Abstract
Background
Independent of current socioeconomic status (SES), past maternal SES might influence asthma outcomes in children.
Objective
We examined associations among the mother’s SES in the first 10 years of her life (maternal childhood SES), increased cord blood IgE levels (upper 20% [1.37 IU/mL]), and repeated wheeze (≥2 episodes by age 2 years) in an urban pregnancy cohort (n = 510).
Methods
Data on sociodemographics, discrimination, financial strain, community violence, interpersonal trauma, and other negative events were obtained prenatally. Prenatal household dust was assayed for cockroach and murine allergens, and traffic-related air pollution was estimated by using spatiotemporal land-use regression. Maternal childhood SES was defined by parental home ownership (birth to 10 years). Maternally reported child wheeze was ascertained at 3-month intervals from birth. Using structural equation models, we examined whether outcomes were dependent on maternal childhood SES directly versus indirect relationships operating through (1) cumulative SES-related adversities, (2) the mother’s socioeconomic trajectory (adult SES), and (3) current prenatal environmental exposures.
Results
Mothers were largely Hispanic (60%) or black (28%), 37% had not completed high school, and 56% reported parental home ownership. When associations between low maternal childhood SES and repeated wheeze were examined, there were significant indirect effects operating through adult SES and prenatal cumulative stress (β = 0.28, P = .003) and pollution (β = 0.24, P = .004; P value for total indirect effects ≤ .04 for both pathways). Low maternal childhood SES was directly related to increased cord blood IgE levels (β = 0.21, P = .003). Maternal cumulative adversity (interpersonal trauma) was also associated with increased cord blood IgE levels (β = 0.19, P = .01), although this did not explain maternal childhood SES effects.
Conclusion
Lower maternal childhood SES was associated with increased cord blood IgE levels and repeated wheeze through both direct and indirect effects, providing new insights into the role of social inequalities as determinants of childhood respiratory risk.
Keywords: Childhood socioeconomic status, intergenerational, cord blood IgE, inner-city, childhood wheeze, structural equation models, life course
Research links childhood factors to adult health, including respiratory disease.1,2 In the context of women’s health, childhood conditions might carry over to the next generation.3 Although we recognize that childhood asthma has its roots prenatally4-6 and that maternal socioeconomic status (SES) during pregnancy and the child’s first years contribute to observed disparities, effects on the next generation might be influenced by maternal risk factors and health disparities rooted in a mother’s own childhood SES. If early childhood experiences related to disadvantage can affect asthma risk in the next generation, then the need for interventions aimed at breaking the cycle of childhood poverty becomes heightened.
Although studies have begun to examine the relationship between maternal SES and correlated exposures assessed before pregnancy on her children’s health,3,7,8 none have examined the influence of maternal childhood SES on childhood wheeze and related phenotypes (eg, atopy). Intergenerational influences of maternal SES might be better understood if we consider how biological and social risks in the mother’s childhood and subsequent life course can influence her perinatal health and behaviors, maternal-fetal exposures, and associated outcomes in her children.9 Grounded in life-course epidemiology, this approach examines theoretical models linking exposures at different life periods and later health. The latent effects model proposes that early-life SES might determine maternal childhood environmental exposures (eg, pollutants, allergens, and stress) that prime her own immune system toward asthma and atopy, contributing to related disorders in her offspring.10 A cumulative risk model suggests that psychosocial experiences related to childhood poverty and associated adversities accumulating over a mother’s life (eg, family violence) might have lasting effects on maternal psychological functioning11 and stress responses,4 which, in turn, might affect the developing infant. Finally, the socioeconomic trajectory model posits that the mother’s childhood SES might be related to her adult socioeconomic position (eg, educational attainment) and associated adverse social and physical environmental exposures during her child’s early development. This might manifest, for example, through perinatal health behaviors, such as maternal smoking,12 contributing to childhood wheeze. Arguably, intergenerational effects of maternal childhood SES can be more fully explored through a life-course approach examining the direct relationship between maternal childhood SES and respiratory outcomes in the child, as well as considering potential pathways.10,13
We examine associations among maternal childhood SES, cord blood IgE levels (an index of early atopic risk7), and repeated wheeze in children enrolled in an urban pregnancy cohort: the Asthma Coalition on Community, Environment, and Social Stress (ACCESS) project. Using structural equation models (SEMs), we examine whether these childhood outcomes are dependent on (1) the direct effects of low maternal childhood SES versus indirect relationships operating through (2) the cumulative risk of SES-related social adversities throughout the mother’s life course and/or (3) the socioeconomic trajectory of the mother (ie, adult SES) as related to environmental exposures and psychological stress experienced more proximate to the index pregnancy.
METHODS
Sample
This prospective cohort was originally funded to recruit 589 pregnant women and their children to study the effects of prenatal maternal and early-life stress on urban childhood asthma risk.14 English- or Spanish-speaking pregnant women (≥18 years old) receiving prenatal care at Brigham & Women’s Hospital, Boston Medical Center, and 3 community health centers were recruited between August 2002 and January 2006. Among 754 women approached who met the eligibility criteria, 78.1% agreed to enroll. There were no significant differences between participants and those who declined based on race/ethnicity, education, and income. Procedures were approved by the Brigham & Women’s Hospital and Boston Medical Center human studies committees; written consent was obtained. Data on maternal childhood SES and childhood repeated wheeze were available for 510 mother-child pairs with follow-up to age 2 years; cord blood IgE measurements were available for 454. Baseline data on sociodemographics and prenatal exposures were obtained at 28.4 ± 7.9 weeks’ gestation.
Maternal childhood SES
Housing status, a robust marker of economic circumstances over the life course, correlates with other conventional SES indicators (eg, income and assets) and is retrospectively reported with accuracy.15,16 Parental home ownership in childhood is associated with later life outcomes, including physiological disruption.15-17 Mothers reported whether their parents ever owned a home over the first 10 years of her childhood. A binary indicator of parental home ownership (ever between ages 0 and 10 years) indexed maternal childhood SES in the first 10 years of her life.
Outcome variables
Wheeze
At approximately 3-month intervals starting from birth, maternally reported wheeze was ascertained up to age 2 years. Caretakers were asked the following: “Since we last spoke with you on (date), has your infant/child had wheezing or whistling in the chest?” Two or more episodes constituted repeated wheeze.
Cord blood IgE
Cord blood total IgE levels (in international units per milliliter) were determined by using the CAP fluorescent enzyme immunoassay (Pharmacia [now Phadia], Uppsala, Sweden; lower limit of detection, 0.2 IU/mL).18 IgE was log transformed and divided into quintiles, with serum levels at or greater than the upper 20% (1.37 IU/mL) considered high.7
Social pathway variables
Cumulative lifetime adversity
Interpersonal trauma (IPT) disproportionally burdens lower-income populations,19 and trauma over the mother’s life course has been associated with increased IgE levels in newborns.7 IPT was assessed based on the mother’s self-report on the Revised Conflict Tactics Scale20 with documented reliability (range, 0.79-0.95) and validity in English and Spanish.21 IPT was assessed during childhood (≤11 years of age), adolescence (12-17 years), adulthood before the pregnancy, and during the pregnancy asking whether anyone had ever pushed, grabbed, or shoved them; kicked, bit, or punched them; hit them with something; choked or burned them; forced them to engage in sexual activities; or physically attacked them in another way. Mothers were considered exposed in each period if they answered yes to any item. IPT was categorized as follows: (1) unexposed, (2) 1 time period (during childhood/teenage years only or adulthood and/or pregnancy only), or (3) chronic (both periods).
