Abstract
Despite significant improvement in therapy, the prognosis of cancer with bone metastasis is generally poor. Therefore, there is a great need for new therapeutic approaches for metastatic disease. It has been appreciated that tumor cells metastasize to bone using similar mechanisms of hematopoietic stem cell (HSC) homing to bone marrow (e.g. CXCL12/CXCR4). It was recently found that prostate cancer (PCa) cells target the bone marrow microenvironment for HSCs, or HSC niche, during metastasis. Importantly, these disseminated PCa cells can be mobilized out of the niche using HSC mobilizing agents. These findings suggest that bone marrow HSC niche is a potential therapeutic target for metastatic disease. Therefore, the hypothesis worth considering is that agents that can disrupt the interactions between tumor cells and the HSC niche may prove efficacious when used in conjunction with standard chemotherapeutic agents. Although further understanding of the tumor-niche interactions is needed, the concept of targeting the niche in conjunction with chemotherapy could open up new windows to eradicate incurable metastatic diseases.
Background
Bone is a common metastatic site in certain cancers including breast and prostate cancer (PCa) (1). Bone metastasis displays complex process such that cancer cells leave from a primary tumor, intravasate into the blood stream, and extravasate through the endothelium into bone marrow. Although conventional treatments, such as surgery, chemotherapy, and radiation, have been improved to treat localized cancer, bone metastasis remains a major cause of death in these cancer patients. These current therapies do not always work for metastatic disease and unfortunately effective therapeutic strategies for metastatic disease are illusive. In PCa, disseminated tumor cells (DTCs) occupy the marrow space that can lead to replacement of hematopoieic tissues and create both osteolytic and osteoblastic lesions in metastatic bone site that can lead to pain, fractures and spinal-cord compression (2). This suggests that the interactions with the microenvironment are crucial for establishing bone metastasis. Therefore, recent attention has focused on alternative strategies whereby tumor cells may be eliminated by manipulating their microenvironment (3). The growth and metastasis of solid tumors are thought to rely on tumor microenvironments (or non-tumor host cells), including endothelial cells, cancer-associated fibroblasts, and tumor-associated macrophages (3). The interaction between tumor cells and their microenvironments is referred to as ‘tumor ecosystem’ (3). Potentially targeting the tumor ecosystem rather than the tumor cells alone may open up new venues in therapy.
Although multiple factors are thought to be involved in the dissemination process of DTCs to the marrow, growing evidence suggest that DTCs gain access to the bone marrow using similar ‘homing’ mechanisms of hematopoietic stem cells (HSC) (4–10). In the marrow, HSCs reside in a unique microenvironment (or niche) (11). While defining the cells responsible for creating the HSC niche is a topic of great debate, cells of the osteoblastic and endothelial lineage are believed to be major components of the niche (11). Chemokine gradients in the marrow and adhesion molecules expressed by the HSC niche are believed to be crucial for HSC homing (11). Chemokine is a small cytokine that binds to G protein-coupled receptors containing 7 transmembrane domains. Its major role is chemotaxis, or cellular trafficking. Several downstream signaling pathways are also activated to regulate survival and proliferate of both normal and malignant cells, when chemokines bind to their receptors. One of the most well studied molecules involved in HSC homing is chemokine CXCL12 (or SDF-1) which is expressed by several cell types in the marrow (11–12). Binding between CXCL12 to its receptor CXCR4 plays an important role in regulating homing, adhesion, and survival of HSCs through the key pathways including phosphatidylinositol 3-kinases (PI3K)/Akt, mitogen-activated protein (MAPK)/extracellular-signal-regulated kinases (ERK), or janus kinase (JAK)/signal transducer and activator of transcription (STAT) (Figure 1) (11–14). In this context, serin-threonine protein phosphatase 2A regulates CXCL12-mediated chemotaxis and adhesion of human cord blood HSCs through Akt signaling pathway (15). Cross-talk between CXCL12 and TGF-β signaling pathways control the cell-cycling of HSCs by activating PI3K/Akt/Foxo3a/mammalian target of rapamycin (mTOR) pathway (16). In addition, by coupling with Flt3 ligand, which is known to promote HSC homing and proliferation, CXCL12 facilitates migration of HSCs via MAPK, cyclic adenosine monophosphate response element binding protein (CREB), and Akt pathway (17). Conversely, CXCL12-mediated cell trafficking of HSCs are prevented by inhibiting JAK2 (18). Consistent with this notion, granulocyte colony-stimulating factor (G-CSF) mobilizes HSCs from the marrow by degrading CXCL12 in the marrow (19). CXCR4 inhibitor AMD3100 has also been appreciated as HSC mobilization agents (20). Conversely, by cleaving CXCL12, the inhibitor of peptidase CD26 (DPPIV/dipeptidylpeptidase IV), which is a glycoprotein expressed on the surface of a variety of cell types and also crucial for tumor progression (21), enhances the engraftment of HSCs during bone marrow transplantation (22).
