Abstract
A series of lobelane analogues has been synthesized and their structure–activity relationships at the vesicular monoamine transporter-2 (VMAT2) have been evaluated. The most potent analogues in this series were the cis-2,6-piperidino analogues, 25b, 27b, 28b, and 30b, with Ki values ranging from 430 to 580 nM.
Keywords: Lobeline, Methamphetamine, Vesicular monoamine transporter, Structure–activity relationships
1. Introduction
Psychostimulant abuse is a serious and escalating worldwide problem.1 Methamphetamine is an addictive psychostimulant drug and chronic use may cause long-term neural damage in humans, with concomitant deleterious effects on cognitive processes such as memory and attention.2 Despite the serious consequences of methamphetamine abuse, currently there are no accepted pharmacological treatments for methamphetamine addiction. As a result, there is increasing interest in identifying underlying mechanisms of methamphetamine action and relevant pharmacological targets to promote the development of novel therapeutic agents to treat methamphetamine abuse.
The abuse liability of psychostimulants is thought to result from alterations in the brain s dopaminergic system, which is generally accepted as being responsible for the rewarding effects of these abused drugs.3 Through an interaction with the vesicular monoamine transporter-2 (VMAT2), amphetamine and methamphetamine promote dopamine (DA) release from the synaptic vesicles into the cytosol of the dopaminergic presynaptic terminals.4,5 These psychostimulants also inhibit monoamine oxidase,6 resulting in an increase in DA concentration in the cytosolic pool, which undergoes reverse transport via the DA transporter, leading to an increase in DA concentrations in the extracellular space.5
(−)-Lobeline (the 2R,6S,10S-stereoisomer, 1; Fig. 1), the major alkaloid in Lobelia inflata, decreases both the stimulant and rewarding effects of methamphetamine, and does not act as a substitute reinforcer.7-10 The mechanism underlying the lobeline-induced inhibition of these effects of methamphetamine has been suggested to be due to a noncompetitive inhibition of VMAT2.11 The observation that lobeline is not self-administered is consistent with findings that lobeline does not evoke DA release.10-12 Furthermore, the observation that lobeline inhibits methamphetamine-evoked DA release from superfused rat striatal slices7 is in agreement with its ability to decrease methamphetamine self-administration.9 These studies clearly suggest the significance of VMAT2 as a potential target for the development of agents to treat methamphetamine abuse. To date, there are very few VMAT2 ligands reported in the literature.13-15 Thus, lobeline analogues with selectivity for VMAT2 would provide a novel structural class of ligands as new tools to probe VMAT2 and as potential leads for therapeutic development.
Figure 1.
Structures of lobeline (1), MTD (2), lobelane (3), and N-methyl-2,6-cis-di-(naphthylene-1-ethyl)piperidine (1-NAP-lobelane, 4).
Due to the high affinity of lobeline for several neuronal nicotinic acetylcholine receptor (nAChR) subtypes,16-18 studies have been conducted in our laboratory, which have focused on structural modification of the lobeline molecule to increase affinity and selectivity for VMAT2.17,19 Systematic structural modification of lobeline afforded meso-transdiene (MTD, 2) (Fig. 1), a more selective ligand than lobeline at VMAT2; however, MTD was slightly less potent than lobeline at VMAT2.17,19 Interestingly, the defunctionalized, saturated lobeline analogue, lobelane (3) (Fig. 1), had higher affinity than lobeline and good selectivity for the [3H]dihydrotetrabenazine ([3H]DTBZ) binding site on VMAT2 compared with lobeline. To further explore the structural features that might improve affinity and selectivity for VMAT2, we prepared compound 4 (1-NAP-lobelane, Fig. 1) where the 2,6-phenyl groups in lobelane have been replaced by 1-naphthyl moieties to determine if enhancing π-π interaction at the VMAT2 binding site would improve affinity.19 While retaining affinity at VMAT2, compound 4 had increased selectivity at VMAT2 over α4β2* and α7* nAChRs, indicating that the VMAT2 binding site can tolerate structural modifications in the phenyl groups of lobelane.
The objective of the present study was to prepare an expanded series of lobelane analogues containing a variety of substituents in the phenyl rings of lobelane, and to assess their affinity and selectivity for VMAT2. In addition, to further develop structure–activity relationships within the lobeline/lobelane series of analogues and to define the structural requirement for VMAT2 binding, we prepared additional analogues. These included (1) structural fragments of the lobeline, MTD, and lobelane molecules, (2) stereoisomeric analogues at the piperidino C2 and C6 positions, (3) replacement of the piperidine ring with a piperizino ring, and (4) replacement of the N-methyl group of lobelane with either a hydrogen, ethyl, or propyl group.
2. Results and discussion
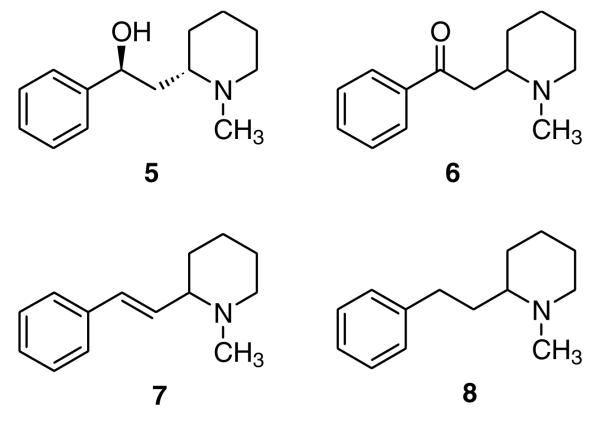
Fragments of the lobeline structure, such as compounds 5 and 6, have been shown previously to have diminished affinity at nAChRs.20-22 Additionally, MTD (2) and lobelane (3), which are defunctionalized lobeline analogues, had markedly diminished nAChR affinity. Importantly, both MTD and lobelane had good affinity at VMAT2. Furthermore, MTD and lobelane were more selective ligands than lobeline at this transporter. Lobelane exhibited a significant, but small increase in affinity at VMAT2, compared to lobeline.17 In order to determine if the structure of the whole lobeline/MTD/lobelane molecule is required for potent VMAT2 interaction, we synthesized analogues 5–8 (Fig. 2), which are structural fragments of lobeline (5 and 6), MTD (7), and lobelane (8). Compounds 523 and 824 were synthesized as previous reported. Compounds 625 and 726 were synthesized via initial Sonogashira cross-coupling of 2-bromopyridine (9) and phenylacetylene to form compound 10.27 Compound 10 was then hydrolyzed to the keto compound 11 with 60% sulfuric acid under reflux. Hydrogenation of 11 with Adam’s catalyst and then N-methylation afforded compound 6. The keto group in compound 6 was reduced to a hydroxyl group and the resulting product dehydrated to compound 7 (Scheme 1). Compounds 5 and 6, which represent the hydroxyl containing fragment and the keto containing fragment of lobeline (1), showed diminished affinity at both α4β2* and α7* nAChRs. Interestingly, compound 8, in which one of the piperidine ring side chains of lobelane (3) has been removed, showed 3-fold higher affinity at α4β2* compared to lobelane, and exhibited no affinity at α7*. Compound 7, in which a double bond was introduced, showed no affinity at either α4β2* or α7* nAC-hRs. None of these fragmented compounds showed any affinity at VMAT2 (Table 1). These results suggest that both the C2 and C6 side chains of the piperidine ring of lobeline, MTD, or lobelane are essential for VMAT2 affinity and selectivity.
Figure 2.
Structural fragments of the lobeline (5 and 6), MTD (7), and lobelane (8) molecules.
Scheme 1.
Reagents and conditions: (a) phenylacetylene, Pd(PPh3)2Cl2, CuI, Et3N, THF, rt; (b) 60% H2SO4, reflux; (c) (i) HCl; (ii) H2, PtO2, MeOH; (d) HCOOH, 37% HCHO, reflux; (e) NaBH4, EtOH; (f) 85% H3PO4, 60 °C.
Table 1.
Inhibition constants (Ki) for lobelane analogues at the [3H]NIC binding site (α4β2* nAChR) and the [3H]MLA binding site (α7* nAChR) on rat brain membranes, and at the [3H]DTBZ binding site (VMAT2) on rat synaptic vesicle membranes
| Compound |
Ki (μM) ± SEMa |
||
|---|---|---|---|
| [3H]NIC binding |
[3H]MLA binding |
[3H]DTBZ binding |
|
| 1 | 0.004 ± 0.000 | 6.26 ± 1.30 | 2.76 ± 0.64 |
| 2 | 11.6 ± 2.01 | >100 | 9.88 ± 2.22 |
| 3 | 14.9 ± 1.67 | 26.0 ± 6.57 | 0.97 ± 0.19 |
| 4 | >100 | >100 | 0.63 ± 0.16 |
| 5 | >100 | >100 | >100 |
| 6 | 25.9 ± 1.62 | >100 | >100 |
| 7 | 4.52 ± 0.58 | >100 | >100 |
| 8 | >100 | >100 | >100 |
| 14a | >100 | >100 | 6.11 ± 0.92 |
| 15a | >100 | >100 | 11.7 ± 0.65 |
| 14b | >100 | >100 | 1.36 ± 0.11 |
| 15b | >100 | >100 | 2.62 ± 0.51 |
| 14c | >100 | >100 | 36.7 ± 20.3 |
| 15c | 5.06 ± 0.70 | >100 | 6.09 ± 0.19 |
| 18a | >100 | 66.9 ± 27.4 | 3.32 ± 0.74 |
| 19a | >100 | >100 | 3.11 ± 0.51 |
| 20a | >100 | 9.02 ± 1.54 | 3.35 ± 0.80 |
| 18b | >100 | >100 | 3.95 ± 0.61 |
| 19b | >100 | >100 | 10.5 ± 1.58 |
| 20b | >100 | >100 | 4.04 ± 1.76 |
| 18c | 7.62 ± 1.47 | >100 | 1.87 ± 0.25 |
| 19c | >100 | >100 | 3.28 ± 1.65 |
| 20c | >100 | >100 | 2.47 ± 0.70 |
Each Ki value represents data from at least four independent experiments, each performed in duplicate.
