Abstract
Background
Neocortical development represents more than a simple unfolding of a genetic blueprint but rather represents a complex dance of genetic and environmental events that interact to adapt the brain to fit a particular environmental context. Although most cortical regions are sensitive to a wide range of experiential factors during development and later in life, the prefrontal cortex appears to be unusually sensitive to perinatal experiences and relatively immune to many adulthood experiences relative to other neocortical regions.
Methods and results
One way to examine experience-dependent prefrontal development is to conduct studies in which experiential perturbations are related neuronal morphology. This review of the research reveals both pre- and post-natal factors have important effects on prefrontal development and behaviour. Such factors include psychoactive drugs, including both illicit drugs and prescription drugs, stress, gonadal hormones and sensory and motor stimulation. A second method of study is to examine both the effects of perinatal prefrontal injury on the development of the remaining cerebral mantle and correlated behaviours as well as the effects of post-injury rehabilitation programmes on the anatomical and behavioural measures.
Conclusions
Prefrontal injury alters cerebral development in a developmental-stage dependent manner with perinatal injuries having far more deleterious effects than similar injuries later in infancy. The outcome of perinatal injuries can be modified, however, by rehabilitation with many of the factors shown to influence prefrontal development in the otherwise normal brain.
Introduction
An emerging theme in developmental neuroscience is that brain development is profoundly influenced by a wide range of pre- and post-natal experiences that act upon a basic genetic blueprint. Many of these experiences are well known, such as the effects of pre-natal exposure to alcohol, but others are less obvious, such as the pre-natal effects of prescription drugs. Even less known are the interactions between such perinatal experiences and recovery from perinatal cerebral injury. This study considers first how perinatal experiences influence brain and behavioural development and secondly how these experiences interact with perinatal injury to the prefrontal cortex.
The prefrontal cortex of the rat can be functionally sub-divided into many discrete regions [1], but for the purpose of this study it will focus on two regions: a medial region (Cg3) and a ventrolateral (orbital) region (AID). These regions both show impressive plasticity in response to gonadal hormones [2] and psychotrophic drugs [3]. For the brain injury studies, this study focused on the effects of treatments on recovery from medial prefrontal injuries that include Cg3 as well as several other medial regions.
The challenge of studying brain and behavioural development
Brain development can be studied at many levels of analysis. Historically, human brain development was studied by the analysis of the gross neuroanatomy of postmortem tissue. Although much was learned from these studies, they say little about the functional implications of anatomical change. At the other end of the analysis spectrum, brain development could be inferred from global measures of brain activity, such as in non-invasive in vivo imaging, but whereas imaging is easily adapted to studying adult brain function, it is very difficult to adapt to the study of infants. Thus, although it is relatively simple to study behavioural changes in the developing infant, it is difficult to infer specific neuronal changes of the behavioural changes. Such changes can be studied more easily in laboratory animal models, however, because it is possible to tie neuronal change and behaviour together with more certainty than in human studies.
Identification of the neuronal correlates of behaviour still has its problems. Some of these changes are directly associated with behaviour but others are ambiguous. Consider an example. If a pregnant mum is given an anti-depressant drug, one may see an obvious acute behavioural change in the infant such as increased anxiety. If one were to look for changes in the brain that were related to the increased anxiety one might find a change in synapse number in some discrete brain region such as the amygdala or prefrontal cortex. Both the behavioural change and the synaptic change are correlates of the drug administration. However, what is the causal relationship between the behavioural and synaptic change? It seems reasonable to conclude that the drug caused the behavioural change, but it is less clear that the drug directly caused the neuronal change. It is possible that the behavioural change caused the neuronal change or that both were related to some other change in the brain. Thus, a common criticism of studies trying to link neuronal changes to behaviour is that ‘they are only correlates’. This is true enough, but it is hardly a reason not to do such studies. The task of the researcher is to try to break the correlation by showing that one change can occur without the other. The presence of such evidence would disconfirm causality, but, unfortunately, the failure to break the correlation is not proof of causation. Ultimately the proof would be in showing how the synaptic changes arose, which would presumably involve molecular analysis such as a change in gene transcription. For many studies this would be an extremely difficult challenge and is often impractical. It is the authors’ view that once the ‘rules’ that govern neuronal and behavioural change are understood, one will be better able to look for molecular changes. Furthermore, it is argued that a certain level of ambiguity in the degree of causation is perfectly justifiable. An example might be seen in using a drug to facilitate recovery from stroke. It is known that repeated doses of nicotine lead to increased synaptogenesis in motor cortex [4]. If there is a partial loss of motor cortex after a stroke, it is reasonable to suppose that increasing synapse number in the remaining motor cortex might facilitate recovery and indeed this is the case [5]. Understanding the mechanism whereby the synaptic changes might occur is not necessary to proceed with further studies aimed at improving functional outcome.
Thus, as one looks for therapies to facilitate functional improvement after perinatal brain injury, one’s gaze is cast towards experiences that are known to influence brain development in the ‘normal brain’. At this point one is less concerned with underlying mechanisms than with the functional outcome. As in studies of brain development, one is interested in demonstrating neuronal correlates of the behavioural changes but do not pretend that one understands how or why these neuronal changes occur. The goal is to demonstrate that the treatments do induce behavioural changes and that there are correlated neuronal changes.
