Abstract
Purpose of review
Human fat consists of white and brown adipose tissue (WAT and BAT). Though most fat is energy-storing WAT, the thermogenic capacity of even small amounts of BAT makes it an attractive therapeutic target for inducing weight loss through energy expenditure. This review evaluates the recent discoveries regarding the identification of functional BAT in adult humans and its potential as a therapy for obesity and diabetes.
Recent findings
Over the past year, several independent research teams used a combination of positron-emission tomography and computed tomography (PET/CT) imaging, immunohistochemistry, and gene and protein expression assays to prove conclusively that adult humans have functional BAT. This has occurred against a backdrop of basic studies defining the origins of BAT, new components of its transcriptional regulation, and the role of hormones in stimulation of BAT growth and differentiation.
Summary
Adult humans have functional BAT, a new target for antiobesity and antidiabetes therapies focusing on increasing energy expenditure. Future studies will refine the methodologies used to measure BAT mass and activity, expand our knowledge of critical-control points in BAT regulation, and focus on testing pharmacological agents that increase BAT thermogenesis and help achieve long-lasting weight loss and an improved metabolic profile.
Keywords: adult humans, antiobesity therapy, brown adipose tissue, clinical and basic science research, PET/CT
Introduction
The contemporary pandemics of obesity and diabetes are devastating in size, breadth, and rate of growth. Over 1 billion adults are either overweight BMI >25 or obese BMI>30, and more than 150 million adults have diabetes, most of which is type 2 diabetes driven by obesity-associated insulin resistance. Even more problematic is the fact that about 25% of children in the USA are also now overweight or obese leading to the appearance of type 2 diabetes in this previously unaffected population. These numbers are expected to increase by more than half again by the year 2025 worldwide, with especially severe impact in less developed countries [1].
The emerging role of adipose tissue
Obesity develops when energy intake exceeds energy expenditure. This is the result of an imbalance between the ingestion of energy-dense foods, decreased physical activity, and the inability of the CNS to suppress appetite appropriately. In this classical view, adipose tissue is considered a passive storage depot for the excess calories. Over the past two decades, however, this view has dramatically changed. Adipose tissue is now known to be an active endocrine organ, releasing free fatty acids (FFA) and adipokines such as leptin, adiponectin, TNFα, interleukin-6 (IL-6), and retinol binding protein-4 (RBP-4), all of which can act on other tissues, including the brain, liver, and muscle to regulate food intake, energy balance, and insulin sensitivity [2,3].
White adipose tissue (WAT) distribution greatly affects metabolic risk. Increased intra-abdominal/visceral fat is associated with a high risk of metabolic disease, whereas increased subcutaneous fat in the thighs and hips exerts little or no risk of metabolic disease. Recent evidence indicates that these functional differences may be developmental in origin [4], and perhaps to the degree of infiltration of these different fat depots with other cells, which release cytokines that contribute to the insulin resistance seen in obesity [5]. In addition, adipose tissue is heterogeneous. Thus, in addition to WAT, which stores energy, mammals including humans have brown adipose tissue (BAT), which burns energy for thermogenesis.
Over the past 2 to 3 years, there has been an explosion of knowledge about BAT, both at the cell biological level and at the clinical level.
Features of the whole-body adipose tissue depots
In the rodent, the macroscopic view suggests that the adipose organ is composed of a number of depots of WAT but only one major BAT depot that is concentrated in the interscapular area. In addition, however, small collections of BAT have been identified in other areas, including admixed with WAT and interspersed between bundles of skeletal muscle [6,7]. The composition of these sites is influenced by age, strain/genetics, environment, nutrition, and drugs [8]. For example, in some obesity-resistant strains of mice, there is more BAT admixed in the hindlimb skeletal muscle, suggesting a role for these depots in control of body weight [6,7].
BAT is important for thermogenesis and energy balance in small mammals, and its induction in mice promotes energy expenditure, reduces adiposity, and protects mice from diet-induced obesity [9,10]. Conversely, BAT ablation reduces energy expenditure and increases obesity in response to high-fat diets [11]. At the cellular level, brown adipocytes regulate energy expenditure through their numerous, large mitochondria. In the inner mitochondrial membrane is the BAT-specific uncoupling protein 1 (UCP-1), which when activated dissipates the intermembrane proton-motive force and generates heat instead of ATP [8]. The thermogenic capacity of BAT is impressive. In a cold-acclimatized rat, oxygen consumption by BAT is approximately twice the normal whole-body basal metabolic rate [12]. In humans, it has been estimated that as little as 50 g of BAT could utilize up to 20% of basal caloric needs if maximally stimulated [13].
UCP-1 is unique to BAT and is necessary to mediate BAT thermogenesis [14]. In addition, to UCP-1, brown adipocytes can be distinguished from WAT at the molecular level by high-levels of expression of type 2 iodothyronine deiodinase (DIO2), the transcription coregulators PRDM16 and PGC-1α, and the regulator of lipolysis Cidea [4]. Despite the immense contribution that BAT plays in rodents, it has generally been regarded as nonexistent in adult humans, and except under uncommon circumstances, such as chronic cold exposure, hyper-adrenergic stimulation in pheochromocytoma, and in tumors [15–17]. The latter, termed hibernomas, are rare, benign tumors usually composed of mixtures of BAT and WAT that bear resemblance to the brown fat of hibernating animals [18]. The importance and function of BAT in normal adult humans otherwise had been considered minimal.