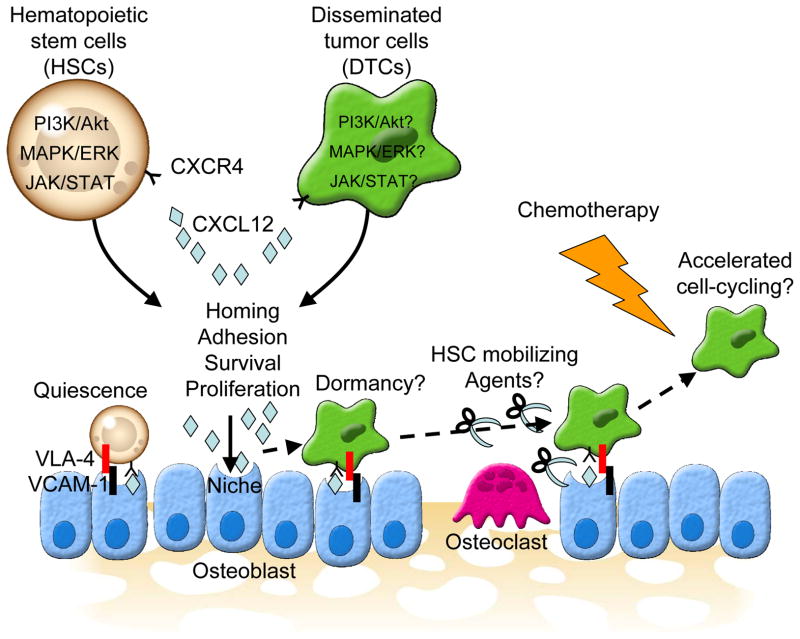
Figure 1. A conceptual scheme of a niche target therapy for bone metastatic disease.
Bone metastatic disseminated tumor cells (DTCs) target osteoblastic hematopoietic stem cell (HSC) niche where HSCs reside, and compete for occupancy of the niche with HSCs. Both DTCs and HSCs are believed to use similar mechanisms to gain access to the HSC niche (e.g. CXCL12/CXCR4 axis, VLA-4/VCAM-1). The CXCL12/CXCR4 axis is believed to play major roles in the homing, adhesion, survival, and proliferation of HSCs. Similar to HSC, accumulating evidence suggest that the CXCL12/CXCR4 axis is also involved in dissemination, adhesion, survival, and growth of DTCs. CXCL12 activates several key survival signaling pathways, phosphatidylinositol 3-kinases (PI3K)/Akt, mitogen-activated protein (MAPK)/extracellular-signal-regulated kinases (ERK), or Janus kinase (JAK)/signal transducer and activator of transcription (STAT), in both HSCs and DTCs by binding to its receptor CXCR4. Herein, both HSCs and DTCs undergo growth arrest, also known as quiescence or dormancy, while preventing them from apoptosis when they bind to the niche. This is why current therapies that target the proliferating cells fail to eradicate DTCs. If the cell-cycle of dormant DTCs are accelerated by mobilizing them out of the niche using HSC mobilizing agents (e.g. G-CSF, AMD 3100), the current existing chemotherapies can be used to treat metastatic disease.
