Abstract
The prefrontal cortex occupies the anterior portion of the frontal lobes and is thought to be one of the most complex anatomical and functional structures of the mammalian brain. Its major role is to integrate and interpret inputs from cortical and sub-cortical structures and use this information to develop purposeful responses that reflect both present and future circumstances. This includes both action-oriented sequences involved in obtaining rewards and inhibition of behaviors that pose undue risk or harm to the individual. Given the central role in initiating and regulating these often complex cognitive and behavioral responses, it is no surprise that alcohol has profound effects on the function of the prefrontal cortex. In this chapter, we review the basic anatomy and physiology of the prefrontal cortex and discuss what is known about the actions of alcohol on the function of this brain region. This includes a review of both the human and animal literature including information on the electrophysiological and behavioral effects that follow acute and chronic exposure to alcohol. The chapter concludes with a discussion of unanswered questions and areas needing further investigation.
Keywords: Executive function, Orbitofrontal cortex, Working memory, Persistent Activity, Up-States
I. Introduction
It is now widely accepted that alcohol and other addictive drugs act within the mesolimbic dopamine (DA) system of the brain. This system originates in the ventral tegmental area (VTA) and projects to various limbic structures, including the nucleus accumbens (NAc), amygdala, and hippocampus. Most notably, it is thought that the positive reinforcing effects of drugs of abuse relate to enhanced DA neurotransmission particularly within the NAc. Therefore, virtually all models of the addiction neurocircuitry feature the mesolimbic DA system as central to the addictive process. However, evidence gained over the past decade or more suggests that drug-induced changes in the prefrontal cortex (PFC) also critically regulate drug and alcohol addiction (Everitt and Robbins, 2005; Kalivas and Volkow, 2005; Kalivas, 2009). This evidence comes from diverse studies that include human and animal behavioral work, brain imaging, electrophysiology, and molecular and cellular observations. Whereas a comprehensive review of the role of the PFC in addiction is beyond the scope of this chapter, our aim is to provide a review of the literature regarding the effects of acute and chronic alcohol exposure on PFC structure and function. Consistent with the accumulating evidence implicating the PFC in addiction to opiates, cocaine, and other psychostimulants, it is our contention that current models of the neurocircuitry of alcohol addiction should prominently include ethanol-induced alterations in PFC structure and function. We further suggest that long-lasting alterations in the executive function of the PFC and its associated networks may play an equal or even greater role in alcohol addiction and relapse to drinking than changes within the mesolimbic DA system.
II. Anatomy of the Prefrontal Cortex
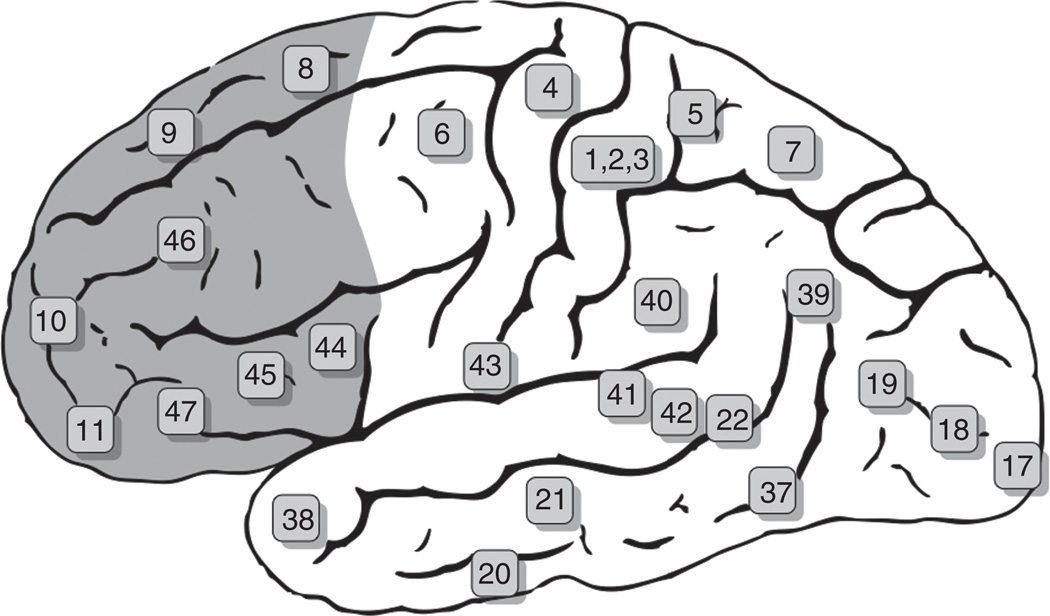
The cerebral cortex of humans and nonhuman primates is separated into a frontal and posterior region by the Rolandic fissure. The primary motor cortex (area 4) is a relatively narrow cortical area that lies immediately anterior to this fissure, and immediately anterior to the primary motor cortex is the premotor cortex (area 6). The PFC makes up the remaining anterior pole of the frontal cortex (Fig. 1, shaded area) and is divided into three interconnected subregions known as the lateral PFC, the medial/cingulate PFC, and the orbital PFC (commonly referred to as the orbitofrontal cortex (OFC)). Although overlapping but not identical, the term ventromedial PFC is sometimes encountered in the literature instead of OFC.
FIG. 1.
Brodmann areas of the human brain. Schematic shows the Brodmann areas of the human brain with those of the prefrontal cortex shaded in orange. From Grays Anatomy.
The PFC was originally termed the “frontal granular cortex” based upon the presence of a cortical granular layer IV and a location rostral to the agranular premotor cortex. However, subsequent comparisons across species of other cortical areas with known functional homology soon revealed that defining cortical regions based upon a granular versus agranular cytoarchitecture was invalid, and current definitions of the PFC utilize functional homology and anatomical connectivity across species. With regard to connectivity, the presence of dense reciprocal projections between the mediodorsal nucleus of the thalamus along with certain cortico-cortical connections is now considered the major defining structural feature of the PFC that is applicable to all mammalian species (Fuster, 2008).
While it must be kept in mind that there are generally no neat boundaries separating the subregions of the PFC, the human PFC can be grossly localized to specific Brodmann areas of the frontal cortex. The lateral PFC is composed principally of parts or all of Brodmann areas 8, 9, 10, and 46, and the medial PFC is composed principally of parts of areas 8, 9, 10, and 12, with areas 24, 25, and 32 forming the anterior cingulate (ACC). The orbitofrontal cortex (OFC) is the ventral portion of the frontal cortex on the dorsal surface of the orbit and is comprised of areas 11, 12, 13, and 14.
The PFC is extensively interconnected not only between cortical layers but also between subregions, and this connectivity helps to define distinct neural networks that exert behavioral control. The portion of the lateral PFC dorsal to the fundus of the principal sulcus receives afferent projections mainly from the medial PFC and belongs to the dorsolateral PFC network, whereas that portion that lies ventral to the principal sulcus receives afferent projections mostly from the OFC and belongs to the ventrolateral (sometimes referred to as orbitoventral) PFC network (Barbas and Pandya, 1989; Ongur and Price, 2000). In addition to reciprocal connections with the thalamus, the lateral PFC is connected either directly or indirectly with virtually all areas of the neocortex and hippocampus and has dense efferent projections to the dorsal caudate nucleus (e.g., dorsal striatum). There are distinct medial and orbital PFC networks that are also characterized by cortico-cortical connections and connections with brain regions outside the PFC. The medial PFC is connected with the thalamus and sends efferents to the hypothalamus and periaqueductal gray, and belongs to a network that plays a major role in autonomic and somatic responses to emotional stimuli. The ACC receives similar reciprocal projection as the medial PFC but is thought to belong to a network involved in attentional processing and conflict monitoring. The OFC is densely connected with the basal ganglia, amygdala, and other prefrontal areas, and belongs to a network that mediates motivational and affective aspects of behavior. The medial and OFC networks are sometimes grouped together as the orbitomedial PFC (OMPFC). The medial PFC and OFC are phylogenetically older regions, whereas the lateral PFC is a phylogenetically newer structure and provide substrates for executive cognitive functions. The role of the PFC in behavioral control is discussed in greater detail in the following section.
The extent to which rats have a PFC has been the subject of debate (Uylings et al., 2003; Seamans et al., 2008). The main issue of controversy is whether rats have a prefrontal area that is comparable with the dorsolateral PFC of primates. In their review of the extant human, nonhuman primate, and rodent literature, Uylings et al. (2003) concluded that, similar to primates, rodents do in fact have a region of the frontal cortex that can be defined both anatomically and functionally as PFC. They provide a strong argument that rats have a functionally divided prefrontal cortex that includes not only features of the medial and orbital areas in primates, but also some features of the primate dorsolateral PFC. In a more recent review, Seamans et al. (2008) took this a step further by suggesting that the rat medial PFC combines elements of the primate dorsolateral PFC and ACC at a rudimentary level that in primates may have formed the building blocks required for abstract rule encoding during evolutionary expansion of the PFC dorsally.
As in primates, the rodent PFC is located in the anterior pole of the frontal cortex and is loosely defined as the ACC, the medial PFC, and the OFC, as well as portions of the agranular insular cortex. The rodent medial PFC is subdivided into a dorsally located prelimbic mPFC (PrL-mPFC) and ventrally located infralimbic mPFC (IfL-mPFC) subregion. The OFC is also subdivided into the medial, ventral, lateral, and dorsolateral orbitofrontal regions. The connectivity of the rodent PFC has common features with that of primates, including a dorsal-to-ventral gradient of projection patterns. For example, the PrL-mPFC sends dense projections to the dorsal striatum that transitions to the ventral stratum (nucleus accumbens) as one moves ventrally through the IfL-mPFC to the OFC.
III. The Executive Function of the Prefrontal Cortex
The perception–action cycle, originally described by Arbib in 1985, is a behavioral construct of circular information flow that describes the interaction of an organism with its environment allowing it to carry out the orderly sequencing of goal-directed actions (Arbib, 1985). As pointed out by Fuster (2008), an important element of this construct is that it includes internal feedback from effectors to sensors to provide representations of current actions to sensory structures to modulate further input. In anatomical terms, there are two cognitive networks of this cycle (Fuster, 2008). One is the perceptual network of the posterior cortex and the other is the executive network of the frontal cortex. Although these networks are structurally distinct, they are not functionally independent and there is a great deal of information exchange between them. Furthermore, the cortical components of these networks do not act independent of subcortical structures. At the apex of the hierarchical organization of the perception–action cycle is the PFC.
Before proceeding further with a discussion of executive function, we should note there are different models of cognitive networks of the PFC. Prominent are variations of modular concepts where cognitive representations are essentially ascribed to different neural connections and anatomical locations (e.g., localization of working memory to the dorsolateral PFC) (Norman and Shallice, 1986). In contrast is the model of large-scale cortical networks first suggested by Bressler and expanded upon by Fuster (Bressler, 1995; Fuster, 2008). In this model, while cognitive networks may have foci, to one degree or another, in specific subregions of the PFC, they are widely distributed and bind together neuronal assemblies from widespread regions of the cortex during information processing. Importantly, a network can subserve different cognitive needs (e.g., memories), and different neurons or groups of neurons can participate in, or belong to, different networks depending upon the particular ongoing cognitive demands. The formation of these networks is presumably dependent upon attractor dynamics of cell assemblies that encode specific actions or memories.
