Abstract
Objectives
To elucidate the mechanism through which vitamin D is associated with decreased falls.
Design
This was a convenience sample from a larger observational study examining correlations between vitamin D and 1) falls, 2) motor function, and 3) cognition (n=159).
Setting
Falls data were collected via weekly on-line surveys completed in the participants' homes. Yearly evaluations of motor and cognitive function were conducted in an out-patient setting of a large tertiary medical center.
Participants
Participants from the Intelligent Systems for Assessment of Aging Changes Study (ISAAC), a community-based cohort study of independently living older adults over age 70, who had vitamin D concentration within 6 months of clinical evaluations were included in the analysis.
Results
Participants mean age was 85 years and 74% were women. Fallers (n=37) had significantly lower vitamin D concentration (32.9 ng/ml) compared to non-fallers (39.2 ng/ml) (p<0.01). The relationship between vitamin D and falls remained significant after adjusting for age, health status (via CIRS), and supplement use (p=0.004). Vitamin D concentration were significantly associated with cognitive impairment (Clinical Dementia Rating = 0.5) (p=0.02) and MMSE (p<0.01) after adjusting for age, gender, and education. Vitamin D concentrations did not correlate with any motor measures.
Conclusion
Vitamin D concentrations correlated with cognition and falls, but not with motor measures. Further research is needed to demonstrate a causal relationship between vitamin D and cognitive function and determine if cognition plays a role in falls reduction.
Keywords: Accidental falls, cognitive function, vitamin D
Introduction
Vitamin D is traditionally associated with bone health, but more recent research demonstrates an effect of vitamin D on multiple other organ systems (1). Of particular interest for physicians working with an older population is the fact that vitamin D supplementation greater than 700 IU a day appears to decrease falls by approximately 20% (2). Since one in three adults over age 65 fall annually with a total direct cost for fall injuries expected to climb as high as $54.9 billion by 2020, this has major public health implications (3, 4). The NIH conference “Vitamin D and Health in the 21st Century: an Update” identified determining the mechanism through which vitamin D decreases falls as a key research need (5). We proposed to look for correlations of vitamin D concentration with measures of motor function (gait, balance, and strength) and cognitive function to better inform mechanisms of action for falls reduction. We hypothesized significant correlations between motor function and vitamin D concentration.
Methods
Participants
The Intelligent Systems for Assessment of Aging Changes Study (ISAAC) is a community-based cohort study that examines changes in motor and cognitive function among independently living older adults over age 70. Participants have yearly evaluations and have computer systems in the home to complete questionnaires about falls and other health measures. Inclusion criteria include age of 80 years and older (70 years and older for minorities and spouses of participants), living independently, not demented with a Clinical Dementia Rating score < 0.5, Mini-Mental State Examination (MMSE) score > 24, and being of average health for age. Full details of study enrollment and assessment procedures have previously been described (6). We describe the 159 ISAAC participants with vitamin D concentration (within 6 month of clinical evaluations) and complete clinical data here.
Assessments
Participants were assessed with standardized health and function questionnaires, physical and neurological examinations, and a variety of tests of motor and cognitive function. Motor tests included: Tinetti Gait and Balance Instrument, UPDRS, chair stands, grip strength, and gait speed (7–10). The neuropsychological testing included the following domains and tests: Attention and concentration (Digit Span Forward, Digit-Symbol and Trail Making Part A); Processing speed (Simple & Choice Reaction Time and Crossing-off test); Working memory (Digit Span Backward, Letter-Number Sequencing, and Digit Sequencing); Memory (Logical Memory Delayed Visual Reproduction, CERAD Word List, and CERAD Visual Figures Recall; Language WRAT-R and Boston Naming Test); Executive function (Category Fluency, Trail Making Part B, Stroop Test, Letter Fluency, and Odd Man Out task); and Visuospatial construction (Picture Completion and Block Design) (11–16). Z-normalized scores were calculated for each of the 5 cognitive domains as well as a global score. Falls were self-reported via weekly, computerized questionnaires.
