Abstract
Spermatogonial stem cells (SSCs) are at the foundation of mammalian spermatogenesis. While rare Asingle spermatogonia comprise the rodent SSC pool, primate spermatogenesis arises from more abundant Adark and Apale spermatogonia and the identity of the stem cell is subject to debate. The fundamental differences between these models highlight the need to investigate the biology of primate SSCs, which have greater relevance to human physiology. The alkylating chemotherapeutic agent, busulfan, ablates spermatogenesis in rodents and causes infertility in humans. We treated adult rhesus macaques with busulfan to gain insights about its effects on SSCs and spermatogenesis. Busulfan treatment caused acute declines in testis volume and sperm counts, indicating a disruption of spermatogenesis. One year after high-dose busulfan treatment sperm counts remained undetectable and testes were depleted of germ cells. Similar to rodents, rhesus spermatogonia expressed markers of germ cells (VASA, DAZL) and stem/progenitor spermatogonia (PLZF and GFRα1), and cells expressing these markers were depleted following high-dose busulfan treatment. Furthermore, fresh or cryopreserved germ cells from normal rhesus testes produced colonies of spermatogonia, which persisted as chains on the basement membrane of mouse seminiferous tubules in the primate to nude mouse xenotransplant assay. In contrast, testis cells from animals that received high-dose busulfan produced no colonies. These studies provide basic information about rhesus SSC activity and the impact of busulfan on the stem cell pool. In addition, the germ cell depleted testis model will enable autologous/homologous transplantation to study stem cell/niche interactions in nonhuman primate testes.
Keywords: Busulfan, chemotherapy, infertility, spermatogenesis, spermatogonial stem cells, xenotransplantation
Introduction
The stem cell population that balances self-renewal and differentiation to maintain sperm production throughout adult life is at the foundation of spermatogenesis in the mammalian testis. Despite their critical importance to male fertility, the cellular and molecular characteristics of spermatogonial stem cells (SSCs) remain largely undefined. Currently, the only way to identify a SSC is to observe its biological capacity to initiate and maintain spermatogenesis. A SSC transplantation technique was developed for mice over twelve years ago and enabled tremendous progress investigating the phenotypic and functional characteristics of these adult tissue stem cells (1, 2). The results have broad implications for understanding the regulation of germ cell development and spermatogenesis, stem cell biology in adult self-renewing tissues, and etiology/treatment of male infertility (3). Since mammalian spermatogenesis is a highly conserved process (4), it is tempting to extrapolate that the characteristics and regenerative potential of SSCs will be conserved in higher species, including humans. Here, we develop research tools and begin characterizing primate SSCs
In primates, human and nonhuman alike, classical histological studies of nuclear morphology indicate that two types of undifferentiated spermatogonia are present on the basement membrane of testicular seminiferous tubules, designated Adark and Apale (5, 6). The prevailing model of spermatogonial proliferation and differentiation is that Adark and Apale represent reserve and active stem cells, respectively. According to this model, Adark spermatogonia rarely divide and are activated by cytotoxic insult, while Apale spermatogonia undergo regular self-renewing divisions to maintain a pool of undifferentiated germ cells, which support spermatogenesis under normal circumstances (7-12). However, these stem cell designations in primates are subject to debate and are clearly different from rodents, in which the entire spermatogenic lineage is derived from Asingle spermatogonia, the rodent SSC (13, 14). Thus, there is justification for studying the biology of SSCs in a nonhuman primate model that exhibits germ cell organization similar to humans.
While tools and reagents for studying SSCs in rodents are well established (e.g., SSC transplantation), the resources for studying these cells in primates are limited. Establishment of a germ cell depleted model of male infertility in nonhuman primates will enhance investigation of SSCs by facilitating experiments that evaluate their regenerative potential and stem cell/niche interactions. Furthermore, depletion of spermatogenesis and infertility is a common side effect experienced by cancer survivors who have undergone chemotherapy and radiation treatments (15, 16). Therefore, a nonhuman primate model of chemotherapy-induced infertility constitutes a valuable tool for both fundamental and applied investigations [reviewed in (17)].
In the current study, we validated antibodies for germ cell and stem/progenitor markers in the rhesus testis and optimized rhesus-to-nude mouse xenotransplantation as a routine biological assay to study rhesus SSCs. We used these tools to obtain baseline information about stem cell activity in normal rhesus testes, evaluate the effect of cryopreservation on SSC biological activity, and test the effect of busulfan treatment on spermatogenesis and the stem cell pool to identify a treatment regimen that causes long-term infertility.
Materials and Methods
Animal Experiments
All animal experiments were approved by the Institutional Animal Care and Use Committee at the Magee-Womens Research Institute and Foundation (Assurance # A3654-01) and were conducted in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals.
Experimental design
Six adult rhesus macaques were assigned to three treatment groups (two animals per group; 4, 8, and 12 mg/kg busulfan). Two additional adult males were used as unmanipulated controls (i.e., 0 mg/ml treatment group) and provided a baseline for weekly and seasonal fluctuations in sperm counts, testis volume measurements, and hematological parameters. For the purpose of histological evaluation and isolation of testicular cells, testis tissue was recovered by biopsy or hemicastration, before and after busulfan treatment (Fig. 1A & 1E). A small piece of each hemicastrated testis was used for morphological analyses and the remainder was used to generate a single cell suspension by two-step enzymatic digestion (see below, Fig. 1A).
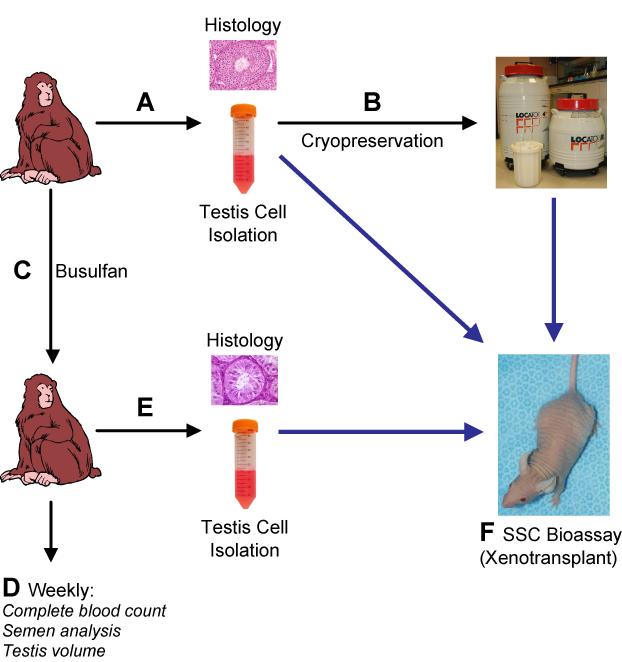
Figure 1. Experimental design.