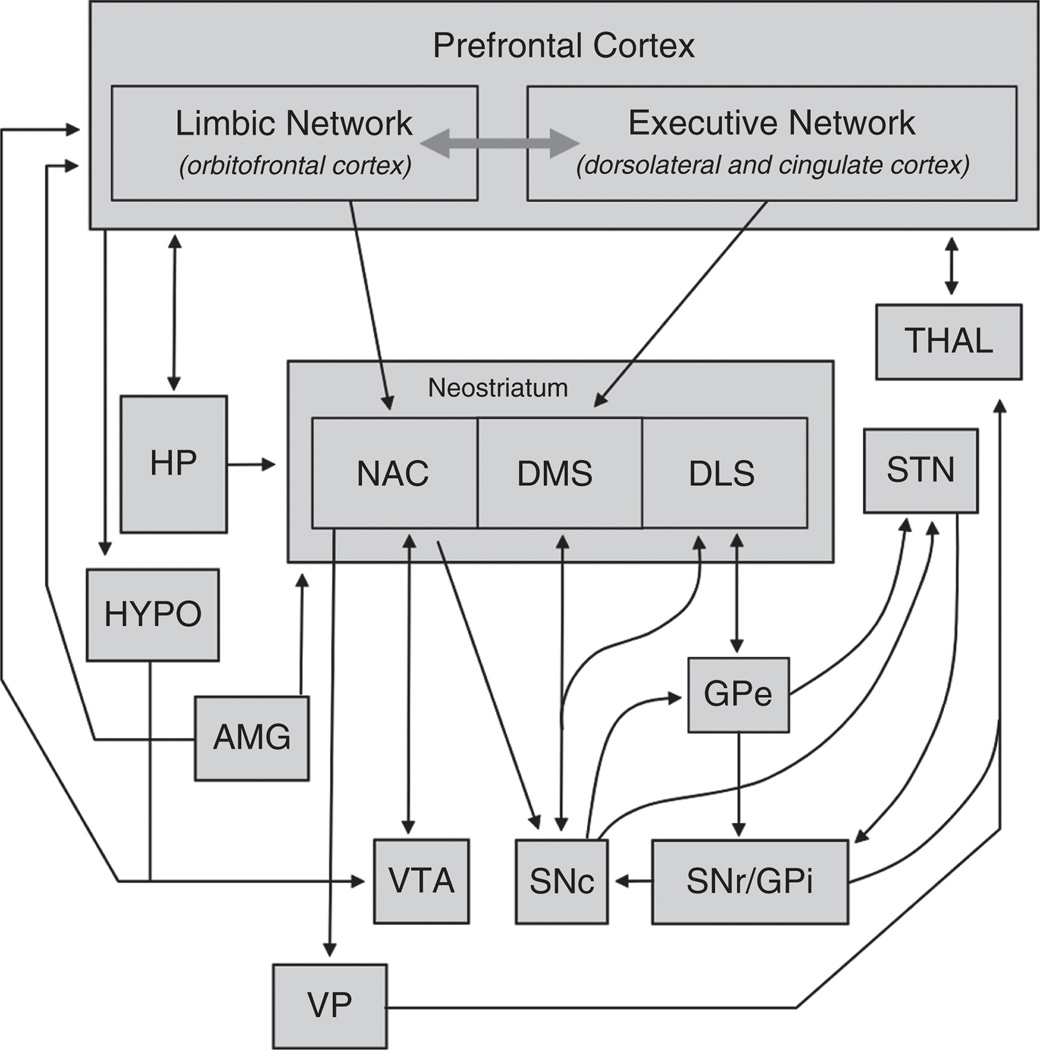
The primary function of the PFC can be summarized as the temporal organization of behavior. Executive function involves attention, planning, and decision making, and is how the PFC organizes behavior in a sequential manner. This occurs in large part through the dynamic interaction of two parallel networks of the PFC—an “executive” network with a primary location in the dorsolateral PFC (or medial PFC in rodents) and a “limbic” network primarily contained in the OFC (Fig. 2). These networks mediate higher-order behaviors such as purposeful goal-directed actions, language, and reasoning. These networks are required to perform a number of functions for appropriate control of goal-directed behavior (outlined by Moghaddam and Homayoun, 2008). In summary, they must (1) detect situations that demand mediation, (2) direct selective attention to stimuli relevant to this situation, (3) suppress distractions caused by irrelevant stimuli, (4) bring on line relevant past memories, (5) plan behavioral sequence based upon these memories and the present relevant stimuli, and (6) encode the preparatory set that leads to motor execution of the appropriate behavior. As noted above, the executive network is thought to be primarily localized within the dorsolateral PFC, and as such this structure is considered to be the ultimate regulator of goal-directed actions. However, the ACC also critically contributes to many aspects of executive functioning by mediating attention to action and conflict monitoring. Also as noted previously, the OFC is extensively connected with sensory cortical areas and limbic structures, and it is thought that this network plays a role in integrating and determining the salience of information about environmental contingencies. It plays a particularly important role in inhibitory control of inappropriate behaviors by relaying processed information to the executive control network of the dorsolateral PFC. Thus, the executive and limbic networks function in concert to orchestrate behaviors particularly related to context and expected outcomes (e.g., expectation of a goal-directed action).
FIG. 2.
Network organization of the PFC. Schematic shows the important connections of the limbic (orbitofrontal) and executive (dorsolateral and cingulate) divisions of the PFC. Abbreviations: HP (hippocampus), NAC (nucleus accumbens), DMS (dorsomedial striatum), DLS (dorsolateral striatum), THAL (thalamus), STN (sub-thalamic nuclei), HYPO (hypothalamus), AMG (amygdala), GPe (globus pallidus-external), VTA (ventral tegmental area), SNc (substantia nigra-compacta), SNr/ GPi (substantia nigra-reticulata/globus pallidus-internal), VP (ventral pallidum).
Any discussion of the executive function of the PFC would be remiss without at least mentioning working memory, one of the major executive cognitive functions of the PFC. Working memory is a form of sustained attention for the processing of prospective action. Thus, working memory involves the maintenance and manipulation of task-relevant information in the service of planning, problem solving, and predicting forthcoming events (Unterrainer and Owen, 2006; D’Esposito, 2007). Working memory is therefore a form of “active” memory involving sustained attention that is focused on an internal representation and can be distinguished from short-term memory. Although short-term memory often functions in the service of working memory (e.g., bringing a short-term memory online for the purpose of planning and predicting forthcoming events), it is classically viewed as temporary memory storage prior to its storage in long-term memory. Closely related to working memory is attentional “set”, which is essentially attention to motor activity and is used to plan a sequence of forthcoming actions. In a sense, working memory is the representation of the near past whereas set is a representation of the near future (Fuster, 2008), and together they are critical for the temporal organization of behavior.
Glutamatergic neurotransmission is the primary regulator of activity-dependent synaptic modifications that underlie experience-dependent brain plasticity (Chandler, 2003). As will be discussed in the next section, both acute and chronic alcohol exposure significantly impact glutamatergic neurotransmission in the PFC. In addition, results from several studies suggest that plastic processes of associative learning overlap and interact with those that underlie the development of addictive behaviors (Berke and Hyman, 2000). Addiction is clearly a complex disease that involves motivational and higher-order cognitive processes that initiate and control goal-directed behaviors, and accumulating evidence implicates altered glutamatergic neurotransmission mediated by projections to and from the prefrontal cortex in the neuroplasticity of addiction (Jentsch and Taylor, 1999; Kalivas, 2009). The PFC is highly integrated into the addiction neurocircuitry. Addictive behaviors, such as those associated with alcohol abuse and alcoholism, include loss of control over consumption and relapse to drinking. The PFC normally exerts “top-down” (e.g., information derived from prior experience) inhibitory control over internal and external sensory-driven compulsive behaviors. Increasing evidence suggests that continued drug exposure leads to attenuation of the ability of the PFC to monitor and inhibit these behaviors, with eventual loss of inhibitory control over drinking. Volkow and colleagues have conceptualized this loss of inhibitory control by the PFC as a syndrome of “impaired response inhibition and salience attribution” (Goldstein and Volkow, 2002; Volkow et al., 2003). This syndrome is envisioned to encompass an integrated cluster of addictive behaviors that depend upon interaction with the PFC. An important feature of the PFC is that it is functionally and structurally adaptable, and a major component of cortical cognitive processing is that it is highly influenced by “knowledge” of past experiences. Thus, reward information such as that provided by the VTA dopaminergic system has a pervasive and lasting influence on the activity of the PFC.
IV. Electrophysiological Properties of the PFC
A. Firing Properties of Cortical Pyramidal and Interneurons
1. Pyramidal Neurons
In the neocortex, glutamatergic pyramidal neurons represent approximately 80% of the neuronal population and have been best studied in sensory cortices, especially the visual cortex. Neocortical pyramidal neurons are arranged in layers (I–VI in most parts of cortex, rodent PFC lacks a well-defined layer IV) and make connections with both glutamatergic and γ-amino-butyric acid (GABA) neurons within the cortex and those in subcortical areas. Although less well characterized than interneurons (see below), cortical pyramidal neurons also show diversity with respect to size, dendritic morphology, and firing properties. In general, cortical pyramidal neurons are grouped into two general classes, regular spiking (RS) neurons that show little or slow adaptation during repetitive firing and intrinsically bursting (IB) neurons that display more complex periods of firing. In the prefrontal cortex of both rodents and primates, most RS neurons (RS1 subtype) respond to current injection by smooth increases in action potential (AP) frequency with little change in AP threshold or amplitude (Zhang, 2004; Chang and Luebke, 2007). RS2 neurons show decreasing action-potential height and an increase in AP firing threshold whereas fast-accommodating (FA) RS neurons show similar changes in AP characteristics along with a robust accommodation in spiking. Intrinsically bursting (IB) neurons make up only a small percentage of neocortical pyramidal neurons and generate three to six spikes during threshold amounts of stimulation.
In rodents, these patterns of activity develop during the first two postnatal weeks and are not complete until adulthood (Zhang, 2004). With respect to morphology, two general classes of pyramidal neurons have been proposed, with Type I neurons having thick apical dendrites that terminate in tufts in superficial cortical layers. These neurons project axonally to primarily sub-cortical structures. Type II neurons have less apical branching and project intracortically as well as to the striatum. Generally, Type II neurons are regular spiking whereas Type I neurons show bursting patterns of action potentials (Molnar and Cheung, 2006). All cortical pyramidal neurons show complex dendritic branching patterns and asymmetrical synapses characterized by the dendritic spine, the specialized structure that receives excitatory input. In general, PFC pyramidal neurons show more branching and greater density of spines compared with those in visual cortex and other sensory cortices (Elston, 2003).
2. Interneurons
Although GABAergic neurons within neocortex represent only 20% of the neuronal population, there is a great deal of diversity with respect to their anatomical, biochemical, and functional properties. The Petilla Interneuron Nomenclature Group (named in honor of Ramon Y Cajal’s hometown) has recently suggested a set of common terms to classify the various subtypes of interneurons (Ascoli et al., 2008). Although beyond the scope of the present review, the conclusions of this group are summarized below. GABAergic neurons within the neocortex (interneurons) usually have short axons that bifurcate within a cortical column but can project across columns. Despite this general scheme, there are long-axon GABA neurons that project to distal subcortical structures although less is known about these types of neurons. Cortical GABAergic interneurons show a wide variety of axonal and dendritic branching patterns that have historically been grouped into several categories, including basket cells, chandelier cells, bipolar cells, double bouquet cells, bitufted cells, and neurogliaform cells (Markram et al., 2004). These different cell types specialize in their location of axon innervation of target neurons, including soma and proximal dendrites (basket cells), more distal dendrites (double bouquet, bipolar, bitufted, neurogliaform, Martinotti cell), and axon initial segments (chandelier). These distinct patterns of target neuron innervation allow for precise control of both input (dendritic processing and integration) and output (somatic and initial segment) functions of the postsynaptic neuron. Interneurons are also classified according to their expression of different calcium binding proteins (parvalbumin, calbindin, and calretinin) and neuropeptides (neuropeptide Y, vasoactive intestinal peptide, somatostatin, and cholecystokinin). In terms of electrophysiological responses, neocortical interneurons display a wide range of spiking patterns in response to steady-state current injection. These include non-accommodating, accommodating, stuttering, irregular spiking, and bursting. Within the prefrontal cortex, most studies have recognized four different classes of interneurons based on spiking patterns during current injection (Kawaguchi, 1995; Gorelova et al., 2002). Fast-spiking (FS) neurons are the most numerous and display very rapid action potentials (~0.5ms half-width) each followed by a deep after-hyperpolarization. FS neuron firing is non-accommodating and rarely do these neurons show bursting type of activity. Late spiking (LS) interneurons show a slowly developing depolarization during current injection that eventually triggers spikes. LS cells also are non-accommodating and have spike widths approximately twice that of FS neurons. Low threshold spike (LTS) neurons generate action potentials upon current injection only when cells are held at fairly hyperpolarized potentials (< –70mV). Action potentials also arise at the end of a hyperpolarizing pulse. Regular spiking nonpyramidal (RSNP) neurons show a slow adaptation to firing and have prominent hyperpolarization-induced inward currents (IH). When combined with morphological and biochemical analysis, FS neurons include some basket cells, PV positive neurons that are also positive for GABA, and some chandelier cells (Kawaguchi, 1995; Markram et al., 2004). LS neurons are neurogliaform cells while LTS neurons have axons terminating on Layer I neurons. RSNP neurons are not easily classified into any of these groups and likely are comprised of multiple and diverse members.
