Abstract
Background
African Americans are at greater risk to reach end stage renal disease and this risk may carry over in a kidney transplant recipient after kidney transplantation.
Methods
Linking the 5-year patient data of a large dialysis organization to the Scientific Registry of Transplant Recipients, we identified 13,692 hemodialysis patients who underwent first kidney transplantation. Mortality or graft failure and delayed graft function (DGF) risks were estimated by Cox regression (hazard ratio [HR] and 95% CI) and logistic regression, respectively.
Results
Patients were 48±14 years old and included 39% women and 26% diabetics. After adjusting for several relevant clinical and transplant-related variables, African American donor race was associated with higher all-cause mortality, with hazard ratios of 1.39 (1.09–1.78) for all-cause mortality, 1.80 (1.17–2.76) for cardiovascular mortality, 1.30 (1.03–1.64) for death-censored graft loss and 1.31 (1.10–1.57) for combined outcome over the 6-year observation period. In the non-African American recipient sub-cohort, but not in the African American recipient sub-cohort, African American donor race was associated with higher risk of death-censored graft loss (2.24(1.44–3.49)) in our fully adjusted model.
Conclusions
African American donor race was associated with increased all-cause and cardiovascular mortality and graft loss.
Keywords: Donor race/ethnicity, kidney transplantation, malnutrition-inflammation complex, mortality, cardiovascular death, graft failure, delayed graft function
Introduction
The United States Renal Data System data indicates that 32% of Americans with end stage renal disease (ESRD) are African American, even though black individuals represent only 13% of the US population.1,2 In addition to many sociocultural and environmental influences, over the last few years genetic factors were identified that could explain this disproportionality. Polymorphisms in the nonmuscle myosin heavy chain 9 (MYH9) gene exhibit strong association with non-diabetic chronic kidney disease (CKD) in African Americans.3 Recently Genovese et al. observed that genetic variation in the APOL1 gene, located immediately centromeric of MYH9, is also strongly associated with non-diabetic CKD, and they presented evidence that the statistical association is stronger than that of MYH9. They suggested that these coding variants are in fact causally related to kidney disease and provided an explanation for selection of APOL1 kidney disease risk polymorphisms as protective from an infectious disease common in Africa.4–6 MYH9 and APOL1 have been suggested to account for a large proportion of the excess risk of ESRD observed in African Americans.
These contributions to ESRD risk could be expressed in transplant recipients who originally don’t carry these risks when they undergo kidney transplantation, especially genetic risks. Accordingly, one would expect that a kidney transplanted from an African American donor displayed more graft loss and worse residual renal function, which could also result in higher mortality. However, data examining the association of donor race/ethnicity with outcomes are controversial. A recent study of 72,945 patients from OPTN/UNOS reported that African American donor kidneys had lower graft survival when transplanted into white Americans or African Americans and were also associated with lower patient survival when transplanted into white American recipients.7 Increased risk of hypertension and diabetes was reported in African American donors.8 Along the same lines, Swanson et al. found that kidneys from African American deceased donors had a 1.64-fold higher risk of graft loss compared to those from white donors.9 Contradicting these studies, Locke et al. reported that among African American recipients of kidneys obtained after cardiac death those who received kidneys from African American donors had better long-term graft and patient survival than those who received kidneys from white American donors. In addition, compared with standard-criteria kidneys from white American donors after brain death, kidneys from African American donors after cardiac death conferred a 70% reduction in the risk of graft loss and a 59% reduction in risk of death among African American recipients.10 These findings suggest that kidneys obtained from African American donors after cardiac death may afford the best long-term survival for African American recipients.10
As an initial step toward exploring this issue, we examined associations of donor race/ethnicity with post-transplant short term and long term outcomes in a large national cohort of kidney transplant recipients. We hypothesized that African American donor race is associated with worse post-transplant patient and graft survival in a large prospective cohort of incident kidney transplant recipients from the United States.
Patients and Methods
Patients
We linked data on all kidney transplant recipients listed in the Scientific Registry of Transplant Recipients (SRTR) up to June 2007 to a list of individuals with CKD who underwent maintenance hemodialysis treatment from July 2001 to June 2006 in one of the outpatient dialysis facilities of a US-based large dialysis organization (DaVita Inc, prior to its acquisition of former Gambro dialysis facilities) using patients’ social security numbers. The study was approved by the Institutional Review Boards of both Los Angeles Biomedical Research Institute at Harbor-UCLA and DaVita Clinical Research.
Clinical and Demographic Measures
The creation of the national DaVita hemodialysis patient cohort has been described previously.11–16 Demographic data and details of medical history were collected, with information on age, gender, race/ethnicity, type of insurance, marital status, presence of diabetes, height, post-hemodialysis dry weight (to calculate averaged body mass index [BMI]) and dialysis vintage. Dialysis vintage was defined as the duration of time between the first day of dialysis treatment and the day of kidney transplantation.
Race/ethnicity
The DaVita national database, similar to the USRDS database, includes race/ethnicityfor over 98% of all patients as “self-identified” data. Race/ethnicity determinations were based on “self-identification” data, in that dialysis patients chose the race/ethnicitywith which they most closely identified according to the definitions set forth by the United States Census Bureau and the Federal Office of Management and Budget. In this study mutually exclusive ethnic categories were created according to the donorracedata from the SRTR.
Laboratory Measures
Blood samples were drawn using uniform techniques in all of the DaVita dialysis clinics and were transported to the DaVita Laboratory in Deland, Florida, typically within 24 hours. All laboratory values were measured by automated and standardized methods in the DaVita Laboratory. Most laboratory values were measured monthly, including serum urea, creatinine, albumin, calcium, phosphorus, bicarbonate, and total iron binding capacity (TIBC). Serum ferritin was measured at least quarterly. Hemoglobin was measured at least monthly in essentially all patients and weekly to biweekly in most patients. Most blood samples were collected pre-dialysis with the exception of post-dialysis serum urea nitrogen to calculate urea kinetics. Kt/V (single pool) was calculated using urea kinetic modeling equations as described elsewhere.13
Statistical Methods
Data were summarized using proportions, means (±standard deviation [SD]) or medians (interquartile range [IQR]) as appropriate. Categorical variables were compared using chi-square tests, and continuous variables were compared using t tests or Mann-Whitney U tests, Kruskal- Wallis H tests, or analyses of variance, as appropriate. For all-cause and cardiovascular mortality and graft failure, defined as re-initiation of dialysis treatment or re-transplantation, time to event was used in all survival analyses. For delayed graft function (DGF), defined as the need for any dialysis therapy in the first week after transplantation,17 time to event was not accounted for. The association between donor race and outcomes was assessed using Cox regression analysis and Kaplan-Meier plots with log-rank test. Survival analyses to calculate hazard ratios (HR) and 95% confidence interval (95%CI) of death or graft failure employed Cox proportional hazards regression. In the mortality analyses the patients were followed until event (death) or censoring (graft failure or end of follow-up period) whichever happened first. In the graft failure analyses the patients were followed until event (graft failure) or censoring (death or end of follow-up period) whichever happened first. In the combined outcome analyses patients were followed until event (death or graft failure) or censoring (end of follow-up period) whichever happened first. Logistic regression models were employed to estimate the odds ratio (OR) and 95%CI of post-transplant DGF.