Educational attainment
Current maternal SES was indexed as less than high school, high school, and some college or more.
Cumulative prenatal stress
Lower maternal adult SES might be associated with increased chronic stressors experienced more proximate to the pregnancy.22 Constructs assessed included discrimination, financial strain, community violence, and other negative life events.
The Experiences of Discrimination scale23 ascertained maternal reports of ever experiencing racially motivated unfair treatment (eg, at school, getting a job, or from the police or courts). Items were summed, with higher scores indicating greater discrimination.
Financial strain was assessed through a 3-item index of economic difficulties in the past 3 months24 scored on a 5-point scale and summed; higher scores indicated greater difficulties.
Community violence was obtained by using the My Exposure to Violence survey25 assessing events over the past year, including hearing gunshots and witnessing or experiencing shoving or hitting, knife attacks, and shootings. Multi-item responses were summarized into a continuous score.26
Other negative life events in the past 6 months were assessed by using the Crisis in Family Systems survey,27 encompassing 11 domains (ie, financial, legal, career, relationships, medical problems, safety in the community and home, other home issues, difficulty with authority, and discrimination), with multiple items in each domain. Women endorsed items as positive, negative, or neutral. The number of domains with 1 or more negative events were summed, creating a negative domains score, with higher scores indicating greater stress.
Physical pathway variables
Because lower-SES populations might be more likely to live in poor housing with increased indoor allergens28 or communities with greater air pollution,29 we also assessed the following.
Prenatal household allergens
Cockroach allergens (Blattella germanica: Bla g 1 and Bla g 2) and mouse urinary protein (MUP) were measured in settled dust collected within 2 weeks of the baseline questionnaire from the mother’s bed and bedroom floor by using an mAb-based ELISA (Indoor Biotechnologies, Charlottesville, Va).30 Increased cockroach levels were defined as Bla g 1, Bla g 2, or both levels of greater than 2 U/g,30 whereas MUP levels of greater than the median were considered high.31
Housing deterioration
Because housing deterioration is predictive of household allergens,28 an indicator of housing disrepair was defined by the number of adverse characteristics endorsed (eg, water damage, other evidence of leaks, and holes or cracks in the floor) categorized from 0 to 4.
Prenatal ambient pollution
Exposure to traffic-related air pollution based on residence over the entire pregnancy was estimated by using a validated spatiotemporal land-use regression model accounting for cumulative traffic density within 100 m, distance to roadway, percentage of urbanization, and meteorology.32
Maternal-fetal factors
Childhood and adult SES might be associated with maternal health behaviors and fetal factors linked to offspring asthma risk, including maternal age, preterm delivery, low birth weight,33 and maternal smoking.34 Continuous measures of maternal age (in years), birth weight for gestational age,35 and maternal prenatal smoking (yes/no) based on self-report were thus considered. Maternal lifetime history of atopy was defined as clinician-diagnosed asthma, hay fever, and/or eczema.
Statistical analyses
We examined descriptive statistics for the overall sample and then relative to maternal childhood SES. Relationships between home ownership and covariates were examined by using Kruskal-Wallis ANOVA to test differences in means and the Pearson χ2 statistic for differences in proportions. The Bonferroni adjustment was applied to the Pearson χ2 test and the Wilcox rank sum test for 2-group pairings within multigroup comparisons.
Next we used confirmatory factor analysis to test the fit of 2 latent constructs: (1) discrimination, financial strain, community violence, and other negative life events formed the composite measure of prenatal stress (factor loadings, 0.3-0.8), and (2) we also used a composite measure of prenatal household allergen exposure, including cockroach and MUP, and deteriorating housing scores (factor loadings, 0.5-0.7).
Finally, we tested the direct and indirect effects of maternal childhood SES on the offspring’s outcomes by using path analyses, a special case of SEMs, implemented in MPLUS.36 This allowed us to test both direct associations between maternal childhood SES and child outcomes (repeated wheeze and cord blood IgE level), as well as whether associations were operating through a number of pathways: cumulative adversity (lifetime IPT); current physical exposures (housing-related exposures and ambient pollution) and/or psychosocial factors (prenatal stress) operating through adult SES (maternal educational attainment); and/or maternal-fetal factors (maternal age at the child’s birth, prenatal maternal smoking, and birth weight). All SEMs were adjusted for the child’s sex, maternal race/ethnicity, and maternal atopy. Given that home ownership might not have the same meaning related to social status or wealth in those born in the United States versus those born elsewhere, we also adjusted for nativity status (US born vs foreign born). Because season of birth and maternal smoking were not significantly related to the exposure, intermediate variables, or the outcomes, these were excluded from analyses.
Similar to traditional logistic regression models for binary responses,37 the asymptotic distribution of statistics testing absolute goodness of fit can be difficult to approximate. Tests reflecting the relative fit of 2 competing models are less problematic. Therefore we compared the fit of our proposed SEMs with the null model. Our models fit the data significantly better than the null model (P < .0001 for all).
RESULTS
Descriptive statistics
Tables I and II21,23-25,27,32 describe sample characteristics stratified by maternal childhood SES and outcomes. Most were Hispanic (60%) or black (28%) and foreign born (67%). More than half (56%) reported parental home ownership, 14% had repeated wheeze, and 21% had increased cord blood IgE levels. Mothers with high childhood SES (mother’s reporting parental home ownership in the first 10 years of her life) were older at the time of the child’s birth. Women with low childhood SES were more likely US born, atopic, currently living in areas with higher community violence, and smokers.
TABLE I.
