Abstract
Bone destruction is a common feature of inflammatory arthritis and is mediated by osteoclasts, the only specialized cells to carry out bone resorption. Aberrant expression of receptor activator of nuclear factor kappa β ligand (RANKL), an inducer of osteoclast differentiation has been linked with bone pathology and the synovial fibroblast in rheumatoid arthritis (RA). In this manuscript, we challenge the current concept that an increase in RANKL expression governs osteoclastogenesis and bone destruction in autoimmune arthritis.
We isolated human fibroblasts from RA, pyrophosphate arthropathy (PPA) and osteoarthritis (OA) patients and analyzed their RANKL/OPG expression profile and the capacity of their secreted factors to induce osteoclastogenesis. We determined a 10-fold increase of RANKL mRNA and protein in fibroblasts isolated from RA relative to PPA and OA patients. Peripheral blood mononuclear cells (PBMC) from healthy volunteers were cultured in the presence of RA, PPA and OA synovial fibroblast conditioned medium. Osteoclast differentiation was assessed by expression of tartrate-resistant acid phosphatase (TRAP), vitronectin receptor (VNR), F-actin ring formation and bone resorption assays. The formation of TRAP+, VNR+ multinucleated cells, capable of F-actin ring formation and lacunar resorption in synovial fibroblast conditioned medium cultures occured in the presence of osteoprotegerin (OPG) a RANKL antagonist. Osteoclasts did not form in these cultures in the absence of macrophage colony stimulating factor (M-CSF).
Our data suggest that the conditioned medium of pure synovial fibroblast cultures contain inflammatory mediators that can induce osteoclast formation in human PBMC independently of RANKL. Moreover inhibition of the TNF or IL-6 pathway was not sufficient to abolish osteoclastogenic signals derived from arthritic synovial fibroblasts. Collectively, our data clearly show that alternate osteoclastogenic pathways exist in inflammatory arthritis and place the synovial fibroblast as a key regulatory cell in bone and joint destruction, which is a hallmark of autoimmune arthritis.
Keywords: Synovial fibroblast, Arthritis, RANKL, Osteoclast, Rheumatoid arthritis
1. Introduction
Synovial fibroblasts, including type B synoviocytes, are mesenchymal, nonvascular, nonepithelial, CD45-negative cells that display heterogeneous tissue localization (intimal and subintimal) [1,2]. The physiological function of the synovial fibroblast is to provide nutrients for cartilage and to produce proteoglycans that lubricate the articular surfaces [3]. The synovial fibroblast has been closely associated with joint pathology in rheumatic diseases, as aberrant cytokine production often leads to bone and cartilage destruction [4,5]. Isolated human RA synovial fibroblasts can induce arthritis upon transfer to the knee of healthy severe combined immunodeficiency (SCID) mice even in the absence of a functioning adaptive immune system [6]. T and B cell independent induction of arthritis is not dependent on cell-contact mechanisms but on secretion of soluble-factors, namely RANKL, IL-6, and TNF [6–9]. In these experiments we investigated the effect of secreted factors from human fibroblasts isolated from inflammatory arthritis patients, including RA, pyrophosphate arthropathy (PPA) and osteoarthritis (OA). The ability of these isolated fibroblasts to directly induce osteoclastogenesis was evaluated using in vitro osteoclast functional assays.
Bone destruction is a common feature of inflammatory arthritis and is mediated by osteoclasts, the only specialized cells that carry out bone resorption [10,11]. Osteoclast precursors express the receptor activator for nuclear factor-κB (RANK) and differentiate into mature functional osteoclasts in the presence of macrophage colony stimulating factor (M-CSF) and receptor activator for nuclear factor-κB ligand (RANKL) [12,13]. Once terminally differentiated, the mature osteoclast is a multinucleated cell that attaches onto the bone surface and forms a sealing zone with the aid of vitronectin receptor (VNR), in which it acidifies the extracellular matrix using a proton pump and with the aid of enzymes such as tartrate-resistant acid phosphatase (TRAP), matrix metallopeptidase 9 (MMP9) and Cathepsin K (CTSK) resorbs bone [10,14,15]. Activation of the RANKL pathway is inhibited by osteoprotegerin (OPG), a soluble decoy receptor for RANKL. Both RANKL and OPG are expressed in RA synovial tissue and disturbance in the RANKL/OPG ratio is associated with the formation of marginal erosions [16–18]. Increased RANKL/OPG ratio in synovial fluid may result in increased osteoclast differentiation of synovial macrophages present in inflamed joint tissues [18–20]. In addition to RANKL, the release of other pro-inflammatory factors have been linked with pathology of the joints. CD14+ human peripheral blood mononuclear cells (PBMC) cultured with IL-6, IL-11 or TNF induced the formation of multinucleated cells which were positive for TRAP, VNR and CTSK and showed evidence of experimental bone resorption, although at much lower levels than RANKL [21–23]. Additionally, TNF and IL-6 have been shown to induce osteoclast formation in both mononuclear precursor (CD14+/CD14−) and macrophage (CD14+) synovial cell populations [24,25].
A strong correlation exists between the extent of macrophage infiltration and the degree of bone destruction that occurs in arthritis [26]. Therefore a complex interplay between synovial fibroblasts and synovial macrophages may regulate the amount of osteoclastogenesis and subsequently the amount of bone destruction [27]. In this study, we analyzed the capacity of arthritic synovial fibroblasts to support osteoclast formation and we investigated the osteoclastogenic pathways by blocking common effectors and transducers of the RANKL, TNF and IL-6 signaling pathways.
2. Materials and methods
2.1. Materials
For all incubations α-minimal essential medium (MEM) (Invitrogen, USA) was supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 10 mM l-glutamine (Invitrogen, USA) and 10% fetal bovine serum (FBS; Invitrogen, USA). Recombinant human M-CSF (25 ng/ml), RANKL (30 ng/ml), OPG (100 ng/ml), anti-TNF (MAB 210, clone, 1825) and anti-gp130 (MAB 628, clone, 28105) up to (50 μg/ml) were obtained from R & D Systems (USA). β-actin monoclonal antibody was purchased from Abcam (Cambridge, UK), monoclonal mouse anti-human proline-4-hydroxylase clone 5B5 and monoclonal mouse anti-human vimentin clone V9 were purchased from Dako (Carpenteria, CA, USA). All other antibodies were purchased from R & D Systems (USA). All incubations were carried out at 37° C in 5% CO2.