For each regression analysis, four level of multivariate adjustment were examined: (I) A minimally adjusted (referred to as “unadjusted”) model that included donor race as the predictor and entry calendar quarter (q1 through q20) as the covariate; (II) Case-mix adjusted models that included the above plus age, gender, recipient race (African Americans and other self-categorized Blacks, Non-Hispanic Whites, Asians, Hispanics and others), diabetes mellitus, dialysis vintage (<6 mo, 6 mo to 2 yrs, 2–<5 yrs and ≥5 yrs), primary insurance (Medicare, Medicaid, private and others), marital status (married, single, divorced, widowed and other or unknown), standardized mortality ratio of the dialysis clinic during entry quarter, dialysis dose as indicated by Kt/V (single pool), presence or absence of a dialysis catheter, and residual renal function during the entry quarter and 8 co-morbidities (atherosclerotic heart disease, congestive heart failure, cancer, chronic obstructive pulmonary disease, cerebrovascular disease, hypertension, peripheral vascular disease, tobacco use); (III) The malnutrition-inflammation-complex syndrome (MICS) adjusted models which included all of the covariates plus 11 surrogates of nutritional status and inflammation measured during the last calendar quarter before transplantation including body mass index (BMI) and 9 laboratory variables, i.e. nPCR as an indicator of daily protein intake, also known as the normalized protein nitrogen appearance (nPNA)18, and serum or blood concentrations of TIBC, ferritin, phosphorus, calcium, bicarbonate, peripheral white blood cell count (WBC), lymphocyte percentage, albumin and hemoglobin; and (IV) Case-mix, MICS and transplant data adjusted models included all of the above plus 6 transplant-related variables: (1) donor type (deceased or living), (2) donor age, (3) donor gender, (4) panel reactive antibody (PRA) titer (last value prior to transplant), (5) number of HLA mismatches and (6) cold ischemia time. All analyses were carried out with SAS version 9.1, SAS Institute Inc., Cary, North Carolina and STATA version 11.1 (STATA Corporation, College Station, TX).
Results
The original 5-year (7/2001–6/2006) national database of all DaVita patients included 164,789 adult subjects. Out of 65,386 DaVita patients who were identified in the SRTR database, 17,629 had undergone one or more kidney transplantation during their life time, but only 14,508 dialysis patients had undergone kidney transplantation for the first time. After excluding those without electronically recorded donor race/ethnicity (n=816), there were 13,692 hemodialysis patients who underwent a first kidney transplantation during the observation period and who were followed until death, graft failure, loss of follow up, or survival until June 30th 2007 (Figure S1). There were 986 deaths (7.2%) and 1,351 graft failures (9.9%) irrespective of subsequent deaths. The median follow-up time was 730 days (interquartile range was: 365–1219 days). The basic characteristics of waitlisted, but non-transplanted, patients have been described elsewhere.19 Table 1 shows the clinical, demographic and laboratory data of the 13,692 transplanted hemodialysis patients. Recipients who received a kidney from an African American donor were more likely to be African American, to have received a kidney from a living, younger donor with shorter cold ischemic time and had higher crude DGF rates. Table 2 shows the clinical, demographic and laboratory data of the 13,187 transplanted hemodialysis patients across different donor and recipient races. The crude mortality and cardiovascular death rate was the highest in non-African American recipients who received a kidney from an African American donor and the crude graft loss and delayed graft function rate was the highest in African American recipients who received a kidney from a non-African American donor.
Table 1.
Baseline characteristics of 13,692 hemodialysis patients who underwent renal transplantation between 7/2001 and 6/2006
| Total population | African American donor | Non-African American donor | p-value* | |
|---|---|---|---|---|
| N [%] | 13,692 [100] | 1,936 [14] | 11,756 [86] | N/A |
| Deaths (n) [Crude Death Rate %] | 986 [7.2] | 141 [7.3] | 845 [7.2] | 0.88 |
| Cardiovascular Deaths (n) [Crude Death Rate %] | 266 [1.9] | 47 [2.4] | 219 [1.9] | 0.10 |
| Graft failure (n) [Crude Death Rate %] | 1,351 [9.9] | 247 [12.8] | 1,104 [9.4] | <0.001 |
| DGF (n) [Crude DFG %] | 2,769 [20.2] | 371 [19.2] | 2,398 [20.4] | 0.21 |
| Recipient baseline characteristics: | ||||
| Recipient’s Age (years), (mean ± SD) | 48±14 | 47±14 | 49±14 | <0.001 |
| Recipient’s Gender (% women) | 39 | 43 | 39 | 0.001 |
| Recipient’s race (% African-American) | 37 | 65 | 20 | <0.001 |
| Recipient’s Diabetes mellitus (%) | 26 | 25 | 26 | 0.19 |
| Recipient’s BMI (kg/m2), (mean ± SD) | 26.8±6.1 | 27.1±6.8 | 26.7±5.9 | 0.006 |
| Kt/V (dialysis dose), (mean ± SD) | 1.60±0.36 | 1.56±0.37 | 1.61±0.36 | <0.001 |
| Dialysis Vintage (%): | ||||
| 0–6 months | 12 | 9 | 13 | <0.001 |
| 6–24 months | 30 | 29 | 30 | 0.61 |
| 2–5 years | 37 | 36 | 37 | 0.44 |
| >5 years | 21 | 26 | 21 | <0.001 |
| nPCR (g/kg/day), (mean ± SD) | 1.04±0.26 | 1.02±0.26 | 1.04±0.26 | <0.001 |
| KRU (ml/min), (median (min.–max.)) | 0 (0–25) | 0 (0–25) | 0 (0–20) | 0.001 |
| Dialysis Catheter (%) | 18 | 21 | 17 | 0.01 |
| Serum creatinine (mg/dL), (mean ± SD) | 10.6±3.4 | 11.4±3.5 | 10.4±3.3 | <0.001 |
| Serum albumin (g/dL), (mean ± SD) | 3.98±0.40 | 3.97±0.40 | 3.98±0.41 | 0.55 |
| Blood hemoglobin (g/dL), (mean ± SD) | 12.2±1.3 | 12.2±1.3 | 12.2±1.3 | 0.12 |
| WBC (×103/l), (mean ± SD) | 6.9±2.1 | 6.7±2.1 | 7.0±2.1 | <0.001 |
| Transplantation related data: | ||||
| Number of HLA mismatch, (median (IQR)) | 4 (3–5) | 4 (3–5) | 4 (3–5) | <0.001 |
| PRA (%), (median (IQR)) | 0 (0–2) | 0 (0–5) | 0 (0–2) | <0.001 |
| EDC kidney (%) | 19 | 16 | 19 | 0.02 |
| Cold Ischemia time(hours), (median (IQR)) | 14 (3–21) | 12 (2–21) | 14 (3–21) | <0.001 |
| Donor baseline characteristics: | ||||
| Donor age (years), (mean ± SD) | 39±15 | 36±14 | 40±15 | <0.001 |
| Donor Type (% Living) | 34 | 41 | 33 | <0.001 |
| Donor’s hypertension (%) | 27 | 34 | 26 | <0.001 |
| Donor’s diabetes (%) | 5 | 6 | 5 | 0.08 |
| Donor’s BMI (kg/m2), (mean ± SD) | 26.6±5.8 | 27.1±6.4 | 26.5±5.7 | <0.001 |
| Donor after cardiac death (%) | 6 | 3 | 7 | <0.001 |
| Cerebrovascular cause of death (%) | 43 | 45 | 43 | 0.23 |
Data are presented in percent or mean ± SD or median (IQR) as appropriate.
p-value: comparing between Non-African American donor vs African American donor categories
Values in parentheses represent the proportion of the HD patients in both categories. Values in brackets indicate the crude death rate or crude graft failure rate or crude delayed graft function rate in the indicated group during the 6 years of observation.
PRA: panel reactive antibody (last value prior to transplant). DGF: Delayed Graft Function. WBC: White Blood Cell count. BMI: Body Mass Index. EDC: Extended Donor Criteria. KRU: residual renal function. nPCR: normalized protein catabolic rate. IQR: Interquartile Range.
Table 2.
