Abstract
In dilated cardiomyopathy, a condition characterized by chamber enlargement and reduced myocardial contractility, decreases in β-adrenergic receptor density and increases in Gαi and β-adrenergic receptor kinase activities attenuate the stimulation of adenylyl cyclase in response to catecholamines. PDE3 inhibitors have been used to ‘overcome’ the reduction in cAMP generation by blocking cAMP hydrolysis. These drugs increase contractility in the short-term, but long-term administration leads to an increase in mortality that correlates with an increase in sudden cardiac death. Whether separate mechanisms account for these beneficial and harmful effects, and, if so, whether PDE3 can be targeted so as to increase contractility without increasing mortality are questions that remain unanswered.
Introduction
Dilated cardiomyopathy is a syndrome characterized by enlargement of cardiac chambers, especially the left ventricle, and a reduction in myocardial contractility. Its primary causes are diverse, including coronary artery disease, hypertensive vascular disease, diabetes, viral illnesses, valvular heart disease, congenital abnormalities, amyloidosis and pregnancy. In perhaps a third of cases, no underlying disease can be identified, and the disease is classified as idiopathic, though genetic factors are increasingly recognized. Hemodynamic consequences may include decreased cardiac output and decreased systemic blood pressure, while a decreased ability of the ventricle to empty during systole can result in increased filling pressures, pulmonary edema and pulmonary arterial hypertension. Dilated cardiomyopathy is also characterized by ventricular arrhythmias that account for much of the mortality in this syndrome.
From this perspective, dilated cardiomyopathy is not generally thought of as an endocrine disease. Yet agents that modify endocrine signaling have proven useful in the treatment of dilated cardiomyopathy, and the benefits of angiotensin-converting-enzyme inhibitors, angiotensin-receptor antagonists and aldosterone antagonists in this syndrome extend beyond what can be attributed to their vasodilatory and diuretic actions [1–5]. But perhaps the most well-characterized endocrine abnormalities in the syndrome relate to cAMP-mediated signaling, and agents that modify cAMP-mediated signaling – β-adrenergic receptor agonists, which stimulate cAMP production, β-adrenergic receptor antagonists, which do the opposite, and inhibitors of the cyclic nucleotide phosphodiesterase PDE3, which block cAMP hydrolysis – are used at different stages in the treatment of this disease. In this review, we have focused specifically on PDE3 inhibition and the challenges it has posed in the treatment of dilated cardiomyopathy.
The role of cAMP in myocardial contractility
Agents that increase intracellular cAMP content activate cAMP-dependent protein kinase (PKA). As in other cells, many proteins in cardiac myocytes are phosphorylated by PKA. Inotropic effects are likely to result from increases in the phosphorylation of several membrane-bound PKA substrates involved in intracellular Ca2+ cycling. Phosphorylation of L-type Ca2+ channels increases Ca2+ influx during systole [6]; phosphorylation of ryanodine-sensitive Ca2+ channels increases Ca2+ release by the sarcoplasmic reticulum [7]; and phosphorylation of phospholamban blocks its inhibitory interaction with SERCA2, the Ca2+-transporting ATPase of the sarcoplasmic reticulum, resulting in an increase in Ca2+ accumulation during diastole [8]. These actions increase the amplitude of intracellular Ca2+ transients, which are attenuated in dilated cardiomyopathy [9]. Studies in animal models suggest that the phosphorylation of phospholamban may be the most therapeutically relevant of these mechanisms. Depletion of phospholamban and expression of a non-functional mutant form of the protein – which mimics the stimulation of SERCA2 activity seen with phospholamban phosphorylation – increase contractility in cultured cardiac myocytes, while germ-line ablation of phospholamban, knockdown with antisense RNA and expression of anti-phospholamban antibody-derived proteins improve contractile function and prevent pathologic remodeling [10–18].
Diminished cAMP generation in failing human myocardium
Comparative studies of tissue obtained from the explanted failing hearts of heart transplant recipients with dilated cardiomyopathy and from presumably ‘normal’ hearts obtained from organ donors that were not transplanted because a suitable recipient was not identified at the time of organ procurement have identified several changes in the expression of proteins involved in receptor-stimulated cAMP generation in this disease. Among these changes are a reduction in β1-adrenergic receptor levels in failing hearts [19••,20]; an increase in the expression and activity of β-adrenergic receptor kinase (phosphorylation of β-adrenergic receptors leads to their binding to β-arrestins, which ‘uncouple’ them from G proteins) [21••,22]; and an increase in the expression and activity of the inhibitory G protein Gαi [23••,24]. These changes combine to reduce the stimulation of adenylyl cyclase activity in response to β-adrenergic receptor agonists and intracellular cAMP content – in particular, membrane-bound cAMP content – in failing hearts [25••,26,27•].