Health status was documented via the modified Cumulative Illness Rating Scale (17). Falls were defined as any fall, including a slip or trip, in which the subject came to rest on the floor, ground or on a lower level (6). The number of falls each subject reported were summed for the 3 months before, and the 3 months after the date of the vitamin D blood draw. Blood draws were non-fasting and were all completed between 9/26/08 and 2/11/09. Ninety percent of plasma collections were done in the Fall. Vitamin D assays were completed in October 2009. Vitamin D was measured as 25-hydroxy vitamin D in the serum using a radioimmunoassay (RIA) from IDS (Immunodiagnostic Systems Inc, Fountain Hills, AZ 85269-7063). The sensitivity, defined as the concentration corresponding to the mean minus 2 standard deviations of 10 replicates of the zero calibrator, is < 1.2 ng/mL. Our in-house laboratory quality control program produces interassay %CV’s of 5.1% at 13.7 ng/mL and 9.1% at 51.3 ng/mL. The antibody recognizes 100% of 25 hydroxyvitamin D3 and 75% of 25-hydroxyvitamin D2 when assessed at 50% binding of the zero calibrator according to the manufacturer.
Statistical analysis
Neuropsychological and motor measures obtained at the annual evaluation closest to the vitamin D blood draw date were used. Participant characteristics of fallers and non-fallers were compared using Student’s t-test or Wilcoxon Rank Sum Test for continuous variables and Pearson Chi-square test for categorical variables. Multivariate logistic regression was used to estimate risk of falls by vitamin D level, controlling for gender and supplementation use. Interaction terms were introduced to investigate whether the relation between vitamin D and falls risk varied by cognitive status (CDR = 0 vs. 0.5). Analysis of variance was used to compare vitamin D concentration among three fall categories (non-faller, single faller, multiple faller). Correlations between Vitamin D concentration and clinical variables are reported as Spearman’s or Pearson’s coefficient as appropriate. Multivariate linear regression was used to model the relationships between Vitamin D level and each cognitive domain z-score, after adjusting for age, sex and education. All analyses were performed using SAS 9.2 software (SAS Institute Inc., Cary, NC).
Results
Subject Characteristics
Subjects averaged 85 years old, were generally highly educated (average of 15 years of education), and largely white females (92% and 74% respectively). The mean serum vitamin D level was 37.7 ng/ml (range: 9–90 ng/ml). Twenty-three percent of subjects (n=37) reported at least one fall within 6 months surrounding the vitamin D collection (3 months before and 3 months after date of blood draw) and only 5% (n=8) reported multiple falls. Table 1 presents demographic, clinical, and cognitive characteristics among fallers and non-fallers.
Table 1.
Subject Characteristics
| Variables | Total | No Falls | Fallers | p-value |
|---|---|---|---|---|
| N | 159 | 122 | 37 | |
| Age (years) | 85.5 | 85.1 | 86.6 | 0.09 |
| Gender (% female) | 74% | 74% | 76% | 0.82 |
| Education (years) | 15.5 | 15.5 | 15.4 | 0.68 |
| Race (% Non-White) | 8% | 8% | 5% | 0.73 |
| Cognitive Status (% CDR=0.5) | 13% | 12% | 17% | 0.4 |
| Vitamin D (ng/ml) | 37.7 | 39.2 | 32.9 | <0.01** |
| Vit D supplementation (%) | 41% | 43% | 32% | 0.23 |
| Geriatric Depression Score (0–30) | 1.6 | 1.4 | 2.2 | 0.39 |
| Functional Assessment Scale (0–30) | 0.9 | 0.8 | 1.5 | 0.08 |
| Cumulative Illness Rating Scale (14–70) | 22 | 21.6 | 23.2 | 0.01** |
| MMSE (0–30) | 28.4 | 28.4 | 28.5 | 0.85 |
| Cognitive Domain z-scores | ||||
| Global Score | −0.12 | −0.12 | −0.14 | 0.87 |
