Abstract
Aims
To update our prior meta-analysis that showed past major depression (MD+) to be unrelated to smoking cessation outcome [Hitsman et al. J Consult Clin Psychol 2003; 71:657–63].
Methods
Eligible trials included 14 from our original review and 28 identified through an updated systematic review (2000–2009). We coded for assessment of past MD, exclusion for recent MD episode (MDE; ≤6 months versus no exclusion), duration/modality of cognitive behavioral treatment (CBT; face-to-face versus self-help), and other factors. To minimize influence of experimental treatments that may selectively benefit MD+ smokers, we analyzed placebo/lowest intensity control arms only. Study-specific odds ratios (ORs) for the effect of past MD on short-term (≤3 months) and long-term (≥6 months) abstinence were estimated and combined using random effects. Two-way interaction models of past MD with study methodology and treatment factors were used to evaluate hypothesized moderators of the past MD-abstinence association.
Results
MD+ smokers had 17% lower odds of short-term abstinence (n=35, OR=0.83, 95% CI=0.72–0.95, p=0.009) and 19% lower odds of long-term abstinence (n=38, OR=0.81, 95% CI=0.67–0.97, p=0.023) than MD− smokers after excluding the sole study of varenicline because of its antidepressant properties. The association between past MD and abstinence was affected by methodological (recent MDE exclusion, type of MD assessment) and treatment (CBT modality) factors.
Conclusions
Past major depression has a modest adverse effect on abstinence during and after smoking cessation treatment. An increased focus on the identification of effective treatments or treatment adaptations that eliminate this disparity in smoking cessation for MD+ smokers is needed.
Keywords: Systematic review, meta-analysis, major depression, smoking cessation
INTRODUCTION
Given the high co-occurrence of smoking and major depression (MD) across adulthood [1, 2], there has been substantial interest in determining whether past MD interferes with smoking cessation [3–7]. In 2003, we published a meta-analysis of the association between past MD and cessation outcome [8]. Among the 13 trials published between 1988 and 2000 that met our eligibility requirements, smokers with past MD (MD+) were as likely as those with no past MD (MD−) to achieve abstinence. This was the case whether we analyzed abstinence data from experimental and control treatment arms or, as was done in Hitsman et al. [8], when we analyzed data from the placebo/alternative lowest intensity arms alone. The latter finding was replicated by Covey et al. [9] in an independent meta-analysis.
Despite these results, it continues to be widely accepted that past MD constitutes an impediment to smoking cessation. Many recent trials have been mixed; reporting either lower abstinence rates [10–13] or comparable rates of abstinence [14–19] between MD+ and MD-smokers. These diverse findings suggest that differences among trials in study factors may be important moderators of an association between past MD and smoking cessation. Studies vary by exclusion criteria for use of antidepressant medication and time since last major depressive episode (MDE). Japuntich et al. [10] tested the association between past MD and abstinence in a large trial permitting use of antidepressants and recent MDE, and past MD was not associated with smoking at either 3 or 6 months post-quit.
Differences also exist between trials in the assessment of past MD. A validated diagnostic interview, such as the Structured Clinical Interview for DSM-IV (SCID-IV) [20], is the gold standard, but increasingly questionnaires and brief 1–2 item scales adapted from validated interviews are being used. Brief scales show good predictive value, as long as it is required that past depressed mood and(or) anhedonia persisted for two weeks or longer [21, 22]. Some trials, however, have required only the endorsement of past depressed mood and (or) anhedonia, without specifying a duration requirement [5, 23]. Others have classified past MD based only on the endorsement of a “history of depression” [24] or on whether the smoker was ever told by a health care provider that s/he had “depression” [25].
Trials also vary on treatment factors that could moderate an association between past MD and smoking cessation. Studies evaluating similar types of standard interventions, such as CBT plus TNP, can differ in treatment duration, CBT modality (e.g., face-to-face or self-help), and amount of contact with a therapist. Any disproportionate benefit of longer duration treatment, CBT face-to-face, or greater therapist contact time for MD+ smokers would minimize any adverse effect of MD+ on smoking cessation.
The primary aim of this review was to re-evaluate whether past MD is associated with smoking cessation by updating our prior review to include trials published since 2000. As done before [9, 26], we focused on smokers randomized to the placebo/alternative lowest intensity control arms of the trials under consideration. Isolating the control treatments removes from the analyses the potentially disproportionate influence of the experimental treatments that might selectively benefit MD+ smokers. A secondary aim was to extend our review to evaluate whether certain study factors moderate an association between past MD and cessation.
METHODS
Data sources and systematic search
Two authors performed the electronic literature search in November and December of 2008. Databases included PubMed and Ovid (MEDLINE), EMBASE, CINAHL, PsychINFO, Cochrane Database of Systematic Reviews, and Cochrane Central Register of Controlled Trials (CENTRAL). Two searches of each were performed for the period 2000–2008. One search used the terms “mood AND smoking cessation” and the other “depression AND smoking cessation.” Search delimiters were “English” and “human.” In addition, a manual online search was conducted in January, March, and August of 2009 for published and in-press studies appearing since December 2008 in the following journals because eligible studies had been published in them: Addiction, Addictive Behaviors, Alcoholism: Clinical and Experimental Research, American Journal of Psychiatry, Archives of General Psychiatry, Archives of Internal Medicine, Journal of Consulting and Clinical Psychology, Journal of General Internal Medicine, Nicotine & Tobacco Research, Psychology of Addictive Behaviors, American Journal on Addictions, and Drug and Alcohol Dependence. As a final step, the authors searched the reference lists of included articles and a relevant Cochrane Protocol [27].
Study selection
Selection requirements were the same as those in Hitsman et al. [8], i.e., the scope of the review was limited to trials that included a measure of past MD and involved adults.1 Trials in which the target population had a psychiatric disorder other than alcohol/substance dependence were excluded, so that other major psychiatric comorbidities would not obscure any association between past MD and smoking cessation. All studies were reviewed independently by two reviewers; a third reviewer reconciled disagreements on an as-needed basis.
Data extraction
Study characteristics and outcomes were extracted independently by two authors, with discrepancies resolved by consensus among four authors. Abstinence status was extracted by past MD status and treatment arm. Methodological characteristics included: type of MD assessment (clinical interview, questionnaire, 1–2 item scale considering duration of depressed mood and anhedonia, 1–2 item scale of symptoms only–non-DSM based), MDE exclusion criterion (exclusion for MDE within 6 months of enrollment, no exclusion for recent MDE), abstinence bioverified (yes, no), treatment randomization stratified by past MD (yes, no/not reported), and study exclusion for antidepressant medication use (yes, no/not reported). Treatment characteristics included: modality of CBT for smoking cessation (face-to-face, self-help), total therapist-patient contact time (minimal <3 hours, intensive ≥3 hours), type of smoking cessation pharmacotherapy (none, placebo, active standard medication, e.g., TNP, active standard + placebo, active standard antidepressant, e.g., bupropion), and treatment duration (standard ≤12 weeks, extended >12 weeks).
As noted, we selected the subset of participants in the placebo/alternative lowest intensity control treatment arms to minimize any influence of the experimental treatment arms that could have selectively benefited MD+ smokers. For example, we only included data from the standard CBT alone arm of the Patten et al. trial [28] that compared standard CBT plus CBT for depression versus standard CBT alone. Excluded from this review are data from smokers who received experimental smoking cessation treatment involving CBT for depression or antidepressant medication (e.g., bupropion or nortriptyline). An exception was made for Swan et al. [12], which contrasted bupropion (150 mg/day versus 300 mg/day) and CBT (tailored self-help versus proactive telephone counseling). We included the data from the smokers treated with low dose bupropion 150 mg/day and tailored self-help, as these were the control arms. For the 6 trials that did not screen out for recent MDE (i.e., within 6 months of enrollment) and measured current MDE [10, 29–33], we excluded participants classified as having current MDE. Thus, the MD+ participants from these studies who provided data for the meta-analysis could have had a MDE within 6 months of enrollment, but not within two weeks.