Adult male rhesus macaques were used to test the effects of busulfan on spermatogenesis and the SSC pool. (A) Testis samples from experimental animals were used for histological evaluation and to generate a single cell suspension by a two-step enzymatic digestion procedure. Aliquots of the resulting suspension were used immediately for xenotransplantation to nude mice (blue arrow) and the remaining volume was (B) cryopreserved for use in later experiments. (C) Experimental males were then treated with the chemotherapeutic drug busulfan (Busulfex IV). (D) Semen analysis, blood samples, and testis volume measurements taken at weekly intervals were used to demonstrate the effect of busulfan on spermatogenesis and hematopoiesis. (E) Testis samples obtained after busulfan treatment were used for histological evaluation and to isolate testis cells for immediate xenotransplantation to nude mice (blue arrow). (F) To evaluate baseline stem cell activity, as well as the effects of cryopreservation and busulfan treatment, rhesus testis cells were xenotransplanted [at each step (indicated by blue arrows)] into the testes of busulfan-treated nude mouse recipients.
Busulfan Treatment
Following initial testis tissue collection (hemicastration or biopsy), animals were treated with the chemotherapeutic agent busulfan (Busulfex IV; PDL BioPharma, Fremont, CA), at doses of 4, 8, and 12 mg/kg (2 animals per group; Fig. 1C). Busulfex was diluted in physiological saline and administered at 1 mg/ml in a single bolus infused over 5 minutes.
Semen collection and analysis
Semen samples were collected from experimental and control animals at weekly intervals (Fig. 1D), as described (18). Total sperm count per ejaculate was determined by hemocytometer.
Testis volume measurements
At weekly intervals, calipers were used to measure the longest diameter (height) and shortest diameter (width) of each testis through the scrotum of each experimental and control animal (Fig. 1D). Testis volume was estimated using the equation for the volume of a prolate spheroid . For animals with both testes, testis volumes were averaged.
Blood collection and analysis
Blood was collected at weekly intervals and processed for complete blood count (CBC; Antech Diagnostics, Lake Success, NY) to measure hematopoietic parameters and determine the general health status of experimental animals (Fig. 1D).
Histology
Portions of testicular tissue collected above were fixed with Bouin’s solution (Accustain; Sigma-Aldrich, St. Louis, MO), paraffin embedded, sectioned (5μm), and stained with hematoxylin and eosin (Fig. 1A & 1E). Stained seminiferous tubule cross-sections were scored for the extent of spermatogenesis as empty (few or no germ cells, Sertoli cell only), multiple germ cell layers (at least two layers of germ cells, but no elongating/elongated spermatids), or complete spermatogenesis (presence of elongating or elongated spermatids). A minimum of 100 seminiferous tubule cross-sections were evaluated per animal, except where noted.
Immunofluorescent detection of germ cell and SSC markers
Biopsies of rhesus testes collected above were fixed in 4% paraformaldehyde, paraffin embedded, and sectioned (5 μm) (Fig. 1A & 1E). Following high-temperature citrate buffer antigen retrieval, sections were stained with rabbit anti-DDX4 IgG (VASA, 1:200 dilution; Abcam, Cambridge, MA), goat anti-human DAZL (1:200; Novus Biologicals, Littleton, CO), mouse anti-human GFRα1 IgG (1:250; R&D Systems, Minneapolis, MN), or Armenian hamster anti-PLZF IgG (1:200 dilution; Hobbs and Pandolfi). Primary antibodies were detected with goat anti-rabbit IgG AlexaFluor488 (1:200; Invitrogen), donkey anti-goat IgG AlexaFluor568 (1:200; Invitrogen), goat anti-mouse IgG AlexaFluor488 (1:200; Invitrogen), or goat anti-Armenian hamster IgG FITC (1:50; Jackson ImmunoResearch Laboratories, West Grove, PA). Samples were mounted with VectaShield mounting media containing DAPI (Vector Laboratories, Burlingame, CA). For quantification of rhesus spermatogonia expressing PLZF, positive nuclei and seminiferous tubule cross-sections were counted from four normal adult rhesus macaques.
Preparation of donor rhesus macaque testis cell suspensions
Donor cells were recovered from rhesus macaque testes by modification of a previously published two-step enzymatic digestion procedure [(19, 20), Fig. 1A & 1E]. Upon castration, seminiferous tubules were removed from the tunica albuginea and digested with collagenase type IV (1 mg/ml; Sigma) in Hanks balanced salt solution (HBSS; Invitrogen, Carlsbad, CA) for 5-10 min at 37°C with vigorous shaking. Dispersed seminiferous tubules were sedimented by gentle centrifugation at 100 x g, and washed three times in HBSS to remove interstitial cells. Isolated seminiferous tubules were further digested with trypsin (2.0 mg/ml trypsin + 1.04 mM EDTA, Invitrogen) and DNase I (1.4 mg/ml, Sigma) in HBSS for 10-15 min at 37°C with mild trituration every five minutes. Digestions were quenched with 10% fetal bovine serum (FBS; HyClone, Logan, UT) and filtered through a nylon mesh (70 μm Cell Strainer; BD, Franklin Lakes, NJ), producing a single cell suspension. Cells were pelleted by centrifugation at 600 x g and resuspended in minimum essential medium alpha (MEMα, Invitrogen) containing 10% FBS. In some experiments, testis cells were xenotransplanted immediately (Fig. 1F), while the remaining cells were cryopreserved for future studies (Fig. 1B). For cryopreservation, cells were resuspended at 40 × 106/ml in medium (MEMα + 10% FBS), aliquoted in cryovials and an equal volume of freezing medium (MEMα + 20% FBS + 20% DMSO) was added drop-wise. Vials were frozen in −1°C/minute controlled-rate freezing containers (Nalgene-Nunc International, Rochester, NY) to −80°C and stored in liquid nitrogen (final cryopreserved cell concentration was 20 × 106/ml in MEMα + 15% FBS + 10% DMSO). For experiments using cryopreserved cells, vials were thawed rapidly at 37°C, excess medium (MEMα + 10% FBS) was added to the cell mixture drop-wise, cells were washed three times in medium, and were used for xenotransplantation (Fig. 1F).
Recipient mouse preparation and xenotransplantation procedure
Nude mice (NCr nu/nu; Taconic, Germantown, NY) were treated with busulfan (40 mg/kg; Sigma) at 6 wk of age to eliminate endogenous spermatogenesis, as described (1, 21). Six weeks after busulfan treatment, approximately 7μl of fresh or cryopreserved donor testis cell suspension containing 10% trypan blue (Invitrogen) at 100-200 × 106 cells/ml were injected into the seminiferous tubules of recipient testes via the efferent ducts [Fig. 1F, (22)].
Antibody generation
For generation of the rhesus testis cell polyclonal antiserum, testis cell suspensions from two juvenile rhesus macaques (ages 1.5 & 1.33 years) were prepared as above and used to inoculate immune-naïve rabbits (Covance Research Products, Denver, PA). The IgG fraction of the resulting rabbit antiserum was purified by affinity chromatography (MAbTrap kit; GE Healthcare Bio-Sciences Corp, Piscataway, NJ) and pre-absorbed against acetone-precipitated mouse testis proteins (1:10; IgG:mouse testis acetone powder, w/w) to minimize cross-reactivity to mouse antigens.