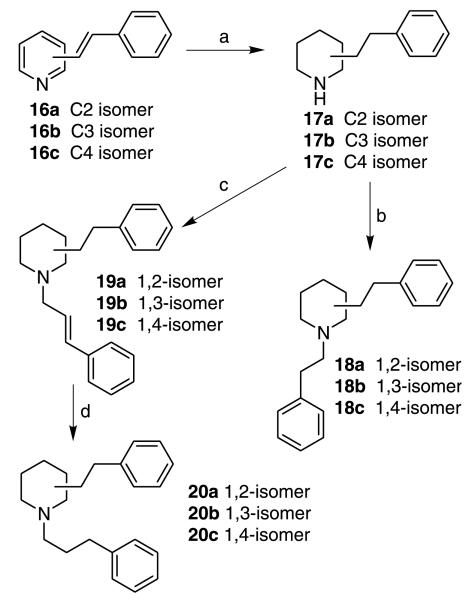
To further understand the importance of the juxtaposition and stereochemistry of the piperidino C2 and C6 substituents in the lobelane molecule, and the importance of the intramolecular distance between the nitrogen atom and the phenyl rings of lobelane, compounds 14a,b, 15a,b, 18a–c, 19a–c, and 20a–c were synthesized. In these compounds, the C2, C6 phenethyl groups of lobelane (3) were moved to the C3, C5; C2, C4; N1, C2; N1, C3; or N1, C4 positions of the piperidine ring. The synthesis of 14b and 15b was initiated from the condensation reaction of 2,4-lutidine with benzaldehyde.28 The resulting highly conjugated product, 13b, was reduced to compound 14b under Adam s reduction conditions.28 N-Methylation was then carried out utilizing NaCNBH3/para-formaldehyde to form compound 15b (Scheme 2). Similar procedures were employed for the synthesis of compounds 14a and 15a. N-Benzylidene-4-chloroaniline was used as an activated form of benzaldehyde in the condensation reaction, due to the low activity of the pyridinyl 3-methyl group.29 Compounds 17a–c, which were synthesized from 2-, 3-, or 4-picoline utilizing a similar procedure as that utilized for the synthesis of 14a and 14b (Scheme 3), were transformed into the N-phenethyl compounds 18a, 18b, and 18c via reductive amination, or transformed into 19a, 19b, and 19c by alkylation with trans-cinnamyl bromide. Catalytic hydrogenation of 19a, 19b, or 19c afforded compound 20a, 20b, or 20c, respectively (Scheme 3). [3H]Nicotine ([3H]NIC), [3H]methyllycaconitine ([3H]MLA), and [3H]dihydrotetrabenazine ([3H]DTBZ) binding data for compounds 14a–20c are summarized in Table 1. As expected, most of these compounds showed no affinity at either α4β2* or α7* nAChRs. All of these analogues retained affinity at VMAT2 but were generally less potent than lobelane; affinity for the [3H]DTBZ binding site on VMAT2 was within one order of magnitude of that for lobelane (3) (Table 1). Analogues bearing a side chain at the piperidino C3 position, that is, 14a, 15a, 18b, 19b, and 20b, exhibited slightly lower potency at VMAT2 compared with lobelane (3). Thus, the position of the piperidine N atom relative to the C2 and C6 side chains does not appear to be critical for VMAT2 interaction. The best compound in this series was 14b (Ki = 1.36 μM), in which the two side chains are located on the piperidino C2 and C4 positions. These results also indicate that the VMAT2 binding site can tolerate apparent changes in distance between the piperidine nitrogen and the two phenyl rings in these molecules. However, the above molecules are very flexible, and can exist in a number of conformational forms. Thus, it is possible that the VMAT2 pharmacophore may require a fixed phenyl ring-piperidine nitrogen distance, and that the above molecules may undergo conformational changes to accommodate this pharmacophoric requirement.
Scheme 2.
Reagents and conditions: (a) 12a: N-benzylidene-4-chloroaniline, t-BuOK, DMF, 90 °C; 12b and 12c: benzaldehyde, Ac2O, reflux; (b) H2, PtO2, HOAc, 45 psi, rt; (c) (CH2O)n, NaCNBH3, MeOH, rt.
Scheme 3.
Reagents and conditions: (a) H2, PtO2, HOAc, 45 psi, rt; (b) phenylacetaldehyde, NaCNBH3, MeOH, rt; (c) trans-cinnamyl bromide, K2CO3, acetone; (d) H2, 10% Pd/C, EtOH, 15 psi, rt.
Compounds 14c and 15c, in which the piperidine ring of lobelane has been replaced by a piperazine ring, were synthesized by employment of a similar procedure as was utilized for the preparation of 14b and 15b (Scheme 2). Compounds 14c and 15c were about 36- and 6-fold less potent than lobelane at VMAT2, respectively. In addition, compound 15c had 3-fold higher affinity than lobelane at α4β2* (Table 1).
In a previous SAR study, we prepared compound 4 (Fig. 1, Table 1) in which the phenyl groups in lobelane were replaced with 1-naphthyl moieties.19 We found that compound 4 had increased affinity and selectivity at VMAT2 compared with lobeline, and had slightly better affinity than lobelane at VMAT2. This led us to conclude that modification of the phenyl rings in lobelane, and specifically introduction of an extended π system might offer improved potency and selectivity at VMAT2. Unfortunately, compound 4 has very low water solubility, which precluded further evaluation of the pharmacological properties of this compound. Nevertheless, the pharmacological properties of compound 4 strongly suggest that the phenyl groups of lobelane might be amenable to structural modification. Thus, a series of phenyl ring substituted lobelane analogues were synthesized and evaluated at the [3H]NIC binding site (α4β2* nAChR) and the [3H]MLA binding site (α7* nAChR) on rat brain membranes, and at the [3H]DTBZ binding site (VMAT2) on rat synaptic vesicle membranes. These data are summarized in Table 2. The synthesis of these compounds was carried out using an approach similar to that utilized for compounds 14b and 15b (see Scheme 2). The general structures of these compounds are given in Table 2. All the compounds listed in Table 2 showed little or no affinity for both α4β2* and α7* nAChRs, but retained VMAT2 affinity, which was comparable to lobelane. All these phenyl substituted lobelane analogues exhibited Ki values in the VMAT2 assay within a range of about one order of magnitude. The 2-methoxy substituted analogue 28b had the highest affinity (Ki = 0.43 μM) at the [3H]DTBZ binding site on VMAT2. Interestingly, in these analogues, there were no marked differences in VMAT2 affinity between analogues bearing electron donating or withdrawing substituents. However, compounds 34a and 34b, in which a phenyl group is attached to the para position of the lobelane phenyl rings, showed reduced affinity, and a lack of affinity at VMAT2, respectively. This may indicate that the VMAT2 binding site can not accommodate bulky substituents at the para position of the phenyl rings.
Table 2.
Inhibition constants (Ki) for lobelane analogue at [3H]NIC (α4β2* nAChR) and [3H]MLA (α7* nAChR) binding sites on rat brain membranes, and inhibition of the [3H]DTBZ binding site on VMAT2 on rat synaptic vesicle membranes

| Compound | R | R1 | R2 | R3 |
Ki (μM) ± SEMa |
||
|---|---|---|---|---|---|---|---|
| [3H]NIC binding | [3H]MLA binding | [3H]DTBZ binding | |||||
| 22 | CH3CH2 | H | H | H | >100 | >100 | 3.41 ± 0.67 |
| 23 | CH3CH2CH2 | H | H | H | >100 | 18.6 ± 2.87 | 1.87 ± 0.25 |
| 24a | H | F | H | H | >100 | >100 | 1.60 ± 0.10 |
| 24b | CH3 | F | H | H | >100 | >100 | 1.07 ± 0.07 |
| 25a | H | H | F | H | >100 | 49.2 ± 21.3 | 1.60 ± 0.08 |
| 25b | CH3 | H | F | H | >100 | 16.6 ± 3.47 | 0.57 ± 0.07 |
| 26a | H | H | H | F | >100 | >100 | 1.25 ± 0.08 |
| 26b | CH3 | H | H | F | >100 | >100 | 0.98 ± 0.31 |
| 27a | H | CH3O | H | H | >100 | >100 | 1.87 ± 0.19 |
| 27b | CH3 | CH3O | H | H | >100 | 29.7 ± 7.73 | 0.58 ± 0.04 |
| 28a | H | H | CH3O | H | >100 | >100 | 1.67 ± 0.07 |
| 28b | CH3 | H | CH3O | H | >100 | 25.1 ± 2.23 | 0.43 ± 0.03 |
| 29a | H | H | H | CH3O | >100 | >100 | 3.32 ± 0.82 |
| 29b | CH3 | H | H | CH3O | >100 | >100 | 1.73 ± 0.20 |
| 30a | H | H | –OCH2O– | >100 | >100 | 2.34 ± 0.16 | |
| 30b | CH3 | H | –OCH2O– | >100 | 18.2 ± 6.87 | 0.52 ± 0.25 | |
| 31a | H | H | H | CH3 | >100 | >100 | 3.23 ± 0.10 |
| 31b | CH3 | H | H | CH3 | >100 | >100 | 4.36 ± 0.18 |
| 32a | H | H | CF3 | H | >100 | >100 | 9.90 ± 2.01 |
| 32b | CH3 | H | CF3 | H | >100 | >100 | 1.51 ± 0.07 |
| 33a | H | Cl | H | Cl | >100 | >100 | 1.32 ± 0.18 |
| 33b | CH3 | Cl | H | Cl | >100 | >100 | 1.04 ± 0.06 |
| 34a | H | H | H | Ph | >100 | >100 | >100 |
| 34b | CH3 | H | H | Ph | >100 | >100 | 10.7 ± 6.60 |
| 35 | H | H | H | CH3COO | >100 | >100 | 5.96 ± 1.76 |
| 36 | H | H | H | OH | >100 | >100 | 5.26 ± 0.47 |
Each Ki value represents data from at least four independent experiments, each performed in duplicate.