Rediscovery of brown fat in humans
During 2009, several studies led to a paradigm shift in our understanding of the potential role of BAT in adult humans. Although it has been known that adult humans may have small pockets of BAT at postmortem examination [19], attempts to find functional BAT during life [20] or utilize its thermogenesis for weight loss [21,22] have been largely unsuccessful [17,23], so BAT was thought to be biologically irrelevant in adult humans. Despite this view in the endocrine community, reports in the radiological literature using positron-emission tomography and computed tomography (PET/CT) began to suggest a different point of view. PET, or positron emission tomography, uses radiotracers such as 18F-fluorodeoxyglucose (18F-FDG) to measure the metabolic activity of tissues. CT provides high-resolution anatomical detail. Fusion of the PET and CT images provides both functional and structural information in a single image (Fig. 1). In the course of using PET/CT to detect and stage tumors [24], radiologists noted small, but distinct, nontumor collections of adipose tissue, that is, fat with high uptake of 18F-FDG [25,26]. Although this tissue was often called ‘BAT’ in the radiological literature, internists, and endocrinologists doubted these findings and persisted with the view that adult humans have no functional BAT.
Figure 1. The presence and absence of detectable BAT via 18 F-FDG PECT/CT.
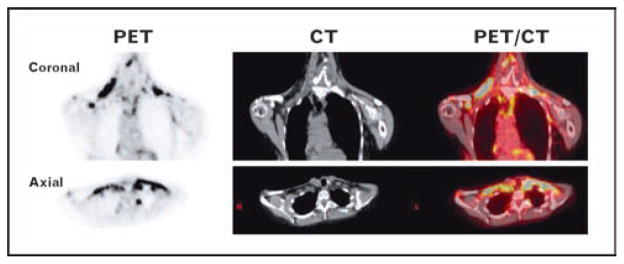
Shown are the attenuation-corrected coronal and axial PET (left), CT (center), and fused PET/CT (right) images of a 60 year-old woman with substantial amounts of BAT. The intense yellow regions in the PET/CT images correspond to the cervical and supraclavicular BAT depots.
Then, in the span of 1 month in the first half of 2009, five independent groups used 18F-FDG PET/CT to identify and characterize the presence and relevance of BAT in adult humans [27• •–31• •]. All five showed major depots of metabolically active fat in the cervical–supraclavicular region that had UCP-1 and histological characteristics of BAT. Virtanen et al. [29• •] also demonstrated the expression of UCP-1, DIO2, and the β3-adrenergic receptor, indicating the potential responsiveness of human BAT to both hormonal and pharmacological stimuli. In our retrospective study of almost 2000 individuals undergoing PET/CT, we showed that BAT is detectable in a substantial fraction of the population and that there is an inverse correlation between BAT activity and average outdoor temperature, beta-blocker medication use, and BMI, indicating that adult human BAT responds to stimulation by the sympathetic nervous system and likely participates in both cold-induced and diet-induced thermogenesis. Both Marken Lichtenbelt, et al. [28• •] and Saito et al. [31• •] found a similar relationship to BMI and body fat. Zingaretti et al. [30• •] added that many adults had brown adipocyte precursors within the depots carrying mature brown adipocytes.
The location of BAT in adult humans was unexpected. In rodents and human infants, the predominant BAT depot is interscapular. On the contrary, in adult humans the most common location for metabolically active BAT is the cervical–supraclavicular depot, in a distinct fascial plane in the front of the neck, extending into the supraclavicular and thoracic region in some people [27• •]. In addition, the percentage of adult humans with BAT that can be activated and detected may be quite high, as recent prospective studies using cold stimulation to increase BAT activity and detection via PET/CT show that among younger people, 96% have functional BAT [28• •]. The overall conclusion of these studies is that BAT is present and can be activated in most adult humans and that total BAT activity is inversely associated with adiposity and indexes of the metabolic syndrome suggesting that increasing BAT mass and/or activity may be a target for pharmacologic and nutritional interventions that modulate energy expenditure to treat obesity [32].