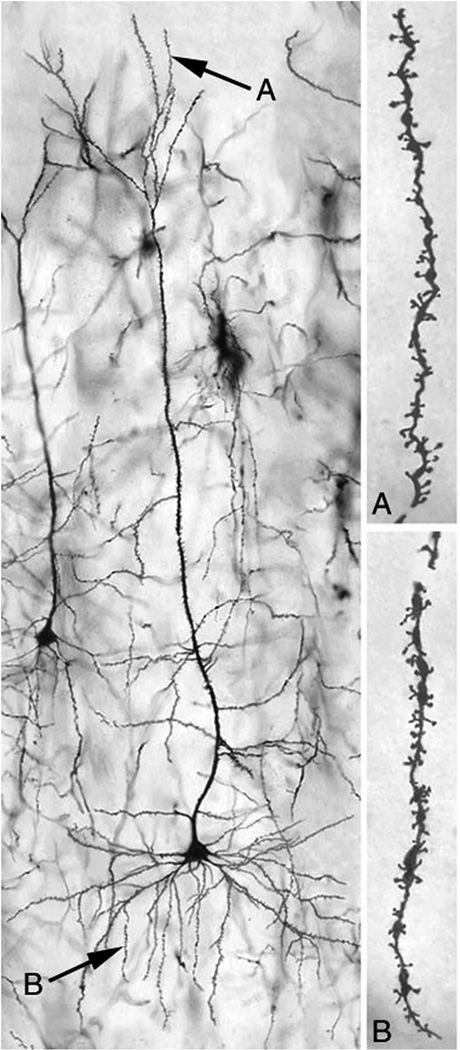
One challenge in all studies of brain-behavioural relationships is to identify a surrogate marker for the change in brain organization. Over the past 20 years one has chosen to use the morphology of individual neurons that are identified by using Golgi-stained tissue. Although this technique can be criticized as being one that belongs to the 19th century, it is found that the technique remains useful in the 21st century. Entire neurons are coated with a heavy metal (gold, silver or mercury) and multiple, functionally significant characteristics of the dendritic arbor can be quantitatively analysed (Figure 1). By knowing these measures, one can infer the relative number and distribution of synapses on neurons and their weighting and correlate this with behavioural change. The studies of Jacobs et al. [6] provide a good example. These researchers examined the dendritic morphology of pyramidal neurons in postmortem brains of people whose educational and employment history was known. In one study comparison of synapse numbers in the somatosensory finger representation of people with occupations requiring dextrous finger use (typists) vs those with other jobs (e.g. sales) showed that there were more synapses on the neurons in the finger regions from brains from typists. The study cannot tell why this correlation is present, but it tells that there is some relationship between experience and synaptic organization. The authors have used a similar logic in studies of the actions of perinatal experiences and treatments for brain injury.
Figure 1.
A neuron from the Cg 3 region of the medial prefrontal cortex of a rat.
Post-natal experiential determinants of prefrontal development
The simplest way to manipulate experience during development is to compare brain structure in animals socially living in standard laboratory caging to animals placed either in relatively impoverished environments with restricted social or sensory stimulation or so-called ‘enriched’ environments, which will be referred to as complex environments. Such studies in adults have identified a large range of cortical neural changes associated with complex housing. These include changes in cortical thickness, spine density, synapses per neuron, glial numbers and complexity and vascular arborization [7,8]. Curiously, however, these changes are not observed in the prefrontal cortex [9]. When animals are placed in complex environments for 30 or more days during the periadolescent period one might anticipate greater changes in prefrontal cortex, but there is only a small effect on dendritic branching, no effect on total dendritic length, in the basilar dendritic field of medial prefrontal neurons and no effect on orbital prefrontal dendrites [10]. Other cortical regions, such as parietal and occipital cortex, show clear increases in dendritic branching and length, although early complex housing has paradoxical effects on spine density: animals housed from weaning until adulthood show a decrease in spine density, whereas animals housed only during the periadolescent period (days 40–70) show no change in spine density [9,10].
The failure to find persistent effects of complex housing in the prefrontal cortex led one to look for transient effects by placing animals in the complex environments for only 4 days. The hypothesis was that perhaps the prefrontal cortex changes in response to novelty but once the novelty is gone, there is a regression to baseline. Indeed, adult rats with such treatment showed an increase in spine density in medial prefrontal cortex and a decrease in spine density in orbital cortex [11]. In contrast, rats with similar experience at 40–44 days (periadolescence) showed only an increase in spine density in orbital cortex. The general conclusion from both the transient and chronic complex housing studies is that the prefrontal cortex does not show much change in response to complex housing during development, even though sensory and motor cortical regions do.
The absence of an effect of global experience, namely complex housing, on the prefrontal cortex led one to consider the effects of more selective, but biologically significant, experiences (Table I). Bell et al. [12] raised weanling rats either with siblings or adults. The idea was that in the former case the animals would have multiple play partners whereas in the latter they would have conspecifics to live with but their opportunities for play behaviour would be limited. The results showed that the prefrontal cortex does respond to experience during development: the medial frontal neurons had more dendritic arbor in the rats raised with adults, whereas the orbital neurons had more arbor in the rats raised with siblings.
Table I.
Modification of prefrontal cortical development.
| Treatment | Result | Basic reference |
|---|---|---|
| Complex housing at weaning | No effect on dendritic length or spine density | [10] |
| Play behaviour | Sibling play increased OFC dendritic length and spine density; adult ’play’ increased mPFC dendritic length | [12] |
| Prenatal stress | Decreased dendritic length and spine density in OFC | [16] |
| Psychomotor stimulants | Increased denritic length and spine density in mPFC; no change in OFC | [18,52] |
| Antipsychotic drugs | Decreased dendritic length and spine density in both mPFC and OFC | [20] |
| Gonadal hormones | Longer mPFC dendritic length in males; longer OFC dendritic length in females | [2] |
One does not yet know if the prefrontal cortex will be altered by other forms of specific experiences, but one has begun to investigate this. It is known, for example, that tactile stimulation 45 min daily with a soft brush decreases dendritic length and spine density in parietal cortex and increases cortical thickness. These morphological changes are associated with enhanced motor and cognitive skills in adulthood [13]. It seems likely that the treatment decreases programmed cell death (apoptosis), thus resulting in more parietal neurons that have a simpler dendritic arborization than in animals with fewer neurons. Similar studies are now looking at the prefrontal regions.
Prenatal experiential determinants of prefrontal development
Although not well studied to date, it seems likely that pre-natal experiences may also influence pre-natal development. It has been shown, for example, that pre-natal complex housing (i.e. pregnant dams in complex environments) changes the development of the parietal cortex: as adults the offspring show increases in spine density and decreased dendritic length in the parietal cortex [14]. Although one does not yet know if such treatments alter prefrontal development, it is known that they do profoundly affect recovery from perinatal prefrontal lesions, as shall be seen below.