B. Network Activity
1. Persistent Activity-In Vitro Models of PFC Function
Recordings from prefrontal cortex in awake behaving monkeys revealed sustained periods of firing during delay periods in visual-guided tasks designed to assess working memory demands (Williams and Goldman-Rakic, 1995; Ichihara-Takeda and Funahashi, 2007). These persistent patterns of firing are maintained in the absence of visual cues and were interpreted as a cellular correlate of the PFC’s ability to hold and manipulate information in anticipation of a future action. Work from Steriade and others also described a slow oscillation (0.5–2Hz) in prefrontal cortex during periods of quiet wakefulness or sleep (Steriade et al., 1993). The slow oscillation consisted of periods of hyperpolarized down-states interrupted by brief transitions into depolarized up-states during which action potentials often arose. One hallmark of this activity is the relatively constant amount of depolarization produced during an up-state (~10–15 mV) that persists for several seconds. During active waking periods or REM sleep, neurons reside mostly at up-state membrane potentials consistent with enhanced sensory input and synaptic drive. In acute slices of prefrontal cortex, neurons usually do not display spontaneous oscillations of membrane potentials (termed bistability) or action potential firing although these patterns can be elicited under certain conditions. For example, acute slices from the ferret visual or prefrontal cortex showed spontaneous oscillations in membrane potential when the ionic composition of the bathing solution was modified to mimic that of brain cerebrospinal fluid (Sanchez-Vives and McCormick, 2000). In rodents, slices of prefrontal cortex that are maintained in organotypic cell culture develop spontaneous oscillations in membrane potential during the first 2 weeks that are maintained for the life of the culture (Seamans et al., 2003; Tu et al., 2007). Similar patterns of activity can be induced in acute rodent slices by supplementing the bathing solution with optimal concentrations of NMDA and dopamine (Tseng and O’Donnell, 2004). Up-states in organotypic PFC cultures can also be evoked by applying brief trains of electrical pulses through a stimulating electrode and are accompanied by robust action potential firing. Paired recordings of neurons in PFC cultures show that the initiation and termination of up-states are highly synchronized between pyramidal neurons and fast-spiking interneurons although action potential firing is typically asynchronous (Seamans et al., 2003; Woodward and Pava, 2009). Pharmacological analysis of PFC up-states in PFC cultures reveals several components including an initial EPSP:IPSP sequence driven by AMPA and GABAA receptors followed a prolonged inward current composed of both AMPA- and NMDA-mediated events (Seamans et al., 2003).
V. Neuromodulation of PFC Neuron Activity
A. Dopamine
Dopamine modulation of PFC function has long been known to be important for optimizing cognitive performance, especially in tasks requiring working memory. Disruption of this important modulatory input is associated with various neurological and psychiatric illnesses. PFC dopamine arises from terminals provided by dopaminergic neurons whose cell bodies reside in the ventral tegmental area (VTA). These neurons also provide dopaminergic tone to other limbic structures including nucleus accumbens and recent evidence suggests that there are discrete subpopulations of VTA dopamine neurons that display both anatomical and functional specializations depending on their pattern of innervation (Lammel et al., 2008). There is a rich literature regarding the effects of dopamine on PFC-associated behaviors and neuronal function and the reader is referred to several excellent reviews of this material (Tzschentke, 2001; Seamans and Yang, 2004). A brief summary of the key findings as they relate to PFC electrophysiology is presented here. In the prefrontal cortex, dopamine D1 receptors are expressed to a higher degree than other subtypes and are located primarily on dendritic spines and shafts of target neurons. D2 dopamine receptors are expressed both pre- and postsynaptically, and receptors on axon terminals reduce the release of both glutamate and GABA. In general, most studies have shown that dopamine transiently reduces neuronal excitability followed by a long-lasting enhancement in firing activity. These effects arise from both modulation of intrinsic mediators of excitability (voltage-sensitive Na+ and K+ channels) and changes in glutamatergic and GABAergic synaptic transmission. D1-mediated responses are associated primarily with a long-lasting increase in excitability, with NMDA currents being particularly sensitive to low concentrations of dopamine that potentiate NMDA EPSCs. At higher doses, dopamine and certain D1 agonists may actually inhibit NMDA function via direct channel blockade suggesting caution in interpreting results when high concentrations of these compounds are used (Cui et al., 2006). Dopamine also enhances the firing activity of fast-spiking but not other types of interneurons in the prefrontal cortex (Gorelova et al., 2002). This effect is blocked by D1 antagonists and involves the cAMP:PKA signaling pathway (Trantham-Davidson et al., 2004). Higher concentrations of dopamine lead to suppression of interneuron excitability via a D2-receptor-mediated pathway that leads to enhanced phosphatase activity and reduced release of GABA (Trantham-Davidson et al., 2004, 2008). In a recent study that utilized the slice culture model described above, Kroener et al. (2009) investigated the impact of dopamine on persistent activity of deep-layer pyramidal neurons. These cultures contained slices of PFC and VTA, and dopaminergic neurons established a strong innervation of the PFC slice consistent with that observed in vivo. Adding 1 uM or more exogenous dopamine reduced up-state duration and spiking activity whereas dopamine enhanced up-state duration and firing in cultures lacking endogenous DA tone. Action potentials induced by direct current injection during down-state periods were enhanced by dopamine even at concentrations as low as 100nM. Dopamine also enhanced the ability of stimulus-evoked EPSPs to induce firing when these events were generated during an up-state. This effect was concentration dependent as up-state duration and spiking were unaffected by 10 nM DA but were inhibited by 10 µM DA. These data are consistent with dopamine’s role in tuning PFC function during working memory tasks and support the idea that dopamine’s actions are optimal over a narrow concentration range (Goldman-Rakic, 1999).
B. Norepinephrine
Norepinephrine (NE) is also an important catecholamine modulator of PFC function and NE neurons that innervate the PFC originate in the locus ceruleus. NE content is generally lower in the PFC than dopamine and NE fibers appear to target more superficial layers as compared to dopamine. NE’s effects on PFC neuronal function are mediated primarily by α1, α2a, and β1 adrenergic receptors. Its affinity for these subtypes is greatest for α2a receptors and this subtype is thought to underlie most of NE’s modulation of PFC function during normal, nonstressed waking conditions. The effects of methylphenidate, an indirect NE agonist, on the intrinsic excitability of deep-layer PFC pyramidal neurons have recently been studied using whole-cell patch clamp electrophysiology (Andrews and Lavin, 2006). Methylphenidate enhanced current-evoked spike activity and this effect was blocked by α2a antagonists but not those selective for α1 or β1 receptors. Interestingly, the effect of methylphenidate on firing rate was abolished when blockers of synaptic transmission were added to the bath suggesting that NE may suppress the activity of fast-spiking interneurons that normally regulate pyramidal neuron excitability. In addition to these findings, the effect of activating adrenergic receptors on NMDA EPSCs recorded from deep-layer pyramidal neurons in the rodent PFC has also been investigated. Norepinephrine itself reduced stimulus-evoked NMDA EPSCs and this effect was mimicked by both α1 and α2 receptors agonists (Liu et al., 2006). This study also demonstrated that adrenergic receptor suppression of NMDA responses involved different intracellular signaling pathways with α1 effects mediated via the PLC-IP3-Ca2+ pathway and α2 effects occurring through inhibition of the cAMP-PKA signal transduction process. The α1 calcium-mediated inhibition of PFC activity may also arise via activation of calcium-sensitive potassium channels (SK) that contribute to the regulation of the after-hyperpolarization. Hyperpolarization-activated cyclic-nucleotide gated channels (HCN) may reduce activity through shunting inhibition when levels of cAMP are high. Modulating these channels pharmacologically improves working memory performance in rodents consistent with the idea that optimal levels of NE signaling are required for proper PFC function (Arnsten, 2009).
C. Serotonin
Serotonin (5-hydroxytryptamine, 5-HT) neurons originate in the raphe nuclei of the pons and cerebellum and project widely to subcortical and cortical structures, including both medial and orbital aspects of the prefrontal cortex. The 5-HT2A receptor subtype is thought to mediate many of the effects of psychedelic hallucinogens such as lysergic acid diethylamide, and dysregulation of 5-HT signaling in PFC is thought to contribute to the pathology of schizophrenia and mood disorders. Although 5-HT activates a large number of receptors including an ion channel subtype (5-HT3), 5-HT1A and 5-HT2A receptors are thought to be the predominant forms expressed by PFC neurons (Kia et al., 1996; Willins et al., 1997; Santana et al., 2004). Activation of 5-HT2A receptors enhances the frequency of both inhibitory and excitatory postsynaptic currents recorded in pyramidal neurons, with less effect noted for interneurons (Zhou and Hablitz, 1999). The 5-HT2A-dependent increase in spontaneous events was not observed in the presence of TTX suggesting that these receptors do not alter presynaptic release of glutamate. Whereas 5-HT or 5-HT2A agonists generate only small, subthreshold depolarizations in most PFC pyramidal neurons tested, approximately a third of large pyramidal neurons in layer V/VI were strongly depolarized by these treatments (Beique et al., 2007). This is consistent with data from in situ hybridization studies showing that approximately 50% of glutamatergic neurons in prelimbic PFC express 5-HT2A mRNA with 26% of layer VIa showing strong expression (Santana et al., 2004). These findings suggest that the 5-HT2A-dependent increase in spontaneous events recorded in PFC neurons is due to direct excitation of a small number of excitatory neurons that spreads throughout the PFC due to the strong recurrent connections among deep-layer pyramidal neurons.
In contrast to these results, activation of 5-HT1A receptors inhibits neuronal excitation through enhancement of potassium currents and inhibition of calcium channels (Araneda and Andrade, 1991). 5-HT receptors also appear to directly influence GABA and NMDA-mediated conductances with 5-HT2A/C receptors inhibiting GABA-induced currents via a PKC-dependent pathway (Liu et al., 2006). NMDA receptor currents are inhibited by 5-HT1A receptors whereas 5-HT2A activation enhances NMDA function. These effects appear to result from changes in NMDA receptor trafficking with inhibition and activation, respectively, of ERK phosphorylation of the MAP kinase pathway (Yuen et al., 2008). Although PFC neurons express both 5-HT1A and 5-HT2A receptors, these subtypes have a differential affinity for serotonin suggesting that activation of raphe serotonin neurons may initiate opposing changes in NMDA function in the PFC that depends on the extent and rate of firing. 5-HT also influences glutamatergic plasticity of PFC pyramidal neurons. Activation of 5-HT2A/C receptors during tetanic stimulation of deep-layer pyramidal neurons led to long-term depression of AMPA-mediated synaptic EPSCs (Zhong et al., 2008). This effect was NMDA independent but was blocked by antagonists of group I metabotropic glutamate receptors as well as inhibitors of P38 MAP kinase.
D. Endocannabinoids
Endocannabinoids (ECs) are neuromodulators derived from membrane phospholipids during periods of neuronal activity. Synthesis occurs in the postsynaptic neuron and is calcium dependent while EC action takes place on the presynaptic terminal, thus linking neuronal activity to the production of a retrograde messenger. ECs suppress the presynaptic release of GABA and glutamate through a process termed depolarization-induced suppression of inhibition or excitation (DSI, DSE) that requires activation of cannabinoid-type I receptors (CB1). ECs are rapidly degraded by enzymatic pathways involving fatty acid amide hydrolase (FAAH) or monoacylglycerol lipase (MGL) that preferentially inactivate anandamide and 2-acylglycerol (2-AG), the two best characterized endocannabinoids in the brain. EC modulation of synaptic transmission has been studied in most brain regions examined, including the cerebral cortex where both DSE and DSI have been described. However, most of these studies are confined to somatosensory cortex and show that in this region, EC modulation of signaling is layer specific with DSE and DSI occurring in layer II/III neurons while only DSE is observed in layer V pyramidal neurons (Fortin and Levine, 2007). In one study of layer V PFC neurons from juvenile rats, bath application of the CB1 agonist WIN55212-1 significantly decreased stimulus-evoked EPSCs and this effect was blocked by the CB1 antagonist SR141716A (Auclair et al., 2000). The decrease in excitatory transmission was mediated by a reduction in presynaptic release as WIN reduced the frequency but not the amplitude of evoked asynchronous EPSCs. WIN also shifted the plasticity of layer V PFC neurons toward long-term depression, consistent with its effect on presynaptic glutamate release. Endogenous cannabinoids also mediate PFC neuronal plasticity as stimulation of layer II/III afferents induces a long-lasting LTD in layer V pyramidal neurons from mouse PFC (Lafourcade et al., 2007). This depression was prevented by AM251, a CB1 antagonist, and was again mediated by a reduction in the frequency but not the amplitude of spontaneous EPSCs. The mechanism underlying the induction of EC synthesis was also investigated and was found to require mGluR5 receptors and activation of intracellular phospholipase C. Taken together, the results of these studies suggest that the endocannabinoid system is an important regulator of PFC neuronal activity and support results from behavioral studies showing impairments in working memory following use of cannabis (Riedel and Davies, 2005).