Recent advances in brown-adipose tissue biology
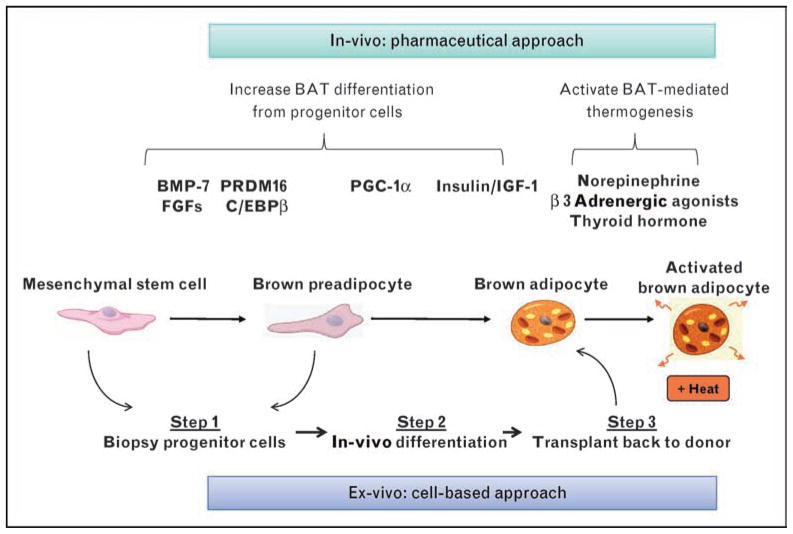
Over the past several years, there has also been important progress in understanding the origin and control of BAT development. BAT is derived from multipotent mesenchymal stems cells [4]. However, brown adipocytes in different anatomical locations appear to have distinct lineages. Thus, interscapular and perirenal BAT appear to be derived from myf5-expressing, myogenic precursors [33,34•], but systemic brown fat cells seen within white fat tissue and interspersed within muscle appear to derive from myf5 negative cells and have different features, including greater sensitivity to β3-adrenergic stimulation and cold exposure [6,7,34•,35,36]. The theoretical scheme of brown adipogenesis is shown in Fig. 2 and is composed of both positive and negative regulators A key transition begins with the triggering of differentiation suppressed by proteins of the retinoblastoma (Rb) family and necdin, a growth repressor functionally resembling Rb [37–39]. After derepression by insulin/IGF-1, a transcriptional cascade begins [40], leading ultimately to lipid synthesis, mitochondrial biogenesis, and expression of UCP-1 [39]. Among the critical players are the zinc-finger containing transcription co-factor PRDM16 [41], which in brown preadipocytes interacts with transcription factors, PPARγ, and C/EBPβ and their coactivators PGC-1α/β [42,43].
Figure 2. Therapies for obesity and diabetes that target the BAT differentiation and activation pathway.
The development of a fully functional brown adipocyte can be divided into three phases: the multipotent mesenchymal stem cell; the brown preadipocyte; and the mature brown adipocytes. Shown in the upper part of the panel are key hormonal regulators of each phase. There are two principal approaches to utilizing BAT as a therapy for obesity and diabetes. One is the conventional in-vitro pharmaceutical approach of developing hormones or drugs that activate components of the pathway leading from the precursor cells to mature, activated brown adipocytes. The other is a novel ex-vivo strategy whereby progenitor cells are isolated from patients surgically, treated with factors that promote brown adipocyte differentiation, and then transplanted back into the same individuals to establish functional BAT that can be activated to burn off excess calories.
The best-known and effective inducer of brown adipogenesis and function is norepinephrine, which plays an important role in stimulating proliferation and differentiation of brown preadipocytes, as well as directly modulating the thermogenic function in mature brown adipocytes [44]. Of particular interest from the perspective of novel therapeutics for obesity and diabetes, there have now been identified additional specific-secreted signaling molecules that trigger brown adipocyte development, including nodal, wingless, and members of the fibroblast growth factor (FGF) and bone morphogenetic protein (BMP) families [4]. The BMPs are secreted proteins in the TGF-β superfamily that modify mesenchymal-stem cell development and can promote both brown and white adipogenesis [45]. BMP-7 in particular is a powerful inducer of brown adipogenesis and is of therapeutic interest, as it has been shown to preferentially support differentiation of BAT over WAT and increase mitochondrial biogenesis and expression of UCP1 [46• •]. Adenoviral-mediated expression of BMP-7 in obesity-prone mice significantly increases brown fat mass and energy expenditure and reduced weight gain [46• •].
Another factor affecting brown adipogenesis from the TGF-β/BMP superfamily is growth differentiation factor (GDF)3. Mice lacking GDF3 are protected from diet-induced obesity due to an increased basal metabolism rate, which results from the development of brown adipocytes within white-adipose tissue [47]. Several members of the FGF family, including FGF-16, FGF-19, and FGF-21, together with their coreceptors from the klotho family have also been shown to increase BAT growth, possibly via autocrine mechanisms [48–51].
Using brown adipose tissue as a therapy for obesity and diabetes
With the recognition that adult humans have in BAT an organ with substantial capacity to dissipate energy, targeting BAT thermogenesis may now be viewed as an appealing way to treat or prevent obesity and its associated diseases. This is especially important because there are currently only three drugs approved by the FDA specifically for weight loss (sibutramine, phentermine, and orlistat), all of which focus primarily on the reduction of energy intake [52], and none of which have provided adequate long-term clinical efficacy [53].
Looking forward to the future, there are two general approaches that could be used to increase BAT mass and activity: an in-vivo pharmacological approach using small molecules and growth factors (Table 1) [54–61] to stimulate BAT growth, differentiation, and activation, and an ex-vivo cell-based approach, in which recent advances in brown adipogenesis could be used to increase brown fat differentiation from progenitor cells that in-vitro could then be implanted in patients seeking weight loss (Fig. 2). The pharmacological approach is very attractive because increasing energy expenditure through thermogenesis has already proved to be very effective in achieving weight loss. For example, dinitrophenol (DNP), a nonselective uncoupler of mitochondrial oxidation, has been shown to increase energy expenditure in a sustained fashion without tolerance [54]. However, the myriad of life-threatening adverse effects precludes its use as therapy. Second, increasing energy expenditure may support the body’s own adaptive response to weight gain. Interventions designed to increase BAT-mediated energy expenditure may be able to reset an obese individual’s settling point for body weight back to a lower, healthier range [62•]. Whether treatment with drugs that increase BAT activity would need to be sustained or could be used for only defined periods of time, needs to be examined. Also, it is not clear to what extent there might be even further increases in energy intake to offset energy expenditure by activated BAT in obese individuals.