| Attention | −0.14 | −0.16 | −0.1 | 0.65 |
| Executive Function | −0.03 | 0.00 | −0.13 | 0.37 |
| Memory | −0.18 | −0.16 | −0.26 | 0.62 |
| Working Memory | −0.2 | −0.25 | −0.04 | 0.19 |
| Visuospatial | 0.01 | 0.01 | 0.04 | 0.82 |
| Motor Measures | ||||
| Stopwatch walk | 75.8 | 77 | 71.3 | 0.21 |
| Tinetti gait (0–16) | 1.2 | 1.1 | 1.5 | 0.08 |
| Tinetti balance (0–16) | 4.5 | 4.2 | 5.5 | 0.12 |
| mUPDRS (0–108) | 2.3 | 2.1 | 3.1 | 0.11 |
| Grip strength (dynes) | 17.7 | 18.1 | 16.6 | 0.32 |
| Chair stands: Unable (%) | 23% | 19% | 33% | 0.08 |
Fallers had lower serum vitamin D
Fallers had a significantly lower vitamin D level (32.9 ng/ml) as compared to non-fallers (39.2 ng/ml) (p=<.01). Fallers had slightly higher cumulative illness ratings (CIRS) at 23.2 compared to 21.6 (p=0.01). There were no statistically significant differences in motor measures or cognitive domain z-scores between groups. The adjusted OR and 95% CI for the association between vitamin D and falls is OR 0.96, 95% CI 0.93 – 0.99, p=0.02. Using logistic regression we modeled the risk of falling by vitamin D after adjustment for covariates: age, health status (via CIRS), and supplement use. Vitamin D remains a significant predictor of falls risk, p=0.004. A 5 ng/ml increase in vitamin D corresponds to a 20% decrease in odds of falling. Looking exclusively at the 111 participants with generally considered sufficient vitamin concentration D (>30 ng/ml) mean vitamin D among fallers was lower at 40.0 ng/ml than non-fallers at 45.1 ng/ml; this did not reach statistical significance possibly due to lower sample size (p=0.07). Vitamin D supplement use (based on patient self-report for the 2 weeks prior to evaluations) did not modify the relationship between vitamin D and falls risk. Cognitive status (CDR=0 vs. 0.5) did not modify the relationship between vitamin D and falls risk (p=0.12). A post-hoc analysis was done for falls and vitamin D including covariates: BMI, depression (GDS), autonomy (FAS), grip strength, and race, although none of these variables were different among fallers and non-fallers (see Table 1). Vitamin D remained the only variable in the model significantly associated with fall status, p=0.01.
Serum vitamin D and motor function
We investigated the relationships between vitamin D and motor measures (see Table 2). There were no significant correlations between vitamin D concentration and the following motor measures: stopwatch walk, Tinetti gait and balance, UPDRS, grip strength, or chair stands.
Table 2.
Correlations between clinical variables and vitamin D concentration
| Motor Measure | Correlation | p value |
|---|---|---|
| Stopwatch walk | −0.08 | 0.33 |
| Tinetti gait | 0.1 | 0.21 |
| Tinetti balance | 0.02 | 0.89 |
| UPDRS | −0.01 | 0.88 |
| Grip strength | −0.14 | 0.09 |
| Chair stands (n=120) | 0.04 | 0.7 |
| Cognitive Measure |
Correlation | p value | Regression coefficient** |
95% confidence interval |
p value |
|---|---|---|---|---|---|
| Global Score | 0.14 | 0.07 | 3.30 | −0.50, 7.10 | 0.09 |
| Attention | 0.15 | 0.06 | 2.68 | −0.68, 6.04 | 0.12 |
| Executive Function | 0.10 | 0.21 | 1.73 | −1.35, 4.80 | 0.27 |
| Memory | 0.12 | 0.13 | 1.35 | −0.90, 3.60 | 0.24 |
| Working Memory | 0.14 | 0.08 | 2.30 | −0.44, 5.04 | 0.10 |
| Visuospatial | 0.08 | 0.35 | 1.63 | −1.18, 4.44 | 0.25 |
| MMSE* | 0.24 | <0.01 | 1.50 | −0.01, 3.01 | 0.05 |
| Cognitive Status | Mean vitamin D concentration | p value |
|---|---|---|
| CDR=0.5 | 30.9 ng/ml | |
| 0.02 | ||
| CDR=0 | 38.8 ng/ml |
Spearman’s rank correlation coefficient was used since MMSE scores are not normally distributed.
Individual multivariate linear models for vitamin D adjusted for age, gender, and education.