VI. Effects of Alcohol on PFC Neuron Function—In Vitro Studies
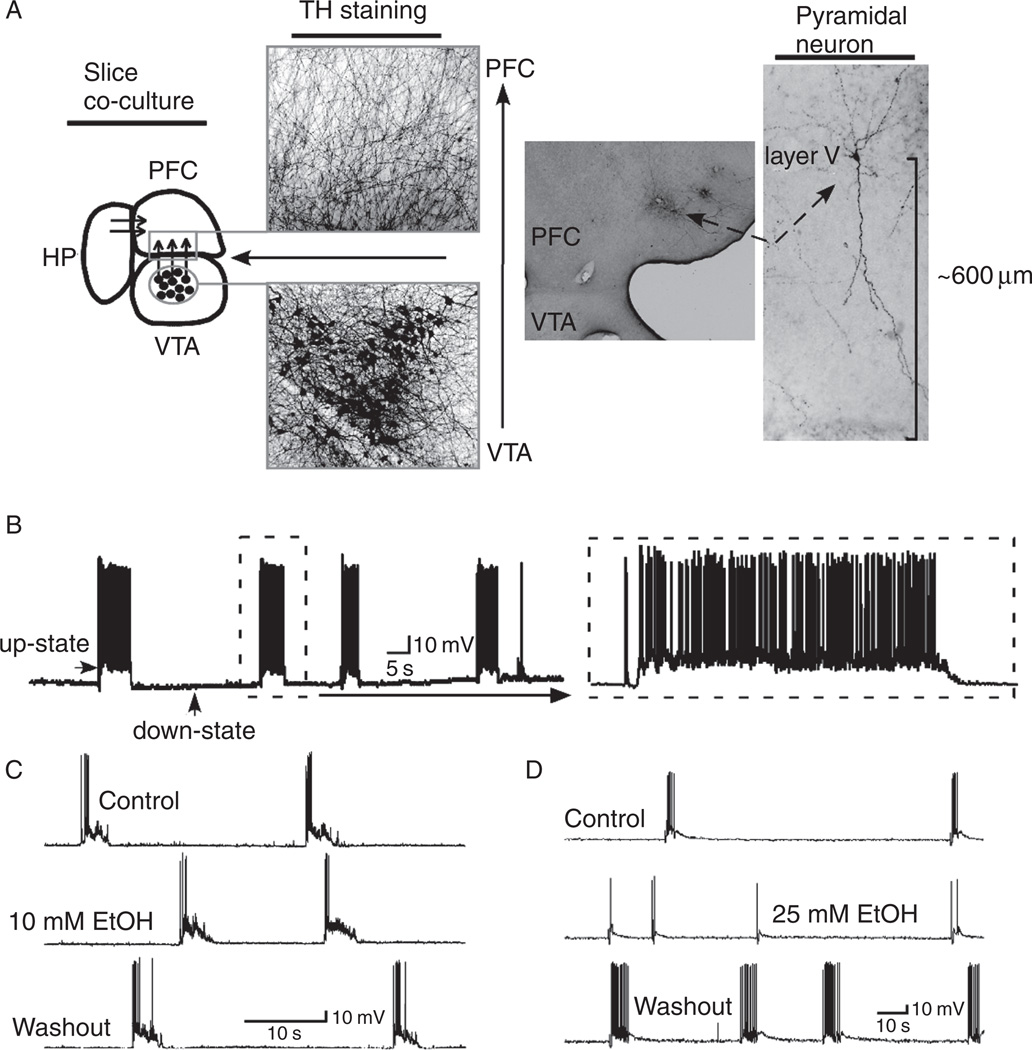
Although the effects of biogenic amines and endogenous cannabinoids on PFC neuron excitability have been examined using in vitro electrophysiological techniques, there is much less known about the actions of alcohol on these neurons. Recent studies from the authors’ laboratories have investigated the effects of acute ethanol exposure on persistent activity patterns in PFC neurons in organotypic cell culture and on synaptic transmission in slices from rat prefrontal cortex. Tu et al. (2007) used triple-slice organotypic cultures that contained prefrontal cortex, hippocampus, and ventral tegmental area. As described earlier, pyramidal neurons in the prefrontal cortex portion of these cultures develop spontaneous patterns of persistent activity within 2 weeks of culture, and up-states can also be evoked by direct electrical stimulation of these cultures (Fig. 3). Ethanol, applied via the bath solution, had no effect on firing of PFC pyramidal neurons induced by direct current injection but reduced the duration, amplitude, and spike activity of spontaneous up-states. These effects occurred at the beginning at 17mM (~0.08% blood ethanol concentration) and persistent activity of PFC neurons was almost totally suppressed at 50mM ethanol. Up-state duration was affected at lower concentrations of ethanol (25 mM) than was up-state amplitude, although both measures were reduced as ethanol concentrations were increased. Following washout of the ethanol solution, up-state duration and spiking were often enhanced as compared to pre-ethanol control values and this rebound in activity persisted for 10–15min post washout.
FIG. 3.
Up-states in PFC slice cultures are inhibited by ethanol. Panel A shows arrangement of prefrontal cortex (PFC), hippocampus (HP), and ventral tegmental area (VTA) in brain slice co-culture. Picture shows dopamine neurons stained for tyrosine hydroxylase (TH). Panel B shows example of up-states recorded from a deep-layer PFC pyramidal neuron. Ethanol reversibly inhibits up-state activity at a concentration of 25 mM (panel D) but not 10 mM (panel C). Taken from Tu et al. (2007).
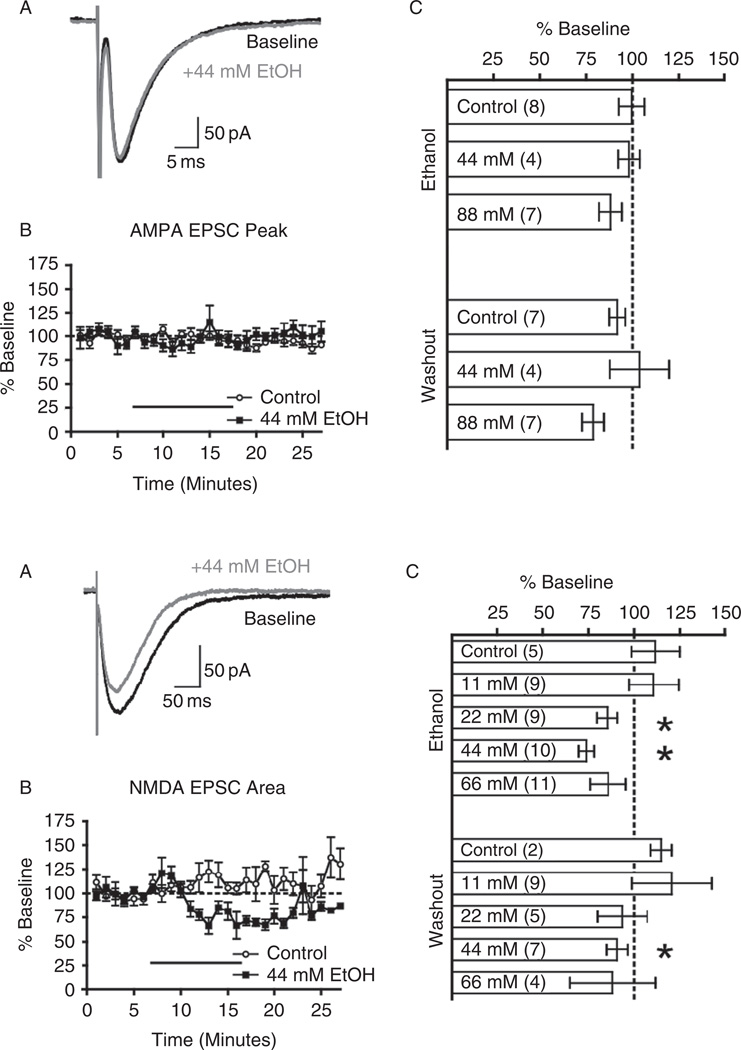
Ethanol also inhibited up-states evoked by brief electrical stimulation of the VTA designed to mimic the in vivo bursting pattern of DA neurons upon presentation of rewarding stimuli (Schultz, 1986; Bayer et al., 2007). As compared to spontaneous up-states, there was less inhibition of VTA-stimulated persistent activity and this difference was eliminated when the D1 receptor antagonist SCH23390 was included in the bath solution. These results suggest that the phasic release of dopamine induced by DA neuron bursting can partially offset the inhibitory actions of ethanol on prefrontal neuron function. The mechanism underlying this effect is not known but may involve potentiation of NMDA receptors that are an important component of up-states. In a follow-up study, we examined the effects of acute ethanol on pharmacologically isolated synaptic currents in deep-layer pyramidal neurons of rat prefrontal cortex (Weitlauf and Woodward, 2008). Ethanol, at concentrations up to 88mM (~0.4% blood ethanol concentration), had no significant effect on evoked or spontaneous AMPA-mediated EPSCs (Fig. 4). Inhibitory currents mediated by the synaptic release of GABA were also largely insensitive to high concentrations of ethanol and no effects were observed in the paired pulse ratio of evoked GABA responses or in the frequency or amplitude of spontaneous GABAA IPSCs. These studies also showed no evidence for a tonic GABA-mediated current in deep-layer PFC pyramidal neurons or any effect of ethanol on the holding current that would have been expected if ethanol-sensitive extrasynaptic GABAA receptors were present. In contrast to these effects, ethanol inhibited stimulus-evoked NMDA-mediated EPSCs with significant effects occurring at concentrations of 22mM (~0.1% blood ethanol concentration) and above. Unlike that found in the slice cultures, there was no evidence for any rebound in activity following ethanol washout for any of the synaptic currents examined. These results suggest that NMDA receptors are a particularly important target for the acute effects of ethanol on PFC pyramidal neuron function and that ethanol’s inhibition of persistent activity in slice cultures is a result of this effect. This is consistent with the ability of a low concentration of the NMDA antagonist AP5 to reduce up-state duration, amplitude, and spiking in PFC cultures (Tu et al., 2007).
FIG. 4.
Acute ethanol inhibits NMDA but not AMPA-mediated EPSCs in PFC pyramidal neurons. Left panels (A–C) show lack of significant effect of ethanol on AMPA-mediated EPSCs from deep-layer PFC pyramidal neurons. Right panels (A–C) shows that ethanol inhibits NMDA-mediated EPSCs from deep-layer PFC pyramidal neurons. Taken from Weitlauf and Woodward, 2008.
In a recent study, we recorded from both pyramidal and fast-spiking GABA interneurons in PFC cultures and examined how ethanol affects up-state activity in these neurons (Woodward and Pava, 2009). During dual patch-clamp recordings, neurons entered and exited the up-state simultaneously regardless of the type of neuron pairs recorded (pyramidal:pyramidal; interneuron:interneuron; pyramidal:interneuron). These findings are consistent with the idea of highly interconnected recurrent networks of excitatory and inhibitory neurons in the PFC. In FS GABAergic inteneurons, fast EPSPs could be observed during up-states whereas the up-state depolarization in pyramidal neurons was smooth and relatively constant as previously reported (McCormick et al., 2003; Seamans et al., 2003). However, individual EPSPs in pyramidal neurons were revealed by intracellular perfusion of the NMDA channel blocker MK801 supporting the suggestion from Seamans et al. (2003) that the slower kinetics of NMDA receptors bridge the fast transients gated by AMPA receptors. Intracellular MK801 had little effect on the up-state dynamics of FS neurons consistent with the reduced expression of NMDA receptors expression in this subpopulation of PFC interneurons (Wang and Gao, 2009). Despite these differences in functional NMDA receptors, bath application of ethanol reduced up-state duration by approximately the same amount in both pyramidal and interneurons. This finding again underscores the highly interconnected nature of prefrontal neurons and how they contribute to network activity.