The PLZF antibody was raised in Armenian hamsters to a peptide corresponding to sequence within the hinge domain of mouse Plzf. Details of PLZF antibody derivation will be described elsewhere (Hobbs and Pandolfi).
Whole-mount immunofluorescent staining of xenotransplanted testes
For quantitative analysis of donor rhesus testis cell colonization, intact seminiferous tubules were prepared from nude mouse recipient testes, collected two months after transplantation, as described (19, 20, 23). Several improvements to previously reported staining procedures were employed. Donor-derived colonies of spermatogonia were detected in intact seminiferous tubules by whole-mount immunofluorescent staining with the rhesus testis cell antibody, as described with modifications (24). Samples were dehydrated in a graded methanol dilution series prior to incubation in MeOH:DMSO:H2O2 (4:1:1), which was limited to 2-3 hours. All dehydration, rehydration, blocking and washing steps were performed in 12mm Transwell baskets (12μm pore size, Corning Life Sciences, Corning, NY) to facilitate washing and prevent loss of seminiferous tubules and data. The rhesus testis cell antibody was used at a 1:200 dilution and detected with goat anti-rabbit IgG conjugated to AlexaFluor 488 (1:200 dilution, Invitrogen). Following staining, samples were mounted with VectaShield mounting media containing DAPI (Vector Laboratories) on glass-slides with raised coverslips (to preserve seminiferous tubule dimensions). The DAPI staining was used to locate donor spermatogonia nuclei in relation to recipient Sertoli cell and peritubular myoid cell nuclei, and thus, to determine whether they were positioned on the basement membrane. Samples were visualized by fluorescent microscopy using a FITC/TRITC dual-emission filter (Nikon) to distinguish specific signal and tissue autofluorescence. In co-staining experiments, the rhesus testis cell antibody was detected with donkey anti-rabbit IgG AlexaFluor 488 (Invitrogen), and VASA was detected with a goat anti-human VASA IgG (1:200; R&D Systems) together with donkey anti-goat IgG AlexaFluor 568 (Invitrogen).
For histological analysis of rhesus spermatogonia in xenotransplant colonies, a recipient nude mouse testis was fixed, embedded, and serial sectioned five months after transplantation with a rhesus testis cell suspension. Recipient nude mouse testis sections were stained with the rhesus testis cell antibody (1:200 dilution), mounted and analyzed as described above.
Results
Busulfan treatment leads to spermatogenic arrest and long-term male infertility in rhesus macaques
In rodents, treatment with the alkylating agent busulfan disrupts spermatogenesis and leads to long-term infertility (2, 21). To identify a busulfan treatment regimen that would elicit similar effects in nonhuman primates, we treated six adult rhesus macaques with busulfan (Busulfex IV) at doses of 4, 8, and 12 mg/kg (two animals per dose) and followed the consequences on spermatogenesis for up to one year (see Fig. 1). Busulfan also elicits myelosuppressive effects (see Supplemental Fig. 1) and two animals were compassionately euthanized, based on veterinary advice, due to hematopoietic complications (one animal from each of the 8 and 12 mg/kg treatment groups, euthanized 10 weeks and 7 weeks after treatment, respectively). These outcomes highlight one of the challenges of working with large animal models and prompted modifications to the supportive care in future studies, including prophylactic autologous hematopoietic stem cell transplantation following busulfan administration.
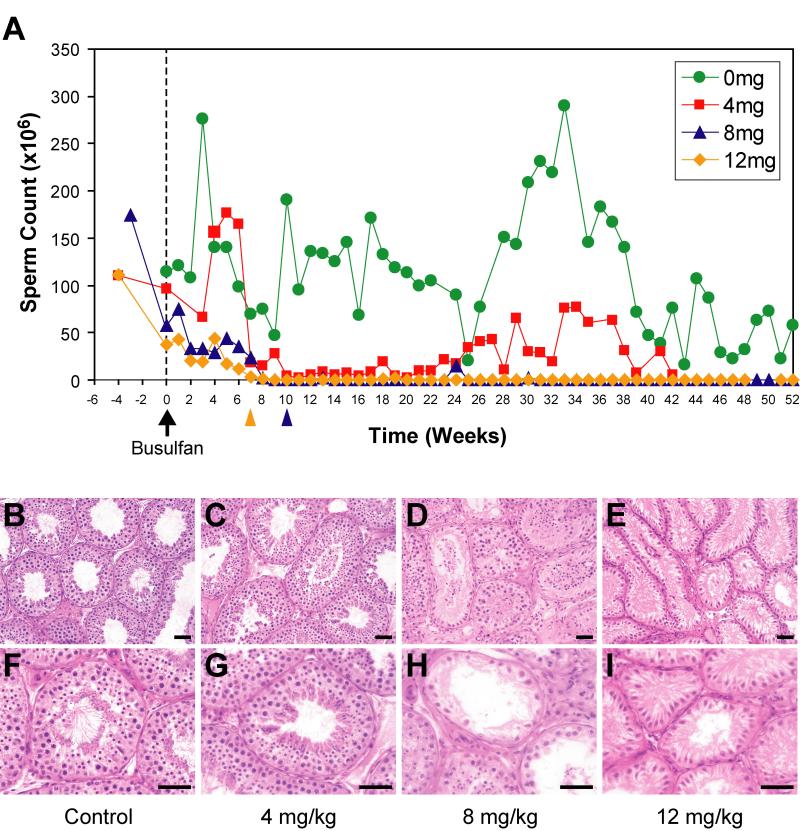
Compared to two untreated control males, ejaculated sperm counts in experimental animals sharply declined with all three doses of busulfan and reached 0 by 10 weeks after treatment, indicating that busulfan treatment acutely disrupted spermatogenesis (Fig. 2A). While sperm counts returned to a normal range by 24 weeks in animals treated with 4 mg/kg busulfan, sperm counts remained undetectable one year following high-dose treatment with 8 and 12 mg/kg busulfan, suggesting long-term infertility (Fig. 2A). Testis volume measurements closely mirrored the sperm counts, indicating changes in sperm production were due to spermatogenic defects, not ductile obstruction (Supplemental Fig. 2). Importantly, RBC, WBC and platelet counts declined acutely with 8 and 12 mg/kg busulfan, but returned to baseline in surviving animals within 3 months, indicating that these doses can be compatible with long-term hematopoietic recovery (Supplemental Fig. 1).
Figure 2. Busulfan treatment leads to long-term infertility in male rhesus macaques.