2.2. Samples
2.2.1. Patients
Fresh specimens of the synovial membrane were obtained from 12 patients undergoing total knee replacement, including four patients with OA (age 62–78 years), five patients with RA (age 51–70 years), and three patients with PPA (age 63–75 years). The diagnosis of OA, RA and PPA was established clinically and by radiological and laboratory investigations as previously described [23].
2.2.2. Isolation and long-term culture of synovial fibroblasts
The cell suspension derived from synovial tissues was prepared as described previously [23]. Briefly, tissue specimens werewashed thoroughly with PBS, cut into small fragments, and digested in MEM containing 1 mg/ml collagenase for 30 min at 37° C; this was followed by 1 h incubation in Versene (Invitrogen, USA). The digested tissue was filtered with a 70 μm cell strainer (Falcon, USA) and the filtrate centrifuged at 800 g for 10 min. The cell pellet was re-suspended in MEM/FBS and then placed in Falcon tissue culture flasks. The medium was changed after 24 h and then at 3–5 day intervals. Cultures were characterized after 5 passages.
2.2.3. Isolation and culture of human PBMCs
PBMCs were isolated from the peripheral blood of healthy male subjects aged 25 and 35 years. Blood was collected and diluted 1:1 in MEM, layered over Histopaque (Sigma–Aldrich, USA), centrifuged at 1800 rpm for 15 min, washed, and then re-suspended in MEM/FBS. The cells in the resultant suspension of PBMCs were counted using a haemocytometer after lysis of red blood cells using a 5% (v/v) acetic acid solution. The cell suspension of human PBMCs (5 × 105 cells/well) was added to 96 well culture plates containing dentine slices and coverslips. After a 2 h incubation, dentine slices and coverslips were removed from the wells and washed vigorously in MEM/FBS to remove non-adherent cells; they were then placed in a 24 well culture plate containing 1 ml of MEM/FBS supplemented with 25 ng/ml M-CSF.
This is an established method of selecting for adherent cells and ensures that approximately 95% of the cells are CD14+ [23].
2.3. Immunohistochemistry
The synovial membrane was fixed in formalin and embedded in paraffin wax. 5 μm sections were cut, dewaxed, and rehydrated by successive immersion in xylene, graded ethanol and water, followed by microwave treatment (700 W, 2 × 4 min) in target retrieval solution (Dako, Carpenteria, USA). Sections were stained using an indirect immunoperoxidase technique. Endogenous peroxidase was blocked by 0.3% (v/v) hydrogen peroxide and protein block serum (Dako). Sections were then incubated with mouse-anti-human RANKL monoclonal antibody (1:100 in PBS) or mouse-anti-human OPG monoclonal antibody (1:100 in PBS). Antigens were detected by incubation with labeled polymer and diaminobenzidine (Envision+ kit; Dako). The sections were then counterstained with hematoxylin, dehydrated, cleared, and mounted. Negative controls consisted of sections stained with primary antibody diluent alone and substitution of a control isotype IgG antibody (Jackson Immunoresearch, USA).
2.4. Western blotting
Total cell lysate was prepared from sub-confluent (90%) cells harvested in ice-cold PBS and lysed in lysis buffer (LB: 10 mM Tris–HCl, pH 7.4, 1% Triton X-100, 0.5% Nonidet P-40,150 mM NaCl, 0.2 mM sodium orthovanadate, 1 mM Phenylmethyl sulphonyl fluoride (PMSF), 40 g/ml leupeptin and 45 g/ml aprotinin). 25 μg of total cell protein extract were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). After membrane transfer (Hybond-P, Amersham Pharmacia Biotech, Little Chalfont, UK) proteins of interest were detected with antibodies and reactive species were visualized using an enhanced chemiluminescence (ECL) kit (Pierce). Blots for further probing were stripped in 62.5 mM Tris–HCl, pH 6.7, containing 2% SDS and 100 mM β-mercaptoethanol, for 30 min at 50° C. Immunoreactive bands were analyzed by densitometry using ImageJ software (National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/index.html).
2.5. qRT-PCR
RNA was extracted from synovial fibroblast cultures using a commercially available kit (Qiagen, UK). cDNA was synthesized using random hexamers as recommended by the manufacturer (Invitrogen, UK). qRT-PCR was performed in a Rotor-gene 3000 (Corbett Research, Sydney, Australia). qRT-PCR reactions for RANKL and OPG were performed in a total volume of 20 μl, containing 10 μl of SYBR® Green PCR Master Mix (Applied Biosystems, CA, USA), 20 pmol of forward and reverse primer and 1 μl of cDNA. The PCR reaction commenced with an initial denaturing step at 95 °C for 10 min followed by 50 cycles of 95°C for 30 s, 57 °C for 30 s, 72 °C for 30 s, and finally a melting curve starting at 60 °C for 30 s and then raising to 99 °C by increasing one degree every 5 s. Hypoxanthine phosphoribosyltransferase (HPRT) was used as a housekeeper gene. Reactions were performed in a total volume of 10 μl, consisting of 5 μl universal qPCR mastermix (Eurogentec, Hampshire, UK), 0.5 μl of Assay on Demand (Applied Biosystems, CA, USA) and 1 μl of cDNA template. The PCR commenced with a 50 °C incubation for 2 min, denatured at 95 °C for 10 min followed by 45 cycles of 95 °C for 15 s and 60 °C for 60 s, using the following primers: RANKL Forward 5′-CAGATGGATCCTAATAGAAT-3′, Reverse 5′-ATGGGAACCAGATGGGATGTC-3′, OPG, Forward 5′ATGAACAA GTTGCTG TGCTG-3′, Reverse, 5′-GCAGAACTCTATCTCAAGGTA-3′. All samples were used in triplicate and a two standard curve method was used to normalize the data.
2.6. Effect of synovial fibroblast conditioned medium on osteoclast formation
In order to determine whether soluble-factors secreted by isolated synovial fibroblasts can induce osteoclast formation, PBMCs were cultured in the presence of M-CSF with various combinations of OPG anti-TNF and anti-gp130. 20% Conditioned media (CM) derived from cultured OA, RA, or PAA synovial fibroblastswas added to the cultures and maintained for 14 or 21 days in the presence or absence of the factors as indicated below. The culture medium and added factors were replenished every 3 days for each CM.
M-CSF (negative control).