Baseline characteristics of 13,187 hemodialysis patients who underwent renal transplantation between 7/2001 and 6/2006 across different donor’ and recipients’ races categories
| Donor Race | African American | Non-African American | |||
|---|---|---|---|---|---|
| Recipient Race | African American | Non-African American | African American | Non-African American | p-value* |
| N [%] | 1,221 [9.3] | 661 [5.0] | 2,281 [17.3] | 9,024 [68.4] | N/A |
| Deaths (n) [Crude Death Rate %] | 85 [7.0] | 56 [8.5] | 168 [7.4] | 657 [7.3] | 0.67 |
| Cardiovascular Deaths (n) [Crude Rate %] | 29 [2.4] | 18 [2.7] | 48 [2.1] | 161 [1.8] | 0.19 |
| Graft failure (n) [Crude Death Rate %] | 156 [12.8] | 83 [12.6] | 328 [14.4] | 745 [8.3] | <0.001 |
| DGF (n) [Crude DFG %] | 212 [17.4] | 150 [22.7] | 725 [31.8] | 1.627 [18.0] | <0.001 |
| Recipient baseline characteristics: | |||||
| Recipient’s Age (years), (mean ± SD) | 46±14 | 49±13 | 47±13 | 49±14 | <0.001 |
| Recipient’s Gender (% women) | 45 | 38 | 38 | 39 | <0.001 |
| Recipient’s Diabetes mellitus (%) | 26 | 23 | 25 | 27 | 0.068 |
| Recipient’s BMI (kg/m2), (mean ± SD) | 27.7±7.3 | 26.2±5.6 | 27.6±5.6 | 26.5±5.9 | <0.001 |
| Kt/V (dialysis dose), (mean ± SD) | 1.53±0.35 | 1.63±0.39 | 1.56±0.30 | 1.63±0.37 | <0.001 |
| Dialysis Vintage (%): | |||||
| <6 months | 10 | 8 | 5 | 15 | <0.001 |
| 6–24 months | 31 | 26 | 16 | 33 | <0.001 |
| 2–5 years | 35 | 38 | 42 | 35 | <0.001 |
| >5 years | 24 | 28 | 37 | 17 | <0.001 |
| nPCR (g/kg/day), (mean ± SD) | 0.99±0.25 | 1.08±0.26 | 1.00±0.24 | 1.06±0.26 | <0.001 |
| KRU (ml/min), (median (min.–max.)) | 0 (0–22) | 0 (0–25) | 0 (0–10) | 0 (0–20) | <0.001 |
| Dialysis Catheter (%) | 24 | 16 | 16 | 18 | <0.001 |
| Serum creatinine (mg/dL), (mean ± SD) | 11.8±3.5 | 10.7±3.5 | 12.3±3.4 | 10.1±3.2 | <0.001 |
| Serum albumin (g/dL), (mean ± SD) | 3.98±0.39 | 3.98±0.40 | 4.00±0.37 | 3.98±0.41 | <0.001 |
| Blood hemoglobin (g/dL), (mean ± SD) | 12.2±1.3 | 12.2±1.3 | 12.2±1.3 | 12.3±1.3 | 0.98 |
| WBC (×103/l), (mean ± SD) | 6.6±2.1 | 6.9±2.0 | 6.4±1.9 | 7.1±2.1 | <0.001 |
| Transplantation related data: | |||||
| EDC kidney (%) | 15 | 17 | 20 | 19 | 0.045 |
| Cold Ischemia time(hours), (median (IQR)) | 8 (1–18) | 17 (10–24) | 17 (12–23) | 13 (2–21) | <0.001 |
| Number of HLA mismatch, (median (IQR)) | 4 (3–5) | 5 (4–5) | 5 (4–5) | 4 (2–5) | <0.001 |
| PRA (%), (median (IQR)) | 0 (0–6) | 0 (0–4) | 0 (0–3) | 0 (0–2) | 0.001 |
| Donor baseline characteristics: | |||||
| Donor age (years), (mean ± SD) | 36±13 | 36±16 | 40±16 | 40±15 | <0.001 |
| Donor Type (% Living) | 55 | 13 | 6 | 39 | <0.001 |
| Donor’s hypertension (%) | 33 | 35 | 27 | 25 | <0.001 |
| Donor’s diabetes (%) | 6 | 7 | 5 | 5 | 0.31 |
| Donor’s BMI (kg/m2), (mean ± SD) | 27.3±6.1 | 26.5±6.8 | 26.6±6.1 | 26.5±5.6 | <0.001 |
| Donor after cardiac death (%) | 3 | 3 | 8 | 6 | <0.001 |
| Cerebrovascular cause of death (%) | 44 | 45 | 43 | 43 | 0.67 |
Data are presented in percent or mean ± SD or median (IQR) as appropriate.
p-value: comparing between different subgroups using chi-square test, ANOVA and Kruskal-Wallis test
Values in parentheses represent the proportion of the HD patients across categories. Values in brackets indicate the crude death rate or crude graft failure rate or crude delayed graft function rate in the indicated group during the 6 years of observation.
PRA: panel reactive antibody (last value prior to transplant). DGF: Delayed Graft Function. WBC: White Blood Cell count. BMI: Body Mass Index. EDC: Extended Donor Criteria. KRU: residual renal function. nPCR: normalized protein catabolic rate. IQR: Interquartile Range.
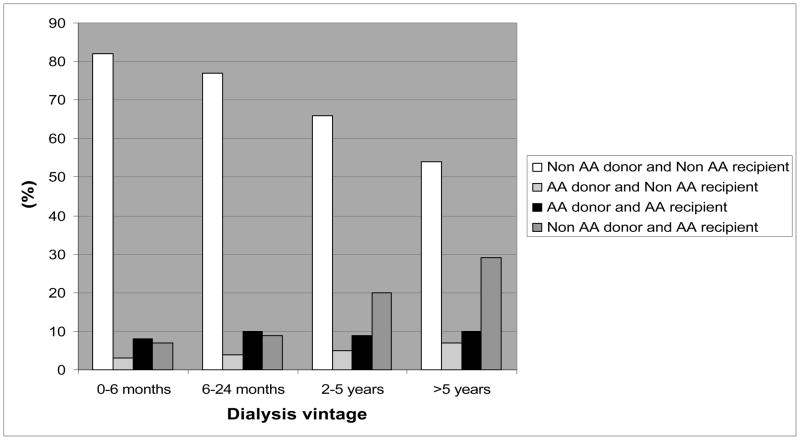
We also noticed changes over time in racial proportions of donors and recipients according to time on dialysis or vintage (Figure 1). The proportion of non-African American recipients received kidney from non-African American donors showed a decreasing trend (p<0.001), whereas the proportion of African American recipients received kidney from non-African American donors increased over time of dialysis treatment (p<0.001) according to longer time on dialysis (Figure 1).
Figure 1.