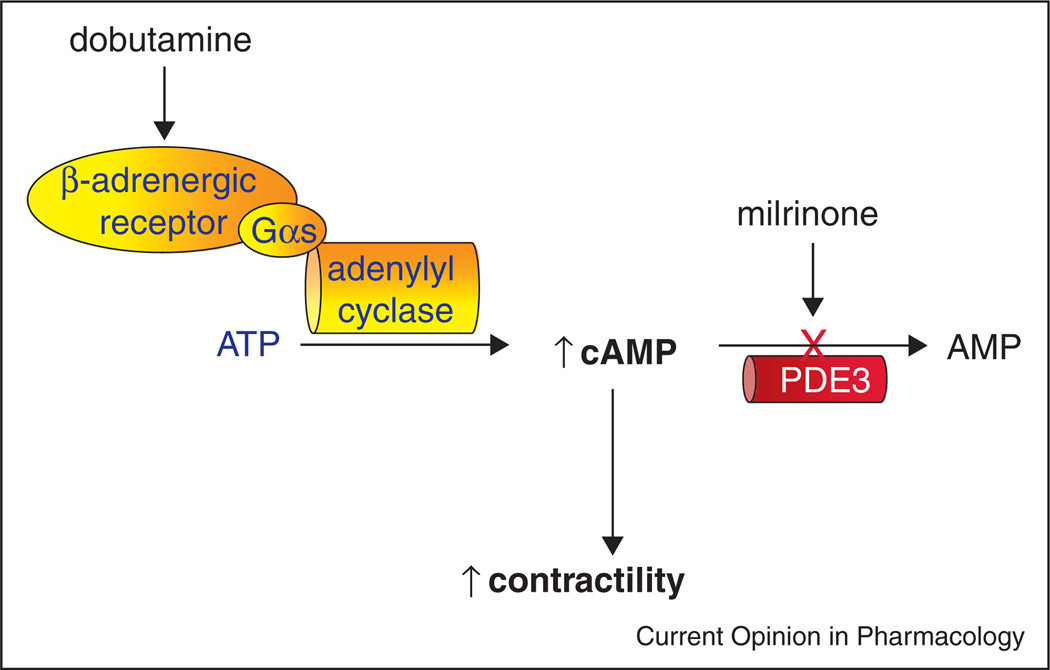
Given the role of cAMP in stimulating increasing contractility in cardiac myocytes, one would expect agents that inhibit hydrolysis of cAMP by cyclic nucleotide phosphodiesterases to bypass receptors, transducers and effectors and ‘overcome’ the impairment in cAMP generation in dilated cardiomyopathy (Figure 1). Raising cAMP content in this manner would, in turn, reverse the reduction in myocardial contractility.
Figure 1.
Effects of phosphodiesterase (specifically, PDE3) inhibition on cAMP content in cardiac myocytes.
cAMP phosphodiesterase activity in human myocardium
Eleven families of cyclic nucleotide phosphodiesterases have been described. Two seem especially important with respect to regulating cAMP content in human myocardium. Most of the membrane-associated cAMP-hydrolytic activity is attributable to enzymes in the PDE3 family, which are characterized by their high affinity for both cAMP and cGMP, with Km values of ≤100 nanomolar [28,29]. Owing to their much higher catalytic rates for cAMP than for cGMP, PDE3 isoforms are generally regarded principally as cGMP-inhibited cAMP-phosphodiesterases. Most of the cytoplasmic cAMP-hydrolytic activity in human is attributable to PDE1C1, a Ca2+/calmodulin-activated enzyme that hydrolyses both cAMP and cGMP with Km values in the micromolar range. Inasmuch as PDE1 inhibitors have, to our knowledge, never been used as therapeutic agents in patients with dilated cardiomyopathy, PDE1 is given no further attention in this review.
(It is worth pointing out that the profile of phosphodiesterase activity in humans differs significantly from that seen in animal models. Enzymes in the PDE4 family of phosphodiesterases constitute a large fraction of the cAMP-hydrolytic activity in mouse myocardium but a very small fraction of this activity in human myocardium [30]. Similarly, PDE5 activity, which constitutes a large fraction of the cGMP-activity in mouse myocardium, is present at very low levels in human myocardium [31]. And levels of cAMP-hydrolytic activity in human myocardium are several-fold higher than in mouse myocardium [30]. There is therefore reason for restraint in drawing inferences regarding dilated cardiomyopathy and its treatment from findings involving the use of phosphodiesterase inhibitors in mouse models.)