(A) Sperm counts were measured at weekly intervals and were averaged for animals within each group. Due to adverse reactions to busulfan, two animals were compassionately euthanized based on veterinary advice; 204 (8 mg/kg; 10 weeks after treatment, noted by blue arrowhead below X-axis) and 70 (12 mg/kg; 7 weeks after treatment, noted by yellow arrowhead below X-axis). Hematoxylin & eosin staining of adult rhesus macaque testes demonstrated the extent of spermatogenesis in experimental animals (B, F) before and (C, G) after 4 mg/kg busulfan (46 weeks), (D, H) after 8 mg/kg (60 weeks), or (E, I) after 12 mg/kg busulfan treatment (63 weeks). Quantification of testis morphology in each group is shown in Supplemental Table 1. Scale bar = 50μm. As noted (black arrow below x-axis), busulfan was administered at week 0. Note: samples for week 0 were collected prior to busulfan administration.
The dose-dependent busulfan-induced disruption of spermatogenesis correlated with a loss of germ cells (Fig. 2B-2I, Supplemental Table 1). As expected, nearly all seminiferous tubule cross-sections from animals treated with the 4 mg/kg dose demonstrated complete spermatogenesis and appeared normal 46 weeks after treatment [compare Fig. 2B & 2F (pretreatment control) to Fig. 2C & 2G (animal 215, 4 mg/kg), Supplemental Table 1]. In contrast, significant spermatogenic depletion (46.6% of seminiferous tubules were empty from animal 72) was observed 60 weeks following 8 mg/kg busulfan treatment (Fig. 2D & 2H, Supplemental Table 1) and the majority of seminiferous tubules were empty 63 weeks after treatment of animal 73 with 12 mg/kg busulfan (Fig. 2E & 2I, Supplemental Table 1). Analysis of the testes from animals 70 and 204 (which were euthanized 7 and 10 weeks after busulfan treatment, respectively) provided insight into the progression of spermatogenic depletion. In particular, 30% of seminiferous tubules were empty 7 weeks after busulfan treatment (animal 70) and 99.6% of seminiferous tubules were empty by 10 weeks after busulfan treatment (animal 204, Supplemental Table 1). However, complete spermatogenic recovery was observed in 23.4% of seminiferous tubules 60 weeks after 8 mg/kg busulfan treatment (animal 72), suggesting that some stem cells survived that chemotherapeutic dose and regenerated spermatogenesis (Supplemental Table 1).
Immunohistochemical studies demonstrated that adult rhesus macaque germ cells expressed two proteins known to be present in germ cells from a variety of species, VASA [(DDX4) (25)] and DAZL (26), and both proteins were absent following 12 mg/kg busulfan treatment (Fig. 3A-3D). Furthermore, GFRα1 (GDNF receptor) (27-29) and PLZF (30, 31) are consensus markers of stem and progenitor spermatogonia, which comprise the undifferentiated pool [defined as As, Apr and Aaligned4-16, (13)] in rodents and exhibit a staining pattern consistent with this designation in rhesus testes (Fig. 3E & 3G). There were 1.84 ± 0.59 PLZF expressing spermatogonia per seminiferous tubule cross-section (Fig. 3G, Supplemental Table 2), which is slightly less than the reported combined frequency of Adark and Apale spermatogonia [~2.2 per seminiferous tubule (32)]. Notably, expression of GFRα1 and PLZF were absent following 12 mg/kg busulfan treatment, suggesting that high-dose busulfan depleted the SSC pool (Fig. 3F & 3H). To gain insight into the progression of spermatogenic depletion caused by busulfan treatment, we analyzed the testis of animal 204, which was euthanized 10 weeks after 8 mg/kg busulfan treatment. At this time point, 99.6% of seminiferous tubules were empty (Supplemental Table 1). In the 0.4% of seminiferous tubules that contained two or more layers of germ cells (Supplemental Table 1), no GFRα1 expression was observed (Supplemental Fig. 3D-F), suggesting that those tubules also would eventually become empty. However, a few empty seminiferous tubules had GFRα1+ cells (Supplemental Fig. 3G-I). It is tempting to speculate that these tubules would eventually exhibit spermatogenic recovery, as observed for animal 72, 60 weeks after busulfan treatment (See Fig. 2D and H). While these staining results are compelling, a functional assay that measures the biological potential of SSCs to colonize and persist in recipient seminiferous tubules provides a more definitive assessment stem cell activity.
Figure 3. Busulfan treatment of rhesus testes caused a loss of germ cell and SSC markers.
To further characterize the loss of germ cells observed following busulfan treatment, we detected expression of (A, B) VASA, (C, D) DAZL, (E, F) GFRα1, and (G, H) PLZF by immunofluorescent staining in paraffin-embedded tissue sections. Expression of each marker is merged with DAPI counterstain (Blue) indicating the nuclei of all cells in the section. Staining was performed using adult rhesus testis (A, C, E, G) before treatment and (B, D, F, H) 63 weeks after 12 mg/kg busulfan treatment (animal 73). Arrowheads mark PLZF-stained nuclei in (G). An asterisk notes a single residual VASA-positive cell in the lumen of one seminiferous tubule in (B). Scale bar = 50μm.
A biological assay for rhesus macaque SSCs
In order to demonstrate that busulfan treatment depleted SSCs, we optimized an assay to evaluate stem cell activity in the rhesus macaque testis. In this assay, efferent duct injection introduced donor rhesus testis cell suspensions into the testes of busulfan-treated nude mice. Whole mount analysis of mouse recipient seminiferous tubules using the rhesus testis cell antibody, two months after transplantation, revealed rhesus testis cell engraftment. Western blot analysis comparing mouse and monkey testis proteins demonstrated the specificity of this antibody for rhesus antigens (Fig. 4A). Further, no immunoreactivity was observed in untransplanted busulfan-treated nude mouse testes (Fig. 4B). In transplanted mouse testes, rhesus donor-derived foci of colonization, ranging in size from 4 to 145 cells, were present on the basement membrane of recipient seminiferous tubules (Fig. 4C-4D). Cells within donor-derived colonies exhibited characteristic spermatogonial features, including ovoid shape, with high nuclear to cytoplasmic ratios, and were arranged in chains connected by intracytoplasmic bridges. Histological evaluation of recipient nude mouse testes confirmed that donor rhesus testis cells with spermatogonial appearance were located on the seminiferous tubule basement membrane (Fig. 4E-F). It is important to interpret results of engraftment assays cautiously and in this assay many foci of colonization (positive immunoreactivity with the rhesus testis cell antibody) failed to exhibit spermatogonial features (see Supplemental Fig. 4). Therefore, we developed strict criteria to identify putative, stem cell-derived foci of colonization. These colonies exhibited spermatogonial morphology, were located on the basement membrane, and constituted 4 or more cells in a continuous area of recipient seminiferous tubule (≤100μm between cells). In addition, we confirmed that colonies of rhesus cells expressed VASA (DDX4), a consensus germ cell marker (Fig. 4G-4I).
Figure 4. Rhesus testis cell xenotransplantation assay detects donor-derived spermatogonial colonies.