N-Methyl lobelane analogues were often slightly more potent than the corresponding nor-lobelane (R1 = R2 = R3 = R = H, see structure in Table 2) analogues. This encouraged us to synthesize N-ethyl lobelane (22) and N-propyl lobelane (23) in order to determine if the methyl group is critical for recognition by the VMAT2 bind site. Compounds 22 and 23 were equipotent, but slightly less potent than lobelane at VMAT2, indicating that the N-methyl group plays a role in VMAT2 binding, but is not critical for binding site recognition.
3. Summary
Defunctionalization of the lobeline molecule markedly decreases affinity for α4β2* and α7* nAChRs, while increasing affinity for VMAT2. The most potent and selective compounds were lobelane analogues bearing methoxy, methylenedioxy, and fluoro substituents in the aromatic rings. The complete lobelane structure appears to be critical for high affinity binding at VMAT2, since fragments of lobelane or MTD exhibited significantly decreased affinity for VMAT2. Modification of the phenyl rings in lobelane by introduction of an extended p system also appears to afford an improvement in potency and selectivity at VMAT2. Compound 28b appears to be a promising lead compound for further development of second generation of VMAT2 ligands.
4. Experimental
All reagents and chemicals were purchased from Aldrich Chemical Co., Milwaukee, WI; Acros Organics, Somerville, NJ; or Lancaster Synthesis, Windham, NH and were used without further purification. Flash column chromatography was carried out using ICN SILITECH 32–63, 60Å silica gel. TLC analyses were carried out on EMD Chemicals Inc. glass plates precoated with 250 μm silica gel 60 F254. Melting points were determined on a Fisher Scientific melting point apparatus and are uncorrected. NMR spectra were recorded in CDCl3 on a Varian 300 MHz instrument and are reported in ppm relative to TMS as internal standard. Mass spectra were recorded on a JEOL JMS-700T MStation or on a Bruker Autoflex MALDI-TOF MS. GC–mass spectra were recorded on an Agilent 6890 GC incorporating an Agilent 7683 autosampler and an Agilent 5973 MSD. All of the final amine compounds were converted to their hydrochloride salts with 2 N HCl in Et2O. Elemental analyses were carried out on a COSTECH elemental combustion system and are within ±0.4% of theoretical values. Compounds 5,23 6,25 7,24 and 826 have been previously reported, and their characterization data are in agreement with reported values.
4.1. General procedure for synthesis of compounds 14a–c, 17a–c, nor-lobelane, 24a–34a, and 35
The synthetic procedure utilized was a modification of a previously reported general procedure.28 2,4-Lutidine (1 equiv for synthesis of 14b), or 2,6-dimethylpyrazine (for synthesis of 14c), or 2-picoline (for synthesis of 17a), or 4-picoline (for synthesis of 17c), or 2,6-lutidine (for synthesis of nor-lobelane, 24a–34a, and 35), was mixed with the appropriate aryl aldehyde (1.05 equiv for picolines and 2.1 equiv for 2,6-dimethylpyrazine and lutidines) (for compound 35, 4-hydroxybenzaldehyde was used) and acetic anhydride. The mixture was refluxed under N2 for 48–72 h and then cooled to room temperature. The solidified mixture was transferred to a suction filter and the filter cake was washed with 95% ethanol. If the product did not solidify during cooling, then excessive ethanol (95%) was added and the mixture stirred until the product was precipitated. The crude product was recrystallized from benzene or ethanol to afford compounds 13b,c, 16a,c, and 21. For compound 13a or 16b, N-benzylidene-4-chloroaniline was used as an activated form of benzaldehyde, t-BuOK was used as base, as reported previously.29
Each of the above conjugated products (10 mmol) was dissolved in glacial acetic acid (30 mL) and PtO2 (1–2% w/w) was added. The resulting mixture was hydrogenated on a Parr hydrogenation apparatus (45 psi) for 12–48 h. The catalyst was removed by filtration through a Celite pad. The filter cake was rinsed with methanol, and the combined organic liquors were concentrated under reduced pressure. The resulting residue was basified with saturated aqueous K2CO3 and the aqueous solution was extracted with CHCl3 (3 × 50 mL). The combined organic extracts were dried (Na2SO4), filtered, and concentrated. The crude product was purified by column chromatography to afford the title compounds.
4.1.1. 3,5-cis-Diphenethylpiperidine (14a)
Mp 98–99 °C (HCl salt); 1H NMR (300 MHz, CDCl3): δ 0.73 (m, 1H), 1.40–1.60 (m, 6H), 2.00 (br d, J = 12.9 Hz, 1H), 2.19 (dd, J = 11.7, 10.5 Hz, 2H), 2.50–2.71 (m, 5H), 3.13 (br d, J = 9.0 Hz, 2H), 7.13–7.32 (m, 10H) ppm; 13C NMR (75 MHz, CDCl3): δ 33.3, 36.8, 37.0, 38.7, 52.8, 125.8, 128.4, 128.5, 142.6 ppm; MS (EI) m/z 293 (M+). Anal. Calcd for C21H27N·HCl·1/3H2O: C, 75.09; H, 8.60; N, 4.17. Found: C, 74.86; H, 8.79; N, 4.15.
4.1.2. 2,4-cis-Diphenethylpiperidine (14b)
1H NMR (300 MHz, CDCl3): δ 0.83 (dd, J = 24.0, 11.7 Hz, 1H), 1.10 (ddd, J = 24.3, 12.3, 4.2 Hz, 1H), 1.39 (m, 1H), 1.55 (m, 2H), 1.62–1.98 (m, 5H), 2.40–2.76 (m, 6H), 3.11 (ddd, J = 12.0, 4.2, 2.4 Hz, 1H), 7.10–7.36 (m, 10H) ppm; 13C NMR (75 MHz, CDCl3): δ 32.6, 33.1, 33.4, 36.1, 39.3, 39.4, 39.9, 47.0, 56.4, 125.7, 125.9, 128.4, 128.5, 142.3, 142.8 ppm; MS (EI) m/z 293 (M+). Anal. Calcd for C21H27N·HCl·0.5H2O: C, 74.42; H, 8.62; N, 4.13. Found: C, 74.44; H, 8.50; N, 4.08.
4.1.3. 2,6-cis-Diphenethylpiperizine (14c)
Mp 251–252 °C (HCl salt); 1H NMR (300 MHz, DMSO-d6): δ 1.95 (m, 2H), 2.13 (m, 2H), 2.62–2.86 (m, 4H), 3.14 (t, J = 12.3 Hz, 2H), 3.34–3.68 (m, 4H), 7.17–7.38 (m, 10H) ppm; 13C NMR (75 MHz, DMSO-d6): δ 30.2, 31.4, 43.0, 52.0, 126.0, 128.1, 128.3, 140.2 ppm; MS (EI) m/z 294 (M+). Anal. Calcd for C20H26N2·2HCl·0.25H2O: C, 64.60; H, 7.73; N, 7.53. Found: C, 64.56; H, 7.44; N, 7.61.
4.1.4. 2,6-cis-Di-(o-fluorophenethyl)piperidine (24a)
Mp 228–229 °C (HCl salt); 1H NMR (300 MHz, CDCl3): δ 1.08 (ddd, J = 12.9, 4.0, 3.9 Hz, 2H), 1.33 (ddt, J = 25.8, 12.9, 3.9 Hz, 1H), 1.59–1.75 (m, 6H), 1.79 (ddd, J = 12.9, 6.0, 3.0 Hz, 1H), 2.50 (m, 1H), 2.69 (t, J = 7.8 Hz, 4H), 6.96–7.09 (m, 4H), 7.12–7.22 (m, 4H) ppm; 13C NMR (75 MHz, CDCl3): δ 25.0, 25.7, 32.8, 37.9, 56.8, d (115.150, 115.454), d (124.050, 125.095), d (127.497, 127.603), d (129.031, 129.243), d (130.534, 130.595), d (159.525, 162.760) ppm; MS (EI) m/z 329 (M+). Anal. Calcd for C21H25F2N·HCl: C, 68.94; H, 7.16; N, 3.83. Found: C, 69.46; H, 7.13; N, 4.05.
4.1.5. 2,6-cis-Di-(m-fluorophenethyl)piperidine (25a)
Mp 202–203 °C (HCl salt); 1H NMR (300 MHz, CDCl3): δ 1.07 (ddd, J = 24.0, 13.2, 3.6 Hz, 2H), 1.33 (ddt, J = 25.8, 12.9, 3.6 Hz, 1H), 1.58–1.75 (m, 6H), 1.80 (ddd, J = 12.9, 6.3, 3.3 Hz, 1H), 2.50 (m, 2H), 2.64 (t, J = 7.8 Hz, 4H), 6.83–7.00 (m, 6H), 7.23 (m, 2H) ppm; 13C NMR (75 MHz, CDCl3): δ 24.9, 32.4, 32.8, 38.9, 56.7, d (112.645, 112.918), d (115.135, 115.409), d (124.065, 124.095), d (129.805, 129.912), d (144.855, 144.961), d (164.317, 164.567) ppm; MS (EI) m/z 329 (M+). Anal. Calcd for C21H25F2N·HCl: C, 68.94; H, 7.16; N, 3.83. Found: C, 68.98; H, 7.42; N, 4.07.
4.1.6. 2,6-cis-Di-(p-fluorophenethyl)piperidine (26a)
Mp 194–195 °C (HCl salt); 1H NMR (300 MHz, CDCl3): δ 1.08 (ddd, J = 24.0, 12.9, 3.6 Hz, 2H), 1.33 (ddt, J = 26.4, 12.9, 3.6 Hz, 1H), 1.58–1.74 (m, 6H), 1.80 (ddd, J = 13.2, 5.7, 3.6 Hz, 1H), 2.49 (m, 2H), 2.61 (t, J = 7.8 Hz, 4H), 6.96 (tt, J = 9.0, 2.4 Hz, 4H), 7.12 (dd, J = 9.0, 5.4 Hz, 4H) ppm; 13C NMR (75 MHz, CDCl3): δ 25.0, 31.9, 32.9, 39.4, 56.8, 115.1, 115.4, 129.7, 129.8, 137.8, 137.9, 159.6, 162.9 ppm; MS (EI) m/z 329 (M+). Anal. Calcd for C21H25F2N·HCl·0.2H2O: C, 68.26; H, 7.20; N, 3.79. Found: C, 68.35; H, 7.42; N, 4.07.