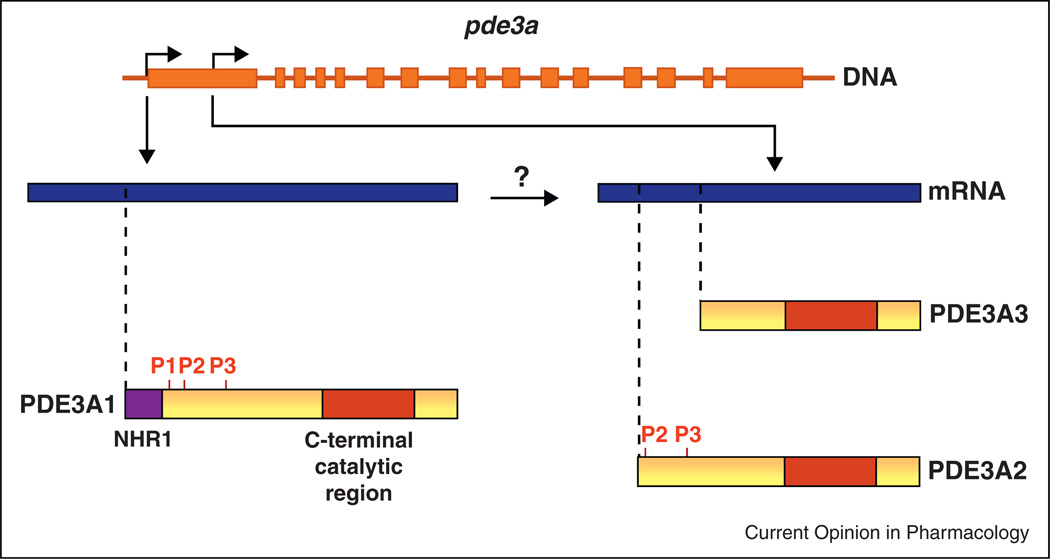
In the PDE3 family, two genes – PDE3A and PDE3B – are known to exist. Both are expressed in cardiac muscle, but experiments in PDE3A-knockout and PDE3Bknockout mice have shown that inotropic effects result specifically from inhibition of PDE3A [32]. Three isoforms are generated from the PDE3A gene through a combination of transcription and translation from alternative initiation sites (Figure 2) [33•]. Their amino-acid sequences are identical except for the presence of different lengths of N-terminal sequence, in which membranelocalizing domains and phosphorylation sites are found. PDE3A1 contains NHR1, which consists of hydrophobic loops that insert into intracellular membranes [34]. PDE3A1 also contains three sites – S293, S312 and S428 – that are phosphorylated by PKA, PKB and PKC [35,36]. PDE3A2 lacks NHR1 and S293, while PDE3A3 lacks NHR1 and all three phosphorylation sites. The three isoforms all contain the same C-terminal catalytic region, and are identical with respect to catalytic activity and inhibitor sensitivity [29], but their N-terminal differences lead to differences in their intracellular distribution. PDE3A1 is an obligatory membrane protein recovered only in microsomal fractions of human myocardium, while PDE3A2 and PDE3A3 are recovered in microsomal as well as cytosolic fractions [33•]. Owing to these differences in intracellular localization, the three isoforms are likely to have distinct roles in modulating cAMP-mediated signaling in cardiac myocytes, though these roles have not been defined. Moreover, there are no agents available that can selectively target either PDE3A versus PDE3B or PDE3A1 versus PDE3A2 or PDE3A3.
Figure 2.
Generation of PDE3A isoforms in human cardiac myocytes.
Whether PDE3 levels are altered in patients with dilated cardiomyopathy is a matter of controversy [31,37,38]. The high efficiency with which donors and recipients are matched nowadays has meant that the availability of ‘normal’ hearts for comparative studies is extremely low, making it difficult to achieve statistically significant results that can exclude the possibility of small differences in protein expression between normal and failing hearts.
PDE3 inhibition in the treatment of heart failure
The use of PDE3 inhibitors to raise intracellular cAMP content appeared to be a promising approach to the decrease in receptor-stimulated cAMP generation and intracellular cAMP content in failing myocardium. The beneficial short-term effects of PDE3 inhibition were readily apparent in early investigations, which showed inotropic, lusitropic and vasodilatory responses that resulted in increased cardiac output, decreased left ventricular end-diastolic pressures and decreased pulmonary artery pressures [39–45]. These changes were typically observed within the first fifteen minutes of treatment and were sustained over at least 48 h. PDE3 inhibition did not result in as large an increase in heart rate or myocardial oxygen consumption as did treatment with the β-adrenergic receptor agonist dobutamine [46,47]. An additive effect on cardiac performance, through cAMP augmentation by independent mechanisms, was demonstrated with the combined use of PDE3 inhibitors and dobutamine [48,49]. This would be of benefit in patients in whom responses to one agent alone were inadequate; alternatively, combination therapy could be used to decrease the effective dose of both agents and to reduce dose-dependent side effects. Whether short-term PDE3 inhibition led to longer-term benefits was less obvious: a large trial of milrinone infused for 48 h in patients with advanced heart failure showed no reduction (versus placebo-treated patients) with respect to rehospitalization from cardiovascular causes within 60 days of randomization, while the incidence of atrial arrhythmias and hypotension was increased [50].
A critical question was whether long-term PDE3 inhibition would have a favorable impact on quality of life and survival. A large number of clinical trials were carried out, at great expense, in the hope of demonstrating such a benefit. The results, which were examined in a comprehensive meta-analysis, were immensely disappointing. No important quality-of-life benefits were observed, and annual mortality, which was ~16% in the patients treated with placebo, increased to ~19% in patients taking PDE3 inhibitors. This increase appeared to be attributable to an increase in sudden cardiac death, which was ~6% per year in placebo-treated patients and ~9% per year in patients taking PDE3 inhibitors [51••].