Distribution of covariates by maternal childhood SES (n = 510)*
| All (n = 510) |
Parental home ownership† |
P value‡ |
||
|---|---|---|---|---|
| No (n = 223) | Yes(n = 287) | |||
| Maternal characteristics | ||||
| Age at child’s birth (y [mean ± SD]) |
27 ± 6.1 | 25.3 ± 5.5 | 28.6 ± 6.1 | <.001 |
| Nativity status§ | <.001 | |||
| American born | 169 (33) | 113 (51) | 56 (20) | |
| Foreign born | 341 (67) | 110 (49) | 231 (81) | |
| Education, no. (%) | .11 | |||
| <High school | 188 (37) | 90 (40) | 98 (34) | |
| High school degree | 149 (29) | 71 (32) | 78 (27) | |
| ≥Some college | 157 (31) | 59 (27) | 98 (34) | |
| Missing | 16(3) | 3 (1) | 13 (5) | |
| Race, no. (%) | .08 | |||
| Hispanic | 302 (60) | 118 (53) | 184 (64) | |
| Black | 141 (28) | 73 (33) | 68 (24) | |
| White | 42 (8) | 20 (9) | 22 (8) | |
| Other/mixed | 25 (5) | 12 (5) | 13 (5) | |
| Asthma/atopy, no. (%) | <.01 | |||
| No | 317 (62) | 123 (55) | 194 (68) | |
| Yes | 178 (35) | 91 (41) | 87 (30) | |
| Missing | 15 (3) | 9 (4) | 6(2) | |
| Lifetime IPT∥ | .15 | |||
| Never experienced | 266 (52) | 105 (47) | 161 (56) | |
| One period | 161 (32) | 74 (33) | 87 (30) | |
| Chronic IPT | 64 (13) | 33 (15) | 31 (11) | |
| Missing | 19 (4) | 11 (5) | 8 (3) | |
| Prenatal maternal smoking, no. (%) |
<.01 | |||
| No | 430 (84) | 176 (79) | 254 (89) | |
| Yes | 77 (15) | 45 (20) | 32 (11) | |
| Missing | 3 (1) | 2 (1) | 1 (0) | |
| Childhood characteristics | ||||
| Sex, no. (%) | .18 | |||
| Male | 248 (49) | 116 (52) | 132 (46) | |
| Female | 262 (51) | 107 (48) | 155 (54) | |
| Season of birth | .53 | |||
| Winter | 139 (27) | 63 (29) | 76 (27) | |
| Summer | 111 (22) | 54 (24) | 57 (20) | |
| Spring | 105 (21) | 42 (19) | 63 (22) | |
| Fall | 155 (30) | 64 (29) | 91 (32) | |
| Birth weight, z value for gestational age (mean ± SD) |
− 0.26 ± 1.2 | − 0.27 ± 1.1 | − 0.25 ± 1.2 | .87 |
| Prenatal exposures | ||||
| Discrimination (mean ± SD)¶ |
1.0 ± 1.6 | 0.94 ± 1.5 | 1.1 ± 1.6 | .34 |
| Financial strain# | 5.9 ± 2.6 | 5.9 ± 2.7 | 5.9 ± 2.5 | .96 |
| Community violence (mean ± SD)** |
0.006 ± 0.92 | 0.16 ± 0.96 | −0.11 ± 0.87 | <.001 |
| Other negative life events (mean ± SD)†† |
1.8 ± 1.8 | 1.9 ± 1.9 | 1.7 ± 1.8 | .23 |
| Allergens (cockroach or mouse), no. (%)‡‡ |
.98 | |||
| No | 262 (51) | 110 (49) | 152 (53) | |
| Yes | 191 (38) | 80 (36) | 111 (39) | |
| Missing | 57 (11) | 33 (15) | 24 (8) | |
| Signs of housing deterioration |
.24 | |||
| None | 247 (48) | 99 (44) | 148 (52) | |
| 1 | 161 (32) | 71 (32) | 90 (31) | |
| 2 | 80 (16) | 42 (19) | 38 (13) | |
| ≥3 | 22 (4) | 11 (5) | 11 (4) | |
| Traffic-related air pollution (mean ± SD)11 |
1.8 ± 0.7 | 1.9 ± 0.8 | 1.8 ± 0.7 | .16 |
Percentage might add to more than 100 because numbers were rounded up.
The mother’s parents owned their home during her childhood (birth to age 10 years).
For differences between nonmissing multigroup comparisons using ANOVA and χ2 distribution when applicable.
Less than 4% of the Latino sample was born in Puerto Rico. They were considered US born.
Derived from the Revised Conflict Tactics Scale.21 IPT was a 3-category interval variable: (1) unexposed, (2) 1 time period (during childhood or teenage years only or during adulthood and/or the index pregnancy only), or (3) exposure in both time periods.
Assessed by using the Experiences of Discrimination Scale.23
Based on a 3-item index of economic difficulties.24
Assessed through the modified version of the My Exposure to Violence survey.25
Assessed through the Crisis in Family Systems (CRISYS) survey.27
High MUP values were defined as greater than the median: 246 U/g for wheeze and 332 U/g for IgE. An increased cockroach allergen level was defined as Bla g 1, Bla g 2, or both at greater than 2 U/g.
Black carbon exposure was estimated on the basis of the mother’s residence during pregnancy by using a validated spatiotemporal land-use regression model.32
TABLE II.
Distribution of covariates by childhood wheeze and cord blood IgE level*
| Repeated wheeze in offspring† (n = 510) |
Increased IgE level in offspring‡ (n = 455) |
|||||||
|---|---|---|---|---|---|---|---|---|
| All (n = 510) | No (n = 437) | Yes (n = 73) | P value§ | All (n = 455) | No (n = 358) | Yes (n = 95) | P value§ | |
| Maternal characteristics | ||||||||
| Age at birth (y [mean ± SD]) |
27 ± 6.1 | 27.2 ± 6.1 | 26.9 ± 5.5 | .65 | 27 ± 5.7 | 26.7 ± 5.7 | 25.8 ± 6.0 | .15 |
| Nativity status ∥ | .07 | .01 | ||||||
| American born | 169 (33) | 138 (32) | 31 (42) | 159 (35) | 115 (32) | 44 (46) | ||
| Foreign born | 341 (67) | 299 (68) | 42 (58) | 296 (65) | 245 (68) | 51 (54) | ||
| Education, no. (%) | .12 | .26 | ||||||
| <High school | 188 (37) | 168 (38) | 20 (27) | 167 (37) | 139 (39) | 28 (30) | ||
| <High school degree | 149 (29) | 127 (29) | 22 (30) | 133 (29) | 102 (28) | 31 (33) | ||
| ≥Some college | 157 (31) | 128 (29) | 29 (40) | 132 (29) | 101 (28) | 31 (33) | ||
| Missing | 16 (3) | 14 (3) | 2(3) | 23 (5) | 18 (5) | 5(5) | ||
| Race, no. (%) | .81 | .003 | ||||||
| Hispanic | 302 (60) | 260 (60) | 42 (58) | 271 (60) | 230 (64) | 41 (43) | ||
| Black | 141 (28) | 122 (28) | 19 (26) | 135 (30) | 95 (26) | 40 (42) | ||
