Abstract
Purpose of review
Children and adults have two major types of adipocytes, which represent the predominant cells in white adipose tissue (WAT), which is involved in energy-storage, and brown adipose tissue (BAT) which is responsible for thermogenesis and energy expenditure. This review discusses BAT physiology and evaluates the recent discoveries regarding its development, identification, and function.
Recent findings
This past year multiple independent research teams using combined positron-emission tomography and computed tomography (PET/CT) imaging, immunohistochemistry, and gene and protein expression have proven conclusively that adult humans have functional BAT. In parallel, basic studies defined BAT origins, its transcriptional regulation, and the role of hormones in BAT growth and activation. These methods have begun to be applied to children to understand pediatric BAT anatomy and physiology.
Summary
Adult humans have functional BAT, which plays a role in energy balance. BAT is more prevalent in children, suggesting an even greater physiological role than that seen in adults. Future studies will identify safe ways to quantify BAT mass and activity and which interventions might be used to increase BAT mass and/or thermogenesis to treat obesity.
Key words/phrases: brown adipose tissue, energy balance, UCP-1, PET/CT
1. Introduction
The current obesity pandemic is a having a disproportionately large effect on the pediatric population. In the US, the prevalence of overweight children and adolescents has tripled in the past four decades to over 16%, with even higher percentages in ethnic minorities [1]. Coupled with this is the alarming growth in obesity-related diseases rarely seen previously in children, including type 2 diabetes, non-alcoholic fatty liver disease and sleep apnea [2]. We now face the possibility in the coming century that this disease may actually lower life-expectancy. The situation has become so dire that in February 2010, President Obama established a task force on childhood obesity to “develop an interagency action plan to solve the problem of obesity among our Nation’s children within a generation” [3].
2. The Evolving Understanding of Adipose Tissue
Obesity develops when energy intake exceeds energy expenditure. Classically, adipose tissue has been viewed as playing a passive role as a storage depot for excess calories. This view has changed dramatically over the past two decades, as adipose tissue is now known to be an active endocrine organ, releasing free fatty acids (FFA) and adipokines such as leptin, adiponectin, TNFα, interleukin-6 (IL-6), and retinol binding protein-4 (RBP-4), all of which can act on other tissues, including the brain, liver, and muscle to regulate food intake, energy balance, insulin sensitivity, and even reproduction [4;5].
It is not just the size but the distribution of adipose tissue that matters. An increase in intra-abdominal/visceral fat is associated with a high risk of metabolic disease, while excess subcutaneous fat in the thighs and hips exerts little or no risk. Recent evidence indicates that these functional differences may be inherent to the different types of adipocytes residing in the various depots [6]. In addition to white adipose tissue (WAT), mammals also have brown adipose tissue (BAT), which burns energy for thermogenesis. In the last two to three years we have witnessed a revolution in our understanding of BAT from the molecular to the clinical level.
3. The Nature of the Whole-body Adipose Tissue Depot
Our knowledge of fat, especially BAT, has been influenced significantly by studies in rodent models. There, the macroscopic view suggests a simple dichotomy, with multiple WAT depots throughout the body and one BAT depot situated between the scapulae. However, small collections of brown adipocytes have been identified intermixed with white adipocytes and between skeletal muscle bundles, in some obesity-resistant strains of mice suggesting a role for BAT in the control of body weight [7;8]. Close histological analysis has shown that many adipose tissue depots are a mix of white and brown adipocytes where the balance is influenced by age, strain/genetics, environment, nutrition, and drugs [9].
In contrast to muscle, which generates heat by reactions within its cytoplasm, BAT thermogenesis takes place in its numerous, densely-packed mitochondria containing the BAT-specific inner membrane protein uncoupling protein 1 (UCP-1). At the molecular level, norepinephrine triggers a signaling cascade that activates UCP-1, which then uncouples aerobic respiration by dissipating the intermembrane proton-motive force, creating a futile cycling of ions, thus generating heat instead of ATP [9]. In a cold-acclimatized rat, oxygen consumption by BAT, which represents <2% of body weight, is approximately twice the whole-body basal metabolic rate under thermoneutral conditions [10]. UCP-1 is unique to brown adipocytes and is necessary for BAT thermogenesis [11]. However, brown adipocytes can also be distinguished from white adipocytes at the molecular level by their higher levels of expression of type 2 iodothyronine deiodinase (DIO2), the transcription co-regulators PRDM16 and PGC-1α, and Cidea, a regulator of lipolysis [6].
In rodents, BAT generates heat for two principal reasons: 1) to protect against cold exposure via non-shivering thermogenesis and 2) to burn off excess calories and reduces adiposity [12;13]. Thus, BAT has a central role in protecting mice from diet-induced obesity, and ablation of BAT reduces energy expenditure and increases obesity in response to high-fat diets [14]. In adult humans, the potential contribution of BAT to metabolism is debated, but it has been estimated that as little as 50 g of BAT could utilize up to 20% of basal caloric needs if maximally stimulated [15].