4.1.7. 2,6-cis-Di-(o-methoxyphenethyl)piperidine (27a)
Mp 141–142 °C (HCl salt); 1H NMR (300 MHz, CDCl3): δ 1.09 (ddd, J = 24.0, 12.6, 3.6 Hz, 2H), 1.29 (ddt, J = 25.5, 12.6, 3.6 Hz, 1H), 1.58–1.76 (m, 6H), 1.76 (ddd, J = 12.6, 6.3, 3.0 Hz, 1H), 2.45 (m, 2H), 2.65 (m, 4H), 3.79 (s, 6H), 6.82 (d, J = 8.1 Hz, 2H), 6.87 (dt, J = 7.5, 1.2 Hz, 2H), 7.10–7.20 (m, 4H) ppm; 13C NMR (75 MHz, CDCl3): δ 25.0, 26.6, 32.8, 37.8, 55.3, 56.8, 110.2, 120.5, 127.0, 129.8, 130.7, 157.3 ppm; MS m/z 353 (M+). Anal. Calcd for C23H31NO2·HCl·1/3H2O: C, 69.77; H, 8.32; N, 3.54. Found: C, 69.71; H, 8.44; N, 3.88.
4.1.8. 2,6-cis-Di-(m-methoxyphenethyl)piperidine (28a)
Mp 113–114 °C (HCl salt); 1H NMR (300 MHz, CDCl3): δ 1.08 (ddd, J = 24.0, 12.9, 3.6 Hz, 2H), 1.32 (ddt, J = 25.8, 12.9, 3.6 Hz, 1H), 1.62–1.75 (m, 6H), 1.79 (ddd, J = 13.2, 6.3, 3.0 Hz, 1H), 2.50 (m, 2H), 2.61 (t, J = 8.1 Hz, 4H), 3.78 (s, 6H), 6.70–6.80 (m, 6H), 7.16–7.26 (m, 4H) ppm; 13C NMR (75 MHz, CDCl3): δ 25.0, 32.8, 32.9, 39.1, 55.3, 56.9, 111.2, 114.2, 120.9, 129.4, 144.0, 159.7 ppm; MS (EI) m/z 353 (M+). Anal. Calcd for C23H31NO2·HCl·1/3H2O: C, 69.77; H, 8.32; N, 3.54. Found: C, 69.65; H, 8.54; N, 3.72.
4.1.9. 2,6-cis-Di-(p-methoxyphenethyl)piperidine (29a)
Mp 205–206 °C (lit.28 mp 209–211 °C) (HCl salt); 1H NMR (300 MHz, CDCl3): δ 1.08 (ddd, J = 23.7, 13.2, 3.3 Hz, 2H), 1.31 (ddt, J = 25.8, 13.2, 3.3 Hz, 1H), 1.41 (br s, 1H), 1.58–1.73 (m, 6H), 1.78 (ddd, J = 12.9, 6.3, 3.3 Hz, 1H), 2.48 (m, 2H), 2.57 (t, J = 8.1 Hz, 4H), 3.77 (s, 6H), 6.82 (dd, J = 8.7, 3.0 Hz, 4H), 7.09 (dd, J = 8.7, 3.3 Hz, 4H) ppm; 13C NMR (75 MHz, CDCl3): δ 25.0, 31.8, 32.9, 39.4, 55.4, 56.8, 113.9, 129.3, 134.4, 157.8 ppm; MS (EI) m/z 353 (M+). Anal. Calcd for C23H31NO2·HCl·1/3H2O: C, 69.77; H, 8.32; N, 3.54. Found: C, 69.82; H, 8.77; N, 3.81.
4.1.10. 2,6-cis-Di-(3,4-methylenedioxyphenethyl)piperidine (30a)
Mp 207–208 °C (lit.28 215–216 °C) (HCl salt); 1H NMR (300 MHz, CDCl3): δ 1.06 (ddd, J = 24.0, 12.9, 3.6 Hz, 2H), 1.31 (ddt, J = 5.8, 12.9, 3.6 Hz, 1H), 1.52-1.73 (m, 6H), 1.78 (ddd, J = 13.2, 6.3, 3.0 Hz, 1H), 2.47 (m, 2H), 2.55 (t, J = 8.1 Hz, 4H), 5.90 (s, 4H), 6.62 (dd, J = 7.8, 1.8 Hz, 2H), 6.67 (d, J = 1.8 Hz, 2H), 6.72 (d, J = 7.8 Hz, 2H) ppm; 13C NMR (75 MHz, CDCl3): δ 25.0, 32.4, 32.9, 39.4, 56.7, 100.9, 108.3, 108.9, 121.1, 136.1, 145.6, 147.6 ppm; MS (EI) m/z 381 (M+). Anal. Calcd for C23H27NO4·HCl·1/3H2O: C, 65.16; H, 6.82; N, 3.30. Found: C, 65.35; H, 7.20; N, 3.52.
4.1.11. 2,6-cis-Di-(p-methylphenethyl)piperidine (31a)
Mp 221–222 °C (HCl salt); 1H NMR (300 MHz, CDCl3): δ 1.08 (ddd, J = 24.0, 12.9, 3.3 Hz, 2H), 1.32 (m, 1H), 1.58–1.73 (m, 6H), 1.78 (m, 1H), 2.31 (s, 6H), 2.49 (m, 2H), 2.59 (t, J = 8.1 Hz, 4H), 7.08 (s, 8H) ppm; 13C NMR (75 MHz, CDCl3): δ 21.3, 25.1, 32.3, 32.9, 39.3, 56.9, 128.3, 129.2, 135.3, 139.3 ppm; MS (EI) m/z 321 (M+). Anal. Calcd for C23H31N·HCl·0.5-H2O: C, 75.28; H, 9.06; N, 3.82. Found: C, 75.49; H, 9.09; N, 4.19.
4.1.12. 2,6-cis-Di-(m-trifluoromethylphenethyl)piperidine (32a)
Mp 162–163 °C (HCl salt); 1H NMR (300 MHz, CDCl3): δ 1.08 (ddd, J = 24.3, 12.9, 3.9 Hz, 2H), 1.35 (ddt, J = 26.1, 12.9, 3.9 Hz, 1H), 1.61–1.78 (m, 6H), 1.82 (ddd, J = 13.2, 6.3, 3.0 Hz, 1H), 2.52 (m, 2H), 2.71 (t, J = 8.1 Hz, 4H), 7.34–7.58 (m, 8H) ppm; 13C NMR (75 MHz, CDCl3): δ 24.9, 32.5, 32.9, 39.1, 56.7, q (118.961, 122.560, 126.174, 129.773), q (122.757, 122.803, 122.848, 122.909), q (125.081, 125.126, 125.187, 125.233), 128.9, q (130.138, 130.563, 130.988, 131.398), 131.8, 143.3 ppm; MS (EI) m/z 430 (M−1)+. Anal. Calcd for C23H25F6N·HCl·1/3H2O: C, 58.54; H, 5.70; N, 2.97. Found: C, 58.34; H, 5.97; N, 3.21.
4.1.13. 2,6-cis-Di-(2,4-dichlorophenethyl)piperidine (33a)
Mp 183–184 °C (HCl salt); 1H NMR (300 MHz, CDCl3): δ 1.09 (ddd, J = 24.0, 12.6, 3.6 Hz, 2H), 1.35 (ddt, J = 26.1, 12.9, 3.6 Hz, 1H), 1.56–1.77 (m, 6H), 1.83 (ddd, J = 13.2, 6.6, 3.3 Hz, 1H), 2.53 (m, 2H), 2.74 (dd, J = 10.7, 7.2 Hz, 4H), 7.15 (s, 2H), 7.16 (d, J = 1.8 Hz, 2H), 7.35 (dd, J = 1.8, 0.6 Hz, 2H) ppm; 13C NMR (75 MHz, CDCl3): δ 24.9, 29.8, 32.7, 37.5, 56.8, 127.2, 129.3, 129.4, 131.1, 132.2, 134.6, 138.5 ppm; MS (EI) m/z 428/430/432/434/436 (M−1)+. Anal. Calcd for C21H23Cl4N·HCl: C, 53.93; H, 5.17; N, 2.99. Found: C, 54.33; H, 5.25; N, 3.14.
4.1.14. 2,6-cis-Di-(p-phenylphenethyl)piperidine (34a)
Mp 253–254 °C (HCl salt); 1H NMR (300 MHz, CDCl3): δ 1.11 (ddd, J = 23.7, 12.6, 3.3 Hz, 2H), 1.34 (ddt, J = 26.1, 12.6, 3.3 Hz, 1H), 1.58–1.88 (m, 7H), 2.54 (m, 2H), 2.68 (t, J = 7.8 Hz, 4H), 7.22–7.59 (m, 18H) ppm; 13C NMR (75 MHz, CDCl3): δ 25.0, 32.3, 32.9, 39.1, 56.9, 127.0, 127.1, 127.2, 128.8, 128.9, 138.8, 141.1, 141.4 ppm; MS (EI) m/z 445 (M+). Anal. Calcd for C33H35N·HCl: C, 82.21; H, 7.53; N, 2.91. Found: C, 82.51; H, 7.46; N, 2.87.