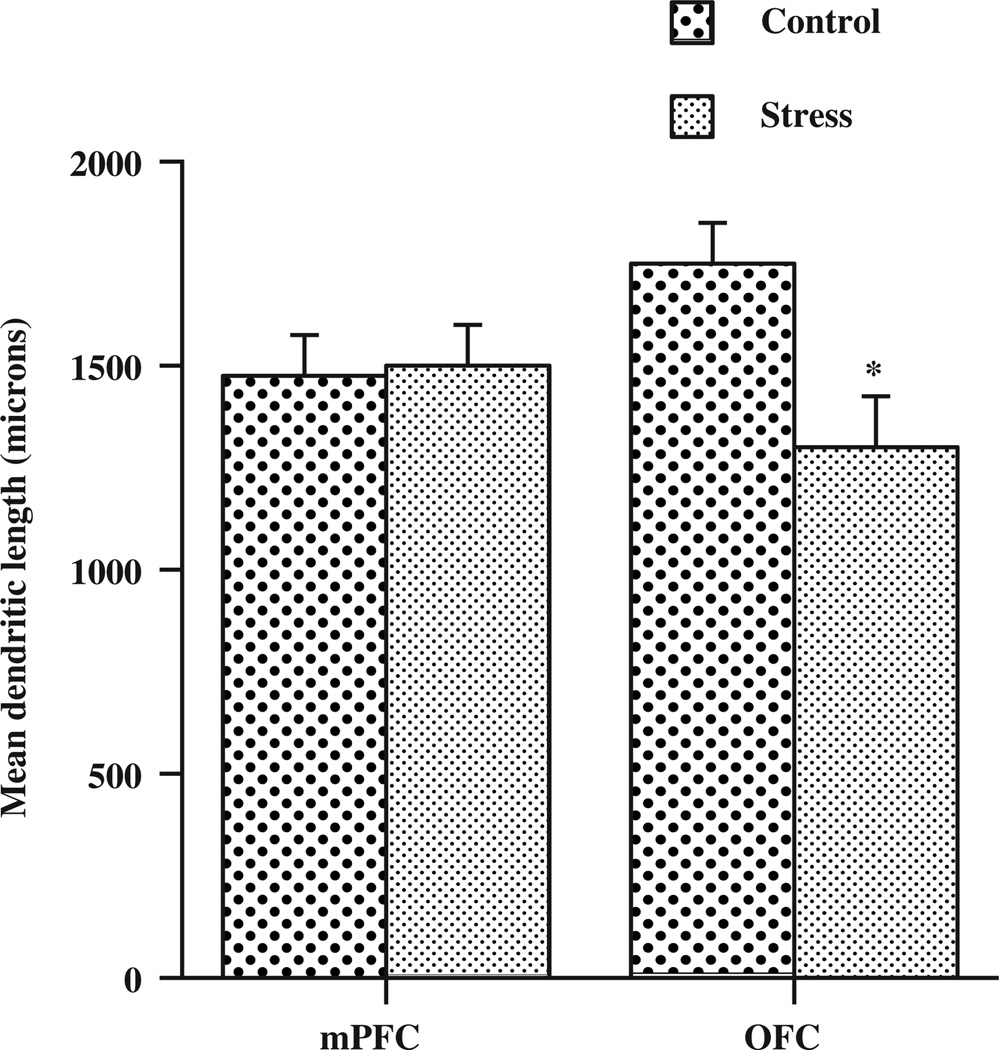
One pre-natal experience that does alter prefrontal development is stress. Stress in adulthood reduces dendritic length and spine density in medial prefrontal cortex and increases the same measures in orbital frontal cortex [15]. The authors have shown a different result during development, namely that pre-natal stress does not alter dendritic growth in medial prefrontal cortex but reduces dendritic length and spine density in orbital prefrontal regions (Figure 2) [16]. These anatomical effects are correlated with behavioural impairments in prefrontal-sensitive cognitive and motor tests.
Figure 2.
The effects of prenatal stress on dendritic length. *Differs significantly from control.
Post-natal drug effects on prefrontal development
Robinson and Kolb [3] found that exposure to psychomotor stimulants in adulthood produced large changes in the structure of cells in prefrontal cortex and nucleus accumbens. Specifically, whereas these drugs (amphetamine, cocaine, nicotine) produced increases in dendritic length and spine density in Cg3 and nucleus accumbens, there was either a decrease in these measures in AID or, in some cases, no change. They subsequently showed that virtually every class of psychoactive drugs also produces changes in prefrontal cortex and that the effects are consistently different in the two prefrontal regions. For example, morphine decreased dendritic length and spine density in Cg3 but increased these measures in AID. Given that the developing brain is often exposed to psychoactive drugs, either in utero or during post-natal development, this study asked what effects these drugs would have on cortical development.
In the first study, rats were given therapeutic doses (for ADHD) of amphetamine twice daily during post-natal days 22–34 and the brains of the animals were evaluated 2 weeks after the termination of drug administration [17]. This treatment produced an increase in dendritic length and branches of pyramidal neurons of the medial prefrontal cortex, but not in the nucleus accumbens. Orbital cortex was not available in this study.
The next study repeated the experiment using a comparable dose of methylphenidate (Ritalin) [18]. The brains of one group of rats were evaluated 1 week after drug termination, whereas the brains of a second group were studied in adulthood after the play and cognitive behaviour of the rats was examined.
Regardless of the age when the brains were collected there was an increase in dendritic length and spine density in medial prefrontal cortex, although there were differences in the details of the changes at the different ages. In contrast there was a decrease in dendritic length but no change in spine density in orbital cortex. These dendritic changes were associated with abnormal play behaviour in the drug-treated rats, as they displayed reduced play initiation compared to saline-treated playmates as well as impaired performance on a test of working memory. Psychomotor stimulants thus appear to alter the development of the prefrontal cortex and this is manifested in behavioural abnormalities on prefrontal-related behaviours later in life.
It was wondered if the early drug exposure was specifically altering behaviour or having a more general effect on cortical plasticity later in life. It had been shown previously that exposure to psychomotor stimulants in adulthood completely blocked the later effect of complex housing on neuronal morphology [19]. This experiment was repeated with rats given amphetamine during adolescence and the same result found: the prior exposure to a low dose of amphetamine blocked experience-dependent plasticity weeks later in adulthood. Taken together, these data suggest that early psychomotor stimulant exposure can have significant consequences for both behavioural and anatomical development of the prefrontal cortex. The authors are currently looking at the effects of two other stimulants to which children have a high risk of pre- and/or post-natal exposure: caffeine and nicotine.