Mechanisms responsible for the adverse consequences of PDE3 inhibition
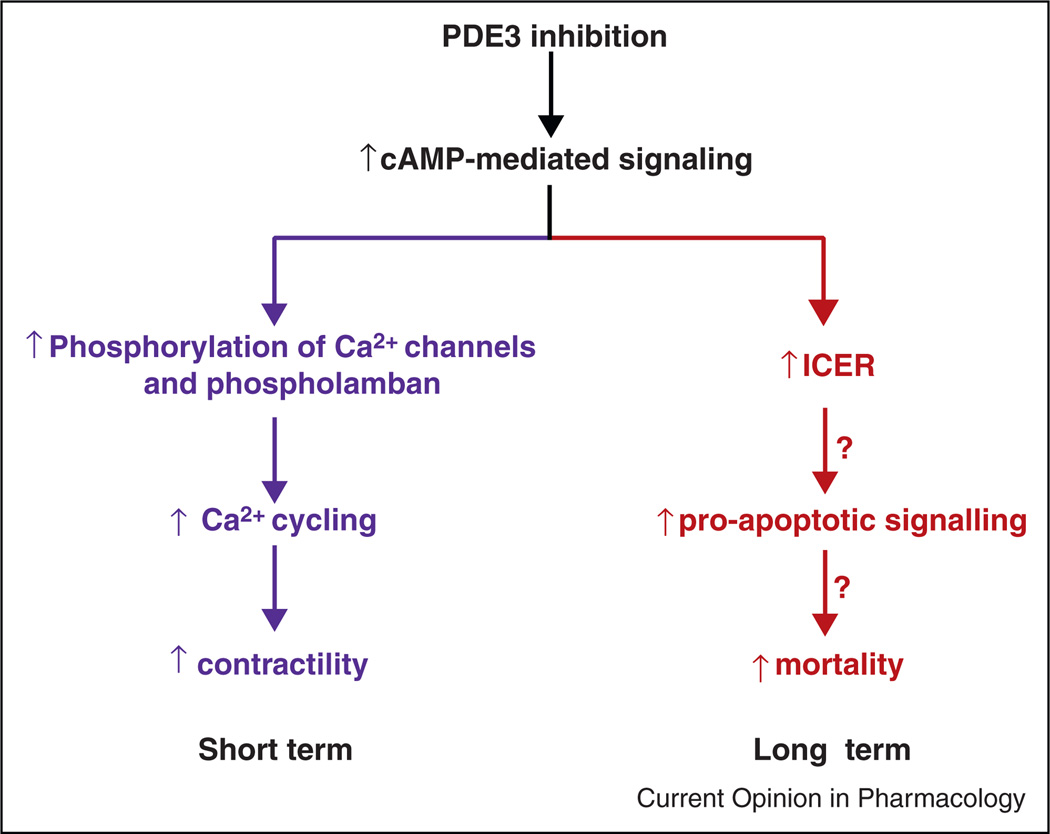
The mechanisms responsible for the increase in arrhythmias and sudden death observed in clinical trials of PDE3 inhibitors have not been clearly defined. One possibility is that these adverse outcomes are consequences of the same changes in intracellular Ca2+ handling that result in increased contractility. A secondary effect attributable to a cAMP-mediated acceleration of pathologic remodeling is also possible. The fact that reductions in sudden death have been seen in clinical trials of ACE inhibitors and isosorbide dinitrate/hydralazine, which act through separate molecular mechanisms, can be regarded as evidence for secondary effects [51••]. Studies in rats have documented pro-apoptotic consequences of PDE3 inhibition associated with an increase in the expression of inducible cAMP early repressors (‘ICERs’), an effect THAT occurs via phosphorylation of the transcription factor CREB by PKA (Figure 3) [37,52,53]. Inasmuch as pathologic remodeling has been associated with an increase in apoptosis in human disease and in animal models [54], the possibility that this mechanism is a proximal step in the pro-arrhythmic effects of PDE3 inhibition seems plausible. Another relevant question, in view of the different roles of PDE3A and PDE3B in regulating myocardial contractility [32], is the extent to which adverse effects may be attributable to PDE3B inhibition rather than PDE3A inhibition (as noted earlier, existing therapeutic agents do not select for one or the other of these isoforms).
Figure 3.
Mechanisms of inotropic actions and adverse consequences of increasing intracellular cAMP content in failing myocardium.
Prospects for long-term PDE3 inhibition in heart failure
At this time, American College of Cardiology/American Heart Association and Heart Failure Society of America guidelines recommend against the long-term use of PDE3 inhibitors in patients with heart failure, except for continuous infusions used as palliative long-term therapy and, in the acute setting, for patients with heart failure exacerbations and low-output states [55]. There may, however, be situations in which the long-term use of these agents is beneficial. Patients in whom PDE3 inhibitors are started because of decompensated heart failure and in whom subsequent efforts to discontinue these drugs fail because of hypotension, low cardiac output or pulmonary edema comprise a self-defined subset in whom mortality without PDE3 inhibitors is so overwhelmingly high that their long-term use is appropriate, regardless of the results of the large clinical trials.
Another situation involves chronic intermittent outpatient infusion of milrinone. In nonrandomized trials with a limited number of subjects, patients treated with milrinone had significant functional improvement, a reduced number of emergency room visits and hospital admissions, and a marked reduction in hospitalization days, without evidence of increased mortality [56,57]. In other studies, patients treated with intermittent infusions of inotropic agents while receiving continuous treatment with antiarrhythmic drugs had largely favorable outcomes [58,59]. These latter results raise an important issue: Trials of PDE3 inhibitors were carried out before the implantation of cardiac defibrillators in patients with advanced dilated cardiomyopathy became standard therapy [60]. Since the increased mortality in patients treated with PDE3 inhibitors derived from an increase in sudden cardiac death, it is possible that the implantation of defibrillators would have addressed this problem. At this point, however, in view of the disappointing results of the many clinical trials of PDE3 inhibitors, pharmaceutical companies are understandably reluctant to commit more resources to clinical trials that would address this issue.
Conclusion
The inotropic consequences of PDE3 inhibition are helpful in the management of acutely decompensated dilated cardiomyopathy. With long-term adminstration, however, these agents have been found to increase mortality in this syndrome, owing to an increase in sudden cardiac death, and their use is generally recommended only when withdrawal of PDE3 inhibitors leads to levels of contractile dysfunction incompatible with life. Whether the long-term adverse consequences of PDE3 inhibition can be mitigated through the concomitant use of anti-arrhythmic drugs or implantable cardiac defibrillators is unknown. The fact that several forms of PDE3 differing in their intracellular localization are found in cardiac myocytes raises the possibility that agents that could target individual PDE3 isoforms selectively might produce inotropic responses without increasing mortality, but at this writing this remains solely a theoretical consideration.