4. Anatomical Distribution of Pediatric BAT
BAT has been known to exist in children for over 50 years from two pivotal autopsy studies: one [16], reviewing over 400 necropsies on infants from 29 weeks of gestation to 4 weeks postpartum and the other of 52 humans ages 0 to over 80 years [17]. These revealed that the distribution of BAT in infants is largely similar to that of adults. The primary depots surround the cervical muscles and vasculature, particularly the carotid sheath, and extend below the clavicles into the axillae and sometimes the thorax following the great vessels, esophagus and trachea. In the abdomen, discrete masses of BAT accompany the aorta and lie near posterior structures such as the pancreas, autonomic ganglia, kidneys, and adrenal glands. There also is an interscapular depot, thought to correspond to the major depot in rodents, which lies in a diamond-shaped sheet separated from the subcutaneous WAT by a discontinuous fibrous layer [16].
Immature brown adipocytes can be seen as early as 29 weeks’ gestation. Growth during the third trimester appears to be by the expansion of cell volume from mitochondriogenesis. Postpartum, cells continue to grow and can double in size by week 5. During this period, cellular lipid content declines, particularly in premature infants, suggesting a role for BAT in newborns in thermogenesis. [16]. Human newborns have an estimated 30 g of BAT, representing 1% of body weight [18]. As children leave the first decade of life, the wide distribution of BAT declines such that the more peripherally situated areas, such as interscapular and abdominal wall depots disappear. However, the deeper depots are maintained even into the ninth decade [17]. Histologically BAT in the mediastinal and para-aortic depots in infants is more homogeneous tissue, intermixed with few white adipocytes, whereas brown and white adipocytes are intermixed in other sites, including the interscapular, cervical, axillary, and perirenal depots. Brown adipocytes in infants have a rich capillary and neuronal supply, suggesting that both pathways may be involved in BAT activation [16].
While the data support a role for BAT in children in non-shivering thermogenesis, the relative contribution of BAT in various depots in children is unclear. Many studies suggest that interscapular BAT is the major source of thermogenesis, implying similarities to rodent physiology. However, there was little direct evidence for this except of increases in skin-surface temperature which overlie the interscapular region [19;20]. A similar assumption was made in the adult, but biopsies of these warmer sites show no BAT, suggesting that this temperature gradient is due to a lower insulating fat thickness and increased blood flow, rather than BAT [21]. Indeed, this and similar studies led to the conclusion among many that there are no significant amounts of functional BAT anywhere in human adults – a conclusion which has been clearly disproven by recent PET/CT studies discussed below.
5. BAT physiology in children
Given its wide and scattered distribution, measuring the contribution of BAT to energy expenditure in children is a theoretical and practical challenge that has been addressed differently over the past decades. Initially, investigators relied on histological appearance to assess the functional state of brown adipocytes with the assumption the less lipid, the more active. This approach was supported by findings in children exposed to cold [19] and rodent studies [22;23]. It also appears that as humans age, BAT becomes less functional since lipid accumulates to the point where BAT becomes indistinguishable from WAT (reviewed in [24]). The discovery of UCP-1 in 1976 [25] followed by the development of sensitive ELISAs and RIAs to UCP-1 [26][27] permitted a more accurate assessment of BAT content and indicated that older infants and young children had considerably more BAT than either pre-term infants, stillborns, or human adults [28]. In newborns BAT can contribute significantly to metabolism. In a study of children 6 to 30 hours old, cooling to 25 – 26 °C for 20 minutes led to a doubling of oxygen consumption, thought to be due to BAT [20].
BAT functionality is also dependent on nutritional status. In a study of malnourished Jamaican children aged 4–16 months, there was an impaired response to cold exposure as measured by oxygen consumption, rectal temperature, and skin surface temperature overlying the interscapular region compared to when they recovered [19]. In a second part of that study, the cervical and interscapular BAT in a different set of malnourished children aged 1–24 months showed lipid depletion, suggesting that normal caloric intake is necessary to maintain proper BAT function. Also of note is that there was no shivering seen in these children, making it likely that BAT is a major form of thermogenesis until at least the second year of life [19].
One circumstance that has led to a greater understanding of the potential contribution of BAT to thermogenesis in children has been the search for a connection between hyperactive BAT and “cot death,” now referred to as sudden infant death syndrome (SIDS). One case report described two infants, aged 5 months, who were found with core body temperatures above 40°C. At autopsy BAT from the cervical, axiallary, interscapular and perirenal depots showed normal amounts of UCP-1, cytochrome oxidase, and GDP binding activity compared to the BAT in other species [29]. Based on an oxygen consumption of maximally-stimulated murine BAT of 1.5 mL O2/minute, and assuming 30 g of BAT in human neonates, the maximal heat output of neonatal BAT could be 250 cal/min, which is twice the normal basal metabolic rate and could raise core temperature 5–6 °C/h [29]. Though suggestive of a connection between malfunctioning BAT and SIDS, a larger study of 88 infants who died of SIDS showed no differences in periadrenal BAT when compared to 62 children who died of other causes. However, these authors extended previous studies by showing that BAT activity, as defined by histological appearance, increased after birth, peaked at 4–8 weeks, and then slowly declined into adolescence [30]. They also described the heterogeneous nature of periadrenal fat, which contained a range of brown and white adipocytes [30]. Unfortunately no studies have true measures of BAT metabolic activity.
