Abstract
Young infants use caregivers’ emotional expressions to guide their behavior in novel, ambiguous situations. This skill, known as social referencing, likely involves at least 3 separate abilities: (a) looking at an adult in an unfamiliar situation, (b) associating that adult’s emotion with the novel situation, and (c) regulating their own emotions in response to the adult’s emotional display. The authors measured each of these elements individually as well as how they related to each other. The results revealed that 12-month-olds allocated more attention, as indicated by event-related potential measures, to stimuli associated with negative adult emotion than to those associated with positive or neutral emotion. Infants’ interaction with their caregiver was affected by adult emotional displays. In addition, how quickly infants referenced an adult predicted both their brain activity in response to pictures of stimuli associated with negative emotion as well as some aspects of their behavior regulation. The results are discussed with respect to their significance for understanding why infants reference and regulate their behavior in response to adult emotion. Suggestions for further research are provided.
Keywords: social referencing, emotion, brain activity
By the 2nd year of life, infants use the emotional expressions of familiar adults to regulate their behavior in novel situations (Gunnar & Stone, 1984), a skill known as social referencing. This ability to respond to adults’ emotional signals is imperative to the survival of children. Caregivers’ emotional expressions convey information about what children may consume, play with, and approach safely. Social referencing also may be a precursor to other social–cognitive abilities, particularly the ability to understand that other people possess thoughts, feelings, and attitudes, or “theory of mind” (Baron-Cohen, 1995; Stone, Baron-Cohen, & Knight, 1998). Failure to develop these social–cognitive abilities is a feature of such disorders as autism.
Feinman and colleagues (Feinman, Roberts, Hsieh, Sawyer, & Swanson, 1992) described three elements that are involved in social referencing. The first element involves the infant seeking information from adults in a novel or ambiguous situation. This usually takes the form of a triadic interaction among the infant, adult, and object and may be related to infant joint attention. The second element involves the infant associating the referent with the social message. The third element involves the infant regulating his or her behavior in response to the information provided by the referee (the adult providing the information). Although each of these three social referencing elements is important in early social cognition, most studies have focused primarily on infants’ regulation of their behavior in response to the referee’s signal. The purpose of the present research was to examine these three aspects of social referencing and how they interact in 12-month-old infants by examining their behavior in a context designed to elicit social referencing and their brain activity in response to referents.
Studies of the first element (i.e., joint attention) of social referencing as it relates to social referencing are rare. Some research on early joint attention has documented relations between joint attention and general cognitive abilities. For example, imitative play, referential language, and the ability to follow an adult’s bid for joint attention emerge at a similar age and are correlated with one another (Carpenter, Nagell, & Tomasello, 1998; Slaughter & Mc-Connell, 2003). However, we know relatively little about how joint attention is related to skills involved in social referencing, such as emotion–referent associations and behavior regulation (Moore & Corkum, 1994).
Few investigations have been conducted to specifically examine the second social referencing element: how infants form relations between emotional signals and novel objects. In one study, Hertenstein and Campos (2004) examined infants’ behavior directed toward novel objects after a delay. This study established that 14-month-olds’, but not 11-month-olds’, behavior was influenced by an adult emotional signal after a delay of 1 hr. However, although retention of an association between the emotional information and the novel object is probably required for infants to be affected by the emotional information after a delay, this study measured only the third aspect of social referencing, behavior regulation. An important question that remains unaddressed is how infants’ associative abilities are involved in social referencing. Presumably, development in memory and attention influences how infants form these associations.
In contrast to the small number of studies of the role of joint attention and associations between emotions and referents in social referencing, many studies have examined how infants regulate their behavior in response to emotional information. In a classic social referencing study, 12-month-old infants were placed on a visual cliff while their caregiver posed emotional expressions at the opposite end. Infants whose caregiver demonstrated negative emotional expressions were less likely to cross the deep end of the visual cliff than were infants whose caregivers provided positive emotional information (Sorce, Emde, Campos, & Klinnert, 1985). This finding established the idea that infants can use familiar adults’ emotions to regulate their behavior.
Gunnar and Stone (1984) evaluated infants’ reactions to the presentation of maternal displays of positive versus neutral affect after the presentation of novel objects to which infants’ baseline response was positive, negative, or ambiguous. Their investigation revealed that, under the positive maternal display condition, infants responded congruently only for the ambiguous toy. These findings provide important evidence that infants’ use of others’ behavior to appraise situations is somewhat selective. Infants apparently use the behavior of others to assess events specifically when they cannot assess events on their own.
Apparently, young infants can regulate their behavior in response to emotional cues even without seeking such information. In one recent study, infants responded to emotions posed by televised actresses (Mumme & Fernald, 2003). In this case, contrary to typical social referencing paradigms, infants were not seeking information from others; rather, they were participating only as passive observers. Yet, even in the absence of active social referencing, infants were attentive to the emotional expressions of others and used the affective responses they observed to guide their behavior. Infants touched and interacted less with objects toward which a televised actress appeared to be gazing while she displayed negative emotion than they did objects toward which she was not looking or displayed positive emotion. Evidently, adult emotional expressions have an impact on infants’ behavior by 12 months of age, regardless of whether infants understand the adult’s mental state accompanying that display of emotion.
Thus, we have a fairly complete understanding of infants’ ability to regulate their behavior on the basis of the emotional displays of others. Nevertheless, several questions remain unanswered regarding the relation between joint attention and social referencing and the role of infants’ developing associative abilities in social referencing. In addition, how the three social referencing elements (i.e., joint attention, association, and behavior regulation) are related is unclear. Addressing this question is important for understanding how infants’ changing cognitive abilities drive social referencing.
For infants to have a complete, adult-like ability to interpret others’ emotions and use them to regulate behavior, they must be able to combine the functions of each of the three social referencing elements. The development of these abilities requires the maturation of a complex set of brain areas, each of which can be associated with one or more of the three elements involved in social referencing. Frontal areas likely involved in the development of joint attention (Mundy, Card, & Fox, 2000; Mundy, Fox, & Card, 2003) are probably important for infants’ developing ability to seek social information. Structures in the medial temporal lobe are important for recognition of emotions (Adolphs, Baron-Cohen, & Tranel, 2002; Cahill, Babinsky, Markowitsch, & McGaugh, 1995; Thomas et al., 2001), but to associate these emotions with novel objects also requires participation of medial temporal lobe areas involved in memory (Nelson, 1995). Both the ability to recognize specific emotions (de Haan & Nelson, 1998; Nelson & de Haan, 1996; Schwartz, Izard, & Ansul, 1985) and long-term memory (Barr, Dowden, & Hayne, 1996; Bauer, Wiebe, Carver, Waters, & Nelson, 2003; Carver & Bauer, 1999, 2001; Meltzoff, 1988a, 1988b) appear to undergo significant development late in the 1st year of life. Finally, areas in the insula and anterior cingulate may be involved in observing and understanding the emotions of others (Carr, Iacoboni, Dubeau, Mazziotta, & Lenzi, 2003; Singer et al., 2004). These areas may be important for both associating emotion with referents and emotion regulation. In addition to joint attention and information seeking, frontal areas may be involved in emotion regulation (Davidson & Fox, 1982, 1988; Fox & Davidson, 1987).
Although we can speculate on the development of these brain systems and their possible contribution to the emergence of social referencing abilities, to date no studies have attempted to examine brain activity related to social referencing or any of its three elements. This absence of research probably is due to constraints on experiments that can be used to measure brain–behavior relations. The most appropriate method for localizing function (functional brain imaging) requires behavior and tasks (e.g., lying still in a magnet and pressing buttons) that are impractical for use with young infants. Event-related potentials (ERPs), which are noninvasive and can be used with even young infants, require the presentation of many trials of briefly repeated static stimuli. This constraint is not conducive to naturalistic studies of infants in social interactions. Behavioral studies, although more naturalistic, cannot tell us about the development of the neural correlates of the aspects of social referencing behavior.
The present study was a first attempt at understanding development of the neural circuitry involved in the second aspect of social referencing (associating emotion with referents) and relating that activity to the other aspects of social referencing (i.e., joint attention and behavior regulation). We measured the three elements involved in social referencing, including measures of brain activity in response to novel stimuli that previously had been tagged with emotional information.
We measured the first aspect of social referencing, joint attention, by examining how quickly infants looked at an adult in the presence of a novel, ambiguous object. We related this variable to measures of brain activity and behavior regulation.
To measure the second social referencing element, we used ERPs to examine infants’ brain responses to pictures of novel objects that previously had been associated with adult emotion. Of particular interest in this study were two components that have been identified in the infant ERP literature. The Nc component is a middle latency, centrally and frontally distributed component that is negative in polarity and has been associated with attention (Courchesne, 1978; Courchesne, Ganz, & Norcia, 1981). Differences in the amplitude and latency of the Nc component have been identified in studies of memory, attention, and emotion recognition (Bauer et al., 2003; Carver, Bauer, & Nelson, 2000; de Haan & Nelson, 1999; Nelson & de Haan, 1996; Richards, 2003). Richards (2003) found that the Nc component increased in response to stimuli that were different from ongoing background information and that any stimulus that stood out as different from the ongoing background stimuli elicited a large Nc component. This explanation fits well with the fact that, in studies using an oddball paradigm in which one stimulus was presented on 80% of trials and another stimulus was presented on the remaining 20% of trials, the Nc component in response to the infrequent trials was larger than to the frequent trials (e.g., Courchesne et al., 1981; Nikkel & Karrer, 1994). However, in other studies (Carver et al., 2000; Dawson et al., 2002; de Haan & Nelson, 1997, 1999) in which stimuli were presented with equal probability, the amplitude of the Nc component differentiated familiar from unfamiliar stimuli (Carver et al., 2000; de Haan & Nelson, 1997, 1999) or one emotion from another (Nelson & de Haan, 1996). Thus, the Nc component appears to reflect aspects of attention that are related to memory and emotion as well as attention to stimuli that are different from the ongoing background information.