Data synthesis and meta-analysis
Following Hitsman et al. [8], outcome was classified as either short-term (≤ 3 months) or long-term (≥ 6 months) abstinence post-quit date. When a study offered multiple measurements that met criteria for either short- or long-term abstinence, we selected for analysis the furthest endpoint that fit within our definitions. As a result, only one effect size estimate was used from each study for each abstinence category. To circumvent complications due to within-study dependence, short- and long-term abstinence rates were analyzed separately, with missing outcomes for participants lost to follow-up coded as smoking.
If a study did not provide a short-term endpoint, the 3-month cutoff was extended to 4-months in order to capture a short-term outcome, i.e., [13]. For long-term outcomes, we chose the furthest abstinence assessment available after 6-months. For studies in which the timing of the abstinence measure was linked to the start or end of treatment, abstinence endpoints were recalculated based upon the target quit date. For example, Killen et al. [34] defined 11-, 25-, and 52-week outcome endpoints relative to start of treatment; given a 2-week target quit date, we revised these endpoints to 9, 23, and 50 weeks post-quit date.
For each type of outcome, we estimated study-specific odds ratios (ORs) for the effect of past MD on abstinence, and combined them across studies using the random effects approach recommended by DerSimonian & Laird [35]. Unlike fixed effects approaches that assume that a common OR is being measured by all studies under consideration [35], random effects models are more flexible, in that they allow the OR of interest to vary across studies. To examine the influence of each study on the pooled OR, we calculated study-specific weights. Under random effects, smaller studies tend to receive more weight than warranted by their precision when there is significant between-study heterogeneity, as indicated by larger values of Cochran’s Q statistic [36]. We used the I2 statistic to quantify the extent of between-study heterogeneity, with cutoffs of 30% and 50% used to distinguish mild, moderate, and severe levels [37].
Funnel plots of study-level estimates against their standard error (SE) are typically used to assess publication bias in studies of continuous outcomes. However, the mean-variance relationship that characterizes binary outcomes introduces asymmetry in the funnel plot of ORs versus SEs [38], making such plots potentially misleading. For this reason, we do not present funnel plots. Instead, we assess bias using a modified version of a well-known test [39] tailored to the analysis of binary outcomes [40].
To further explain the between-study heterogeneity in the effect of past MD on abstinence, we followed this traditional meta-analysis focused on study-level summaries by an analysis of individual participant data (IPD). Such IPD-level analyses are known to be more powerful than meta-regression methods for detecting interactions [41], and are not susceptible to ecological bias that can arise when examining study-level aggregates of participant characteristics [42]. However, they do require that we account for the nesting of participants within studies. This was accomplished by estimating our logistic regression model using the Generalized Estimating Equations (GEE) capabilities built into PROC GENMOD of SAS/STAT 9.2 [43] with study ID as the cluster identifier. Although the same aim could have been achieved via a random effects model, an advantage of the GEE approach [44] is that it produces consistent estimates of the regression coefficients, as long as the mean model is correctly specified, and does not require potentially unwarranted normality assumptions about the distribution of the random effects. Further, odds ratios have a marginal interpretation that applies to all studies, and do not need to be interpreted conditionally on the study identifier [45].
Our logistic regression model specification included a past MD term, main effects of the extracted study characteristics (i.e., type of MD assessment, MDE exclusion criterion, abstinence bioverified, treatment randomization stratified by past MD, study exclusion for antidepressant medication use, modality of CBT for smoking cessation, total therapist-patient contact time, type of smoking cessation pharmacotherapy, and treatment duration in long-term model only), and their two-way interactions with past MD2,3. Main effects terms are useful in explaining between-study variation in the odds of abstinence among MD− participants (our reference group), while interaction terms capture between-study variation in the past MD odds ratio for MD+ versus MD− subjects. Significance levels were calculated based upon robust standard errors, calculated under a working independence correlation structure.
A backwards elimination procedure with a 5% significance threshold was used to simplify the interaction effects. To partly compensate for low power in detecting interactions with past MD involving multi-category study level predictors, all interaction contrasts pertaining to such a predictor were retained in the model, if at least one of these contrasts attained statistical significance. Methodological/treatment characteristics that failed to moderate the association between past MD and abstinence were dropped altogether, as their main effects were of little interest in themselves. Exceptions were characteristics expected a priori to exert independent effects on abstinence, i.e., smoking cessation pharmacotherapy and exclusion for antidepressant medication use; their main effects were retained, irrespective of statistical significance, so as to provide effect size estimates.
RESULTS
Sample for meta-analysis
Our search captured 3,330 candidate articles (see Figure 1). Two independent reviews of the abstracts returned 333 articles. Excluding instances of articles appearing more than once resulted in 103 candidate articles. In addition, five studies were identified through the online search of selected journals; four studies were found through searching reference sections of qualitative reviews; and two were offered by investigators familiar with this ongoing work, raising the total to 114. Of the 114 candidates for full-text review, 28 studies were determined to be eligible. Combined with the 13 trials in Hitsman et al. [8] and another trial [23] excluded from that review, the final sample comprised 42 studies. Data not reported in the articles were obtained from authors of 18 of these studies (see Acknowledgements).
Figure 1.
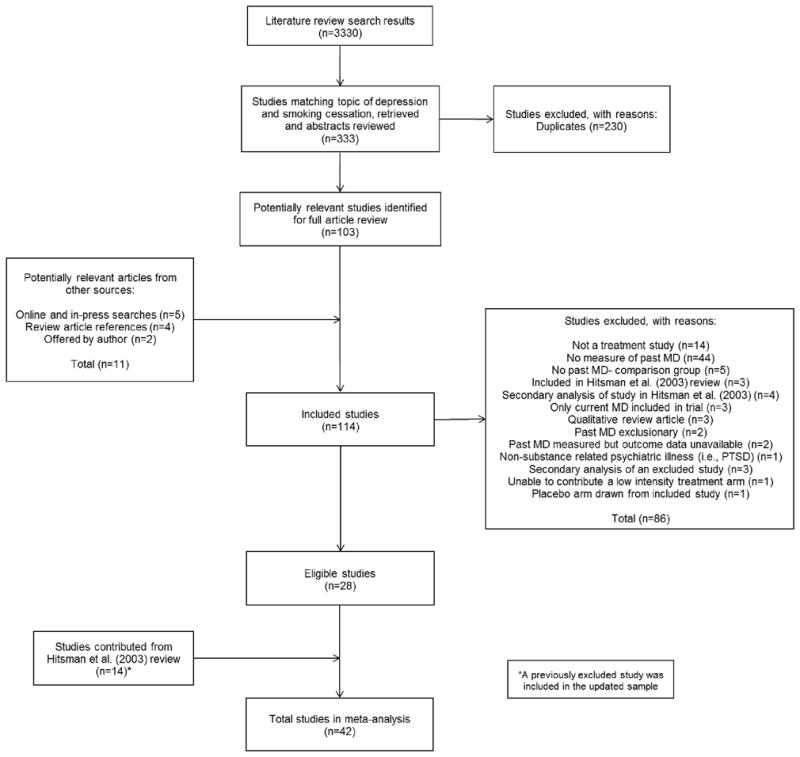
Flow diagram summarizing the study selection process.
Treatment modality across these 42 studies was as follows: 34 involved combination CBT and pharmacotherapy, four involved CBT face-to-face only, and four involved CBT self-help only. Twenty-seven studies evaluated experimental cessation treatment with antidepressant therapy. Classification of past MD was based on clinical interview in 27 studies, DSM-based questionnaire in seven studies, and 1–2 item scales in eight studies (DSM and non-DSM based). Thirty-one studies (74%) excluded smokers with recent MDE; the remaining 11 did not screen out for recent MDE. Seven-day point prevalence abstinence with missing equals smoking was assessed in 41 studies and biochemically-verified in 36 studies. The control treatment arms chosen for the 42 studies are shown in Table 1. The sample size, proportion MD+, and overall rates of abstinence for each study are available from the authors upon request.
Table 1.