Table 1.
Antiobesity treatments that may increase BAT mass and/or energy expenditure
| Drug | Principal mechanism |
|---|---|
| Ephedrine, Ma Huang (herb) | Mixed sympathomimetic [54] |
| BRL-26830, L-796568, N-5984 | Selective cell-surface b3 adrenergic receptor activators [55•] |
| BMP-7 | Cell-surface BMP receptor activator [46••] |
| FGFs | Cell-surface FGF receptor activator signaling [48–51] |
| Bile acids, INT-777 | Cell-surface TGR5 receptor activator [56,57•] |
| Glitazars | PPAR alpha/gamma agonist [58,59] |
| Resveratrol | SIRT1 activator [60] |
| GW501516 | PPAR delta agonist [61] |
| GDF3 antagonist | Cell-surface GDF receptor blocker [47] |
Previous therapies directed at brown adipose tissue and what they tell us
Despite only recent proof that adult humans have functional BAT and may be involved in weight regulation [27• •–31• •], attempts to exploit energy expenditure in either occult depots of BAT or muscle as an approach for treating obesity have been considered for decades. The mixed sympathomimetic ephedrine is an alkaloid derived from the Ephedra spp. that induces central nervous system stimulation, bronchodilation, and vasoconstriction [63•], and has been used for weight loss. A meta-analysis of several dozen trials found that ephedrine does promote modest short-term weight loss, but there are no data on longer-term efficacy [64]. Exactly, how and where Ephedra works is unknown, but PET/CT in rats shows that ephedrine’s effects on metabolism are mediated at least in part by activation of BAT [65].
Approaches to activate BAT by selective sympathetic activation have thus far had only limited success. In rodents, the β3-adrenergic receptor is found nearly exclusively on adipocytes, and this led to development of β3-specific agonists, such as CL-316 243, which could be used to increase energy expenditure [54]. Human brown fat also expresses β3-adrenergic receptors [29• •]. However, early human trials using β3 receptor agonists were not successful. This is, at least in part, because human β3-adrenergic receptors have different binding characteristics than those in rodents [66] and did not recognize some of the early ligands used in the clinical studies. Second generation β3 agonists, which do bind human β3 receptors, have poor oral bioavailability or unfavorable pharmacokinetics [55•]. One new-generation β3 agonist, L-796568, has been shown to acutely increase lipolysis and energy expenditure in overweight men [67], but its effect seemed to be lost after 28 days of treatment [22,55•]. However, all of these studies were done before PET/CT scanning was available to specifically measure BAT function and mass and quantify the BAT activation in a particular therapy. Given the new ability to quantify human BAT activity, attempts to develop β3-adrenergic agonists need re-evaluation as a potential treatment for obesity.
Novel therapies to increase brown-adipose tissue activation
A second approach for pharmacologic stimulation of BAT thermogenesis relies not on the traditional adrenergic pathways, but on newly discovered mechanisms involving BAT-specific growth factors (Fig. 2). For example, as noted earlier, the mesodermal growth factor BMP-7 can stimulate BAT growth and reduce weight gain when expressed systemically in mice [46• •]. Originally identified as a hormone that increased bone formation, BMP-7 has now been recognized in murine models as a potential treatment for cardiovascular, metabolic, and degenerative diseases [68] and may be effective for certain chronic-kidney diseases, such as diabetic nephropathy [69,70]. Studies are already underway to determine if spinal-implanted BMP-7 can have a systemic effect on BAT growth and activation leading to weight loss in humans. The nexus between nutritional and pharmacological activators of BAT activity can be seen with the FGFs. Mice overexpressing FGF-21 are lean and have increased brown fat [50]. FGF-21 can also be induced by a ketogenic diet [71] suggesting that some of the beneficial metabolic effects induced by a ketogenic diet are brought about by increases in BAT mass. Though further details of this family’s function remain to be determined, development of recombinant FGFs designed to stimulate BAT growth and activation hold promise as means for treating obesity.
In addition to these hormones, small-molecule regulators of proteins known to control brown adipogenesis, such as PGC-1α, Rb, necdin, or PRDM16 [37,39,41,72], may be developed. They could fill a niche similar to the thiazolidinediones, which may themselves improve insulin sensitivity through increased brown adipogenesis [73]. The caveat of this approach is that there are no known small molecule activators of these transcriptional regulators, and transcription factors can have pleiotropic effects in many tissues of the body, suggesting that the immediate focus should be on pharmaceutical agents that more purely regulate UCP-1 activity.
Although certainly in its earliest phases, recent studies [43] have suggested a new model for antiobesity treatment: ex-vivo manipulation to form functional BAT. The underlying principle that makes this an alluring option is that only gram-amounts of BAT are needed to have a meaningful metabolic impact [13]. As noted earlier, at least two different routes of differentiation have been suggested to give rise to BAT [4] (Fig. 2): one leading to the major preformed depot of BAT in rodents, that is, the interscapular brown fat pad, which are derived from myf-5 expressing precursors [34•] and the second involving systemic brown adipocytes that appear to be derived from myf-5 negative precursors [6,7] (T. Schulz et al., unpublished). Which of these pathways, if either, leads to the deposits of BAT found in adult humans in the cervical and supraclavicular area remains unknown, but as both pathways are driven by BMPs and FGFs, there may be common external regulators that could be used to therapeutic advantage. Thus, in an ex-vivo approach, one would envision-isolating progenitor cells from an individual by liposuction or muscle biopsy, then inducing brown adipogenesis using appropriate growth factors such as the BMPs, and transplanting them back into the original donor for in-vivo expansion. This methodology of autotransplantation could avoid unwarranted side effects of differentiating agents or growth factors and require only minimal surgical intervention. One challenge related to this novel methodology is whether increasing BAT mass will also result in increased activated BAT, which is required for thermogenesis and weight loss to occur.