The association between donors’ and recipients’ mismatches and time on dialysis
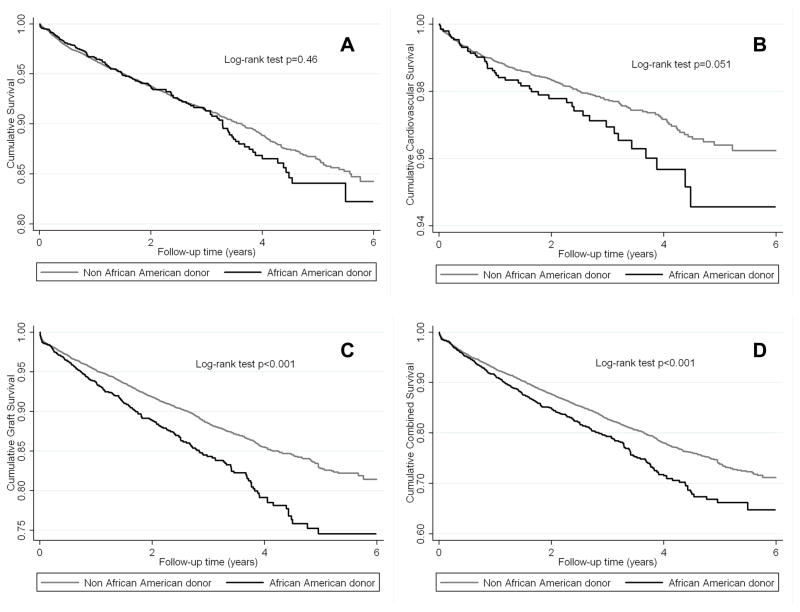
Figure 2–4 and Table 3 shows the association of African American donor race with various outcomes in the entire cohort and separately among non-African American recipients and African American recipients. In the entire cohort African American donor race was associated with higher cardiovascular mortality (Figure 2B), death-censored transplant loss (Figure 2C), and combined outcome (Figure 2D). The all-cause mortality curves were parallel during the first 3 years, but separated thereafter representing worse survival in African American donor group (Figure 2A). Table 3A shows the calculated HR and OR of all-cause and cardiovascular death, graft failure and delayed graft function comparing African American versus non-African American donor races in the entire cohort. African American donor race was associated with 39%, 80%, 30% and 31% higher all-cause mortality (HR and 95%CI: 1.39 (1.09–1.78)), cardiovascular mortality (HR and 95%CI: 1.80 (1.17–2.76)), death-censored graft loss (HR and 95%CI: 1.30 (1.03–1.64)) and combined outcome (HR and 95%CI: 1.31 (1.10–1.57)), respectively over the 6-year observation period after adjusting for several relevant clinical and transplant-related variables. The risk of delayed graft function did not show association with African American donor race.
Figure 2.
The crude association between African-American donor race and all-cause mortality (A), cardiovascular mortality (B), death-censored graft loss (C) and combined outcome (D) according to Kaplan-Meier analysis among 13,692 kidney transplanted patients
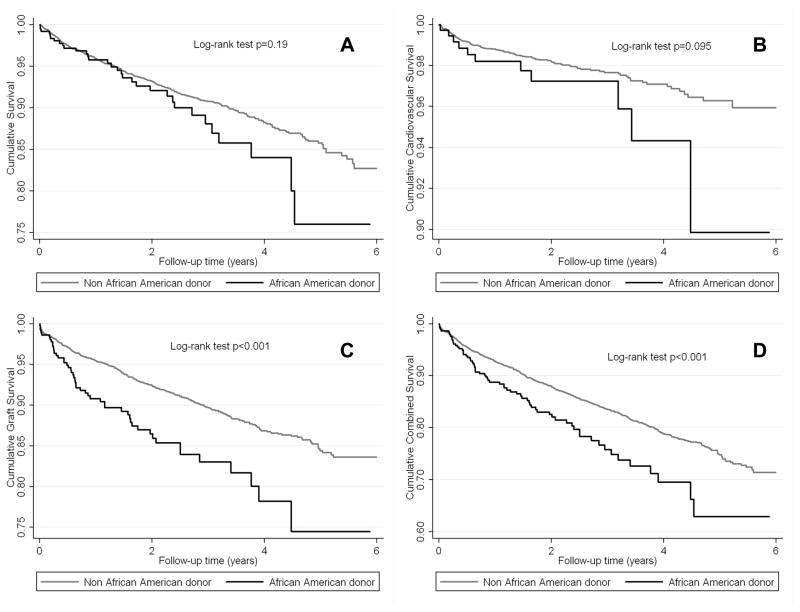
Figure 4.
The crude association between African-American donor race and all-cause mortality (A), cardiovascular mortality (B), death-censored graft loss (C) and combined outcome (D) according to Kaplan-Meier analysis among 3,052 African-American kidney transplanted recipients
Table 3.
Hazard ratio (HR) and Odds ratio (OR) (95% confidence intervals) of post-transplant death (all-cause or cardiovascular), graft failure, combined outcome and delayed graft function for African American donor versus non-African American donor (reference) using Cox regression analyses for survival, graft failure and combined outcome and logistic regression analysis for delayed graft function in hemodialysis patients who underwent renal transplantation and observed for up to 6 years (7/2001–6/2007)
| unadjusted | + case-mix adjusted* | + MICS adjusted** | + transplant data adjusted *** | |||||
|---|---|---|---|---|---|---|---|---|
| A | 13,692 hemodialysis patients who underwent renal transplantation | |||||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Graft failure censored all-cause death | 1.07 (0.89–1.28) | 0.46 | 1.19 (0.95–1.49) | 0.13 | 1.25 (0.99–1.58) | 0.059 | 1.39 (1.09–1.78) | 0.008 |
| Graft failure censored cardiovascular death | 1.37 (0.99–1.87) | 0.052 | 1.56 (1.04–2.33) | 0.031 | 1.73 (1.15–2.61) | 0.009 | 1.80 (1.17–2.76) | 0.007 |
| All-cause death censored graft failure | 1.43 (1.24–1.64) | <0.001 | 1.21 (0.99–1.49) | 0.066 | 1.20 (0.97–1.48) | 0.092 | 1.30 (1.03–1.64) | 0.024 |
| Combined all-cause death or graft failure | 1.28 (1.14–1.44) | <0.001 | 1.21 (1.03–1.42) | 0.021 | 1.22 (1.04–1.44) | 0.017 | 1.31 (1.10–1.57) | 0.003 |
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Delayed graft function | 0.93 (0.82–1.04) | 0.21 | 0.78 (0.66–0.92) | 0.003 | 0.78 (0.66–0.92) | 0.003 | 1.08 (0.90–1.29) | 0.40 |
| B | 6,056 White hemodialysis patients who underwent renal transplantation | |||||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Graft failure censored all-cause death | 1.27 (0.89–1.82) | 0.19 | 0.95 (0.58–1.53) | 0.82 | 0.91 (0.55–1.52) | 0.72 | 0.92 (0.54–1.55) | 0.75 |
| Graft failure censored cardiovascular death | 1.68 (0.91–3.63) | 0.10 | 1.19 (0.51–2.76) | 0.69 | 1.33 (0.57–3.12) | 0.51 | 1.09 (0.45–2.65) | 0.84 |
| All-cause death censored graft failure | 1.79 (1.34–2.40) | <0.001 | 2.03 (1.36–3.01) | <0.001 | 1.91 (1.27–2.89) | 0.002 | 2.24 (1.44–3.49) | <0.001 |
| Combined all-cause death or graft failure | 1.51 (1.18–1.93) | 0.001 | 1.49 (1.09–2.05) | 0.014 | 1.41 (1.01–1.97) | 0.043 | 1.49 (1.05–2.13) | 0.025 |
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Delayed graft function | 1.60 (1.25–2.05) | <0.001 | 1.28 (0.92–1.78) | 0.14 | 1.17 (0.82–1.65) | 0.39 | 0.91 (0.63–1.33) | 0.64 |
| C | 3,052 African-American hemodialysis patients who underwent renal transplantation | |||||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Graft failure censored all-cause death | 0.90 (0.69–1.17) | 0.43 | 1.21 (0.88–1.67) | 0.24 | 1.32 (0.95–1.83) | 0.10 | 1.50 (1.01–2.23) | 0.043 |
| Graft failure censored cardiovascular death | 1.08 (0.68–1.71) | 0.75 | 1.83 (1.04–3.21) | 0.036 | 1.91 (1.08–3.40) | 0.027 | 1.47 (0.73–2.97) | 0.29 |
| All-cause death censored graft failure | 0.85 (0.70–1.03) | 0.092 | 0.89 (0.67–1.18) | 0.41 | 0.86 (0.65–1.15) | 0.31 | 0.90 (0.63–1.29) | 0.57 |