Placebo or alternative lowest intensity control arms† that contributed to the meta-analysis of short-term (≤ 3 months post-quit date) and long-term (≥ 6 months post-quit date) abstinence.
| Study | Short-term Abstinence | Long-term Abstinence |
|---|---|---|
| Covey et al. (1993) [4] | Placebo clonidine + standard CBT | Assessment unavailable |
| Glassman et al. (1993) [57] | Placebo clonidine + standard CBT | Same as short-term |
| Hall et al. (1994) [58] | Nicotine gum (2 mg) + standard CBT | Same as short-term |
| Ginsberg et al. (1995) [59] | Aversive smoking + standard CBT | Same as short-term |
| Hall et al. (1996) [60] | Placebo or active nicotine gum (2 mg)a + standard CBT | Same as short-term |
| Muñoz et al. (1997) [31] | Standard CBT self-help material via mail | No low intensity arm |
| Hall et al. (1998) [61] | Placebo nortriptyline + standard CBT | Same as short-term |
| Covey et al. (1999) [62] | Placebo naltrexone + standard CBT | Same as short-term |
| Hayford et al. (1999) [63] | Placebo bupropion + standard CBT | Same as short-term |
| Niaura et al. (1999) [5] | Standard CBT self-help material | Assessment unavailable |
| Wetter et al. (1999) [23] | Assessment unavailable | Placebo or active TNP (22 mg/day)b + standard CBT |
| Keuthen et al. (2000) [64] | Placebo fluoxetine + standard CBT | Same as short-term |
| Killen et al. (2000) [65] | Placebo paroxetine + TNP (21 mg/day) + standard CBT | Same as short-term |
| Hall et al. (2002) [16] | Placebo medicationc (pooled) + standard CBT | Same as short-term |
| Patten et al. (2002) [28] | Standard CBT | Same as short-term |
| Levine et al. (2003) [18] | Non-specific social support + standard CBT | Same as short-term |
| Mermelstein et al. (2003) [13] | Proactive supportive or tailored telephone counseling (pooled) + standard CBTd | Same as short-term |
| Smith et al. (2003) [6] | Placebo bupropion + placebo TNP + standard CBT | Same as short-term |
| Swan et al. (2003) [12] | Bupropion (150 mg/day) + tailored CBT self-help material via mail | Same as short-term |
| Cox et al. (2004) [66] | No low intensity arm | Placebo bupropion + standard CBTe |
| Hall et al. (2004) [17] | Placebo nortriptyline + TNP (21 mg/day) + standard CBT | No treatmentf |
| Saules et al. (2004) [67] | Placebo fluoxetine + TNP (15 mg/day) + standard CBT | Same as short-term |
| Cinciripini et al. (2005) [68] | Placebo venlafaxine + TNP (22 mg/day) + standard CBT | Same as short-term |
| Killen et al. (2006) [34] | No low intensity arm | Placebo bupropion + standard CBTg |
| King et al. (2006) [69] | Placebo naltrexone + TNP (21 mg/day) + standard CBT | Same as short-term |
| Muñoz et al. (2006)h [30] | Standard CBT self-help material via Internet | Same as short-term |
| Brown et al. (2007) [14] | Placebo bupropion + standard CBT | Same as short-term |
| Japuntich et al. (2007) [10] | TNP (22 mg/day) + standard CBT | Same as short-term |
| Oncken et al. (2007) [11] | Placebo TNP + standard CBT | Same as short-term |
| Spring et al. (2007) [7] | Placebo fluoxetine + standard CBT | Same as short-term |
| Aveyard et al. (2008) [70] | Placebo nortriptyline + NRT (various)i + standard CBT | Same as short-term |
| Carmody et al. (2008) [24] | Assessment unavailable | TNP (21mg/day) + standard CBT |
| Killen et al. (2008) [71] | No low intensity arm | Supportive counseling via telephonej |
| Kodl et al. (2008) [29] | Concurrent or delayed TNP (21 mg/day)k + standard CBT | Same as short-term |
| Leventhal et al. (2008) [72] | Standard CBT | Same as short-term |
| Hall et al. (2009) [73] | No low intensity arm | No treatmentl |
| Hays et al. (2009) [74] | Tailored TNP (22, 33, or 44 mg/day)m + standard CBT | Placebo bupropion + standard CBTn |
| McClure et al. (2009) [19] | Varenicline (2 mg/day) + standard CBT self-help material via Internet | Same as short-term |
| Muñoz et al. (2009) [32] | Standard CBT self-help material via Internet | Same as short-term |
| Parsons et al. (2009) [75] | Placebo St John’s Wort + placebo chromium + standard CBT | Same as short-term |
| Simon et al. (2009) [25] | Placebo bupropion + standard CBT | Same as short-term |
| Piper et al. (2010) [33] | Placebo NRTo (pooled) + standard CBT | Same as short-term |
Note.
In single arm studies, a study was included if there was author consensus that the treatment was of comparable efficacy to the placebo or alternative control treatment arms of multi-arm studies. Assessment unavailable = abstinence assessment or abstinence not reported separately for past MD+ and MD− smokers; Pooled = treatment arms were combined because they were judged to have comparable efficacy; Standard CBT = standard cognitive-behavioral treatment for smoking cessation; TNP= transdermal nicotine patch (doses indicated are the starting dose of step-down therapy); NRT=nicotine replacement therapy
Stratified on active versus placebo gum but abstinence status was not reported separately for past MD+ and MD− participants
Pooled participants of three randomized trials of TNP; each study stratified on placebo versus active TNP but abstinence status was not reported separately for past MD+ and MD− participants
Placebo bupropion or placebo nortriptyline
7 weeks of group CBT followed by randomized maintenance treatment (10 weeks) with either proactive supportive or tailored telephone counseling; only completers of group CBT were randomized to individual relapse prevention phase
Randomized placebo-controlled relapse prevention phase after 7 weeks of treatment with bupropion (300 mg/day) + standard CBT
12 weeks of randomized treatment with placebo nortriptyline + TNP (21mg/day) + standard CBT followed by randomized extended duration treatment phase (40 weeks) during which this control arm received no further treatment
11 weeks of treatment with bupropion (300 mg/day) + TNP (21mg/day) + standard CBT followed by randomized placebo controlled extended duration treatment phase (14 weeks) during which this control arm received placebo bupropion and standard CBT
Studies 3 (English) and 4 (Spanish) were pooled and included in the meta-analysis
Employed a flexible design in which participants could choose type of NRT (and combination NRT if desired) and switch between products
8 weeks of treatment with bupropion (300 mg/day) + TNP (21 mg/day) + standard CBT followed by randomized extended duration treatment phase (12 weeks) during which this control arm received brief supportive counseling via telephone
Evaluated concurrent versus delayed smoking cessation treatment for patients in alcohol dependence treatment; pharmacotherapy also included nicotine gum if > 20 cigarettes per day
12 weeks of treatment with bupropion (300 mg/day) + nicotine gum (2 or 4 mg dose depending on ≥ 25 cigarettes per day) + standard CBT followed by randomized extended duration treatment phase (40 weeks) during which this control arm received no further treatment
Tailored to baseline level serum cotinine
8 weeks of treatment with TNP (22, 33, or 44 mg/day) and standard CBT followed by randomized maintenance treatment (44 weeks) with placebo bupropion and standard CBT; only abstainers were randomized
Placebo TNP (8 weeks) or placebo nicotine lozenge (12 weeks)
Past MD and short-term abstinence
A forest plot of the past MD ORs for the 36 studies (n=5,447) that provided short-term abstinence data is given in Figure 2. Point estimates and 95% confidence intervals were obtained separately for each study, and are plotted relative to the point of no association indicated by the solid vertical line (OR=1); their exact values presented in the logarithmic scale and study-level weights are available from the authors upon request.
Figure 2.
