/m | + | – | + | Nail | 3 | 1 | Amputated | |||
| 15 | 12/m | − | I | − | Never fractured | ||||||
| 16 | 12/m | − | II | − | Ilizarov | 6 | 1 | Healed, orthosis | 5 | ||
| 17 | 11/f | + | IV | + | Ilizarov | 5 | 1 | 1 | Refracture at lengthening site, plate | 5 | |
| 18 | 11/m | + | IV | + | Ilizarov | 5 | 1 | Healed, orthosis | 5.5 | ||
| 19 | 10/f | + | IV | − | Ilizarov | 6 | 1 | Healed, orthosis | 3.5 | ||
| 20 | 9/f | + | III | + | Ilizarov | 4 | 2 | Healed, telescopic nail | 1 | ||
| 21 | 9/m | + | III | − | Ilizarov | 5 | 1 | 1 | Ilizarov + telescopic nail | ||
| 22 | 8/f | + | III | − | Ilizarov | 4 | 1 | Healed | 1.6 |
m male, f female
We classified all cases of CPT included in this study according to Herring’s [3] and Crawford and Bagamery’s [7] classifications. Herring’s classification relies on two simple criteria: (1) the presence or absence of fracture and (2) the age at which fracture first occurs (“early onset” before 4 years of age, “delayed onset” after 4 years). The classification of Crawford and Bagamery describes the untreated appearance of the diseased bone [7].
In 15 patients primary surgical treatment was pseudarthrosis resection and a proximal metaphyseal osteotomy by use of the Ilizarov method, whereby six patients were treated with segmental transport (Fig. 3) and nine patients with acute compression and subsequent proximal tibial lengthening (Fig. 4). In these nine patients with proximal lengthening of the tibia, an osteotomy was also performed in the middle third of the fibula. The resection of the pseudarthrosis included removal of the sclerotic bone ends and the surrounding periosteum. In three patients primary treatment was intramedullary nailing with Fassier–Duval telescopic nails. In two of these patients a resection of the pseudarthrosis was done combined with bone grafting and periostal transplantation from the iliac crest.
Fig. 3.
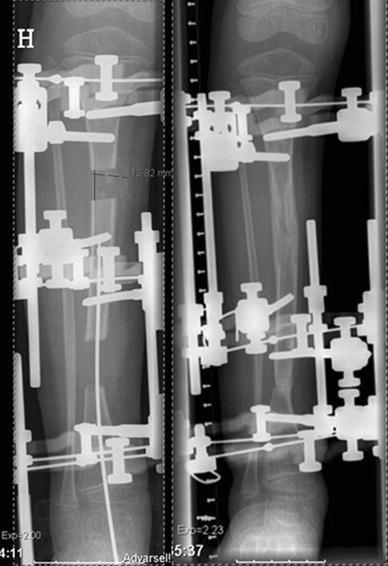
Boy at age 6 years (patient no. 16). Primary treatment with resection of the pseudarthrosis and segmental transport guided by an intramedullary pin using the Ilizarov method. Note the constricted diaphyseal diameter at the “docking zone” and sclerosis. The patient refractured at the “docking zone” 2 months after frame removal
Fig. 4.
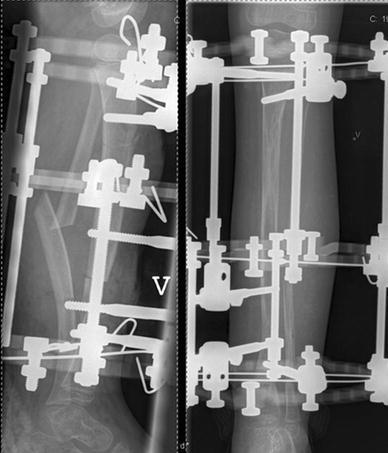
Girl at age 4 years (patient no. 20). Primary treatment with resection of the pseudarthrosis, fibular osteotomy, and acute compression using the Ilizarov method followed by proximal metaphyseal lengthening
In two patients primary treatment was pseudarthrosis resection and osteosynthesis with plate and screws. Mean age at first surgery, including all operated patients in this study, was 4.6 (range 3–13) years. Two patients never developed a fracture and were treated with an orthosis. All children, including those with fractures and those with an unfractured bowed tibia, used prophylactic bracing until skeletal maturity once the child began weight bearing. A knee-ankle–foot-orthosis was used for shorter periods, such as after proximal tibial lengthening procedures.