| Combined all-cause death or graft failure | 0.88 (0.75–1.04) | 0.13 | 1.03 (0.82–1.28) | 0.81 | 1.04 (0.83–1.30) | 0.75 | 1.10 (0.83–1.46) | 0.49 |
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Delayed graft function | 0.45 (0.38–0.54) | <0.001 | 0.57 (0.46–0.71) | <0.001 | 0.57 (0.46–0.71) | <0.001 | 1.26 (0.96–1.64) | 0.09 |
Case-mix adjusted models adjusted for: age, gender, race/ethnicity, diabetes mellitus, dialysis vintage, primary insurance, marital status, standardized mortality ratio of the dialysis clinic during entry quarter, dialysis dose as indicated by Kt/V (single pool), presence or absence of a dialysis catheter, residual renal function during the entry quarter, and 8 co-morbidities (atherosclerotic heart disease, congestive heart failure, cancer, chronic obstructive pulmonary disease, cerebrovascular disease, hypertension, peripheral vascular disease, tobacco use)
MICS adjusted models which included all of the covariates plus; BMI, nPCR, serum or blood concentrations of TIBC, ferritin, phosphorus, calcium, bicarbonate, peripheral white blood cell count (WBC), lymphocyte percentage, albumin and hemoglobin
Case-mix, MICS and transplant data adjusted models included all of the above plus donor type, donor age, donor gender, panel reactive antibody (PRA) titer (last value prior to transplant), number of HLA mismatches and cold ischemia time
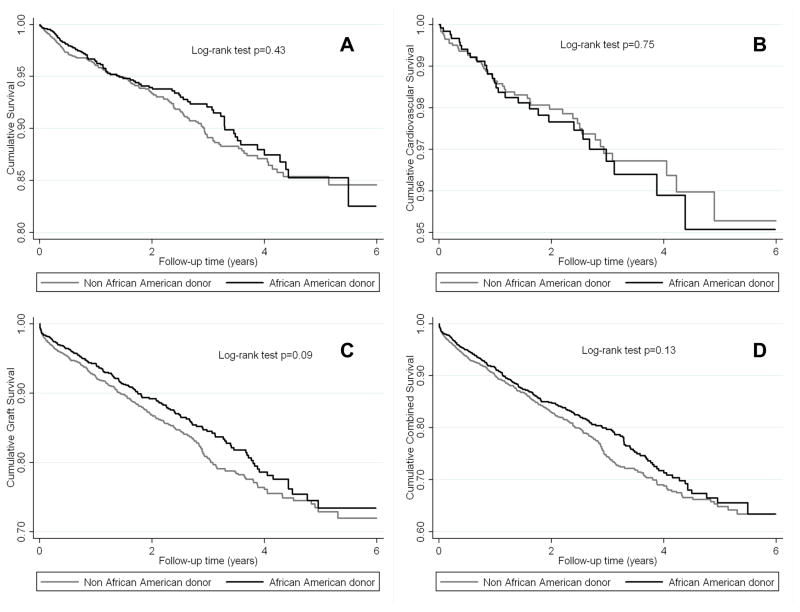
Among non-African American recipients, African American donor race was associated with higher death-censored graft loss (Figure 3C), and combined outcome (Figure 3D). The all-cause mortality curves were parallel during the first year, but separated thereafter representing worse survival in the African American donor race group (Figure 3A). A similar trend was observed for cardiovascular mortality (Figure 3B). Moreover, in this sub-cohort African American donor race was associated with more than two times and 49% higher death-censored graft loss (HR and 95%CI: 2.24 (1.44–3.49)) and combined outcome (HR and 95%CI: 1.49 (1.05–2.13)) after adjusting for several relevant clinical and transplant-related variables (Table 3B).
Figure 3.
The crude association between African-American donor race and all-cause mortality (A), cardiovascular mortality (B), death-censored graft loss (C) and combined outcome (D) according to Kaplan-Meier analysis among 6,056 White kidney transplanted recipients
Among African American recipients, all survival curves were parallel (Figure 4A–D). In this sub-cohort African American donor race was associated with higher all-cause mortality (HR and 95%CI: 1.50 (1.01–2.23)) only after adjusting for several relevant clinical and transplant-related variables (Table 3C).
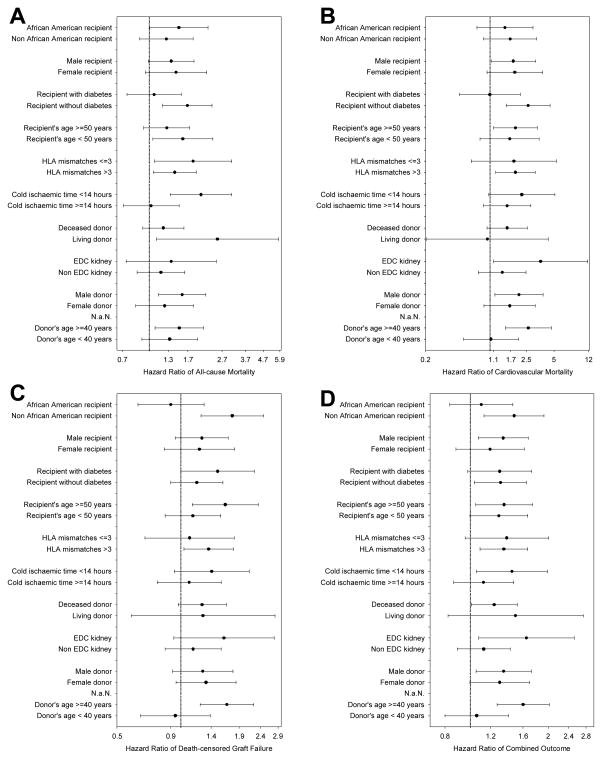
Figure 5 shows fully adjusted hazard ratios of all-cause mortality (A), cardiovascular mortality (B), death-censored graft loss (C) and combined outcome (D) associated with African American donor race in selected patient subgroups. The hazard ratios were above unity in almost all examined subgroups in different outcome measures, indicating a higher risk of poor outcomes in kidney transplantation using African American donor.
Figure 5.
Multivariate analysis of fully adjusted (for case-mix, MICS and transplant covariates) Cox regression models showing the African American donor and HR (and 95% CI as error bars) of all-cause mortality (A) cardiovascular mortality (B), death-censored graft loss (C) and combined outcome (D) in different sub-group of patients
Figure S3–S5 and Table S1 shows the association the non-African American donor and outcomes in the entire cohort and among non-African American recipients and African American recipients. In all plots the survival curves were running parallel during the follow-up time, representing similar risk of outcomes as clearly shown using Kaplan-Meier plot (Figure S3). Table S1A shows the non-African American donor race was associated with 24%, 32%, 17% and 18% lower all-cause mortality (HR and 95%CI: 0.76 (0.63–0.92)), cardiovascular mortality (HR and 95%CI: 0.68 (0.47–0.98)), death-censored graft loss (HR and 95%CI: 0.83 (0.69–1.00)) and combined outcome (HR and 95%CI: 0.82 (0.71–95)), respectively over the 6-year observation period after adjusting for several relevant clinical and transplant-related variables. The risk of delayed graft function did not show association with non-African American donor race.
Table 4 shows the calculated HR and OR of post-transplant outcomes across different donor-recipient races. Comparing to non-African American recipients and donors sub-group, the African American recipients and donors sub-group reported 49%, more than two times and 38% higher risk of all-cause (HR and 95%CI: 1.49 (1.10–2.03)), cardiovascular death (HR and 95%CI: 2.30 (1.37–3.87)) and delayed graft function (OR and 95%CI: 1.38 (1.10–1.74)), respectively (Table 4A–B and Table 4E). Comparing to non-African American recipients and donors sub-group, all other sub-groups reported higher risk of death-censored graft loss and combined outcome (Table 4C–D).