Vitamin D and cognitive measures
MMSE scores correlated significantly with vitamin D levels (p<0.01). Subjects with very mild dementia (CDR=0.5) had significantly lower vitamin D concentration as compared to cognitively intact subjects (CDR=0), 30.9 vs. 38.8 (p=0.02). These relationships remained significant after adjustment for age, gender, and education (p-values<0.05). There was a trend towards a correlation with the global cognition, as well as the attention and working memory subscores, but these did not reach significance. Multivariate linear regression between vitamin D concentration and each cognitive domain z-score (adjusted for age, gender, and education) did not reach statistical significance.
Discussion
These data are consistent with other studies showing that higher plasma vitamin D concentrations are associated with reduced falls (2). In this group of independently living older adults, fallers had significantly lower vitamin D concentration than non-fallers. Correction for age and health status did not modify this relationship. A “dose response” was also evident, with multiple-fallers having lower serum vitamin D than single fallers and non-fallers. In our study, a 5 ng/ml increase in vitamin D decreased odds of falling by 20%.
One possible mechanism by which vitamin D might reduce fall risk is via a direct effect of vitamin D on muscle, hypothesized because vitamin D receptors are in muscle and severe vitamin D deficiency causes a myopathy (18). However, our data did not show a correlation between vitamin D and strength. It is possible that our measure of muscle function (grip strength) was not sensitive enough or that other mentions of muscle power, such as speed of contraction is more important than absolute strength (19). Central nervous system effects of vitamin D on gait and balance regulation have also been postulated (20). Again, our data did not show a significant correlation between vitamin D and gait or balance measures. Our data did however show a significant correlation of serum vitamin D concentration with MMSE scores and cognitive status. A number of cross-sectional studies show a similar relationship, but none have included falls data (21, 22). Two small, short term intervention studies showed no significant change in cognitive measures (23, 24).
Our data suggest that cognitive effects may play a role in vitamin D’s influence on falls while motor function may play a lesser role. This however does seem to explain the entire mechanism as our data showed the relationship between vitamin D and falls remained after correcting for cognitive status, suggesting there are additional factors at play. There are clear limitations to this study. With a cross-sectional study causality cannot be concluded. Certainly one concern is reverse causation – those that are more cognitively intact remember to take their supplements and those who don’t fall get outside to obtain more sun exposure. This cohort is also particularly healthy so results may not be generalisable to other populations. Season of blood draw may also affect vitamin D concentration, but samples were collected over a relatively narrow window (90% in the Fall) and there was no significant variation by season was seen. This study by no means is conclusive. The relevance of these data is in informing future studies of vitamin D and falls. It is well known that cognitive function plays a role in falls (25, 26). These data suggest that future intervention studies should includ in-depth cognitive measures to properly elucidate the mechanism via which vitamin D results in falls reduction. It adds further supports that intervention studies to specifically look at the effects of vitamin D supplementation on cognitive function, are warranted.
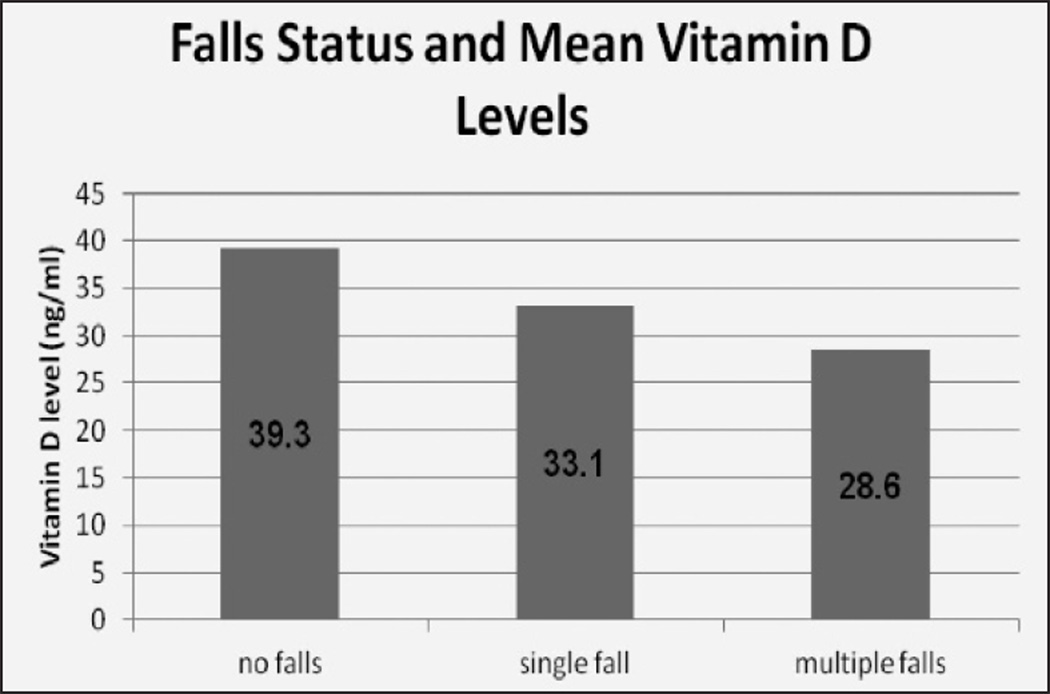
Figure 1.