4.1.15. 2,6-cis-Di-(p-acetoxyphenethyl)piperidine (35)
Mp 238–239 °C (HCl salt); 1H NMR (300 MHz, CDCl3): δ 1.07 (ddd, J = 23.4, 12.6, 3.6 Hz, 2H), 1.33 (ddt, J = 25.8, 12.9, 3.6 Hz, 1H), 1.80 (ddd, J = 10.2, 5.7, 3.0 Hz, 1H), 2.28 (s, 6H), 2.51 (m, 2H), 2.64 (t, J = 7.8 Hz, 4H), 6.99 (dt, J = 9.0, 2.4 Hz, 4H), 7.18 (dt, J = 9.0, 2.4 Hz, 4H) ppm; 13C NMR (75 MHz, CDCl3): δ 21.4, 24.9, 32.0, 32.8, 39.2, 56.8, 121.5, 129.3, 139.9, 148.8, 169.7 ppm; MS (EI) m/z 408 (M−1)+. Anal. Calcd for C25H31NO4·HCl: C, 67.33; H, 7.23; N, 3.14. Found: C, 67.15; H, 7.54; N, 3.40.
4.2. General procedure for synthesis of compounds 15a–c, 18a–c, and 24b–34b
NaCNBH3 (188 mg, 3.00 mmol) was added to a mixture of the appropriate nor-compound (1.00 mmol), para-formaldehyde (150 mg, 5.00 mmol) (for 18a–c, phenylacetaldehyde was used) and methanol (10 mL). The mixture was stirred at room temperature for 2–12 h. The solvent was then evaporated under reduced pressure. The residue was dissolved in water (30 mL) and the aqueous solution was extracted with CHCl3 (3 × 20 mL). The combined organic extracts were dried (Na2SO4), filtered, and concentrated. The crude product was purified by column chromatography to give the title compounds.
4.2.1. N-Methyl-3,5-cis-diphenethylpiperidine (15a)
Mp 151–152 °C (HCl salt); 1H NMR (300 MHz, CDCl3): δ 0.57 (dd, J = 24.0, 12.0 Hz, 1H), 1.44–1.61 (m, 6H), 1.68 (m, 2H), 1.93 (br d, J = 13.2 Hz, 1H), 2.30 (s, 3H), 2.56–2.72 (m, 4H), 2.93 (br d, J = 10.2 Hz, 2H), 7.12–7.35 (m, 10H) ppm; 13C NMR (75 MHz, CDCl3): δ 33.5, 36.0, 36.9, 37.7, 46.7, 62.4, 125.8, 128.4, 128.5, 142.6 ppm; MS (EI) m/z 307 (M+). Anal. Calcd for C22H29N·HCl: C, 76.83; H, 8.79; N, 4.07. Found: C, 76.55; H, 8.83; N, 4.12.
4.2.2. N-Methyl-2,4-cis-diphenethylpiperidine (15b)
1H NMR (300 MHz, CDCl3): δ 1.14 (dd, J = 23.4, 11.7 Hz, 1H), 1.24–1.46 (m, 2H), 1.59 (dd, J = 15.3, 6.6 Hz, 2H), 1.66–1.98 (m, 5H), 2.11 (dt, J = 12.0, 2.4 Hz, 1H), 2.30 (s, 3H), 2.50–2.82 (m, 4H), 2.95 (m, 1H), 7.12–7.40 (m, 10H) ppm; 13C NMR (75 MHz, CDCl3): δ 31.4, 32.4, 33.3, 35.7, 36.0, 37.7, 38.9, 42.8, 57.7, 63.5, 125.77, 125.83, 128.38, 128.43, 128.5, 142.8 ppm; MS (EI) m/z 307 (M+). Anal. Calcd for C22H29N·HCl·0.2H2O: C, 76.03; H, 8.82; N, 4.03. Found: C, 76.38; H, 8.73; N, 4.19.
4.2.3. 1,4-Dimethyl-3,5-cis-diphenethylpiperizine (15c)
Mp 179–180 °C (HCl salt); 1H NMR (300 MHz, CDCl3): δ 1.70 (m, 2H), 1.93 (m, 2H), 2.01 (t, J = 11.4 Hz, 2H), 2.25 (s, 3H), 2.30 (s, 3H), 2.40 (m, 2H), 2.55–2.83 (m, 6H), 7.13–7.39 (m, 10H) ppm; 13C NMR (75 MHz, CDCl3): δ 32.2, 33.8, 35.7, 46.3, 58.9, 61.9, 125.9, 128.3, 128.4, 142.3 ppm. MS (EI) m/z 308 (M+). Anal. Calcd for C22H30N2·2HCl·H2O: C, 63.91; H, 8.29; N, 6.78. Found: C, 64.05; H, 8.43; N, 6.76.
4.2.4. 1,2-Diphenethylpiperidine (18a)
Mp 157–158 °C (HCl salt); 1H NMR (300 MHz, CDCl3): δ 1.27–1.98 (m, 8H), 2.35–3.00 (m, 9H), 7.10–7.39 (m, 10H) ppm; 13C NMR (75 MHz, CDCl3): δ 23.8, 25.7, 30.3, 32.1, 32.4, 33.3, 52.1, 55.7, 59.4, 125.9, 126.0, 128.4, 128.5, 128.8, 140.8, 142.8 ppm; MS (EI) m/z 292 (M−1)+. Anal. Calcd for C21H27N·HCl: C, 76.45; H, 8.55; N, 4.25. Found: C, 76.34; H, 8.82; N, 4.21.
4.2.5. 1,3-Diphenethylpiperidine (18b)
Mp 187–188 °C (HCl salt); 1H NMR (300 MHz, CDCl3): δ 0.91 (m, 1H), 1.42-1.76 (m, 6H), 1.84 (dd, J = 12.9, 1.5 Hz, 1H), 1.94 (dt, J = 11.4, 2.4 Hz, 1H), 2.52–2.68 (m, 4H), 2.80 (m, 2H), 2.97 (m, 2H), 7.10–7.35 (m, 10H) ppm; 13C NMR (75 MHz, CDCl3): δ 25.6, 31.2, 33.5, 33.8, 36.1, 36.8, 54.5, 60.5, 61.3, 125.8, 126.1, 128.4, 128.5, 128.8, 140.6, 142.7 ppm; MS (EI) m/z 292 (M−1)+. Anal. Calcd for C21H27N·HCl: C, 76.45; H, 8.55; N, 4.25. Found: C, 76.21; H, 8.63; N, 4.07.
4.2.6. 1,4-Diphenethylpiperidine (18c)
Mp 178–179 °C (HCl salt); 1H NMR (300 MHz, CDCl3): δ 1.48 (m, 1H), 1.69 (dd, J = 15.3, 6.9 Hz, 2H), 1.84-2.18 (m, 4H), 2.54–2.72 (m, 4H), 3.06–3.34 (m, 4H), 3.62 (br d, J = 11.4 Hz, 2H), 7.08–7.40 (m, 10H) ppm; 13C NMR (75 MHz, CDCl3): δ 29.2, 30.5, 32.8, 33.8, 37.1, 53.4, 59.0, 126.1, 127.4, 128.3, 128.6, 128.8, 129.1, 136.3, 141.5 ppm; MS (EI) m/z 292 (M−1)+. Anal. Calcd for C21H27N·HCl: C, 76.45; H, 8.55; N, 4.25. Found: C, 76.68; H, 8.74; N, 4.21.
4.2.7. N-Methyl-2,6-cis-di-(o-fluorophenethyl)piperidine (24b)
Mp 181–182 °C (HCl salt); 1H NMR (300 MHz, CDCl3): δ 1.32–1.52 (m, 5H), 1.68 (m, 2H), 1.74–1.87 (m, 3H), 2.18 (s, 3H), 2.37 (m, 2H), 2.70 (m, 4H), 6.96–7.06 (m, 4H), 7.12–7.24 (m, 4H) ppm; 13C NMR (75 MHz, CDCl3): δ 25.2, 25.7, 27.0, 30.9, 35.0, 62.7, d (115.214, 115.435), d (124.035, 124.073), d (127.443, 127.527), d (129.849, 130.009), d (130.889, 130.943), d (160.167, 162.604) ppm; MS (EI) m/z 343 (M+). Anal. Calcd for C22H27F2N·HCl: C, 69.55; H, 7.43; N, 3.69. Found: C, 69.18; H, 7.21; N, 3.89.
4.2.8. N-Methyl-2,6-cis-di-(m-fluorophenethyl)piperidine (25b)
Mp 140–141 °C (HCl salt); 1H NMR (300 MHz, CDCl3) δ 1.33–1.50 (m, 5H), 1.65 (m, 2H), 1.78 (m, 1H), 1.84 (m, 2H), 2.15 (s, 3H), 2.37 (m, 2H), 2.67 (m, 4H), 6.84–6.94 (m, 4H), 6.97 (d, J = 5.7 Hz, 2H), 7.22 (dt, J = 5.7, 4.5 Hz, 2H) ppm; 13C NMR (75 MHz, CDCl3): δ 25.2, 26.7, 30.3, 32.1, 36.1, 62.3, d (112.558, 112.770), d (115.351, 115.556), d (124.270, 124.293), d (129.781, 129.865), d (145.707, 145.775), d (161.883, 164.319) ppm; MS (EI) m/z 343 (M+). Anal. Calcd for C22H27F2N·HCl·H2O: C, 66.40; H, 7.60; N, 3.52. Found: C, 66.50; H, 7.68; N, 3.70.
4.2.9. N-Methyl-2,6-cis-di-(p-fluorophenethyl)piperidine (26b)
Mp 185–186 °C (HCl salt); 1H NMR (300 MHz, CDCl3): δ 1.30–1.51 (m, 5H), 1.63 (m, 2H), 1.72–1.91 (m, 3H), 2.15 (s, 3H), 2.37 (m, 2H), 2.64 (m, 4H), 6.95 (tt, J = 9.0, 2.4 Hz, 4H), 7.15 (dt, J = 9.0, 3.0 Hz, 4H); 13C NMR (75 MHz, CDCl3): δ 25.3, 26.8, 30.5, 31.7, 36.7, 62.4, d (114.9, 115.2), d (129.8, 129.9), 138.5, d (159.6, 162.8); MS (EI) m/z 343 (M+). Anal. Calcd for C22H27F2N·HCl·2/3H2O: C, 67.42; H, 7.59; N, 3.57. Found: C, 67.50; H, 7.44; N, 3.94.