VII. Effects of Alcohol on PFC Neuron Function—In Vivo Animal Studies
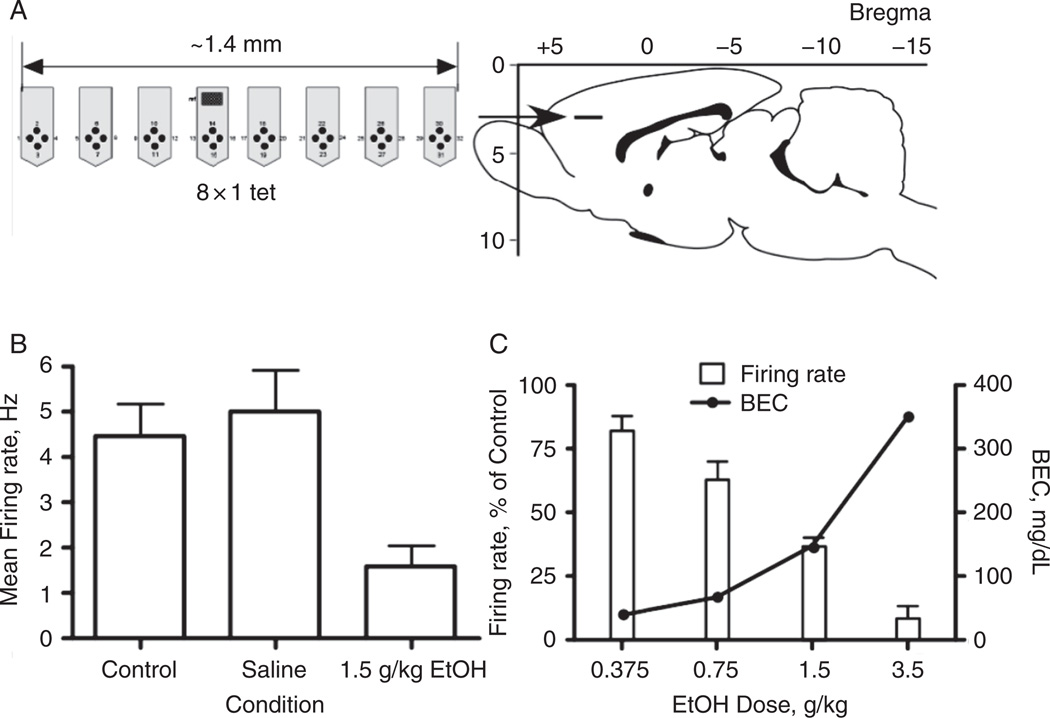
Acute administration of ethanol to experimental animals is associated with changes in various neurotransmitter systems in the PFC. Several studies have examined these effects using outbred strains of rats. For example, µ-opioid receptors were upregulated in frontal cortex 2 h after intragastric administration of 2.5 g/kg ethanol (Mendez et al., 2001). In Wistar rats, ethanol (1 g/kg) increased the extracellular levels of 5-HT as measured by in vivo microdialysis (Langen et al., 2002). One day of exposure to an ethanol-containing liquid diet decreased CB1 receptor levels in rat PFC (Rubio et al., 2009). Measures of the immediate-early gene Fos have been measured in rat PFC neurons following acute administration of ethanol to gauge its effects on activity. Ethanol increased Fos staining with apparently equal increases in both GABAergic and glutamatergic neurons (Leriche et al., 2008). In contrast to these results, spontaneous spike activity of prefrontal neurons in Sprague-Dawley rats as measured by in vivo multi-array electrodes (Fig. 5) was decreased in a dose-dependent manner by ethanol (Tu et al., 2007). The differences between the results of the Fos and spike activity studies may be due to the use of different sampling times or the use of an anesthetized animal in the recording studies.
FIG. 5.
Ethanol inhibits spike firing of PFC neurons in vivo. Panel A shows the arrangement of multi-electrode array and location in the brain. Panel B shows effects of saline or ethanol on spike firing of PFC neurons. Panel C demonstrates that ethanol inhibits PFC firing in a dose-dependent manner and is correlated with blood ethanol concentration (BEC). Taken from Tu et al. (2007).
In addition to these studies with outbred rats, a variety of rodent models have been developed through selective breeding to generate animals with a high propensity for ethanol self-administration and dependence. These animals may better mimic the drinking patterns of human alcoholics and provide an opportunity to investigate how genetic, structural, and functional differences in individuals may predispose them to alcohol dependence. For example, the alcohol-preferring P rat consumes high amounts of alcohol naturally unlike outbred strains that require time-consuming behavioral training sessions to overcome their initial resistance to alcohol consumption. P rats show a higher level of glucose utilization in PFC than non-alcohol-preferring rats in a drug-naive state (Smith et al., 2001). In response to an acute EtOH challenge, P rats show a divergent response with low doses increasing PFC glucose utilization, and higher doses producing a decrease. Alcohol non-preferring rats, on the other hand, showed no change to the acute ethanol challenge (Strother et al., 2005), indicating that enhanced sensitivity to ethanol in PFC may play a role in the development of alcohol dependence.
With respect to mice, the DBA/2J (DBA) strain shows relatively low levels of voluntary ethanol consumption as compared to C57BL/6J (C57) mice. Microarray analysis has demonstrated very different patterns of gene activation in PFC of these two strains in response to acute alcohol exposure. DBA mice showed a greater change in the number of genes that were upregulated in response to EtOH than their C57 counterparts (Kerns et al., 2005). Among those transcripts upregulated were a number of genes that encode for glucocorticoid-binding proteins and cortisol levels are known to increase after acute alcohol consumption (King et al., 2006). This suggests that the divergent response in PFC gene expression between the two strains following ethanol challenge may indicate a differential response to circulating glucocorticoids. Other genes that were upregulated in the microarray study included those related to synaptic plasticity and myelination. Interestingly, there was grouping of upregulated genes around specific chromosomal regions suggesting some common element that underlies ethanol-responsive genes. Chronic EtOH has been shown to alter the acetylation of histones (Pascual et al., 2009), so that chromatin rearrangement following ethanol exposure may play a role in these changes in gene expression. Alcohol-preferring AA rats show increased phosphorylation and subsequent inhibition of GSK-3β, involved in metabolism and possibly neurotransmission, in response to increased phosphorylation of AKT, a regulator of GSK-3β (Neznanova et al., 2009).
Other studies suggest differences in neurotransmission in the PFC between different rat strains. The Lewis rat strain self-administers alcohol at greater levels than the Fisher rat. The Lewis rats demonstrated lower basal levels of glutamate in the PFC than did the Fisher strain (Selim and Bradberry, 1996). In addition, alcohol-naive P rats have a lower extracellular concentration of DA in medial PFC than do non-EtOH preferring Wistar rats (Engleman et al., 2006). In the same study, however, acute EtOH administration did not alter DA levels in the PFC of either group. By contrast, the Sardinian alcohol-preferring rat (sP) showed greater basal levels of DA in the mPFC than did the Sardinian alcohol-nonpreferring (sNP) strain (Leggio et al., 2003). Although this does not rule out a role for PFC DA basal levels in alcohol seeking or the development of alcohol dependence, it is clear that factors other than basal DA levels are involved. For example, alcohol-preferring AA rats showed higher levels of mu-opioid binding in PFC under drug-naive conditions than did alcohol-avoiding ANA rats, as well as higher levels of proenkephalin mRNA (Marinelli et al., 2000). Differences have also been observed in PFC 5-HT receptor levels in PFC between strains and sP rats demonstrated lower levels of basal 5-HT2A-binding sites than sNP animals (Ciccocioppo et al., 1997). Given the powerful effects of these neurotransmitters on PFC function, it is likely that differences in expression or functional activity of these neuromodulators is important in shaping the PFC’s response to ethanol.
VIII. Effects of Alcohol on PFC Neuron Function—Human Studies
A. Acute Ethanol
Acute ethanol administration in humans has been shown to cause deficits in executive activities that are thought to require the PFC. For example, performance in a spatial recognition task and a planning task was decreased in social drinkers while inebriated (Weissenborn and Duka, 2003) and acute EtOH has been shown to cause poorer decision making using a gambling task to assess PFC function (George et al., 2005). Working memory (WM) tasks are also commonly used to test executive function given the role that the PFC has been shown to play in mediating this activity. However, the effects of acute EtOH on working memory performance in humans are mixed. One study observed alcohol-induced WM deficits in a backward digit span task only among those who demonstrated a high baseline WM performance when sober (Finn et al., 1999). The study that demonstrated deficits in a spatial recognition task (Weissenborn and Duka, 2003) failed to show a decrease in spatial WM performance in alcohol-exposed subjects, whereas impairments in a memory scanning task following acute ethanol challenge have been reported (Grattan-Miscio and Vogel-Sprott, 2005). To try and reconcile these conflicting data, Saults and colleagues examined the effects of ethanol on different types of working memory tasks that varied in two dimensions of substrates that needed to be retained for completion of the task. These authors showed that consumption of alcohol impaired memory for auditory and visual sequences but not that for simultaneous presentations of auditory or visual stimuli (Saults et al., 2007). They concluded that ethanol impaired certain strategies for holding information online rather than causing a general decrease in the size of the working memory buffer.
A number of studies have sought to examine the effects of EtOH on PFC function in humans using brain imaging techniques. Acute administration of alcohol increases blood flow in the right prefrontal cortex of healthy patients (Tiihonen et al., 1994) and this effect appears dose dependent. PFC blood flow was significantly increased after doses of 0.7 g/kg or 1 g/kg EtOH (Volkow et al., 1988; Sano et al., 1993) whereas flow was reduced when 1.5 g/kg EtOH was administered. Although cerebral blood flow is a useful proxy for measuring ongoing neuronal activity in awake humans, it can be problematic when studying alcohol, as alcohol acts as a direct vasodilator. An alternative approach for studying changes in brain activity during ethanol exposure is to measure changes in glucose metabolism. Whole brain glucose utilization has been shown to decrease during experiments in which the subject has self-reported feelings of “highness” (Wang et al., 2000) and mood alteration (de Wit et al., 1990). This decrease is observed in cortical areas, including PFC, with little change seen in basal ganglia and corpus callosum (Volkow et al., 1990). While these studies were performed with moderate doses of EtOH, low doses have also been shown to reduce glucose metabolism, although behavioral deficits were not reported at this low dose (Volkow et al., 2006). Transcranial magnetic stimulation (TMS) has been combined with brain imaging to probe changes in PFC activity, and healthy social drinkers show decreased TMS-induced prefrontal activity after 0.8 g/kg EtOH (Kahkonen et al., 2003). These results are congruent with studies that have shown decreases in prefrontal glucose metabolism in the PFC after acute EtOH administration (de Wit et al., 1990; Volkow et al., 1990). The deleterious effects of acute EtOH on PFC-dependent behaviors may also result from the breakdown of functional specificity in different brain regions. Indeed, recent work has demonstrated a decrease in functional heterogeneity in right prefrontal cortex following ethanol administration (Volkow et al., 2008). A decrease in asymmetry has also been observed while inebriated patients participated in a verbal fluency task (Wendt and Risberg, 2001). In this case, the decrease in lateral activation of the left dorsolateral PFC, a region associated with language performance, correlated with decreased performance on the task. Another study examined the effects of acute EtOH on memory encoding that ordinarily depends upon activation of the right PFC (Soderlund et al., 2007). Again, ethanol administration decreased performance on the task, and this was associated with reduced bilateral PFC activation.
