Abstract
The purpose of this study was to evaluate the prognostic value of stress echocardiography in patients with angiographically significant coronary artery disease (CAD). Two hundred sixty patients (mean age 63 ± 10 years, 58% men) who underwent stress echocardiography (41% treadmill, 59% dobutamine) and coronary angiography within 3 months and without intervening coronary revascularization were evaluated. All patients had significant CAD as defined by coronary stenosis ≥70% in major epicardial vessels or branches (45% had single-vessel disease, and 55% had multivessel disease). The left ventricle was divided into 16 segments and scored on a 5-point scale of wall motion. Patients with abnormal results on stress echocardiography were defined as those with stress-induced ischemia (increase in wall motion score of ≥1 grade). Follow-up (3.1 ± 1.2 years) for nonfatal myocardial infarction (n = 23) and cardiac death (n = 6) was obtained. In patients with angiographically significant CAD, stress echocardiography effectively risk stratified normal (no ischemia, n = 91) in contrast to abnormal (ischemia, n = 169) groups for cardiac events (event rate 1.0%/year vs 4.9%/year, p = 0.01). Multivariate logistic regression analysis identified multivessel CAD (hazard ratio 2.53, 95% confidence interval 1.16 to 5.51, p = 0.02) and number of segments in which ischemia was present (hazard ratio 4.31, 95% confidence interval 1.29 to 14.38, p = 0.01) as predictors of cardiac events. A Cox proportional-hazards model for cardiac events showed small, significant incremental value of stress echocardiography over coronary angiography (p = 0.02) and the highest global chi-square value for both (p = 0.004). In conclusion, in patients with angiographically significant CAD, (1) normal results on stress echocardiography conferred a benign prognosis (event rate 1.0%/year), and (2) stress echocardiographic results (no ischemia vs ischemia) added incremental prognostic value to coronary angiographic results, and (3) stress echocardiography and coronary angiography together provided additive prognostic value, with the highest global chi-square value.
The prognostic value of stress echocardiography is routinely incorporated into clinical practice.1– 8 Survival rates for patients with stable obstructive coronary artery disease (CAD) are correlated with the extent of disease.9 The objectives of the present stress echocardiographic study were twofold: (1) to define the prognostic value of stress echocardiography in patients with angiographic CAD and (2) to evaluate the incremental value of clinical findings, stress electrocardiography, stress echocardiography, and coronary angiography separately and together in predicting cardiac events.
Methods
We identified 260 nonconsecutive patients who were referred for exercise or pharmacologic stress echocardiography from March 21, 2000, to December 31, 2008. Successful follow-up (100%) for cardiac events ≥1 year after testing was obtained.
Maximal symptom-limited treadmill exercise (electrocardiographic [ECG]) testing was performed using a standard Bruce protocol. Patients exercised to general fatigue, with premature termination for severe angina, ventricular tachycardia, hemodynamically significant arrhythmias, or hemodynamic instability. Postexercise echocardiographic images were acquired <30 to 60 seconds after the termination of treadmill exercise. In pharmacologic stress, dobut-amine was administered intravenously beginning at a dose of 5 to 10 μg/kg/min and increased by 10 μg/kg/min every 3 minutes to a maximum of 40 μg/kg/min or until a study end point was achieved. The end points for termination of the dobutamine infusion included the development of new segmental wall motion abnormalities, attainment of >85% of age-predicted maximum heart rate, or development of significant adverse effects related to dobutamine infusion.