Given that children have more BAT than adults, one question is whether this difference is manifested physiologically, for example by greater resistance to cooling, such that children could tolerate cold exposure better than adults. One measure of this might be the cold pressor test (CPT) in which a hand or forearm is placed in water at 10 °C. This produces slowly increasing pain of mild to moderate intensity, so the primary outcome measure is the duration for which the individual can maintain the limb in the cold water. The CPT has been used in many studies of pain, autonomic reactivity, and hormonal stress responses (reviewed in [31]). Moreover, it is now known that this kind of cold exposure activates human BAT [32;33]. Studies to date have found that cold pain intensity ratings are predicted by sex and gender [34] and distractability [35], but there is little information comparing children to adults, integrating this response to measures of BAT activity. Other quantitative measures of cold tolerance or intolerance are lacking at present.
6. A Connection Between BAT and Obesity?
The central role BAT plays in normal metabolism suggests that mutations in certain genes, especially BAT-specific UCP-1, could have significant effects on whole-body energy homeostasis. Though sequence variations have been found in the human UCP-1 gene, to date no profound effect has been identified. In a study of French Canadians, a rare allele of a nucleotide polymorphism was more frequent among people who gained more weight over a 12-year interval [36]. A more extensive study of the human UCP-1 coding region uncovered five substitutions that altered the UCP-1 protein’s primary amino acid sequence. The frequencies of the mutations were compared among Danish males but were not associated with obesity [37]. A -3826 A → G nucleotide variant in the UCP-1 promoter has been associated with a reduction in the thermic effect of food in spite of increased sympathetic output in a cohort of Japanese boys aged 8–11y [38]. Other physiological studies have suggested that genetic differences in thermogenesis may play a role in predisposition toward obesity. For example, children of obese parents have, on average, a 20% lower energy requirement compared with those from normal-weight parents [39]. These and similar data suggest that reduced diet-induced thermogenesis from BAT, either through mutation or reduced function, may contribute to why obesity-prone children are found to have lower energy requirements and be in positive-energy balance on a “normal” food intake [40]. These findings also imply that at least in children, BAT may play a role in diet-induced thermogenesis, and impaired UCP-1 function may contribute to pediatric obesity.
7. Recent Advances in BAT Biology
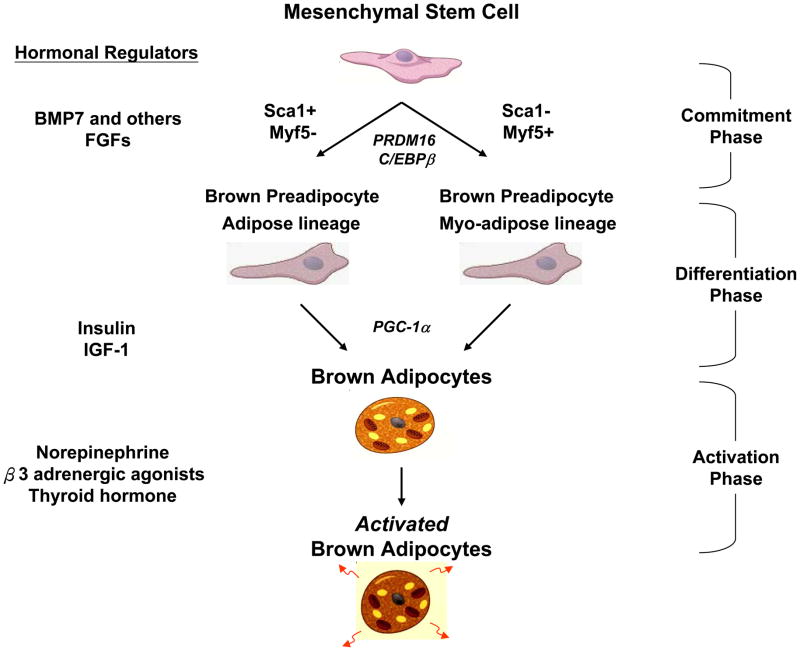
The past several years have seen tremendous progress in understanding the origin of brown adipocytes and the regulation of its development. Brown adipocytes are derived from multipotent mesenchymal stems cells [6], but different anatomical locations may have distinct lineages. For example, in the mouse, interscapular and perirenal BAT develop from myf5-expressing myogenic precursors [41;42], while systemic brown fat cells seen within white fat tissue and interspersed within muscle appear to derive from myf5 negative cells and have different features, including greater sensitivity to β3-adrenergic stimulation and cold exposure [7;8;42–44]. A current scheme for brown adipogenesis is shown in Figure 1.
Figure 1. The Brown Adipocyte Differentiation and Activation Pathway.
The development of a fully functional brown adipocyte can be divided into three phases: the multipotent mesenchymal stem cell; the brown preadipocytes; and the mature brown adipocytes. Shown for each phase are key hormonal regulators (left) and transcriptional regulators (italics, center).