4.2.10. N-Methyl-2,6-cis-di-(o-methoxyphenethyl)piperidine (27b)
Mp 92–93 °C (HCl salt); 1H NMR (300 MHz, CDCl3): δ 1.36–1.56 (m, 5H), 1.64 (m, 2H), 1.74–1.90 (m, 3H), 2.21 (s, 3H), 2.35 (br s, 2H), 2.66 (t, J = 8.4 Hz, 4H), 3.81 (s, 6H), 6.80-6.92 (m, 4H), 7.10–7.20 (m, 4H) ppm; 13C NMR (75 MHz, CDCl3): δ 25.4, 27.2, 27.7, 31.8 34.9, 55.5, 63.4, 110.3, 120.5, 126.9, 129.9, 131.5, 157.5 ppm; MS (EI) m/z 367 (M+). Anal. Calcd for C24H33NO2·HCl·1.25H2O: C, 67.59; H, 8.63; N, 3.28. Found: C, 67.67; H, 8.77; N, 3.73.
4.2.11. N-Methyl-2,6-cis-di-(m-methoxyphenethyl)piperidine (28b)
Mp 157–158 °C (HCl salt); 1H NMR (300 MHz, CDCl3): δ 1.40–1.62 (m, 3H), 1.73 (m, 2H), 1.82–1.95 (m, 3H), 2.17 (m, 2H), 2.50 (s, 3H), 2.67 (m, 4H), 3.81 (s, 6H), 6.73–6.82 (m, 6H), 7.21 (dd, J = 9.0, 7.5 Hz, 2H) ppm; 13C NMR (75 MHz, CDCl3): δ 22.8, 24.1, 32.4, 33.6, 55.6, 64.7, 112.4, 113.8, 120.7, 129.8, 141.2, 159.9 ppm; MS (EI) m/z 367 (M+). Anal. Calcd for C24H33NO2·HCl·H2O: C, 68.31; H, 8.60; N, 3.32. Found: C, 68.25; H, 9.03; N, 3.43.
4.2.12. N-Methyl-2,6-cis-di-(p-methoxyphenethyl)piperidine (29b)
Mp 175–176 °C (lit.28 mp 176-178 °C) (HCl salt); 1H NMR (300 MHz, CDCl3): δ 1.40 (m, 4H), 1.55–1.70 (m, 3H), 1.70–1.90 (m, 3H), 2.16 (s, 3H), 2.37 (m, 2H), 2.60 (m, 4H), 3.78 (s, 6H), 6.82 (d, J = 8.4 Hz, 4H), 7.12 (d, J = 8.4 Hz, 4H) ppm; 13C NMR (75 MHz, CDCl3): δ 25.4, 27.0, 30.9, 31.6, 36.9, 55.5, 62.6, 113.8, 129.4, 135.1, 157.6 ppm; MS (EI) m/z 367 (M+). Anal. Calcd for C24H33NO2·HCl·0.5H2O: C, 69.80; H, 8.54; N, 3.39. Found: C, 69.60; H, 8.66; N, 3.53.
4.2.13. N-Methyl-2,6-cis-di-(3,4-methylenedioxyphenethyl)piperidine (30b)
Mp 195–196 °C (HCl salt); 1H NMR (300 MHz, CDCl3): δ 1.34–1.48 (m, 6H), 1.44–1.68 (m, 2H), 1.74–1.91 (m, 2H), 2.18 (s, 3H), 2.37–2.49 (m, 2H), 2.54–2.64 (m, 4H), 5.91 (s, 4H), 6.64 (dd, J = 7.8, 1.8 Hz, 2H), 6.70 (d, J = 1.8 Hz, 2H), 6.72 (d, J = 7.8 Hz, 2H) ppm; 13C NMR (75 MHz, CDCl3): δ 25.1, 26.7, 30.4, 32.3, 36.6, 62.6, 100.9, 108.2, 109.1, 121.2, 136.6, 145.6, 147.6 ppm; MS (MALDI) m/z 396 (M+1)+. Anal. Calcd for C24H29NO4·HCl·0.5H2O: C, 65.37; H, 7.09; N, 3.18. Found: C, 65.13; H, 7.38; N, 3.57.
4.2.14. N-Methyl-2,6-cis-di-(p-methylphenethyl)piperidine (31b)
Mp 203–204 °C (HCl salt); 1H NMR (300 MHz, CDCl3): δ 1.26–1.48 (m, 5H), 1.63 (m, 2H), 1.75–1.92 (m, 3H), 2.16 (s, 3H), 2.31 (s, 6H), 2.36 (br t, J = 6.0 Hz, 2H), 2.50–2.71 (m, 4H), 7.09 (s, 8H) ppm; 13C NMR (75 MHz, CDCl3): δ 21.3, 25.3, 27.1, 31.0, 32.1, 36.8, 62.7, 128.4, 129.1, 135.1, 139.9 ppm; MS (EI) m/z 335 (M+). Anal. Calcd for C24H33N·HCl·2/3H2O: C, 75.07; H, 9.27; N, 3.65. Found: C, 74.73; H, 9.27; N, 4.05.
4.2.15. N-Methyl-2,6-cis-di-(m-trifluoromethylphenethyl)piperidine (32b)
Mp 152–153 °C (HCl salt); 1H NMR (300 MHz, CDCl3): δ 1.39–1.57 (m, 5H), 1.67 (m, 2H), 1.74–1.96 (m, 3H), 2.17 (s, 3H), 2.42 (m, 2H), 2.75 (t, J = 8.4 Hz, 4H), 7.36–7.50 (m, 8H) ppm; 13C NMR (75 MHz, CDCl3): δ 25.4, 26.4, 29.7, 32.2, 36.2, 62.1, q (122.575, 122.621, 122.666, 122.727), q (125.233, 125.293, 125.339, 125.384), 126.2, 128.8, q (130.001, 130.411, 130.836, 131.261), 132.0, 143.8 ppm; MS (EI) m/z 444 (M−1)+. Anal. Calcd for C24H27F6N·HCl·1/3H2O: C, 59.32; H, 5.95; N, 2.88. Found: C, 59.24; H, 6.32; N, 3.14.
4.2.16. N-Methyl-2,6-cis-di-(2,4-dichlorophenethyl)piperidine (33b)
Mp 181–182 °C (HCl salt); 1H NMR (300 MHz, CDCl3): δ 1.30–1.56 (m, 5H), 1.65 (m, 6H), 1.71–1.86 (m, 3H), 2.18 (s, 3H), 2.38 (m, 2H), 2.66–2.84 (m, 4H), 7.14–7.22 (m, 4H), 7.35 (dd, J = 1.8, 0.6 Hz, 2H) ppm; 13C NMR (75 MHz, CDCl3): δ 25.3, 26.9, 29.8, 30.6, 34.4, 62.5, 127.1, 129.3, 131.4, 132.1, 134.6, 139.1 ppm; MS (EI) m/z 442/444/446/448/450 (M−1)+. Anal. Calcd for C22H25Cl4N·HCl: C, 54.85; H, 5.44; N, 2.91. Found: C, 55.04; H, 5.61; N, 3.11.
4.2.17. N-Methyl-2,6-cis-di-(p-phenylphenethyl)piperidine (34b)
Mp 215–216 °C (HCl salt); 1H NMR (300 MHz, CDCl3): δ 1.32–1.47 (m, 4H), 1.64–1.95 (m, 6H), 2.21 (s, 3H), 2.42 (m, 2H), 2.62–2.80 (m, 4H), 7.26–7.60 (m, 18H) ppm; 13C NMR (75 MHz, CDCl3): δ 25.3, 26.9, 30.7, 32.0, 36.4, 62.6, 126.9, 127.2, 127.3, 128.9, 129.0, 138.8, 141.3, 142.3 ppm; MS (MALDI) m/z 460 (M+1)+. Anal. Calcd for C34H37N·HCl·1.5H2O: C, 78.06; H, 7.90; N, 2.68. Found: C, 78.20; H, 8.42; N, 3.05.
4.3. General procedure for synthesis of compounds 19a–c, and 20a–c
trans-Cinnamyl bromide (394 mg, 2.00 mmol) was added dropwise to a mixture of the appropriate nor-compound (17a,b, or 17c) (378 mg, 2.00 mmol), K2CO3 (552 mg, 4.00 mmol) and acetone (15 mL). The mixture was stirred at room temperature for 4 h. The solvent was then evaporated under reduced pressure. The residue was dissolved in CHCl3 (40 mL) and washed with water (20 mL) and saturated aqueous NaCl (20 mL). The organic phase was dried (Na2SO4), filtered, and concentrated. The crude product was purified by column chromatography to give the title compounds 19a–c. Compounds 19a,b, or 19c (305 mg, 1 mmol) in ethanol (20 mL) with 10% Pd/C (20 mg) was hydrogenated on a Parr hydrogenation apparatus (15 psi) for 4 h. The catalyst was removed by filtration through a Celite pad. The filter cake was rinsed with ethanol, and the combined organic liquors were concentrated under reduced pressure. The resulting residue was purified by column chromatography to give the title compounds 20a–c.
4.3.1. 1-trans-Cinnamyl-2-phenethylpiperidine (19a)
1H NMR (300 MHz, CDCl3): δ 1.35 (m, 1H), 1.52–2.10 (m, 7H), 2.40 (m, 1H), 2.48–2.63 (m, 2H), 2.76 (m, 1H), 2.99 (dt, J = 11.4, 4.5 Hz, 1H), 3.31 (ddd, J = 13.8, 7.8, 1.2 Hz, 1H), 3.57 (ddd, J = 13.8, 6.9, 2.1 Hz, 1H), 6.27 (ddd, J = 15.9, 7.5, 6.3 Hz, 1H), 6.54 (d, J = 15.9 Hz, 1H), 7.14–7.39 (m, 10H) ppm; 13C NMR (75 MHz, CDCl3): δ 23.2, 24.9, 29.6, 31.9, 32.6, 52.0, 55.8, 60.0, 124.4, 126.0, 126.5, 127.8, 128.3, 128.5, 128.6, 134.3, 136.6, 142.0 ppm; MS (EI) m/z 305 (M+). Anal. Calcd for C22H27N·HCl: C, 73.41; H, 8.40; N, 3.89. Found: C, 73.08; H, 8.54; N, 3.87.