An additional component of interest in this study was a middle latency, positive component (P500) that peaked over occipital electrode sites. This component has been described in recent studies of face and object processing in which an average reference was used (Dawson et al., 2002; de Haan, Pascalis, & Johnson, 2002; Halit, Csibra, Volein, & Johnson, 2004; Halit, de Haan, & Johnson, 2003). Although exactly what this component reflects is not entirely clear, apparently it responds differently to face than nonface stimuli (de Haan et al., 2002; Halit et al., 2003). In addition, this ERP component has been shown to reflect differences in responses to social and nonsocial stimuli in typical and clinical populations (Dawson et al., 2002). Thus, we expected P500 to be sensitive to differences in response to emotionally tagged stimuli.
Given that this study was the first of its kind, we did not predict a specific pattern of relations between brain activity and associated emotion. Several possibilities were evident on the basis of previous research. First, infants might show larger ERP responses to objects associated with positive emotion. This pattern would be consistent with previous behavioral studies that have shown that infants attend more to and interact more with stimuli associated with positive emotion (Gunnar & Stone, 1984; Mumme & Fernald, 2003; Mumme, Fernald, & Herrera, 1996). Given that the Nc component is thought to differentially reflect attention to stimuli that infants find more or less interesting, one possibility is that an increase in behavioral attention would be accompanied by a concomitant increase in attention in the ERP, manifested as an increased amplitude in the Nc component. However, we might also predict the opposite pattern. Studies of infants’ processing of facial expressions of emotion have suggested that infants and young children show an increased ERP response to negative stimuli (de Haan & Nelson, 1998; Kestenbaum & Nelson, 1992; Nelson, 1993; Nelson & Nugent, 1990). One explanation for this pattern is that infants generally see negative emotions less frequently than positive emotions and, thus, respond to them with increased attention because they are relatively novel (Nelson, 1993). This pattern may extend to stimuli that have been associated with negative emotion as well. Regardless of which pattern emerges, we expected that differences in attention would be revealed in differential ERP activity to stimuli associated with different emotions.
Finally, to examine the third element of social referencing, we measured infants’ emotion regulation behavior in response to the novel object after an adult provided facial and verbal responses to it. Consistent with previous research, we expected infants to regulate their behavior in response to emotional signals provided by adults. Specifically, we expected infants to be less likely to approach or interact with toys associated with negative adult emotion than toys associated with positive emotion. In addition, we expected infants to use their caregiver’s presence to regulate their emotion. We expected infants to move closer to their caregiver after hearing and seeing negative emotional information about a novel object and to be more willing to move away from their caregiver and explore the novel object after hearing and seeing positive emotional information about it.
In addition to measuring these aspects of social referencing, we examined how individual differences in each of these elements related to the other elements. If the hypothesis that joint attention is a developmental precursor to social referencing (Baron-Cohen, 1991; Charman, Baron-Cohen, Swettenham, Baird, & Cox, 2000) is accurate, then infants’ looking behavior toward adults should predict both their behavior regulation and the associations they form between adult emotional signals and novel objects. If joint attention does not predict associations between adult emotional signals and novel objects or behavior regulation, then the elements involved in social referencing are probably not linked as closely as previously thought. An alternative hypothesis is that the elements involved in social referencing develop separately but interact at the point in development when social referencing emerges as an adult-like skill.
Another possibility is that some aspects of social referencing are related, but others are not. For example, if infants’ joint attention behavior is related only to their interaction with their caregiver but not with other aspects of behavior regulation, such as their attention to or interaction with the novel object, both joint attention and interaction with the caregiver might be driven by a similar mechanism. However, that mechanism may not relate to social referencing. For example, both looking at the caregiver and interaction with her as a result of her emotional expression may reflect aspects of the attachment relationship rather than social understanding (Ainsworth, 1992). If infants’ association between emotional signals and referents, as reflected in ERP activity, is related only to their emotion regulation but not to joint attention, then infants’ behavior and attention to novel stimuli may be simply a consequence of emotional contagion rather than true social referencing.
Method
Participants
Fifty-five 12-month-old infants were tested. A total of 34 infants (20 boys, 14 girls) provided interpretable behavioral and ERP data and were included in the final sample. The average age of the infants included in the final sample was 12.0 months (SD = .25). Most of the infants were from White, middle-class homes, although we did not collect specific data on socioeconomic status or parent education for this sample. Five infants’ parents identified them as biracial (Latino/White or Latino/Asian). The remaining infants were White (n = 20) or their parents did not wish to report their ethnicity (n = 9). Two infants were tested with their fathers; the rest of the infants were tested with their mothers. For each infant, the parent with whom they were tested was the primary caregiver. Of the infants for whom ERP data were not available, 10 infants did not tolerate the ERP testing procedure, and 11 infants did not provide sufficient artifact-free trials for analysis. Data from 4 infants were not included because their caregivers did not provide the target emotional verbal and facial expressions or provided such expressions at inappropriate times (e.g., caregivers provided the emotional signal even when their infant did not reference or provided a neutral verbal or facial expression in the positive or negative condition). Infants received a small toy in appreciation for their participation.
Stimuli
Three novel toys were used to elicit infant social referencing behavior. These toys were chosen from a set of six lab toys (see Table 1), and the toys were counterbalanced so that no toy was used more often than any other toy and no toy was associated with any emotion more often than any other toy. The toys were battery operated and remote controlled. Parents were interviewed to ensure that the toys were unknown to the infants. Pilot testing was conducted to ensure that 12-month-olds responded with neutral interest to them.
Table 1.
Description of Novel Objects Used in the Social Referencing Procedure
| Toy | Dimensions | Description |
|---|---|---|
| Dragon | 30 × 30 × 28 cm | Remote-controlled dragon; moves forward, backward, and turns |
| Robot | 11 × 11 × 15.5 cm | Remote-controlled robot; eyes light up; beeps and makes other noises |
| Dinosaur | 12 × 18.5 × 27 cm | Remote-controlled dinosaur; moves and spins, beeps, and makes other noises |
| Bumble ball | Diameter = 11.78 cm | Battery-operated ball; bounces in random patterns |
| Circumference = 37 cm | ||
| Weasel ball | 29 × 7 × 6 cm | Ball with furry stuffed animal attached; moves in random patterns |
| Spider | 13 × 20 × 8.5 cm | Remote-controlled spider; moves and makes noises |
For the ERP portion of the experiment, infants were seated on their caregivers’ laps approximately 75 cm in front of a computer monitor and were shown digital images of the toys in each of three conditions (positive, negative, neutral). The size of the images was manipulated to be consistent across conditions. Each image was approximately 10 cm × 20 cm (for primarily rectangular stimuli) or 15 cm × 15 cm (for primarily square or circular stimuli).
Behavioral Procedure
Infants were tested in a single session. During the first part of the test session, infants’ behavioral responses to adults’ emotional signals were recorded. Infant behavior was coded from videotapes after testing.
Before testing began, infants’ caregivers were brought into an adjacent room and trained to make positive (happy), negative (disgust), and neutral (calm) facial expressions. Caregivers were instructed on how to make these expressions using examples from a standardized set of photographs of facial affect (Tottenham, Borscheid, Ellertsen, Marcus, & Nelson, 2002). In addition, caregivers were trained to make a verbal signal following a specific script (“What a pretty [nasty, ordinary] toy”). The experimenter then demonstrated each of the facial and verbal signals. Finally, the caregiver was given a practice visualization exercise (e.g., for the disgust condition, the caregiver was instructed to “imagine the toy is dirty and broken”) and was asked to practice the signals. During coding, the caregivers’ production of signals was rated to confirm that caregivers accurately demonstrated the target emotional signals. The caregivers were consistent in providing the target emotional signals. The average score assigned by coders for the facial expression on a 5-point scale was 4.12 (SD = 1.05) for the happy expression, 4.0 (SD = .79) for the disgust condition, and 3.88 (SD = 1.11) for the neutral condition. The coders assessed the match between the caregivers’ facial expressions and the expressions used during training. For the vocal statements, coders evaluated the degree of emotion in the caregiver’s verbal statements (i.e., the extent to which caregivers’ tone and intensity matched the emotion being modeled). For positive and negative emotions, caregivers were expected to portray intense verbal and facial emotion to score a 5 on this rating scale. For neutral, they were expected to portray neutral vocal expression without extreme positive or negative emotion to receive a score of 5. The average score for vocalizations, scored on the same 5-point scale, was 3.56 (SD = 1.29) for the positive condition, 3.61 (SD = 1.29) for the negative condition, and 3.61 (SD = 1.38) for the neutral condition.