For years, the best known inducer of brown adipogenesis and function has been norepinephrine [45], but recent studies have identified additional specific secreted signaling molecules including nodal, wingless, and members of the fibroblast growth factor (FGF) and bone morphogenetic protein (BMP) families [6]. The BMPs in particular are of interest because they are secreted proteins in the TGF-β superfamily that can promote both brown and white adipogenesis [46]. BMP-7 has been shown to be a powerful inducer of brown adipogenesis, while BMP-2 and BMP-4 are strong inducers of WAT. Adenoviral-mediated expression of BMP-7 in liver of mice has been shown to increase BAT mass and energy expenditure and to reduce weight gain [47].
8. Rediscovery of Brown Fat in Adult Humans
Though BAT was known to exist and have a physiological role in children, BAT was thought to be nonexistent and nonfunctional in adults because attempts to find functional BAT during life [48] or utilize its thermogenesis for weight loss [49;50] had been largely unsuccessful [51;52]. Beginning in 2002, reports in the radiological literature using PET/CT suggested this may not be true. PET, or positron emission tomography, uses radiotracers such as 18F-fluorodeoxyglucose (18F-FDG) to measure the metabolic activity of tissues in living organisms. CT, or computed tomography, provides high-resolution anatomical detail. Fusion of the PET and CT images provides both functional and structural information in a single image (Figure 2). In the course of using PET/CT to detect and stage tumors [53], radiologists noted FDG-avid adipose tissue in the same sites seen thirty years previously [17;54;55]. This tissue was often called “BAT” in the radiological literature, but endocrinologists doubted these findings, in part because of the lack of immunohistochemical evidence.
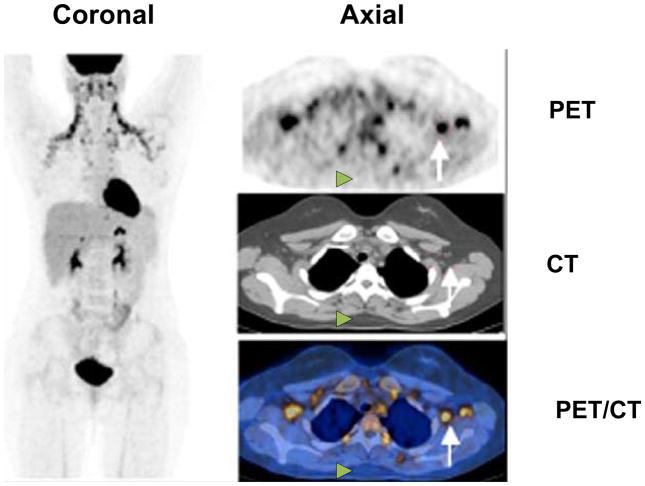
Figure 2. The typical pattern of pediatric BAT detectable via 18F-FDG PET/CT.
PET/CT of a 16-year-old girl with Hodgkin’s disease after two courses of chemotherapy. The maximal intensity projection 18F-FDG PET image (left) shows symmetrical FDG uptake in both sides of the neck and supraclavicular regions typical of BAT activity. Transaxial PET, CT, and fused PET/CT images (right) show examples of supraclavicular BAT (white arrows) and the absence of activity within the interscapular adipose tissue (white arrowheads).
Then, in 2009 several studies fundamentally altered our understanding of adult human BAT, with implications for pediatric BAT as well. Five independent groups used 18F-FDG PET/CT to identify and characterize the presence and relevance of BAT in adult humans [32;33;56–58]. All showed major depots of metabolically active fat in the cervical-supraclavicular region that had UCP-1 and histological characteristics of BAT, and one group also demonstrated the gene and protein expression of UCP-1 [32]. In our retrospective study of almost 2000 adults undergoing PET/CT, we found an inverse correlation between BAT activity and average outdoor temperature, beta-blocker medication use, and body-mass index (BMI), indicating that adult human BAT responds to stimulation by the sympathetic nervous system and likely participates in both cold-induced and diet-induced thermogenesis. The location of the most highly active BAT in adult humans was in the cervical–supraclavicular depot [56].
The wide use of FDG-PET also permits an assessment of BAT activity in children as well. In a study of children age 1.2 to 22.6 y, metabolically active BAT was found in cervical and supraclavicular depots as well as paraspinally, but not interscapularly [59]. Thus, BAT in children is in essentially the same depots identified histologically decades before [16;17]. In addition, studies have shown that interscapular BAT in children, if it exists, does not take up much FDG and is not likely metabolically active [59–63], which stands in direct contrast to rodent studies showing high uptake even under basal conditions [22;64;65].
BAT in children and adults differs significantly in terms of overall activity when measured by PET. In children, the detection rate in non-stimulated individuals is much higher – up to 31% [63] compared to 5% in adults [56], and the frequency is equal in girls and boys [59] instead of displaying the marked female predominance seen in adults [56]. BAT activity is also modifiable, as pretreatment of children with opiates such as fentanyl or anxiolytics such as diazepam reduces FDG uptake [59]. Simply increasing the ambient room temperature from 21 °C to 24 °C also reduces FDG uptake by BAT in children [63]. The physiological implications of these findings are now an exciting area for discovery.
9. Conclusion
The obesity pandemic in children presents a unique set of challenges. Harnessing the thermogenic, fat-burning power of BAT is clearly an attractive new therapeutic target. Since treatments with growth factors, such as BMP’s and FGF’s, or adrenergic agents are not likely to be employed because of their pleiotropic effects, further research will be required to find safe methods, such as mild cold exposure, to increase BAT function for treatment or prevention of obesity. For now, combating childhood obesity will rely on reducing caloric intake and increasing energy expenditure through exercise.