B. Chronic Alcohol
Chronic alcohol use in humans has been linked to deficits in executive function that depend on the PFC. One study showed that a significant number of substance-dependent individuals, including alcohol-dependent subjects, showed deficits in a gambling task that were similar to patients with lesions of the ventromedial PFC (Bechara et al., 2001). Long-term use of alcohol has been shown to be more detrimental than cocaine in attention and executive functioning tasks (Goldstein et al., 2004). Even in alcoholics who do not demonstrate deficits in certain executive tasks, differential patterns of activity in PFC are observed that suggest changes in the way that the brain performs the tasks (Pfefferbaum et al., 2001). Similarly, adolescents who engage in binge drinking performed adequately on a visual working memory task, yet there was hypoactivation of the right anterior PFC when compared with controls, suggesting a reorganization or compensatory mechanism in problem solving (Crego et al., 2010). These alterations in PFC activity may go unnoticed under low-level cognitive demands but may underlie deficits associated with higher-order cognitive function. Reduced glucose metabolism in alcohol-dependent subjects has been identified in mediofrontal and left dorsolateral PFC (Dao-Castellana et al., 1998). Decreases in medial frontal cortex glucose metabolism have been correlated with poor performance on the Wisconson Card Sorting Task in alcoholic subjects (Adams et al., 1993). It seems, though, that frontal glucose metabolism increases after a period of abstinence, suggesting that some behavioral consequences of chronic alcohol use may be reversible (Volkow et al., 1994). Indeed, abstinent subjects in one study demonstrated an increase in cognitive and executive functioning that was correlated with an increase in frontal glucose metabolism (Johnson-Greene et al., 1997).
Changes in the structural morphology and integrity of the PFC have also been observed that may underlie the cognitive deficits associated with chronic alcohol exposure. Alcohol-dependent subjects show reduced gray matter in the dorsolateral PFC (Jernigan et al., 1991). Chronic alcohol use is associated with reduced white matter volume throughout the cortex (de la Monte, 1988). Frontal lobes seem to be especially susceptible to volume loss following long-term chronic alcohol exposure (Pfefferbaum et al., 1997). Reductions in levels of N-acetylaspartate, an abundant CNS metabolite, have also been observed in prefrontal white matter of detoxified alcoholic subjects (Schweinsburg et al., 2001), perhaps indicating an atrophy of white matter during withdrawal. Additionally, the integrity of white matter in the right orbitofrontal cortex is significantly impaired in chronic alcoholics (Pfefferbaum and Sullivan, 2005; Harris et al., 2008). A study using stereology to monitor cell number demonstrated no significant cell loss through the neocortex, even though overall cortical volume was decreased, suggesting atrophy or dendritic retraction rather than frank cell death (Jensen and Pakkenberg, 1993). Significant reduction of soma size in frontal cingulate cortex of alcoholics, without a significant change in cell number, has also been observed (Kril et al., 1989). Changes in glial cell count following chronic ethanol exposure have also been reported and indicate decreases in both density and size of glia in dorsolateral PFC (Miguel-Hidalgo et al., 2002) and orbitofrontal cortex (Miguel-Hidalgo et al., 2006). Whether the loss of volume in PFC in alcohol-dependent subjects is reversible is still undetermined. In one study, subjects who were abstinent for 6–9 months after entering a rehab program showed decreased ventricle size, but there was no increase in frontal lobe volume when compared to their entry into the rehab program (Wobrock et al., 2009).
Genomic and proteomic studies in humans have demonstrated alterations in gene expression and protein levels in alcoholics as opposed to nonalcoholic controls. In one study, genes for mitochondrial proteins were downregulated in the PFC of alcoholics and this was paralleled by an increase in repair proteins that are activated due to oxidative damage, possibly in response to mitochondrial dysfunction (Flatscher-Bader et al., 2005). There were also region-specific alterations in transcription factors and genes that encode DNA-binding proteins, protein trafficking, and apoptotic factors. Reverse transcription polymerase chain reaction (RT-PCR) analysis has demonstrated a downregulation in PCP4/PEP19, a gene that encodes for a protein that may have anti-apoptotic properties (Iwamoto et al., 2004). This study also showed alterations in expression of NEFH, which encodes a neurofilament protein, suggesting that changes in the expression of this protein could lead to altered organization of the cytoskeleton. Similar to animal studies, PFC tissue collected from chronic alcoholics shows changes in the expression of a number of genes involved in myelination (Mayfield et al., 2002). Levels of key energy-regulating and metabolism proteins are also downregulated in PFC white matter of alcoholics as opposed to nonalcoholic controls (Alexander-Kaufman et al., 2006). Other proteins show enhanced expression, including midkine, a repair protein, and EAAT1, a glutamate transporter (Flatscher-Bader and Wilce, 2008). Changes in midkine may indicate a response to oxidative damage after chronic alcohol use, whereas the change in transporter levels may be a compensatory mechanism in response to elevated levels of extracellular glutamate during withdrawal. Proteins related to the thiamine pathway that are involved in carbohydrate metabolism were down-regulated in the PFC of non-Wernicke-Korsokoff alcoholics (Matsumoto, 2009). The transcription factor NF-kappaB and its p50 subunit, which inhibit gene transcription, were also downregulated in postmortem prefrontal tissue of alcoholics as compared to controls, suggesting increased expression of genes related to synaptic plasticity that may play a role in the development and maintenance of alcohol dependence (Okvist et al., 2007). Taken together, these postmortem studies indicate a number of differences in the PFC of alcoholics that could lead to compromised function of the PFC and increased cognitive deficits.
IX. Summary
It is clear from the above discussion that exposure to alcohol, either acutely or chronically, has significant effects on the functional and structural status of the prefrontal cortex. Given the key role that this brain region plays in the integration, manipulation, and evaluation of incoming sensory and cognitive information, it is not surprising that alcoholics display deficits in executive control, decision making, and risk management. Despite these observations, however, there are still many unanswered questions that need to be addressed regarding the role of the PFC in the etiology and treatment of alcoholism. At a basic cellular level, there is still a paucity of data with respect to the acute effects of ethanol on subtypes of excitatory and inhibitory neurons within the diverse anatomical structures that make up the prefrontal cortex. Functional mapping studies using both electrophysiological and multineuron imaging techniques are needed to assess the relative ethanol sensitivity of neurons within prefrontal cortical microciruits and across the various subdivisions of this area (e.g., anterior cingulate, prelimbic, infralimbic, and orbitofrontal). There is also little known at the network level about how alcohol disrupts normal patterns of activity that are thought to be critical for processes such as working memory and decision making and whether the chronically alcohol-exposed brain can recover from deficits observed in these behaviors. The continuing development of awake animal array recording techniques coupled with sophisticated multidimensional analytical approaches promises to provide information that can address this key question. In terms of human PFC function and involvement in alcohol-related behaviors, results from imaging studies have provided a great deal of information regarding the effects of chronic alcohol exposure on brain structure and processing. However, these approaches are limited by the rather coarse level of anatomical, cellular, and temporal resolution of functional imaging. Advances in small animal imaging techniques offer at least a chance to address some of these limitations and multi-investigator teams with expertise in both animal and human imaging need to be established to help bridge the gap between clinical and preclinical studies. Finally, it is clear that advances in cognitive neuroscience, neuropsychology, and psychiatry need to be coordinated with basic science findings in order to spur development of new approaches to treat alcohol-induced alterations in prefrontal cortical function. Recent findings in the fear and post-traumatic stress disorder literature suggest that neural mechanisms of plasticity involved in learning can be used to overcome or reset maladaptive behaviors associated with prior emotional, physical, or chemical alterations in brain function. Applying these concepts and ideas to the treatment of alcoholics and those with alcohol-use disorders may augment the few pharmacological approaches that are currently available for treating these alcohol-related problems.
References
- Adams KM, Gilman S, Koeppe RA, Kluin KJ, Brunberg JA, Dede D, Berent S, Kroll PD. Neuropsychological deficits are correlated with frontal hypometabolism in positron emission tomography studies of older alcoholic patients. Alcohol. Clin. Exp. Res. 1993;17:205–210. doi: 10.1111/j.1530-0277.1993.tb00750.x. [DOI] [PubMed] [Google Scholar]
- Alexander-Kaufman K, James G, Sheedy D, Harper C, Matsumoto I. Differential protein expression in the prefrontal white matter of human alcoholics: A proteomics study. Mol. Psychiatry. 2006;11:56–65. doi: 10.1038/sj.mp.4001741. [DOI] [PubMed] [Google Scholar]
- Andrews GD, Lavin A. Methylphenidate increases cortical excitability via activation of alpha-2 noradrenergic receptors. Neuropsychopharmacology. 2006;31:594–601. doi: 10.1038/sj.npp.1300818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araneda R, Andrade R. 5-Hydroxytryptamine2 and 5-hydroxytryptamine 1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience. 1991;40:399–412. doi: 10.1016/0306-4522(91)90128-b. [DOI] [PubMed] [Google Scholar]
- Arbib MA. Schemas for the temporal organization of behaviour. Hum. Neurobiol. 1985;4:63–72. [PubMed] [Google Scholar]
- Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascoli GA, Alonso-Nanclares L, Anderson SA, Barrionuevo G, Benavides-Piccione R, Burkhalter A, Buzsaki G, Cauli B, Defelipe J, Fairen A, Feldmeyer D, Fishell G, Fregnac Y, Freund TF, Gardner D, Gardner EP, Goldberg JH, Helmstaedter M, Hestrin S, Karube F, Kisvarday ZF, Lambolez B, Lewis DA, Marin O, Markram H, Munoz A, Packer A, Petersen CC, Rockland KS, Rossier J, Rudy B, Somogyi P, Staiger JF, Tamas G, Thomson AM, Toledo-Rodriguez M, Wang Y, West DC, Yuste R. Petilla terminology: Nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat. Rev. Neurosci. 2008;9:557–568. doi: 10.1038/nrn2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auclair N, Otani S, Soubrie P, Crepel F. Cannabinoids modulate synaptic strength and plasticity at glutamatergic synapses of rat prefrontal cortex pyramidal neurons. J. Neurophysiol. 2000;83:3287–3293. doi: 10.1152/jn.2000.83.6.3287. [DOI] [PubMed] [Google Scholar]
- Barbas H, Pandya DN. Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. J. Comp. Neurol. 1989;286:353–375. doi: 10.1002/cne.902860306. [DOI] [PubMed] [Google Scholar]
- Bayer HM, Lau B, Glimcher PW. Statistics of midbrain dopamine neuron spike trains in the awake primate. J. Neurophysiol. 2007;98:1428–1439. doi: 10.1152/jn.01140.2006. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Beique JC, Imad M, Mladenovic L, Gingrich JA, Andrade R. Mechanism of the 5-hydroxytryptamine 2A receptor-mediated facilitation of synaptic activity in prefrontal cortex. Proc. Natl. Acad. Sci. U.S.A. 2007;104:9870–9875. doi: 10.1073/pnas.0700436104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- Bressler SL. Large-scale cortical networks and cognition. Brain Res. Brain Res. Rev. 1995;20:288–304. doi: 10.1016/0165-0173(94)00016-i. [DOI] [PubMed] [Google Scholar]