(A) Western blot analysis demonstrating immunoreactivity of the preabsorbed (against mouse antigens) rhesus testis cell antibody with mouse and rhesus testis proteins. Testis proteins were from postnatal day 7 mouse (d7), adult mouse (A) and adult rhesus (nHP). Whole-mount immunofluorescent staining with the rhesus testis cell antibody of intact recipient nude mouse seminiferous tubules that were (B) untransplanted or (C-D) xenotransplanted with donor rhesus testis cells. (E-F) Immunohistochemical evaluation using the rhesus testis cell antibody (green) of sections (5 μm) of a recipient nude mouse testis transplanted with rhesus testis cells. Sections were counterstained with DAPI (blue). White arrowheads mark rhesus cells in recipient nude mouse seminiferous tubule cross-sections and white asterisks mark peritubular myoid cell nuclei. Xenotransplant recipient seminiferous tubules were also co-stained in whole-mount with (G) the rhesus testis cell antibody and (H) an antibody for the germ cell marker VASA. (I) Overlay of rhesus and VASA fluorescent signals. White arrows note a cluster of three autofluorescent interstitial cells located in a focal plane immediately above (outside) the seminiferous tubule. Dashed white lines mark seminiferous tubule margins in (B-D) and (G-I), and the seminiferous tubule basement membrane in (E-F). Scale bars = 50μm.
Busulfan treatment depletes rhesus macaque SSCs
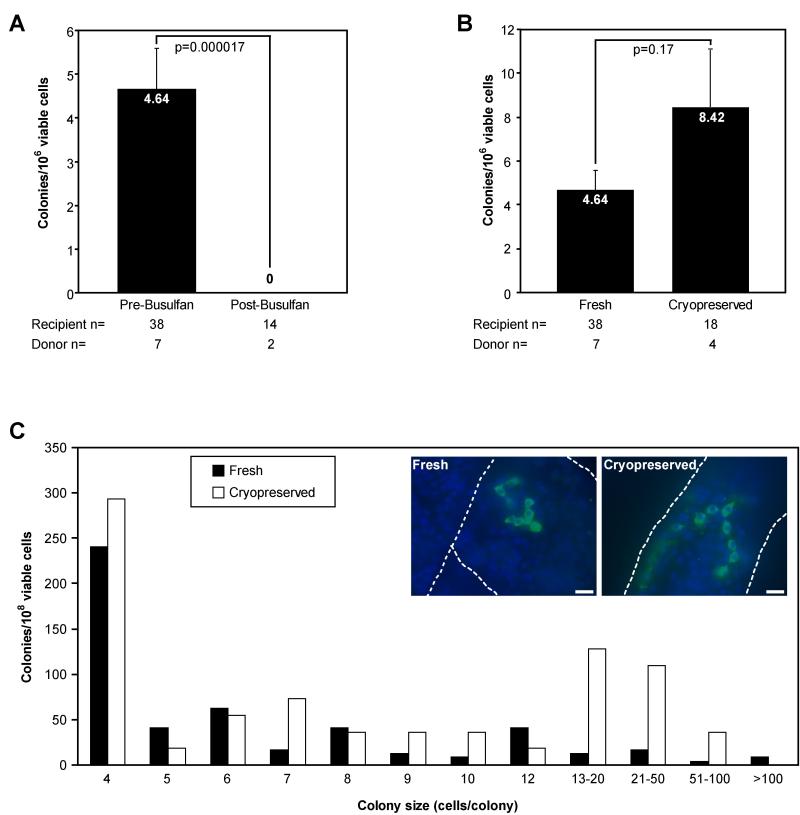
Using the rhesus-to-nude mouse xenotransplantation assay, we quantified colonization activity in suspensions of adult rhesus macaque testis cells. Prior to busulfan treatment, rhesus testis cells produced 4.64 colonies/106 viable cells xenotransplanted (Fig. 5A). Rhesus testis cells isolated from males 60-63 weeks following treatment with 8 or 12 mg/kg busulfan, however, failed to produce detectable colonies of spermatogonia in the xenotransplantation assay (Fig. 5A). These data indicate that high-dose busulfan treatment depleted rhesus SSCs to below the limit of detection more than one year after treatment. These results also partially validate the xenotransplant assay because colonizing activity correlates with expression of germ cell and SSC markers (see Fig. 3).
Figure 5. Rhesus SSC colonization activity is lost after busulfan treatment but retained after cryopreservation.
Xenotransplant colonization activity was measured in testis cell suspensions from adult rhesus macaques (A) before and after busulfan treatment or (B) before and after cryopreservation. Data are presented as the total number of colonies meeting the inclusion criteria (≥4 rhesus cells with spermatogonial morphology on the basement membrane of recipient mouse seminiferous tubules) per 106 viable transplanted cells. Cryopreserved cells in four replicate experiments from different animals were maintained in liquid nitrogen for 336, 289, 216, or 214 days prior to thawing and xenotransplantation. The number of individual rhesus donors (animals) and nude mouse recipient testes is indicated below the bars. Statistical significance is noted above each set of bars and was determined by a two-tailed heteroscedastic student’s T-Test. The histogram in (C) displays the distribution of colony sizes from fresh (white bars) and cryopreserved (black bars) rhesus testis cells reported in (B). Inset images in (C) show representative xenotransplant colonies two months after transplantation from fresh and cryopreserved rhesus testis cells. Colonies were detected by whole-mount staining with the rhesus testis cell antibody (green) and counterstained with DAPI (blue). Dashed white lines mark seminiferous tubule margins. Scale bars = 25μm.
Rhesus SSCs retain qualitative and quantitative colonization capacity following cryopreservation
Autologous transplantation of SSCs to study stem cell/niche interactions in nonhuman primates or for clinical treatment of infertility may require preservation of cells between the times of collection and transplant. By modifying standard cryopreservation techniques developed for SSCs (33, 34), we tested the colonization potential of cryopreserved rhesus testis cells using the xenotransplantation assay. After cryopreservation, viability of testis cell suspensions was significantly lower than fresh cells (58 ± 4.4% vs. 94.6 ± 2.4%, p=0.000014). However, cryopreserved rhesus testis cells produced more colonies of spermatogonia (8.42 colonies/106 viable cells) than fresh cells (4.64 colonies/106 viable cells), although this difference was not statistically significant (p=0.17, Fig. 5B). Based on the distribution of colony size and appearance, the quality of colonies derived from cryopreserved cells was not different from those obtained using fresh cells (Fig. 5C). Thus, rhesus SSCs appear to retain normal functional attributes after cryogenic storage.
Discussion
While SSC transplantation provided a powerful assay for studying stem cells in rodent testes, monkey-to-monkey SSC transplantation would be cost-prohibitive and subject to complications associated with variability between out-bred animals. A routine biological assay in a small animal model would provide a valuable tool to begin studying rhesus SSCs. Thus, we developed and refined the rhesus-to-nude mouse xenotransplantation assay based on the pioneering work of Ralph Brinster and colleagues, who previously reported the transplantation of testis cells from rats (35), hamsters (36), rabbits (23), dogs (23), pigs (37), bulls (37), horses (37), baboons (20), and humans (19) into recipient mouse testes.