4.3.2. 1-(3-Phenylpropyl)-2-phenethylpiperidine (20a)
1H NMR (300 MHz, CDCl3): δ 1.47 (m, 1H), 1.74–2.18 (m, 9H), 2.47–3.03 (m, 8H), 3.15 (m, 1H), 7.08–7.32 (m, 10H) ppm; 13C NMR (75 MHz, CDCl3): δ 21.3, 22.6, 24.8, 26.9, 30.4, 31.8, 33.1, 51.6, 60.6, 126.5, 128.3, 128.71, 128.74, 139.8, 140.0 ppm; MS (EI) m/z 307 (M+). Anal. Calcd for C22H29N·HCl·0.5H2O: C, 74.87; H, 8.85; N, 3.97. Found: C, 75.02; H, 8.96; N, 3.77.
4.3.3. 1-trans-Cinnamyl-3-phenethylpiperidine (19b)
Mp 170–171 °C (HCl salt); 1H NMR (300 MHz, CDCl3): δ 0.91 (ddd, J = 22.8, 12.3, 4.5 Hz, 1H), 1.42-1.74 (m, 6H), 1.78–1.96 (m, 2H), 2.61 (m, 2H), 2.96 (m, 2H), 3.14 (d, J = 6.6 Hz, 2H), 6.30 (dt, J = 15.9, 6.6 Hz, 1H), 6.50 (d, J = 15.9 Hz, 1H), 7.10–7.40 (m, 10H) ppm; 13C NMR (75 MHz, CDCl3): δ 25.7, 31.2, 33.4, 36.1, 36.8, 54.5, 60.6, 61.8, 125.7, 126.4, 127.0, 127.5, 128.4, 128.6, 132.9, 137.1, 142.7 ppm; MS (EI) m/z 305 (M+). Anal. Calcd for C22H27N·HCl·1/3H2O: C, 75.95; H, 8.30; N, 4.03. Found: C, 75.58; H, 8.62; N, 4.11.
4.3.4. 1-(3-Phenylpropyl)-3-phenethylpiperidine (20b)
Mp 158–159 °C (HCl salt); 1H NMR (300 MHz, CDCl3): δ 0.89 (ddd, J = 22.8, 12.3, 4.8 Hz, 1H), 1.40–1.72 (m, 6H), 1.74–1.92 (m, 4H), 2.36 (m, 2H), 2.52–2.66 (m, 4H), 2.90 (m, 2H), 7.10–7.34 (m, 10H) ppm; 13C NMR (75 MHz, CDCl3): δ 25.5, 28.7, 31.1, 33.4, 34.0, 35.9, 36.7, 54.4, 58.7, 60.4, 125.7, 125.8, 128.3, 128.4, 142.1, 142.6 ppm; MS (EI) m/z 307 (M+). Anal. Calcd for C22H29N·HCl·0.5H2O: C, 74.87; H, 8.85; N, 3.97. Found: C, 74.68; H, 8.75; N, 3.74.
4.3.5. 1-trans-Cinnamyl-4-phenethylpiperidine (19c)
Mp 160–161 °C (HCl salt); 1H NMR (300 MHz, CDCl3): δ 1.26–1.65 (m, 5H), 1.79 (br d, J = 13.2 Hz, 2H), 2.10 (m, 2H), 2.63 (t, J = 7.8 Hz, 2H), 3.10 (br d, J = 12.0 Hz, 2H), 3.26 (dd, J = 6.9, 0.9 Hz, 2H), 6.35 (dt, J = 15.9, 6.9 Hz, 1H), 6.54 (d, J = 15.9 Hz, 1H), 7.10–7.43 (m, 10H) ppm; 13CNMR (75 MHz, CDCl3): δ 31.8, 33.3, 35.0, 38.3, 53.8, 61.3, 124.9, 125.8, 126.6, 127.9, 128.40, 128.43, 128.7, 134.4, 136.6, 142.5 ppm; MS (EI) m/z 305 (M+). Anal. Calcd for C22H27N·HCl: C, 73.41; H, 8.40; N, 3.89. Found: C, 73.46; H, 8.73; N, 3.68.
4.3.6. 1-(3-Phenylpropyl)-4-phenethylpiperidine (20c)
Mp 152–153 °C (HCl salt); 1H NMR (300 MHz, CDCl3): δ 1.24–1.43 (m, 3H), 1.56 (m, 2H), 2.38 (m, 2H), 1.73 (br d, J = 10.8 Hz, 2H), 1.80–1.98 (m, 4H), 2.56-2.66 (m, 4H), 2.95 (br d, J = 12.0 Hz, 2H), 7.08–7.32 (m, 10H) ppm; 13C NMR (75 MHz, CDCl3): δ 28.7, 32.3, 33.3, 34.1, 35.5, 38.5, 54.1, 58.6, 125.7, 125.8, 128.38, 128.41, 142.1, 142.7 ppm; MS (EI) m/z 307 (M+). Anal. Calcd for C22H29N·HCl: C, 76.83; H, 8.79; N, 4.07. Found: C, 76.79; H, 8.76; N, 3.98.
4.4. N-Ethyl-2,6-cis-diphenethylpiperidine (22)
A mixture of nor-lobelane (440 mg, 1.50 mmol), iodoethane (2.34 g, 15.0 mmol), and K2CO3 (414 mg, 3.0 mmol) in THF (10 mL) was stirred under reflux for 3 days. The reaction mixture was allowed to cool and the solvent was removed under reduced pressure. The residue was dissolved in water (40 mL) and the aqueous solution was extracted with CHCl3 (3 × 20 mL). The combined organic extracts were dried (Na2SO4), filtered, and concentrated. The crude product was purified by column chromatography (40:1 CHCl3–CH3OH) to give 22 (356 mg, 74%) as a white solid. Mp 186–187 °C (lit.28 187–188 °C) (HCl salt); 1H NMR (300 MHz, CDCl3): δ 0.94 (t, J = 7.2 Hz, 3H), 1.28–1.48 (m, 3H), 1.56–1.82 (m, 5H), 1.86–2.02 (m, 2H), 2.46–2.80 (m, 8H), 7.13–7.31 (m, 10H) ppm; 13C NMR (75 MHz, CDCl3): δ 12.6, 24.5, 28.8, 32.7, 36.0, 39.6, 60.7, 125.8, 128.4, 142.7 ppm; MS (EI) m/z 321 (M+). Anal. Calcd for C23H31N·HCl·0.5H2O: C, 75.28; H, 9.06; N, 3.82. Found: C, 75.26; H, 9.16; N, 4.08.
4.5. N-(n-Propyl)-2,6-cis-diphenethylpiperidine (23)
Compound 23 was prepared as described for compound 22 from nor-lobelane and 1-iodopropane. Mp 176–177 °C (lit.28 174–176 °C) (HCl salt); 1H NMR (300 MHz, CDCl3): δ 0.78 (t, J = 7.5 Hz, 3H), 1.28–1.47 (m, 5H), 1.52–1.89 (m, 7H), 2.41–2.78 (m, 8H), 7.14–7.33 (m, 10H) ppm; 13C NMR (75 MHz, CDCl3): δ 12.1, 22.3, 24.6, 28.1, 33.1, 36.5, 48.3, 61.6, 125.8, 128.46, 128.51, 142.8 ppm; MS (EI) m/z 335 (M+). Anal. Calcd for C24H33N·HCl·2/3H2O: C, 75.07; H, 9.27; N, 3.65. Found: C, 74.83; H, 9.65; N, 3.87.
4.6. 2,6-cis-Di-(p-hydroxyphenethyl)piperidine (36)
A solution of compound 35 (710 mg, 1.73 mmol) in methanol (30 mL) was treated with NaHCO3 (1.45 g, 17.30 mmol) for 4 h at ambient temperature. The solvent was then evaporated under reduced pressure. The residue was dissolved in water (30 mL) and the resulting solution was acidified with 2 N HCl to pH 2.0. The aqueous solution was extracted with CHCl3 (3 × 20 mL). The combined organic extracts were dried (Na2SO4), filtered, and concentrated. The crude product was purified by recrystallization from methanol/diethyl ether to give 36 (480 mg, 77 %) as a white solid. Mp 263–264 °C (HCl salt); 1H NMR (300 MHz, DMSO-d6) (HCl salt): δ 1.26–1.46 (m, 3H), 1.64–1.85 (m, 3H), 1.85–2.06 (m, 4H), 2.41–2.66 (m, 4H), 2.92 (br s, 2H), 6.69 (dt, J = 8.7, 2.5 Hz, 4H), 6.99 (dt, J = 8.7, 2.4 Hz, 4H), 8.64 (m, 2H), 9.23 (s, 2H) ppm; 13C NMR (75 MHz, DMSO-d6): δ 21.9, 27.4, 29.7, 34.8, 56.3, 115.0, 128.8, 130.6, 155.3 ppm; MS (MALDI) m/z 326 (M+1)+. Anal. Calcd for C21H27NO2·HCl·1.2H2O: C, 65.77; H, 7.99; N, 3.65. Found: C, 65.91; H, 8.12; N, 4.03.