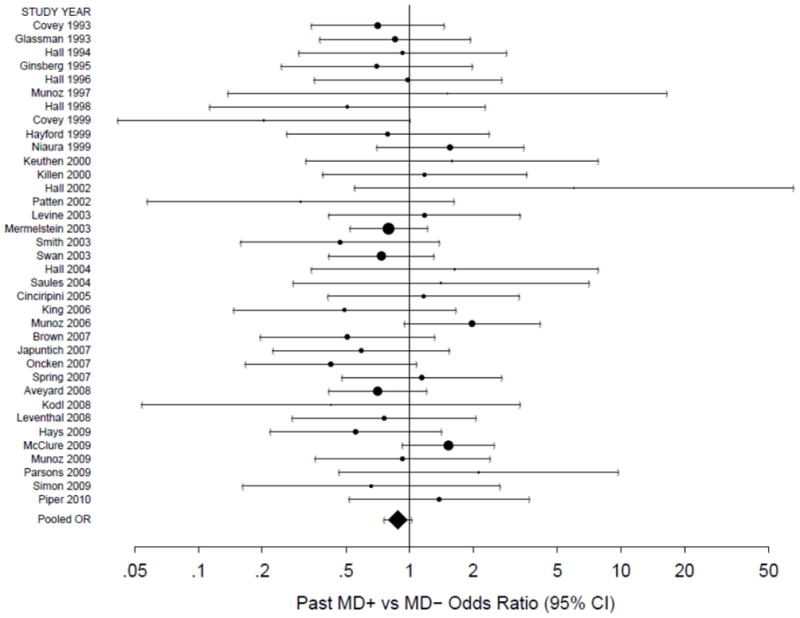
A forest plot of the odds ratios (OR) comparing short-term abstinence rates for past MD+ versus MD− smokers in the placebo/lowest intensity control arms. The size of the circle for each individual effect is proportional to the study’s weight in the analysis. Area inversely proportional to the variance of the log-odds ratio estimator. Error bars show the 95% confidence interval. The diamond indicates the overall short-term random effect. The point of no association is indicated by the solid vertical line. An OR less than 1 indicates a lower abstinence rate among past MD+ smokers.
As seen in Figure 2, the overall association between past MD and short-term abstinence was not statistically significant (OR=0.88, 95% CI=0.76–1.02, p=0.096). The I2 statistic indicated modest levels of between-study variability (I2=0.11, 95% CI=0.00–0.40) and the heterogeneity test statistic (Q=39.15 on 35 d.f., p=0.289) failed to attain statistical significance. However, the heterogeneity test is known to have low power [46], and we sought to further investigate the possible sources of between-study variation in past MD effects on short-term abstinence via a GEE logistic regression model presented in Table 2. The use of placebo medication produced 39% lower abstinence rates compared to no medication (OR=0.61, 95% CI=0.42–0.87), whereas exclusion for use of antidepressant medication was not significant (OR=1.25, 95% CI=0.75–2.08). In addition, two study-level covariates moderated the effect of past MD on the odds of short-term abstinence: CBT modality (p=0.006) and MDE exclusion (p=0.015). The joint impact of these two 2-way interactions is explored further in Table 3, where ORs for the effect of MD+ vs. MD− on short-term abstinence are stratified by CBT modality and MDE exclusion criterion.
Table 2.
Logistic regression model for short-term abstinence in placebo/lowest intensity control arms
| Odds Ratio | 95% CI | Robust P-value | |
|---|---|---|---|
|
|
|||
| Intercept | 0.75 | (0.46, 1.22) | 0.247 |
| Past MD+ | 0.69 | (0.56, 0.84) | <0.001 |
| (Past MD+):(Recent MDE Possible) | 1.52 | (1.09, 2.13) | 0.015 |
| CBT = Self-Help | 0.35 | (0.30, 0.42) | <0.001 |
| (Past MD+):(CBT = Self-Help) | 1.48 | (1.12, 1.96) | 0.006 |
| No Antidepressant Exclusion | 0.80 | (0.48, 1.34) | 0.401 |
| Pharmacological Tx = Placebo | 0.61 | (0.42, 0.87) | 0.007 |
| Pharmacological Tx = Active Std | 0.77 | (0.33, 1.79) | 0.537 |
| Pharmacological Tx = Active Std + Placebo | 1.84 | (0.98, 3.49) | 0.060 |
| Pharmacological Tx = Active Std Antidepressant | 1.72 | (0.98, 2.99) | 0.057 |
Note. Intercept = odds of abstinence for past MD− smokers treated with CBT face-to-face with no smoking cessation medication among studies that excluded for both recent MDE and anti-depressant use; Antidepressant Exclusion = exclusion for antidepressant medication use; Tx = treatment; Active Std = standard smoking cessation pharmacotherapy (e.g., nicotine patch); Active Std Antidepressant = standard smoking cessation pharmacotherapy involving antidepressant medication (n = 1) or medication with antidepressant effects (n = 1).
Table 3.
Odds ratios and 95% confidence intervals for the effect of past MD on short-term abstinence rates. Results stratified by CBT treatment modality and MDE exclusion criterion for study entry.
| CBT Treatment Modality | MDE Exclusion | |
|---|---|---|
| No Recent MDE | Recent MDE Possible | |
| Face-to-face | 0.69 (0.56, 0.84) | 1.05 (0.79, 1.39) |
| N=24; n=2,159 | N=6; n=1,832 | |
| (29.4% Past MD+) | (30.0% Past MD+) | |
| Self-help† | 1.02 (0.75, 1.38) | 1.55 (1.24, 1.93) |
| N=2; n=564 | N=4; n=892 | |
| (38.7% Past MD+) | (39.5% Past MD+) | |
Note. Odds ratios are with respect to past MD− participants receiving the same CBT modality in studies applying a common MDE exclusion criterion. CBT Face-to-Face = CBT delivered face-to-face in either individual or group format; CBT Self-Help = CBT self-help materials only; No Recent MDE = studies that screened out MDE within 6 months of enrollment; Recent MDE Possible = studies that did not exclude for recent MDE. N = number of studies, n = number of participants across studies.
Results show that among studies not permitting recent MDE, MD+ smokers had 31% lower odds of abstinence than MD− smokers (OR=0.69, 95% CI=0.56–0.84) in studies delivering CBT face-to-face, but showed no such disadvantage (OR=1.02, 95% CI=0.75–1.38) in studies that delivered CBT via self-help. In contrast, among studies permitting recent MDE, MD+ smokers showed no differences from MD− smokers (OR=1.05, 95% CI=0.79–1.39) in studies delivering CBT face-to-face, but had 55% higher odds of abstinence (OR=1.55, 95% CI=1.24–1.93) in studies that delivered CBT via self-help.
Past MD and long-term abstinence
A comparable forest plot of within-study ORs (95% CI) for the 39 studies (n=6,278) that provided long-term abstinence data is given in Figure 3, including the estimate of the statistically significant overall association with past MD (OR=0.82, 95% CI=0.69–0.97, p=0.024). Exact study-specific estimates and study-level weights are available from authors upon request. Between-study variability was again modest in magnitude (I2=0.16, 95% CI=0.00–0.44), as also indicated by the lack of significance of the heterogeneity statistic (Q=45.37 on 38 d.f., p=0.192).
Figure 3.
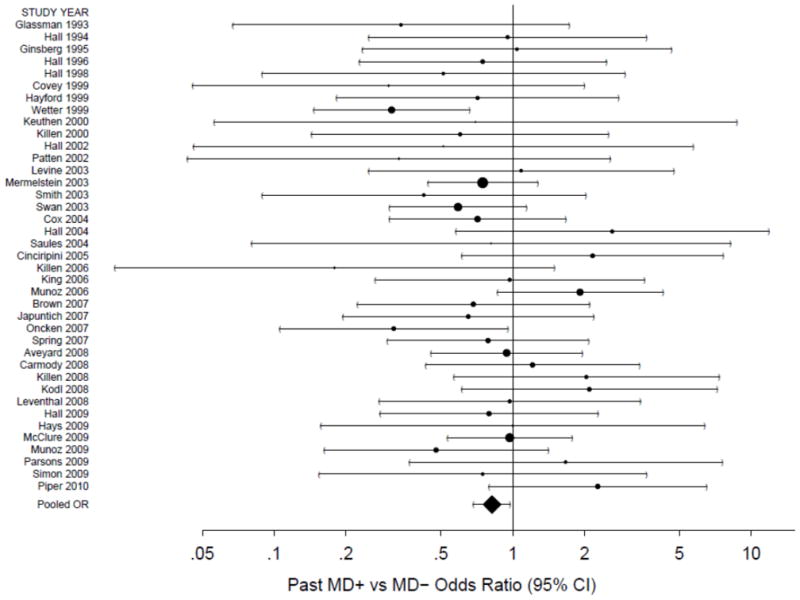
A forest plot of the odds ratios (OR) comparing long-term abstinence rates for past MD+ versus MD− smokers in the placebo/lowest intensity control arms. The size of the circle for each individual effect is proportional to the study’s weight in the analysis. Area inversely proportional to the variance of the log-odds ratio estimator. Error bars show the 95% confidence interval. The diamond indicates the overall long-term random effect. The point of no association is indicated by the solid vertical line. An OR less than 1 indicates a lower abstinence rate among past MD+ smokers.