M-CSF+ RANKL (positive control).
20% CM.
M-CSF + 20% CM.
M-CSF + 20% CM + OPG + anti-TNF + anti-gp130.
2.7. Cytochemical and functional evidence of osteoclast differentiation
After 14 days of incubation, human PBMC and synovial fibroblast co-cultures on coverslips were characterized histochemically for TRAP [28] expression using a commercially available kit (Sigmae–Aldrich Chemicals, USA). Coverslips were also stained immunohistochemically by an indirect immunoperoxidase technique with the monoclonal antibodies JML-H14 and 23C6 (Serotec); these antibodies are directed, respectively, against CD14, a monocyte/macrophage-antigen that is not expressed by osteoclasts, and CD51, VNR that is an osteoclast-associated antigen [29,30]. Presence of the vimentin intermediate filaments and the fibroblast-associated antigen, were detected by immunoperoxidase staining of synovial fibroblasts using the monoclonal antibodies, V9 and 5B5, respectively (Dako, UK). The presence of T cells and B cells, were also evaluated by the use of CD3, and CD20 antibodies, respectively (Dako, UK).
Functional evidence of osteoclast formation was determined by a lacunar resorption assay using cell cultures on dentine slices. This provides a smooth-surfaced mineralized substrate for the assessment of lacunar resorption. After cells had been cultured on dentine slices for 21 days, the slices were removed from the wells, placed in 1Mammonium hydroxide for 2 h and cleaned by ultrasonication to remove adherent cells. The slices were then washed in distilled water, stained with 0.5% toluidine blue and examined by light microscopy. The surface of each dentine slice was examined for evidence of lacunar resorption. Osteoclast morphology and percentage area of lacunar resorption was estimated by scanning electron microscopy as previously described [23].
2.8. Statistical significance
The extent of lacunar resorption was expressed as the percentage of surface area resorbed ± standard error of the mean. Each experiment was carried out in triplicate. Statistical significance was determined using Student's t-test and p-values lower than 0.05 were considered significant.
3. Results
3.1. Phenotypic characteristics of synovial fibroblasts
Synovial membrane isolated from RA and PPA patients stained positive for both RANKL and OPG, whereas synovial membrane isolated from OA were weakly positive (Fig. 1a–i). Synovial membrane explants of these patients were cultured and a heterogeneous population of mainly spindle-shaped mononuclear cells was isolated. Multinucleated cells were not present in these cultures. After reaching confluence the cells were passaged 5 times, before being characterized phenotypically. The synovial fibroblast cultures were negative for adaptive immune T and B cells, CD3 and CD20, cells (Fig. 2a–i). These cells were also negative for CD14, VNR, and TRAP (data not shown), and positive for vimentin and the fibroblast-associated antigen, prolyl-4-hydroxylase (Fig. 2j–o).
Fig. 1.
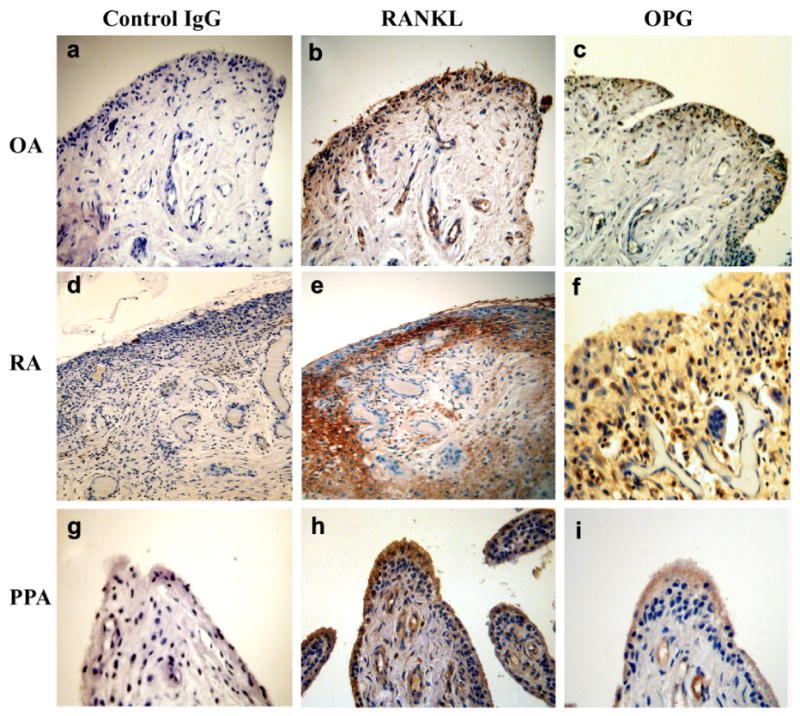
Immunohistochemistry of serial sections of synovial tissue from OA, RA and PPA patients showing RANKL and OPG expression. OA (a) control (b) RANKL (c) OPG, RA (d) control (e) RANKL (f) OPG and PPA (g) control (h) RANKL (i) OPG. (Immunoperoxidase: Original magnification (a, b, c, d, e, g, h, i), ×200 and (f) ×300.
Fig. 2.