| White | 42 (8) | 35 (8) | 7(10) | 25 (6) | 19(5) | 6(6) | ||
| Other/mixed | 25 (5) | 20 (5) | 5 (7) | 24(5) | 16(5) | 8(8) | ||
| Asthma/atopy, no. (%) | <.01 | .03 | ||||||
| No | 317 (62) | 284 (65) | 33 (45) | 260 (58) | 218 (61) | 47 (50) | ||
| Yes | 178 (35) | 142 (33) | 36 (49) | 160 (35) | 117 (33) | 43 (45) | ||
| Missing | 15 (3) | 11 (3) | 4(6) | 30 (7) | 25 (7) | 5(5) | ||
| Lifetime IPT¶ | .07 | <.005 | ||||||
| Never experienced | 266 (54) | 234 (54) | 32 (44) | 228 (50) | 194 (54) | 34 (36) | ||
| One period | 161 (32) | 137 (31) | 24 (33) | 130 (29) | 96 (27) | 34 (36) | ||
| Chronic IPT | 64 (13) | 49 (11) | 15 (21) | 55 (12) | 38 (11) | 17 (18) | ||
| Missing | 19 (4) | 17 (4) | 2(3) | 42 (9) | 32 (9) | 10(11) | ||
| Smoking, no. (%) | .98 | .91 | ||||||
| No | 430 (84) | 66 (15) | 11 (15) | 388 (85) | 307 (85) | 81 (85) | ||
| Yes | 77 (15) | 368 (84) | 62 (85) | 59 (13) | 47 (13) | 12 (13) | ||
| Missing | 3 (1) | 3 (1) | – | 8 (2) | 6(2) | 2 (2) | ||
| Parental home ownership |
.07 | <.001 | ||||||
| No | 223 (44) | 184 (42) | 39 (53) | 213 (47) | 152 (42) | 61 (64) | ||
| Yes | 287 (56) | 253 (58) | 34 (47) | 242 (53) | 208 (58) | 34 (36) | ||
| Child’s characteristics | ||||||||
| Sex, no. (%) | .01 | .65 | ||||||
| Male | 248 (49) | 214 (49) | 48 (66) | 230 (51) | 180 (50) | 50 (53) | ||
| Female | 262 (51) | 223 (51) | 25 (34) | 225 (50) | 180 (50) | 45 (47) | ||
| Season of birth | .69 | .34 | ||||||
| Winter | 139 (27) | 122 (28) | 17 (23) | 131 (29) | 98 (27) | 33 (35) | ||
| Summer | 111 (22) | 97 (22) | 14 (19) | 89 (20) | 75 (21) | 14 (15) | ||
| Spring | 103 (21) | 88 (20) | 17 (23) | 111 (24) | 87 (24) | 24 (25) | ||
| Fall | 155 (30) | 130 (30) | 25 (34) | 124 (27) | 100 (28) | 24 (25) | ||
| Birth weight, z value for gestational age (mean ± SD) |
−0.26 ± 1.2 | −0.25 ± 1.2 | −0.32 ± 1.3 | .63 | −0.43 ± 1.2 | −0.41 ± 1.2 | 0.53 ± 1.3 | .54 |
| Prenatal exposures | ||||||||
| Discrimination (mean ± SD)# |
1.0 ± 1.6 | 0.99 ± 1.6 | 1.2 ± 1.5 | .41 | 1.2 ± 1.7 | 1.1 ± 1.6 | 1.3 ± 1.9 | .41 |
| Financial strain** | 5.9 ± 2.6 | 5.8 ± 2.5 | 6.3 ± 2.9 | .10 | 5.9 ± 2.7 | 5.8 ± 2.6 | 6.2 ± 2.8 | .26 |
| Community violence (mean ± SD)†† |
0.005 ± 1 | −0.1 ± 0.9 | 0.37 ± 0.9 | <.01 | 0.04 ± 1.0 | −0.03 ± 0.9 | 0.28 ± 1.0 | .01 |
| Other current negative life events (mean ± SD)‡‡ |
1.8 ± 1.8 | 1.7 ± 1.8 | 2.4 ± 2.1 | .001 | 1.8 ± 1.9 | 1.8 ± 1.8 | 1.9 ± 2.1 | .03 |
| Allergens (cockroach or mouse), no. % |
1.00 | .81 | ||||||
| No | 262 (51) | 225 (52) | 37 (51) | 239 (53) | 190 (53) | 49 (52) | ||
| Yes | 191 (38) | 164 (38) | 27 (37) | 165 (36) | 133 (37) | 32 (34) | ||
| Missing | 57 (11) | 48 (11) | 9(12) | 51 (11) | 37 (10) | 14 (15) | ||
| Signs of housing deterioration |
.49 | .43 | ||||||
| None | 247 (48) | 217 (50) | 30 (41) | 215 (47) | 166 (46) | 49 (52) | ||
| 1 | 161 (32) | 136 (31) | 25 (34) | 149 (33) | 117 (33) | 32 (34) | ||
| 2 | 80 (16) | 65 (15) | 15 (21) | 70 (15) | 58 (16) | 12 (13) | ||
| ≥3 | 22 (4) | 19 (4) | 3 (4) | 21 (5) | 19 (5) | 2(2) | ||
| Traffic-related air pol- lution (mean ± SD)§§ |
1.8 ± 0.7 | 1.8 ± 0.7 | 2.0 ± 0.8 | .02 | 2.1 ± 1.5 | 2.0 ± 1.3 | 2.3 ± 1.9 | <.001 |
Percentage might add to more than 100 because numbers were rounded up.
Defined as maternal reports of repeated incidents of wheeze or whistling in the chest in children up to age 2 years.
Defined as cord blood level at or greater than 1.37 IU/mL.
For differences between nonmissing multigroup comparisons using ANOVA and χ2 distribution when applicable.
Less than 4% of the Latino sample were born in Puerto Rico. These were considered US born.
Derived from the Revised Conflict Tactics Scale.21 IPT was a 3-category interval variable: (1) unexposed, (2) 1 time period (during childhood or teenage years only or during adulthood and/or the index pregnancy only), or (3) exposure in both time periods.
Assessed by using the Experiences of Discrimination Scale.23
Based on a 3-item index of economic difficulties.24
Assessed through the modified version of the My Exposure to Violence survey.25
Assessed through the Crisis in Family Systems (CRISYS) survey.27
Black carbon exposure was estimated on the basis of the mother’s residence during pregnancy by using a validated spatiotemporal land-use regression model.32
More offspring of mothers with lower childhood SES had increased cord blood IgE levels (28% vs 14%, P < .0001). More children of atopic versus nonatopic mothers had repeated wheeze (20% vs 11%, P <.003) and increased cord blood IgE levels (27% vs 18%, P < .009). A lower percentage of Hispanics versus all others (15% vs 30%, P < .0001) and more children of foreignborn mothers versus their US-born counterparts (28% vs 17%, P < .005) had increased cord blood IgE levels. More boys than girls had repeated wheeze (18% vs 10%, P < .004). There were higher mean negative domain scores among children with versus those without repeated wheeze and increased IgE levels (Table II). Mean community violence levels were higher among children with repeated wheeze and increased cord blood IgE levels. Mean traffic-related pollution values were higher among children with repeated wheeze and higher cord blood IgE levels.