All patients who were treated with an Ilizarov frame received a plaster-cast for the first 6–8 weeks after frame removal. Hence, an eventual lack of healing would not become evident before removal of the cast and weight bearing in an orthosis. Therefore, healing of the pseudarthrosis after primary treatment, examined on standard anterior–posterior and lateral radiographs, was defined as radiological healing of the pseudarthrosis and no refracture within at least 2 months following frame removal.
Residual deformities, which refer to deformities still present after treatment, were analyzed at the latest follow-up. These were evaluated in the treated extremity based on long-standing radiographs, which were available in 20 patients (Tables 3, 4). Alignment and joint orientation of the affected limb were compared with the patient’s own healthy contralateral side. Analysis of joint alignment in the frontal plane was based on the mechanical axis and the position of the articular surfaces in the knee and ankle joint relative to the axes of the individual limb segments (femur and tibia). The following joint orientation angles, as described by Paley [22], were analyzed on the affected and the contralateral side: mechanical lateral distal femoral angle (mLDFA), medial proximal tibial angle (MPTA) and the lateral distal tibial angle (LDTA).
Table 3.
Frontal plane and sagittal plane alignment
| Patient | Frontal plane alignment | Sagittal plane alignment | ||||||
|---|---|---|---|---|---|---|---|---|
| Side | MAD (mm) | mLDFA (°) | MPTA (°) | LDTA (°) | PDFA (°) | PPTA (°) | ADTA (°) | |
| 1 | R | −27 | 3 valgus | 20 valgus | 11 procurvatum | 10 procurvatum | ||
| 2 | L | −24 | 6 valgus | 23 valgus | 2 recurvatum | 18 procurvatum | ||
| 3 | R | −7 | 3 valgus | 34 valgus | 3 procurvatum | 3 recurvatum | 6 procurvatum | |
| 4 | R | −10 | 3 valgus | 18 valgus | NA | 32 procurvatum | ||
| 5 | L | −14 | 6 valgus | 11 valgus | NA | NA | NA | |
| 6 | L | −15 | 3 valgus | 4 recurvatum | 4 procurvatum | |||
| 7 | R | −14 | 3 valgus | 23 valgus | 2 recurvatum | 3 procurvatum | ||
| 8 | L | 40 | 7 varus | 3 varus | 6 recurvatum | 8 procurvatum | 11 procurvatum | |
| 9 | R | −8 | 2 valgus | 19 valgus | 5 recurvatum | 3 procurvatum | 3 procurvatum | |
| 10 | L | 4 valgus | 4 varus | 8 recurvatum | 6 procurvatum | |||
| 11 | R | NA | NA | NA | NA | NA | NA | NA |
| 12 | R | −7 | 2 valgus | 15 valgus | 3 recurvatum | 5 procurvatum | 29 procurvatum | |
| 13 | R | 10 valgus | 3 recurvatum | 3 procurvatum | 17 procurvatum | |||
| 14 | L | NA | NA | NA | NA | NA | NA | NA |
| 15 | R | |||||||
| 16 | R | 10 valgus | 11 procurvatum | |||||
| 17 | R | −3 | 2 valgus | 9 valgus | 17 procurvatum | |||
| 18 | L | −15 | 4 valgus | 19 valgus | 38 procurvatum | |||
| 19 | R | 5 valgus | 18 procurvatum | |||||
| 20 | L | −15 | 3 valgus | 22 valgus | 5 procurvatum | 33 procurvatum | ||
| 21 | R | 14 valgus | 4 procurvatum | 10 procurvatum | ||||
| 22 | L | −14 | 2 valgus | 2 valgus | 15 valgus | 11 procurvatum | ||
Radiological results are from last follow-up. In the frontal plane, alignment and joint orientation of the affected limb were compared with the patient’s own healthy contralateral side. In the sagittal plane, normal values, as described by Paley [22], were used for analysis
Only data that fall outside the normal range are given in this table
NA Radiographs not available, R right, L left, MAD mechanical axis deviation [−, MAD to lateral (knee valgus); +, MAD to medial (knee varus)], mLDFA mechanical lateral distal femoral angle, MPTA medial proximal tibial angle, LDTA lateral distal tibia angle, PDFA posterior distal femoral angle, PPTA posterior proximal tibial angle, ADTA anterior distal tibial angle
Table 4.