4.7. [3H]NIC and [3H]MLA binding assay
For the [3H]NIC and [3H]MLA binding assays, rat whole brain (excluding cerebellum) from two to four rats was homogenized using a Tekmar polytron (Tekmar-Dohrmann, Mason, OH) in 10 vol of ice-cold modified Krebs-HEPES buffer (20 mM HEPES, 118 mM NaCl, 4.8 mM KCl, 2.5 mM CaCl2, and 1.2 mM MgSO4, pH 7.5). Homogenates were incubated in a water bath for 5 min at 37 °C and centrifuged (16,000g for 17 min at 4 °C). Resulting pellets were resuspended in 20 vol of ice-cold MilliQ water (Millipore Corporation, Molsheim, France), incubated for 10 min at 37 °C and centrifuged (16,000g for 17 min at 4 °C). Final pellets were stored at −20 °C in Krebs-HEPES buffer until use. Prior to assay, pellets were resuspended in 20 vol of Krebs-HEPES buffer. Samples (100 μL containing 100–140 μg of membrane protein) were added to assay tubes containing analogue (7–9 concentrations, 1 nM–1 mM) and 3 nM [3H]NIC or [3H]MLA for a final assay vol of 200–250 μL, and incubated for 90 and 120 min, respectively. Nonspecific binding was determined in the presence of 10 μM NIC for the [3H]NIC binding assay and in the presence of 10 μM MLA or 1 mM NIC for the [3H]MLA binding assays. Reactions were terminated by addition of ice-cold buffer and rapid filtration onto either Whatman GF/B glass fiber filters presoaked in 0.5% polyethylenimine using a Brandel Harvester (Biomedical Research and Development Laboratory, Inc., Gaithersburg, MD or onto Unifilter-96 GF/B 96-well filter plates also presoaked in 0.5% polyethylenimine using a Packard Filter Mate Harvester (Packard BioScience Co., Meriden, CT). Bound radioactivity was determined via liquid scintillation spectrometry. Specific [3H]NIC and [3H]MLA binding were determined by subtracting nonspecific binding from total binding. Concentrations of inhibitor that produced 50% inhibition (IC50 values) were determined from the concentration effect curves via an iterative curve-fitting program (Prism 3.0; GraphPad Software Inc., San Diego, CA). Inhibition constants (Ki values) were determined using the Cheng-Prusoff equation.30
4.8. [3H]DTBZ binding assay
Synaptic vesicles were prepared as previously described.12 Briefly, fresh whole rat brain (excluding cerebellum and brain stem) was homogenized in 20 vol of ice-cold 0.32 M sucrose using a glass homogenizer (seven strokes of a Teflon pestle, clearance = 0.003 in.). Homogenates were centrifuged at 1000g for 12 min at 4 °C. Resulting supernatants were centrifuged at 22,000g for 10 min. Resulting pellets, containing the synaptosomes, were resuspended in 18 mL ice-cold Milli-Q water for 5 min with seven strokes of the Teflon pestle homogenizer. Osmolarity was restored by immediate addition of 2 mL of 25 mM HEPES and 100 mM K2-tartrate buffer (pH 7.5). Samples were centrifuged at 20,000g for 20 min. MgSO4 (final concentration, 1 mM) was added to the resulting supernatants. Final centrifugations were performed at 100,000g for 45 min. Pellets were resuspended immediately in ice-cold buffer (25 mM HEPES, 100 mM K2-tartrate, 5 mM MgSO4, 0.1 mM EDTA, and 0.05 mM EGTA, pH 7.5, 25 °C) providing ~15 μg protein/100 μL. [3H]DTBZ binding to synaptic vesicle membranes was performed according to previously described procedures.31 Briefly, 100 μL of vesicles suspension was incubated in assay buffer (see above) in the presence of 5 nM [3H]DTBZ and 1 nM–1 mM lobeline analogues (final concentrations) for 30 min at room temperature. Nonspecific binding was determined in the presence of 20 μM tetrabenazine (TBZ). Assays were performed in duplicate using Unifilter 96-well GF/B filter plates (presoaked in 0.5% polyethylenimine) and terminated by harvesting using the FilterMate harvester. After washing 5 times with 350 μL of the ice-cold wash buffer (25 mM HEPES, 100 mM K2-tartrate, 5 mM MgSO4, and 10 mM NaCl, pH 7.5), filter plates were dried, bottoms-sealed and each well filled with 40 μL Micro-Scint 20 cocktail (Packard). Bound [3H]DTBZ was measured using a Packard TopCount NXT scintillation counter and a Packard Windows NT-based operating system. Data were analyzed as described above.
Acknowledgements
This research was supported by NIH grants DA 00399 and DA 13519. The authors also gratefully acknowledge the generous gift of [3H]DTBZ from Dr. Michael R. Kilbourn (supported by NIH Grant MH 47611). The authors also thank Mr. Robert King of the Environmental Research and Training Laboratory, University of Kentucky for performing the elemental analyses. For purposes of full disclosure, the University of Kentucky holds patents on lobeline and lobeline analogues, which have been licensed by Yaupon Therapeutics Inc. (Lexington, KY). A potential royalty stream to G.Z., L.P.D., M.D.J., and P.A.C. may occur consistent with University of Kentucky policy. Both L.P.D. and P.A.C. are founders of, and have financial interest in Yaupon Therapeutics Inc.
References and notes
- 1.United Nations Office on Drugs and Crimes (UNODC) World Drug Report. 2004. http://www.unodc.org. [Google Scholar]
- 2.Nordahl TE, Salo R, Leamon M. J. Neuropsych. Clin. Neurosci. 2003;15:317. doi: 10.1176/jnp.15.3.317. [DOI] [PubMed] [Google Scholar]
- 3.Wise RA. Neuron. 2002;36:229. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- 4.Pifl C, Drobny H, Hornykiewicz O, Singer EA. Mol. Pharmacol. 1995;47:368. [PubMed] [Google Scholar]
- 5.Sulzer D, Chen TK, Lau Y, Kristensen H, Rayport S, Ewing A. J. Neurosci. 1995;15:4102. doi: 10.1523/JNEUROSCI.15-05-04102.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mantle TH, Tipton KF, Garrett N. J. Biochem. Pharmacol. 1976;25:2073. doi: 10.1016/0006-2952(76)90432-9. [DOI] [PubMed] [Google Scholar]
- 7.Miller DK, Crooks PA, Teng L, Witkin JM, Munzar P, Goldberg SR, Acri JB, Dwoskin LP. J. Pharmacol. Exp. Ther. 2001;296:1023. [PubMed] [Google Scholar]
- 8.Miller DK, Harrod SB, Green TA, Wong MY, Bardo MT, Dwoskin LP. Pharmacol. Biochem. Behav. 2002;74:279. doi: 10.1016/s0091-3057(02)00996-6. [DOI] [PubMed] [Google Scholar]
- 9.Harrod SB, Dwoskin LP, Crooks PA, Klebaur JE, Bardo MT. J. Pharmacol. Exp. Ther. 2001;298:172. [PubMed] [Google Scholar]
- 10.Harrod SB, Dwoskin LP, Green TA, Gehrke BJ, Bardo MT. Psychopharmacology. 2003;165:397. doi: 10.1007/s00213-002-1289-6. [DOI] [PubMed] [Google Scholar]
- 11.Dwoskin LP, Crooks PA. Biochem. Pharmacol. 2002;63:89. doi: 10.1016/s0006-2952(01)00899-1. [DOI] [PubMed] [Google Scholar]
- 12.Teng L, Crooks PA, Sonsalla PK, Dwoskin LP. J. Pharmacol. Exp. Ther. 1997;280:1432. [PubMed] [Google Scholar]
- 13.Perera RP, Wimalasena DS, Wimalasena K. J. Med. Chem. 2003;46:2599. doi: 10.1021/jm030004p. [DOI] [PubMed] [Google Scholar]
- 14.Canney DJ, Kung M, Kung HF. Nucl. Med. Biol. 1995;22:527. doi: 10.1016/0969-8051(94)00118-4. [DOI] [PubMed] [Google Scholar]
- 15.Lee LC, Vander BT, Sherman PS, Frey KA, Kilbourn MR. J. Med. Chem. 1996;39:191. doi: 10.1021/jm950117b. [DOI] [PubMed] [Google Scholar]
- 16.Miller DK, Crooks PA, Dwoskin LP. Neuropharmacology. 2000;39:2654. doi: 10.1016/s0028-3908(00)00140-4. [DOI] [PubMed] [Google Scholar]
- 17.Miller DK, Crooks PA, Zheng G, Grinevich VP, Norrholm S, Dwoskin LP. J. Pharmacol. Exp. Ther. 2004;310:1035. doi: 10.1124/jpet.104.068098. [DOI] [PubMed] [Google Scholar]
- 18.Briggs CA, McKenna DG. Mol. Pharmacol. 1998;54:1095. [Google Scholar]
- 19.Zheng G, Dwoskin LP, Deaciuc AG, Crooks PA. J. Med. Chem. under review. [Google Scholar]
- 20.Crooks PA, Jones MD, Chesnut MD, Jaromczyk AM, Dwoskin LP. NIDA Res. Monograph. 1999;180:234. [Google Scholar]
- 21.Terry AV, Jr., Williamson R, Gattu M, Beach JW, McCurdy CR, Sparks JR. Neuropharmacology. 1998;37:93. doi: 10.1016/s0028-3908(97)00142-1. [DOI] [PubMed] [Google Scholar]
- 22.Flammia D, Malgorzata D, Damaj MI, Martin B, Glennon RA. J. Med. Chem. 1999;42:3726. doi: 10.1021/jm990286m. [DOI] [PubMed] [Google Scholar]
- 23.Zheng G, Dwoskin LP, Crooks PA. J. Org. Chem. 2004;69:8514. doi: 10.1021/jo048848j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phillips AP. J. Am. Chem. Soc. 1950;72:1850. [Google Scholar]
- 25.Howton DR, Golding DRV. J. Org. Chem. 1950;15:1. [Google Scholar]
- 26.Thompson CM, Green DLC, Kubas R. J. Org. Chem. 1988;53:5389. [Google Scholar]
- 27.Lautens M, Yoshida M. J. Org. Chem. 2003;68:762. doi: 10.1021/jo0205255. [DOI] [PubMed] [Google Scholar]
- 28.Lee J, Freudenberg W. J. Org. Chem. 1944;9:537. [Google Scholar]
- 29.Newkome GR, Robinson JM. Tetrahedron Lett. 1974;9:691. [Google Scholar]
- 30.Cheng YC, Prusoff WH. Biochem. Pharmacol. 1973;22:3099. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 31.Teng L, Crooks PA, Dwoskin LP. J. Neurochem. 1998;71:258–265. doi: 10.1046/j.1471-4159.1998.71010258.x. [DOI] [PubMed] [Google Scholar]