DTCs are frequently found in the bone marrow (23). Based upon the molecular mechanisms related to the HSC homing, it has been recently reported that CXCL12/CXCR4, or alternative receptor CXCR7, chemokine axis also plays a major role in the bone metastasis of PCa (5–6, 8–10). CXCL12 signaling through CXCR4 may trigger the dissemination of PCa by activating αvβ3 integrins (a cell surface receptor play a role in adhesion, migration, invasion, growth, and angiogenesis of tumor cells) (9) and CD164 (a sialomucin protein mediate adhesive function and regulate hematopoiesis) (24) expression in PCa. In addition, the CXCL12/CXCR4 signaling participates in both metastasis and angiogenesis process of PCa by down-regulating the expression and secretion of the glycolytic enzyme phosphoglycerate kinase 1 (PGK1) and angiostatin (25). Importantly, CXCL12 regulates the angiogenic phenotype in PCa through CXCR4 (26). VEGF and TIMP-2 secretion are induced through PI3K/Akt pathway, while IL-6 and IL-8 secretion are stimulated through MAPK/ERK pathway (26). In fact, the metastasis and growth of PCa in bone were prevented by blocking the CXCL12/CXCR4 pathway (6, 8, 10). Therefore, this pathway is also likely to be involved in the bone metastasis of other solid tumors (Figure 1) (4). Additionally, both HSCs and PCa both bind to adhesion molecule annexin II expressed by osteoblasts, and blocking annexin II or the receptor for annexin II prevents homing of both cell types to the marrow (7, 27).
Another major role of niche is to maintain HSC quiescence (11). Quiescence is important for HSCs to retain their self-renewal ability. It was demonstrated that endosteal (or osteoblastic) HSC niche maintains quiescent HSCs through Tie2 (the cell surface receptor for angiopoietin) and its ligand angiopoietin-1 (Ang-1, a growth factor regulates angiogenesis) pathway in the marrow (28). Moreover, quiescent HSCs are thought to be localized closely to endosteal region that is extremely hypoxic (29). Recent studies have shown that hypoxia-inducible factor-1α (HIF-1α) is crucial for HSCs to undergo quiescent in endosteal region (30–31). Likewise, metastatic tumors can exist in dormant state within such hypoxic microenvironment (32). Tumor cells are believed to become dormant to escape from apoptosis and eventually proliferate (33–34). Several lines of evidence also demonstrated that bone marrow cells facilitate drug-resistance of DTCs (35–36). Recent study revealed that the bone marrow microenvironment facilitates the drug resistance in multiple myeloma by enhancing IL-6-mediated STAT3 signaling pathway following the adhesion to β1 integrin (37). Once tumors become dormant, they acquire an ability to evade current chemotherapeutic agent or radiation that targets proliferating (or dividing) cells. Although mechanisms responsible for this remain unknown, these observations indicate that both HSC quiescence and tumor dormancy are regulated by the niche in similar fashion.
Using an in vivo micro-metastatic model (38), it has been demonstrated that disseminated PCa cells compete for occupancy of osteoblastic HSC niche with HSCs to crate metastatic foci (39). In this study, disseminated PCa cells directly compete with transplanted HSCs for occupancy of the osteoblastic HSC niche, and HSCs and PCa co-localize to the endosteal bone surfaces (39). It was possible to recruit greater number of disseminated PCa cells into the vacant niche following the HSCs mobilization out of the niche (39). In addition, the number of DTCs correlated closely with the number of the osteoblastic niche. When the osteoblastic niche was conditionally compromised using skeletal tissues obtained from a transgenicmouse line in which the herpes thymidine kinase gene is fused with the 2.3-kb fragment of the rat type I collagen α1 promoter (40), fewer disseminated PCa cells were observed in micro-metastatic assays (39). Conversely, the expansion of osteoblastic niche with parathyroid hormone (PTH) boosted the dissemination of PCa cells (39). Intriguingly, disseminated PCa cells pushed HSCs outwards from the niche and the cell cycle in HSCs were accelerated (39). Consequently, the number of progenitor cells was increased (39). More importantly, disseminated PCa cells could be mobilized back into the peripheral blood using HSCs mobilizing agents such as granulocyte colony-stimulating factor (G-CSF) and a CXCR4 inhibitor AMD3100 (39).
These findings suggest that the osteoblastic HSC niche serves as a specific component of tumor ecosystem in the marrow, and that the niche may be able to support tumor dormancy as they do to HSCs, and regulate eventual tumor recurrence.