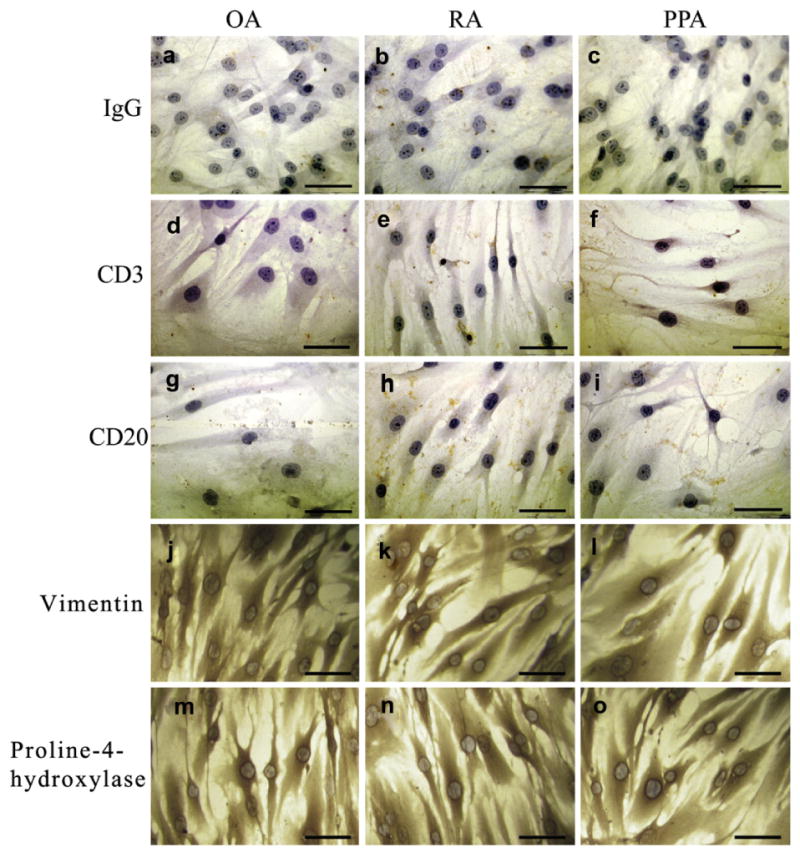
Immunohistochemistry of isolated synovial fibroblasts. Fibroblasts cultured on coverslips showing the (a–c) IgG control and lack of expression of (d–f) the T cell marker CD3 and (g–i) the B cell marker CD20 and positive expression of (j–l) vimentin and (m–o) proline-4-hydroxylase. Scale bar represents 50 μm.
3.2. RANKL and OPG mRNA and protein expression by synovial fibroblasts
To investigate the capacity of the isolated synovial fibroblasts to induce osteoclastogenic signals we evaluated RANKL and OPG mRNA expression by qRT-PCR. Since OPG is a decoy receptor for RANKL and would therefore influence osteoclastogenesis we examined the relationship between these genes by comparing the RANKL/OPG ratio. In inflammatory arthritis, an increased RANKL/ OPG ratio has been observed in the synovial tissue [18]. We observed increased RANKL expression in RA-synovial fibroblasts, which was 10-fold higher than that of OA-derived fibroblasts, and a 3-fold increase in RANKL expression in PPA compared to OA-derived samples. Overall the RANKL/OPG ratio was increased in RA fibroblasts compared to OA or PPA fibroblasts (Fig. 3a). We investigated the protein expression of RANKL and OPG on isolated synovial fibroblasts cultured on coverslips using immunohistochemistry (data not shown). These results were confirmed by Western blotting analysis that showed OPG and RANKL protein is synthesized by synovial fibroblasts (Fig. 3b,c).
Fig. 3.
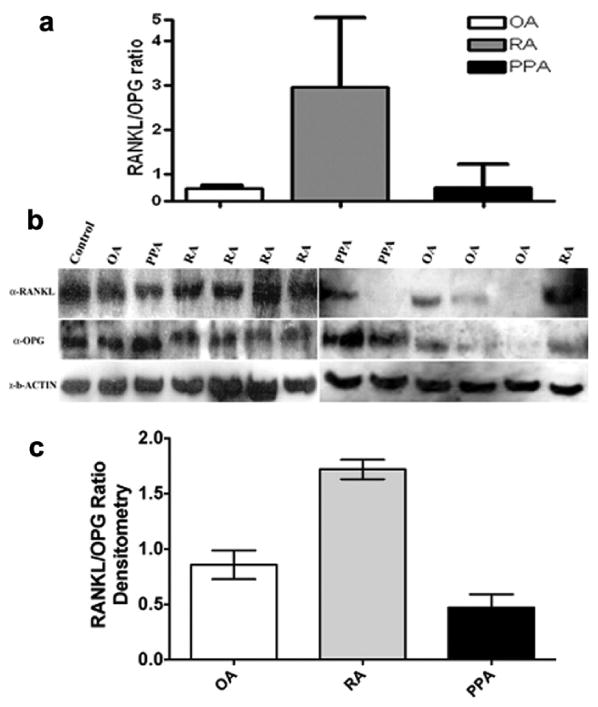
RANKL/OPG expression in OA, RA, and PPA. (a) Normalized average data of the mRNA RANKL/OPG ratio for OA, RA, and PPA. (b) Western blotting analysis of synovial fibroblast total cell lysates showed expression of RANKL and OPG protein. A human osteosarcoma cell line MG63 known to express RANKL and OPG was used as a positive control. (c) Densitometric analyses of bands for RANKL and OPG were obtained using ImageJ software, and results were normalized to basal level.
3.3. Synovial fibroblast conditioned medium supports osteoclast formation
In control cultures where PBMC were incubated with M-CSF for 21 days, there were no multinucleated VNR+ or/and TRAP+ cells. Evidence of lacunar resorption was also not present on dentine slices in these cultures. Similarly there was no observed osteoclastogenesis in PBMC cultures incubated with synovial fibroblast CM in the absence of M-CSF.
In contrast, TRAP+ cells were observed in PBMC cultures on glass coverslips, after 16 days of incubation in synovial fibroblast CM and M-CSF (Fig. 4a–d). We observed that the number of multinucleated TRAP cells was higher in the RA and PPA synovial fibroblast CM compared toOAcontrols. However TRAP expression is not a defining osteoclast marker since macrophage polykaryons, immature dendritic cells, and mononuclear macrophages also express TRAP [23,28,31]. In the lack of a unique osteoclast antigen we also analyzed the expression of VNR+ multinucleated cells capable of F-actin ring formation in PBMC cultures on glass coverslips, after 21 days of incubation in synovial fibroblast CM and M-CSF (Fig. 4e–g).
Fig. 4.