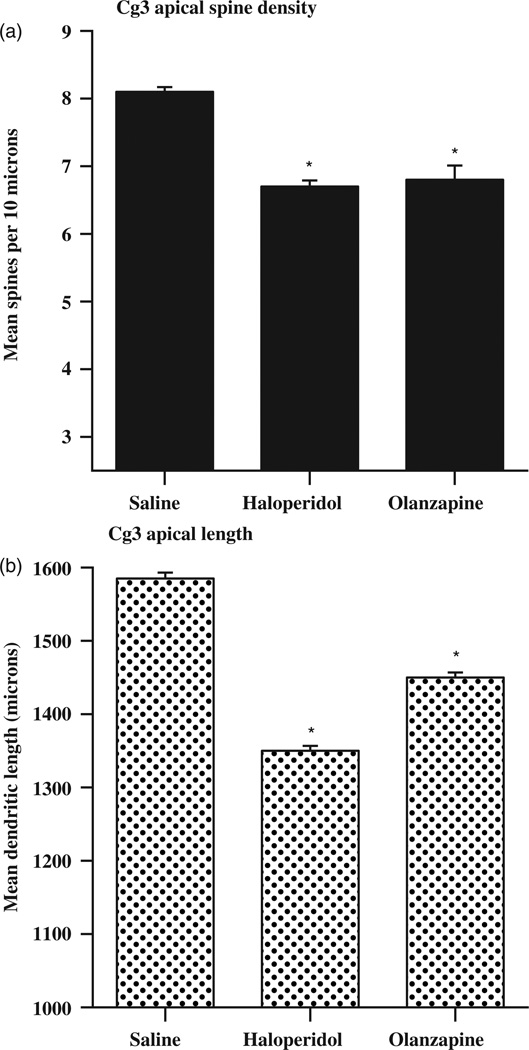
Children may also be exposed to prescription medications either in utero or post-natally. Three commonly prescribed classes of drugs are antipsychotics, antidepressants, and anxiolytics. All three have dramatic effects on cortical development. Frost et al. [20] analysed dendritic architecture in adult mice treated with paradigmatic typical- (haloperidol) or atypical (olanzapine) antipsychotic drugs at developmental stages corresponding to foetal (post-natal days 3–10) or foetal and early childhood (post-natal days 3–20) stages in humans. Both drugs produced reductions in dendritic length, dendritic branching complexity and spine density in both medial prefrontal and orbital cortex, as illustrated in Figure 3. It did not matter whether the drugs were given from post-natal day 3–10 or 3–20, the results were essentially the same, suggesting that the key time for morphological effects of the drugs might be in the earlier time period.
Figure 3.
Effects of antipsychotic drugs administered from post-natal day 3 to 10 on dendritic length.
Although one has not yet completed the analysis of prefrontal cortex neurons, one has examined the effect of fluoxetine (Prozac), a commonly prescribed antidepressant, and diazepam (Valium), an anxiolytic, given to pregnant rat dams beginning pre-natally and continuing until weaning [21]. It had already been found that this type of fluoxetine exposure produced smaller than normal brains. Thus, it was anticipated that there would be reductions in dendritic length and spine density and this was confirmed. There were reductions in dendritic branching complexity and length in pyramidal neurons in the parietal cortex of adult rats perinatally treated with fluoxetine but, to the authors’ surprise, diazepam had an opposite effect—there was an increase. Behavioural analysis of these rats showed cognitive deficits in the fluoxetine-treated rats, but an advantage in motor tasks for the diazepam-treated rats. Given that the behavioural tests used were sensitive to prefrontal functions, chronic changes were anticipated in the prefrontal cortex as well.
In sum, exposure to both prescription drugs and drugs of abuse has a profound effect on prefrontal development and prefrontal-related behaviours. These effects appear to be long lasting or permanent and can influence brain plasticity in adulthood.
Gonadal hormonal effects on prefrontal development
Goldstein et al. [22] did a comprehensive evaluation of the volume of 45 different brain regions from MRI scans of healthy adult subjects. There were sex differences in volume, relative to total cerebral volume, and this was especially true in prefrontal cortex: females had a relatively larger volume of dorsolateral prefrontal cortex, whereas males had a relatively larger volume of orbitofrontal cortex. This sexual dimorphism is correlated relatively highly to regional levels of sex steroid receptors during early life in laboratory animals. Kolb and Stewart [2] showed in rats that neurons in the mPFC had larger dendritic fields in males and that neurons in OFC had larger cells in females. These differences vanished when animals were gonadectomized at birth. It thus appears in both humans and laboratory animals that gonadal hormones alter prefrontal cortical development. This is particularly important when considering that the effects of other experiences such as exposure to complex housing or psychomotor stimulants are also sexually dimorphic. It seems likely that many other developmental experiences may differentially alter the female and male brains, although few studies have actually made this comparison.
The effects of early prefrontal injury
It was generally assumed up until the 1970s that the earlier brain injury occurred during development, the better the outcome would be, although this common wisdom came to be questioned [23,24]. Until 25 years ago, there were few studies of children or laboratory animals with prefrontal injuries. Since then, extensive studies of cats, rats and monkeys with prefrontal cortical injuries have shown a critical relationship between age at injury and functional outcome [25–29]. These results can be illustrated by the studies in rats with prefrontal lesions at different developmental ages. The key finding is that the functional outcome is always best after injury during the 2nd week of life [30]. A similar pattern of results can be seen in parallel studies of the effects of cortical lesions in kittens by Villablanca et al. [29], although because the rat and cat develop at different rates the precise ages are different in the two species. Specifically, damage in the first few weeks after birth in the cat is equivalent to damage in the second postnatal week in the rat and thus is associated with a relatively good outcome, whereas damage in the last few pre-natal weeks in the cat is equivalent to damage in the first post-natal week in the rat and is associated with a very poor functional outcome. Thus, birth date is irrelevant—it is the stage of neural development that is important. Similarly, monkeys with pre-natal injuries that are about the same developmental stage as the 7 day old rat show better outcome than with injuries later in development [25].