A GEE logistic regression model that helps to explain further this between-study variability in past MD effects on long-term abstinence is presented in Table 4. Neither pharmacological treatment nor exclusion for antidepressant medication use were associated with long-term abstinence, whereas extended duration treatment (>12 weeks) raised the odds of abstinence by about half (OR=1.48, 95% CI=1.01–2.16). The use of active standard antidepressant medication more than tripled abstinence rates compared to no medication (OR=3.25, 95% CI=1.52–6.97). Finally, studies that delivered CBT via self-help had 40% lower odds of abstinence than those delivering it face-to-face (OR=0.60, 95% CI=0.50–0.72). Our model simplification procedure stipulated that all interaction contrasts pertaining to a study characteristic would be retained, if at least one of these contrasts attained statistical significance. This resulted in the retention of a MD assessment by past MD interaction, driven by the 44% amplification in the adverse effect of past MD on the odds of long-term abstinence in studies that used a 1–2 item non-DSM scale to classify past MD rather than clinical interview (OR=0.56, 95% CI=0.36–0.87). In addition, the association between past MD and long-term abstinence was moderated by MDE exclusion (p<0.001).
Table 4.
Logistic regression model for long-term abstinence in placebo/lowest intensity control arms.
| Odds Ratio | 95% CI | Robust P-value | |
|---|---|---|---|
|
|
|||
| Intercept | 0.30 | (0.23, 0.40) | <0.001 |
| Past MD+ | 0.70 | (0.57, 0.87) | 0.001 |
| (Past MD+):(Recent MDE Possible) | 1.81 | (1.33, 2.48) | <0.001 |
| CBT = Self-Help | 0.60 | (0.50, 0.72) | <0.001 |
| No Antidepressant Exclusion | 0.82 | (0.63, 1.07) | 0.139 |
| Pharmacological Tx = Placebo | 0.96 | (0.68, 1.35) | 0.800 |
| Pharmacological Tx = Active Std | 0.68 | (0.39, 1.20) | 0.187 |
| Pharmacological Tx = Active Std + Placebo | 0.73 | (0.39, 1.37) | 0.329 |
| Pharmacological Tx = Active Std Antidepressant | 3.25 | (1.52, 6.97) | 0.002 |
| Tx Duration > 12 Weeks | 1.48 | (1.01, 2.16) | 0.045 |
| MD Assessment = 1–2 Item Scale Non-DSM | 1.19 | (0.71, 2.01) | 0.504 |
| MD Assessment = 1–2 Item Scale | 0.77 | (0.39, 1.50) | 0.439 |
| MD Assessment = Questionnaire | 1.22 | (0.81, 1.84) | 0.331 |
| (Past MD+):(MD Assessment = 1–2 Item Non-DSM) | 0.56 | (0.36, 0.87) | 0.010 |
| (Past MD+):(MD Assessment = 1–2 Item) | 0.85 | (0.62, 1.16) | 0.309 |
| (Past MD+):(MD Assessment = Questionnaire) | 0.69 | (0.45, 1.07) | 0.096 |
Note. Intercept = odds of abstinence for past MD− smokers ascertained via interview and treated with CBT face-to-face for standard duration (≤12 weeks) with no smoking cessation medication among studies that excluded for both recent MDE and antidepressant use; Antidepressant Exclusion = exclusion for antidepressant medication use; Tx = treatment; Active Std = standard smoking cessation pharmacotherapy (e.g., nicotine patch); Active Std Antidepressant = standard smoking cessation pharmacotherapy involving antidepressant medication (n = 1) or medication with antidepressant effects (n = 1); MD Assessment: 1–2 Item Non-DSM = past MD classification based on the endorsement of past depressed mood and (or) anhedonia without specifying a duration requirement (i.e., 2 weeks or longer).
The joint impact of these two 2-way interactions with past MD is explored further in Table 5, where ORs for the effect of MD+ versus MD− on long-term abstinence are stratified by MD assessment and MDE exclusion criterion. Results show that among studies not permitting recent MDE, past MD+ had deleterious effects on long-term abstinence, irrespective of MD assessment method (ORs=0.39–0.70, all ps <0.001). In contrast, among studies permitting recent MDE, past MD+ had no effects on long-term abstinence, irrespective of MD assessment method (ORs= 0.71–1.27, all ps>0.12).
Table 5.
Odds ratios and 95% confidence intervals for the effect of past MD on long-term abstinence rates. Results stratified by type of MD assessment and MDE exclusion criterion for study entry.
| MD Assessment | MDE Exclusion | |
|---|---|---|
| No Recent MDE | Recent MDE Possible | |
| Clinical interview | 0.70 (0.57, 0.87) | 1.27 (0.86, 1.88) |
| N=22; n=2,085 | N=3; n=680 | |
| (24.9 % Past MD+) | (9.6% Past MD+) | |
| Questionnaire | 0.49 (0.34, 0.70) | 0.88 (0.64, 1.22) |
| N=4; n=320 | N=3; n=1,215 | |
| (31.3% Past MD+) | (32.2% Past MD+) | |
| 1–2 item scale | 0.60 (0.47, 0.75) | 1.08 (0.94, 1.25) |
| N=2; n=416 | N=2; n=746 | |
| (40.1% Past MD+) | (54% Past MD+) | |
| 1–2 item non-DSM scale | 0.39 (0.27, 0.58) | 0.71 (0.46, 1.10) |
| N=1; n=632 | N=2; n=184 | |
| (26.4% Past MD+) | (38.0% Past MD+) | |
Note. Odds ratios are with respect to past MD− participants with the same type of MD assessment in studies applying a common MDE exclusion criterion. N = number of studies, n = number of participants across studies.
Publication bias
Harbord’s [40] test produced two-tailed p-values that failed to attain statistical significance for either short-term (t=0.04, d.f.=34, p=0.967) or long-term (t=1.01, d.f.=37, p=0.321) abstinence, providing evidence against the possibility that small negative studies may have been inadvertently excluded from our review.
Sensitivity Analysis
One treatment arm in the included studies involved low dose antidepressant medication (i.e., bupropion [12]), and another involved varenicline [19]. Varenicline is not FDA-approved for MD, but pre-clinical studies have documented antidepressant properties, resulting in it being evaluated for the treatment of MD [47]. Some limited evidence indicates that nicotine replacement therapy (NRT) may also have antidepressant-like effects [48, 49].
Excluding the 11 NRT trials did not change the results for either short-term (OR=0.85, 95% CI=0.70–1.03, p=0.107) or long-term abstinence (OR=0.80, 95% CI=0.65–0.99, p=0.037). Similarly, exclusion of the bupropion trial did not affect the pooled OR for either short-term (OR=0.89, 95% CI=0.76–1.05, p=0.157) or long-term abstinence (OR=0.84, 95% CI=0.70–1.01, p=0.058), although it did deflate the statistical significance of the long-term findings. Exclusion of the varenicline trial strengthened the pooled OR for short-term abstinence and made this effect statistically significant (OR=0.83, 95% CI=0.72–0.95, p=0.009), leaving that for long-term abstinence unchanged (OR=0.81, 95% CI=0.67–0.97, p=0.023). Additional analyses showed that exclusion of the bupropion and varenicline studies did not alter our GEE findings.
DISCUSSION
Our updated systematic review and meta-analysis helps to settle the controversy that has existed over the past decade about whether or not past MD, in the absence of current MD, interferes with smoking cessation. Findings showed that past MD was associated with a statistically significant, but modest, decrease in both short-term and long-term abstinence rates after the sole varenicline study [19] had been excluded because of its possible antidepressant properties.