Conclusion
The obesity pandemic requires new and novel treatments. The past few years have witnessed multiple studies conclusively showing that adult humans have functional BAT, a tissue that has a tremendous capacity for obesity-reducing thermogenesis. It is now clear that adult human BAT responds to cold stimulation under both acute and chronic conditions, is regulated by adrenergic stimulation, and can be monitored using noninvasive imaging via PET/CT. Most importantly, nearly every adult human has or can make activated BAT. Combining this knowledge with recent advances in understanding BAT differentiation has created new interest in this tissue as a possible therapeutic approach for metabolic diseases. Although many questions remain regarding efficacy, safety, practicality, and durability of such treatments, we are encouraged that both classical and novel therapies targeting BAT thermogenesis may be available in the near future as therapies for obesity and diabetes.
Acknowledgments
This work was supported in part by the Eli Lilly Foundation and NIH grants DK082659 (C.R.K), DK046200, DK081604, DK087317, RR025757 (A.M.C.), and P30 DK036836 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National NIDDK or the NIH.
Footnotes
Dr Cypess reports being the sole inventor on a pending patent application to use IR thermography to monitor brown adipose tissue. Dr Kahn reports being an advisory board member for Sirtris, Plexxicon, Fiveprime, and Dicerna; owns equity in GSK, Plexxicon, and Fiveprime; receiving lecture fees from Wyeth, Novartis, and Novo-Nordisk; and has a pending patent in the area of stimulating brown fat growth with BMPs. No other conflicts of interest were reported.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 179).
- 1.Haslam DW, James WP. Obesity. Lancet. 2006;366:1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 2.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–853. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Puri V, Virbasius JV, Guilherme A, Czech MP. RNAi screens reveal novel metabolic regulators: RIP140, MAP4k4 and the lipid droplet associated fat specific protein (FSP) 27. Acta Physiol (Oxf) 2008;192:103–115. doi: 10.1111/j.1748-1716.2007.01786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131:242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Arkan MC, Hevener AL, Greten FR, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- 6.Almind K, Manieri M, Sivitz WI, et al. Ectopic brown adipose tissue in muscle provides a mechanism for differences in risk of metabolic syndrome in mice. Proc Natl Acad Sci U S A. 2007;104:2366–2371. doi: 10.1073/pnas.0610416104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xue B, Rim JS, Hogan JC, et al. Genetic variability affects the development of brown adipocytes in white fat but not in interscapular brown fat. J Lipid Res. 2007;48:41–51. doi: 10.1194/jlr.M600287-JLR200. [DOI] [PubMed] [Google Scholar]
- 8.Cinti S. The adipose organ. Prostaglandins leukot essent fatty acids. 2005;73:9–15. doi: 10.1016/j.plefa.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Ghorbani M, Claus TH, Himms-Hagen J. Hypertrophy of brown adipocytes in brown and white adipose tissues and reversal of diet-induced obesity in rats treated with a beta3-adrenoceptor agonist. Biochem Pharmacol. 1997;54:121–131. doi: 10.1016/s0006-2952(97)00162-7. [DOI] [PubMed] [Google Scholar]
- 10.Guerra C, Koza RA, Yamashita H, et al. Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity. J Clin Invest. 1998;102:412–420. doi: 10.1172/JCI3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lowell BB, Susulic V, Hamann A, et al. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature. 1993;366:740–742. doi: 10.1038/366740a0. [DOI] [PubMed] [Google Scholar]
- 12.Foster DO, Frydman ML. Tissue distribution of cold-induced thermogenesis in conscious warm or cold-acclimated rats reevaluated from changes in tissue blood flow: the dominant role of brown adipose tissue in the replacement of shivering by nonshivering thermogenesis. Can J Physiol Pharmacol. 1979;57:257–270. doi: 10.1139/y79-039. [DOI] [PubMed] [Google Scholar]
- 13.Rothwell NJ, Stock MJ. Luxuskonsumption, diet-induced thermogenesis and brown fat: the case in favour. Clin Sci (Lond) 1983;64:19–23. doi: 10.1042/cs0640019. [DOI] [PubMed] [Google Scholar]
- 14.Golozoubova V, Hohtola E, Matthias A, et al. Only UCP1 can mediate adaptive nonshivering thermogenesis in the cold. FASEB J. 2001;15:2048–2050. doi: 10.1096/fj.00-0536fje. [DOI] [PubMed] [Google Scholar]
- 15.Huttunen P, Hirvonen J, Kinnula V. The occurrence of brown adipose tissue in outdoor workers. Eur J Appl Physiol Occup Physiol. 1981;46:339–345. doi: 10.1007/BF00422121. [DOI] [PubMed] [Google Scholar]
- 16.English JT, Patel SK, Flanagan MJ. Association of pheochromocytomas with brown fat tumors. Radiology. 1973;107:279–281. doi: 10.1148/107.2.279. [DOI] [PubMed] [Google Scholar]