Studies of children with acquired brain injuries such as head trauma have similarly concluded that recovery depends upon the developmental stage at which the injury was sustained. Anderson et al. [31] have shown, for example, that the best outcome from brain injury during development is ~8–10 years of age and that very early injuries are associated with poor outcomes. It is difficult to compare the timelines in the laboratory animals and children for two reasons. First, the children have much more diffuse injuries that likely include a lot of white matter damage, whereas the laboratory animals have focal lesions that are largely restricted to gray matter. Secondly, there are very few studies of laboratory animals with injuries during the juvenile period (but see Kolb and Whishaw [32]). The former problem can be addressed by making more diffuse lesions in the lab animals, such as by hypoxia/ischemia, and preliminary results do show an age-related pattern of recovery as in the focal lesions (Williams and Kolb, unpublished observations). The latter problem can be studied by making lesions, focal or ischemic, at later time points. This work is in progress in the laboratories.
Brain development after early brain injury
Regardless of the time of injury to the prefrontal cortex, there is always a reduction in overall brain size and a reduction in cortical thickness relative to similar injuries later in life. Thus, brain size and cortical thickness are unlikely to underlie age at lesion-dependent behavioural outcomes, whereas changes in synaptic organization that are inevitably produced as a consequence of age at lesion-dependent alterations in dendritic architecture would be likely causes.
Golgi analyses of cortical neurons in rats with bilateral perinatal prefrontal lesions consistently show a general atrophy of dendritic arborization and a decrease in spine density across the cortical mantle [33]. In contrast, rats with cortical lesions produced on P10 show an increase in dendritic arborization and an increase in spine density throughout the cerebral cortex relative to control littermates. Thus, animals with the best functional outcomes show the greatest synaptic increases, whereas animals with the worst functional outcomes have a synaptic decrease relative to control animals.
Analysis of the brains of animals with unilateral medial prefrontal lesions shows a somewhat different pattern of results. There is atrophy of cortical pyramidal cells in the anterior portion of the hemisphere ipsilateral to the lesion, the atrophy being larger with earlier injuries, and little evidence of hypertrophy after injury at any age [34,35]. More interesting, however, there is an increase in cortical thickness in the contralateral medial prefrontal cortex, the effect being largest in animals with injuries ~day 10. Golgi analysis of the cells in this region shows them to have less dendritic arborization, the effect being largest in the day 10 operates. This paradoxical result may imply that there are more neurons in the contralteral cortex, but they are simpler in structure [36].
The changes in dendritic organizaton must be related to changes in cortical connectivity, but it is somewhat easier to identify the former than the latter changes. Nonetheless, there is large literature showing that rewiring in sensory systems after early injuries is correlated with both sparing of function [37,38] and abnormalities in function [24]. Parallel studies in rats and cats with unilateral motor cortex injuries have shown there is a major expansion of the ipsilateral corticospinal pathway from the undamaged hemisphere, which is correlated with partial recovery of skilled forelimb use [39–41]. It appears, however, that the anomalous corticospinal projections sometimes may be formed at a significant cost. For example, when comparing the effects of motor cortex lesions on postnatal days 4, 10 and 90 it was found that although it was only the youngest animals that showed the enhanced ipsilateral connections, it was the rats subjected to lesions on day 10 that showed the best functional outcomes. Furthermore, animals with lesions made on day 4 showed unexpected deficits on cognitive tasks (e.g. a spatial navigation task). It thus seems likely that the aberrant corticospinal pathway interfered with the normal functioning of cortical areas that would not ordinarily be involved in motor function. Similarly, when examining in adulthood the effects on cortical connectivity of medial prefrontal lesions made on days 1 or 10 a wide range of abnormal connections were found in the early operates but none in the day 10 animals. For example, there were anomalous thalamocortial connections in both the auditory and visual systems such that the lateral geniculate nucleus sent projections to the visual and auditory cortex and the medial geniculate nucleus sent connections to the auditory and visual cortex. Such abnormal connections may be the reason that the rat operated on on day 1 had the worst behavioural outcomes.
In sum, early prefrontal lesions have significant effects on the development of the rest of the brain and, like the behavioural effects, the spectrum of changes varies with the developmental stage at which the injury is sustained. The expectation thus has been that the effects of early perturbations that influence brain and behavioural development, including injury, pre-natal stress and drugs, will depend upon the developmental stage at which the brain is perturbed.
Rehabilitation after early prefrontal injury
Given that a wide range of experiences can alter cortical development, it is reasonable to expect that at least some of them will modulate the effects of early prefrontal injury, especially very early injury. Table II summarizes a range of different treatments that have proven effective in stimulating functional recovery and correlated dendritic change. Kolb and Gibb [13] showed, for example, that tactile stimulation for 15 min, three times per day for 2 weeks after medial prefrontal lesions made on day 3 stimulated functional recovery in adulthood and this was correlated with increased spine density in the sensorimotor cortex adjacent to the injury. One other effect of the tactile stimulation was an increase in Fibroblast Factor-2 (FGF-2) expression in skin and cerebral cortex [14]. Subsequently, FGF-2 was administered subcutaneously and increased spine density was found that was correlated with functional recovery [42]. A subsequent study gave FGF-2 to rats with motor cortex lesions on day 10 and not only was facilitation of functional recovery found but also that the FGF-2 stimulated the genesis of new neurons that migrated to the lesion site and subsequently made connections with the spinal cord [43,44]. It is the authors’ working hypothesis that experiential treatments modulate cerebral development and recovery from cortical injury at least partly by stimulating the production of FGF-2, which acts to facilitate neurogenesis and synaptogenesis.
Table II.
Modification of the effects of early medial prefrontal cortical injury by post-injury treatments.
| Treatment | Result | Basic reference |
|---|---|---|
| A. Experiential modification | ||
| Complex housing at weaning | Enhanced recovery after P1–7 injury | [53] |
| Post-injury tactile stimulation | Enhanced recovery after P3–4 injury | [14] |
| B. Neurotrophic modification | ||
| Post-injury FGF-2 | Functional recovery after P4 injury; dendritic growth | [42] |
| C. Diet modification | ||
| High choline diet after P4 lesion | Stimulated recovery; enhanced dendritic growth | [54] |
| Vitamin supplement | Stimulated recovery; enhanced dendritic growth | [51] |
Two other treatments warrant some mention here. It is known that dietary modification can alter cortical development. For example, dietary choline supplementation during the perinatal period produces a variety of changes in both behaviour and brain [45], including enhanced spatial memory [46,47] and increased levels of nerve growth factor (NGF) in hippocampus and neocortex [48]. Finally, pre-natal choline supplementation also protects against the effects of pre-natal alcohol exposure on memory [49].