Presents mean vitamin D concentration for the 122 non-fallers, 29 single fallers, and 8 multi-fallers. There was a significant difference across groups (ANOVA, p=0.0387). Post –hoc pair-wise analysis between each group showed a significant difference in vitamin concentration between non-fallers vs. multiple fallers (p=0.04). Analysis in non-fallers vs. single fallers and single fallers vs. multiple fallers did not reach statistical significant (p=0.08 and p=0.33 respectively)
Acknowledgments
Supported by National Institute of Health grants P30-AG008017, P30-AG024978, R01-AG024059, K01-AG23014, K23- AT004777, the Department of Veterans Affairs P30-AG008017 and M01-RR000334, and Intel Corporation.
References
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007 Jul 19;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, Orav JE, Stuck AE, Theiler R, Wong JB, Egli A, Kiel DP, Henschkowski J. Fall prevention with supplemental and active forms ofvitamin D: A meta-analysis of randomised controlled trials. BMJ. 2009;339:b3692. doi: 10.1136/bmj.b3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hausdorff JM, Rios DA, Edelberg HK. Gait variability and fall risk in community-living older adults: A 1-year prospective study. Archives of Physical Medicine & Rehabilitation. 2001 Aug;82(8):1050–1056. doi: 10.1053/apmr.2001.24893. [DOI] [PubMed] [Google Scholar]
- 4.Englander F, Hodson TJ, Terregrossa RA. Economic dimensions of slip and fall injuries. J Forensic Sci. 1996 Sep;41(5):733–746. [PubMed] [Google Scholar]
- 5.Raiten DJ, Picciano MF. Vitamin D and health in the 21st century: Bone and beyond executive summary. Am J Clin Nutr. 2004 Dec;80(6 Suppl):1673S–1677S. doi: 10.1093/ajcn/80.6.1673S. [DOI] [PubMed] [Google Scholar]
- 6.Kaye JA, Maxwell SA, Mattek N, Hayes TL, Dodge H, Pavel M, Jimison HB, Wild K, Boise L, Zitzelberger TA. Intelligent systems for assessing aging changes: Home-based, unobtrusive, and continuous assessment of aging. J Gerontol B Psychol Sci Soc Sci. 2011 Jul;66(Suppl 1):180–190. doi: 10.1093/geronb/gbq095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tinetti ME. Performance-oriented assessment of mobility problems in elderly patients. J Am Geriatr Soc. 1986 Feb;34(2):119–126. doi: 10.1111/j.1532-5415.1986.tb05480.x. [DOI] [PubMed] [Google Scholar]
- 8.Kopke S, Meyer G. The tinetti test: Babylon in geriatric assessment. Zeitschrift Fur Gerontologie Und Geriatrie. 2006 Aug;39(4):288–291. doi: 10.1007/s00391-006-0398-y. [DOI] [PubMed] [Google Scholar]
- 9.Fahn S. UPDRS program members. Unified parkinson's disease rating scale. In: Fahn S, Goldstein M, Calne DB, editors. Recent developments in parkinson's disease. Macmillan Healthcare Information; 1987. [Google Scholar]
- 10.Buracchio T, Dodge HH, Howieson D, Wasserman D, Kaye J. The trajectory of gait speed preceding mild cognitive impairment. Arch Neurol. 2010 Aug;67(8):980–986. doi: 10.1001/archneurol.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wechsler D. Manual for the wechsler adult intelligence scale - revised. New York: Psychological Corporation; 1981. [Google Scholar]
- 12.Wechsler D. Wechsler memory scale - revised. 1987 [Google Scholar]