- 17.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293:E444–E452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- 18.Furlong MA, Fanburg-Smith JC, Miettinen M. The morphologic spectrum of hibernoma: a clinicopathologic study of 170 cases. Am J Surg Pathol. 2001;25:809–814. doi: 10.1097/00000478-200106000-00014. [DOI] [PubMed] [Google Scholar]
- 19.Heaton JM. The distribution of brown adipose tissue in the human. J Anat. 1972;112:35–39. [PMC free article] [PubMed] [Google Scholar]
- 20.Astrup A. Thermogenesis in human brown adipose tissue and skeletal muscle induced by sympathomimetic stimulation. Acta Endocrinol Suppl (Copenh) 1986;278:1–32. [PubMed] [Google Scholar]
- 21.Weyer C, Tataranni PA, Snitker S, et al. Increase in insulin action and fat oxidation after treatment with CL 316,243, a highly selective beta3-adrenoceptor agonist in humans. Diabetes. 1998;47:1555–1561. doi: 10.2337/diabetes.47.10.1555. [DOI] [PubMed] [Google Scholar]
- 22.Larsen TM, Toubro S, van Baak MA, et al. Effect of a 28-d treatment with L-796568, a novel beta(3)-adrenergic receptor agonist, on energy expenditure and body composition in obese men. Am J Clin Nutr. 2002;76:780–788. doi: 10.1093/ajcn/76.4.780. [DOI] [PubMed] [Google Scholar]
- 23.Cunningham S, Leslie P, Hopwood D, et al. The characterization and energetic potential of brown adipose tissue in man. Clin Sci (Lond) 1985;69:343–348. doi: 10.1042/cs0690343. [DOI] [PubMed] [Google Scholar]
- 24.Schoder H, Larson SM, Yeung HW. PET/CT in oncology: integration into clinical management of lymphoma, melanoma, and gastrointestinal malignancies. J Nucl Med. 2004;45 (Suppl 1):72S–81S. [PubMed] [Google Scholar]
- 25.Hany TF, Gharehpapagh E, Kamel EM, et al. Brown adipose tissue: a factor to consider in symmetrical tracer uptake in the neck and upper chest region. Eur J Nucl Med Mol Imaging. 2002;29:1393–1398. doi: 10.1007/s00259-002-0902-6. [DOI] [PubMed] [Google Scholar]
- 26.Cohade C, Osman M, Pannu HK, Wahl RL. Uptake in supraclavicular area fat (“USA-Fat”):description on 18F-FDGPET/CT. J Nucl Med. 2003;44:170–176. [PubMed] [Google Scholar]
- 27••.Cypess AM, Lehman S, Williams G, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. PET/CT scans from 1972 patients were reviewed, and it was shown that whole-body BAT could be quantified noninvasively. Active BAT was more frequent in women than in men, and the amount of BAT was inversely correlated with age, outdoor temperature, beta-blocker use, and BMI in older adults. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28••.Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. This prospective study involving young men found that BAT was not observed at 22°C but was detectable in 23/24 individuals at 16°C, demonstrating that functional adult human BAT is highly prevalent. BAT activity correlated positively with resting metabolic rate but inversely with both BMI and percentage of body fat. [DOI] [PubMed] [Google Scholar]
- 29••.Virtanen KA, Lidell ME, Orava J, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. Acute cold exposure induced a 15-fold increase in FDG uptake in adult human BAT. Tissue shown to be FDG-avid adipose tissue via PET/CT was biopsied and shown definitively to contain brown adipocytes and tissue-specific, thermogenic UCP1 via mRNA, protein, and morphological assessments. [DOI] [PubMed] [Google Scholar]
- 30••.Zingaretti MC, Crosta F, Vitali A, et al. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J. 2009;23:3113–3120. doi: 10.1096/fj.09-133546. Adipose tissue was sampled from the necks of 35 patients undergoing surgery. In 1/3, the younger and leaner, distinct islands of richly sympathetically innervated brown adipocytes were identified. These islands had a high capillary density, and the pericapillary regions demonstrated brown adipocyte precursors, indicating that adult humans had the capacity to grow more BAT as well. [DOI] [PubMed] [Google Scholar]
- 31••.Saito M, Okamatsu-Ogura Y, Matsushita M, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526–1531. doi: 10.2337/db09-0530. Acute cold exposure increased BAT activity in the supraclavicular and paraspinal regions of healthy volunteers, and this uptake was increased in the winter compared with summer months. FDG uptake also inversely correlated with BMI and both total and visceral fat. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Celi FS. Brown adipose tissue: when it pays to be inefficient. N Engl J Med. 2009;360:1553–1556. doi: 10.1056/NEJMe0900466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Timmons JA, Wennmalm K, Larsson O, et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci U S A. 2007;104:4401–4406. doi: 10.1073/pnas.0610615104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34•.Seale P, Bjork B, Yang W, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. Using in-vivo fate mapping, this group showed that brown, but not white, fat cells arise from precursors that express Myf5, a myogenic gene. Moreover, the transcriptional regulator PRDM16 controls a bidirectional cell fate switch between skeletal myoblasts and brown-fat cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kopecky J, Clarke G, Enerback S, et al. Expression of the mitochondrial uncoupling protein gene from the aP2 gene promoter prevents genetic obesity. J Clin Invest. 