It was hypothesized that increases in plasma choline and the subsequent storage of choline as phosphatidylcholine in mature cholinergic neurons augment the available supply of choline for acetylcholine synthesis, which increases NGF synthesis. It has been shown elsewhere that NGF stimulates recovery from cortical injury in adult rats [50]; thus choline supplementation’ enhancement of NGF might promote increased dendritic arborization, which could inhibit the dendritic atrophy associated with perinatal cortical injuries.
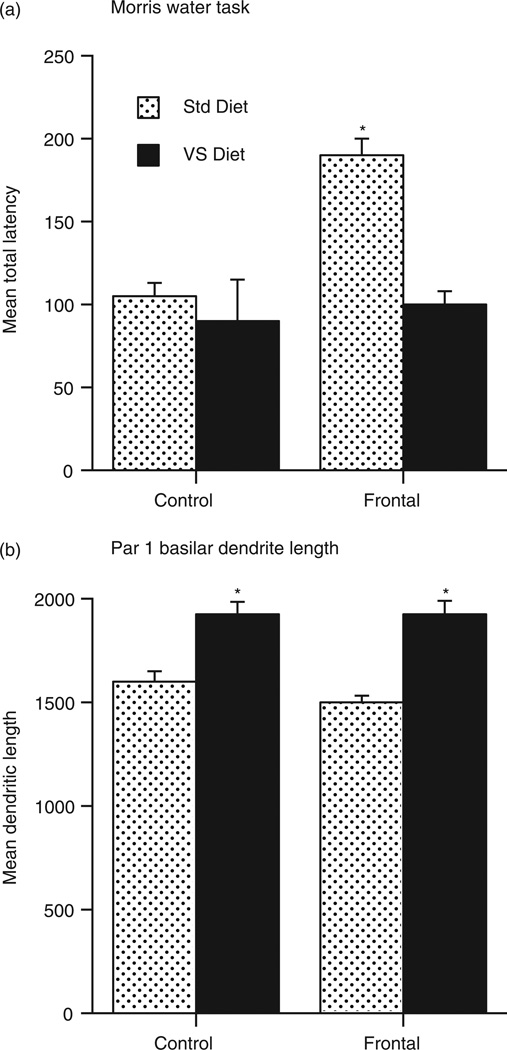
The results show that choline supplementation beginning pre-natally via drinking water given to pregnant dams and continuing until weaning dramatically facilitates recovery from medial frontal lesions and is correlated with increased dendritic arborization in the perilesional sensorimotor cortex [16]. This experiment was subsequently repeated using a broad spectrum concoction of vitamins and minerals (including choline) that was added to the feed of the pregnant dam and continued until weaning. Even more impressive behavioural and anatomical changes were found, as illustrated in Figure 4 [51].
Figure 4.
Effects of vitamin supplements on recovery from medial prefrontal lesions on post-natal day 3 in rats. (a) Performance on the Morris water task. (b) Dendritic length in perilesional cortex.
Conclusions
This study has summarized plastic changes in the development of the prefrontal cortex in response to a range of early life experiences as well as the remarkable capacity of the developing brain to recover from injury either spontaneously or in response to rehabilitative treatments. A key question that remains unanswered, however, is how this happens. At this point, one’s understanding of how morphological changes are produced is only in its infancy. It seems likely that the important molecular events leading to the neuroplastic changes will include changes in gene expression, perhaps mediated by epigenetic mechanisms. It seems unlikely that a single change in gene expression can tie together the effects of environmental experiences, psychoactive drugs, play, diet, stress and so on. There is preliminary evidence that perinatal experiences can decrease gene methylation in the cortex, but one is still ignorant as to how the gene methylation might be translated into changes in spine density. This is clearly grist for future studies.
Acknowledgements
This research is supported by Natural Science and Engineering Research Council of Canada grants to RG and BK, a Canadian Institutes of Health Research Grant to BK and a National Institute of Health grant to DO. The authors gratefully acknowledge the histological help from Dawn Danka, Cathy Carroll and the late Grazyna Gorny.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Uylings H, Groenewegen H, Kolb B. Does the rat have a prefrontal cortex? Behavioural Brain Research. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 2.Kolb B, Stewart J. Sex-related differences in dendritic branching of cells in the prefrontal cortex of rats. Journal of Neuroendocrinology. 1991;3:95–99. doi: 10.1111/j.1365-2826.1991.tb00245.x. [DOI] [PubMed] [Google Scholar]
- 3.Robinson TE, Kolb B. Structural plasticity associated with drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 4.Brown, Kolb 2000 [Google Scholar]
- 5.Gonzalez, et al. 2004 [Google Scholar]
- 6.Jacobs B, Schall M, Scheibel AB. A quantitative dendritic analysis of Wernicke’s area in humans: II. Gender, hemispheric, and environmental factors. Journal of Comparative Neurology. 1993;327:97–111. doi: 10.1002/cne.903270108. [DOI] [PubMed] [Google Scholar]
- 7.Greenough WT, Chang FF. Plasticity of synapse structure and pattern in the cerebral cortex. In: Peters A, Jones EG, editors. Cerebral cortex. Vol. 7. New York: Plenum Press; 1989. pp. 391–440. [Google Scholar]
- 8.Siervaag AM, Greenough WT. A multivariate statistical summary of synaptic plasticity meaures in rats exposed to complex, social, and individual environments. Brain Research. 1988;441:386–392. doi: 10.1016/0006-8993(88)91420-5. [DOI] [PubMed] [Google Scholar]
- 9.Kolb B, Gibb R, Gorny G. Experience-dependent changes in dendritic arbor and spine density in neocortex vary with age and sex. Neurobiology of Learning and Memory. 2003;79:1–10. doi: 10.1016/s1074-7427(02)00021-7. [DOI] [PubMed] [Google Scholar]
- 10.Comeau, Kolb 2008 [Google Scholar]
- 11.Comeau WL, McDonald R, Kolb B. Learning-induced alterations in prefrontal cortical circuitry. Manuscript in submission. 2009 doi: 10.1016/j.bbr.2010.04.033. [DOI] [PubMed] [Google Scholar]
- 12.Bell H, Pellis S, Kolb B. Number of social partners and juvenile peer play experience affect brain development in orbital and medial prefrontal cortices in rats. 2009 doi: 10.1016/j.bbr.2009.09.029. Manuscript in submission. [DOI] [PubMed] [Google Scholar]
- 13.Kolb B, Gibb R. Tactile stimulation after posterior parietal cortical injury in infant rats stimulates functional recovery and altered cortical morphology. 2009 doi: 10.1016/j.bbr.2010.04.024. Manuscript in submission. [DOI] [PubMed] [Google Scholar]