The left ventricle was divided into 16 segments as recommended by the American Society of Echocardiography,10 and a score was assigned to each segment at baseline, with each stage of stress (dobutamine only) and during recovery. Each segment was scored as 1 = normal, 2 = mild to moderate hypokinesia (reduced wall thickening and excursion), 3 = severe hypokinesia (markedly reduced wall thickening and excursion), 4 = akinesia (no wall thickening and excursion), or 5 = dyskinesia (paradoxical wall motion away from the center of the left ventricle during systole).11 A normal response to stress was defined as normal wall motion at rest with an increase in wall thickening and excursion during stress. An abnormal (ischemic) response to stress was defined as (1) a left ventricular (LV) wall segment that did not increase in thickening and excursion during stress (lack of a hyperdynamic wall motion response) or (2) a deterioration in LV wall segment thickening and excursion during stress (increase in wall motion score of ≥1 grade) and (3) a biphasic response with dobutamine stress. Maximal severity was the score of the LV wall segments with the greatest value (worst wall motion grade) at postexercise stress (range 0 to 5). Peak wall motion score index after stress was derived from the cumulative sum score of 16 LV wall segments divided by the number of visualized segments. The rest ejection fraction used in the study analysis was an average visual estimation12 from 2 experienced echocardiographers.
All cardiac catheterizations were performed using a standard Judkins technique. Significant coronary stenosis was defined as a luminal diameter narrowing ≥70% in either a main epicardial artery or a major branch.
Follow-up was obtained in all patients by means of physician-directed telephone interviews using a standardized questionnaire. The hard end points of the study were nonfatal myocardial infarction or cardiac death. Nonfatal myocardial infarction was documented when diagnostic changes in cardiac enzymes (troponin) were accompanied by appropriate clinical symptoms, ECG findings, or both. Cardiac death was confirmed by review of hospital medical records, death certificate, and autopsy records when available.
All analyses were performed using commercially available statistical software (SPSS for Windows version 10.0.5; SPSS, Inc., Chicago, Illinois). Continuous variables are expressed as mean ± SD. Patient groups were compared using Student’s t tests. Differences in categorical variables among groups were assessed using chi-square analysis. Univariate analysis was performed to determine the relation between clinical and echocardiographic variables and cardiac events. Univariate variables that were predictive of cardiac events were considered in multivariate logistic regression analysis. Kaplan-Meier cumulative survival analysis with stratification by normal or abnormal stress echocardiographic results was performed. The comparison of survival between groups was made using the Mantel-Cox test. Statistical significance was defined as p <0.05.
A forward conditional (Wald) Cox proportional-hazards model with all assumptions tested was used to determine the incremental prognostic value of stress echocardiographic variables over clinical, stress ECG, and coronary angiographic variables. The stepwise selection or removal of variables for inclusion was based on clinical judgment and univariate statistical significance.
Results
In the study cohort of 260 patients, 107 (41%) underwent treadmill exercise and 153 (59%) underwent pharmacologic stress. The patient characteristics, stress echocardiographic, and coronary angiographic results are listed in Table 1. Patients were followed for up to 5 years (mean 3.1 ± 1.2), and all patients were followed for ≥1 year. Among the study cohort of 260 patients, 29 cardiac events (11%) occurred during the follow-up period. These included 18 non-fatal myocardial infarctions (7%) and 11 cardiac deaths (4%). There were 7 cardiac events in patients who underwent treadmill stress and 22 events in those who underwent dobutamine stress (6.5%/year vs 14.4%/year, p = 0.11). Forty-eight patients underwent early coronary revascularization <60 days after stress echocardiography (30 percutaneous coronary interventions and 18 bypass procedures). There were no hard cardiac events in these patients up to 1 year after revascularization.
Table 1.