Clinical-translational advances
It is currently clear that existing mono-therapies are not sufficient to eradicate tumor cells once they metastasize to organs. Tumor cells are thought to acquire drug-resistance by interacting with the distant microenvironment. Based upon our recent observation (39), tumor cells which favorably metastasize to bone target the bone marrow microenvironment for HSCs (or HSC niche) and may be parasitic on such microenvironment to survive for an extended period of time. Therefore, the engagement of the HSC niche by DTCs may induce dormancy which protects DTCs from the majority of the existing chemotherapeutic agents. Potentially, interfering with adhesion molecules that link tumor cells to the niche could be an attractive target to reverse their drug resistance. A critical implication of the strategy that target HSC niche is that agents that induce HSCs to leave the niche also stimulate cell cycle progression of the released cells. If similar agents can be used to release dormant DTCs out of the marrow niche, then they too are likely to be susceptible to existing chemotherapeutic agents that target cells in cell cycle.
The CXCL12 and its receptor CXCR4 are believed to play a major role in the HSC mobilization. CXCL12 is known as a molecule associated with HSC homing and osteoblastic niche is one of the major sources of CXCL12 in the marrow (11). The hematopoietic growth factors, such as G-CSF, have been widely used to mobilize HSCs into peripheral blood (19, 41). G-CSF is thought to induce the HSC mobilization by degrading CXCL12 through the stimulation of protease activities (e.g. neutrophil serine proteases, cathepsins, elastase, MMPs, CD26) (41). G-CSF appears to regulate HSC mobilization through actions on bone remodeling. Recent study demonstrated that G-CSF cleaves CXCL12 in the marrow by enhancing the expression of MMP9 and cathepsin K in the osteoclasts (42). It has been also shown that G-CSF reduces the levels of CXCL12 in the marrow by directly inhibiting osteoblastic activity (43–44). The blockade of the receptor for CXCL12 has also been approved as an agent for the HSC mobilization. AMD3100, a CXCR4 antagonist, induces HSC mobilization in mouse and human and exerts synergistic effect on mobilization when administrated with G-CSF (20). In addition, growing evidence suggest that the central nervous system participates in the HSC mobilization from osteoblastic niche via CXCL12/CXCR4 axis (45–47). As well as the blocking of CXCL12/CXCR4 axis, the degradation of other adhesive interactions between HSC and niche has been utilized to mobilize HSCs from the marrow (41). Therefore, a combination of blockade of very late antigen-4 (VLA-4) with G-CSF and/or AMD3100 dramatically augmented the effects of both G-CSF and AMD3100 on HSC mobilization (48–49). VLA-4 is an integrin family protein known to bind to vascular cell adhesion protein 1 (VCAM-1) which appears to play an important role in HSC homing. VLA-4/VCAM-1 interactions appear to be independent of the CXCL12/CXCR4 axis. Likewise, G-CSF mobilized disseminated PCa cells from the niche into the peripheral blood following the induction of MMP2/9 and the enhancement of osteoclastic activity, and so did AMD3100 (39). These findings suggest that therapies that target HSC niche to interfere the tumor-niche interaction are promising.
There are, however, few therapies that target the bone marrow microenvironment, or HSC niche. AMD3100 enhances chemosensitivity of acute myeloid leukemia (AML) (50) and multiple myeloma (51). Consistent with this notion and our findings (39), AMD3100 increases the mobilization of niche-engaged leukemia and myeloma cells into the circulation and enhances their sensitivity to chemotherapy (50–51). Treatment with anti-VLA-4 antibodies is also able to minimize tumor burden in AML in conjunction with chemotherapy (52). Bone metastases of breast and ovarian cancer were significantly prevented by blocking integrin αvβ3 in in vivo model (53). The others target osteoblastic IL-6 activity (54–55), the RANK/RANKL axis (56), and TGF-β signaling in bone marrow stromal cells (57–58).
Conclusions
The tumor ecosystem is a dynamic, complex and evolving environment. Interactions between tumor cells and the surrounding host components provide multiple options as therapeutic targets. Since one of the major functions of the HSC niche is to induce its occupants into growth arrest while supporting their self-renewal (11), tumor cells that metastasize to bone may usurp the HSC niche to become and stay dormant for years. If true, this partially explains why DTCs are sequestered from current therapies. Although the mechanisms that regulate the induction and release of tumor dormancy are poorly understood, the strategy that targets HSC niche to mobilize DTCs using agents used to mobilize HSCs would open up a new window to eradicate incurable DTCs (Figure 1).