Length measures
| Patient | LLD (mm) | Femoral length (mm) | Tibial length (mm) | Foot height (mm) |
|---|---|---|---|---|
| 1 | +20 | +4 | −21 | |
| 2 | +10 | +14 | +6 | −10 |
| 3 | −20 | +14 | −34 | |
| 4 | +29 | −14 | −15 | |
| 5 | −8 | NA | NA | NA |
| 6 | +20 | −11 | −9 | |
| 7 | −14 | +22 | −13 | −7 |
| 8 | −45 | +2 | −53 | |
| 9 | −25 | +14 | −43 | |
| 10 | NA | NA | NA | |
| 11 | NA | NA | NA | NA |
| 12 | +28 | −18 | −11 | |
| 13 | −15 | +11 | −29 | |
| 14 | NA | NA | NA | NA |
| 15 | +5 | −5 | ||
| 16 | +4 | −4 | ||
| 17 | −50 | −50 | ||
| 18 | −20 | +23 | −33 | −4 |
| 19 | +19 | +5 | −30 | |
| 20 | −20 | +8 | −12 | −17 |
| 21 | −60 | −62 | −4 | |
| 22 | +18 | −20 | ||
| Mean (range) | −13 (−60 to −10) | 12 (2–29) | −18 (−62 to −18) | −9 (−30 to −4) |
Leg length discrepancy (LLD) and differences in femoral length, tibial length and foot height between affected and non-affected side [+, longer on affected side; −, shorter on affected side; blank, no difference]. Joint space width was not assessed
Only data that fall outside the normal range are given in this table
NA radiographs not available
In the sagittal plane radiographs of the contralateral side were not available in a large number of patients; therefore, normal values as described by Paley [22] were used for the analysis in the lateral view. Thus, the following joint orientation angles were measured in the sagittal plane on the affected limb: posterior distal femoral angle (PDFA), proximal posterior tibial angle (PPTA), and the anterior distal tibial angle (ADTA). The sagittal plane analysis of the femur was based on the anatomical axis. In the tibia, most of the patients had a diaphyseal deformity in the sagittal plane; therefore, analysis was done based on a modified tibial mechanical axis line as described by Paley [22].
As most of the patients showed an apparent overgrowth of the femur on the side with the congenital tibia pseudarthrosis, femoral length, tibial length, and foot height were measured in both extremities on long-standing radiographs, if available.
Results
Epidemiology and classification
A total of 1,183,380 live-births were registered by the Norwegian Birth Register [21] in the time period 1987–2006 (Table 2). Twenty-two patients with CPT were registered during the same time, of which 20 were ethnic Norwegian, one was the child of immigrants from Asia, and one was from the Middle East. The incidence of CPT based on the time period 1987–2006 was 1:60,000 for ethnic Norwegian patients and 1:53,000 when all patients born with a congenital tibia pseudarthrosis in Norway were included (Table 2). Fourteen patients (63 %) had definite signs of neurofibromatosis.
Table 2.
Live-births based on data from the Norwegian birth register and registered cases of congenital pseudarthrosis of the tibia
| Time period | No. of live births | Cases of CPT |
|---|---|---|
| 1987–1990 | 233,938 | 3 |
| 1991–1994 | 243,190 | 8 |
| 1995–1998 | 240,178 | 3 |
| 1999–2002 | 233,583 | 7 |
| 2003–2006 | 232,491 | 1 |
| Total | 1,183,380 | 22 |
CPT Congenital pseudarthrosis of the tibia
According to Herring’s classification system [3], 20 of the children developed a fracture/pseudarthrosis of the tibia, of which 18 were early-onset cases since they occurred before the age of 4 years (mean age 16 months, age range 0–36 months). The two late-onset cases occurred at the age of 5 and 13 years, respectively. Two patients showed characteristic bowing of the tibia but never developed a fracture. One patient with this anterolateral bowing received a corrective osteotomy and fixation with Fassier–Duval telescopic nails. The healing period was significantly prolonged, although the osteotomy eventually healed after 18 months at the same time the patient reached skeletal maturity. Due to the characteristic bowing and the prolonged healing after osteotomy, this case was considered to be a late-onset type CPT.
Based on the classification of Crawford and Bagamery [7], we found three type I, one type II, four type III, and nine type IV cases. Five cases could not be classified according to the Crawford and Bagamery system since radiographs before primary surgery were not available. However, these cases could be classified according to Herring’s system since the occurrence of fracture was documented in the patients’ hospital records.