Clinical characteristics in patients with coronary artery disease by angiography
| Variable | Stress Echocardiographic Results
|
p Value | |
|---|---|---|---|
| Normal
|
Abnormal
|
||
| (n = 91) | (n = 169) | ||
| Age (years) | 62 ± 11 | 65 ± 10 | 0.02 |
| Men | 46 (50%) | 106 (63%) | 0.05 |
| Abnormal rest ECG results | 55 (60%) | 84 (50%) | 0.10 |
| Previous myocardial infarction | 35 (38%) | 56 (33%) | 0.39 |
| Previous percutaneous coronary intervention | 27 (30%) | 37 (22%) | 0.16 |
| Previous bypass surgery | 19 (21%) | 27 (16%) | 0.32 |
| History of hypertension | 64 (70%) | 126 (74%) | 0.36 |
| History of diabetes | 32 (35%) | 67 (40%) | 0.48 |
| Number of cardiac risk factors | 2.4 ± 1.1 | 2.38 ± 1.13 | 0.91 |
| Aspirin | 63 (69%) | 95 (56%) | 0.04 |
| β blockers | 45 (49%) | 80 (47%) | 0.74 |
| % maximum age-predicted heart rate | 86 ± 16 | 88 ± 11 | 0.37 |
| Treadmill exercise | 31 (34%) | 76 (45%) | 0.12 |
| Abnormal stress ECG results | 14 (15%) | 45 (26%) | 0.03 |
| Rest wall motion score index | 1.3 ± 0.4 | 1.2 ± 0.3 | 0.19 |
| Number of new ischemic wall motion abnormalities | — | 4.5 ± 3.1 | <0.0001 |
| Peak wall motion score index | 1.2 ± 0.3 | 1.6 ± 0.4 | <0.0001 |
| Ejection fraction (%) | 56 ± 6 | 55 ± 5 | 0.36 |
| 1-vessel disease | 45 (50%) | 71 (42%) | 0.25 |
| 2- or 3-vessel disease | 46 (50%) | 98 (58%) | 0.25 |
| Cardiac events | |||
| Myocardial infarction | 3 (3.3%) | 15 (8.8%) | 0.09 |
| Cardiac death | 0 (0.0%) | 11 (6.5%) | 0.01 |
Data are presented as mean ± SD or as number (percentage).
Descriptive patient characteristics and exercise and stress echocardiographic variables in patients with and without cardiac events on follow-up are listed in Table 2.
Table 2.
Clinical characteristics in patients with cardiac events and no events
| Variable | Cardiac Events
|
No Events
|
p Value |
|---|---|---|---|
| (n = 29) | (n = 231) | ||
| Age (years) | 66 ± 12 | 63 ± 10 | 0.38 |
| Men | 18 (62%) | 134 (58%) | 0.69 |
| Abnormal rest ECG results | 19 (66%) | 120 (55%) | 0.16 |
| Previous myocardial infarction | 16 (55%) | 75 (32%) | 0.01 |
| Previous percutaneous coronary intervention | 4 (14%) | 60 (26%) | 0.16 |
| Previous bypass surgery | 6 (21%) | 40 (17%) | 0.62 |
| History of hypertension | 25 (86%) | 165 (71%) | 0.12 |
| History of diabetes | 14 (48%) | 85 (36%) | 0.28 |
| Number of cardiac risk factors | 2.2 ± 1.01 | 2.4 ± 1.13 | 0.37 |
| Aspirin | 17 (59%) | 141 (61%) | 0.78 |
| β blockers | 17 (59%) | 108 (47%) | 0.22 |
| % maximum age-predicted heart rate | 87 ± 9 | 87 ± 13 | 0.94 |
| Treadmill exercise | 7 (24%) | 100 (43%) | 0.08 |
| Abnormal stress ECG results | 6 (21%) | 53 (23%) | 0.77 |
| Rest wall motion score index | 1.1 ± 0.3 | 1.2 ± 0.4 | 0.20 |
| Number of new ischemic wall motion abnormalities | 3.6 ± 2.6 | 2.9 ± 3.4 | 0.005 |
| Peak wall motion score index | 1.4 ± 0.4 | 1.4 ± 0.5 | 0.51 |
| Ejection fraction (%) | 56 ± 5 | 55 ± 5 | 0.67 |
| 1-vessel disease | 8 (28%) | 108 (47%) | 0.04 |
| 2- or 3-vessel disease | 21 (72%) | 123 (53%) | 0.04 |
Data are presented as mean ± SD or as number (percentage).