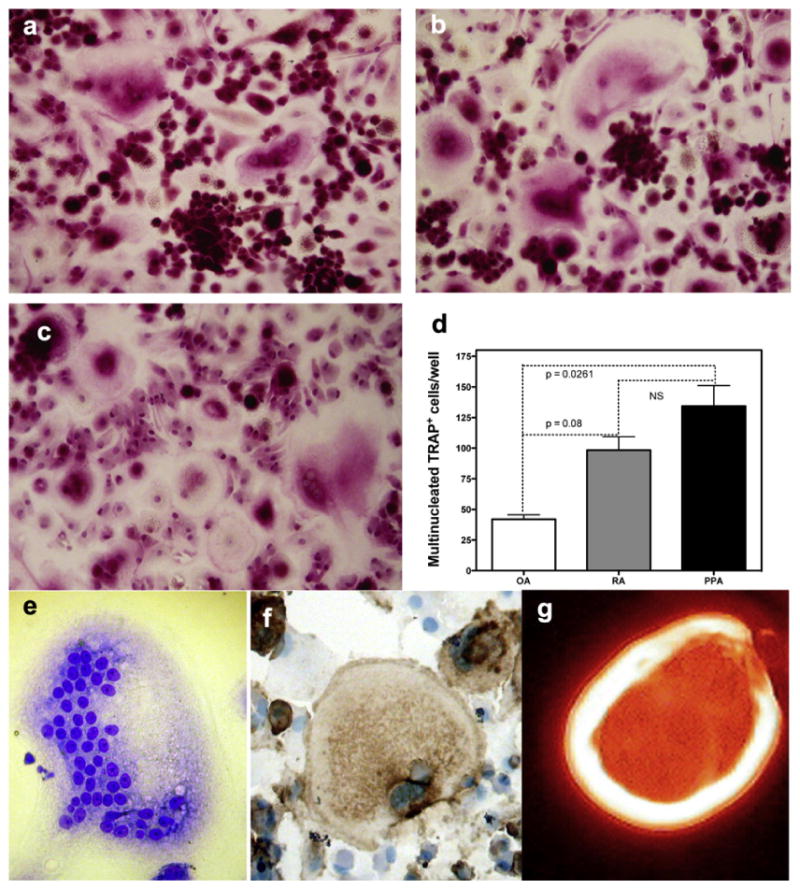
TRAP cytochemical stain images of human PBMC cultured for 16 days in the presence of M-CSF and conditioned medium from a) OA, b) RA and c) PPA and results expressed d) graphically, (p < 0.05 Manne–Whitney nonparametric test, error bars standard error of the mean) data pooled from 3 experiments. Osteoclasts were also determined by the presence of multinucleated cells (e) expressing the specific osteoclast markers (f) VNR, and capable of (g) F-actin ring formation. (Representative pictures of at least three experiments M-CSF and CM OA, RA and PPA cultures that induced osteoclast formation).
The only unique characteristic of an osteoclast is its ability to resorb bone and parallel cultures of synovial fibroblast CM and M-CSF on dentine slices showed extensive lacunar resorption pit formation after 21 days of incubation (Fig. 5a, b). The mean percentage area of bone resorption was between 0.1 and 2.0% of the total area in all cases of OA, RA, and PPA in conditioned medium experiments (Fig. 5c).
Fig. 5.
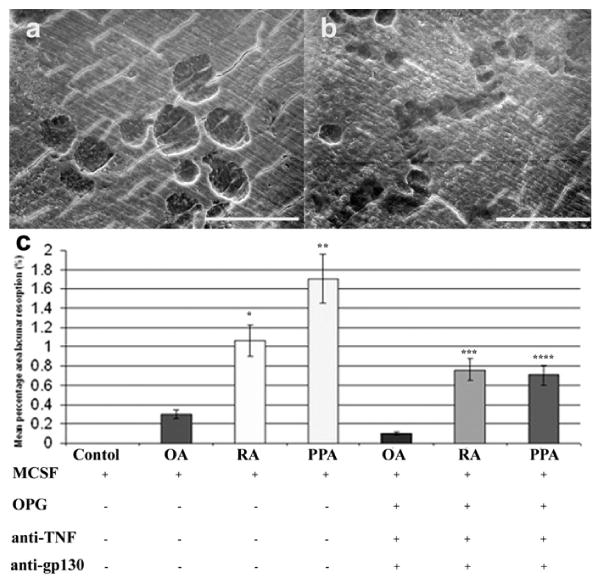
Scanning electron micrographs of dentine slices after PBMC culture for 21 days in the presence of M-CSF, OPG, and CM of cultured arthritic synovial fibroblasts. Fibroblasts isolated from (a) OA and (b) RA showed evidence of lacunar resorption formation. (Image representative of average lacunar resorption formation.) (c) Mean percentage area of lacunar resorption in OA, RA, and PPA conditioned medium in cultures of PBMCs incubated for 21 days in the presence of M-CSF, with or without OPG, anti-TNF, and anti-gp130. Scale bars represent 100 μm. (Representative data of at least three experiments.)
3.4. Effect of OPG, anti-TNF and anti-gp130 antibody on synovial fibroblast-induced osteoclast formation
To identify whether osteoclast formation induced by synovial fibroblast CM occurred by a RANKL-dependent/independent mechanism, PBMC cultures incubated in the presence of M-CSF and CM from OA, RA, and PPA synovial fibroblasts were also treated with OPG, anti-TNF, and anti-gp130. We measured lacunar resorption on dentine slices and found that although reduced, resorption was not completely abolished by the addition of OPG, anti-TNF antibody, and anti-gp130 in any of the synovial fibroblast CM cultures (Fig. 5c).
4. Discussion
In this study we investigated the effect of secreted factors from human fibroblasts isolated from RA, PPA and OA patients. The ability of these fibroblasts to directly induce osteoclastogenesis was evaluated using in vitro osteoclast functional assays. Analysis of RANKL and OPG expression in OA, RA and PPA synovium revealed that RANKL was more highly expressed in synovium isolated from inflammatory arthritis (RA and PPA) as compared to noninflammatory OA. Therefore the extent of inflammatory infiltrate in the synovium directly correlates with the expression of RANKL. Inflammatory mediators from T cells, such as IL-17 induce RANKL expression in stromal cells, and Th17 cells directly secrete RANKL [32–34]. Therefore, to study the contribution of synovial fibroblasts in RANKL expression we employed long-term pure fibroblast cultures devoid of T and B cells.
By qRT-PCR analysis of purified long-term synovial fibroblast cultures, we showed that RANKL mRNA expression was 10-fold higher in RA samples and 3-fold higher in PPA samples, whilst at the same time the average OPG expression was not significantly different between pathology groups. We also confirmed by immunohistochemistry and Western blotting higher RANKL protein expression in RA and PPA than OA, whereas again expression of OPG was not significantly different between pathology groups. Interestingly, although the amount of inflammatory infiltrate is similar in the RA and PPA synovium, RANKL expression is higher in RA. RA and PPA synovial fibroblasts may exhibit distinct expression profiles due to intrinsic functional differences or more likely due to the nature and composition of the inflammatory infiltrate in their anatomical microenvironment.