Primary treatment results
In the Ilizarov group primary healing was achieved in ten patients (66 %), which means that they showed apparent healing before frame removal and did not refracture within the first 2 months after frame removal. Five patients refractured within 2 months after frame removal. However, all patients except one who were initially treated successfully with the Ilizarov frame refractured within 4 years (mean refracture time 9 months, range 6–48 months) after primary treatment (Table 1). Neither the type of Ilizarov treatment (segmental transport or acute compression and lengthening) nor the presence or absence of an intact fibula influenced the time point of refracture. At the time of refracture all patients had some form of malalignment of the tibia; the main deformities were a mean of 15° (range 10–20°) valgus and 21° (range 5–45°) procurvatum.
The two patients who primarily were treated with a telescopic nail and grafting of bone and periosteum from the iliac crest achieved primary healing. However, both patients refractured within 5 years. One patient who was treated with telescopic nailing alone (late-onset CPT) achieved healing and did not require any further surgery. Primary treatment with pseudarthrosis resection and plate fixation combined with bone grafting did not lead to healing. These patients were successfully treated with removal of the plates and an Ilizarov procedure.
As far as the treatment outcome in relation to the different classification system is concerned, two patients in this study population were late-onset cases according to Herring’s classification (patient no. 10 and 16; Table 1). However, the number of late-onset cases in this study population was too small to draw any conclusions about the usefulness of Herring’s classification to predict treatment outcome.
All but one Crawford and Bagamery Type II, III, and IV cases refractured at least once at the pseudarthrosis site. Several patients refractured several times and/or required additional surgery for lengthening or axis correction. However, no apparent correlation between the different Crawford and Bagamery types and the outcome could be observed.
Further treatment and long-term results
Within 5 years after initial treatment all patients but three required further surgery due to refracture at the pseudarthrosis site (Table 1). Eight patients refractured more than one time (mean no. of refractures at the pseudarthrosis 2, range 1–5). Two of these patients received a vascularized fibula graft, one patient was operated on with an intramedullary nail, and all other patients were treated with Ilizarov procedures. Two patients received an Ilizarov procedure combined with Fassier–Duval telescopic nails (Fig. 5), and one patient received an Ilizarov procedure combined with a rush-pin. A total of 27 lengthening procedures were performed in 17 patients of our study population; mean lengthening was 42 mm (range 7–75 cm). Seven patients fractured at the lengthening site, of whom one patient refractured two times and one patient refractured three times. Nine patients required an average of two (range 1–4) additional surgical procedures for lengthening or axis correction (Table 1). At the present time, all 12 skeletally mature patients are considered to be healed, with an average of 8 (range 0.5–16) years without refracture, and of the ten skeletally immature patients, two never developed a fracture and one patient was amputated below the knee. Of the remaining seven skeletally immature patients, five are considered to be healed, with a mean of 3 (range 1–5) years without refracture. One patient is under treatment with an Ilizarov procedure in combination with a Fassier–Duval nail, and one patient has been recently operated on with a plate osteosynthesis for a fracture at the lengthening site.
Fig. 5.
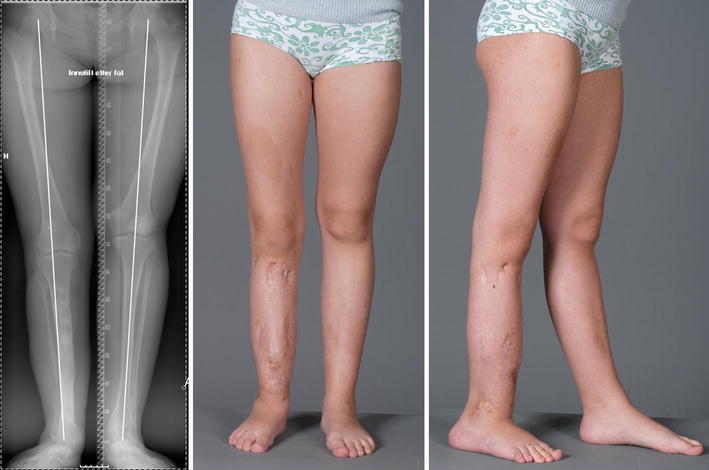
A 16-year-old girl (patient no. 12) with neurofibromatosis and congenital pseudarthrosis of the tibia (CPT) of the right leg after a total of three Ilizarov procedures. Present residual deformities are: slightly knee valgus (9 mm mechanical axis deviation to the lateral), significant ankle valgus (lateral distal tibial angle 67°). Note the ipsilateral overgrowth of the femur (28 mm)
Residual deformities
Frontal plane alignment
The alignment and joint orientation of the affected limb in the frontal plane were compared with the patient’s own healthy contralateral side. Overall malalignment in the frontal plane based on the mechanical axis deviation (MAD) was 9.5 mm to lateral (range 27 lateral–40 medial), whereas 13 patients had a valgus deformity with a mean MAD of 13 (range 3–27) mm to the lateral. Six patients had a neutral mechanical axis and one patient a varus deformity with a MAD of 40 mm to the medial side.