Acknowledgments
This work was supported in part by the Eli Lilly Foundation and NIH grants DK082659 (C.R.K), DK046200, DK081604, DK087317, RR025757 (A.M.C.), and P30 DK036836 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National NIDDK or the NIH.
Footnotes
“The author should describe the differentiation and development of brown adipose tissue (brown fat) and discuss the physiological and biochemical differences between brown and white fat. He might also comment upon the potential therapeutic utility of this tissue as a target for the regulation of energy metabolism.”
Dr. Cypess reports being the sole inventor on a pending patent application to use IR thermography to monitor brown adipose tissue. Dr. Kahn reports being an advisory board member for Sirtris, Plexxicon, Fiveprime, and Dicerna; owns equity in GSK, Plexxicon, and Fiveprime; receiving lecture fees from Wyeth, Novartis, and Novo-Nordisk; and has a pending patent in the area of stimulating brown fat growth with BMPs. No other conflicts of interest were reported.
Reference List
*of special interest
**of outstanding interest
- *1.Ogden CL, Carroll MD, Flegal KM. High body mass index for age among US children and adolescents, 2003–2006. JAMA. 2008;299:2401–2405. doi: 10.1001/jama.299.20.2401. The authors report that pediatric obesity, while considerably elevated, has not increased in prevalence between 1999–2000 and 2005–2006. Nevertheless, the pre-existing racial/ethnic disparities have not improved. [DOI] [PubMed] [Google Scholar]
- *2.Crocker MK, Yanovski JA. Pediatric obesity: etiology and treatment. Endocrinol Metab Clin North Am. 2009;38:525–548. doi: 10.1016/j.ecl.2009.06.007. This review describes the factors contributing to excessive weight gain in children and illustrates the current approaches for treating pediatric obesity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The White House Office of the Press Secretary. Presidential Memorandum - Establishing a Task Force on Childhood Obesity. 2010 [Google Scholar]
- 4.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–853. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puri V, Virbasius JV, Guilherme A, Czech MP. RNAi screens reveal novel metabolic regulators: RIP140, MAP4k4 and the lipid droplet associated fat specific protein (FSP) 27. Acta Physiol (Oxf) 2008;192:103–115. doi: 10.1111/j.1748-1716.2007.01786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131:242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Almind K, Manieri M, Sivitz WI, Cinti S, Kahn CR. Ectopic brown adipose tissue in muscle provides a mechanism for differences in risk of metabolic syndrome in mice. Proc Natl Acad Sci U S A. 2007;104:2366–2371. doi: 10.1073/pnas.0610416104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xue B, Rim JS, Hogan JC, Coulter AA, Koza RA, Kozak LP. Genetic variability affects the development of brown adipocytes in white fat but not in interscapular brown fat. J Lipid Res. 2007;48:41–51. doi: 10.1194/jlr.M600287-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Cinti S. The adipose organ. Prostaglandins Leukot Essent Fatty Acids. 2005;73:9–15. doi: 10.1016/j.plefa.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 10.Foster DO, Frydman ML. Tissue distribution of cold-induced thermogenesis in conscious warm- or cold-acclimated rats reevaluated from changes in tissue blood flow: the dominant role of brown adipose tissue in the replacement of shivering by nonshivering thermogenesis. Can J Physiol Pharmacol. 1979;57:257–270. doi: 10.1139/y79-039. [DOI] [PubMed] [Google Scholar]
- 11.Golozoubova V, Hohtola E, Matthias A, Jacobsson A, Cannon B, Nedergaard J. Only UCP1 can mediate adaptive nonshivering thermogenesis in the cold. FASEB J. 2001;15:2048–2050. doi: 10.1096/fj.00-0536fje. [DOI] [PubMed] [Google Scholar]
- 12.Ghorbani M, Claus TH, Himms-Hagen J. Hypertrophy of brown adipocytes in brown and white adipose tissues and reversal of diet-induced obesity in rats treated with a beta3-adrenoceptor agonist. Biochem Pharmacol. 1997;54:121–131. doi: 10.1016/s0006-2952(97)00162-7. [DOI] [PubMed] [Google Scholar]
- 13.Guerra C, Koza RA, Yamashita H, Walsh K, Kozak LP. Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity. J Clin Invest. 1998;102:412–420. doi: 10.1172/JCI3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lowell BB, Susulic V, Hamann A, Lawitts JA, Himms-Hagen J, Boyer BB, Kozak LP, Flier JS. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature. 1993;366:740–742. doi: 10.1038/366740a0. [DOI] [PubMed] [Google Scholar]
- 15.Rothwell NJ, Stock MJ. Luxuskonsumption, diet-induced thermogenesis and brown fat: the case in favour. Clin Sci (Lond) 1983;64:19–23. doi: 10.1042/cs0640019. [DOI] [PubMed] [Google Scholar]