1995;96:2914–2923. doi: 10.1172/JCI118363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leonardsson G, Steel JH, Christian M, et al. Nuclear receptor corepressor RIP140 regulates fat accumulation. Proc Natl Acad Sci U S A. 2004;101:8437–8442. doi: 10.1073/pnas.0401013101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hansen JB, Jorgensen C, Petersen RK, et al. Retinoblastoma protein functions as a molecular switch determining white versus brown adipocyte differentiation. Proc Natl Acad Sci U S A. 2004;101:4112–4117. doi: 10.1073/pnas.0301964101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scime A, Grenier G, Huh MS, et al. Rb and p107 regulate preadipocyte differentiation into white versus brown fat through repression of PGC-1alpha. Cell Metab. 2005;2:283–295. doi: 10.1016/j.cmet.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 39.Tseng YH, Butte AJ, Kokkotou E, et al. Prediction of preadipocyte differentiation by gene expression reveals role of insulin receptor substrates and necdin. Nat Cell Biol. 2005;7:601–611. doi: 10.1038/ncb1259. [DOI] [PubMed] [Google Scholar]
- 40.Cowherd RM, Lyle RE, McGehee REJ. Molecular regulation of adipocyte differentiation. PMID. 1999;10:3–10. doi: 10.1006/scdb.1998.0276. [DOI] [PubMed] [Google Scholar]
- 41.Seale P, Kajimura S, Yang W, et al. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007;6:38–54. doi: 10.1016/j.cmet.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kajimura S, Seale P, Tomaru T, et al. Regulation of the brown and white fat gene programs through a PRDM16/CtBP transcriptional complex. Genes Dev. 2008;22:1397–1409. doi: 10.1101/gad.1666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kajimura S, Seale P, Kubota K, et al. Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-beta transcriptional complex. Nature. 2009;460:1154–1158. doi: 10.1038/nature08262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 45.Schulz TJ, Tseng YH. Emerging role of bone morphogenetic proteins in adipogenesis and energy metabolism. Cytokine Growth Factor Rev. 2009;20:523–531. doi: 10.1016/j.cytogfr.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46••.Tseng YH, Kokkotou E, Schulz TJ, et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008;454:1000–1004. doi: 10.1038/nature07221. Of the family of bone morphogenetic proteins (BMP), BMP-7 was shown to activate a full program of brown adipogenesis in precursor cells. Implantation of treated cells into nude mice led to development of BAT. In other mice, adenoviral-mediated expression of BMP-7 increased brown, but not white, fat mass and increased energy expenditure and a reduction in weight gain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shen JJ, Huang L, Li L, et al. Deficiency of growth differentiation factor 3 protects against diet-induced obesity by selectively acting on white adipose. Mol Endocrinol. 2009;23:113–123. doi: 10.1210/me.2007-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Konishi M, Mikami T, Yamasaki M, et al. Fibroblast growth factor-16 is a growth factor for embryonic brown adipocytes. J Biol Chem. 2000;275:12119–12122. doi: 10.1074/jbc.275.16.12119. [DOI] [PubMed] [Google Scholar]
- 49.Tomlinson E, Fu L, John L, et al. Transgenic mice expressing human fibroblast growth factor-19 display increased metabolic rate and decreased adiposity. Endocrinology. 2002;143:1741–1747. doi: 10.1210/endo.143.5.8850. [DOI] [PubMed] [Google Scholar]
- 50.Kharitonenkov A, Shiyanova TL, Koester A, et al. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115:1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mori K, Yahata K, Mukoyama M, et al. Disruption of klotho gene causes an abnormal energy homeostasis in mice. Biochem Biophys Res Commun. 2000;278:665–670. doi: 10.1006/bbrc.2000.3864. [DOI] [PubMed] [Google Scholar]
- 52.Kaplan LM. Pharmacological therapies for obesity. Gastroenterol Clin North Am. 2005;34:91–104. doi: 10.1016/j.gtc.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 53.Melnikova I, Wages D. Antiobesity therapies. Nat Rev Drug Discov. 2006;5:369–370. doi: 10.1038/nrd2037. [DOI] [PubMed] [Google Scholar]
- 54.Shekelle PG, Hardy ML, Morton SC, et al. Efficacy and safety of ephedra and ephedrine for weight loss and athletic performance: a meta-analysis. JAMA. 2003;289:1537–1545. doi: 10.1001/jama.289.12.1537. [DOI] [PubMed] [Google Scholar]
- 55•.Arch JR. The discovery of drugs for obesity, the metabolic effects of leptin and variable receptor pharmacology: perspectives from beta3-adrenoceptor agonists. Naunyn Schmiedebergs Arch Pharmacol. 2008;378:225–240. doi: 10.1007/s00210-008-0271-1. This review addresses the effects of leptin on weight loss and gives a comprehensive discussion of the attempts to develop β3-adrenergic receptor agonists to treat obesity. [DOI] [PubMed] [Google Scholar]