Analysis of joint orientation of the knee joint in the frontal plane showed that 14 patients had a mLDFA within the normal range, whereas five patients had a mild valgus deformity in the femur with a mean of 4.2° (range 2–7°) valgus and one patient had 7° of varus. Seven patients had a MPTA within the normal range, whereas ten patients had a valgus deformity in the proximal tibia with a mean of 3.4° (2–6°) of valgus. Based on the MPTA measurements, two patients had a varus deformity of 3° and 4°, respectively.
Of the 20 patients for X-rays were available, 16 had a valgus deformity in the ankle joint (Fig. 5) on the affected side, with a mean LDTA of 16.7° (range 5–34°), whereas four patients showed ankle joint orientation within the normal range (Table 3).
Sagittal plane alignment
In the sagittal plane, normal values, as described by Paley [22], were used for evaluation. Analysis of PDFA showed that six patients had a mild recurvatum deformity in the distal femur on the affected side, with mean of 3.8° (2–6°) recurvatum, one patient showed a procurvatum of 3°, whereas all other patients with available X-rays had normal sagittal alignment in the distal femur.
Based on measurements of the PPTA, seven patients had a procurvatum deformity in the proximal tibia (mean: 6.7°, range 3–11°), whereas three patients had a mild recurvatum deformity (range 2°–8°) and nine patients showed normal sagittal alignment in the proximal tibia. Sagittal alignment measures in the distal tibia based on the ADTA showed that 18 patients had an average procurvatum deformity of 15° (range 3–38°) with respect to the ankle joint, whereas only one of 20 patients had normal sagittal alignment in the distal tibia (Table 3).
Length measurements
Mean leg length discrepancy (LLD) in our patient group was 13 (range 0–60) mm, and the affected extremity was shorter in all patients, but one. Of the 19 measured patients, 11 showed a femoral overgrowth of >10 mm (mean 12 mm, range 11–29 mm) on the affected side. Mean length discrepancy in the tibia was 18 (range 4–62) mm. In 14 of 18 measured cases the affected tibia was shorter. In 11 patients, foot height, from the level of the floor to the top of the talus, was reduced on the affected side compared to the healthy side (mean 9 mm, range 4–30 mm), whereas seven patients showed no difference in foot height (Table 4).
For both frontal and sagittal plane alignment and length measures, only data that fall outside the normal range are given in Tables 3 and 4.
Discussion
The incidence of CPT of 1:60,000 live births in Norway based on a 20-year period seems to be notably higher compared to epidemiological data from other countries [9]—more precisely almost threefold higher than in Denmark [4]—but epidemiological data on these patients are limited [9]. However, due to the centralized treatment of the disease in Norway, more cases might have been registered than in other investigations.
Our study is one of the few published studies to include more than 20 patients and a follow-up time of up to 16 years [19, 23–26].
Neither the Herring nor the Crawford and Bagamery classification could provide guidance to management or long-term outcome of the condition, which confirms findings by other authors [3]. However, our study population was small, with only two patients fulfilling the criteria for “late-onset” cases according to Herring. Therefore, the possible usefulness of any of the used classifications might not have become apparent in our study.
With the Ilizarov method, healing in primary treatment was achieved in more than half of the patients. However, all patients but one refractured within 4 years and required multiple surgeries in order to achieve permanent healing. The young age at primary surgery and the high degree of residual deformities might explain the high refracture rate. The fact that all skeletally mature patients ultimately healed after repeated treatment shows that results are satisfying in the long term. The outcome of a surgical procedure improved with increasing age, suggesting, as has been noted in other studies, that primary treatment below the age of 5–6 years might not be advantageous [13, 15, 24].
There is some controversy regarding the role of the fibula in CPT [15]. However, in our study the presence or absence of an intact fibula did not influence the rate of refracture in the tibia. Furthermore, as observed by others [27], the presence of neurofibromatosis did not appear to affect the incidence of union or the ultimate outcome. Three cases were primarily treated with intramedullary telescopic nails. One of these patients was treated with a corrective osteotomy, and another was later amputated below the knee due to persisting pain and pseudarthrosis. The number of patients primarily treated with intramedullary fixation alone is too small to draw any conclusions with respect to the treatment results. Two patients were treated with plate and screw fixation, which resulted in a persisting pseudarthrosis. This treatment option has been abandoned by most authors for many years [15].