- 16.Aherne W, Hull D. Brown adipose tissue and heat production in the newborn infant. J Pathol Bacteriol. 1966;91:223–234. doi: 10.1002/path.1700910126. [DOI] [PubMed] [Google Scholar]
- 17.Heaton JM. The distribution of brown adipose tissue in the human. J Anat. 1972;112:35–39. [PMC free article] [PubMed] [Google Scholar]
- 18.Hull D. The function of brown adipose tissue in the newborn. Biochem Soc Trans. 1976;4:226–228. doi: 10.1042/bst0040226. [DOI] [PubMed] [Google Scholar]
- 19.Brooke OG, Harris M, Salvosa CB. The response of malnourished babies to cold. J Physiol. 1973;233:75–91. doi: 10.1113/jphysiol.1973.sp010298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dawkins MJ, Scopes JW. Non-shivering thermogenesis and brown adipose tissue in the human new-born infant. Nature. 1965;206:201–202. doi: 10.1038/206201b0. [DOI] [PubMed] [Google Scholar]
- 21.Astrup A, Bulow J, Christensen NJ, Madsen J. Ephedrine-induced thermogenesis in man: no role for interscapular brown adipose tissue. Clin Sci (Lond) 1984;66:179–186. doi: 10.1042/cs0660179. [DOI] [PubMed] [Google Scholar]
- 22.Baba S, Tatsumi M, Ishimori T, Lilien DL, Engles JM, Wahl RL. Effect of nicotine and ephedrine on the accumulation of 18F-FDG in brown adipose tissue. J Nucl Med. 2007;48:981–986. doi: 10.2967/jnumed.106.039065. [DOI] [PubMed] [Google Scholar]
- *23.Baba S, Jacene HA, Engles JM, Honda H, Wahl RL. CT Hounsfield units of brown adipose tissue increase with activation: preclinical and clinical studies. J Nucl Med. 2010;51:246–250. doi: 10.2967/jnumed.109.068775. An important correlation is demonstrated between histological appearance and both the radiodensity and FDG uptake of basal and cold-activated BAT in rats as measured by PET/CT. These data support the position that the lipid-depleted BAT taken from humans reflects increased metabolic activity and that CT alone may identify collections of human BAT. [DOI] [PubMed] [Google Scholar]
- 24.Lean ME. Brown adipose tissue in humans. Proc Nutr Soc. 1989;48:243–256. doi: 10.1079/pns19890036. [DOI] [PubMed] [Google Scholar]
- 25.Ricquier D, Kader JC. Mitochondrial protein alteration in active brown fat: a soidum dodecyl sulfate-polyacrylamide gel electrophoretic study. Biochem Biophys Res Commun. 1976;73:577–583. doi: 10.1016/0006-291x(76)90849-4. [DOI] [PubMed] [Google Scholar]
- 26.Cannon B, Hedin A, Nedergaard J. Exclusive occurrence of thermogenin antigen in brown adipose tissue. FEBS Lett. 1982;150:129–132. doi: 10.1016/0014-5793(82)81319-7. [DOI] [PubMed] [Google Scholar]
- 27.Lean ME, Branch WJ, James WP, Jennings G, Ashwell M. Measurement of rat brown-adipose-tissue mitochondrial uncoupling protein by radioimmunoassay: increased concentration after cold acclimation. Biosci Rep. 1983;3:61–71. doi: 10.1007/BF01121572. [DOI] [PubMed] [Google Scholar]
- 28.Lean ME, James WP, Jennings G, Trayhurn P. Brown adipose tissue uncoupling protein content in human infants, children and adults. Clin Sci (Lond) 1986;71:291–297. doi: 10.1042/cs0710291. [DOI] [PubMed] [Google Scholar]
- 29.Lean ME, Jennings G. Brown adipose tissue activity in pyrexial cases of cot death. J Clin Pathol. 1989;42:1153–1156. doi: 10.1136/jcp.42.11.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Emery JL, Dinsdale F. Structure of periadrenal brown fat in childhood in both expected and cot deaths. Arch Dis Child. 1978;53:154–158. doi: 10.1136/adc.53.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Baeyer CL, Piira T, Chambers CT, Trapanotto M, Zeltzer LK. Guidelines for the cold pressor task as an experimental pain stimulus for use with children. J Pain. 2005;6:218–227. doi: 10.1016/j.jpain.2005.01.349. [DOI] [PubMed] [Google Scholar]
- **32.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, Nuutila P. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. Acute cold exposure induced a 15-fold increase in FDG uptake in adult human BAT. Tissue shown to be FDG-avid adipose tissue via PET/CT was biopsied and shown definitively to contain brown adipocytes and tissue-specific, thermogenic UCP1 via mRNA, protein, and morphological assessments. [DOI] [PubMed] [Google Scholar]
- **33.Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K, Kawai Y, Tsujisaki M. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526–1531. doi: 10.2337/db09-0530. Acute cold exposure increased BAT activity in the supraclavicular and paraspinal regions of healthy volunteers, and this uptake was increased in the winter compared with summer months. FDG uptake also inversely correlated with BMI and both total and visceral fat. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Myers CD, Tsao JC, Glover DA, Kim SC, Turk N, Zeltzer LK. Sex, gender, and age: contributions to laboratory pain responding in children and adolescents. J Pain. 2006;7:556–564. doi: 10.1016/j.jpain.2006.01.454. [DOI] [PubMed] [Google Scholar]