Structural equation models
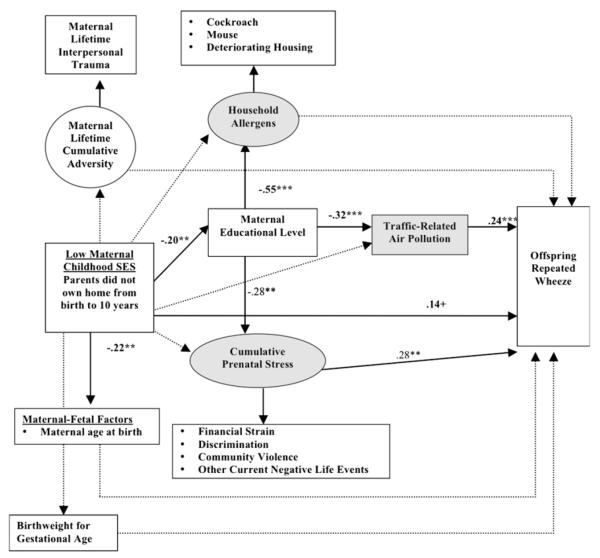
Figs 1 and 2 show significant path coefficients between low maternal childhood SES and repeated wheeze in offspring and increased IgE levels, respectively. Although the direct path between low maternal childhood SES and repeated wheeze in offspring was not significant (β = 0.14, P = .09), there were significant indirect effects operating through adult SES and related prenatal environmental exposures (Fig 1). Adjusting for standard control variables (ie, maternal atopy, nativity status, race/ethnicity, and child’s sex), as well as other covariates (maternal age at child’s birth, birth weight for gestational age, and life-course IPT), low maternal childhood SES was associated with lower maternal educational attainment, and, in turn, lower education in mothers was associated with increased prenatal environmental exposures (allergens, air pollution, and cumulative stress). There were significant indirect effects of low maternal childhood SES operating through lower adult SES and increased prenatal cumulative stress (β = 0.03, P = .03) and increased pollution (β = 0.03, P = .04), ie, P values for total indirect effects. Although maternal childhood SES was inversely related to maternal age at birth, the latter was not significantly associated with wheeze (see Table E1 in this article’s Online Repository at www.jacionline.org).
FIG 1.
Significant pathways linking low maternal childhood SES to repeated wheeze in offspring. Ovals represent unmeasured latent variables, and boxes represent measured variables. Shaded areas represent prenatal social and physical environmental exposures considered in analyses. All models adjust for maternal atopy/asthma, nativity status, child’s sex, and race. Bolded lines represent significant pathways, and dotted lines represent nonsignificant pathways. Coefficients are listed only for significant pathways. Effect estimates for all tested direct and indirect pathways are provided in the Online Repository (see Table E1). A positive sign indicates that the probability of the categorical dependent variable is increased when the predictor increases. ***P < .001, **P < .01, *P < .05, and +P < .10.
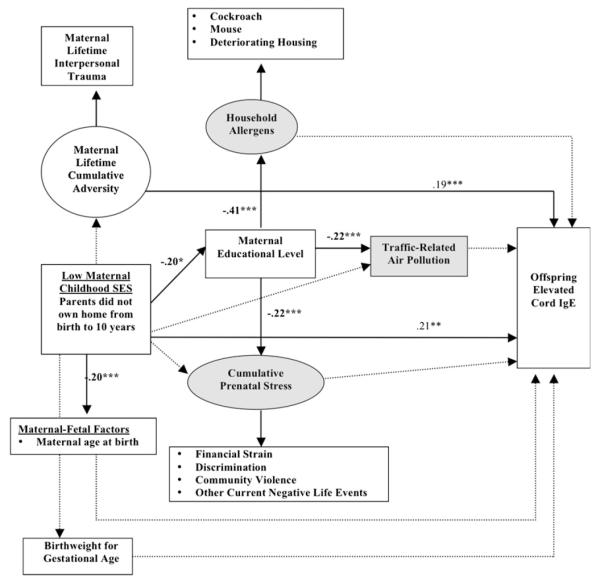
FIG 2.
Significant pathways linking low maternal childhood SES to increased cord blood IgE levels. Ovals represent unmeasured latent variables, and boxes represent measured variables. Shaded areas represent prenatal social and physical environmental exposures considered in analyses. All models adjust for maternal asthma/atopy, nativity status, child’s sex, and race. Bolded lines represent significant pathways, and dotted lines represent non-significant pathways. Coefficients are listed only for significant pathways. Effect estimates for all tested direct and indirect pathways are provided in the Online Repository (see Table E1). A positive sign indicates that the probability of the categorical dependent variable is increased when the predictor variable increases. ***P < .001, **P < .01, *P < .05.
TABLE E1.
Standardized path coefficients for all SEMs examining relationships among low maternal childhood SES and asthma-related outcomes in offspring
| Coefficient within analytic group* |
||||
|---|---|---|---|---|
| Tested pathway | Repeated wheeze† (n = 510) |
Increased IgE level‡ (n = 455) |
||
| Direct effects | B | P value | β | P value |
| Low maternal childhood SES§ → outcome | 0.14 | .09 | 0.21 | .01 |
| Effects of low maternal childhood SES on pathway variables | ||||
| Low childhood SES → adult SES (higher-level maternal education)∥ | −0.20 | <.001 | −0.13 | .03 |
| Low childhood SES → cumulative adversity (IPT) | 0.01 | .89 | 0.01 | .88 |
| Low childhood SES → household allergens | −0.06 | .47 | −0.05 | .59 |
| Low childhood SES → cumulative prenatal stress | −0.05 | .42 | −0.03 | .70 |
| Low childhood SES → air pollution | −0.04 | .47 | −0.03 | .69 |
| Low childhood SES → maternal age at birth | −0.22 | <.001 | −0.20 | <.001 |
| Low childhood SES → birth weight for gestational age | 0.02 | .77 | 0.03 | .54 |
| Effects of adult SES (mother’s education) on prenatal pathway variables | ||||
| Adult SES → household allergens | −0.55 | <.001 | −0.41 | <.001 |
| Adult SES → cumulative prenatal stress | −0.28 | .003 | −0.20 | <.001 |
| Adult SES → traffic-related air pollution | −0.32 | <.001 | −0.22 | <.001 |
| Effect of pathway variables on outcome | ||||
| Maternal lifetime cumulative adversity (IPT) → outcome | 0.10 | .19 | 0.19 | .01 |
| Household allergens → outcome | 0.16 | .31 | −0.13 | .29 |
| Prenatal stress → outcome | 0.28 | .003 | 0.06 | .41 |
| Traffic-related air pollution → outcome | 0.24 | .004 | 0.06 | .17 |
| Maternal age at child’s birth → outcome | −0.01 | .85 | −0.02 | .73 |
| Infant’s birth weight for gestational age → outcome | −0.01 | .85 | −0.05 | .58 |
| Total indirect effects | ||||
| Low childhood SES → adult SES → cumulative prenatal stress → outcome | 0.03 | .03 | 0.002 | .46 |
| Low childhood SES → adult SES → traffic-related pollution → outcome | 0.03 | .04 | 0.002 | .24 |
All models were also adjusted for maternal history of asthma/atopy, nativity status, child’s sex, and child’s race.
Defined as maternal reports of repeated incidents of wheeze or whistling in the chest in children up to age 2 years.
Defined as a cord blood IgE level at or greater than 1.37 IU/mL.
Defined as no parental home ownership during the mother’s childhood (ages 0-10 years).
Measured through an ordinal variable (<high school, high school, and ≥some college).
A significant direct path was found between low maternal childhood SES and increased cord blood IgE levels (Fig 2). Controlling for other covariates (maternal atopy, nativity status, race/ethnicity, child’s sex, maternal age at child’s birth, birth weight for gestational age, life-course IPT, maternal education, prenatal stress, air pollution, and allergens), mothers with low childhood SES were significantly more likely to have newborns with increased IgE levels compared with those with higher maternal childhood SES. Additionally, mothers reporting IPT experienced in a greater number of life stages had a higher risk of having a child with increased IgE levels, although lower childhood SES was not significantly related with increased maternal IPT. Prenatal stress, household allergens, and traffic-related pollution were not significantly associated with cord blood IgE levels. Effect estimates for all tested direct and indirect pathways are provided in this article’s Online Repository (see Table E1).