A significant number of additional surgeries, not only due to refracture at the pseudarthrosis, but also because of malalignment and complications related to lengthening, were required in all patients. A total of nine fractures through the callotasis regenerated after lengthening were observed in our patients, and an additional 17 surgical procedures for lengthening or deformity correction were required. There was no obvious correlation between the length of the lengthening and the risk of refracture, since about half of the patients refractured at the lengthening site and the other half did not, with similar amounts of lengthening in all patient’s. However, refracture rates at the lengthening site in the proximal tibia were high, indicating that pathological changes of the tibial bone in CPT might not only affect the bone around the pseudarthrosis site. Considering the number of refractures at the lengthening zone, excessive lengthening increases the number of complications and the time in the Ilizarov frame for the children with eventual consequences for their psychosocial development [28]. Excessive resection of bone around the pseudarthrosis might not be necessary and, therefore, excessive lengthening procedures might not always be required. There is some evidence that the periosteum at the pseudarthrosis site shows pathological changes, and resection might be recommended [29]. When a medullary canal is re-established in the sclerotic bone ends and kept open with an intramedullary nail, less bone resection might be required. However, problems associated with intramedullary nails, such as the need for nail changes and an eventual lack of telescoping in telescopic nails due to procurvatum and valgus deformity in the tibia, have to be considered. Leg length discrepancies might be addressed by epiphysiodesis on the contralateral side, which according to Herring [3] is the best management for a discrepancy of ≤5 cm. Fortunately, most of the CPT patients in our study showed a compensatory overgrowth in the femur on the affected side, a phenomenon which has been described before [30, 31].
Analysis of residual deformities in our patients showed that the majority of the patients had a valgus deformity in the affected extremity with a lateral MAD. The source of the frontal plane valgus malalignment affecting the mechanical axis was mainly localized on the tibial segment (Table 3). Most patients showed significant ankle valgus deformity. The development of a valgus deformity in the ankle joint is common in CPT. Supramalleolar distal tibia osteotomy to correct ankle valgus is considered to be the least attractive treatment option, since recurrent pseudarthrosis may develop in up to 50 % of the patients after this procedure [3]. Hence, ankle valgus should probably be addressed by a medial hemi-epiphysiodesis at an appropriate time, a technique which is described as being effective for a variety of congenital conditions [32].
We also found a procurvatum angulation in the proximal tibia and a significant procurvatum deformity in almost all patients in the distal tibia with respect to the ankle joint (Fig. 5). Procurvatum in the tibia is a characteristic of the disease, but it seems challenging to adequately address all deformities in CPT, especially when considering the fact that patients often weight bear for a few years on the affected limb before primary surgery with resulting adaptations in the foot. Our findings on the residual deformities in CPT confirm those of other authors [17, 26].
From a biomechanical point of view, residual malalignment in patients with CPT might lead to unfavorable stress distribution in the lower leg and increase the risk of refracture and impaired function [33]. Primary treatment should therefore not only intend to achieve healing of the pseudarthrosis, but also to restore alignment of the bone segment [34].
Permanent intramedullary fixation to maintain alignment and to provide internal bracing for a united tibia might be considered [3] (Fig. 6). However, in all cases external bracing with an orthosis is advised until skeletal maturity. One weakness of our study is that we did not include the functional outcome of the patients. However, due to the complexity of the disease with numerous surgical procedures to be evaluated, we concentrated on the radiographic outcome. Furthermore, conclusions or recommendations regarding the treatment can only be given with reservation as the disease is rare and complex.
Fig. 6.