The events that occurred during the social referencing assessment are described in Table 2. Briefly, infants were exposed to emotional signals provided by the caregiver or an experimenter each time that the infants referenced. If infants did not reference within a minute of the presentation of the novel toy, caregivers were instructed to elicit infants’ attention and provide the appropriate emotional signal. All infants referenced either the experimenter or the caregiver spontaneously on at least one of the toy presentations. Fifteen infants referenced spontaneously for all three toy presentations. Nine infants referenced spontaneously for two of the three stimuli, and 10 infants referenced spontaneously for one of the three stimuli. In this way, all infants were exposed to the emotional information, although for some toys, some of the infants received this information by referencing and others received the information without referencing. In addition, infants who referenced more often received more emotional information than infants who referenced fewer times. The order of the toy presentations, the order of the facial expressions, and the toys with which each expression was associated were counterbalanced across infants such that no toy or emotion occurred more often in any serial position than any other toy or emotion and no toy was associated with any particular affective signal more often than any other toy.1 After infants viewed three toys, one in each affective category, they were taken into an adjacent room for ERP testing.
Table 2.
Sequence of Events During Behavioral Social Referencing Assessment
| Time (min) | Event | Parent and experimenter behavior |
|---|---|---|
| 0:00–1:00 | Infant plays with familiar toys | Talk quietly with each other |
| 1:00–2:00 | Introduction of Novel Toy 1 | Provide emotional signal when infant references |
| 2:00–3:00 | Infant plays with familiar toys | Talk quietly with each other |
| 3:00–4:00 | Introduction of Novel Toy 2 | Provide emotional signal when infant references |
| 4:00–5:00 | Infant plays with familiar toys | Talk quietly with each other |
| 5:00–6:00 | Introduction of Novel Toy 3 | Provide emotional signal when infant references |
Note. If infants failed to reference during the 1st minute after the toy was introduced, the caregiver was instructed to get the infants’ attention and provide him or her with the emotional signal. For these infants, the interval during which the toy was present was extended by an additional 15 s. This timing was essential to ensure that every infant had some experience with the emotional signals.
ERP Testing
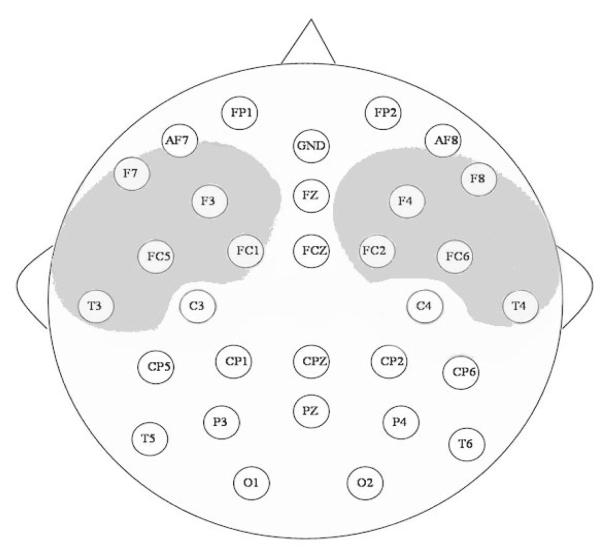
After the behavioral social referencing test, infants were taken into an adjoining room, and ERPs were recorded in response to pictures of the three toys seen during the behavioral testing. There was a delay of about 20 min between the end of the social referencing procedure and the beginning of ERP recordings, during which time the electrode cap was applied and impedances were measured. Data were collected from 32 channels placed according to the 10/20 system of electrode placement (Electrocap, Inc., Eaton, OH). A schematic of the location of the electrodes is shown in Figure 1. Data were amplified 20,000 times using a 32-channel Sensorium amplifier (Sensorium, Inc., Charlotte, VT). Infants were seated on their caregiver’s lap and saw 33 randomly ordered presentations of each condition. Caregivers were not blind to the visual display. Images were presented for 500 ms. Data were acquired for a 100-ms baseline interval preceding stimulus presentation and for 1,200 ms following stimulus offset. The intertrial interval varied between 1,000 and 1,500 ms. The sampling rate was 284 Hz (one sample every 3.52 ms). Impedances were measured before and after testing and were maintained at 10 kΩ (M = 6.5 kΩ, SD = 2.56, before testing; M = 5.18 kΩ, SD = 8.26, after testing).
Figure 1.
Electrode configuration. Shaded areas indicate electrodes used to measure lateral and frontal Nc activity.
Data were collected and referenced to the vertex electrode (Cz) and were rereferenced offline to an average reference. This reference was chosen for two reasons. First, in this study, we sought to examine the effects of different emotions on ERP activity at lateral electrode locations. Hemispheric differences in lateralization of the ERP are seen more easily with the use of central or average references than with lateral (e.g., linked mastoid) references. Second, we also sought to compare the data acquired with this method as closely as possible with data from recently published studies of social stimuli, such as faces, all of which used an average reference (Dawson et al., 2002; de Haan et al., 2002; Halit et al., 2003). Although some controversy exists about what constitutes the proper reference in ERP research, each method has advantages and disadvantages (Dien, 1998), and the average reference seems to have the best utility for the questions addressed in this study.
During testing, an experimenter who was hidden behind the screen through which the stimuli were presented observed the infant and indicated, via mouse button press, when the infant was not looking at the screen. Trials on which the infant did not attend to the stimuli were excluded from further analysis. The data were collected with a .01-Hz high-pass and 50-Hz low-pass filter in place. Trials that included eye blinks, defined as amplitude greater than 150 μV that resolved within 150 ms over frontal and EOG electrodes, were removed from further analysis. Trials were also excluded when activity exceeded A to D values or when the amplitude of the activity deviated more than 200 μV from the baseline. Offline digital filters (low pass = 15 Hz) were used to smooth averaged data (Instep Systems, Ottawa, Ontario, Canada).2
Behavioral Data Coding
Videotapes of the behavioral sessions were viewed by coders who were naive to the main hypotheses. For the first element (i.e., joint attention) involved in social referencing, infants’ latency to the first look to an adult after initially looking at the novel object was measured. To measure the third social referencing element, behavior regulation, several aspects of infants’ behavior were assessed on a 5-point rating scale ranging from 1 (least positive behavior) to 5 (most positive behavior). Definitions of the behaviors that were coded are shown in Table 3. Tapes for 25% of the participants were coded by a second coder for reliability purposes. Coders agreed on 90.83% of behaviors for these tapes.
Table 3.
Definitions and Scoring for Behaviors in the Social Referencing Evaluation
| Score |
|||||
|---|---|---|---|---|---|
| Measure | 1 | 2 | 3 | 4 | 5 |
| Affect | Clear negative emotion, crying | Apparent negative emotion; fussy but not crying |
Neutral emotion | Positive, smiling, minimal vocalization |
Very positive, laughing, vocalizing |
| Distance from caregiver | At caregiver’s feet | Between caregiver and experimenter |
At experimenter’s feet | Middle of room, within about 4 ft of caregiver and experimenter |
Not near either caregiver or experimenter, near edges of room |
| Behavior toward caregiver | Ignores | Reaches for, looks at caregiver | Approaches caregiver | Touches caregiver | Clings to caregiver |
| Behavior toward experimenter | Ignores | Reaches for, looks at experimenter |
Approaches experimenter | Touches experimenter | Clings to experimenter |
| Reaction to toy | Withdraws from toy | Ignores toy | Look or points at toy | Approaches or reaches for toy | Touches or explores toy |
| Interest in toy | Ignores | Looks occasionally, does not track |
Tracks toy for less than 5 s | Tracks toy for 5–10 s | Very interested, tracks toy for more than 10 s |
ERP Data Reduction and Analysis
Artifact-free trials were averaged by condition (positive, negative, neutral). Participants who provided more than 8 artifact-free trials in each condition were included in the final analysis. The average number of trials included per participant was 19.26 (SD = 5.85) for the positive condition, 18.03 (SD = 5.57) for the negative condition, and 19.06 (SD = 5.38) for the neutral condition.
ERP waveforms of interest were identified on the basis of inspection of grand average data and individual subjects’ data. Two ERP waveforms of interest were identified on the basis of inspection of the grand averages and previous research on social–cognitive function in children in the same age range as those studied here. The Nc component was identified over central and frontal electrodes, which was defined as the most negative peak in the window between 400 and 1,200 ms after stimulus onset. This interval was determined by examining the data for each infant and selecting a time window that captured the deviation from baseline, peak, and return to baseline of the Nc component at each frontal and central electrode. A computer-assisted program then calculated the peak amplitude and latency for each infant. The average latency of the Nc component in the present study at the FCz electrode was 595 ms (SD = 193.93). The peak latency of the Nc component at all electrodes at which it appeared for individual infants ranged from 575 ms to 944 ms after stimulus onset. The electrodes over which we analyzed the Nc component were determined by selecting those electrodes at which the waveform morphology was noted and by using electrode sites that were comparable to those used in previous research (e.g., Dawson et al., 2002).
A second component that was identified was the P500 component. This component peaked at an average of 550 ms (SD = 115.61; range = 447 to 697 ms) after stimulus onset and was apparent primarily at the occipital (O1 and O2) electrodes. The window used for identifying the P500 ranged from 370 to 842 ms after stimulus onset. The peak amplitude and latency of the P500 component were found as described for the Nc component.
Results
The three elements of social referencing were examined separately, including joint attention, forming an association between emotion and object, and emotion regulation. Relations between these elements were analyzed using linear regression.