DISCUSSION
These analyses are the first to demonstrate an association between low maternal childhood SES (mothers’ SES in the first 10 years of her life) and adverse respiratory outcomes in children (ie, intergenerational effects).
Lower maternal childhood SES was directly associated with increased cord blood IgE levels, supporting the latent effects model that early-life environmental stressors (psychological and physical) related to the mother’s own lower childhood SES might have a programming effect on key physiological systems that carries through to her adulthood and pregnancy and consequently influence her child’s development.4,38 For example, a woman’s exposure to SES-related stressors in her early life might imprint lasting biological changes in her neuroendocrine and immune systems that continue to exert their effects into adulthood.15,39 Dysregulated immunocompetence38 and stress responses (eg, disrupted hypothalamic-pituitary-adrenal axis) experienced during pregnancy (albeit rooted in the mother’s childhood) might affect fetal immune40 and lung41 development.
Although our analysis with SEM corroborated our earlier finding that maternal lifetime IPT predicts increased cord blood IgE levels in children,7 lower maternal childhood SES was not associated with maternal IPT. This finding does not support a cumulative risk/adversity model. It might be that parental home ownership does not account for other important factors contributing to lifetime IPT (eg, frequent moving, and parental separation/divorce). More detailed assessment of other components of the mother’s childhood SES (eg, parental occupation or employment status and parental education) when they were growing up, as well as other measures of ongoing adversity, might be needed in future work.42
In relation to repeated wheeze, evidence supported the socioeconomic trajectory model. The association between maternal childhood SES and repeated wheeze in her offspring operated through increased prenatal environmental exposure to stress and traffic-related pollution, which in turn was inversely related to the mother’s education. These urban, largely low-income women experienced significant psychosocial stress across several domains (ie, abuse, financial strain, discrimination, and community violence), which might influence their own prenatal physiology, that then affects the fetal environment. Although other studies have shown a relationship between early postnatal stress and repeated wheeze43 and increased IgE levels,44 this is the first to demonstrate an association between maternal prenatal stress and repeated wheeze in early childhood. Again, mechanisms that alter the course of neuroendocrine,45 immune,46 or lung/airway development40,41 can be implicated. Prenatal exposure to air pollutants might alter the normal course of lung morphogenesis and maturation, affecting both the structure and function of the respiratory system.47 These findings corroborate others demonstrating an association between prenatal air pollution exposure and pediatric wheeze in the first 2 years.48,49 We did not identify a significant effect of maternal smoking on children’s respiratory health, possibly because of low smoking prevalence in this largely Latina, immigrant population, factors related to lower smoking rates.50,51 Finally, our analyses suggest that birth weight, maternal smoking, and maternal atopy were neither significant mediators nor confounders.
The inability to find a significant direct association between maternal childhood SES and child wheeze might be related to differential reporting of wheeze in lower-SES, ethnic/minority subjects. Although repeated wheeze, as defined here, is a reasonable surrogate for who goes on to have asthma,43 it will be important to examine the relationship between maternal childhood SES and more definitive outcomes as these children age (ie, physician-diagnosed asthma and lung function). It is also plausible that these effects operate indirectly through pathways unmeasured in the analysis. For example, despite having information on adult SES and a number of physical and social environmental exposures during pregnancy, these analyses did not include measures of SES during later childhood/adolescence or comparable physical and psychosocial exposures in earlier life (with the exception of maternal IPT). Future work with more complete information on SES over the life course, as well as measures of the social and physical environment, could better test these alternative pathways.42
The differences in results between cord blood IgE levels and wheeze underscore the complexity of asthma etiology. Early wheeze and asthma is a heterogeneous syndrome associated with diverse factors beyond allergen sensitization (eg, air pollution, tobacco smoke, and stress).41 In our urban sample repeated wheeze was associated with prenatal stress and pollution exposure, whereas these environmental exposures in pregnancy did not predict cord blood IgE levels. Although lower adult SES was associated with increased household allergen exposure, the latter was not significantly related to wheeze or IgE levels. Notably, although evidence suggests children are exposed to allergens prenatally, it is not clear that this is a risk factor for asthma or allergic disease.52 Data on the effects of prenatal air pollution are even more sparse. One study documented a relationship between intrauterine exposure to fine-particulate matter and early wheeze but did not consider IgE.49 Another European study found a relationship between prenatal exposure to air pollution and increased cord blood IgE levels only in neonates with nonatopic mothers.53 Finding stronger effects for wheeze as opposed to cord blood IgE also suggests nonatopic pathways might be operating. One proposed pathway linking SES-related exposure to nonatopic wheeze is altered somatic growth (prematurity and low birth weight); however, adjusting for birth weight did not change these relationships. Alternative mechanisms could include environmental exposures (air pollution and stress) that produce altered intrinsic airway responses and immunomodulation occurring in utero (not mediated through IgE) that affect airway inflammation.41
The timing of SES-related exposures might also vary in importance, depending on the asthma phenotype. It is plausible that the latent programming effects of maternal childhood SES and the cumulative effects of maternal adversity might operate primarily through atopic pathways (as indexed by increased cord blood IgE levels), whereas the more proximate effects of current SES-related exposures (eg, fetal exposure to air pollution and stress) might operate through nonatopic mechanisms.
Some limitations are noteworthy. Although home ownership is a robust marker of wealth and stability with high recall accuracy,15 SES is a multidimensional construct, operating at different levels (individual, family, and neighborhood) and capturing material, social, and status-related resources.25 Home ownership does not account for each parent’s contribution to the family’s SES or the larger neighborhood context.42 Moreover, home ownership might not have the same meaning related to social status or wealth for US-born versus foreign-born women. Adjusting for nativity status did not influence results. In sensitivity analyses (not shown) we also stratified SEM models by nativity status, and effect sizes were in the same direction and of similar magnitude (although not significant for all models likely given reduced sample size within strata). Future studies incorporating multiple socioeconomic indicators assessed over the mother’s lifetime might better delineate the range of SES-related health consequences to the next generation and allow for more definitive exploration of possible nativity effects.
Maternal childhood SES might have intergenerational implications for respiratory disorders. Unless the cycle is broken, childhood poverty will not only contribute to disparate risk for adverse outcomes in the index children but also might have implications for the health of their future children. From a policy perspective, these results suggest that early-life interventions, especially those broadly related to childhood poverty reduction and more specifically related to housing policies, might be powerful tools in reducing asthma disparities by potentially breaking the intergenerational propagation of respiratory disease risk.
Clinical implications: : Childhood asthma risk might be influenced by maternal risk factors and health disparities rooted in her own childhood SES, heightening the call for public health interventions designed to eliminate childhood poverty.
Acknowledgments
The Asthma Coalition on Community, Environment, and Social Stress study is funded by grants R01 ES10932, U01 HL072494, and R01 HL080674 (R.J.W., principal investigator). M. J. Sternthal was supported by grant T32-ES07069-29 and the Leaves of Grass Foundation.