To evaluate any functional significance of the discrepancy observed between RA and PPA synovial fibroblasts we performed in vitro functional osteoclast assays. In these experiments we determined whether isolated synovial fibroblasts from RA and PPA equally stimulate osteoclastogenesis. Synovial fibroblast conditioned medium isolated from both RA and PPA patients was able to support osteoclast formation in human PBMC cultures in the presence of M-CSF. These cultures resulted in the formation of TRAP+ and VNR+ cells capable of F-actin ring formation that showed evidence of lacunar resorption. Suprisingly, the CM-induced osteoclastogenesis observed was not abolished with addition of OPG at doses as high as 100 ng/ml. Unexpectedly subsequent addition of OPG at high doses (up to 500 ng/ml) did not inhibit osteoclastogenesis. Our data indicate that although the RANKL/OPG ratio is indeed higher in RA than in PPA this is not so significant as it is not the only pathway of osteoclast formation. We reasoned that other factor(s) may be present in synovial fibroblast CM that induce osteoclast formation independently of the RANKL pathway.
RANKL-independent mechanisms of osteoclast formation have been described, whereby TNF can directly induce NF-κB and substitute for RANKL [22,23,35]. Fibroblasts derived from different anatomical locations and different disease conditions express TNF [36,37]. In RA synovial fibroblasts TNF also induces OPG expression thereby blocking RANKL-dependent osteoclastogenesis [38]. To block TNF stimulation of osteoclastogenesis we neutralized TNF in our synovial fibroblast cultures. The addition of anti-TNF antibody alone minimally inhibited osteoclastogenesis in our study.
Bone marrow stromal fibroblasts also express IL-6 and GM-CSF in the absence of inflammatory signals [39]. GM-CSF can promote osteoclastogenesis via transiently increasing M-CSF mRNA expression, whereas long exposure of GM-CSF strongly induces DC differentiation from monocytes [40–42]. In our in vitro system we did not see osteoclast formation in the absence of M-CSF so the function of GM-CSF should be negligible. However, various interleukins including IL-6 and IL-11, are known to stimulate osteoclast formation and resorption and can be produced and secreted by synovial fibroblasts [21,39,43–45]. IL-6 cytokine family shares a common ß -receptor, known as gp130, a signal-transducing receptor. In this receptor system, a ligand/receptor complex associates with gp130 to induce homodimerization. Formation of the gp130 homodimer activates kinases and transduces signals to the nucleus [46–49]. To inhibit the actions of IL-6 and block the downstream activation of gp130 we treated PBMC cultures with M-CSF and synovial fibroblast CM in the presence of an anti-gp130 antibody. Again the addition of anti-gp130 antibody alone minimally inhibited osteoclastogenesis in our study. The combination of anti-TNF anti-gp130, and OPG substantially reduced bone resorption, but did not completely abolish it (Fig. 5c). Therefore, it is evident that other cytokines and growth factors not yet identified act directly on osteoclast differentiation and are responsible for bone destruction in arthritis. Our data indicates that proosteoclastogenic conditions are prominent within the microenvironment of the inflamed arthritic tissue and are independent of the RANKL, TNF and IL-6 signaling pathways.
As continuously novel cytokines and growth factors are being discovered and alternate osteoclastogenic pathways in inflammatory arthritis are rapidly emerging [50,51] the synovial fibroblast may well be the key regulatory cell in bone and joint destruction, which is a hallmark of autoimmune arthritis.
Acknowledgments
Authors wish to thank Nick Athanasou for providing the samples and pathology expertise and Carles Villarino Guell for technical assistance on qRT-PCR and data analysis. IEA is the recipient of the Sontag Fellowship of Arthritis National Research Foundation. Research was partly supported by NIAMS R01 AR062173-01 to IEA.
Abbreviations
- RANKL
receptor activator for nuclear factor- κB ligand
- RA
rheumatoid arthritis
- PPA
pyrophosphate arthropathy
- OA
osteoarthritis
- OPG
osteoprotegerin
- qRT-PCR
quantitative real time-PCR
- PBMC
peripheral blood mononuclear cells
- TRAP
tartrate-resistant acid phosphatase
- VNR
vitronectin receptor
- CM
conditioned medium
- M-CSF
macrophage colony stimulating factor
References
- 1.Aidinis V, Plows D, Haralambous S, Armaka M, Papadopoulos P, Kanaki MZ, et al. Functional analysis of an arthritogenic synovial fibroblast. Arthritis Res Ther. 2003;5:R140–57. doi: 10.1186/ar749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edwards JC. Fibroblast biology. Development and differentiation of synovial fibroblasts in arthritis. Arthritis Res. 2000;2:344–7. doi: 10.1186/ar110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muller-Ladner U, Gay RE, Gay S. Activation of synoviocytes. Curr Opin Rheumatol. 2000;12:186–94. doi: 10.1097/00002281-200005000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Pap T, Muller-Ladner U, Gay RE, Gay S. Fibroblast biology. Role of synovial fibroblasts in the pathogenesis of rheumatoid arthritis. Arthritis Res. 2000;2:361–7. doi: 10.1186/ar113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kontoyiannis D, Kollias G. Fibroblast biology. Synovial fibroblasts in rheumatoid arthritis: leading role or chorus line? Arthritis Res. 2000;2:342–3. doi: 10.1186/ar109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lehmann J, Jungel A, Lehmann I, Busse F, Biskop M, Saalbach A, et al. Grafting of fibroblasts isolated from the synovial membrane of rheumatoid arthritis (RA) patients induces chronic arthritis in SCID mice-A novel model for studying the arthritogenic role of RA fibroblasts in vivo. J Autoimmun. 2000;15:301–13. doi: 10.1006/jaut.2000.0435. [DOI] [PubMed] [Google Scholar]