Referencing Behavior
The first element, joint attention or looking to the caregiver in the novel situation, was measured as the latency from when the novel object was first presented to the infant and the infant’s first look to an adult. This measure was used in analyses of the relation between social referencing elements. A preliminary analysis was conducted to ensure that latency to reference did not vary as a function of emotional condition. A difference in this analysis was not anticipated because, before the signal was given (which, by definition, occurred when the infant referenced), infants could not differentiate the conditions. As expected, we found no difference between conditions for the latency to reference, F(2, 32) = .671, p = .52. The mean latency to reference in the positive condition was 38.76 s (SD = 23.9); in the negative condition, it was 38.29 s (SD = 26.74); and in the neutral condition, it was 44.59 s (SD = 23.27). A condition (positive, negative, neutral) by adult (caregiver, experimenter) repeated measures analysis of variance (ANOVA) revealed no difference in how many emotional signals were given between the different emotional conditions, F(2, 32) = 1.60, p = .22. The results reflected a significant main effect of adult, F(1, 33) = 5.51, p < .05. Infants were more likely to look at their caregiver than at an unfamiliar adult (mean looks to caregiver = 1.8, SD = 1.07; mean looks to experimenter = 1.1, SD = .82). The interaction between experimenter and condition was not significant, F(2, 34) = .04. Thus, infants saw the same number of adult emotional displays for each condition but saw these signals more frequently from the caregiver than from the experimenter.
ERP Activity
We observed both an anterior Nc component and posterior P500 component (see Figure 2). For each ERP waveform, we used repeated measures ANOVAs to compare activity across three conditions (positive, negative, neutral) and for lateral electrodes between hemisphere (left, right). Greenhouse–Geissler corrections for sphericity were applied as appropriate.
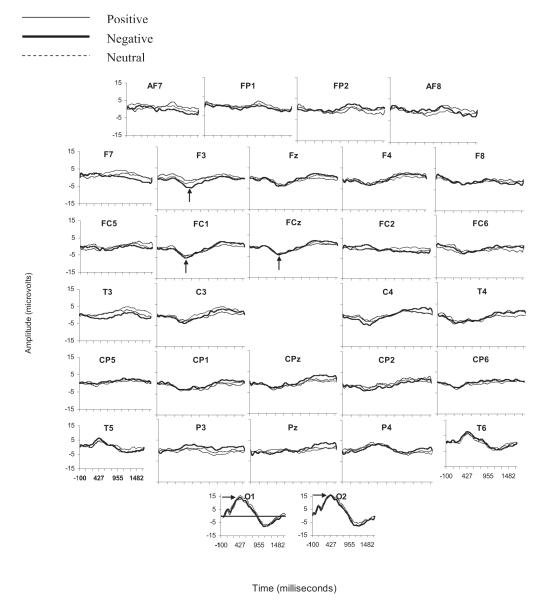
Figure 2.
Grand mean event-related potential data for each of the 30 electroencephalograph channels. The arrows indicate the Nc and P500 components.
Nc Component
A 3 (condition: positive, negative, neutral) × 5 (lead: T3/4, F3/4, FC1/2, FC5/6, F7/8) × 2 (hemisphere: left, right) repeated measures ANOVA revealed a main effect of condition for the amplitude of the Nc component. The amplitude of the Nc component was larger to pictures of toys associated with negative caregiver affect than to toys associated with positive and neutral adult affect, F(2, 32) = 4.08, p < .05 (see Figure 3). Post hoc Bonferroni-corrected pairwise comparisons indicated that infants showed larger peak amplitude activity for the negative condition than for the positive condition (mean difference = .83 V, 95% confidence interval [CI] = .012, 1.638, p < .05) and a marginally larger response for the negative than the neutral condition (mean difference = 1.1 V, 95% CI = −2.23, .04, p = .06).3 No difference between the positive and neutral conditions was observed (mean difference = .27, 95% CI = −.75, 1.29, p > .90). A main effect of hemisphere that approached significance was also observed, F(1, 33) = 3.89, p = .057. The amplitude of the Nc component was larger over the right (M = −6.38 V, SD = 2.71) than the left hemisphere (M = −5.2 V, SD = 3.19). No main effects or interactions for the amplitude of the Nc component at midline electrode sites were found (all ps > .10).
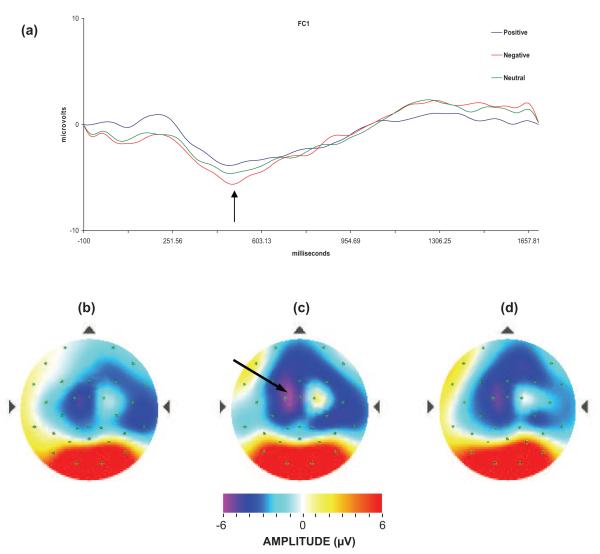
Figure 3.
(a) Event-related potential waveform (the arrow indicates the Nc component) and spline maps of activity occurring 587 ms after stimulus onset (Nc component peak); (b) positive condition; (c) negative condition (arrow indicates the peak of the Nc activity); and (d) neutral condition.
For the latency of the Nc component, a Condition × Lead × Hemisphere interaction that approached significance was found, F(8, 24) = 2.142, p = .07. This interaction was the result of a difference in the relative latency between conditions at different leads. At most lateral frontal leads, no significant difference in latency between conditions was obtained, and the trend in each case was a slower response to neutral stimuli than for positive and negative stimuli (follow-up ANOVAs for leads T3/4, FC1/2, FC5/6, and F7/8 yielded ps > .11). However, at frontal sites F3 and F4, a Condition × Hemisphere interaction was observed, F(2, 32) = 5.79, p < .007. At the left hemisphere site F3, the response was faster for the neutral than for the positive condition (mean difference = 129.36 ms, 95% CI = 10.36, 248.35). A significant difference in latency between positive and negative (mean difference = 63.90 ms, 95% CI = −57.41, 185.21) or between negative and neutral (mean difference = 65.45 ms, 95% CI = −178.40, 47.5) was not observed. There were no effects of condition or lead on the latency of the Nc component at midline leads (all ps > .10).
P500 Component
No effects of condition or electrode on the amplitude or latency of the P500 component at occipital (O1 and O2) electrodes were found.
Behavior Regulation
Infants’ behavior was coded before and after the adult emotional signal for several aspects of behavior regulation. These included the infants’ affect, their interest in the toy, their proximity to the toy, the distance they maintained to their caregiver, and their interactions with the caregiver and the adult experimenter (e.g., smiling and looking at the adult). Because infants’ behavior before the presentation of the adult emotional signal was an important baseline for each of these measures, we calculated a score for each behavior on the basis of the rating of the behavior before versus after the emotional signal. This score was calculated as the difference in the log ratios of infants’ behavior compared between two emotional conditions before and after the adult emotional signal, or (LOG [positive after signal/negative after signal]–LOG [positive before signal/negative before signal]). Log ratio scores were used to normalize the data and to compare behavior after the emotional signal while accounting for behavior before it. The result of this calculation was compared to zero using a one-sample t test. A significantly nonzero result indicates that in one emotional condition, infants’ behavior changed more from before to after the emotional signal than it did in the comparison emotional condition.
Affect
Analysis of infants’ emotional behavior revealed a significant difference between affect before and after the negative versus neutral adult emotional signals, t(33) = −2.23, p < .05. Infants displayed a greater increase in negative emotion after a negative signal than after a neutral signal. No effect of the positive condition on infant emotion when compared with either the negative or neutral conditions was observed (ps > .10).
Proximity to the Caregiver
No effect of emotion was seen on infants’ proximity to their caregiver (ps > .10).
Interaction With the Caregiver
Infants’ behavior directed toward their caregiver was differently affected by the negative and neutral emotional signals. Infants were more likely to increase looking at and interacting with their caregiver following a negative emotion than following a neutral emotion, t(33) = 2.597, p < .05. No difference in interaction with the caregiver between positive and neutral or between positive and negative conditions was found (ps > .10).
Interaction With the Toy and Experimenter
No effect of adult emotional signal was observed for infants’ interest in the toy, their proximity to the toy, or their interactions with the adult experimenter (ps > .10).
Relations Between Social Referencing Elements
We also examined the relations between each of the elements that make up social referencing. Because we conducted a large number of regression analyses, we first present those analyses that were significant on the basis of a relatively more conservative p < .01 criterion. Analyses that met a less stringent criterion (p < .05) are also mentioned but should be considered preliminary given the large number of comparisons used.