Once tumor spreads to distant organs such as bone, survival rates of cancer patients drastically decline. Although significant progress has been made in the early diagnosis and treatment of localized of tumors, we are still losing the battle against metastatic disease. Patients with bone metastases and their families suffer for a long time physically, emotionally and financially. Simultaneously, the financial burden of health care is growing steadily. Therefore, new approaches to cure cancer with bone metastasis are urgently needed. We believe that the concept of mobilizing DTCs out of the HSC niche using HSC mobilizing agents prior to chemotherapy is highly innovative and directly challenges existing paradigms. However, critical questions remain unsolved:
-
1)
Why do DTCs target the osteblastic HSC niche during dissemination?
-
4)
Do all DTCs become dormant?
-
5)
Do specific osteblastic HSC niche subtypes play a role in tumor dormancy?
-
6)
How do DTCs become dormant?
-
7)
Do DTCs use same mechanisms to become dormant as HSCs become quiescence?
-
8)
Can HSC mobilizing agents accelerate the cell-cycling of dormant DTCs?
-
9)
Does DTC mobilization depend on circadian rhythm?
-
10)
Are there any agents that can mobilize only DTCs but not HSCs?
At present, the role of the bone marrow HSC niche in DTCs is not directly known. However the answers to these questions would provide new cellular and molecular targets that could be used as a niche-related therapy for bone metastasis.
Acknowledgments
We thank Drs. Laurie K. McCauley and Evan T. Keller for scientific discussions. This work is directly supported by a Pediatric Oncology Research Fellowship (Y.S.), the Charles Eliot Ware Memorial Fellowship (A.M.H.), the National Cancer Institute (CA093900, K.J.P. and R.S.T.), the Department of Defense (K.J.P. and R.S.T.), and the Prostate Cancer Foundation (K.J.P. and R.S.T.). K.J.P. receives support as an American Cancer Society Clinical Research Professor, NIH SPORE in prostate cancer grant P50 CA69568, and the Cancer Center support grant P30 CA46592.
References
- 1.Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s–9s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 2.Logothetis CJ, Lin SH. Osteoblasts in prostate cancer metastasis to bone. Nat Rev Cancer. 2005;5:21–8. doi: 10.1038/nrc1528. [DOI] [PubMed] [Google Scholar]
- 3.Pienta KJ, McGregor N, Axelrod R, Axelrod DE. Ecological therapy for cancer: defining tumors using an ecosystem paradigm suggests new opportunities for novel cancer treatments. Transl Oncol. 2008;1:158–64. doi: 10.1593/tlo.08178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–6. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 5.Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 2002;62:1832–7. [PubMed] [Google Scholar]
- 6.Sun YX, Schneider A, Jung Y, Wang J, Dai J, Cook K, et al. Skeletal localization and neutralization of the SDF-1(CXCL12)/CXCR4 axis blocks prostate cancer metastasis and growth in osseous sites in vivo. J Bone Miner Res. 2005;20:318–29. doi: 10.1359/JBMR.041109. [DOI] [PubMed] [Google Scholar]
- 7.Shiozawa Y, Havens AM, Jung Y, Ziegler AM, Pedersen EA, Wang J, et al. Annexin II/annexin II receptor axis regulates adhesion, migration, homing, and growth of prostate cancer. J Cell Biochem. 2008;105:370–80. doi: 10.1002/jcb.21835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun YX, Wang J, Shelburne CE, Lopatin DE, Chinnaiyan AM, Rubin MA, et al. Expression of CXCR4 and CXCL12 (SDF-1) in human prostate cancers (PCa) in vivo. J Cell Biochem. 2003;89:462–73. doi: 10.1002/jcb.10522. [DOI] [PubMed] [Google Scholar]