Acknowledgements
Supported by medical research funds from the United States Department of Veterans Affairs and a grant from the Leducq Foundation (06CVD02 cycAMP) to Matthew A. Movsesian.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 2.Cohn JN, Johnson G, Ziesche S, Cobb F, Francis G, Tristani F, Smith R, Dunkman WB, Loeb H, Wong M, et al. A comparison of enalapril with hydralazine-isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med. 1991;325:303–310. doi: 10.1056/NEJM199108013250502. [DOI] [PubMed] [Google Scholar]
- 3.Cohn JN, Tognoni G. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667–1675. doi: 10.1056/NEJMoa010713. [DOI] [PubMed] [Google Scholar]
- 4.Poole-Wilson PA, Swedberg K, Cleland JG, Di Lenarda A, Hanrath P, Komajda M, Lubsen J, Lutiger B, Metra M, Remme WJ, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the carvedilol or metoprolol european trial (comet): randomised controlled trial. Lancet. 2003;362:7–13. doi: 10.1016/S0140-6736(03)13800-7. [DOI] [PubMed] [Google Scholar]
- 5.Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21. [Google Scholar]
- 6.Sculptoreanu A, Rotman E, Takahashi M, Scheuer T, Catterall WA. Voltage-dependent potentiation of the activity of cardiac l-type calcium channel alpha 1 subunits due to phosphorylation by camp-dependent protein kinase. Proc Natl Acad Sci USA. 1993;90:10135–10139. doi: 10.1073/pnas.90.21.10135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takasago T, Imagawa T, Shigekawa M. Phosphorylation of the cardiac ryanodine receptor by camp-dependent protein kinase. J Biochem (Tokyo) 1989;106:872–877. doi: 10.1093/oxfordjournals.jbchem.a122945. [DOI] [PubMed] [Google Scholar]
- 8.Simmerman HK, Jones LR. Phospholamban: protein structure, mechanism of action, and role in cardiac function. Physiol Rev. 1998;78:921–947. doi: 10.1152/physrev.1998.78.4.921. [DOI] [PubMed] [Google Scholar]
- 9.Beuckelmann DJ, Nabauer M, Erdmann E. Intracellular calcium handling in isolated ventricular myocytes from patients with terminal heart failure. Circulation. 1992;85:1046–1055. doi: 10.1161/01.cir.85.3.1046. [DOI] [PubMed] [Google Scholar]
- 10.Minamisawa S, Hoshijima M, Chu G, Ward CA, Frank K, Gu Y, Martone ME, Wang Y, Ross J, Jr, Kranias EG, et al. Chronic phospholamban-sarcoplasmic reticulum calcium atpase interaction is the critical calcium cycling defect in dilated cardiomyopathy. Cell. 1999;99:313–322. doi: 10.1016/s0092-8674(00)81662-1. [DOI] [PubMed] [Google Scholar]
- 11.He H, Meyer M, Martin JL, McDonough PM, Ho P, Lou X, Lew WY, Hilal-Dandan R, Dillmann WH. Effects of mutant and antisense rna of phospholamban on sr ca(2+)-atpase activity and cardiac myocyte contractility. Circulation. 1999;100:974–980. doi: 10.1161/01.cir.100.9.974. [DOI] [PubMed] [Google Scholar]
- 12.Hoshijima M, Ikeda Y, Iwanaga Y, Minamisawa S, Date MO, Gu Y, Iwatate M, Li M, Wang L, Wilson JM, et al. Chronic suppression of heart-failure progression by a pseudophosphorylated mutant of phospholamban via in vivo cardiac raav gene delivery. Nat Med. 2002;8:864–871. doi: 10.1038/nm739. [DOI] [PubMed] [Google Scholar]
- 13.Iwanaga Y, Hoshijima M, Gu Y, Iwatate M, Dieterle T, Ikeda Y, Date MO, Chrast J, Matsuzaki M, Peterson KL, et al. Chronic phospholamban inhibition prevents progressive cardiac dysfunction and pathological remodeling after infarction in rats. J Clin Invest. 2004;113:727–736. doi: 10.1172/JCI18716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eizema K, Fechner H, Bezstarosti K, Schneider-Rasp S, van der Laarse A, Wang H, Schultheiss HP, Poller WC, Lamers JM. Adenovirus-based phospholamban antisense expression as a novel approach to improve cardiac contractile dysfunction: comparison of a constitutive viral versus an endothelin-1-responsive cardiac promoter. Circulation. 2000;101:2193–2199. doi: 10.1161/01.cir.101.18.2193. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe A, Arai M, Yamazaki M, Koitabashi N, Wuytack F, Kurabayashi M. Phospholamban ablation by rna interference increases Ca2+ uptake into rat cardiac myocyte sarcoplasmic reticulum. J Mol Cell Cardiol. 2004;37:691–698. doi: 10.1016/j.yjmcc.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Suckau L, Fechner H, Chemaly E, Krohn S, Hadri L, Kockskamper J, Westermann D, Bisping E, Ly H, Wang X, et al. Long-term cardiac-targeted rna interference for the treatment of heart failure restores cardiac function and reduces pathological hypertrophy. Circulation. 2009;119:1241–1252. doi: 10.1161/CIRCULATIONAHA.108.783852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dieterle T, Meyer M, Gu Y, Belke DD, Swanson E, Iwatate M, Hollander J, Peterson KL, Ross J, Jr, Dillmann WH. Gene transfer of a phospholamban-targeted antibody improves calcium handling and cardiac function in heart failure. Cardiovasc Res. 2005;67:678–688. doi: 10.1016/j.cardiores.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 18.Meyer M, Belke DD, Trost SU, Swanson E, Dieterle T, Scott B, Cary SP, Ho P, Bluhm WF, McDonough PM, et al. A recombinant antibody increases cardiac contractility by mimicking phospholamban phosphorylation. FASEB J. 2004;18:1312–1314. doi: 10.1096/fj.03-1231fje. [DOI] [PubMed] [Google Scholar]
- 19. Bristow MR, Ginsburg R, Minobe W, Cubicciotti RS, Sageman WS, Lurie K, Billingham ME, Harrison DC, Stinson EB. Decreased catecholamine sensitivity and beta-adrenergic-receptor density in failing human hearts. N Engl J Med. 1982;307:205–211. doi: 10.1056/NEJM198207223070401. This and the following paper defined the changes in β-adrenergic receptors in failing human myocardium.