- 14.Gibb R, Kolb B. Tactile-stimulation that facilitates functional recovery after perinatal cortical injury is mediated by FGF-2. 2009 Manuscript in submission. [Google Scholar]
- 15.Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. Journal of Neuroscience. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halliwell C, Tees RC, Kolb B. Neonatal dietary choline supplementation facilitates functional recovery and morphological change after neonatal frontal or parietal lesions in rats. 2009 Manuscript in submission. [Google Scholar]
- 17.Diaz Heijtz R, Kolb B, Forssberg H. Can a therapeutic dose of amphetamine during pre-adolescence modify the pattern of synaptic organization in the brain? European Journal of Neuroscience. 2003;18:3394–3399. doi: 10.1046/j.0953-816x.2003.03067.x. [DOI] [PubMed] [Google Scholar]
- 18.Comeau, Diaz Heijtz, Kolb 2009 [Google Scholar]
- 19.Kolb B, Gorny G, Li Y, Samaha AN, Robinson TE. Amphetamine or cocaine limits the ability of later experience to promote structural plasticity in the neocortex and nucleus accumbens. Proceedings of the National Academy of Sciences (USA) 2003;100:10523–10528. doi: 10.1073/pnas.1834271100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frost DO, Cerceo S, Carroll C, Kolb B. Early exposure to haloperidol or olanzapine induces long-term alterations of dendritic form. 2009 doi: 10.1002/syn.20715. Manuscript in submission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolb B, Gibb R, Pearce S, Tanguay R. Prenatal exposure to prescription medication alters recovery following early brain injury in rats. Society for Neuroscience Abstracts. 2008 [Google Scholar]
- 22.Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS, Jr, Faraone SV, Tsuang MT. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cerebral Cortex. 2001;11:490–499. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- 23.Goldman 1974 [Google Scholar]
- 24.Schneider GD. Is it really better to have your brain lesion early? A revision of the ‘Kennard principle’. Neuropsychologia. 1979;17:557–583. doi: 10.1016/0028-3932(79)90033-2. [DOI] [PubMed] [Google Scholar]
- 25.Goldman PS, Galkin TW. Prenatal removal of frontal association cortex in the fetal rhesus monkey: Anatomical and functional consequences in postnatal life. Brain Research. 1978;152:451–485. doi: 10.1016/0006-8993(78)91103-4. [DOI] [PubMed] [Google Scholar]
- 26.Kolb B. Recovery from early cortical damage in rats. I. Differential behavioral and anatomical effects of frontal lesions at different ages of neural maturation. Behavioural Brain Research. 1987;25:205–220. doi: 10.1016/0166-4328(87)90069-6. [DOI] [PubMed] [Google Scholar]
- 27.Kolb, et al. 1993 [Google Scholar]
- 28.Schmanke TD, Villablanca JR. A critical maturational period of reduced brain vulnerability to injury. A study of cerebral glucose metabolism in cats. Developmental Brain Research. 2001;26:127–141. doi: 10.1016/s0165-3806(01)00248-6. [DOI] [PubMed] [Google Scholar]
- 29.Villablanca JR, Hovda DA, Jackson GF, Infante C. Neurological and behavioral effects of a unilateral frontal cortical lesion in fetal kittens: II. Visual system tests, and proposing a ‘critical period’ for lesion effects. Behavioural Brain Research. 1993;57:79–92. doi: 10.1016/0166-4328(93)90063-v. [DOI] [PubMed] [Google Scholar]
- 30.Kolb B. Brain plasticity and behavior. Mahwah, NJ: Erlbaum; 1995. [Google Scholar]
- 31.Anderson, et al. 2009 [Google Scholar]
- 32.Kolb B, Whishaw IQ. Neonatal frontal lesions in the rat: Sparing of learned but not species-typical behavior in the presence of reduced brain weight and cortical thickness. Journal of Comparative and Physiological Psychology. 1981;95:863–879. doi: 10.1037/h0077849. [DOI] [PubMed] [Google Scholar]
- 33.Kolb B, Gibb R, van der Kooy D. Neonatal frontal cortical lesions in rats alter cortical structure and connectivity. Brain Research. 1994;645:85–97. doi: 10.1016/0006-8993(94)91641-1. [DOI] [PubMed] [Google Scholar]
- 34.Kolb, Gibb, van der Kooy 1995 [Google Scholar]
- 35.Kolb B, Cioe J, Whishaw IQ. Is there an optimal age for recovery from unilateral motor cortex lesions? Behavioural and anatomical sequelae of unilateral motor cortex lesions in rats on postnatal days 1, 10, and in adulthood. Restorative Neurology and Neuroscience. 2000;17:61–70. [PubMed] [Google Scholar]
- 36.Sherren, Kolb in preparation. [Google Scholar]
- 37.Frost DO, Boire D, Gingras G, Ptito M. Surgically created neural pathways mediate visual pattern discrimination. Proceedings of the National Academy of Sciences (USA) 2000;97:11068–11073. doi: 10.1073/pnas.190179997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Payne BR, Lomber S. Reconstructing functional systems afer lesions of the cerebral cortex. Nature Reviews, Neuroscience. 2001;2:911–919. doi: 10.1038/35104085. [DOI] [PubMed] [Google Scholar]
- 39.Castro A. Plasticity in the motor system. In: Kolb B, Tees R, editors. Cerebral cortex of the rat. Cambridge, MA: MIT Press; 1990. pp. 563–588. [Google Scholar]
- 40.Whishaw IQ, Kolb B. Sparing of skilled forelimb reaching and corticospinal projections after neonatal motor cortex removal or hemidecortication in the rat: Support for the Kennard Doctrine. Brain Research. 1988;451:97–114. doi: 10.1016/0006-8993(88)90753-6. [DOI] [PubMed] [Google Scholar]