Relations Between Joint Attention and ERP Activity (Elements 1 and 2)
Relations between joint attention and ERP activity are shown in Table 4. To measure the relation between the first social referencing element, joint attention, and the second element, forming an association between emotion and the novel object, we used linear regression. We tested whether the amplitude and latency of ERP components related to infants’ latency to reference the caregiver. We did not predict which specific electrodes would and would not show relations between behavior and ERP activity. However, because some effects that involved lead were apparent in the analysis of ERP data alone, the relations between behavior and ERP activity could be different between electrodes. Thus, we analyzed the relation between behavior and ERP activity at each electrode site separately. Infants’ latency to reference averaged across emotional conditions was expected to reflect joint attention because it was an indication of infants’ overall tendency to look at the adults when presented with a novel object. Relations between latency to reference in a specific emotional condition and the ERP response in that emotional condition could have occurred for reasons that would not necessarily reflect the infants’ joint attention abilities. For example, if infants’ latency to reference within the positive emotional condition predicted infants’ ERP response to the positive stimulus, a true relation between sharing attention and the ERP response to that stimulus might exist. However, another possibility is that infants who referenced more quickly to that particular stimulus also had more time to process it. Thus, only relations between overall latency to reference and ERP activity are interpreted here as indicative of a relation between the first and second elements of social referencing.
Table 4.
Relations of Joint Attention With Event-Related Potential (ERP) Activity and Behavior
| Variable and significant correlation | r |
|---|---|
| ERP component (Nc) Amplitude in response to negative stimulus |
.44** |
| Behavior (proximity to toy) Difference in change from before to after emotional signal for the positive versus negative conditions |
.32* |
| Difference in change from before to after emotional signal for the positive versus neutral conditions |
.42* |
p < .05.
p < .01.
Overall latency to reference predicted the amplitude of the Nc component, r = .45, F(1, 32) = 8.143, p < .01. The faster infants referenced the caregiver, the larger their ERP was to the negative stimulus.
Peak latency of the Nc component in response to the negative toy was also predicted by latency to reference. However, this result was true only for the latency to reference within the negative condition, r = .44, F(1, 32) = 7.64, p < .01. Infants who referenced more quickly when shown the toy associated with negative emotion had a faster peak latency than infants who took longer to reference.
Relations Between ERP Activity and Behavior Regulation (Elements 2 and 3)
Relations between ERP activity and behavior regulation are summarized in Table 5. Linear regression was used to determine whether the amplitude and latency of the ERP activity and the log ratio scores calculated for emotion regulation behaviors were related. The results of these analyses are described below for each emotion regulation behavior measured.
Table 5.
Relations Between Event-Related Potential (ERP) Activity and Behavior Regulation
| Behavior | ERP component | r |
|---|---|---|
| Interest in toys (positive vs. negative) | Nc latency to negative stimulus | .33* |
| Interest in toys (negative vs. neutral) | Nc latency to negative stimulus | .45** |
| Proximity to toy (positive vs. negative) | Nc latency to positive stimulus | .33* |
| Proximity to toy (negative vs. neutral) | Nc latency to positive toy | .40* |
| Nc latency to negative toy | .39* | |
| Proximity to caregiver (positive vs. negative) | Nc latency to neutral stimulus | −.34* |
| Interaction with caregiver (positive vs. negative) | P500 amplitude to negative stimulus | −.35* |
| Interaction with caregiver (negative vs. neutral) | P500 amplitude to negative stimulus | .47*** |
| Interaction with caregiver (negative vs. neutral) | Nc latency to negative stimulus | .35* |
| Interaction with experimenter | P500 latency | −.47** |
p < .05.
p < .01.
p < .005.
Affect
No significant relations were found between infants’ change in emotion after positive, negative, and neutral emotional signals and ERP activity.
Interest in toys
Infants’ interest in the toy after a negative versus a neutral adult emotional signal was also related to the latency of the Nc component to the negative stimulus at the FC1 lead, r = .45, F(1, 32) = 8.11, p < .01. Infants who showed less interest in the toy after a negative versus a neutral emotional signal also showed a faster latency to peak for the Nc component in response to the negative stimulus than infants who showed more interest in the toy following the negative emotional signal.
The change in infants’ interest in toys after positive versus negative adult emotional signals marginally predicted the latency of the Nc response to the negative toy at the midline FCz lead, r = .33, F(1, 33) = 3.91, p =.05. Infants who responded with less interest in the positive toy after the positive emotional signal than after the negative emotional signal showed a faster ERP response to the toy associated with negative emotion than infants who showed more interest in the positive toy and less in the negative toy after the emotional signals.
Infants’ interaction with the caregiver
The amplitude of the P500 component at O2 in response to the negative stimulus also was related to the change in infants’ interaction with their caregiver between the negative and neutral conditions, r = .47, F(1, 32) = 8.92, p < .005. Infants who interacted less with their caregiver following the negative rather than the neutral emotional signal showed a larger ERP response to the negative stimulus than did infants who interacted more after the same signals.
The amplitude of the P500 component at the right posterior lead O2 predicted the difference in the change in infants’ interaction with their caregiver after the positive versus negative emotional signal, r = −.35, F(1, 32) = 4.37, p < .05. Infants who interacted less with their caregiver following a negative signal rather than the positive signal had a larger P500 response to the stimulus associated with negative emotion than did infants who interacted more with their caregiver after the negative emotional signal.
Infants’ interaction with their caregiver after the negative versus the neutral emotional signal was marginally related to the latency of the Nc response to the negative object, r = −.345, F(1, 32) = 4.32, p < .05. Infants who interacted more with their caregiver following the negative emotional signal rather than the neutral emotional signal showed a faster Nc response to the negative object than did infants who interacted less with their caregiver following a negative rather than a neutral emotional signal.
Infants’ behavior toward the experimenter
The amplitude of the P500 component at the O2 lead in response to the negative stimulus was predicted by the change in infants’ behavior toward the experimenter between the positive and neutral conditions, r = −.47, F(1, 32) = 8.75, p < .01. Infants who interacted more positively with the experimenter after the positive rather than after the neutral emotional signal showed a larger P500 response to the negative stimulus.
Infants’ proximity to toys
The latency of the Nc component to the positive stimulus at the midline lead FCz was marginally related to the difference in infants’ proximity to the toy after the positive versus the neutral signal, r = .33, F(1, 32) = 4.004, p = .05. Infants who were more likely to manipulate or touch the toy after the positive versus after the negative emotional signal had a faster Nc response to the picture of the toy associated with the positive emotion in ERP testing.
Infants’ proximity to the negative versus neutral toys was also related to the latency of the Nc response to the positive toy at the FCz lead, r = .397, F(1, 32) = 5.998, p < .05. Infants who were less likely to approach the toy after a negative signal rather than after a neutral signal showed a faster Nc response to the positive toy. This pattern also emerged for the ERP response to the picture of the negative stimulus, r = .39, F(1, 32) = 4.16, p = .05. Infants who were less likely to approach or touch the toy after a negative emotional signal rather than after a neutral emotional signal showed a faster Nc peak in response to the negative stimulus.
Infants’ distance to the caregiver
The change in distance between the infant and the caregiver following the positive versus negative emotional signals predicted the latency of the Nc response to the neutral stimulus at the FC1 lead, r = −.34, F(1, 32) = 4.26, p < .05. Infants who moved closer to the caregiver after the negative rather than after the positive emotional signal showed a faster Nc response to the picture associated with neutral emotion.
Relations Between Joint Attention and Behavior Regulation
Significant relations between joint attention and behavior regulation are summarized in Table 4.
Affect
No significant relations between latency to reference and infants’ emotion following the emotional signals were observed.
Interest in toys
No significant relations between infants’ latency to reference and changes in their interest in the toys following the emotional signals were found.
Proximity to toys
Infants’ overall latency to reference across emotional conditions predicted their proximity to and interaction with toys after positive versus neutral emotional signals, r = .42, F(1, 32) = 6.97, p < .02. Infants who referenced an adult faster after presentation of the novel toy were less likely to approach or touch the toy after the positive emotional signal than after the neutral emotional signal. A similar pattern also approached significance in the comparison of infants’ proximity to positive and negative toys, r = .32, F(1, 32) = 3.57, p = .06. Infants who referenced more quickly in the positive condition were marginally less likely to approach or touch the toy following the positive than they were following the negative emotional signal.
Infants’ distance to the caregiver
No significant relations between infants’ latency to reference and the distance maintained between the infants and their caregiver were found.
Infants’ interaction with the caregiver
No significant relations between infants’ latency to reference and their interaction with their caregiver were observed.
Infants’ interaction with the experimenter
A relation was observed between infants’ latency to reference in the neutral condition only and the change in their interaction with the experimenter after the positive and neutral emotional signals, r = −.339, F(1, 32) = 4.157, p = .05. Infants who were faster to look at an adult after seeing the toys interacted more with the experimenter following the positive rather than the negative signal.
Discussion
This study separately examined the behaviors that make up social referencing (joint attention, association, and emotion regulation). Infants showed an increase in ERP activity in response to stimuli associated with negative adult emotion. In addition, this pattern in individual infants was related to their joint attention behavior: Infants who looked at an adult more quickly after presentation of a novel stimulus showed an increase in ERP amplitude to negative stimuli to a greater degree than infants who referenced more slowly. The present results also provide some evidence of a relation between joint attention abilities and behavior regulation. In addition, this study also is the first to show a relation between patterns of brain activation and social referencing in young infants.