All variables listed in Table 1 were considered in the univariate analysis. Significant univariate predictors of cardiac events are listed in Table 3. Clinical and echocardiographic variables significant on univariate analysis were considered in multivariate analysis. On multivariate logistic regression analysis, multivessel CAD (hazard ratio 2.53, 95% confidence interval 1.16 to 5.51, p = 0.02) and the number of segments in which ischemia was present (hazard ratio 4.31, 95% confidence interval 1.29 to 14.38, p = 0.01) were predictors of cardiac events.
Table 3.
Univariate and multivariate predictors of cardiac events
| Variable | Hazard Ratio | 95% Confidence Interval | p Value |
|---|---|---|---|
| Univariate predictors | |||
| Age (years) | 1.04 | 1.00–1.08 | 0.03 |
| Hypertension | 0.97 | 0.94–1.0 | 0.04 |
| Multivessel disease | 3.66 | 1.50–8.97 | 0.004 |
| Number of new ischemic wall motion abnormalities | 5.05 | 1.53–16.7 | 0.008 |
| Multivariate predictors | |||
| Multivessel disease | 2.53 | 1.16–5.51 | 0.02 |
| Number of new ischemic wall motion abnormalities | 4.31 | 1.29–14.38 | 0.01 |
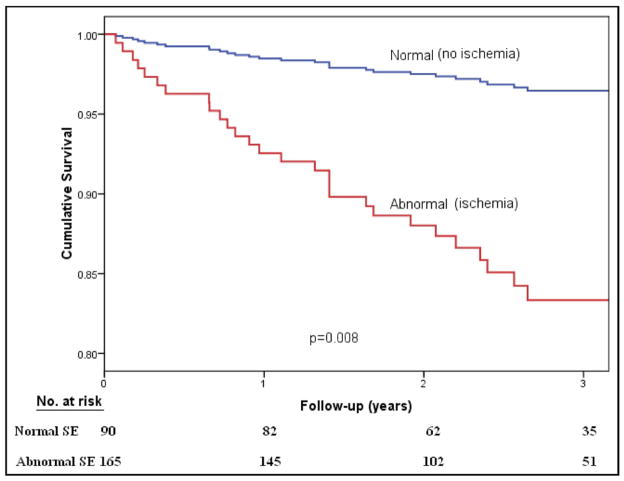
Figure 1 shows cumulative survival curves in patients with angiographic CAD as a function of stress echocardiographic results. Normal results on stress echocardiography identified patients at low risk. Cumulative survival was worse with abnormal stress echocardiographic results.
Figure 1.
Cumulative survival as a function of stress echocardiographic results using cardiac events as an end point.
The chi-square statistic is an index of the predictive power of important variables from 4 major categories (Figure 2). The addition of stress electrocardiography to clinical data minimally improved the chi-square value to 13.3 (p = 0.66). The addition of coronary angiographic data to clinical and stress ECG variables improved the chi-square value to 19.58 (p = 0.002). The addition of stress echocardiographic data to clinical, stress ECG, and angiographic variables improved the chi-square value to 20.8 (p = 0.02). The chi-square value of all variables (clinical, stress ECG, angiographic, and stress echocardiographic) was 26.4 (p = 0.004).
Figure 2.
Independent and incremental prognostic power of clinical (age, gender, and chest pain), stress ECG, angiographic (cath), and stress echocardiographic (SE) variables.
Discussion
The results of this study demonstrate that in patients with angiographically significant CAD, stress echocardiography provides independent and incremental prognostic information over clinical, ECG, and coronary angiographic data. Multivessel CAD and the number of segments in which ischemia was present were predictors of cardiac events. Normal results on stress echocardiography in patients with CAD conferred a benign prognosis.