Our findings also reveal the importance of accounting for sources of methodological and treatment heterogeneity. These factors mattered even though our meta-analyses were restricted to smokers in the placebo/alternative control arms (i.e., standard non-antidepressant smoking cessation therapies) that we determined to be statistically homogeneous. The short-term association between past MD and smoking cessation depended on whether recent MDE was an exclusion criterion for the trial and on CBT modality. Short-term abstinence was significantly lower for MD+ smokers than for MD− smokers among 24/36 studies that screened out smokers with recent MDE and involved CBT face-to-face. The deleterious effect of past MD+ among face-to-face trials not permitting recent MDE extended to both CBT modalities for long-term abstinence, but results were equivocal for studies permitting recent MDE.
An unexpected finding for past MD and short-term abstinence, which is challenging to explain, was that MD+ smokers performed better than MD− smokers when treated with CBT self-help among trials permitting recent MDE. To the extent that excluding smokers with recent MDE resulted in samples of MD+ smokers with lower levels of emotional distress, acute nicotine withdrawal may have been especially distressing in that it provoked a return of affective symptoms that had previously been under control. For these MD+ smokers, the demands of manualized CBT delivered face-to-face amidst re-occurring emotional distress may have been sufficiently challenging to interfere with refining their self-management skills. By contrast, smokers with possible recent MDE (i.e., within 6 months of enrollment) may maintain some affective symptoms leading up to smoking cessation that may work to their advantage especially in the short-term and in treatment involving CBT self-help material. Their ongoing emotional distress may render acute withdrawal less emotionally “shocking” and skills training more manageable because their CBT is self-paced.
Our findings also suggest that the magnitude of the long-term association between past MD and abstinence may depend on the type of assessment that is used to classify past MD in smokers. Controlling for recent MDE exclusion criterion, use of non-validated 1–2 item self-report scales of past MD [23–25] significantly amplified the association between MD+ and long-term abstinence compared with clinical interview. The potential influence of MD assessment method on the association between past MD and smoking cessation is important to be mindful of in future studies, given the increasing reliance on brief assessments in community-based effectiveness trials of large populations of smokers.
Among the many strengths of our review are the large sample, in terms of number of studies and number of participants across studies, comparability among trials in their definition and measurement of abstinence, and IPD abstinence outcomes. Our database also has limitations. With the exception of three large studies focused on Spanish speaking populations worldwide [30–32], participants in the included trials were mostly European-American. The extent to which our results generalize to other racial/ethnic groups needs to be studied. We also were unable to re-evaluate whether or not gender moderates an association between past MD and cessation due to the lack of new IPD. It remains possible that MD+ conveys greater relapse risk among women, given that women are nearly twice as likely as men to experience MD [50].
Until now, researchers have largely stopped short at the post-hoc identification of vulnerable subgroups that show lower smoking cessation rates. The current need is to move beyond by identifying effective treatments or treatment adaptations that eliminate disparities in cessation outcomes [51]. The 2008 U.S. Public Health Service Clinical Practice Guideline recommends either bupropion or nortriptyline in particular for smokers with past MD based on a Guideline meta-analysis of four studies comparing bupropion SR or nortriptyline versus placebo, which indicated that both medications are effective for this population (page 146) [52]. Nicotine replacement therapy, such as nicotine patch or gum, also appear to be effective in smokers with past MD [52]. Though not reviewed in the Guideline, certain psychological interventions, such as cognitive behavioral mood management skills training [31, 53–56], may improve abstinence rates for past MD smokers when added to standard CBT for smoking cessation.
In conclusion, MD+ has a modest adverse effect on abstinence during and after smoking cessation treatment, and certain study characteristics appear to intensify the risk conveyed by MD+. Past MD among smokers who have not experienced a recent MDE appears to make achieving long-term abstinence even harder. To a certain extent, the inconsistent findings over the last decade may reflect methodological and treatment variation among studies. Comparative effectiveness research focused on smokers with past MD is needed to determine whether certain treatments are differentially effective for this large underserved population.
Acknowledgments
We thank the following persons for providing us with data not presented in their published articles: Paul Aveyard & Amanda C. Parsons, University of Birmingham, United Kingdom; Richard A. Brown & David R. Strong, The Warren Alpert Medical School of Brown University and Butler Hospital; Paul M. Cinciripini, University of Texas M.D. Anderson Cancer Center; Lirio S. Covey, Columbia University Medical Center; Sharon M. Hall, Ricardo F. Muñoz, & Kevin L. Delucchi, University of California San Francisco; J. Taylor Hays, Mayo Clinic; Sandra J. Japuntich, Massachusetts General Hospital and Harvard University Medical School; Joel D. Killen, Stanford University School of Medicine; Andrea King, University of Chicago Medical Center; Molly M. Kodl, Minneapolis VA Health Care System; Adam M. Leventhal, University of Southern California; Michele D. Levine, University of Pittsburgh; Jennifer B. McClure, Group Health Research Institute; Cheryl Oncken, University of Connecticut Health Center; Christi A. Patten, Mayo Clinic; Megan E. Piper, University of Wisconsin School of Medicine and Public Health; Joel A. Simon, University of California San Francisco and San Francisco VA Medical Center; Karen K. Saules, Eastern Michigan University; and Gary E. Swan, SRI International.
Footnotes
A single item measure of past MD was exclusionary in Hitsman et al. [8] but allowed in this review because brief screeners of past MD have become widely used in smoking cessation effectiveness studies.
Smoking cessation treatment duration was only included in the long-term abstinence model, due to the choice of coding scheme (standard duration treatment ≤12 weeks, extended duration treatment >12 weeks).
Only a pure interaction of MDE exclusion with past MD was included in these models, as its main effect on the reference group of MD− smokers was expected to be zero a priori.
Declarations: This review was supported in part by a NIH Mentored Clinical Scientist Research Career Development Award (K08 DA017145) to Brian Hitsman. The funding organization had no role in the review process or preparation of this manuscript.
Contributor Information
Brian Hitsman, Department of Preventive Medicine, Northwestern University Feinberg School of Medicine, Chicago, Illinois.
George D. Papandonatos, Center for Statistical Sciences, Brown University, Providence, Rhode Island
Dennis E. McChargue, Department of Psychology, University of Nebraska, Lincoln, Nebraska
Andrew DeMott, Department of Preventive Medicine, Northwestern University Feinberg School of Medicine, Chicago, Illinois.
María José Herrera, Department of Psychology, University of Nebraska, Lincoln, Nebraska.
Bonnie Spring, Department of Preventive Medicine, Northwestern University Feinberg School of Medicine, Chicago, Illinois.
Belinda Borrelli, Department of Psychiatry and Human Behavior, The Warren Alpert Medical School of Brown University & The Miriam Hospital, Providence, Rhode Island.
Raymond Niaura, The Schroeder Institute for Tobacco Research and Policy Studies, American Legacy Foundation, Washington, DC.