An important aspect of this study is that it is the first to show a relation between infants’ ability to associate emotions with novel referents, as indicated by their brain activity, and the other aspects of social referencing, joint attention and emotion regulation. This finding is important because, although many theorists have speculated about the brain basis of early social–cognitive development (e.g., Baron-Cohen, 1991, 1995), few studies have addressed this topic. Furthermore, because of methodological constraints, the majority of the studies of the brain basis of social cognition have focused on a small number of domains (e.g., face processing). Measures of brain activity as it relates to social referencing offer information that measures of behavior alone cannot provide. Measures that require behavioral responses on the part of the infant provide only an indirect indication of the underlying causes of change in infants’ behavior. Because infants cannot communicate through language, we must interpret their behavior and assume that it reflects the processes we expected when we designed the study. ERP activity, in contrast, is a direct physiological measure and, thus, is less susceptible to observer bias and interpretation. In addition, ERP measures will likely have utility for understanding change in the brain basis of social cognition as well as impairments in social cognition (e.g., autism).
The use of ERP responses allowed us to view the functions of the network that is likely to be involved in social referencing. In addition, because ERP is a direct response to a specific stimulus, it can allow us to see the effects of adult emotion on infants’ responses to specific novel objects. Thus, the present method provided us with the ability to examine the second element of social referencing (i.e., association) specifically and separately from the other elements.
Few attempts have been undertaken in the literature to evaluate the specific elements that are involved in social referencing. Many studies have examined only the third element, behavior regulation (Gunnar & Stone, 1984; Mumme & Fernald, 2003; Mumme et al., 1996; but see Carpenter et al., 1998, for a discussion of the relation between joint attention and communicative development). This study represents a first step in relating the elements involved in social referencing to each other. One theoretical position regarding social–cognitive development is that joint attention is a developmental precursor to social referencing (Baron-Cohen, 1991, 1995); however, few, if any, attempts have been made to relate joint attention to either infants’ association of the adult emotional expression with the referent or their behavioral regulation.
Perhaps the most interesting finding from the current study is that, as a group, infants displayed a larger amplitude in the Nc component in response to objects associated with negative than with either positive or neutral emotion. This finding suggests that when infants form associations between novel objects and negative emotional signals, attention networks in the brain are affected. This pattern is consistent with the idea that negative emotion is relatively novel to infants because they see adults display negative emotion much less frequently than positive emotion (de Haan & Nelson, 1998; Nelson, 1993; Nelson & de Haan, 1996). Previous research has provided evidence that infants show larger ERP responses to negative than to positive facial expressions (de Haan & Nelson, 1998; Nelson, 1993). In addition, caregivers are likely to direct more positive than negative emotions toward young infants. Thus, infants may show larger ERP responses to negative expressions because they occur less frequently in the infants’ natural environment. The present results suggest that this effect may carry over to stimuli that are associated with negative emotion as well.
Infants may also show increased attention to the negative stimulus because the caregiver’s response rendered it salient. An evolutionary–biological argument would suggest that infants attend more to stimuli associated with negative caregiver signals because being aware of negative stimuli and avoiding them is in the best interest for the survival of the infant. Infants must be aware of potentially dangerous stimuli to avoid them. Because infants lack an understanding of explicit verbal instructions about what to avoid, a separate mechanism is required to ensure that infants avoid stimuli that might endanger them. Increased attention to stimuli associated with negative parental vocal and facial emotion may provide a mechanism for infants to learn the association between the novel stimulus and potential negative outcomes.
The current data are inconsistent with the alternative prediction that infants’ ERP activity would increase to positively tagged stimuli. In studies of behavior, infants tend to attend more to and interact more with objects associated with positive emotion and less with objects associated with negative emotion. If this pattern had held true in this ERP paradigm, infants would have shown an increase in ERP activity toward the positive stimulus (the object to which the infants preferentially attend behaviorally) and a decrease in the response to the negative stimulus (the object toward which infants behaviorally attend less). The fact that this pattern did not emerge suggests that infants’ ERP activity may not reflect the same thing as their behavior. Infants’ behavior may be determined by a “what to do with this information” response, whereas their ERP activity may be affected by a change in salience that is conferred by the signal, but it may be unrelated to any knowledge of the conveyed emotion as a mechanism by which they can regulate their behavior.
An alternative explanation for these data is that the infants’ representation of the object associated with negative emotion was disrupted because of the negative emotion, but the representations of the positive and neutral object were not disrupted. This interference would lead to the observed pattern of an increase in ERP activity to the negative stimulus, not because of the emotion associated with it per se but because the negative stimulus would be perceived as more novel by the infant. Data from a recent study (Hertenstein & Campos, 2004) suggest that 14-month-olds, but not 11-month-olds, continue to regulate their behavior according to the adult’s emotional displays after a delay of 1 hr. If the 12-month-olds tested here are more like the 11-month-olds tested by Hertenstein and Campos (2004), this finding may provide an explanation for infants’ failures to relate emotion to specific stimuli after a delay. In contrast, if 12-month-olds are more like 14-month-olds, the disruption of the memory trace would less likely explain the current results. A strong conclusion on this point cannot be drawn from the current data, but future studies could address this question using converging measures of memory, attention, and emotion regulation. For example, infants’ memory for the negative stimulus could be measured using looking time or imitation measures. However, including emotional information may also have the effect of interfering with memory with either of those measures as well. Longitudinal or cross-sectional studies in the age range described by Hertenstein and Campos (2004) may also shed light on this issue.
Although the Nc activity that differentiated between conditions appeared to be left lateralized (see Figure 3), the interaction between hemisphere and condition for this component was not statistically significant. Across conditions, the amplitude of the Nc component was larger over the right hemisphere. This pattern is consistent with studies that suggest a right-hemisphere bias in emotion processing and assert that such a bias is at least somewhat established fairly early in development. In addition, some research has suggested that the right hemisphere, especially in the frontal cortex, may be especially sensitive to negative emotion, and the left hemisphere may be especially sensitive to positive emotion (e.g., Davidson & Fox, 1988; Fox, 1991). Although such results were not found here (the effect of hemisphere was across all emotions rather than limited to any specific emotion), the fact that the negative emotion appeared to be more salient might have led to a right-hemisphere bias for the task as a whole. In effect, because of the increased salience of the negative condition, the ERP test could have elicited greater right-hemisphere activation consistent with previous studies of negative affect.
Although a difference between positive, negative, and neutral conditions for the P500 component was expected, we observed no such differences. Previous research has suggested that the P500 component may be responsive to social stimuli (Dawson et al., 2002; de Haan et al., 2002; Halit et al., 2003). However, the present results suggest that this responsiveness may be specific to faces, at least in the population of infants we tested. Further research might be undertaken to reach a definitive conclusion on the function of this ERP component.
Several aspects of infants’ behavior regulation were influenced by the emotional signal. Infants displayed more negative emotion following the negative signal relative to the neutral signal. Two explanations for this finding are plausible. First, infants may have understood the negative emotion and behaved negatively as a consequence. Alternatively, infants’ negative affect after the negative signal may have been a consequence of negative emotional contagion. The present data cannot inform the question of why infants responded with negative emotion following the negative signal. However, the fact that infants’ emotional response to the signal was unrelated to either their latency to look at an adult after presentation of the emotional stimulus or to their ERP response suggests that the emotional response may not be related to social referencing but may be related instead to a more general mechanism such as emotional contagion.
Infants’ interaction with their caregiver was also affected by the emotional valence of the signals. Infants were more likely to interact with their caregiver after a negative than after a neutral signal. This finding is consistent with interpretations from attachment theory. Attachment theory suggests that, in threatening situations, infants rely on the caregiver as a secure base from which to explore the novel object. Infants’ behavior in response to negative emotion in this study suggests that infants use the caregiver more as a secure base when the emotional context is negative. One interesting question for future research is whether this pattern of behavior is a consequence of the infants’ understanding of their caregiver’s negative emotion or simply a reaction to it.
The relation between the three elements involved in social referencing was also examined. Infant joint attention (as indicated by how quickly they referenced an adult) after presentation of the novel stimulus predicted the amplitude of their Nc response to the negative stimulus. Several possible explanations exist for this outcome. First, infants who are faster to reference might have a greater understanding that they should look to adults in novel situations. This interpretation is consistent with theories that suggest that 12-month-olds engage in “real” social referencing in which they reference because they understand the other person as a potential source of information in a novel situation (see Baldwin & Moses, 1996; Carpenter et al., 1998; Moore & Corkum, 1994). Other possible interpretations must be considered, however. Infants who reference quickly may do so because of their temperament. For example, infants who are temperamentally inhibited may be more likely to look at adults when confronted with an ambiguous situation. These infants also may have a greater tendency to attend more to stimuli associated with negative emotion than to positive or neutral emotion. This hypothesis is consistent with recent data suggesting that infants with fearful temperament show a larger Nc response to negative facial expressions versus happy faces over the right hemisphere than infants with a less fearful temperament (de Haan, Belsky, Reid, Volein, & Johnson, 2004). Future studies would benefit by including measures of infant temperament to test this possibility directly.
Another reason for the relation between latency to reference and ERP activity may come from the attachment relationship. Infants who are more securely attached may have a greater inclination to look at the caregiver in particular. These infants may also be more sensitive to adult emotional information, more reliant on the caregiver for information, and thus more easily affected by the adult emotional signal, or may be more vulnerable to any interference effects of the emotional signal on memory for the negative stimulus. A complete understanding of the reason for the relation between how quickly infants reference and their ERP activity requires developmental (longitudinal or cross-sectional) research. If older infants show the same pattern of relations between latency to reference and ERP activity, these findings would support, at least in part, the idea that 12-month-olds’ ERP activity is driven by the same social mechanisms as activity in older infants.