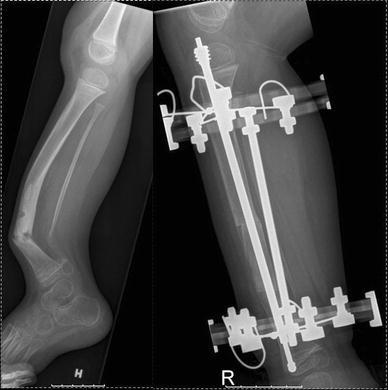
Boy at age 5 years. Primary treatment with minimal resection of the pseudarthrosis, diaphyseal corrective osteotomy, restoration of a medullary canal, and intramedullary nailing with a telescopic nail in combination with Ilizarov external ring fixator
Based on our study, we conclude that the incidence of CPT in Norway seems to be notably higher than that reported in epidemiological data from other authors. Together with earlier investigations aimed at gaining epidemiological data on CPT, our study might contribute to a further understanding of this rare disease. With the Ilizarov method, healing can be achieved in more than half of the patients. However, multiple surgeries are usually required due to refracture, deformities, and leg length discrepancy. The odds of achieving permanent union are more favorable at a higher age. Refractures should be avoided and alignment of the leg maintained. For primary treatment, an Ilizarov frame combined with a permanent intramedullary nail might be considered to maintain alignment and to provide internal bracing for a united tibia. However, protection of the extremity with an orthosis until skeletal maturity is recommended in all patients. Lengthening procedures and other corrective osteotomies increase the rate of complications. Avoidance of excessive resection of the pseudarthrosis might be considered to reduce the demand for lengthening procedures. Furthermore, the possibility for epiphysiodesis and hemi-epiphysiodesis for correction of LLD and angular deformities should be considered at an appropriate age.
Conflict of interest
None.
References
- 1.Hefti F. Malformations of the lower extremities. Orthopade. 2008;37:381–402. doi: 10.1007/s00132-008-1250-4. [DOI] [PubMed] [Google Scholar]
- 2.Paterson D (1989) Congenital pseudarthrosis of the tibia. An overview. Clin Orthop Relat Res 247:44–54 [PubMed]
- 3.Herring J. Disorders of the leg. In: Herring J, editor. Tachdjian’s pediatric orthopaedics. Philadelphia: Saunders Elsevier; 2008. [Google Scholar]
- 4.Andersen KS. Occurrence of congenital tibial pseudoarthrosis in Denmark 1940–1965. Nord Med. 1971;86:1395. [PubMed] [Google Scholar]
- 5.Andersen KS. Congenital pseudarthrosis of the tibia and neurofibromatosis. Acta Orthop Scand. 1976;47:108–111. doi: 10.3109/17453677608998981. [DOI] [PubMed] [Google Scholar]
- 6.Feldman DS, Jordan C, Fonseca L. Orthopaedic manifestations of neurofibromatosis type 1. J Am Acad Orthop Surg. 2010;18:346–357. doi: 10.5435/00124635-201006000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Crawford AH, Jr, Bagamery N. Osseous manifestations of neurofibromatosis in childhood. J Pediatr Orthop. 1986;6:72–88. doi: 10.1097/01241398-198601000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Crawford AH, Schorry EK. Neurofibromatosis in children: the role of the orthopaedist. J Am Acad Orthop Surg. 1999;7:217–230. doi: 10.5435/00124635-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Hefti F, Bollini G, Dungl P, et al. Congenital pseudarthrosis of the tibia: history, etiology, classification, and epidemiologic data. J Pediatr Orthop B. 2000;9:11–15. doi: 10.1097/01202412-200001000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Andersen KS. Radiological classification of congenital pseudarthrosis of the tibia. Acta Orthop Scand. 1973;44:719–727. doi: 10.3109/17453677308989112. [DOI] [PubMed] [Google Scholar]
- 11.Boyd HB, SAGE FP. Congenital pseudarthrosis of the tibia. J Bone Jt Surg Am. 1958;40-A:1245–1270. [PubMed] [Google Scholar]
- 12.Boyd HB (1982) Pathology and natural history of congenital pseudarthrosis of the tibia. Clin Orthop Relat Res 166:5–13 [PubMed]
- 13.Grill F, Bollini G, Dungl P, et al. Treatment approaches for congenital pseudarthrosis of tibia: results of the EPOS multicenter study. European Paediatric Orthopaedic Society (EPOS) J Pediatr Orthop B. 2000;9:75–89. doi: 10.1097/01202412-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Boero S, Catagni M, Donzelli O, et al. Congenital pseudarthrosis of the tibia associated with neurofibromatosis-1: treatment with Ilizarov’s device. J Pediatr Orthop. 1997;17:675–684. doi: 10.1097/01241398-199709000-00019. [DOI] [PubMed] [Google Scholar]