- 20.Bristow MR, Ginsburg R, Umans V, Fowler M, Minobe W, Rasmussen R, Zera P, Menlove R, Shah P, Jamieson S, et al. Beta 1- and beta 2-adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: coupling of both receptor subtypes to muscle contraction and selective beta 1-receptor down-regulation in heart failure. Circ Res. 1986;59:297–309. doi: 10.1161/01.res.59.3.297. [DOI] [PubMed] [Google Scholar]
- 21. Ungerer M, Parruti G, Bohm M, Puzicha M, DeBlasi A, Erdmann E, Lohse MJ. Expression of beta-arrestins and beta-adrenergic receptor kinases in the failing human heart. Circ Res. 1994;74:206–213. doi: 10.1161/01.res.74.2.206. This and the following paper identified mechanisms for the uncoupling of β-adrenergic receptor occupancy from stimulation of adenylyl cyclase activity in failing human myocardium.
- 22.Ungerer M, Bohm M, Elce JS, Erdmann E, Lohse MJ. Altered expression of beta-adrenergic receptor kinase and beta 1-adrenergic receptors in the failing human heart. Circulation. 1993;87:454–463. doi: 10.1161/01.cir.87.2.454. [DOI] [PubMed] [Google Scholar]
- 23. Neumann J, Schmitz W, Scholz H, von Meyerinck L, Doring V, Kalmar P. Increase in myocardial gi-proteins in heart failure. Lancet. 1988;2:936–937. doi: 10.1016/s0140-6736(88)92601-3. This and the following paper identified another mechanism for the uncoupling of β-adrenergic receptor occupancy from stimulation of adenylyl cyclase activity in failing human myocardium.
- 24.Feldman AM, Cates AE, Veazey WB, Hershberger RE, Bristow MR, Baughman KL, Baumgartner WA, Van Dop C. Increase of the 40,000-mol wt pertussis toxin substrate (g protein) in the failing human heart. J Clin Invest. 1988;82:189–197. doi: 10.1172/JCI113569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Feldman MD, Copelas L, Gwathmey JK, Phillips P, Warren SE, Schoen FJ, Grossman W, Morgan JP. Deficient production of cyclic amp: pharmacologic evidence of an important cause of contractile dysfunction in patients with end-stage heart failure. Circulation. 1987;75:331–339. doi: 10.1161/01.cir.75.2.331. This paper described changes in cAMP-mediated signaling in failing human myocardium.
- 26.Danielsen W, v der Leyen H, Meyer W, Neumann J, Schmitz W, Scholz H, Starbatty J, Stein B, Doring V, Kalmar P. Basal and isoprenaline-stimulated camp content in failing versus nonfailing human cardiac preparations. J Cardiovasc Pharmacol. 1989;14:171–173. doi: 10.1097/00005344-198907000-00026. [DOI] [PubMed] [Google Scholar]
- 27. Bohm M, Reiger B, Schwinger RH, Erdmann E. Camp concentrations, camp dependent protein kinase activity, and phospholamban in non-failing and failing myocardium. Cardiovasc Res. 1994;28:1713–1719. doi: 10.1093/cvr/28.11.1713. This paper demonstrated compartment-selective effects on cAMP-mediated signaling in failing human myocardium.