Previous studies have demonstrated that normal stress echocardiographic results are associated with a benign prognosis.5–7 These results compare favorably with those of normal myocardial perfusion findings, which are similarly associated with a benign prognosis.13 Stress echocardiography can also identify an intermediate-risk group (1%/year to 5%/year).7 Stress echocardiography also influences clinical decision making in higher risk patients, with significantly increased referral to coronary angiography and revascularization. Patients with markedly abnormal stress echocardiographic results (peak wall motion score index >1.7) and at highest cardiac risk are most likely to benefit from coronary revascularization.14,15
In this study, the extent of CAD from coronary angiography is an important predictor of prognosis.16 Furthermore, the findings demonstrate that in stable patients with CAD, the number of segments in which ischemia was present from stress echocardiography provided incremental prognostic information over clinical, stress ECG, and coronary angiographic data.
The presence of a normal wall motion response to exercise conferred a benign prognosis. In this study, about 50% of patients with normal stress echocardiographic findings had multivessel CAD. Despite angiographically significant CAD, normal LV function in most patients, the potential presence of coronary collaterals (not assessed in this study), and possible visual overestimation of CAD severity by angiography may explain the absence of myocardial ischemia on stress echocardiography. Although not examined in this study, patients with CAD and stable symptoms are at low risk and may perhaps experience acceptable lowering of cardiac risk by an initial strategy of optimal medical treatment and aggressive risk factor modification alone.17 Additionally, an invasive management strategy with coronary revascularization may be reserved for patients at high risk identified by stress echocardiography, or those with lower risk and refractory symptoms. The role of coronary revascularization is unclear in the absence of documented myocardial ischemia. In contrast, the presence of abnormal stress echocardiographic findings in patients with angiographically significant CAD conferred an intermediate to high cardiac event rate. Coronary revascularization should be considered in conjunction with optimal medical treatment and risk factor modification as an individualized approach to lower cardiac risk. The fact that patients with abnormal stress echocardiographic results were not revascularized implies that such decisions are often complex, incorporating variables of high-risk markers, co-morbidities, the presence of symptoms, patient and physician preferences, and other factors into a comprehensive decision.
Thus, stress echocardiography optimizes clinical decision making by identifying which patients with angiographically significant CAD demonstrate myocardial ischemia to strengthen decision making regarding coronary revascularization. In fact, the underuse of noninvasive testing to document ischemia before elective percutaneous coronary intervention has recently been called into question.18 Stress echocardiography may serve an important role in identifying patients for coronary revascularization within an individualized strategy based on the assessment of patient risks and benefits.15 From this study, physiologic data derived from stress echocardiography may have additive prognostic value to extent of CAD alone by coronary angiography for decision making regarding coronary revascularization.
There has been a long-standing debate with respect to whether stress imaging or coronary angiography (or computed tomographic angiography) is more valuable in CAD assessment. An anatomic approach with superior accuracy (high negative predictive value) may be justified in patients with higher CAD likelihood by saving the added cost of stress imaging to routine evaluations. The exclusion of anatomic CAD also addresses the difficulty in managing patients with normal stress imaging results and recurrent chest pain symptoms. However, stress imaging experts argue that a conservative sequential testing strategy is cost effective and would avoid the invasive nature of coronary angiography and radiation exposure of fluoroscopy (or computed tomographic angiography) in most patients.19 A noninvasive approach would avoid unnecessary coronary angiography, potential revascularizations (the “oculostenotic reflex”), and associated risks and costs incurred.20 Stress echocardiography can also differentiate high-risk patients with severe LV dysfunction into those with extensive scar (severe LV dysfunction) in contrast to hibernating myocardium.7
However, in the present study, we did not prospectively compare different management strategies. The Prospective Imaging Study for Evaluation of Chest Pain (PROMISE) is a large randomized trial funded by the National Heart, Lung, and Blood Institute comparing diagnostic strategies for the assessment of new or worsening but stable symptoms suspicious for CAD and is currently recruiting patients.21 This trial will determine whether the information derived from an anatomic imaging strategy (>64-slice computed tomographic angiography) compared to a functional testing strategy (exercise electrocardiography, nuclear stress testing, stress echocardiography) will provide superior clinical outcomes (death, myocardial infarction, medical costs, quality of life) in patients with symptoms concerning for CAD. The Fractional Flow Reserve in Contrast to Angiography for Guiding Percutaneous Coronary Intervention (FAME) study22 supports the findings in the present study. Routine measurement of fractional flow reserve in patients with multivessel CAD who undergo percutaneous coronary intervention with drug-eluting stents significantly reduces the number of stents used per patient (1.9 ± 1.3 and 2.7 ± 1.2, p <0.001) and the composite 1-year event rate (13.2% and 18.3, p = 0.02).