In order for rhesus testis cell xenotransplantation to serve as a quantitative assay, we developed strict criteria to identify putative donor stem cell-derived colonies (see results). These criteria provide a framework for interpreting the results of the current study in the context of previous and future investigations. The xenotransplantation assay detected 4.64±0.95 colonies of rhesus spermatogonia per 106 viable cells transplanted to recipient mouse testes. Colonization foci in recipient nude mouse testes arose from transplanted rhesus testis cells that migrated to the basement membrane of recipient seminiferous tubules and produced chains of spermatogonia (demonstrated by VASA staining) that persisted long-term. These attributes are consistent with spermatogonial stem cell behavior, although evolutionary distance between rhesus and mouse [65-85 million years, (38, 39)] precluded the establishment of complete rhesus spermatogenesis. While colonizing activity correlated with the presence or absence of consensus stem/progenitor markers (i.e., GFRα1 and PLZF), we cannot exclude the possibility that differentiating spermatogonia (e.g., type B) produce some colonization foci. Ultimate validation of the xenotransplant assay will depend on future rhesus-to-rhesus testis cell transplantations where donor cells are expected to produce complete spermatogenesis.
Based on the assumption that xenotransplant colonization data reflect the activity of SSCs, the concentration of putative SSCs identified in this study (4.64 colonies/106 cells transplanted) is comparable to those detected from adult mice [9 colonies per 106 cells; (34)] and baboons [3.9 colonies per 106 cells; (20)], but lower than adult rat [169 colonies per 106 cells; (40)]. Based on the average number of cells obtained per gram of adult rhesus testis (82.1 ± 28.1 × 106 cells/g testis tissue), there are approximately 381 SSCs/g rhesus testis tissue [(4.64 SSCs/106 viable cells transplanted) × (82.1 × 106 cells/g donor testis tissue)] or 8517 SSCs/testis (for the average 22.36 g testis in this study). Since the number of functional rodent stem cells observed by SSC transplantation is thought to represent 5-12% of actual stem cells (i.e., engraftment efficiency), our estimates likely under represent the total rhesus SSC pool (40-43). Previous morphological analyses estimated there are approximately 300 × 106 Adark and 300 × 106 Apale spermatogonia per adult rhesus testis (32). This suggests that either the efficiency of rhesus SSC engraftment in mouse seminiferous tubules is extraordinarily low [e.g., 0.0015%-0.003%, depending on whether stem cell activity resides in one or both subpopulation(s) of rhesus spermatogonia] and/or that rhesus stem cells are a subpopulation of morphologically-defined undifferentiated Adark and/or Apale spermatogonia.
The PLZF staining data suggest that rhesus SSCs represent a subpopulation of Adark and/or Apale spermatogonia. In rodents, Plzf marks undifferentiated spermatogonia [As, Apr, Aal4-16, (30, 31)] and SSCs (As) comprise 10% of the undifferentiated pool (44). The frequency of PLZF+ cells in adult rhesus testes (1.86 per tubule cross section, Supplemental Table 2) is similar to the frequency of undifferentiated spermatogonia in rhesus testes [(Apale = 1.1 per tubule cross section) + (Adark 1.1 per tubules cross section) = 2.2] (32). If PLZF marks stem and progenitor (undifferentiated) spermatogonia in rhesus and stem cells are a small fraction of the stem/progenitor pool (i.e., 10% as for rodents), then the SSC pool is a smaller subpopulation of Adark and/or Apale spermatogonia that remains to be defined.
Cytotoxic cancer treatments (i.e., chemotherapy and radiation) can cause temporary or permanent infertility (15) because these treatments fail to discriminate between cancerous and naturally proliferative tissues in the body (e.g., spermatogenesis). Cryopreservation of SSCs prior to cancer treatment and autologous transplantation of these cells back into the testis after cure may restore fertility and allow male cancer survivors to father their own genetic children (17, 45). Our data demonstrate that rhesus SSCs retain their engraftment potential after cryopreservation. While other fertility preserving options exist for adult male cancer survivors (semen cryopreservation or testicular sperm extraction followed by IVF or ICSI) some adult survivors remain unable to father children because of the advanced stage of their disease at the time of diagnosis (46). In addition, there are currently no fertility-preserving options for preadolescent cancer survivors who are not yet producing sperm at the time of treatment (47). Responsible development of SSC transplantation to treat male infertility requires investigation of the fundamental characteristics of the SSC pool in primates and the consequences of cytotoxic therapies.
The current study investigated the long-term effects of busulfan treatment on spermatogenesis and SSCs in rhesus macaques. Several studies have documented the hematological effects of busulfan in this species (48-52), but its gonadotoxicity has received little attention (53). While intraperitoneal busulfan treatment (10 mg/kg) caused transient disruption of rhesus spermatogenesis in a previous study (53), we observed long-term infertility following intravenous treatment with 8 and 12 mg/kg Busulfex IV. This apparent discrepancy may result from pharmacokinetic differences arising from the route of busulfan administration. In addition, our analysis suggests that busulfan-induced loss of germ cells correlated with SSC depletion because colonizing activity was not detectable in the xenotransplant assay and markers of stem/progenitor spermatogonia (PLZF and GFRα1) were absent. Thus, we have established a nonhuman primate model of chemotherapy-induced infertility.
To maximize the potential for using this rhesus model of infertility to study the fundamental characteristics and regenerative potential of rhesus SSCs, some refinement of the experimental approach is required. Future studies will employ the lowest dose of busulfan that causes long-term infertility (probably between 8 and 10 mg/kg) and use prophylactic peripheral blood stem cell transplant to guard against the hematopoietic deficits caused by busulfan treatment in some animals. High dose busulfan treatment produces an ablated testis model that will facilitate rhesus-to-rhesus transplantation in future studies to evaluate stem cell/niche interactions and optimize SSC transplantation in a primate model.
Conclusion
Our results provide the first quantitative information about spermatogonial stem cells in rhesus testes and suggest that high-dose chemotherapy depletes the stem cell pool and causes infertility. In addition, we demonstrated conserved expression of markers of undifferentiated spermatogonia (PLZF and GFRα1) between mice and nonhuman primates. While the xenotransplantation assay is limited because of the evolutionary distance between rhesus and mouse, it serves as a practical tool that lays the foundation to test future hypotheses. The ablated rhesus testis model established in this study will facilitate future rhesus-to-rhesus testis cell transplantations to validate the xenotransplantation results, investigate the full regenerative potential of nonhuman primate SSCs and study stem cell/niche interactions.
Supplementary Material
Acknowledgements
The authors thank Kevin Grund, Cynthia Oberley, Kari Panza, and Felicity Winkler for assistance with semen collection and analysis. Tony Battelli, Michelle Walsh, and Michael Bodenheimier provided rodent colony maintenance. The rhesus testis cell antibody was produced in collaboration with Dr. Tony Plant, who also assisted with hemicastrations. Dr. Stefan Schlatt assisted with testis biopsy collection.