- 28.Vandeput F, Wolda SL, Krall J, Hambleton R, Uher L, McCaw KN, Radwanski PB, Florio V, Movsesian MA. Cyclic nucleotide phosphodiesterase pde1c1 in human cardiac myocytes. J Biol Chem. 2007;282:32749–32757. doi: 10.1074/jbc.M703173200. [DOI] [PubMed] [Google Scholar]
- 29.Hambleton R, Krall J, Tikishvili E, Honeggar M, Ahmad F, Manganiello VC, Movsesian MA. Isoforms of cyclic nucleotide phosphodiesterase pde3 and their contribution to camp hydrolytic activity in subcellular fractions of human myocardium. J Biol Chem. 2005;280:39168–39174. doi: 10.1074/jbc.M506760200. [DOI] [PubMed] [Google Scholar]
- 30.Richter W, Xie M, Scheitrum C, Krall J, Movsesian MA, Conti M. Conserved expression and functions of pde4 in rodent and human heart. Basic Res Cardiol. 2011;106:249–262. doi: 10.1007/s00395-010-0138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vandeput F, Krall J, Ockaili R, Salloum FN, Florio V, Corbin JD, Francis SH, Kukreja RC, Movsesian MA. Cgmp-hydrolytic activity and its inhibition by sildenafil in normal and failing human and mouse myocardium. J Pharmacol Exp Ther. 2009;330:884–891. doi: 10.1124/jpet.109.154468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun B, Li H, Shakur Y, Hensley J, Hockman S, Kambayashi J, Manganiello VC, Liu Y. Role of phosphodiesterase type 3a and 3b in regulating platelet and cardiac function using subtype-selective knockout mice. Cell Signal. 2007;19:1765–1771. doi: 10.1016/j.cellsig.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 33. Wechsler J, Choi YH, Krall J, Ahmad F, Manganiello VC, Movsesian MA. Isoforms of cyclic nucleotide phosphodiesterase pde3a in cardiac myocytes. J Biol Chem. 2002;277:38072–38078. doi: 10.1074/jbc.M203647200. This paper describes the genesis of isoforms of PDE3A in human myocardium.
- 34.Shakur Y, Takeda K, Kenan Y, Yu ZX, Rena G, Brandt D, Houslay MD, Degerman E, Ferrans VJ, Manganiello VC. Membrane localization of cyclic nucleotide phosphodiesterase 3 (pde3). Two n-terminal domains are required for the efficient targeting to association of, pde3 with endoplasmic reticulum. J Biol Chem. 2000;275:38749–38761. doi: 10.1074/jbc.M001734200. [DOI] [PubMed] [Google Scholar]
- 35.Han SJ, Vaccari S, Nedachi T, Andersen CB, Kovacina KS, Roth RA, Conti M. Protein kinase b/akt phosphorylation of pde3a and its role in mammalian oocyte maturation. EMBO J. 2006;25:5716–5725. doi: 10.1038/sj.emboj.7601431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hunter RW, Mackintosh C, Hers I. Protein kinase c-mediated phosphorylation and activation of pde3a regulate camp levels in human platelets. J Biol Chem. 2009;284:12339–12348. doi: 10.1074/jbc.M807536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ding B, Abe J, Wei H, Huang Q, Walsh RA, Molina CA, Zhao A, Sadoshima J, Blaxall BC, Berk BC, Yan C. Functional role of phosphodiesterase 3 in cardiomyocyte apoptosis: implication in heart failure. Circulation. 2005;111:2469–2476. doi: 10.1161/01.CIR.0000165128.39715.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Movsesian MA, Smith CJ, Krall J, Bristow MR, Manganiello VC. Sarcoplasmic reticulum-associated cyclic adenosine 5’-monophosphate phosphodiesterase activity in normal and failing human hearts. J Clin Invest. 1991;88:15–19. doi: 10.1172/JCI115272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baim DS, McDowell AV, Cherniles J, Monrad ES, Parker JA, Edelson J, Braunwald E, Grossman W. Evaluation of a new bipyridine inotropic agent – milrinone – in patients with severe congestive heart failure. N Engl J Med. 1983;309:748–756. doi: 10.1056/NEJM198309293091302. [DOI] [PubMed] [Google Scholar]
- 40.Benotti JR, Grossman W, Braunwald E, Davolos DD, Alousi AA. Hemodynamic assessment of amrinone. A new inotropic agent. N Engl J Med. 1978;299:1373–1377. doi: 10.1056/NEJM197812212992501. [DOI] [PubMed] [Google Scholar]
- 41.Jaski BE, Fifer MA, Wright RF, Braunwald E, Colucci WS. Positive inotropic and vasodilator actions of milrinone in patients with severe congestive heart failure. Dose-response relationships and comparison to nitroprusside. J Clin Invest. 1985;75:643–649. doi: 10.1172/JCI111742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sinoway LS, Maskin CS, Chadwick B, Forman R, Sonnenblick EH, Le Jemtel TH. Long-term therapy with a new cardiotonic agent, win 47203: drug-dependent improvement in cardiac performance and progression of the underlying disease. J Am Coll Cardiol. 1983;2:327–331. doi: 10.1016/s0735-1097(83)80170-3. [DOI] [PubMed] [Google Scholar]
- 43.Maskin CS, Sinoway L, Chadwick B, Sonnenblick EH, Le Jemtel TH. Sustained hemodynamic and clinical effects of a new cardiotonic agent, win 47203, in patients with severe congestive heart failure. Circulation. 1983;67:1065–1070. doi: 10.1161/01.cir.67.5.1065. [DOI] [PubMed] [Google Scholar]
- 44.Anderson JL. Hemodynamic and clinical benefits with intravenous milrinone in severe chronic heart failure: results of a multicenter study in the united states. Am Heart J. 1991;121(6 Pt 2):1956–1964. doi: 10.1016/0002-8703(91)90832-3. [DOI] [PubMed] [Google Scholar]