Table 4.
Hazard ratio (HR) and Odds ratio (OR) (95% confidence intervals) of post-transplant death (all-cause (A) or cardiovascular (B)), graft failure (C), combined outcome (D) and delayed graft function (E) across different donor-recipient races using Cox regression analyses for A-D and logistic regression analysis for E in hemodialysis patients who underwent renal transplantation and observed for up to 6 years (7/2001–6/2007)
| minimally adjusted | + case-mix adjusted* | + MICS adjusted** | + transplant data adjusted *** | |||||
|---|---|---|---|---|---|---|---|---|
| A | Graft failure censored all-cause death | |||||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Non-African American donor and Non-African American recipient (reference) | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A |
| Non-African American donor and African American recipient | 1.14 (0.96–1.36) | 0.12 | 0.95 (0.77–1.17) | 0.62 | 1.01 (0.80–1.27) | 0.94 | 0.99 (0.78–1.24) | 0.98 |
| African American donor and Non-African American recipient | 1.26 (0.96–1.65) | 0.10 | 1.12 (0.80–1.57) | 0.52 | 1.17 (0.83–1.66) | 0.37 | 1.29 (0.90–1.84) | 0.17 |
| African American donor and African American recipient | 1.03 (0.82–1.29) | 0.79 | 1.17 (0.89–1.53) | 0.25 | 1.32 (0.99–1.75) | 0.051 | 1.49 (1.10–2.03) | 0.01 |
| B | Graft failure censored cardiovascular death | |||||||
| HR (95% CI) | P- value | HR (95% CI) | P- value | HR (95% CI) | P- value | HR (95% CI) | P-value | |
| Non-African American donor and Non-African American recipient (reference) | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A |
| Non-African American donor and African American recipient | 1.32 (0.95–1.82) | 0.09 | 0.99 (0.66–1.51) | 0.98 | 1.13 (0.73–1.76) | 0.58 | 1.22 (0.77–1.94) | 0.40 |
| African American donor and Non-African American recipient | 1.63 (1.01–2.66) | 0.049 | 1.35 (0.72–2.51) | 0.36 | 1.61 (0.86–3.03) | 0.14 | 1.68 (0.87–3.22) | 0.12 |
| African American donor and African American recipient | 1.43 (0.96–2.12) | 0.08 | 1.69 (1.06–2.68) | 0.027 | 2.02 (1.25–3.27) | 0.004 | 2.30 (1.37–3.87) | 0.002 |
| C | All-cause death censored graft failure | |||||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Non-African American donor and Non-African American recipient (reference) | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A |
| Non-African American donor and African American recipient | 1.96 (1.72–2.23) | <0.001 | 1.45 (1.20–1.75) | <0.001 | 1.53 (1.23–1.83) | <0.001 | 1.50 (1.21–1.87) | <0.001 |
| African American donor and Non-African American recipient | 1.63 (1.30–2.05) | <0.001 | 1.63 (1.20–2.19) | 0.002 | 1.67 (1.23–2.27) | 0.001 | 1.77 (1.27–2.49) | 0.001 |
| African American donor and African American recipient | 1.66 (1.40–1.98) | <0.001 | 1.40 (1.09–1.79) | 0.009 | 1.42 (1.09–1.84) | 0.009 | 1.54 (1.14–2.06) | 0.004 |
| D | Combined all-cause death or graft failure | |||||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Non-African American donor and Non-African American recipient (reference) | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A |
| Non-African American donor and African American recipient | 1.60 (1.44–1.79) | <0.001 | 1.21 (1.05–1.41) | 0.01 | 1.26 (1.08–1.48) | 0.004 | 1.26 (1.07–1.50) | 0.007 |
| African American donor and Non-African American recipient | 1.40 (1.16–1.69) | <0.001 | 1.34 (1.06–1.70) | 0.016 | 1.37 (1.07–1.75) | 0.013 | 1.47 (1.13–1.91) | 0.004 |
| African American donor and African American recipient | 1.42 (1.23–1.63) | <0.001 | 1.32 (1.09–1.60) | 0.004 | 1.40 (1.14–1.71) | 0.001 | 1.50 (1.20–1.88) | <0.001 |
| E | Delayed graft function | |||||||
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Non-African American donor and Non-African American recipient (reference) | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A |
| Non-African American donor and African American recipient | 2.12 (1.91–2.35) | <0.001 | 1.57 (1.37–1.79) | <0.001 | 1.54 (1.33–1.77) | <0.001 | 1.12 (0.96–1.31) | 0.15 |
| African American donor and Non- African American recipient | 1.33 (1.10–1.61) | 0.003 | 1.13 (0.89–1.44) | 0.31 | 1.13 (0.88–1.45) | 0.34 | 0.93 (0.71–1.22) | 0.61 |
| African American donor and African American recipient | 0.96 (0.82–1.12) | 0.57 | 0.94 (0.77–1.15) | 0.55 | 0.92 (0.75–1.13) | 0.44 | 1.38 (1.10–1.74) | 0.006 |
Case-mix adjusted models adjusted for: age, gender, diabetes mellitus, dialysis vintage, primary insurance, marital status, standardized mortality ratio of the dialysis clinic during entry quarter, dialysis dose as indicated by Kt/V (single pool), presence or absence of a dialysis catheter, residual renal function during the entry quarter, and 8 co-morbidities (atherosclerotic heart disease, congestive heart failure, cancer, chronic obstructive pulmonary disease, cerebrovascular disease, hypertension, peripheral vascular disease, tobacco use)
MICS adjusted models which included all of the covariates plus; BMI, nPCR, serum or blood concentrations of TIBC, ferritin, phosphorus, calcium, bicarbonate, peripheral white blood cell count (WBC), lymphocyte percentage, albumin and hemoglobin
Case-mix, MICS and transplant data adjusted models included all of the above plus donor type, donor age, donor gender, panel reactive antibody (PRA) titer (last value prior to transplant), number of HLA mismatches and cold ischemia time
Similar results were found when we repeat our survival analyses in model adjusting for case-mix and MICS and transplant data plus donor age, donor hypertension, donor cause of death, donor BMI, donor diabetes, and donor after cardiac death in our sensitivity analyses (not shown).
Discussion
In 13,692 kidney transplant recipients with comprehensive pre-transplant data during hemodialysis treatment who were followed for up to 6 years post-transplantation, African American donor race was associated with increased all-cause and cardiovascular mortality and graft loss. African American donor race was not associated with higher risk of delayed graft function.
A plausible explanation for the observed associations is an unfavorable genetic background of African American donors. MYH9 and APOL1 account for a large proportion of the excess risk of ESRD observed in African Americans compared to non-African Americans.3–6 These genetic traits may result in specific types of kidney injury; upon transplantation such injured kidneys would then carry over the risk imparted by the donor’s genetic trait into the non-African American recipients who were unaffected by such risk before kidney transplantation. A recent study by Reeves-Daniel et al. showed that kidneys from African Americans deceased donors harboring two APOL1 risk variants failed more rapidly after renal transplantation than those with zero or one risk variants.20 In non-African American recipients the African American donor race was associated with increased graft loss, but not with overall increased all-cause or cardiovascular mortality. The survival curves did, however diverge after the first few years of follow-up (Figure 2A–B), which could have been a downstream effect of the lower kidney function in those who received a graft from an African-American donor. Unfortunately, we did not have information about kidney function after transplantation to test this hypothesis.