- 13.Wechsler D. Wechsler memory scale. third edition manual. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- 14.Jastak S, Wilkinson G. The wide range achievement test - revised. Wilmington: Jastak Associates, Inc.; 1984. [Google Scholar]
- 15.Welsh KAPD, Breitner JCSMD, MPH, MagruderHabib KMPD., MPH Detection of dementia in the elderly using telephone screening of cognitive status. Neuropsychiatry, Neuropsychology, & Behavioral Neurology. 1993 Apr;6(2):103–110. [Google Scholar]
- 16.Welsh KA, Butters N, Mohs RC, Beekly D, Edland S, Fillenbaum G, Heyman A. The consortium to establish a registry for alzheimer's disease (CERAD). part V. A normative study of the neuropsychological battery. Neurology. 1994 Apr;44(4):609–614. doi: 10.1212/wnl.44.4.609. [DOI] [PubMed] [Google Scholar]
- 17.Parmelee PA, Thuras PD, Katz IR, Lawton MP. Validation of the cumulative illness rating scale in a geriatric residential population. J Am Geriatr Soc. 1995 Feb;43(2):130–137. doi: 10.1111/j.1532-5415.1995.tb06377.x. [DOI] [PubMed] [Google Scholar]
- 18.Pfeifer M, Begerow B, Minne HW. Vitamin D and muscle function. Osteoporosis Int. 2002 Mar;13(3):187–194. doi: 10.1007/s001980200012. [DOI] [PubMed] [Google Scholar]
- 19.Annweiler C, Bridenbaugh S, Schott AM, Berrut G, Kressig RW, Beauchet O. Vitamin D and muscle function: New prospects? Biofactors. 2009 Jan-Feb;35(1):3–4. doi: 10.1002/biof.4. [DOI] [PubMed] [Google Scholar]
- 20.Beauchet O, Annweiler C, Verghese J, Fantino B, Herrmann FR, Allali G. Biology of gait control Vitamin D involvement. Neurology. 2011 May 10;76(19):1617–1622. doi: 10.1212/WNL.0b013e318219fb08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Annweiler C, Allali G, Allain P, Bridenbaugh S, Schott AM, Kressig RW, Beauchet O. Vitamin D and cognitive performance in adults: A systematic review. Eur J Neurol. 2009 Oct;16(10):1083–1089. doi: 10.1111/j.1468-1331.2009.02755.x. [DOI] [PubMed] [Google Scholar]
- 22.Annweiler C, Schott AM, Allali G, Bridenbaugh SA, Kressig RW, Allain P, Herrmann FR, Beauchet O. Association of vitamin D deficiency with cognitive impairment in older women: Cross-sectional study. Neurology. 2010 Jan 5;74(1):27–32. doi: 10.1212/WNL.0b013e3181beecd3. [DOI] [PubMed] [Google Scholar]
- 23.Przybelski R, Agrawal S, Krueger D, Engelke JA, Walbrun F, Binkley N. Rapid correction of low vitamin D status in nursing home residents. Osteoporosis Int. 2008 Nov;19(11):1621–1628. doi: 10.1007/s00198-008-0619-x. [DOI] [PubMed] [Google Scholar]
- 24.Stein MS, Scherer SC, Ladd KS, Harrison LC. A randomized controlled trial of high-dose vitamin D2 followed by intranasal insulin in alzheimer's disease. J.Alzheimer's Dis. 2011;26:477. doi: 10.3233/JAD-2011-110149. [DOI] [PubMed] [Google Scholar]
- 25.Sheridan PL, Hausdorff JM. The role of higher-level cognitive function in gait: Executive dysfunction contributes to fall risk in alzheimer's disease. Dementia & Geriatric Cognitive Disorders. 2007;24(2):125–137. doi: 10.1159/000105126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rapport LJ, Hanks RA, Millis SR, Deshpande SA. Executive functioning and predictors of falls in the rehabilitation setting. Archives of Physical Medicine & Rehabilitation. 1998 Jun;79(6):629–633. doi: 10.1016/s0003-9993(98)90035-1. [DOI] [PubMed] [Google Scholar]