- 7.Hata H, Sakaguchi N, Yoshitomi H, Iwakura Y, Sekikawa K, Azuma Y, et al. Distinct contribution of IL-6, TNF-alpha, IL-1, and IL-10 to T cell-mediated spontaneous autoimmune arthritis in mice. J Clin Invest. 2004;114:582–8. doi: 10.1172/JCI21795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402:304–9. doi: 10.1038/46303. [DOI] [PubMed] [Google Scholar]
- 9.Kurihara N, Bertolini D, Suda T, Akiyama Y, Roodman GD. IL-6 stimulates osteoclast-like multinucleated cell formation in long term human marrow cultures by inducing IL-1 release. J Immunol. 1990;144:4226–30. [PubMed] [Google Scholar]
- 10.Athanasou NA. Cellular biology of bone-resorbing cells. J Bone Joint Surg Am. 1996;78:1096–112. doi: 10.2106/00004623-199607000-00016. [DOI] [PubMed] [Google Scholar]
- 11.Alexander GM, Dieppe PA, Doherty M, Scott DG. Pyrophosphate arthropathy: a study of metabolic associations and laboratory data. Ann Rheum Dis. 1982;41:377–81. doi: 10.1136/ard.41.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–76. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 13.Quinn JM, Elliott J, Gillespie MT, Martin TJ. A combination of osteoclast differentiation factor and macrophage-colony stimulating factor is sufficient for both human and mouse osteoclast formation in vitro. Endocrinology. 1998;139:4424–7. doi: 10.1210/endo.139.10.6331. [DOI] [PubMed] [Google Scholar]
- 14.Minkin C. Bone acid phosphatase: tartrate-resistant acid phosphatase as a marker of osteoclast function. Calcif Tissue Int. 1982;34:285–90. doi: 10.1007/BF02411252. [DOI] [PubMed] [Google Scholar]
- 15.Blair HC, Teitelbaum SL, Ghiselli R, Gluck S. Osteoclastic bone resorption by a polarized vacuolar proton pump. Science. 1989;245:855–7. doi: 10.1126/science.2528207. [DOI] [PubMed] [Google Scholar]
- 16.Gravallese EM, Manning C, Tsay A, Naito A, Pan C, Amento E, et al. Synovial tissue in rheumatoid arthritis is a source of osteoclast differentiation factor. Arthritis Rheum. 2000;43:250–8. doi: 10.1002/1529-0131(200002)43:2<250::AID-ANR3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 17.Takayanagi H, Iizuka H, Juji T, Nakagawa T, Yamamoto A, Miyazaki T, et al. Involvement of receptor activator of nuclear factor kappaB ligand/osteoclast differentiation factor in osteoclastogenesis from synoviocytes in rheumatoid arthritis. Arthritis Rheum. 2000;43:259–69. doi: 10.1002/1529-0131(200002)43:2<259::AID-ANR4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 18.Haynes DR, Crotti TN, Loric M, Bain GI, Atkins GJ, Findlay DM. Osteoprotegerin and receptor activator of nuclear factor kappaB ligand (RANKL) regulate osteoclast formation by cells in the human rheumatoid arthritic joint. Rheumatology (Oxford) 2001;40:623–30. doi: 10.1093/rheumatology/40.6.623. [DOI] [PubMed] [Google Scholar]
- 19.Pilichou A, Papassotiriou I, Michalakakou K, Fessatou S, Fandridis E, Papachristou G, et al. High levels of synovial fluid osteoprotegerin (OPG) and increased serum ratio of receptor activator of nuclear factor-kappa B ligand (RANKL) to OPG correlate with disease severity in patients with primary knee osteoarthritis. Clin Biochem. 2008;41:746–9. doi: 10.1016/j.clinbiochem.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 20.Wakita T, Mogi M, Kurita K, Kuzushima M, Togari A. Increase in RANKL: OPG ratio in synovia of patients with temporomandibular joint disorder. J Dent Res. 2006;85:627–32. doi: 10.1177/154405910608500709. [DOI] [PubMed] [Google Scholar]
- 21.Kudo O, Sabokbar A, Pocock A, Itonaga I, Fujikawa Y, Athanasou NA. Interleukin-6 and interleukin-11 support human osteoclast formation by a RANKL-independent mechanism. Bone. 2003;32:1–7. doi: 10.1016/s8756-3282(02)00915-8. [DOI] [PubMed] [Google Scholar]
- 22.Kudo O, Fujikawa Y, Itonaga I, Sabokbar A, Torisu T, Athanasou NA. Proinflammatory cytokine (TNFalpha/IL-1alpha) induction of human osteoclast formation. J Pathol. 2002;198:220–7. doi: 10.1002/path.1190. [DOI] [PubMed] [Google Scholar]
- 23.Adamopoulos I, Sabokbar A, Wordsworth B, Carr A, Ferguson D, Athanasou N. Synovial fluid macrophages are capable of osteoclast formation and resorption. J Pathol. 2006;208:35–43. doi: 10.1002/path.1891. [DOI] [PubMed] [Google Scholar]
- 24.Danks L, Sabokbar A, Gundle R, Athanasou NA. Synovial macrophageosteoclast differentiation in inflammatory arthritis. Ann Rheum Dis. 2002;61:916–21. doi: 10.1136/ard.61.10.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jilka RL, Hangoc G, Girasole G, Passeri G, Williams DC, Abrams JS, et al. Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science. 1992;257:88–91. doi: 10.1126/science.1621100. [DOI] [PubMed] [Google Scholar]
- 26.Yanni G, Whelan A, Feighery C, Bresnihan B. Synovial tissue macrophages and joint erosion in rheumatoid arthritis. Ann Rheum Dis. 1994;53:39–44. doi: 10.1136/ard.53.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takayanagi H, Oda H, Yamamoto S, Kawaguchi H, Tanaka S, Nishikawa T, et al. A new mechanism of bone destruction in rheumatoid arthritis: synovial fibroblasts induce osteoclastogenesis. Biochem Biophys Res Commun. 1997;240:279–86. doi: 10.1006/bbrc.1997.7404. [DOI] [PubMed] [Google Scholar]
- 28.Athanasou NA, Quinn J. Immunophenotypic differences between osteoclasts and macrophage polykaryons: immunohistological distinction and implications for osteoclast ontogeny and function. J Clin Pathol. 1990;43:997–1003. doi: 10.1136/jcp.43.12.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davies J, Warwick J, Totty N, Philp R, Helfrich M, Horton M. The osteoclast functional antigen, implicated in the regulation of bone resorption, is biochemically related to the vitronectin receptor. J Cell Biol. 1989;109:1817–26. doi: 10.1083/jcb.109.4.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfeilschifter J, Chenu C, Bird A, Mundy GR, Roodman GD. Interleukin-1 and tumor necrosis factor stimulate the formation of human osteoclastlike cells in vitro. J Bone Miner Res. 1989;4:113–8. doi: 10.1002/jbmr.5650040116. [DOI] [PubMed] [Google Scholar]