Infants who were faster to reference in the negative condition also showed faster Nc peak latency to the negative stimulus. This result could be interpreted as evidence that joint attention is related to the formation of the association between the adult emotional signal and the novel stimulus. However, the fact that the relation was specific to latency to reference in only the negative condition suggests that the perception of the negative stimulus may have been affected perhaps not by the negative emotional signal but rather by how much time or opportunity the infant had to process it. That is, infants who referenced more quickly in the negative condition may have had more time to process that particular emotional stimulus (because they referenced earlier in the window of time in which the novel object was available). Thus, their faster processing to the negative stimulus may have been related to this increase in processing time rather than to their social referencing behavior.
Relations between infants’ ERP activity and several aspects of their behavior regulation were also found. Infants who were less likely to approach the toy following the negative than following the neutral signal also showed a faster Nc amplitude to the negative toy. This finding may indicate that those infants who effectively regulated their behavior by avoiding the negative toy also encoded that object better and, as a result, processed the picture of it more quickly in the ERP test. In addition, recent evidence (Bauer et al., 2003) has suggested that the latency of the Nc component may reflect reintegration in long-term recognition memory. Thus, it may be that infants who were less likely to approach the toy following the negative signal remembered it less well than the neutral toy and may have a less well-consolidated representation of the negative toy, which could be reflected in a shorter Nc latency (Bauer et al., 2003).
A similar pattern that was marginally significant was observed for the positive toy. Infants who were more likely to approach the toy after presentation of the positive emotional signal showed a faster latency to peak of the Nc component to the positive stimulus. Again, this finding suggests that infants who regulate their behavior more congruently with the emotional signal also may process the stimuli associated with that emotional signal more quickly. On the other hand, in this particular case, infant behavior regulation may also be confounded with the amount of time infants interacted with the object. Infants who were more likely to approach the toy after positive emotion would then also spend more time interacting with the toy. Thus, the finding may be the result of improved processing of the novel stimulus when infants interacted more with it after the emotional signal (although this interpretation is inconsistent with the idea that improved memory is reflected in increased Nc latency discussed above; Bauer et al., 2003). These results suggest a mechanism through which adult emotional signals (both positive and negative) may influence infants’ later behavior through the strength of the association formed between the stimulus and the emotional displays.
Infants’ interactions with the adults involved in the experiment were also related to their ERP activity. Infants who were less likely to interact with their caregiver following the negative but not the neutral stimulus showed a larger P500 response to the negative stimulus. Infants may be less likely to interact with their caregiver in the face of negative emotional information because they are distracted by the negatively labeled toy, and the present result suggests that this pattern of attention may carry over into the postreferencing interval.
A marginally significant relation was also observed between infants’ latency to reference and their behavior regulation. Infants who were faster to reference were less likely to interact with the novel object. This pattern was contrary to what was predicted. Infants who were faster to reference overall were less likely to approach the novel object after the positive emotional signal than after the negative emotional signal. The hypothesis that joint attention is a direct precursor to social referencing would suggest that infants who were faster to reference should be more sophisticated with respect to joint attention development. This should lead to a pattern in which infants who were faster to reference would be more likely to approach an object associated with positive emotion and withdraw from an object associated with negative emotion. Our finding suggests that latency to reference, although related to some degree to behavior regulation, may have a less direct relation than previously thought.
It is interesting that several of the most significant relations between social referencing components were between joint attention and ERP responses and between ERP responses and behavior regulation, rather than between joint attention and behavior regulation. This is despite the fact that the ERP measure was taken at a different time and location than the behavior was measured, and joint attention and behavior regulation were measured in the same procedure. If the effects were due to differences in infants’ responses to the test situation, rather than the manipulation itself, it would seem more likely that the behavioral measures would tie together. This pattern suggests that the ability to formulate an association between the emotion and the referent may be key in the development of social referencing. A complete model of these relations and their development is an important goal for future research.
In summary, the present results suggest some patterns that are consistent with the idea that the elements involved in social referencing emerge together and, in 12-month-olds, reflect infants’ understanding of adults’ emotion and intention to convey that emotion. In addition, the results suggest a relation between the elements involved in social referencing, although the exact nature of that relation is not yet clear.
Several caveats should be considered regarding these results. To compare infants’ responses with respect to all three elements involved in social referencing, we followed a fairly flexible experimental procedure in which emotional signals were dependent on infants’ referencing behavior. Under these conditions, we were interested in individual differences in referencing, association, and behavior regulation because they allowed us to examine relations between the variables. However, a necessary effect of this procedure was that the amount of information and its timing varied across infants. In future studies, social referencing in a highly controlled situation (e.g., Mumme & Fernald, 2003) might be examined in which all infants receive emotional information on a consistent schedule and without requiring an active reference by the infant. In this way, infants’ relation of emotion to novel objects as well as behavior regulation could be more accurately assessed.
An additional limitation of the current study has to do with the effects of testing infants using a within-subjects design. Although this design was necessary to compare infants’ ERP activity between emotional conditions and stimuli were carefully counterbalanced to limit effects of one condition on the others, evidence from previous research asserts that exposure to one emotional signal can affect how infants respond to other emotional signals (Klinnert, 1984; see footnote 1). Because the present study compared brain activity and behavior between different emotional conditions, a between-subjects design was impractical. However, in future studies, measuring behavior and brain responses comparing a single emotional condition with a neutral condition would be an interesting undertaking. This design would help determine whether there are carryover effects for specific elements in social referencing.
This study represents one of the first attempts to directly assess the individual elements involved in social referencing, as well as the relations between them, although relations between some elements (e.g., joint attention) and other cognitive functions (e.g., language development) have been examined previously (Carpenter et al., 1998). Finally, the results of this investigation also offer an indication of patterns of brain function that reveal how 12-month-olds relate emotional information to novel objects. This method could be useful in studies across development as well as in special populations in whom social cognition is problematic (e.g., autism).
The present results highlight further the importance of using converging brain and behavioral measures whenever possible to develop a complete picture of the neurobiological basis for behavior.
Acknowledgments
This research was funded by Grant 1 R21 HD43739 from the National Institutes of Health. This research was approved by the University of California, San Diego, Human Subjects Protection Program and by the State of California Committee for the Protection of Human Subjects.
Footnotes
In some studies, order effects are observed when an emotion in one condition carries forward and affects infants’ behavior in a different emotional condition. We conducted preliminary analyses on all behavioral and ERP variables for infants who saw the positive condition before the negative condition versus those who saw the negative condition before the positive condition. No effect of order on any of the behavioral or ERP data was found (all ps > .10). Details are available from Leslie J. Carver.
This smoothing algorithm removes high-frequency noise from averaged data. We analyzed the data without smoothing and confirmed that the results did not change as a result of the smoothing procedure.
A positive peak appearing 200 ms after stimulus onset appeared in some figures to show different responses between conditions. Preliminary analysis revealed that the difference between conditions for this component was not significant, and that the differences found in the Nc component could not be attributed to different amplitude activity in this component. Details are available from Leslie J. Carver.