This work was supported by the Magee-Womens Research Institute and Foundation, Pennsylvania Department of Health grant RFA 02-07-16, a Specialized Cooperative Centers Program in Reproduction and Infertility Research grant (U54 HD008610), NIH grants RR018500 and AG024992 to KEO, NIH grants HD12913, HD47675 and RR13632 to GPS, and an institutional NRSA postdoctoral fellowship HD007332 to BPH.
References
- 1.Brinster RL, Avarbock MR. Germline transmission of donor haplotype following spermatogonial transplantation. Proc. Natl. Acad. Sci. U. S. A. 1994;91:11303–11307. doi: 10.1073/pnas.91.24.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc. Natl. Acad. Sci. U. S. A. 1994;91:11298–11302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brinster RL. Male germline stem cells: from mice to men. Science. 2007;316:404–405. doi: 10.1126/science.1137741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fritz IB. Reflections on the evolution of the regulation of spermatogenesis. Prog. Clin. Biol. Res. 1986;226:371–388. [PubMed] [Google Scholar]
- 5.Clermont Y, Leblond CP. Differentiation and renewal of spermatogonia in the monkey, Macacus rhesus. Am. J. Anat. 1959;104:237–271. doi: 10.1002/aja.1001040204. [DOI] [PubMed] [Google Scholar]
- 6.Clermont Y. Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiological Reviews. 1972;52:198–236. doi: 10.1152/physrev.1972.52.1.198. [DOI] [PubMed] [Google Scholar]
- 7.Clermont Y, Antar M. Duration of the cycle of the seminiferous epithelium and the spermatogonial renewal in the monkey Macaca arctoides. Am. J. Anat. 1973;136:153–165. doi: 10.1002/aja.1001360204. [DOI] [PubMed] [Google Scholar]
- 8.Fouquet JP, Dadoune JP. Renewal of spermatogonia in the monkey (Macaca fascicularis) Biol. Reprod. 1986;35:199–207. doi: 10.1095/biolreprod35.1.199. [DOI] [PubMed] [Google Scholar]
- 9.van Alphen MM, de Rooij DG. Depletion of the seminiferous epithelium of the rhesus monkey, Macaca mulatta, after X-irradiation. Br. J. Cancer Suppl. 1986;7:102–104. [PMC free article] [PubMed] [Google Scholar]
- 10.Plant TM, Marshall GR. The functional significance of FSH in spermatogenesis and the control of its secretion in male primates. Endocr. Rev. 2001;22:764–786. doi: 10.1210/edrv.22.6.0446. [DOI] [PubMed] [Google Scholar]
- 11.Ehmcke J, Luetjens CM, Schlatt S. Clonal organization of proliferating spermatogonial stem cells in adult males of two species of non-human primates, Macaca mulatta and Callithrix jacchus. Biol. Reprod. 2005;72:293–300. doi: 10.1095/biolreprod.104.033092. [DOI] [PubMed] [Google Scholar]
- 12.Simorangkir DR, Marshall GR, Ehmcke J, et al. Prepubertal expansion of dark and pale type A spermatogonia in the rhesus monkey (Macaca mulatta) results from proliferation during infantile and juvenile development in a relatively gonadotropin independent manner. Biol. Reprod. 2005;73:1109–1115. doi: 10.1095/biolreprod.105.044404. [DOI] [PubMed] [Google Scholar]
- 13.Huckins C. The spermatogonial stem cell population in adult rats. I. Their morphology, proliferation and maturation. Anat. Rec. 1971;169:533–557. doi: 10.1002/ar.1091690306. [DOI] [PubMed] [Google Scholar]
- 14.Oakberg EF. Spermatogonial stem-cell renewal in the mouse. Anat. Rec. 1971;169:515–531. doi: 10.1002/ar.1091690305. [DOI] [PubMed] [Google Scholar]
- 15.Gosden RG. Trade-offs in cancer and reproduction. Hum. Reprod. Update. 2001;7:360–362. doi: 10.1093/humupd/7.4.360. [DOI] [PubMed] [Google Scholar]
- 16.Lee SJ, Schover LR, Partridge AH, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J. Clin. Oncol. 2006;24:2917–2931. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 17.Orwig KE, Schlatt S. Cryopreservation and transplantation of spermatogonia and testicular tissue for preservation of male fertility. J. Natl. Cancer Inst. Monogr. 2005;34:51–56. doi: 10.1093/jncimonographs/lgi029. [DOI] [PubMed] [Google Scholar]
- 18.Gould KG, Mann DR. Comparison of electrostimulation methods for semen recovery in the rhesus monkey (Macaca mulatta) J. Med. Primatol. 1988;17:95–103. [PubMed] [Google Scholar]
- 19.Nagano M, Patrizio P, Brinster RL. Long-term survival of human spermatogonial stem cells in mouse testes. Fertil. Steril. 2002;78:1225–1233. doi: 10.1016/s0015-0282(02)04345-5. [DOI] [PubMed] [Google Scholar]
- 20.Nagano M, McCarrey JR, Brinster RL. Primate spermatogonial stem cells colonize mouse testes. Biol. Reprod. 2001;64:1409–1416. doi: 10.1095/biolreprod64.5.1409. [DOI] [PubMed] [Google Scholar]
- 21.Bucci LR, Meistrich ML. Effects of busulfan on murine spermatogenesis: cytotoxicity, sterility, sperm abnormalities, and dominant lethal mutations. Mutat. Res. 1987;176:259–268. doi: 10.1016/0027-5107(87)90057-1. [DOI] [PubMed] [Google Scholar]
- 22.Ogawa T, Aréchaga JM, Avarbock MR, et al. Transplantation of testis germinal cells into mouse seminiferous tubules. Int. J. Dev. Biol. 1997;41:111–122. [PubMed] [Google Scholar]
- 23.Dobrinski I, Avarbock MR, Brinster RL. Transplantation of germ cells from rabbits and dogs into mouse testes. Biol. Reprod. 1999;61:1331–1339. doi: 10.1095/biolreprod61.5.1331. [DOI] [PubMed] [Google Scholar]
- 24.Nagy A, Gertenstein M, Vintersten K, et al. Manipulating the Mouse Embryo: A Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2003. [Google Scholar]
- 25.Toyooka Y, Tsunekawa N, Takahashi Y, et al. Expression and intracellular localization of mouse Vasa-homologue protein during germ cell development. Mech. Dev. 2000;93:139–149. doi: 10.1016/s0925-4773(00)00283-5. [DOI] [PubMed] [Google Scholar]
- 26.Ruggiu M, Speed R, Taggart M, et al. The mouse Dazla gene encodes a cytoplasmic protein essential for gametogenesis. Nature. 1997;389:73–77. doi: 10.1038/37987. [DOI] [PubMed] [Google Scholar]
- 27.Meng X, Lindahl M, Hyvonen ME, et al. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. 2000;287:1489–1493. doi: 10.1126/science.287.5457.1489. [DOI] [PubMed] [Google Scholar]
- 28.Buageaw A, Sukhwani M, Ben-Yehudah A, et al. GDNF family receptor alpha1 phenotype of spermatogonial stem cells in immature mouse testes. Biol. Reprod. 2005;73:1011–1016. doi: 10.1095/biolreprod.105.043810. [DOI] [PubMed] [Google Scholar]