In this study, stress echocardiography added significant incremental prognostic value to coronary angiography and can identify patients with angiographic CAD at highest cardiac risk.
References
- 1.Chaudhry FA. Adenosine stress echocardiography. Am J Cardiol. 1997;79:25–29. doi: 10.1016/s0002-9149(97)00260-9. [DOI] [PubMed] [Google Scholar]
- 2.Marwick TH, Mehta R, Arheart K, Lauer MS. Use of exercise echocardiography for prognostic evaluation of patients with known or suspected coronary artery disease. J Am Coll Cardiol. 1997;30:83–90. doi: 10.1016/s0735-1097(97)00148-4. [DOI] [PubMed] [Google Scholar]
- 3.Chuah SC, Pellikka PA, Roger VL, McCully RB, Seward JB. Role of dobutamine stress echocardiography in predicting outcome in 860 patients with known or suspected coronary artery disease. Circulation. 1998;97:1474–1480. doi: 10.1161/01.cir.97.15.1474. [DOI] [PubMed] [Google Scholar]
- 4.Krivokapich J, Child JS, Walter DO, Garfinkel A. Prognostic value of dobutamine stress echocardiography in predicting cardiac events in patients with known or suspected coronary artery disease. J Am Coll Cardiol. 1999;33:708–716. doi: 10.1016/s0735-1097(98)00632-9. [DOI] [PubMed] [Google Scholar]
- 5.Marwick TH, Case C, Sawada S, Rimmerman C, Brenneman P, Kovacs R, Short L, Lauer M. Prediction of mortality using dobutamine echocardiography. J Am Coll Cardiol. 2001;37:754–760. doi: 10.1016/s0735-1097(00)01191-8. [DOI] [PubMed] [Google Scholar]
- 6.Sicari R, Pasanisi E, Venneri L, Landi P, Cortigiani L, Picano E. Stress echo results predict mortality: a large-scale multicenter prospective international study. J Am Coll Cardiol. 2003;41:589–595. doi: 10.1016/s0735-1097(02)02863-2. [DOI] [PubMed] [Google Scholar]
- 7.Yao S, Qureshi E, Sherrid MV, Chaudhry FA. Practical applications in stress echocardiography: risk stratification and prognosis in patients with known or suspected ischemic heart disease. J Am Coll Cardiol. 2003;42:1084–1090. doi: 10.1016/s0735-1097(03)00923-9. [DOI] [PubMed] [Google Scholar]
- 8.Yao S, Qureshi E, Syed A, Chaudhry FA. Novel stress echocardiographic model incorporating the extent and severity of wall motion abnormality for risk stratification and prognosis. Am J Cardiol. 2004;94:715–719. doi: 10.1016/j.amjcard.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Proudfit WJ, Bruschke AV, MacMillan JP, Williams GW, Sones FM., Jr Fifteen year survival study of patients with obstructive coronary artery disease. Circulation. 1983;68:986–997. doi: 10.1161/01.cir.68.5.986. [DOI] [PubMed] [Google Scholar]
- 10.Shiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I, Silverman NH, Tajik AJ. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 11.Chaudhry FA, Tauke JT, Alessandrini RS, Vardi G, Parker MA, Bonow RO. Prognostic implications of myocardial contractile reserve in patients with coronary artery disease and left ventricular dysfunction. J Am Coll Cardiol. 1999;34:730–738. doi: 10.1016/s0735-1097(99)00252-1. [DOI] [PubMed] [Google Scholar]