References
- 1.Wilhelm K, et al. Grey lungs and blue moods: smoking cessation in the context of lifetime depression history. Aust N Z J Psychiatry. 2004;38(11–12):896–905. doi: 10.1080/j.1440-1614.2004.01489.x. [DOI] [PubMed] [Google Scholar]
- 2.Ziedonis D, et al. Tobacco use and cessation in psychiatric disorders: National Institute of Mental Health report. Nicotine Tob Res. 2008;10(12):1691–715. doi: 10.1080/14622200802443569. [DOI] [PubMed] [Google Scholar]
- 3.Cohn AM, et al. History of single episode and recurrent major depressive disorder among smokers in cessation treatment: Associations with depressive symptomatology and early cessation failure. Addict Disord Their Treat. 2010;9(1):41–52. doi: 10.1097/ADT.0b013e3181b91c6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Covey LS, et al. Effect of history of alcoholism or major depression on smoking cessation. Am J Psychiatry. 1993;150(10):1546–7. doi: 10.1176/ajp.150.10.1546. [DOI] [PubMed] [Google Scholar]
- 5.Niaura R, et al. History and symptoms of depression among smokers during a self-initiated quit attempt. Nicotine Tob Res. 1999;1(3):251–7. doi: 10.1080/14622299050011371. [DOI] [PubMed] [Google Scholar]
- 6.Smith SS, et al. Targeting smokers at increased risk for relapse: treating women and those with a history of depression. Nicotine Tob Res. 2003;5(1):99–109. doi: 10.1080/1462220021000060437. [DOI] [PubMed] [Google Scholar]
- 7.Spring B, et al. Fluoxetine, smoking, and history of major depression: A randomized controlled trial. J Consult Clin Psychol. 2007;75(1):85–94. doi: 10.1037/0022-006X.75.1.85. [DOI] [PubMed] [Google Scholar]
- 8.Hitsman B, et al. History of depression and smoking cessation outcome: a meta-analysis. J Consult Clin Psychol. 2003;71(4):657–63. doi: 10.1037/0022-006x.71.4.657. [DOI] [PubMed] [Google Scholar]
- 9.Covey LS, Bomback A, Yan GW. History of depression and smoking cessation: a rejoinder. Nicotine Tob Res. 2006;8(2):315–9. doi: 10.1080/14622200500485250. [DOI] [PubMed] [Google Scholar]
- 10.Japuntich SJ, et al. Depression predicts smoking early but not late in a quit attempt. Nicotine Tob Res. 2007;9(6):677–86. doi: 10.1080/14622200701365301. [DOI] [PubMed] [Google Scholar]
- 11.Oncken C, et al. Transdermal nicotine for smoking cessation in postmenopausal women. Addict Behav. 2007;32(2):296–309. doi: 10.1016/j.addbeh.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Swan GE, et al. Bupropion SR and counseling for smoking cessation in actual practice: predictors of outcome. Nicotine Tob Res. 2003;5(6):911–21. doi: 10.1080/14622200310001646903. [DOI] [PubMed] [Google Scholar]
- 13.Mermelstein R, Hedeker D, Wong SC. Extended telephone counseling for smoking cessation: does content matter? J Consult Clin Psychol. 2003;71(3):565–74. doi: 10.1037/0022-006x.71.3.565. [DOI] [PubMed] [Google Scholar]
- 14.Brown RA, et al. Bupropion and cognitive-behavioral treatment for depression in smoking cessation. Nicotine Tob Res. 2007;9(7):721–30. doi: 10.1080/14622200701416955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Covey LS, et al. Smokers’ response to combination bupropion, nicotine patch, and counseling treatment by race/ethnicity. Ethn Dis. 2008;18(1):59–64. [PubMed] [Google Scholar]
- 16.Hall SM, et al. Psychological intervention and antidepressant treatment in smoking cessation. Arch Gen Psychiatry. 2002;59(10):930–6. doi: 10.1001/archpsyc.59.10.930. [DOI] [PubMed] [Google Scholar]
- 17.Hall SM, et al. Extended nortriptyline and psychological treatment for cigarette smoking. Am J Psychiatry. 2004;161(11):2100–7. doi: 10.1176/appi.ajp.161.11.2100. [DOI] [PubMed] [Google Scholar]
- 18.Levine MD, Marcus MD, Perkins KA. A history of depression and smoking cessation outcomes among women concerned about post-cessation weight gain. Nicotine Tob Res. 2003;5(1):69–76. doi: 10.1080/1462220021000060455. [DOI] [PubMed] [Google Scholar]
- 19.McClure JB, et al. Mood, side-effects and smoking outcomes among persons with and without probable lifetime depression taking varenicline. J Gen Intern Med. 2009;24(5):563–9. doi: 10.1007/s11606-009-0926-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams JB, et al. The Structured Clinical Interview for DSM-III-R (SCID). II. Multisite test-retest reliability. Arch Gen Psychiatry. 1992;49(8):630–6. doi: 10.1001/archpsyc.1992.01820080038006. [DOI] [PubMed] [Google Scholar]
- 21.Hitsman B, et al. Accuracy of a brief screening scale for lifetime major depression in cigarette smokers. Psychol Addict Behav. 2011;25(3):559–64. doi: 10.1037/a0022772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McChargue DE, Werth Cook J. Depression vulnerability within smoking research: How accurate are one-item screening items? Addict Behav. 2007;32(2):404–9. doi: 10.1016/j.addbeh.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Wetter DW, et al. Gender differences in smoking cessation. J Consult Clin Psychol. 1999;67(4):555–62. doi: 10.1037//0022-006x.67.4.555. [DOI] [PubMed] [Google Scholar]
- 24.Carmody TP, et al. Hypnosis for smoking cessation: a randomized trial. Nicotine Tob Res. 2008;10(5):811–8. doi: 10.1080/14622200802023833. [DOI] [PubMed] [Google Scholar]
- 25.Simon JA, et al. Sustained-release bupropion for hospital-based smoking cessation: a randomized trial. Nicotine Tob Res. 2009;11(6):663–9. doi: 10.1093/ntr/ntp047. [DOI] [PubMed] [Google Scholar]
- 26.Hitsman B, et al. Reply to Covey. Nicotine Tob Res. 2004;6:747–749. [Google Scholar]
- 27.van der Meer RM, Willemsen MC, Smit F, Cuijpers P. Smoking cessation interventions for smokers with current or past depression (Protocol) Cochrane Database Syst Rev. 2007;(Issue 3) doi: 10.1002/14651858.CD006102.pub2. Art. No. CD006102. [DOI] [PubMed] [Google Scholar]
- 28.Patten CA, et al. Effect of depressive symptoms on smoking abstinence and treatment adherence among smokers with a history of alcohol dependence. Psychol Addict Behav. 2002;16(2):135–42. doi: 10.1037//0893-164x.16.2.135. [DOI] [PubMed] [Google Scholar]
- 29.Kodl MM, et al. The impact of depressive symptoms on alcohol and cigarette consumption following treatment for alcohol and nicotine dependence. Alcohol Clin Exp Res. 2008;32(1):92–9. doi: 10.1111/j.1530-0277.2007.00556.x. [DOI] [PubMed] [Google Scholar]
- 30.Munoz RF, et al. Toward evidence-based Internet interventions: A Spanish/English Web site for international smoking cessation trials. Nicotine Tob Res. 2006;8(1):77–87. doi: 10.1080/14622200500431940. [DOI] [PubMed] [Google Scholar]
- 31.Munoz RF, et al. Mood management mail intervention increases abstinence rates for Spanish-speaking Latino smokers. Am J Community Psychol. 1997;25(3):325–43. doi: 10.1023/a:1024676626955. [DOI] [PubMed] [Google Scholar]
- 32.Munoz RF, et al. International Spanish/English Internet smoking cessation trial yields 20% abstinence rates at 1 year. Nicotine Tob Res. 2009;11(9):1025–34. doi: 10.1093/ntr/ntp090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piper ME, et al. Psychiatric disorders in smokers seeking treatment for tobacco dependence: relations with tobacco dependence and cessation. J Consult Clin Psychol. 2010;78(1):13–23. doi: 10.1037/a0018065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Killen JD, et al. Extended treatment with bupropion SR for cigarette smoking cessation. J Consult Clin Psychol. 2006;74(2):286–94. doi: 10.1037/0022-006X.74.2.286. [DOI] [PubMed] [Google Scholar]
- 35.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 36.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. [Google Scholar]
- 37.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 38.Schwarzer G, Antes G, Schumacher M. Inflation of type I error rate in two statistical tests for the detection of publication bias in meta-analyses with binary outcomes. Stat Med. 2002;21(17):2465–77. doi: 10.1002/sim.1224. [DOI] [PubMed] [Google Scholar]