- 29.Ryu BY, Kubota H, Avarbock MR, et al. Conservation of spermatogonial stem cell self-renewal signaling between mouse and rat. Proc. Natl. Acad. Sci. U. S. A. 2005;102:14302–14307. doi: 10.1073/pnas.0506970102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buaas FW, Kirsh AL, Sharma M, et al. Plzf is required in adult male germ cells for stem cell self-renewal. Nat. Genet. 2004;36:647–652. doi: 10.1038/ng1366. [DOI] [PubMed] [Google Scholar]
- 31.Costoya JA, Hobbs RM, Barna M, et al. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat. Genet. 2004;36:653–659. doi: 10.1038/ng1367. [DOI] [PubMed] [Google Scholar]
- 32.Marshall GR, Plant TM. Puberty occurring either spontaneously or induced precociously in rhesus monkey (Macaca mulatta) is associated with a marked proliferation of Sertoli cells. Biol. Reprod. 1996;54:1192–1199. doi: 10.1095/biolreprod54.6.1192. [DOI] [PubMed] [Google Scholar]
- 33.Avarbock MR, Brinster CJ, Brinster RL. Reconstitution of spermatogenesis from frozen spermatogonial stem cells. Nat. Med. 1996;2:693–696. doi: 10.1038/nm0696-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanatsu-Shinohara M, Ogonuki N, Inoue K, et al. Restoration of fertility in infertile mice by transplantation of cryopreserved male germline stem cells. Human Reproduction. 2003;18:2660–2667. doi: 10.1093/humrep/deg483. [DOI] [PubMed] [Google Scholar]
- 35.Clouthier DE, Avarbock MR, Maika SD, et al. Rat spermatogenesis in mouse testis. Nature. 1996;381:418–421. doi: 10.1038/381418a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogawa T, Dobrinski I, Avarbock MR, et al. Xenogeneic spermatogenesis following transplantation of hamster germ cells to mouse testes. Biol. Reprod. 1999;60:515–521. doi: 10.1095/biolreprod60.2.515. [DOI] [PubMed] [Google Scholar]
- 37.Dobrinski I, Avarbock MR, Brinster RL. Germ cell transplantation from large domestic animals into mouse testes. Mol. Reprod. Dev. 2000;57:270–279. doi: 10.1002/1098-2795(200011)57:3<270::AID-MRD9>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 38.Kumar S, Hedges SB. A molecular timescale for vertebrate evolution. Nature. 1998;392:917–920. doi: 10.1038/31927. [DOI] [PubMed] [Google Scholar]
- 39.Eizirik E, Murphy WJ, O’Brien SJ. Molecular dating and biogeography of the early placental mammal radiation. J. Hered. 2001;92:212–219. doi: 10.1093/jhered/92.2.212. [DOI] [PubMed] [Google Scholar]
- 40.Orwig KE, Shinohara T, Avarbock MR, et al. Functional analysis of stem cells in the adult rat testis. Biol. Reprod. 2002;66:944–949. doi: 10.1095/biolreprod66.4.944. [DOI] [PubMed] [Google Scholar]
- 41.Dobrinski I, Ogawa T, Avarbock MR, et al. Computer assisted image analysis to assess colonization of recipient seminiferous tubules by spermatogonial stem cells from transgenic donor mice. Mol Reprod. Dev. 1999;53:142–148. doi: 10.1002/(SICI)1098-2795(199906)53:2<142::AID-MRD3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 42.Nagano M, Avarbock MR, Brinster RL. Pattern and kinetics of mouse donor spermatogonial stem cell colonization in recipient testes. Biol. Reprod. 1999;60:1429–1436. doi: 10.1095/biolreprod60.6.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nagano MC. Homing efficiency and proliferation kinetics of male germ line stem cells following transplantation in mice. Biol. Reprod. 2003;69:701–707. doi: 10.1095/biolreprod.103.016352. [DOI] [PubMed] [Google Scholar]
- 44.Tegelenbosch RAJ, de Rooij DG. A quantitative study of spermatogonial multiplication and stem cell renewal in the C3H/101 F1 hybrid mouse. Mutat. Res. 1993;290:193–200. doi: 10.1016/0027-5107(93)90159-d. [DOI] [PubMed] [Google Scholar]
- 45.Jahnukainen K, Ehmcke J, Soder O, et al. Clinical Potential and Putative Risks of Fertility Preservation in Children Utilizing Gonadal Tissue or Germline Stem Cells. Pediatr. Res. 2006;59:40R–47R. doi: 10.1203/01.pdr.0000205153.18494.3b. [DOI] [PubMed] [Google Scholar]
- 46.Trottmann M, Becker AJ, Stadler T, et al. Semen Quality in Men with Malignant Diseases before and after Therapy and the Role of Cryopreservation. Eur. Urol. 2007 doi: 10.1016/j.eururo.2007.03.085. doi:10.1016/j.eururo.2007.03.085. [DOI] [PubMed] [Google Scholar]
- 47.Brougham MF, Wallace WH. Subfertility in children and young people treated for solid and haematological malignancies. Br. J. Haematol. 2005;131:143–155. doi: 10.1111/j.1365-2141.2005.05740.x. [DOI] [PubMed] [Google Scholar]
- 48.Buckner CD, Dillingham LA, Giddens WE, Jr., et al. Toxicologic and marrow transplantation studies in rhesus monkeys given dimethyl myleran. Exp. Hematol. 1975;3:275–288. [PubMed] [Google Scholar]
- 49.Kuramoto K, Follman D, Hematti P, et al. The impact of low-dose busulfan on clonal dynamics in nonhuman primates. Blood. 2004;104:1273–1280. doi: 10.1182/blood-2003-08-2935. [DOI] [PubMed] [Google Scholar]
- 50.Kahl CA, Tarantal AF, Lee CI, et al. Effects of busulfan dose escalation on engraftment of infant rhesus monkey hematopoietic stem cells after gene marking by a lentiviral vector. Exp. Hematol. 2006;34:369–381. doi: 10.1016/j.exphem.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 51.Kang EM, Hsieh MM, Metzger M, et al. Busulfan pharmacokinetics, toxicity, and low-dose conditioning for autologous transplantation of genetically modified hematopoietic stem cells in the rhesus macaque model. Exp. Hematol. 2006;34:132–139. doi: 10.1016/j.exphem.2005.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kean LS, Adams AB, Strobert E, et al. Induction of Chimerism in Rhesus Macaques through Stem Cell Transplant and Costimulation Blockade-Based Immunosuppression. Am. J. Transplant. 2007;7:320–335. doi: 10.1111/j.1600-6143.2006.01622.x. [DOI] [PubMed] [Google Scholar]
- 53.Kar AB, Kamboj VP, Chandra H. Effect of some chemicals on spermatogenesis in rhesus monkeys. J. Reprod. Fertil. 1968;16:165–170. doi: 10.1530/jrf.0.0160165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.