- 35.Jaaniste T, Hayes B, von Baeyer CL. Effects of preparatory information and distraction on children’s cold-pressor pain outcomes: a randomized controlled trial. Behav Res Ther. 2007;45:2789–2799. doi: 10.1016/j.brat.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 36.Oppert JM, Vohl MC, Chagnon M, Dionne FT, Cassard-Doulcier AM, Ricquier D, Perusse L, Bouchard C. DNA polymorphism in the uncoupling protein (UCP) gene and human body fat. Int J Obes Relat Metab Disord. 1994;18:526–531. [PubMed] [Google Scholar]
- 37.Urhammer SA, Hansen T, Borch-Johnsen K, Pedersen O. Studies of the synergistic effect of the Trp/Arg64 polymorphism of the beta3-adrenergic receptor gene and the -3826 A-->G variant of the uncoupling protein-1 gene on features of obesity and insulin resistance in a population-based sample of 379 young Danish subjects. J Clin Endocrinol Metab. 2000;85:3151–3154. doi: 10.1210/jcem.85.9.6790. [DOI] [PubMed] [Google Scholar]
- 38.Nagai N, Sakane N, Ueno LM, Hamada T, Moritani T. The -3826 A-->G variant of the uncoupling protein-1 gene diminishes postprandial thermogenesis after a high fat meal in healthy boys. J Clin Endocrinol Metab. 2003;88:5661–5667. doi: 10.1210/jc.2003-030672. [DOI] [PubMed] [Google Scholar]
- 39.Griffiths M, Payne PR. Energy expenditure in small children of obese and non-obese parents. Nature. 1976;260:698–700. doi: 10.1038/260698a0. [DOI] [PubMed] [Google Scholar]
- 40.James WPT, Trayhurn P. Thermogenesis and obesity. Br Med Bull. 1981;37:43–48. doi: 10.1093/oxfordjournals.bmb.a071674. [DOI] [PubMed] [Google Scholar]
- 41.Timmons JA, Wennmalm K, Larsson O, Walden TB, Lassmann T, Petrovic N, Hamilton DL, Gimeno RE, Wahlestedt C, Baar K, Nedergaard J, Cannon B. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci U S A. 2007;104:4401–4406. doi: 10.1073/pnas.0610615104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *42.Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scime A, Devarakonda S, Conroe HM, Erdjument-Bromage H, Tempst P, Rudnicki MA, Beier DR, Spiegelman BM. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. Using in vivo fate mapping, this group showed that brown, but not white, fat cells arise from precursors that express Myf5, a myogenic gene. Moreover, the transcriptional regulator PRDM16 controls a bidirectional cell fate switch between skeletal myoblasts and brown fat cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kopecky J, Clarke G, Enerback S, Spiegelman B, Kozak LP. Expression of the mitochondrial uncoupling protein gene from the aP2 gene promoter prevents genetic obesity. J Clin Invest. 1995;96:2914–2923. doi: 10.1172/JCI118363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leonardsson G, Steel JH, Christian M, Pocock V, Milligan S, Bell J, So PW, Medina-Gomez G, Vidal-Puig A, White R, Parker MG. Nuclear receptor corepressor RIP140 regulates fat accumulation. Proc Natl Acad Sci U S A. 2004;101:8437–8442. doi: 10.1073/pnas.0401013101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 46.Schulz TJ, Tseng YH. Emerging role of bone morphogenetic proteins in adipogenesis and energy metabolism. Cytokine Growth Factor Rev. 2009;20:523–531. doi: 10.1016/j.cytogfr.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *47.Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, Tran TT, Suzuki R, Espinoza DO, Yamamoto Y, Ahrens MJ, Dudley AT, Norris AW, Kulkarni RN, Kahn CR. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008;454:1000–1004. doi: 10.1038/nature07221. Of the family of bone morphogenetic proteins (BMP), BMP7 was shown to activate a full program of brown adipogenesis in precursor cells. Implantation of treated cells into nude mice led to development of BAT. In other mice, adenoviral-mediated expression of BMP7 increased brown, but not white, fat mass and increased energy expenditure and a reduction in weight gain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Astrup A. Thermogenesis in human brown adipose tissue and skeletal muscle induced by sympathomimetic stimulation. Acta Endocrinol Suppl (Copenh) 1986;278:1–32. [PubMed] [Google Scholar]
- 49.Weyer C, Tataranni PA, Snitker S, Danforth E, Jr, Ravussin E. Increase in insulin action and fat oxidation after treatment with CL 316,243, a highly selective beta3-adrenoceptor agonist in humans. Diabetes. 1998;47:1555–1561. doi: 10.2337/diabetes.47.10.1555. [DOI] [PubMed] [Google Scholar]