- 45.Monrad ES, McKay RG, Baim DS, Colucci WS, Fifer MA, Heller GV, Royal HD, Grossman W. Improvement in indexes of diastolic performance in patients with congestive heart failure treated with milrinone. Circulation. 1984;70:1030–1037. doi: 10.1161/01.cir.70.6.1030. [DOI] [PubMed] [Google Scholar]
- 46.Shipley JB, Tolman D, Hastillo A, Hess ML. Milrinone: basic and clinical pharmacology and acute and chronic management. Am J Med Sci. 1996;311:286–291. doi: 10.1097/00000441-199606000-00011. [DOI] [PubMed] [Google Scholar]
- 47.Grose R, Strain J, Greenberg M, LeJemtel TH. Systemic and coronary effects of intravenous milrinone and dobutamine in congestive heart failure. J Am Coll Cardiol. 1986;7:1107–1113. doi: 10.1016/s0735-1097(86)80231-5. [DOI] [PubMed] [Google Scholar]
- 48.Gage J, Rutman H, Lucido D, LeJemtel TH. Additive effects of dobutamine and amrinone on myocardial contractility and ventricular performance in patients with severe heart failure. Circulation. 1986;74:367–373. doi: 10.1161/01.cir.74.2.367. [DOI] [PubMed] [Google Scholar]
- 49.Gilbert EM, Hershberger RE, Wiechmann RJ, Movsesian MA, Bristow MR. Pharmacologic and hemodynamic effects of combined beta-agonist stimulation and phosphodiesterase inhibition in the failing human heart. Chest. 1995;108:1524–1532. doi: 10.1378/chest.108.6.1524. [DOI] [PubMed] [Google Scholar]
- 50.Cuffe MS, Califf RM, Adams KF, Jr, Benza R, Bourge R, Colucci WS, Massie BM, O’Connor CM, Pina I, Quigg R, et al. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial. JAMA. 2002;287:1541–1547. doi: 10.1001/jama.287.12.1541. [DOI] [PubMed] [Google Scholar]
- 51. Amsallem E, Kasparian C, Haddour G, Boissel JP, Nony P. Phosphodiesterase III inhibitors for heart failure. Cochrane Database Syst Rev (Online) 2005;1 doi: 10.1002/14651858.CD002230.pub2. CD002230. This paper provides a comprehensive meta-analysis of the clinical trials of PDE3 inhibotors in the treatment of dilated cardiomyopathy.
- 52.Yan C, Miller CL, Abe J. Regulation of phosphodiesterase 3 and inducible camp early repressor in the heart. Circ Res. 2007;100:489–501. doi: 10.1161/01.RES.0000258451.44949.d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ding B, Abe J, Wei H, Xu H, Che W, Aizawa T, Liu W, Molina CA, Sadoshima J, Blaxall BC, et al. A positive feedback loop of phosphodiesterase 3 (pde3) and inducible camp early repressor (icer) leads to cardiomyocyte apoptosis. Proc Natl Acad Sci USA. 2005;102:14771–14776. doi: 10.1073/pnas.0506489102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dorn GW., 2nd Apoptotic and non-apoptotic programmed cardiomyocyte death in ventricular remodelling. Cardiovasc Res. 2009;81:465–473. doi: 10.1093/cvr/cvn243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jessup M, Abraham WT, Casey DE, Feldman AM, Francis GS, Ganiats TG, Konstam MA, Mancini DM, Rahko PS, Silver MA, et al. 2009 focused update: ACCF/AHA guidelines for the diagnosis and management of heart failure in adults: a report of the american college of cardiology foundation/american heart association task force on practice guidelines: developed in collaboration with the international society for heart and lung transplantation. Circulation. 2009;119:1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 56.Marius-Nunez AL, Heaney L, Fernandez RN, Clark WA, Ranganini A, Silber E, Denes P. Intermittent inotropic therapy in an outpatient setting: a cost-effective therapeutic modality in patients with refractory heart failure. Am Heart J. 1996;132:805–808. doi: 10.1016/s0002-8703(96)90315-4. [DOI] [PubMed] [Google Scholar]
- 57.Cesario D, Clark J, Maisel A. Beneficial effects of intermittent home administration of the inotrope/vasodilator milrinone in patients with end-stage congestive heart failure: a preliminary study. Am Heart J. 1998;135:121–129. doi: 10.1016/s0002-8703(98)70352-7. [DOI] [PubMed] [Google Scholar]
- 58.Nanas JN, Tsagalou EP, Kanakakis J, Nanas SN, Terrovitis JV, Moon T, Anastasiou-Nana MI. Long-term intermittent dobutamine infusion, combined with oral amiodarone for end-stage heart failure: a randomized double-blind study. Chest. 2004;125:1198–1204. doi: 10.1378/chest.125.4.1198. [DOI] [PubMed] [Google Scholar]
- 59.Drakos SG, Kanakakis JV, Nanas S, Bonios M, Kaldara E, Katsaros F, Pantsios C, Nanas JN. Intermittent inotropic infusions combined with prophylactic oral amiodarone for patients with decompensated end-stage heart failure. J Cardiovasc Pharmacol. 2009;53:157–161. doi: 10.1097/FJC.0b013e31819846cd. [DOI] [PubMed] [Google Scholar]
- 60.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, 3rd, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, et al. Acc/aha/hrs 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the american college of cardiology/american heart association task force on practice guidelines (writing committee to revise the acc/aha/naspe 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices): developed in collaboration with the american association for thoracic surgery and society of thoracic surgeons. Circulation. 2008;117:e350–e408. doi: 10.1161/CIRCUALTIONAHA.108.189742. [DOI] [PubMed] [Google Scholar]