- 39.Egger M, et al. Bias in meta-analysis detected by a simple graphical test. BMJ. 1997;315(7109):629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. 2006;25(20):3443–57. doi: 10.1002/sim.2380. [DOI] [PubMed] [Google Scholar]
- 41.Simmonds MC, Higgins JP. Covariate heterogeneity in meta-analysis: criteria for deciding between meta-regression and individual patient data. Stat Med. 2007;26(15):2982–99. doi: 10.1002/sim.2768. [DOI] [PubMed] [Google Scholar]
- 42.Thompson SG. Why and how sources of heterogeneity should be investigated. In: Egger M, Smith GD, Altman DG, editors. Systematic reviews in health care: Meta-analysis in context. BMJ Publishing Group; London, UK: 2001. pp. 157–175. [Google Scholar]
- 43.SAS Institute. SAS/STAT users’ guide. Carey, NC: SAS Institute, Inc; 2009. [Google Scholar]
- 44.Griffith LE, et al. Individual participant data meta-analysis of mechanical workplace risk factors and low back pain. Am J Public Health. 2012;102(2):309–18. doi: 10.2105/AJPH.2011.300343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Diggle PJ, Liang KY, Zeger SL. Analysis of longitudinal data. New York, NY: Oxford University Press; 1994. [Google Scholar]
- 46.Takkouche B, Cadarso-Suarez C, Spiegelman D. Evaluation of old and new tests of heterogeneity in epidemiologic meta-analysis. Am J Epidemiol. 1999;150(2):206–15. doi: 10.1093/oxfordjournals.aje.a009981. [DOI] [PubMed] [Google Scholar]
- 47.Philip NS, et al. Nicotinic acetylcholine receptors and depression: a review of the preclinical and clinical literature. Psychopharmacology (Berl) 2010;212(1):1–12. doi: 10.1007/s00213-010-1932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McClernon FJ, et al. Transdermal nicotine attenuates depression symptoms in nonsmokers: a double-blind placebo-controlled trial. Psychopharmacology (Berl) 2006;189(1):125–33. doi: 10.1007/s00213-006-0516-y. [DOI] [PubMed] [Google Scholar]
- 49.Kinnunen T, et al. Depression and smoking cessation: characteristics of depressed smokers and effects of nicotine replacement. J Consult Clin Psychol. 1996;64(4):791–8. doi: 10.1037//0022-006x.64.4.791. [DOI] [PubMed] [Google Scholar]
- 50.Weissman MM, et al. Cross-national epidemiology of major depression and bipolar disorder. JAMA. 1996;276(4):293–9. [PubMed] [Google Scholar]
- 51.Borrelli B. Smoking cessation: next steps for special populations research and innovative treatments. J Consult Clin Psychol. 2010;78(1):1–12. doi: 10.1037/a0018327. [DOI] [PubMed] [Google Scholar]
- 52.Fiore MC, Jaen CR, Baker TB, et al. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; 2008. Treating Tobacco Use and Dependence: 2008 Update. [Google Scholar]
- 53.van der Meer RM, et al. Effectiveness of a mood management component as an adjunct to a telephone counselling smoking cessation intervention for smokers with a past major depression: a pragmatic randomized controlled trial. Addiction. 2010;105(11):1991–9. doi: 10.1111/j.1360-0443.2010.03057.x. [DOI] [PubMed] [Google Scholar]
- 54.Patten CA, et al. Effectiveness of cognitive-behavioral therapy for smokers with histories of alcohol dependence and depression. J Stud Alcohol. 1998;59(3):327–35. doi: 10.15288/jsa.1998.59.327. [DOI] [PubMed] [Google Scholar]
- 55.Haas AL, et al. Influences of mood, depression history, and treatment modality on outcomes in smoking cessation. J Consult Clin Psychol. 2004;72(4):563–70. doi: 10.1037/0022-006X.72.4.563. [DOI] [PubMed] [Google Scholar]
- 56.Brown RA, et al. Cognitive-behavioral treatment for depression in smoking cessation. J Consult Clin Psychol. 2001;69(3):471–80. doi: 10.1037//0022-006x.69.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Glassman AH, et al. Smoking cessation, clonidine, and vulnerability to nicotine among dependent smokers. Clin Pharmacol Ther. 1993;54(6):670–9. doi: 10.1038/clpt.1993.205. [DOI] [PubMed] [Google Scholar]
- 58.Hall SM, Munoz RF, Reus VI. Cognitive-behavioral intervention increases abstinence rates for depressive-history smokers. J Consult Clin Psychol. 1994;62(1):141–6. doi: 10.1037//0022-006x.62.1.141. [DOI] [PubMed] [Google Scholar]
- 59.Ginsberg D, et al. Mood and depression diagnosis in smoking cessation. Exp Clin Psychopharmacol. 1995;3:389–395. [Google Scholar]
- 60.Hall SM, et al. Mood management and nicotine gum in smoking treatment: a therapeutic contact and placebo-controlled study. J Consult Clin Psychol. 1996;64(5):1003–9. doi: 10.1037//0022-006x.64.5.1003. [DOI] [PubMed] [Google Scholar]
- 61.Hall SM, et al. Nortriptyline and cognitive-behavioral therapy in the treatment of cigarette smoking. Arch Gen Psychiatry. 1998;55(8):683–90. doi: 10.1001/archpsyc.55.8.683. [DOI] [PubMed] [Google Scholar]
- 62.Covey LS, Glassman AH, Stetner F. Naltrexone effects on short-term and long-term smoking cessation. J Addict Dis. 1999;18(1):31–40. doi: 10.1300/J069v18n01_04. [DOI] [PubMed] [Google Scholar]
- 63.Hayford KE, et al. Efficacy of bupropion for smoking cessation in smokers with a former history of major depression or alcoholism. Br J Psychiatry. 1999;174:173–8. doi: 10.1192/bjp.174.2.173. [DOI] [PubMed] [Google Scholar]
- 64.Keuthen NJ, et al. Comorbidity, smoking behavior and treatment outcome. Psychother Psychosom. 2000;69(5):244–50. doi: 10.1159/000012403. [DOI] [PubMed] [Google Scholar]
- 65.Killen JD, et al. Nicotine patch and paroxetine for smoking cessation. J Consult Clin Psychol. 2000;68(5):883–9. [PubMed] [Google Scholar]
- 66.Cox LS, et al. Efficacy of bupropion for relapse prevention in smokers with and without a past history of major depression. J Gen Intern Med. 2004;19(8):828–34. doi: 10.1111/j.1525-1497.2004.30423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saules KK, et al. Double-blind placebo-controlled trial of fluoxetine in smoking cessation treatment including nicotine patch and cognitive-behavioral group therapy. Am J Addict. 2004;13(5):438–46. doi: 10.1080/10550490490512762. [DOI] [PubMed] [Google Scholar]
- 68.Cinciripini PM, et al. Combined effects of venlafaxine, nicotine replacement, and brief counseling on smoking cessation. Exp Clin Psychopharmacol. 2005;13(4):282–92. doi: 10.1037/1064-1297.13.4.282. [DOI] [PubMed] [Google Scholar]
- 69.King A, et al. Efficacy of naltrexone in smoking cessation: a preliminary study and an examination of sex differences. Nicotine Tob Res. 2006;8(5):671–82. doi: 10.1080/14622200600789767. [DOI] [PubMed] [Google Scholar]
- 70.Aveyard P, et al. Nortriptyline plus nicotine replacement versus placebo plus nicotine replacement for smoking cessation: pragmatic randomised controlled trial. BMJ. 2008;336(7655):1223–7. doi: 10.1136/bmj.39545.852616.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Killen JD, et al. Extended cognitive behavior therapy for cigarette smoking cessation. Addiction. 2008;103(8):1381–90. doi: 10.1111/j.1360-0443.2008.02273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Leventhal AM, et al. Dimensions of depressive symptoms and smoking cessation. Nicotine Tob Res. 2008;10(3):507–17. doi: 10.1080/14622200801901971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hall SM, et al. Extended treatment of older cigarette smokers. Addiction. 2009;104(6):1043–52. doi: 10.1111/j.1360-0443.2009.02548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hays JT, et al. A randomized, controlled trial of bupropion sustained-release for preventing tobacco relapse in recovering alcoholics. Nicotine Tob Res. 2009;11(7):859–67. doi: 10.1093/ntr/ntp077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Parsons A, et al. A proof of concept randomised placebo controlled factorial trial to examine the efficacy of St John’s wort for smoking cessation and chromium to prevent weight gain on smoking cessation. Drug Alcohol Depend. 2009;102(1–3):116–22. doi: 10.1016/j.drugalcdep.2009.02.006. [DOI] [PubMed] [Google Scholar]