- Chandler LJ. Ethanol and brain plasticity: Receptors and molecular networks of the postsynaptic density as targets of ethanol. Pharmacol. Ther. 2003;99:311–326. doi: 10.1016/s0163-7258(03)00096-2. [DOI] [PubMed] [Google Scholar]
- Chang YM, Luebke JI. Electrophysiological diversity of layer 5 pyramidal cells in the prefrontal cortex of the rhesus monkey: In vitro slice studies. J. Neurophysiol. 2007;98:2622–2632. doi: 10.1152/jn.00585.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Ge J, Barnes NM, Cooper SJ. Autoradiographic mapping of brain 5-HT2A binding sites in P and in AA alcohol-preferring rats. Brain Res. Bull. 1997;44:33–37. doi: 10.1016/s0361-9230(96)00379-6. [DOI] [PubMed] [Google Scholar]
- Crego A, Rodriguez-Holguin S, Parada M, Mota N, Corral M, Cadaveira F. Reduced anterior prefrontal cortex activation in young binge drinkers during a visual working memory task. Drug Alcohol Depend. 2010;109:45–46. doi: 10.1016/j.drugalcdep.2009.11.020. [DOI] [PubMed] [Google Scholar]
- Cui C, Xu M, Atzori M. Voltage-dependent block of N-methyl-D-aspartate receptors by dopamine D1 receptor ligands. Mol. Pharmacol. 2006;70:1761–1770. doi: 10.1124/mol.106.028332. [DOI] [PubMed] [Google Scholar]
- D’Esposito M. From cognitive to neural models of working memory. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2007;362:761–772. doi: 10.1098/rstb.2007.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao-Castellana MH, Samson Y, Legault F, Martinot JL, Aubin HJ, Crouzel C, Feldman L, Barrucand D, Rancurel G, Feline A, Syrota A. Frontal dysfunction in neurologically normal chronic alcoholic subjects: Metabolic and neuropsychological findings. Psychol. Med. 1998;28:1039–1048. doi: 10.1017/s0033291798006849. [DOI] [PubMed] [Google Scholar]
- de la Monte SM. Disproportionate atrophy of cerebral white matter in chronic alcoholics. Arch. Neurol. 1988;45:990–992. doi: 10.1001/archneur.1988.00520330076013. [DOI] [PubMed] [Google Scholar]
- de Wit H, Metz J, Wagner N, Cooper M. Behavioral and subjective effects of ethanol: Relationship to cerebral metabolism using PET. Alcohol. Clin. Exp. Res. 1990;14:482–489. doi: 10.1111/j.1530-0277.1990.tb00508.x. [DOI] [PubMed] [Google Scholar]
- Elston GN. Cortex, cognition and the cell: New insights into the pyramidal neuron and prefrontal function. Cereb. Cortex. 2003;13:1124–1138. doi: 10.1093/cercor/bhg093. [DOI] [PubMed] [Google Scholar]
- Engleman EA, Ingraham CM, McBride WJ, Lumeng L, Murphy JM. Extracellular dopamine levels are lower in the medial prefrontal cortex of alcohol-preferring rats compared to Wistar rats. Alcohol. 2006;38:5–12. doi: 10.1016/j.alcohol.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nat. Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Finn PR, Justus A, Mazas C, Steinmetz JE. Working memory, executive processes and the effects of alcohol on Go/No-Go learning: Testing a model of behavioral regulation and impulsivity. Psychopharmacology (Berl) 1999;146:465–472. doi: 10.1007/pl00005492. [DOI] [PubMed] [Google Scholar]
- Flatscher-Bader T, van der Brug M, Hwang JW, Gochee PA, Matsumoto I, Niwa S, Wilce PA. Alcohol-responsive genes in the frontal cortex and nucleus accumbens of human alcoholics. J. Neurochem. 2005;93:359–370. doi: 10.1111/j.1471-4159.2004.03021.x. [DOI] [PubMed] [Google Scholar]
- Flatscher-Bader T, Wilce PA. Impact of alcohol abuse on protein expression of midkine and excitatory amino acid transporter 1 in the human prefrontal cortex. Alcohol. Clin. Exp. Res. 2008;32:1849–1858. doi: 10.1111/j.1530-0277.2008.00754.x. [DOI] [PubMed] [Google Scholar]
- Fortin DA, Levine ES. Differential effects of endocannabinoids on glutamatergic and GABAergic inputs to layer 5 pyramidal neurons. Cereb. Cortex. 2007;17:163–174. doi: 10.1093/cercor/bhj133. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The Prefrontal Cortex. London: Academic Press; 2008. [Google Scholar]
- George S, Rogers RD, Duka T. The acute effect of alcohol on decision making in social drinkers. Psychopharmacology (Berl) 2005;182:160–169. doi: 10.1007/s00213-005-0057-9. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The physiological approach: Functional architecture of working memory and disordered cognition in schizophrenia. Biol. Psychiatry. 1999;46:650–661. doi: 10.1016/s0006-3223(99)00130-4. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Leskovjan AC, Hoff AL, Hitzemann R, Bashan F, Khalsa SS, Wang GJ, Fowler JS, Volkow ND. Severity of neuropsychological impairment in cocaine and alcohol addiction: Association with metabolism in the prefrontal cortex. Neuropsychologia. 2004;42:1447–1458. doi: 10.1016/j.neuropsychologia.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. Am. J. Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelova N, Seamans JK, Yang CR. Mechanisms of dopamine activation of fastspiking interneurons that exert inhibition in rat prefrontal cortex. J. Neurophysiol. 2002;88:3150–3166. doi: 10.1152/jn.00335.2002. [DOI] [PubMed] [Google Scholar]
- Grattan-Miscio KE, Vogel-Sprott M. Effects of alcohol and performance incentives on immediate working memory. Psychopharmacology (Berl) 2005;181:188–196. doi: 10.1007/s00213-005-2226-2. [DOI] [PubMed] [Google Scholar]
- Harris GJ, Jaffin SK, Hodge SM, Kennedy D, Caviness VS, Marinkovic K, Papadimitriou GM, Makris N, Oscar-Berman M. Frontal white matter and cingulum diffusion tensor imaging deficits in alcoholism. Alcohol. Clin. Exp. Res. 2008;32:1001–1013. doi: 10.1111/j.1530-0277.2008.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihara-Takeda S, Funahashi S. Activity of primate orbitofrontal and dorsolateral prefrontal neurons: Task-related activity during an oculomotor delayed-response task. Exp. Brain Res. 2007;181:409–425. doi: 10.1007/s00221-007-0941-0. [DOI] [PubMed] [Google Scholar]
- Iwamoto K, Bundo M, Yamamoto M, Ozawa H, Saito T, Kato T. Decreased expression of NEFH and PCP4/PEP19 in the prefrontal cortex of alcoholics. Neurosci. Res. 2004;49:379–385. doi: 10.1016/j.neures.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Jensen GB, Pakkenberg B. Do alcoholics drink their neurons away? Lancet. 1993;342:1201–1204. doi: 10.1016/0140-6736(93)92185-v. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: Implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Butters N, DiTraglia G, Schafer K, Smith T, Irwin M, Grant I, Schuckit M, Cermak LS. Reduced cerebral grey matter observed in alcoholics using magnetic resonance imaging. Alcohol. Clin. Exp. Res. 1991;15:418–427. doi: 10.1111/j.1530-0277.1991.tb00540.x. [DOI] [PubMed] [Google Scholar]
- Johnson-Greene D, Adams KM, Gilman S, Koeppe RA, Junck L, Kluin KJ, Martorello S, Heumann M. Effects of abstinence and relapse upon neuropsychological function and cerebral glucose metabolism in severe chronic alcoholism. J. Clin. Exp. Neuropsychol. 1997;19:378–385. doi: 10.1080/01688639708403866. [DOI] [PubMed] [Google Scholar]
- Kahkonen S, Wilenius J, Nikulin VV, Ollikainen M, Ilmoniemi RJ. Alcohol reduces prefrontal cortical excitability in humans: A combined TMS and EEG study. Neuropsychopharmacology. 2003;28:747–754. doi: 10.1038/sj.npp.1300099. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat. Rev. Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: A pathology of motivation and choice. Am. J. Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y. Physiological subgroups of nonpyramidal cells with specific morphological characteristics in layer II/III of rat frontal cortex. J. Neurosci. 1995;15:2638–2655. doi: 10.1523/JNEUROSCI.15-04-02638.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns RT, Ravindranathan A, Hassan S, Cage MP, York T, Sikela JM, Williams RW, Miles MF. Ethanol-responsive brain region expression networks: Implications for behavioral responses to acute ethanol in DBA/2J versus C57BL/6J mice. J. Neurosci. 2005;25:2255–2266. doi: 10.1523/JNEUROSCI.4372-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kia HK, Miquel MC, Brisorgueil MJ, Daval G, Riad M, El Mestikawy S, Hamon M, Verge D. Immunocytochemical localization of serotonin1A receptors in the rat central nervous system. J. Comp. Neurol. 1996;365:289–305. doi: 10.1002/(SICI)1096-9861(19960205)365:2<289::AID-CNE7>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- King A, Munisamy G, de Wit H, Lin S. Attenuated cortisol response to alcohol in heavy social drinkers. Int. J. Psychophysiol. 2006;59:203–209. doi: 10.1016/j.ijpsycho.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Kril JJ, Gundlach AL, Dodd PR, Johnston GA, Harper CG. Cortical dihydropyridine binding sites are unaltered in human alcoholic brain. Ann.Neurol. 1989;26:395–397. doi: 10.1002/ana.410260315. [DOI] [PubMed] [Google Scholar]
- Kroener S, Chandler LJ, Phillips PE, Seamans JK. Dopamine modulates persistent synaptic activity and enhances the signal-to-noise ratio in the prefrontal cortex. PLoS One. 2009;4:e6507. doi: 10.1371/journal.pone.0006507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafourcade M, Elezgarai I, Mato S, Bakiri Y, Grandes P, Manzoni OJ. Molecular components and functions of the endocannabinoid system in mouse prefrontal cortex. PLoS One. 2007;2:e709. doi: 10.1371/journal.pone.0000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Hetzel A, Hackel O, Jones I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57:760–773. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Langen B, Dietze S, Fink H. Acute effect of ethanol on anxiety and 5-HT in the prefrontal cortex of rats. Alcohol. 2002;27:135–141. doi: 10.1016/s0741-8329(02)00219-7. [DOI] [PubMed] [Google Scholar]
- Leggio B, Masi F, Grappi S, Nanni G, Gambarana C, Colombo G, de Montis MG. Sardinian alcohol-preferring and non-preferring rats show different reactivity to aversive stimuli and a similar response to a natural reward. Brain Res. 2003;973:275–284. doi: 10.1016/s0006-8993(03)02533-2. [DOI] [PubMed] [Google Scholar]
- Leriche M, Mendez M, Zimmer L, Berod A. Acute ethanol induces Fos in GABAergic and non-GABAergic forebrain neurons: A double-labeling study in the medial prefrontal cortex and extended amygdala. Neuroscience. 2008;153:259–267. doi: 10.1016/j.neuroscience.2008.01.069. [DOI] [PubMed] [Google Scholar]
- Liu W, Yuen EY, Allen PB, Feng J, Greengard P, Yan Z. Adrenergic modulation of NMDA receptors in prefrontal cortex is differentially regulated by RGS proteins and spinophilin. Proc. Natl. Acad. Sci. U.S.A. 2006;103:18338–18343. doi: 10.1073/pnas.0604560103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli PW, Kiianmaa K, Gianoulakis C. Opioid propeptide mRNA content and receptor density in the brains of AA and ANA rats. Life Sci. 2000;66:1915–1927. doi: 10.1016/s0024-3205(00)00517-8. [DOI] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Matsumoto I. Proteomics approach in the study of the pathophysiology of alcohol-related brain damage. Alcohol Alcohol. 2009;44:171–176. doi: 10.1093/alcalc/agn104. [DOI] [PubMed] [Google Scholar]
- Mayfield RD, Lewohl JM, Dodd PR, Herlihy A, Liu J, Harris RA. Patterns of gene expression are altered in the frontal and motor cortices of human alcoholics. J. Neurochem. 2002;81:802–813. doi: 10.1046/j.1471-4159.2002.00860.x. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Shu Y, Hasenstaub A, Sanchez-Vives M, Badoual M, Bal T. Persistent cortical activity: Mechanisms of generation and effects on neuronal excitability. Cereb. Cortex. 2003;13:1219–1231. doi: 10.1093/cercor/bhg104. [DOI] [PubMed] [Google Scholar]