References
- Adolphs R, Baron-Cohen S, Tranel D. Impaired recognition of social emotions following amygdala damage. Journal of Cognitive Neuroscience. 2002;14:1264–1274. doi: 10.1162/089892902760807258. [DOI] [PubMed] [Google Scholar]
- Ainsworth MDS. A consideration of social referencing in the context of attachment theory and research. In: Feinman S, editor. Social referencing and the social construction of reality in infancy. Plenum Press; New York: 1992. pp. 349–367. [Google Scholar]
- Baldwin DA, Moses LJ. The ontogeny of social information gathering. Child Development. 1996;67:1915–1939. [Google Scholar]
- Baron-Cohen S. Precursors to a theory of mind: Understanding attention in others. In: Whiten A, editor. Natural theories of mind: Evolution, development, and simulation of everyday mindreading. Blackwell Publishers; Oxford, England: 1991. pp. 233–251. [Google Scholar]
- Baron-Cohen S. Mindblindness: An essay on autism and theory of mind. MIT Press; Cambridge, MA: 1995. [Google Scholar]
- Barr R, Dowden A, Hayne H. Developmental changes in deferred imitation by 6- to 24-month-old infants. Infant Behavior & Development. 1996;19:159–170. [Google Scholar]
- Bauer PJ, Wiebe SA, Carver LJ, Waters JM, Nelson CA. Developments in long-term explicit memory late in the first year of life: Behavioral and electrophysiological indices. Psychological Science. 2003;14:629–635. doi: 10.1046/j.0956-7976.2003.psci_1476.x. [DOI] [PubMed] [Google Scholar]
- Cahill L, Babinsky R, Markowitsch HJ, McGaugh JL. The amygdala and emotional memory. Nature. 1995 Sep 28;377:295–296. doi: 10.1038/377295a0. [DOI] [PubMed] [Google Scholar]
- Carpenter M, Nagell K, Tomasello M. Social cognition, joint attention, and communicative competence from 9 to 15 months of age. Monographs of the Society for Research in Child Development. 1998;63 (Serial No. 255) [PubMed] [Google Scholar]
- Carr L, Iacoboni M, Dubeau M-C, Mazziotta JC, Lenzi GL. Neural mechanisms of empathy in humans: A relay from neural systems for imitation to limbic areas. Proceedings of the National Academy of Sciences. 2003;100:5497–5502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver LJ, Bauer PJ. When the event is more than the sum of its parts: 9-month-olds’ long-term ordered recall. Memory. 1999;7:147–174. doi: 10.1080/741944070. [DOI] [PubMed] [Google Scholar]
- Carver LJ, Bauer PJ. The dawning of a past: The emergence of long-term explicit memory in infancy. Journal of Experimental Psychology: General. 2001;130:726–745. [PubMed] [Google Scholar]
- Carver LJ, Bauer PJ, Nelson CA. Associations between infant brain activity and recall memory. Developmental Science. 2000;3:234–246. [Google Scholar]
- Charman T, Baron-Cohen S, Swettenham J, Baird G, Cox A. Testing joint attention, imitation, and play as infancy precursors to language and theory of mind. Cognitive Development. 2000;15:481–498. [Google Scholar]
- Courchesne E. Neurophysiological correlates of cognitive development: Changes in long-latency event-related potentials from childhood to adulthood. Electroencephalography and Clinical Neurophysiology. 1978;45:468–482. doi: 10.1016/0013-4694(78)90291-2. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Ganz L, Norcia AM. Event-related brain potentials to human faces in infants. Child Development. 1981;52:804–811. [PubMed] [Google Scholar]
- Davidson RJ, Fox NA. Asymmetrical brain activity discriminates between positive and negative affective stimuli in human infants. Science. 1982 Dec 17;218:1235–1237. doi: 10.1126/science.7146906. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Fox NA. Cerebral asymmetry and emotion: Developmental and individual differences. In: Molfese D, Segalowitz S, editors. Brain lateralization in children: Developmental implications. Guilford Press; New York: 1988. pp. 191–206. [Google Scholar]
- Dawson G, Carver LJ, Meltzoff AN, Panagiotides H, McPartland J, Webb SJ. Neural correlates of face and object recognition in young children with autism spectrum disorder, developmental delay, and typical development. Child Development. 2002;73:700–717. doi: 10.1111/1467-8624.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan M, Belsky J, Reid V, Volein A, Johnson MH. Maternal personality and infants’ neural and visual responsivity to facial expressions of emotion. Journal of Child Psychology and Psychiatry. 2004;45:1209–1218. doi: 10.1111/j.1469-7610.2004.00320.x. [DOI] [PubMed] [Google Scholar]
- de Haan M, Nelson CA. Recognition of the mother’s face by six-month-old infants: A neurobehavioral study. Child Development. 1997;68:187–210. [PubMed] [Google Scholar]
- de Haan M, Nelson CA. Discrimination and categorisation of facial expressions of emotion during infancy. In: Slater A, editor. Perceptual development. Psychology Press; Hove, England: 1998. pp. 287–309. [Google Scholar]
- de Haan M, Nelson CA. Brain activity differentiates face and object processing in 6-month-old infants. Developmental Psychology. 1999;35:1113–1121. doi: 10.1037//0012-1649.35.4.1113. [DOI] [PubMed] [Google Scholar]
- de Haan M, Pascalis O, Johnson MH. Specialization of neural mechanisms underlying face recognition in human infants. Journal of Cognitive Neuroscience. 2002;14:199–209. doi: 10.1162/089892902317236849. [DOI] [PubMed] [Google Scholar]
- Dien J. Issues in the application of the average reference: Review, critiques, and recommendations. Behavior Research Methods, Instruments, & Computers. 1998;30:34–43. [Google Scholar]
- Feinman S, Roberts D, Hsieh K-F, Sawyer D, Swanson D. A critical review of social referencing in infancy. In: Feinman S, editor. Social referencing and the social construction of reality in infancy. Plenum Press; New York: 1992. pp. 15–54. [Google Scholar]
- Fox NA. If it’s not left, it’s right: Electroencephalograph asymmetry and the development of emotion. American Psychologist. 1991;46:863–872. doi: 10.1037//0003-066x.46.8.863. [DOI] [PubMed] [Google Scholar]
- Fox NA, Davidson RJ. Electroencephalogram asymmetry in response to the approach of a stranger and maternal separation in 10-month-old infants. Developmental Psychology. 1987;23:233–240. [Google Scholar]
- Gunnar MR, Stone CS. The effects of positive maternal affect on infant responses to pleasant, ambiguous, and fear-provoking toys. Child Development. 1984;55:1231–1236. [Google Scholar]
- Halit H, Csibra G, Volein A, Johnson MH. Face-sensitive cortical processing in early infancy. Journal of Child Psychology and Psychiatry. 2004;45:1228–1234. doi: 10.1111/j.1469-7610.2004.00321.x. [DOI] [PubMed] [Google Scholar]
- Halit H, de Haan M, Johnson MH. Cortical specialisation for face processing: Face-sensitive event-related potential components in 3-and 12-month-old infants. NeuroImage. 2003;19:1180–1193. doi: 10.1016/s1053-8119(03)00076-4. [DOI] [PubMed] [Google Scholar]
- Hertenstein MJ, Campos JJ. The retention effects of an adult’s emotional displays on infant behavior. Child Development. 2004;75:595–613. doi: 10.1111/j.1467-8624.2004.00695.x. [DOI] [PubMed] [Google Scholar]
- Kestenbaum R, Nelson CA. Neural and behavioral correlates of emotion regulation in children and adults. Journal of Experimental Child Psychology. 1992;54:1–18. doi: 10.1016/0022-0965(92)90014-w. [DOI] [PubMed] [Google Scholar]
- Klinnert MD. The regulation of infant behavior by maternal facial expression. Infant Behavior & Development. 1984;7:447–465. [Google Scholar]
- Meltzoff AN. Infant imitation after a 1-week delay: Long-term memory for novel acts and multiple stimuli. Child Development. 1988a;24:470–476. doi: 10.1037/0012-1649.24.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff AN. Infant imitation and memory: Nine-month-olds in immediate and deferred tests. Child Development. 1988b;59:217–225. doi: 10.1111/j.1467-8624.1988.tb03210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C, Corkum V. Social understanding at the end of the first year of life. Developmental Review. 1994;14:349–372. [Google Scholar]
- Mumme DL, Fernald A. The infant as onlooker: Learning from emotional reactions observed in a television scenario. Child Development. 2003;74:221–237. doi: 10.1111/1467-8624.00532. [DOI] [PubMed] [Google Scholar]
- Mumme DL, Fernald A, Herrera C. Infants’ responses to facial and vocal emotional signals in a social referencing paradigm. Child Development. 1996;67:3219–3237. [PubMed] [Google Scholar]
- Mundy P, Card J, Fox NA. EEG correlates of the development of infant joint attention skills. Developmental Psychobiology. 2000;69:325–338. [PubMed] [Google Scholar]
- Mundy P, Fox NA, Card J. EEG coherence, joint attention, and language development in the second year. Developmental Science. 2003;6:48–54. [Google Scholar]
- Nelson CA. The recognition of facial expression in infancy: Behavioral and electrophysiological evidence. In: De Boysson-Bardies B, editor. Developmental neurocognition: Speech and face processing in the first year of life. Kluwer Academic; Dordrecht, the Netherlands: 1993. pp. 187–198. [Google Scholar]
- Nelson CA. The ontogeny of human memory: A cognitive neuroscience perspective. Developmental Psychology. 1995;31:723–738. [Google Scholar]
- Nelson CA, de Haan M. Neural correlates of infants’ visual responsiveness to facial expressions of emotion. Developmental Psychobiology. 1996;29:577–595. doi: 10.1002/(SICI)1098-2302(199611)29:7<577::AID-DEV3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Nelson CA, Nugent KM. Recognition memory and resource allocation as revealed by children’s event-related potential responses to happy and angry faces. Developmental Psychology. 1990;26:171–179. [Google Scholar]
- Nikkel L, Karrer R. Differential effects of experience on the ERP and behavior of 6-month-old infants: Trends during repeated stimulus presentations. Developmental Neuropsychology. 1994;10:1–11. [Google Scholar]
- Richards JE. Attention affects the recognition of briefly presented visual stimuli in infants: An ERP study. Developmental Science. 2003;6:312–328. doi: 10.1111/1467-7687.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz GM, Izard CE, Ansul SE. The 5-month-old’s ability to discriminate facial expressions of emotion. Infant Behavior & Development. 1985;8:65–77. [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004 Feb 20;303:1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Slaughter V, McConnell D. Emergence of joint attention: Relationships between gaze following, social referencing, imitation, and naming in infancy. The Journal of Genetic Psychology. 2003;164:54–71. doi: 10.1080/00221320309597503. [DOI] [PubMed] [Google Scholar]
- Sorce JF, Emde RN, Campos JJ, Klinnert MD. Maternal emotional signaling: Its effect on the visual cliff behavior of 1-year-olds. Developmental Psychology. 1985;20:195–200. [Google Scholar]
- Stone VE, Baron-Cohen S, Knight RT. Frontal lobe contributions to theory of mind. Journal of Cognitive Neuroscience. 1998;10:640–656. doi: 10.1162/089892998562942. [DOI] [PubMed] [Google Scholar]
- Thomas KM, Drevets KM, Whalen PJ, Eccard CH, Dahl RE, Ryan ND, Casey BJ. Amygdala response to facial expression in children and adults. Biological Psychiatry. 2001;49:309–316. doi: 10.1016/s0006-3223(00)01066-0. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Borscheid A, Ellertsen K, Marcus D, Nelson CA. Categorization of facial expression in children and adults: Establishing a larger stimulus set. Poster session presented at the annual meeting of the Cognitive Neuroscience Society; San Francisco, CA: Apr, 2002. [Google Scholar]