- 41.Villablanca JR, Gomez-Pinilla F. Novel crossed corticothalamic projections after neonatal cerebral hemispherectomy. A quantitative autoradiography study in cats. Brain Research. 1987;410:2119–2231. doi: 10.1016/0006-8993(87)90319-2. [DOI] [PubMed] [Google Scholar]
- 42.Comeau W, Hastings E, Kolb B. Differential effect of pre and postnatal FGF-2 following medial prefrontal cortical injury. Behavioural Brain Research. 2007;180:18–27. doi: 10.1016/j.bbr.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 43.Monfils M-H, Driscoll I, Kamitakahara H, Wilson B, Flynn C, Teskey GC, Kleim JA, Kolb B. FGF-2-induced cell proliferation stimulates anatomical, neurophysiological, and functional recovery from neonatal motor cortex injury. European Journal of Neuroscience. 2006;24:739–749. doi: 10.1111/j.1460-9568.2006.04939.x. [DOI] [PubMed] [Google Scholar]
- 44.Monfils M-H, Kolb B, Faoud K. FGF-2 promotes restoration of functional corticospinal neurons after neonatal motor cortex injury. Experimental Brain Research. 2008;185:453–460. doi: 10.1007/s00221-007-1172-0. [DOI] [PubMed] [Google Scholar]
- 45.Meck WH, Williams CL. Metabolic imprinting of choline by its availability during gestation: Implications for memory and attentional processing across the lifespan. Neuroscience and Biobehavioral Reviews. 2003;27:385–399. doi: 10.1016/s0149-7634(03)00069-1. [DOI] [PubMed] [Google Scholar]
- 46.Meck WH, Smith RA, Williams CL. Organizational changes in cholinergic activity and enhanced visuospatial memory as a function of choline administered prenatally or postnatally or both. Behavioral Neuroscience. 1988;103:1234–1241. doi: 10.1037//0735-7044.103.6.1234. [DOI] [PubMed] [Google Scholar]
- 47.Tees RC, Mohammadi E. The effects of neonatal choline dietary supplementation on adult spatial and configural memory in rats. Developmental Psychobiology. 1999;35:226–240. [PubMed] [Google Scholar]
- 48.Sandstrom MJ, Loy R, Williams CL. Prentaal choline supplementation increases NGF levels in hippocampus and frontal cortex of young and adult rats. Brain Research. 2002;947:9–16. doi: 10.1016/s0006-8993(02)02900-1. [DOI] [PubMed] [Google Scholar]
- 49.Thomas, et al. 2000 [Google Scholar]
- 50.Kolb B, Gorny G, Cote S, Ribeiro-da-Silva, Cuello AC. Nerve growth factor stimulates growth of cortical pyramidal neurons in young adult rats. Brain Research. 1997;751:289–294. doi: 10.1016/s0006-8993(96)01410-2. [DOI] [PubMed] [Google Scholar]
- 51.Halliwell C, Kolb B. Vitamin and mineral supplementation facilitates functional recovery and morphological change after neonatal medial frontal lesions in rats. 2009 Manuscript in submission. [Google Scholar]
- 52.Heijtz Diaz, et al. 2003 [Google Scholar]
- 53.Kolb B, Elliott W. Recovery from early cortical damage in rats. II. Effects of experience on anatomy and behavior following frontal lesions at 1 or 5 days of age. Behavioural Brain Research. 1987;26:47–56. doi: 10.1016/0166-4328(87)90015-5. [DOI] [PubMed] [Google Scholar]
- 54.Halliwell CI, Checknita D, Kolb B, Gibb R. The effects of vitamin and mineral supplementation on recovery from early cortical injury at postnatal day 10 in prenatally stressed rats. Society for Neuroscience Abstracts. 349:1. [Google Scholar]
- 55.Comeau WL, Butt A, Hess S, Diaz Heijtz R, Kolb B. Enduring effects of early methylphenidate use in rats: Anatomical correlates of modified behavior. 2008 Manuscript in submission. [Google Scholar]
- 56.Comeau W, Gibb R, Hastings E, Cioe J, Kolb B. Therapeutic effects of complex rearing or bFGF after perinatal frontal lesions. Developmental Psychobiology. 2008;50:134–146. doi: 10.1002/dev.20253. [DOI] [PubMed] [Google Scholar]
- 57.Gibb R, Gonzalez CLR, Kolb B. Complex environmental experience during pregnancy facilitates recovery from perinatal cortical injury in the offspring. 2009 Manuscript in submission. [Google Scholar]
- 58.Kolb B, Gibb R, van der Kooy D. Neonatal hemidecortication alters cortical and striatal structure and connectivity. Journal of Comparative Neurology. 1992;322:311–324. doi: 10.1002/cne.903220303. [DOI] [PubMed] [Google Scholar]
- 59.Kolb B, Gorny G, Sonderpalm A, Robinson TE. Environmental complexity has different effects on the structure of neurons in the prefrontal cortex versus the parietal cortex or nucleus accumbens. Synapse. 2003;48:149–153. doi: 10.1002/syn.10196. [DOI] [PubMed] [Google Scholar]
- 60.Kolb B, Petrie B, Cioe J. Recovery from early cortical damage in rats. VII. Comparison of the behavioural and anatomical effects of medial prefrontal lesions at different ages of neural maturation. Behavioural Brain Research. 1996;79:1–13. doi: 10.1016/0166-4328(95)00254-5. [DOI] [PubMed] [Google Scholar]
- 61.Payne BR, Cornwell P. System-wide repercussions of damage of immature visual cortex. Trends in Neuroscience. 1994;17:126–130. doi: 10.1016/0166-2236(94)90122-8. [DOI] [PubMed] [Google Scholar]
- 62.Ryah SH, Williams JK, Thomas JD. Choline supplementation attenuates learning deficits assoiciated with prenatal alcohol exposure in the rat: Effects of varying the timing of choline administration. Brain Research. 2008;1237:91–100. doi: 10.1016/j.brainres.2008.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]