Besides the higher risks associated with African American donor race in non-African American recipients we did not detect a similar pattern in African American recipients, in whom African American donor race was not associated with increased risk of graft failure. The fact that organs received from non-African American donors (and hence devoid of the deleterious effects of the MYH9 and APOL1 mutations) did not impart a favorable outcome in African American recipients indicate that risk factors that are inherent of African American race but unrelated to genetic mutations also play a significant role in determining outcomes in this group. African American recipients suffer from more severe comorbidities and have worse insurance profile.21 Additionally, worse adherence was reported in African American recipients compared to their non-African American counterparts.22 The sickle cell trait is more common in African American patients resulting in more ischemic events and worse outcomes.23 Furthermore, there may be a positive association between transforming growth factor-β1 and several risk factors for CKD progression in African Americans but not in white Americans. 24
Our results confirm and extend the results of previous studies. A recent study of 72,945 patients from OPTN/UNOS indicated that African American donor kidneys are associated with lower graft survival when transplanted into white Americans or African Americans and are only associated with lower patient survival when these kidneys are transplanted into white American recipients.7 Our study does not agree with all these results. We did not find increased graft loss in African American recipients and increased mortality risk in non-African American recipients of kidneys from African American donors. Similarly to Swanson et al. we found that kidneys from African American donors have higher risk of graft loss compared with those from non-African American donors.9 Contradicting our results, Locke et al. reported that among African American recipients of kidneys obtained after cardiac death those who received kidneys from African American donors had better long-term graft and patient survival than those who received kidneys from white American donors. A potential explanation for the different results of our study is that unlike the previous studies we included patient characteristics obtained during the pre-transplant (dialysis) period. The working model for the new kidney allocation policy in the US utilizes the Kidney Donor Profile Index (KDPI), which combines a variety of donor factors to create a numerical risk factor of graft failure after kidney transplant. KDPI includes donor race/ethnicity as a variable; hence our additional validation of donor race/ethnicity as a risk factor is important as it may impact kidney allocation.
The proportion of usage of non-African American donor kidney was different between dialysis vintage categories, while the proportion of usage of African American donor kidney was balanced. More than 80% of recipients with short dialysis vintage (<6 months) who received kidney from non-African American donor were non-African American. However, only 54% of recipients with dialysis vintage more than 5 years who received kidney from non-African American donor were non-African American and 29% of these recipients were African American (Figure 1). One of the potential explanations is that African American dialysis patients live longer than non-African American counterparts.25–27 Another potential explanation is that the healthier non-African American dialysis patients more likely undergo a kidney transplantation in short time than African American counterparts.28,29 In contrast to this we did not find this disproportionality in recipients who received kidney from African American donor. If our findings are verified in additional studies, further explanations are needed.
Our study should be qualified for several potential limitations. We postulated that the observed associations are related to genetic influences such as MYH9 and APOL1 mutations, but we did not have information on these mutations to prove our hypothesis. Nor were we able to test for nontraditional antigentic incompatibilities, sociocultural factors or environmental exposures. Like all observational studies, ours too cannot prove causality. Laboratory variables and immunosuppressive and other medical regimens were not available in the SRTR database, but in the full model we did adjust for a number of transplant-related variables. The median of our follow-up time was 2 years, which to show the poorer graft survival rate in transplantation from African American donor. Generalizability may be limited, given the lower proportion of diabetics patients in our cohort compared to the US CKD population. It is also important to mention that re-transplanted patients were not included to our analyses. In addition, we defined the race according to the definitions set forth by the United States Census Bureau and the Federal Office of Management and Budget, however this definition might be not adequate according to the findings of the Human Genome project.30,31 Our results are not absolutely novel, but supportive of existing data with more robust analyses. To our knowledge this was the first study which included patient data from the pre-transplant period, which have a significant impact on post-transplant outcomes.16,32 Strengths of this study include the high number of patients and multi-level adjustment which include several important pre-transplant measures.
Conclusions
In our large and contemporary national database of 13,692 kidney transplant recipients, African American donor race was associated with increased all-cause and cardiovascular mortality and graft loss. African American donor race was not associated with higher risk of delayed graft function. African American donor race was associated with increased graft loss in non-African American recipients and associated with all-cause mortality in African American recipients. Future studies will have to test specific interventions aimed at abnormalities that are characteristic of allografts harvested from African American donors in order to improve outcomes in recipients of these organs.
Supplementary Material
Figure S1: Flow chart of the patient selection
Figure S2: Proportion of different races in donor (A) and recipients (B)
Figure S3: The crude association between White donor race and all-cause mortality (A), cardiovascular mortality (B), death-censored graft loss (C) and combined outcome (D) according to Kaplan-Meier analysis among 13,692 kidney transplant patients
Figure S4: The crude association between White donor race and all-cause mortality (A), cardiovascular mortality (B), death-censored graft loss (C) and combined outcome (D) according to Kaplan-Meier analysis among 6,056 White kidney transplanted recipients
Figure S5: The crude association between White donor race and all-cause mortality (A), cardiovascular mortality (B), death-censored graft loss (C) and combined outcome (D) according to Kaplan-Meier analysis among 3,052 African-American kidney transplanted recipients
Acknowledgments
Funding Source:
The study was supported by KKZ’s research grant from the American Heart Association grant (0655776Y). KKZ’s other funding sources include the National Institute of Diabetes, Digestive and Kidney Disease of the National Institute of Health (R01 DK078106); a research grant from DaVita Clinical Research and a philanthropic grant from Mr. Harold Simmons. MZM received grants from the National Developmental Agency (KTIA-OTKA-EU 7KP-HUMAN-MB08-A-81231) from the Research and Technological Innovation Fund, and is recipient of the Hungarian Eötvös Scholarship (MÖB/77-2/2012).
We thank DaVita Clinical Research (DCR) for providing the clinical data, analysis and review for this research project.
Footnotes
Relevant Potential Conflict of Interest:
Dr. Krishnan is an employee of DaVita. Dr. Kalantar-Zadeh is the medical director of DaVita Harbor-UCLA/MFI in Long Beach, CA. Other authors have not declared any conflict of interest.