Abbreviations used
- IPT
Interpersonal trauma
- MUP
Mouse urinary protein
- SEM
Structural equation model
- SES
Socioeconomic status
Footnotes
Disclosure of potential conflict of interest: B. A. Coull receives research support from the National Institutes of Health and the US Environmental Protection Agency. R. J. Wright receives research support from the National Institutes of Health. The rest of the authors have declared that they have no conflict of interest.
REFERENCES
- 1.Jackson B, Kubzansky LD, Cohen S, Weiss S, Wright RJ. A matter of life and breath: childhood socioeconomic status is related to young adult pulmonary function in the CARDIA study. Int J Epidemiol. 2004;33:271–8. doi: 10.1093/ije/dyh003. [DOI] [PubMed] [Google Scholar]
- 2.Von Mutius E. Childhood experiences take away your breath as a young adult. Am J Respir Crit Care Med. 2002;165:1467. doi: 10.1164/rccm.2204011. [DOI] [PubMed] [Google Scholar]
- 3.Kahn R, Wilson K, Wise P. Intergenerational health disparities: socioeconomic status, women’s health conditions, and child behavior problems. Public Health Rep. 2005;120:399. doi: 10.1177/003335490512000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright RJ, Enlow MB. Maternal stress and perinatal programming in the expression of atopy. Expert Rev Clin Immunol. 2008;4:535–8. doi: 10.1586/1744666X.4.5.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKeever T, Lewis S, Smith C, Hubbard R. The importance of prenatal exposures on the development of allergic disease: a birth cohort study using the West Midlands general practice database. Am J Respir Crit Care Med. 2002;166:827. doi: 10.1164/rccm.200202-158OC. [DOI] [PubMed] [Google Scholar]
- 6.Prescott S. Early origins of allergic disease: a review of processes and influences during early immune development. Curr Opin Allergy Clin Immunol. 2003;3:125. doi: 10.1097/00130832-200304000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Sternthal MJ, Enlow MB, Cohen S, Canner MJ, Staudenmayer J, Tsang K, et al. Maternal interpersonal trauma and cord blood IgE levels in an inner-city cohort: a life-course perspective. J Allergy Clin Immunol. 2009;124:954–60. doi: 10.1016/j.jaci.2009.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins J, Wambach J, David R, Rankin K. Women’s lifelong exposure to neighborhood poverty and low birth weight: a population-based study. Matern Child Health J. 2009;13:326–33. doi: 10.1007/s10995-008-0354-0. [DOI] [PubMed] [Google Scholar]
- 9.Power C, Hertzman C. Social and biological pathways linking early life and adult disease. Br Med Bull. 1997;53:210. doi: 10.1093/oxfordjournals.bmb.a011601. [DOI] [PubMed] [Google Scholar]
- 10.Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. Int J Epidemiol. 2002;31:285–93. [PubMed] [Google Scholar]
- 11.Clark C, Caldwell T, Power C, Stansfeld SA. Does the influence of childhood adversity on psychopathology persist across the lifecourse? A 45-year prospective epidemiologic study. Ann Epidemiol. 2010;20:385–94. doi: 10.1016/j.annepidem.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 12.Graham H, Hawkins SS, Law C. Lifecourse influences on women’s smoking before, during and after pregnancy. Soc Sci Med. 2010;70:582–7. doi: 10.1016/j.socscimed.2009.10.041. [DOI] [PubMed] [Google Scholar]
- 13.Kuh D, Ben-Shlomo Y, Lynch J, Hallqvist J, Power C. Life course epidemiology. J Epidemiol Community Health. 2003;57:778–83. doi: 10.1136/jech.57.10.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wright RJ, Suglia SF, Levy J, Fortun K, Shields A, Subramanian SV, et al. Transdisciplinary research strategies for understanding socially patterned disease: the Asthma Coalition on Community, Environment, and Social Stress (ACCESS) project as a case study. Cienc Saude Coletiva. 2008;13:1729–42. doi: 10.1590/s1413-81232008000600008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen S, Doyle WJ, Turner RB, Alper CM, Skoner DP. Childhood socioeconomic status and host resistance to infectious illness in adulthood. Psychosom Med. 2004;66:553–8. doi: 10.1097/01.psy.0000126200.05189.d3. [DOI] [PubMed] [Google Scholar]
- 16.Miller G, Chen E. Unfavorable socioeconomic conditions in early life presage expression of proinflammatory phenotype in adolescence. Psychosom Med. 2007;69:402. doi: 10.1097/PSY.0b013e318068fcf9. [DOI] [PubMed] [Google Scholar]
- 17.Van de Mheen H, Stronks K, Looman C, Mackenbach J. Does childhood socioeconomic status influence adult health through behavioural factors? Int J Epidemiol. 1998;27:431. doi: 10.1093/ije/27.3.431. [DOI] [PubMed] [Google Scholar]
- 18.Platts-Mills TAE, Erwin EA, Allison AB, Blumenthal K, Barr M. The relevance of maternal immune responses to inhalant allergens to maternal symptoms, passive transfer to the infant, and development of antibodies in the first 2 years of life. J Allergy Clin Immunol. 2003;111:123. doi: 10.1067/mai.2003.10. [DOI] [PubMed] [Google Scholar]
- 19.Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit area survey of trauma. Arch Gen Psychiatry. 1998;55:626. doi: 10.1001/archpsyc.55.7.626. [DOI] [PubMed] [Google Scholar]
- 20.Straus MA, Douglas EM. A short form of the Revised Conflict Tactics Scale, and typologies for severity and mutuality. Violence Vict. 2004;19:507–20. doi: 10.1891/vivi.19.5.507.63686. [DOI] [PubMed] [Google Scholar]
- 21.Connelly C, Newton R, Aarons G. A psychometric examination of English and Spanish versions of the Revised Conflict Tactics Scale. J Interpers Violence. 2005;20:1560–79. doi: 10.1177/0886260505280341. [DOI] [PubMed] [Google Scholar]
- 22.Myers H. Ethnicity and socioeconomic status-related stresses in context: an integrative review and conceptual model. J Behav Med. 2009;32:9–19. doi: 10.1007/s10865-008-9181-4. [DOI] [PubMed] [Google Scholar]
- 23.Krieger N. Racial and gender discrimination: risk factors for high blood pressure? Soc Sci Med. 1990;30:1273–81. doi: 10.1016/0277-9536(90)90307-e. [DOI] [PubMed] [Google Scholar]
- 24.Braveman PA, Cubbin C, Egerter S, Chideya S, Marchi KS, Metzler M, et al. Socioeconomic status in health research: one size does not fit all. JAMA. 2005;294:2879–88. doi: 10.1001/jama.294.22.2879. [DOI] [PubMed] [Google Scholar]
- 25.Selner-O’Hagan M, Kindlon D, Buka S, Raudenbush S, Earls F. Assessing exposure to violence in urban youth. J Child Psychol Psychiatr. 1998;39:215–24. [PubMed] [Google Scholar]