- 15.Choi IH, Cho TJ, Moon HJ. Ilizarov treatment of congenital pseudarthrosis of the tibia: a multi-targeted approach using the Ilizarov technique. Clin Orthop Surg. 2011;3:1–8. doi: 10.4055/cios.2011.3.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fabry G, Lammens J, Van MJ, Stuyck J. Treatment of congenital pseudarthrosis with the Ilizarov technique. J Pediatr Orthop. 1988;8:67–70. doi: 10.1097/01241398-198801000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Kristiansen LP, Steen H, Terjesen T (2003) Residual challenges after healing of congenital pseudarthrosis in the tibia. Clin Orthop Relat Res 414:228–237 [DOI] [PubMed]
- 18.Romanus B, Bollini G, Dungl P, et al. Free vascular fibular transfer in congenital pseudoarthrosis of the tibia: results of the EPOS multicenter study. European Paediatric Orthopaedic Society (EPOS) J Pediatr Orthop B. 2000;9:90–93. doi: 10.1097/01202412-200004000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Dobbs MB, Rich MM, Gordon JE, et al. Use of an intramedullary rod for treatment of congenital pseudarthrosis of the tibia. A long-term follow-up study. J Bone Jt Surg Am. 2004;86-A:1186–1197. doi: 10.2106/00004623-200406000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Ohnishi I, Sato W, Matsuyama J, et al. Treatment of congenital pseudarthrosis of the tibia: a multicenter study in Japan. J Pediatr Orthop. 2005;25:219–224. doi: 10.1097/01.bpo.0000151054.54732.0b. [DOI] [PubMed] [Google Scholar]
- 21.The Norwegian Institute of Public Health (2012) The Norwegian Institute of Public Health, Oslo
- 22.Paley D. Principles of deformity correction. Berlin: Springer; 2005. [Google Scholar]
- 23.Carney BT, Daniels CL. A retrospective review of congenital pseudarthrosis of the tibia. Iowa Orthop J. 2002;22:57–60. [PMC free article] [PubMed] [Google Scholar]
- 24.Cho TJ, Choi IH, Lee KS, et al. Proximal tibial lengthening by distraction osteogenesis in congenital pseudarthrosis of the tibia. J Pediatr Orthop. 2007;27:915–920. doi: 10.1097/bpo.0b013e31815a6058. [DOI] [PubMed] [Google Scholar]
- 25.Johnston CE. Congenital pseudarthrosis of the tibia: results of technical variations in the Charnley–Williams procedure. J Bone Jt Surg Am. 2002;84-A:1799–1810. [PubMed] [Google Scholar]
- 26.Nguyen NH. Use of an intramedullary Kirschner wire for treatment of congenital pseudarthrosis of the tibia in children. J Pediatr Orthop B. 2009;18:79–85. doi: 10.1097/BPB.0b013e32832942e1. [DOI] [PubMed] [Google Scholar]
- 27.Morrissy RT (1982) Congenital pseudarthrosis of the tibia. Factors that affect results. Clin Orthop Relat Res 166:21–27 [PubMed]
- 28.Patterson M. Impact of external fixation on adolescents: an integrative research review. Orthop Nurs. 2006;25:300–308. doi: 10.1097/00006416-200605000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Ippolito E, Corsi A, Grill F, et al. Pathology of bone lesions associated with congenital pseudarthrosis of the leg. J Pediatr Orthop B. 2000;9:3–10. doi: 10.1097/01202412-200001000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Iamaguchi RB, Fucs PM, da Carlos CA, et al. Congenital pseudoarthrosis of the tibia—results of treatment by free fibular transfer and associated procedures—preliminary study. J Pediatr Orthop B. 2011;20:323–329. doi: 10.1097/BPB.0b013e328347a361. [DOI] [PubMed] [Google Scholar]
- 31.Viehweger E, Pouliquen JC, Kassis B, et al. Bone growth after lengthening of the lower limb in children. J Pediatr Orthop B. 1998;7:154–157. doi: 10.1097/01202412-199804000-00013. [DOI] [PubMed] [Google Scholar]
- 32.Stevens PM, Kennedy JM, Hung M. Guided growth for ankle valgus. J Pediatr Orthop. 2011;31:878–883. doi: 10.1097/BPO.0b013e318236b1df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delgado-Martinez AD, Rodriguez-Merchan EC, Olsen B. Congenital pseudarthrosis of the tibia. Int Orthop. 1996;20:192–199. doi: 10.1007/s002640050062. [DOI] [PubMed] [Google Scholar]
- 34.Paley D, Catagni M, Argnani F et al (1992) Treatment of congenital pseudoarthrosis of the tibia using the Ilizarov technique. Clin Orthop Relat Res 280:81–93 [PubMed]