References
- 1.U.S. Census Bureau: State and County QuickFacts. Data derived from Population Estimates, Census of Population and Housing, Small Area Income and Poverty Estimates, State and County Housing Unit Estimates, County Business Patterns, Nonemployer Statistics, Economic Census, Survey of Business Owners, Building Permits, Consolidated Federal Funds Report [Google Scholar]
- 2.US Renal Data System. USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2010. [Google Scholar]
- 3.Kao WH, Klag MJ, Meoni LA, Reich D, Berthier-Schaad Y, Li M, et al. MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nat Genet. 2008;40:1185. doi: 10.1038/ng.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329:841. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Genovese G, Tonna SJ, Knob AU, Appel GB, Katz A, Bernhardy AJ, et al. A risk allele for focal segmental glomerulosclerosis in African Americans is located within a region containing APOL1 and MYH9. Kidney international. 2010;78:698. doi: 10.1038/ki.2010.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freedman BI, Kopp JB, Langefeld CD, Genovese G, Friedman DJ, Nelson GW, et al. The apolipoprotein L1 (APOL1) gene and nondiabetic nephropathy in African Americans. J Am Soc Nephrol. 2010;21:1422. doi: 10.1681/ASN.2010070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Callender CO, Cherikh WS, Traverso P, Hernandez A, Oyetunji T, Chang D. Effect of donor ethnicity on kidney survival in different recipient pairs: an analysis of the OPTN/UNOS database. Transplantation proceedings. 2009;41:4125. doi: 10.1016/j.transproceed.2009.06.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lentine KL, Schnitzler MA, Xiao H, Saab G, Salvalaggio PR, Axelrod D, et al. Racial variation in medical outcomes among living kidney donors. The New England journal of medicine. 2010;363:724. doi: 10.1056/NEJMoa1000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swanson SJ, Hypolite IO, Agodoa LY, Batty DS, Jr, Hshieh PB, Cruess D, et al. Effect of donor factors on early graft survival in adult cadaveric renal transplantation. Am J Transplant. 2002;2:68. doi: 10.1034/j.1600-6143.2002.020112.x. [DOI] [PubMed] [Google Scholar]
- 10.Locke JE, Warren DS, Dominici F, Cameron AM, Leffell MS, McRann DA, et al. Donor ethnicity influences outcomes following deceased-donor kidney transplantation in black recipients. J Am Soc Nephrol. 2008;19:2011. doi: 10.1681/ASN.2008010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molnar MZ, Lukowsky LR, Streja E, Dukkipati R, Jing J, Nissenson AR, et al. Blood pressure and survival in long-term hemodialysis patients with and without polycystic kidney disease. Journal of hypertension. 2010;28:2475. doi: 10.1097/HJH.0b013e32833e4fd8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller JE, Kovesdy CP, Norris KC, Mehrotra R, Nissenson AR, Kopple JD, et al. Association of cumulatively low or high serum calcium levels with mortality in long-term hemodialysis patients. American journal of nephrology. 2010;32:403. doi: 10.1159/000319861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller JE, Kovesdy CP, Nissenson AR, Mehrotra R, Streja E, Van Wyck D, et al. Association of hemodialysis treatment time and dose with mortality and the role of race and sex. Am J Kidney Dis. 2010;55:100. doi: 10.1053/j.ajkd.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalantar-Zadeh K, Streja E, Kovesdy CP, Oreopoulos A, Noori N, Jing J, et al. The obesity paradox and mortality associated with surrogates of body size and muscle mass in patients receiving hemodialysis. Mayo Clinic proceedings. 2010;85:991. doi: 10.4065/mcp.2010.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalantar-Zadeh K, Miller JE, Kovesdy CP, Mehrotra R, Lukowsky LR, Streja E, et al. Impact of race on hyperparathyroidism, mineral disarrays, administered vitamin D mimetic, and survival in hemodialysis patients. J Bone Miner Res. 2010;25:2448. doi: 10.1002/jbmr.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molnar MZ, Kovesdy CP, Bunnapradist S, Streja E, Mehrotra R, Krishnan M, et al. Associations of Pre-Transplant Serum Albumin with Post-Transplant Outcomes in Kidney Transplant Recipients. Am J Transplant. 2011 doi: 10.1111/j.1600-6143.2011.03480.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yarlagadda SG, Coca SG, Garg AX, Doshi M, Poggio E, Marcus RJ, et al. Marked variation in the definition and diagnosis of delayed graft function: a systematic review. Nephrol Dial Transplant. 2008;23:2995. doi: 10.1093/ndt/gfn158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shinaberger CS, Greenland S, Kopple JD, Van Wyck D, Mehrotra R, Kovesdy CP, et al. Is controlling phosphorus by decreasing dietary protein intake beneficial or harmful in persons with chronic kidney disease? The American journal of clinical nutrition. 2008;88:1511. doi: 10.3945/ajcn.2008.26665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molnar MZ, Streja E, Kovesdy CP, Bunnapradist S, Sampaio MS, Jing J, et al. Associations of Body Mass Index and Weight Loss with Mortality in Transplant-Waitlisted Maintenance Hemodialysis Patients. Am J Transplant. 2011 doi: 10.1111/j.1600-6143.2011.03468.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reeves-Daniel AM, DePalma JA, Bleyer AJ, Rocco MV, Murea M, Adams PL, et al. The APOL1 gene and allograft survival after kidney transplantation. Am J Transplant. 2011;11:1025. doi: 10.1111/j.1600-6143.2011.03513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Obrador GT, Ruthazer R, Arora P, Kausz AT, Pereira BJ. Prevalence of and factors associated with suboptimal care before initiation of dialysis in the United States. J Am Soc Nephrol. 1999;10:1793. doi: 10.1681/ASN.V1081793. [DOI] [PubMed] [Google Scholar]
- 22.Weng FL, Israni AK, Joffe MM, Hoy T, Gaughan CA, Newman M, et al. Race and electronically measured adherence to immunosuppressive medications after deceased donor renal transplantation. J Am Soc Nephrol. 2005;16:1839. doi: 10.1681/ASN.2004121059. [DOI] [PubMed] [Google Scholar]
- 23.Diggs LW, Ahmann CF, Bibb J. The incidence and significance of the sickle cell trait. Ann Intern Med. 1933;7:769. [Google Scholar]
- 24.Suthanthiran M, Gerber LM, Schwartz JE, Sharma VK, Medeiros M, Marion R, et al. Circulating transforming growth factor-beta1 levels and the risk for kidney disease in African Americans. Kidney international. 2009;76:72. doi: 10.1038/ki.2009.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collins AJ, Foley RN, Herzog C, Chavers BM, Gilbertson D, Ishani A, et al. Excerpts from the US Renal Data System 2009 Annual Data Report. Am J Kidney Dis. 2010;55:S1. doi: 10.1053/j.ajkd.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong JS, Port FK, Hulbert-Shearon TE, Carroll CE, Wolfe RA, Agodoa LY, et al. Survival advantage in Asian American end-stage renal disease patients. Kidney international. 1999;55:2515. doi: 10.1046/j.1523-1755.1999.00464.x. [DOI] [PubMed] [Google Scholar]
- 27.Tanna MM, Vonesh EF, Korbet SM. Patient survival among incident peritoneal dialysis and hemodialysis patients in an urban setting. Am J Kidney Dis. 2000;36:1175. doi: 10.1053/ajkd.2000.19832. [DOI] [PubMed] [Google Scholar]
- 28.Epstein AM, Ayanian JZ, Keogh JH, Noonan SJ, Armistead N, Cleary PD, et al. Racial disparities in access to renal transplantation--clinically appropriate or due to underuse or overuse? The New England journal of medicine. 2000;343:1537. doi: 10.1056/NEJM200011233432106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sequist TD, Narva AS, Stiles SK, Karp SK, Cass A, Ayanian JZ. Access to renal transplantation among American Indians and Hispanics. Am J Kidney Dis. 2004;44:344. doi: 10.1053/j.ajkd.2004.04.039. [DOI] [PubMed] [Google Scholar]
- 30.Bonham VL, Warshauer-Baker E, Collins FS. Race and ethnicity in the genome era: the complexity of the constructs. Am Psychol. 2005;60:9. doi: 10.1037/0003-066X.60.1.9. [DOI] [PubMed] [Google Scholar]
- 31.Royal CD, Dunston GM. Changing the paradigm from ‘race’ to human genome variation. Nature genetics. 2004;36:S5. doi: 10.1038/ng1454. [DOI] [PubMed] [Google Scholar]
- 32.Streja E, Molnar MZ, Kovesdy cp, Bunnapradist S, Jing J, Nissenson AR, et al. Associations of Pretransplant Weight and Muscle Mass with Mortality in Renal Transplant Recipients. Clin J Am Soc Nephrol. 2011 doi: 10.2215/CJN.09131010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Flow chart of the patient selection
Figure S2: Proportion of different races in donor (A) and recipients (B)
Figure S3: The crude association between White donor race and all-cause mortality (A), cardiovascular mortality (B), death-censored graft loss (C) and combined outcome (D) according to Kaplan-Meier analysis among 13,692 kidney transplant patients
Figure S4: The crude association between White donor race and all-cause mortality (A), cardiovascular mortality (B), death-censored graft loss (C) and combined outcome (D) according to Kaplan-Meier analysis among 6,056 White kidney transplanted recipients
Figure S5: The crude association between White donor race and all-cause mortality (A), cardiovascular mortality (B), death-censored graft loss (C) and combined outcome (D) according to Kaplan-Meier analysis among 3,052 African-American kidney transplanted recipients