- 26.Suglia S, Ryan L, Wright R. Creation of a community violence exposure scale: accounting for what, who, where, and how often. J Trauma Stress. 2008;21:479–86. doi: 10.1002/jts.20362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shalowitz MU, Berry CA, Rasinski KA, Dannhausen-Brun CA. A new measure of contemporary life stress: development, validation, and reliability of the CRISYS. Health Serv Res. 1998;33:1381–402. [PMC free article] [PubMed] [Google Scholar]
- 28.Rauh VA, Chew GR, Garfinkel RS. Deteriorated housing contributes to high cockroach allergen levels in inner-city households. Environ Health Perspect. 2002;110:323. doi: 10.1289/ehp.02110s2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Neill M, Jerrett M, Kawachi I, Levy J, Cohen A, Gouveia N, et al. Health, wealth, and air pollution: advancing theory and methods. Environ Health Perspect. 2003;111:1861. doi: 10.1289/ehp.6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peters JL, Suglia SF, Platts-Mills TAE, Hosen J, Gold DR, Wright RJ. Relationships among prenatal aeroallergen exposure and maternal and cord blood IgE: Project ACCESS. J Allergy Clin Immunol. 2009;123:1041–6. doi: 10.1016/j.jaci.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phipatanakul W, Eggleston P, Wright E, Wood R. Mouse allergen. The prevalence of mouse allergen in inner-city homes. J Allergy Clin Immunol. 2000;106:1070–4. doi: 10.1067/mai.2000.110796. [DOI] [PubMed] [Google Scholar]
- 32.Gryparis A, Coull B, Schwartz J, Suh H. Semiparametric latent variable regression models for spatiotemporal modelling of mobile source particles in the greater Boston area. J R Stat Soc Ser C Appl Stat. 2007;56:183–209. [Google Scholar]
- 33.Pekkanen J, Xu B, Jarvelin MR. Gestational age and occurrence of atopy at age 31: a prospective birth cohort study in Finland. Clin Exp Allergy. 2001;31:95–102. [PubMed] [Google Scholar]
- 34.Tehranifar P, Liao Y, Ferris J, Terry M. Life course socioeconomic conditions, passive tobacco exposures and cigarette smoking in a multiethnic birth cohort of US women. Cancer Causes Control. 2009;20:867–76. doi: 10.1007/s10552-009-9307-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oken E, Kleinman K, Rich-Edwards J, Gillman M. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muthen L, Muthen B. Mplus user’s guide. Murgwn & Muthen; Los Angeles: 1998. [Google Scholar]
- 37.Agresti A. Categorical data analysis. 2nd ed John Wiley & Sons; New York: 2002. [Google Scholar]
- 38.Prescott S, Clifton V. Asthma and pregnancy: emerging evidence of epigenetic interactions in utero. Curr Opin Allergy Clin Immunol. 2009;9:417–26. doi: 10.1097/ACI.0b013e328330634f. [DOI] [PubMed] [Google Scholar]
- 39.Gustafsson P, Janlert U, Theorell T, Hammarstrom A. Life-course socioeconomic trajectories and diurnal cortisol regulation in adulthood. Psychoneuroendocrinology. 2010;35:613–23. doi: 10.1016/j.psyneuen.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 40.Wright R. Prenatal maternal stress and early caregiving experiences: implications for childhood asthma risk. Paediatr Perinat Epidemiol. 2007;21:8–14. doi: 10.1111/j.1365-3016.2007.00879.x. [DOI] [PubMed] [Google Scholar]
- 41.Wright R. Perinatal stress and early life programming of lung structure and function. Biol Psychol. 2010;84:46–56. doi: 10.1016/j.biopsycho.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen S, Janicki-Deverts D, Chen E, Matthews K. Childhood socioeconomic status and adult health. Ann N Y Acad Sci. 2010;1186:37–55. doi: 10.1111/j.1749-6632.2009.05334.x. [DOI] [PubMed] [Google Scholar]
- 43.Wright R, Cohen S, Carey V, Weiss S, Gold D. Parental stress as a predictor of wheezing in infancy. A prospective birth-cohort study. Am J Respir Crit Care Med. 2002;165:358. doi: 10.1164/ajrccm.165.3.2102016. [DOI] [PubMed] [Google Scholar]
- 44.Wright R, Finn P, Contreras J, Cohen S, Wright R, Staudenmayer J, et al. Chronic caregiver stress and IgE expression, allergen-induced proliferation, and cytokine profiles in a birth cohort predisposed to atopy. J Allergy Clin Immunol. 2004;113:1051–7. doi: 10.1016/j.jaci.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 45.Suglia SF, Staudenmayer J, Cohen S, Bosquet Enlow M, Rich-Edwards JW, Wright RJ. Cumulative stress and cortisol disruption among black and Hispanic pregnant women in an urban cohort. Psychol Trauma. 2010;3:326–34. doi: 10.1037/a0018953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wright RJ, Visness CM, Calatroni A, Grayson MH, Gold DR, Sandel MT, et al. Prenatal maternal stress and cord blood innate and adaptive cytokine responses in an inner-city cohort. Am J Respir Crit Care Med. 2010;182:25–33. doi: 10.1164/rccm.200904-0637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pinkerton K, Joad J. Influence of air pollution on respiratory health during perinatal development. Clin Exp Pharmacol Physiol. 2006;33:269–72. doi: 10.1111/j.1440-1681.2006.04357.x. [DOI] [PubMed] [Google Scholar]
- 48.Miller R, Garfinkel R, Horton M, Camann D, Perera F, Whyatt R, et al. Polycyclic aromatic hydrocarbons, environmental tobacco smoke, and respiratory symptoms in an inner-city birth cohort. Chest. 2004;126:1071–8. doi: 10.1378/chest.126.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jedrychowski W, Perera F, Maugeri U, Mrozek-Budzyn D, Mroz E, Flak E, et al. Early wheezing phenotypes and severity of respiratory illness in very early childhood: study on intrauterine exposure to fine particle matter. Environ Int. 2009;35:877–84. doi: 10.1016/j.envint.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nguyen K, Subramanian SV, Sorensen G, Tsang K, Wright RJ. Influence of experiences of racial discrimination and ethnic identity on prenatal smoking among urban black and Hispanic women. J Epidemiol Community Health. 2010 doi: 10.1136/jech.2009.107516. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 51.Parker ED, Solberg LL, Foldes SS, Walker PF. A surveillance source of tobacco use differences among immigrant populations. Nicotine Tob Res. 2010;12:309–14. doi: 10.1093/ntr/ntp211. [DOI] [PubMed] [Google Scholar]
- 52.Prescott S. Maternal allergen exposure as a risk factor for childhood asthma. Curr Allergy Asthma Rep. 2006;6:75–80. doi: 10.1007/s11882-006-0014-7. [DOI] [PubMed] [Google Scholar]
- 53.Herr CEW, Ghosh R, Dostal M, Skokanova V, Ashwood P, Lipsett M, et al. Exposure to air pollution in critical prenatal time windows and IgE levels in newborns. Pediatr Allergy Immunol. 2011;22:75–84. doi: 10.1111/j.1399-3038.2010.01074.x. [DOI] [PubMed] [Google Scholar]