- 50.Larsen TM, Toubro S, van Baak MA, Gottesdiener KM, Larson P, Saris WH, Astrup A. Effect of a 28-d treatment with L-796568, a novel beta(3)-adrenergic receptor agonist, on energy expenditure and body composition in obese men. Am J Clin Nutr. 2002;76:780–788. doi: 10.1093/ajcn/76.4.780. [DOI] [PubMed] [Google Scholar]
- 51.Cunningham S, Leslie P, Hopwood D, Illingworth P, Jung RT, Nicholls DG, Peden N, Rafael J, Rial E. The characterization and energetic potential of brown adipose tissue in man. Clin Sci (Lond) 1985;69:343–348. doi: 10.1042/cs0690343. [DOI] [PubMed] [Google Scholar]
- 52.Nedergaard J, Bengtsson T, Cannon B. Unexpected Evidence for Active Brown Adipose Tissue in Adult Humans. Am J Physiol Endocrinol Metab. 2007;293:E444–E452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- 53.Schoder H, Larson SM, Yeung HW. PET/CT in oncology: integration into clinical management of lymphoma, melanoma, and gastrointestinal malignancies. J Nucl Med. 2004;45 (Suppl 1):72S–81S. [PubMed] [Google Scholar]
- 54.Hany TF, Gharehpapagh E, Kamel EM, Buck A, Himms-Hagen J, von Schulthess GK. Brown adipose tissue: a factor to consider in symmetrical tracer uptake in the neck and upper chest region. Eur J Nucl Med Mol Imaging. 2002;29:1393–1398. doi: 10.1007/s00259-002-0902-6. [DOI] [PubMed] [Google Scholar]
- 55.Cohade C, Osman M, Pannu HK, Wahl RL. Uptake in supraclavicular area fat (“USA-Fat”): description on 18F-FDG PET/CT. J Nucl Med. 2003;44:170–176. [PubMed] [Google Scholar]
- **56.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. PET/CT scans from 1,972 patients were reviewed, and it was shown that whole-body BAT could be quantified noninvasively. Active BAT was more frequent in women than in men, and the amount of BAT was inversely correlated with age, outdoor temperature, beta-blocker use, and BMI in older adults. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **57.Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. This prospective study involving young men found that BAT was not observed at 22 °C but was detectable in 23/24 subjects at 16 °C, demonstrating that functional adult human BAT is highly prevalent. BAT activity correlated positively with resting metabolic rate but inversely with both BMI and percentage of body fat. [DOI] [PubMed] [Google Scholar]
- **58.Zingaretti MC, Crosta F, Vitali A, Guerrieri M, Frontini A, Cannon B, Nedergaard J, Cinti S. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J. 2009;23:3113–3120. doi: 10.1096/fj.09-133546. Adipose tissue was sampled from the necks of 35 patients undergoing surgery. In 1/3, the younger and leaner, distinct islands of richly sympathetically innervated brown adipocytes were identified. These islands had a high capillary density, and the pericapillary regions demonstrated brown adipocyte precursors, indicating that adult humans had the capacity to grow more BAT as well. [DOI] [PubMed] [Google Scholar]
- 59.Gelfand MJ, O’hara SM, Curtwright LA, Maclean JR. Pre-medication to block [(18)F]FDG uptake in the brown adipose tissue of pediatric and adolescent patients. Pediatr Radiol. 2005;35:984–990. doi: 10.1007/s00247-005-1505-8. [DOI] [PubMed] [Google Scholar]
- 60.Yeung HW, Grewal RK, Gonen M, Schoder H, Larson SM. Patterns of (18)F-FDG uptake in adipose tissue and muscle: a potential source of false-positives for PET. J Nucl Med. 2003;44:1789–1796. [PubMed] [Google Scholar]
- 61.Truong MT, Erasmus JJ, Munden RF, Marom EM, Sabloff BS, Gladish GW, Podoloff DA, Macapinlac HA. Focal FDG uptake in mediastinal brown fat mimicking malignancy: a potential pitfall resolved on PET/CT. AJR Am J Roentgenol. 2004;183:1127–1132. doi: 10.2214/ajr.183.4.1831127. [DOI] [PubMed] [Google Scholar]
- 62.Bar-Sever Z, Keidar Z, Ben Barak A, Bar-Shalom R, Postovsky S, Guralnik L, Ben Arush MW, Israel O. The incremental value of 18F-FDG PET/CT in paediatric malignancies. Eur J Nucl Med Mol Imaging. 2007;34:630–637. doi: 10.1007/s00259-006-0253-9. [DOI] [PubMed] [Google Scholar]
- *63.Zukotynski KA, Fahey FH, Laffin S, Davis R, Treves ST, Grant FD, Drubach LA. Constant ambient temperature of 24 degrees C significantly reduces FDG uptake by brown adipose tissue in children scanned during the winter. Eur J Nucl Med Mol Imaging. 2009;36:602–606. doi: 10.1007/s00259-008-0983-y. [DOI] [PubMed] [Google Scholar]
- 64.Tatsumi M, Engles JM, Ishimori T, Nicely O, Cohade C, Wahl RL. Intense (18)F-FDG uptake in brown fat can be reduced pharmacologically. J Nucl Med. 2004;45:1189–1193. [PubMed] [Google Scholar]
- 65.Baba S, Engles JM, Huso DL, Ishimori T, Wahl RL. Comparison of uptake of multiple clinical radiotracers into brown adipose tissue under cold-stimulated and nonstimulated conditions. J Nucl Med. 2007;48:1715–1723. doi: 10.2967/jnumed.107.041715. [DOI] [PubMed] [Google Scholar]