- 31.Rivollier A, Mazzorana M, Tebib J, Piperno M, Aitsiselmi T, Rabourdin-Combe C, et al. Immature dendritic cell transdifferentiation into osteoclasts: a novel pathway sustained by the rheumatoid arthritis microenvironment. Blood. 2004;104:4029–37. doi: 10.1182/blood-2004-01-0041. [DOI] [PubMed] [Google Scholar]
- 32.Lubberts E, Joosten LAB, Oppers B, van den Bersselaar L, Coenen-de Roo CJJ, Kolls JK, et al. IL-1-Independent role of IL-17 in synovial inflammation and joint destruction during collagen-induced arthritis. J Immunol. 2001;167:1004–13. doi: 10.4049/jimmunol.167.2.1004. [DOI] [PubMed] [Google Scholar]
- 33.Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103:1345–52. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;203:2673–82. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chambers TJ. Regulation of the differentiation and function of osteoclasts. J Pathol. 2000;192:4–13. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH645>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 36.Jaffre F, Callebert J, Sarre A, Etienne N, Nebigil CG, Launay JM, et al. Involvement of the Serotonin 5-HT2B receptor in cardiac hypertrophy linked to sympathetic stimulation. Circulation. 2004;110:969–74. doi: 10.1161/01.CIR.0000139856.20505.57. [DOI] [PubMed] [Google Scholar]
- 37.Werth VP, Bashir MM, Zhang W. IL-12 completely blocks ultraviolet-induced secretion of tumor necrosis factor [alpha] from cultured skin fibroblasts and Keratinocytes. J Invest Dermatol. 2003;120:116–22. doi: 10.1046/j.1523-1747.2003.12012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kubota A, Hasegawa K, Suguro T, Koshihara Y. Tumor necrosis factor-alpha promotes the expression of osteoprotegerin in rheumatoid synovial fibroblasts. J Rheumatol. 2004;31:426–35. [PubMed] [Google Scholar]
- 39.Guba SC, Sartor CI, Gottschalk LR, Jing YH, Mulligan T, Emerson SG. Bone marrow stromal fibroblasts secrete interleukin-6 and granulocyte-macrophage colony-stimulating factor in the absence of inflammatory stimulation: demonstration by serum-free bioassay, enzyme-linked immunosorbent assay, and reverse transcriptase polymerase chain reaction. Blood. 1992;80:1190–8. [PubMed] [Google Scholar]
- 40.Hodge JM, Kirkland MA, Nicholson GC. GM-CSF cannot substitute for M-CSF in human osteoclastogenesis. Biochem Biophys Res Commun. 2004;321:7–12. doi: 10.1016/j.bbrc.2004.06.097. [DOI] [PubMed] [Google Scholar]
- 41.Hodge JM, Kirkland MA, Aitken CJ, Waugh CM, Myers DE, Lopez CM, et al. Osteoclastic potential of human CFU-GM: biphasic effect of GM-CSF. J Bone Miner Res. 2004;19:190–9. doi: 10.1359/JBMR.0301232. [DOI] [PubMed] [Google Scholar]
- 42.Hiasa M, Abe M, Nakano A, Oda A, Amou H, Kido S, et al. GM-CSF and IL-4 induce dendritic cell differentiation and disrupt osteoclastogenesis through M-CSF receptor shedding by up-regulation of TNF-alpha converting enzyme (TACE) Blood. 2009;114:4517–26. doi: 10.1182/blood-2009-04-215020. [DOI] [PubMed] [Google Scholar]
- 43.Schiller C, Gruber R, Ho GM, Redlich K, Gober HJ, Katzgraber F, et al. Interaction of triiodothyronine with 1alpha,25-dihydroxyvitamin D3 on interleukin-6-dependent osteoclast-like cell formation in mouse bone marrow cell cultures. Bone. 1998;22:341–6. doi: 10.1016/s8756-3282(97)00291-3. [DOI] [PubMed] [Google Scholar]
- 44.Gao Y, Morita I, Maruo N, Kubota T, Murota S, Aso T. Expression of IL-6 receptor and GP130 in mouse bone marrow cells during osteoclast differentiation. Bone. 1998;22:487–93. doi: 10.1016/s8756-3282(98)00040-4. [DOI] [PubMed] [Google Scholar]
- 45.Peters M, Muller AM, Rose-John S. Interleukin-6 and soluble interleukin-6 receptor: direct stimulation of gp130 and hematopoiesis. Blood. 1998;92:3495–504. [PubMed] [Google Scholar]
- 46.Benigni F, Fantuzzi G, Sacco S, Sironi M, Pozzi P, Dinarello CA, et al. Six different cytokines that share GP130 as a receptor subunit, induce serum amyloid A and potentiate the induction of interleukin-6 and the activation of the hypothalamus-pituitary-adrenal axis by interleukin-1. Blood. 1996;87:1851–4. [PubMed] [Google Scholar]
- 47.Astry B, Harberts E, Moudgil KD. A cytokine-centric view of the pathogenesis and treatment of autoimmune arthritis. J Interferon Cytokine Res. 2011;31:927–40. doi: 10.1089/jir.2011.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murakami M, Hibi M, Nakagawa N, Nakagawa T, Yasukawa K, Yamanishi K, et al. IL-6-induced homodimerization of gp130 and associated activation of a tyrosine kinase. Science. 1993;260:1808–10. doi: 10.1126/science.8511589. [DOI] [PubMed] [Google Scholar]
- 49.Yawata H, Yasukawa K, Natsuka S, Murakami M, Yamasaki K, Hibi M, et al. Structure-function analysis of human IL-6 receptor: dissociation of amino acid residues required for IL-6-binding and for IL-6 signal transduction through gp130. EMBO J. 1993;12:1705–12. doi: 10.1002/j.1460-2075.1993.tb05815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adamopoulos IE, Tessmer M, Chao CC, Adda S, Gorman D, Petro M, et al. IL-23 is critical for induction of arthritis, osteoclast formation, and maintenance of bone mass. J Immunol. 2011;187:951–9. doi: 10.4049/jimmunol.1003986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hsu YH, Chen WY, Chan CH, Wu CH, Sun ZJ, Chang MS. Anti-IL-20 monoclonal antibody inhibits the differentiation of osteoclasts and protects against osteoporotic bone loss. J Exp Med. 2011;208:1849–61. doi: 10.1084/jem.20102234. [DOI] [PMC free article] [PubMed] [Google Scholar]