- 12.Stamm RB, Carabello BA, Mayers DL, Martin RP. Two-dimensional echocardiographic measurement of left ventricular ejection fraction: prospective analysis of what constitutes an adequate determination. Am Heart J. 1982;104:136–144. doi: 10.1016/0002-8703(82)90651-2. [DOI] [PubMed] [Google Scholar]
- 13.Yao S, Rozanski A. Principal uses of myocardial perfusion scintigraphy in the management of patients with known or suspected coronary artery disease. Prog Cardiovasc Dis. 2001;43:281–302. doi: 10.1053/pcad.2001.20466. [DOI] [PubMed] [Google Scholar]
- 14.Yao S, Shah A, Bangalore S, Chaudhry FA. Transient ischemic left ventricular cavity dilation is a significant predictor of severe and extensive coronary artery disease and adverse outcome in patients undergoing stress echocardiography. J Am Soc Echocardiogr. 2007;20:352–358. doi: 10.1016/j.echo.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Yao S, Bangalore S, Chaudhry FA. Prognostic implications of stress echocardiography and impact on patient outcomes: an effective gatekeeper for coronary angiography and revascularization. J Am Soc Echocardiogr. 2010;23:832– 839. doi: 10.1016/j.echo.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Mock MB, Ringqvist I, Fisher LD, Davis KB, Chaitman BR, Kouchoukos NT, Kaiser GC, Alderman E, Ryan TJ, Russell RO, Jr, Mullin S, Fray D, Killip T., III Survival of medically treated patients in the Coronary Artery Surgery Study (CASS) registry. Circulation. 1982;66:562–568. doi: 10.1161/01.cir.66.3.562. [DOI] [PubMed] [Google Scholar]
- 17.Shaw LJ, Hachamovitch R, Berman DS, Marwick TH, Lauer MS, Heller GV, Iskandrian AE, Kesler KL, Travin MI, Lewin HC, Hendel RC, Borges-Neto S, Miller DD Economics of Noninvasive Diagnosis (END) Multicenter Study Group. The economic consequences of available diagnostic and prognostic strategies for the evaluation of stable angina patients: an observational assessment of the value of precatheterization ischemia. J Am Coll Cardiol. 1999;33:661– 669. doi: 10.1016/s0735-1097(98)00606-8. [DOI] [PubMed] [Google Scholar]
- 18.Lin GA, Dudley RA, Lucas FL, Malenka DJ, Vittinghoff E, Redberg RF. Frequency of stress testing to document ischemia prior to elective percutaneous coronary intervention. JAMA. 2008;300:1765–1773. doi: 10.1001/jama.300.15.1765. [DOI] [PubMed] [Google Scholar]
- 19.Thompson RC, Cullom SJ. Issues regarding radiation dosage of cardiac nuclear and radiography procedures. J Nucl Cardiol. 2006;13:19–23. doi: 10.1016/j.nuclcard.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Topol EJ, Nissen SE. Our preoccupation with coronary luminology: the dissociation between clinical and angiographic findings in ischemic heart disease. Circulation. 1995;92:2333–2342. doi: 10.1161/01.cir.92.8.2333. [DOI] [PubMed] [Google Scholar]
- 21.Douglas P. [Accessed on September 25, 2011];Prospective Imaging Study for Evaluation of Chest Pain (PROMISE) trial. Available at: http://www.nhlbi.nih.gov/recovery/researchers/index.php?id=226.
- 22.Tonino PAL, De Bruyne B, Pijls NHJ, Siebert U, Lkeno F, van’t Veer M, Klauss V, Manoharan G, Engstrom T, Ogdroyd KG, Ver Lee PN, MacCarthy PA, Fearon WF. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213–224. doi: 10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]