- 56.Tiwari A, Maiti P. TGR5: an emerging bile acid G-protein-coupled receptor target for the potential treatment of metabolic disorders. Drug Discov Today. 2009;14:523–530. doi: 10.1016/j.drudis.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 57•.Thomas C, Gioiello A, Noriega L, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167–177. doi: 10.1016/j.cmet.2009.08.001. The compound INT-777, a selective agonist of the TGR5 receptor, induced GLP-1 release and led to improved liver and pancreatic function. Treatment of brown adipocytes isolated from C57BL/6J mice for 12 h with INT-777 increases energy expenditure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kendall DM, Rubin CJ, Mohideen P, et al. Improvement of glycemic control, triglycerides, and HDL cholesterol levels with muraglitazar, a dual (alpha/gamma) peroxisome proliferator-activated receptor activator, in patients with type 2 diabetes inadequately controlled with metformin monotherapy: a double-blind, randomized, pioglitazone-comparative study. Diabetes Care. 2006;29:1016–1023. doi: 10.2337/diacare.2951016. [DOI] [PubMed] [Google Scholar]
- 59.Henry RR, Lincoff AM, Mudaliar S, et al. Effect of the dual peroxisome proliferator-activated receptor-alpha/gamma agonist aleglitazar on risk of cardiovascular disease in patients with type 2 diabetes (SYNCHRONY): a phase II, randomised, dose-ranging study. Lancet. 2009;374:126–135. doi: 10.1016/S0140-6736(09)60870-9. [DOI] [PubMed] [Google Scholar]
- 60.Yamamoto H, Schoonjans K, Auwerx J. Sirtuin functions in health and disease. Mol Endocrinol. 2007;21:1745–1755. doi: 10.1210/me.2007-0079. [DOI] [PubMed] [Google Scholar]
- 61.Wang YX, Lee CH, Tiep S, et al. Peroxisome-proliferator-activated receptor delta activates fat metabolism to prevent obesity. Cell. 2003;113:159–170. doi: 10.1016/s0092-8674(03)00269-1. [DOI] [PubMed] [Google Scholar]
- 62•.Harper ME, Green K, Brand MD. The efficiency of cellular energy transduction and its implications for obesity. Annu Rev Nutr. 2008;28:13–33. doi: 10.1146/annurev.nutr.28.061807.155357. This comprehensive review discusses bioenergetics and the reasons for targeting uncoupling for the treatment of obesity. [DOI] [PubMed] [Google Scholar]
- 63•.Tam J, Fukumura D, Jain RK. A mathematical model of murine metabolic regulation by leptin: energy balance and defense of a stable body weight. Cell Metab. 2009;9:52–63. doi: 10.1016/j.cmet.2008.11.005. Using a mathematical model based on published experimental data regarding leptin function, the authors offer the possibility that the body has multiple steady states for body weight. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nelson DL, Gehlert DR. Central nervous system biogenic amine targets for control of appetite and energy expenditure. Endocrine. 2006;29:49–60. doi: 10.1385/endo:29:1:49. [DOI] [PubMed] [Google Scholar]
- 65.Baba S, Tatsumi M, Ishimori T, et al. Effect of nicotine and ephedrine on the accumulation of 18F-FDG in brown adipose tissue. J Nucl Med. 2007;48:981–986. doi: 10.2967/jnumed.106.039065. [DOI] [PubMed] [Google Scholar]
- 66.Clapham JC, Arch JR. Thermogenic and metabolic antiobesity drugs: rationale and opportunities. Diabetes Obes Metab. 2007;9:259–275. doi: 10.1111/j.1463-1326.2006.00608.x. [DOI] [PubMed] [Google Scholar]
- 67.van Baak MA, Hul GB, Toubro S, et al. Acute effect of L-796568, a novel beta 3-adrenergic receptor agonist, on energy expenditure in obese men. Clin Pharmacol Ther. 2002;71:272–279. doi: 10.1067/mcp.2002.122527. [DOI] [PubMed] [Google Scholar]
- 68.Tobin JF, Celeste AJ. Bone morphogenetic proteins and growth differentiation factors as drug targets in cardiovascular and metabolic disease. Drug Discov Today. 2006;11:405–411. doi: 10.1016/j.drudis.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 69.Li T, Surendran K, Zawaideh MA, et al. Bone morphogenetic protein 7: a novel treatment for chronic renal and bone disease. Curr Opin Nephrol Hypertens. 2004;13:417–422. doi: 10.1097/01.mnh.0000133974.24935.fe. [DOI] [PubMed] [Google Scholar]
- 70.Wang S, Chen Q, Simon TC, et al. Bone morphogenic protein-7 (BMP-7), a novel therapy for diabetic nephropathy. Kidney Int. 2003;63:2037–2049. doi: 10.1046/j.1523-1755.2003.00035.x. [DOI] [PubMed] [Google Scholar]
- 71.Badman MK, Pissios P, Kennedy AR, et al. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007;5:426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 72.Puigserver P, Wu Z, Park CW, et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 73.Vernochet C, Peres SB, Davis KE, et al. C/EBPalpha and the corepressors CtBP1 and CtBP2 regulate repression of select visceral white adipose genes during induction of the brown phenotype in white adipocytes by peroxisome proliferator-activated receptor gamma agonists. Mol Cell Biol. 2009;29:4714–4728. doi: 10.1128/MCB.01899-08. [DOI] [PMC free article] [PubMed] [Google Scholar]