- Mendez M, Leriche M, Calva JC. Acute ethanol administration differentially modulates mu opioid receptors in the rat meso-accumbens and mesocortical pathways. Brain Res. Mol. Brain Res. 2001;94:148–156. doi: 10.1016/s0169-328x(01)00232-7. [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Overholser JC, Meltzer HY, Stockmeier CA, Rajkowska G. Reduced glial and neuronal packing density in the orbitofrontal cortex in alcohol dependence and its relationship with suicide and duration of alcohol dependence. Alcohol. Clin. Exp. Res. 2006;30:1845–1855. doi: 10.1111/j.1530-0277.2006.00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Wei J, Andrew M, Overholser JC, Jurjus G, Stockmeier CA, Rajkowska G. Glia pathology in the prefrontal cortex in alcohol dependence with and without depressive symptoms. Biol. Psychiatry. 2002;52:1121–1133. doi: 10.1016/s0006-3223(02)01439-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Homayoun H. Divergent plasticity of prefrontal cortex networks. Neuropsychopharmacology. 2008;33:42–55. doi: 10.1038/sj.npp.1301554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar Z, Cheung AF. Towards the classification of subpopulations of layer V pyramidal projection neurons. Neurosci. Res. 2006;55:105–115. doi: 10.1016/j.neures.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Neznanova O, Bjork K, Rimondini R, Hansson AC, Hyytia P, Heilig M, Sommer WH. Acute ethanol challenge inhibits glycogen synthase kinase-3beta in the rat prefrontal cortex. Int J Neuropsychopharmacol. 2009;12:275–280. doi: 10.1017/S1461145708009620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman DA, Shallice T. Attention to action. In: Davidson RJ, Schawartz GE, Shapiro D, editors. Consciousness and Self-Regulation. New York: Plenum; 1986. pp. 1–18. [Google Scholar]
- Okvist A, Johansson S, Kuzmin A, Bazov I, Merino-Martinez R, Ponomarev I, Mayfield RD, Harris RA, Sheedy D, Garrick T, Harper C, Hurd YL, Terenius L, Ekstrom TJ, Bakalkin G, Yakovleva T. Neuroadaptations in human chronic alcoholics: Dysregulation of the NF-kappaB system. PLoS ONE. 2007;2:e930. doi: 10.1371/journal.pone.0000930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J. Neurochem. 2009;108:920–931. doi: 10.1111/j.1471-4159.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Desmond JE, Galloway C, Menon V, Glover GH, Sullivan EV. Reorganization of frontal systems used by alcoholics for spatial working memory: An fMRI study. Neuroimage. 2001;14:7–20. doi: 10.1006/nimg.2001.0785. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV. Disruption of brain white matter microstructure by excessive intracellular and extracellular fluid in alcoholism: Evidence from diffusion tensor imaging. Neuropsychopharmacology. 2005;30:423–432. doi: 10.1038/sj.npp.1300623. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol. Clin. Exp. Res. 1997;21:521–529. doi: 10.1111/j.1530-0277.1997.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Riedel G, Davies SN. Cannabinoid function in learning, memory and plasticity. Handb. Exp. Pharmacol. 2005:445–477. doi: 10.1007/3-540-26573-2_15. [DOI] [PubMed] [Google Scholar]
- Rubio M, de Miguel R, Fernandez-Ruiz J, Gutierrez-Lopez D, Carai MA, Ramos JA. Effects of a short-term exposure to alcohol in rats on FAAH enzyme and CB1 receptor in different brain areas. Drug Alcohol Depend. 2009;99:354–358. doi: 10.1016/j.drugalcdep.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Sanchez-Vives MV, McCormick DA. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat. Neurosci. 2000;3:1027–1034. doi: 10.1038/79848. [DOI] [PubMed] [Google Scholar]
- Sano M, Wendt PE, Wirsen A, Stenberg G, Risberg J, Ingvar DH. Acute effects of alcohol on regional cerebral blood flow in man. J. Stud. Alcohol. 1993;54:369–376. doi: 10.15288/jsa.1993.54.369. [DOI] [PubMed] [Google Scholar]
- Santana N, Bortolozzi A, Serrats J, Mengod G, Artigas F. Expression of serotonin1A and serotonin2A receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb. Cortex. 2004;14:1100–1109. doi: 10.1093/cercor/bhh070. [DOI] [PubMed] [Google Scholar]
- Saults JS, Cowan N, Sher KJ, Moreno MV. Differential effects of alcohol on working memory: Distinguishing multiple processes. Exp. Clin. Psychopharmacol. 2007;15:576–587. doi: 10.1037/1064-1297.15.6.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Responses of midbrain dopamine neurons to behavioral trigger stimuli in the monkey. J. Neurophysiol. 1986;56:1439–1461. doi: 10.1152/jn.1986.56.5.1439. [DOI] [PubMed] [Google Scholar]
- Schweinsburg BC, Taylor MJ, Alhassoon OM, Videen JS, Brown GG, Patterson TL, Berger F, Grant I. Chemical pathology in brain white matter of recently detoxified alcoholics: A 1H magnetic resonance spectroscopy investigation of alcohol-associated frontal lobe injury. Alcohol. Clin. Exp. Res. 2001;25:924–934. [PubMed] [Google Scholar]
- Seamans JK, Lapish CC, Durstewitz D. Comparing the prefrontal cortex of rats and primates: Insights from electrophysiology. Neurotox Res. 2008;14:249–262. doi: 10.1007/BF03033814. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Nogueira L, Lavin A. Synaptic basis of persistent activity in prefrontal cortex in vivo and in organotypic cultures. Cereb. Cortex. 2003;13:1242–1250. doi: 10.1093/cercor/bhg094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog. Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Selim M, Bradberry CW. Effect of ethanol on extracellular 5-HT and glutamate in the nucleus accumbens and prefrontal cortex: Comparison between the Lewis and Fischer 344 rat strains. Brain Res. 1996;716:157–164. doi: 10.1016/0006-8993(95)01385-7. [DOI] [PubMed] [Google Scholar]
- Smith DG, Learn JE, McBride WJ, Lumeng L, Li TK, Murphy JM. Alcoholnaive alcohol-preferring (P) rats exhibit higher local cerebral glucose utilization than alcohol-nonpreferring (NP) and Wistar rats. Alcohol. Clin. Exp. Res. 2001;25:1309–1316. [PubMed] [Google Scholar]
- Soderlund H, Grady CL, Easdon C, Tulving E. Acute effects of alcohol on neural correlates of episodic memory encoding. Neuroimage. 2007;35:928–939. doi: 10.1016/j.neuroimage.2006.12.024. [DOI] [PubMed] [Google Scholar]
- Steriade M, Nunez A, Amzica F. A novel slow (< 1 Hz) oscillation of neocortical neurons in vivo: Depolarizing and hyperpolarizing components. J. Neurosci. 1993;13:3252–3265. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strother WN, McBride WJ, Lumeng L, Li TK. Effects of acute administration of ethanol on cerebral glucose utilization in adult alcohol-preferring and alcohol-nonpreferring rats. Alcohol. 2005;35:119–128. doi: 10.1016/j.alcohol.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Kuikka J, Hakola P, Paanila J, Airaksinen J, Eronen M, Hallikainen T. Acute ethanol-induced changes in cerebral blood flow. Am. J. Psychiatry. 1994;151:1505–1508. doi: 10.1176/ajp.151.10.1505. [DOI] [PubMed] [Google Scholar]
- Trantham-Davidson H, Kroner S, Seamans JK. Dopamine modulation of prefrontal cortex interneurons occurs independently of DARPP-32. Cereb. Cortex. 2008;18:951–958. doi: 10.1093/cercor/bhm133. [DOI] [PubMed] [Google Scholar]
- Trantham-Davidson H, Neely LC, Lavin A, Seamans JK. Mechanisms underlying differential D1 versus D2 dopamine receptor regulation of inhibition in prefrontal cortex. J. Neurosci. 2004;24:10652–10659. doi: 10.1523/JNEUROSCI.3179-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, O’Donnell P. Post-pubertal Emergence of Prefrontal Cortical Up States Induced by D1-NMDA Co-activation. Cereb. Cortex. 2004;15:49–57. doi: 10.1093/cercor/bhh107. [DOI] [PubMed] [Google Scholar]
- Tu Y, Kroener S, Abernathy K, Lapish C, Seamans J, Chandler LJ, andWoodward JJ. Ethanol inhibits persistent activity in prefrontal cortical neurons. J. Neurosci. 2007;27:4765–4775. doi: 10.1523/JNEUROSCI.5378-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM. Pharmacology and behavioral pharmacology of the mesocortical dopamine system. Prog. Neurobiol. 2001;63:241–320. doi: 10.1016/s0301-0082(00)00033-2. [DOI] [PubMed] [Google Scholar]
- Unterrainer JM, Owen AM. Planning and problem solving: From neuropsychology to functional neuroimaging. J. Physiol. Paris. 2006;99:308–317. doi: 10.1016/j.jphysparis.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav. Brain Res. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain: Insights from imaging studies. J. Clin. Invest. 2003;111:1444–1451. doi: 10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Hitzemann R, Wolf AP, Logan J, Fowler JS, Christman D, Dewey SL, Schlyer D, Burr G, Vitkun S, et al. Acute effects of ethanol on regional brain glucose metabolism and transport. Psychiatry Res. 1990;35:39–48. doi: 10.1016/0925-4927(90)90007-s. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Ma Y, Zhu W, Fowler JS, Li J, Rao M, Mueller K, Pradhan K, Wong C, Wang GJ. Moderate doses of alcohol disrupt the functional organization of the human brain. Psychiatry Res. 2008;162:205–213. doi: 10.1016/j.pscychresns.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Mullani N, Gould L, Adler SS, Guynn RW, Overall JE, Dewey S. Effects of acute alcohol intoxication on cerebral blood flow measured with PET. Psychiatry Res. 1988;24:201–209. doi: 10.1016/0165-1781(88)90063-7. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Franceschi D, Fowler JS, Thanos PP, Maynard L, Gatley SJ, Wong C, Veech RL, Kunos G, Kai Li T. Low doses of alcohol substantially decrease glucose metabolism in the human brain. Neuroimage. 2006;29:295–301. doi: 10.1016/j.neuroimage.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Hitzemann R, Fowler JS, Overall JE, Burr G, Wolf AP. Recovery of brain glucose metabolism in detoxified alcoholics. Am. J. Psychiatry. 1994;151:178–183. doi: 10.1176/ajp.151.2.178. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Franceschi D, Fowler JS, Thanos PK, Scherbaum N, Pappas N, Wong CT, Hitzemann RJ, Felder CA. Regional brain metabolism during alcohol intoxication. Alcohol. Clin. Exp. Res. 2000;24:822–829. [PubMed] [Google Scholar]
- Wang HX, Gao WJ. Cell Type-Specific Development of NMDA Receptors in the Interneurons of Rat Prefrontal Cortex. Neuropsychopharmacology. 2009;34:2028–2040. doi: 10.1038/npp.2009.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenborn R, Duka T. Acute alcohol effects on cognitive function in social drinkers: Their relationship to drinking habits. Psychopharmacology (Berl) 2003;165:306–312. doi: 10.1007/s00213-002-1281-1. [DOI] [PubMed] [Google Scholar]
- Weitlauf C, Woodward JJ. Ethanol selectively attenuates NMDAR-mediated synaptic transmission in the prefrontal cortex. Alcohol. Clin. Exp. Res. 2008;32:690–698. doi: 10.1111/j.1530-0277.2008.00625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt PE, Risberg J. Ethanol reduces rCFB activation of left dorsolateral prefrontal cortex during a verbal fluency task. Brain Lang. 2001;77:197–215. doi: 10.1006/brln.2000.2434. [DOI] [PubMed] [Google Scholar]
- Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:572–576. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- Willins DL, Deutch AY, Roth BL. Serotonin 5-HT2A receptors are expressed on pyramidal cells and interneurons in the rat cortex. Synapse. 1997;27:79–82. doi: 10.1002/(SICI)1098-2396(199709)27:1<79::AID-SYN8>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Wobrock T, Falkai P, Schneider-Axmann T, Frommann N, Wolwer W, Gaebel W. Effects of abstinence on brain morphology in alcoholism: A MRI study. Eur. Arch. Psychiatry Clin. Neurosci. 2009;259:143–150. doi: 10.1007/s00406-008-0846-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward JJ, Pava MJ. Effects of Ethanol on Persistent Activity and Up-States in Excitatory and Inhibitory Neurons in Prefrontal Cortex. Alcohol. Clin. Exp. Res. 2009;33:2134–2140. doi: 10.1111/j.1530-0277.2009.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Jiang Q, Chen P, Feng J, Yan Z. Activation of 5-HT2A/C receptors counteracts 5-HT1A regulation of n-methyl-D-aspartate receptor channels in pyramidal neurons of prefrontal cortex. J. Biol. Chem. 2008;283:17194–17204. doi: 10.1074/jbc.M801713200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZW. Maturation of layer V pyramidal neurons in the rat prefrontal cortex: Intrinsic properties and synaptic function. J. Neurophysiol. 2004;91:1171–1182. doi: 10.1152/jn.00855.2003. [DOI] [PubMed] [Google Scholar]
- Zhong P, Liu W, Gu Z, Yan Z. Serotonin facilitates long-term depression induction in prefrontal cortex via p38 MAPK/Rab5-mediated enhancement of AMPA receptor internalization. J. Physiol. 2008;586:4465–4479. doi: 10.1113/jphysiol.2008.155143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FM, Hablitz JJ. Activation of serotonin receptors modulates synaptic transmission in rat cerebral cortex. J. Neurophysiol. 1999;82:2989–2999. doi: 10.1152/jn.1999.82.6.2989. [DOI] [PubMed] [Google Scholar]