- 9.Sun YX, Fang M, Wang J, Cooper CR, Pienta KJ, Taichman RS. Expression and activation of alpha v beta 3 integrins by SDF-1/CXC12 increases the aggressiveness of prostate cancer cells. Prostate. 2007;67:61–73. doi: 10.1002/pros.20500. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Shiozawa Y, Wang Y, Jung Y, Pienta KJ, Mehra R, et al. The Role of CXCR7/RDC1 as a Chemokine Receptor for CXCL12/SDF-1 in Prostate Cancer. J Biol Chem. 2008;283:4283–94. doi: 10.1074/jbc.M707465200. [DOI] [PubMed] [Google Scholar]
- 11.Taichman RS. Blood and bone: two tissues whose fates are intertwined to create the hematopoietic stem-cell niche. Blood. 2005;105:2631–9. doi: 10.1182/blood-2004-06-2480. [DOI] [PubMed] [Google Scholar]
- 12.Broxmeyer HE. Chemokines in hematopoiesis. Curr Opin Hematol. 2008;15:49–58. doi: 10.1097/MOH.0b013e3282f29012. [DOI] [PubMed] [Google Scholar]
- 13.Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–8. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 14.Kucia M, Reca R, Miekus K, Wanzeck J, Wojakowski W, Janowska-Wieczorek A, et al. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells. 2005;23:879–94. doi: 10.1634/stemcells.2004-0342. [DOI] [PubMed] [Google Scholar]
- 15.Basu S, Ray NT, Atkinson SJ, Broxmeyer HE. Protein phosphatase 2A plays an important role in stromal cell-derived factor-1/CXC chemokine ligand 12-mediated migration and adhesion of CD34+ cells. J Immunol. 2007;179:3075–85. doi: 10.4049/jimmunol.179.5.3075. [DOI] [PubMed] [Google Scholar]
- 16.Chabanon A, Desterke C, Rodenburger E, Clay D, Guerton B, Boutin L, et al. A cross-talk between stromal cell-derived factor-1 and transforming growth factor-beta controls the quiescence/cycling switch of CD34(+) progenitors through FoxO3 and mammalian target of rapamycin. Stem Cells. 2008;26:3150–61. doi: 10.1634/stemcells.2008-0219. [DOI] [PubMed] [Google Scholar]
- 17.Fukuda S, Broxmeyer HE, Pelus LM. Flt3 ligand and the Flt3 receptor regulate hematopoietic cell migration by modulating the SDF-1alpha(CXCL12)/CXCR4 axis. Blood. 2005;105:3117–26. doi: 10.1182/blood-2004-04-1440. [DOI] [PubMed] [Google Scholar]
- 18.Zhang XF, Wang JF, Matczak E, Proper JA, Groopman JE. Janus kinase 2 is involved in stromal cell-derived factor-1alpha-induced tyrosine phosphorylation of focal adhesion proteins and migration of hematopoietic progenitor cells. Blood. 2001;97:3342–8. doi: 10.1182/blood.v97.11.3342. [DOI] [PubMed] [Google Scholar]
- 19.Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–94. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 20.Broxmeyer HE, Orschell CM, Clapp DW, Hangoc G, Cooper S, Plett PA, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201:1307–18. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun YX, Pedersen EA, Shiozawa Y, Havens AM, Jung Y, Wang J, et al. CD26/dipeptidyl peptidase IV regulates prostate cancer metastasis by degrading SDF-1/CXCL12. Clin Exp Metastasis. 2008;25:765–76. doi: 10.1007/s10585-008-9188-9. [DOI] [PubMed] [Google Scholar]
- 22.Christopherson KW, 2nd, Hangoc G, Mantel CR, Broxmeyer HE. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 2004;305:1000–3. doi: 10.1126/science.1097071. [DOI] [PubMed] [Google Scholar]
- 23.Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer. 2004;4:448–56. doi: 10.1038/nrc1370. [DOI] [PubMed] [Google Scholar]
- 24.Havens AM, Jung Y, Sun YX, Wang J, Shah RB, Buhring HJ, et al. The role of sialomucin CD164 (MGC-24v or endolyn) in prostate cancer metastasis. BMC Cancer. 2006;6:195. doi: 10.1186/1471-2407-6-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang J, Dai J, Jung Y, Wei CL, Wang Y, Havens AM, et al. A glycolytic mechanism regulating an angiogenic switch in prostate cancer. Cancer Res. 2007;67:149–59. doi: 10.1158/0008-5472.CAN-06-2971. [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Sun Y, Song W, Nor JE, Wang CY, Taichman RS. Diverse signaling pathways through the SDF-1/CXCR4 chemokine axis in prostate cancer cell lines leads to altered patterns of cytokine secretion and angiogenesis. Cell Signal. 2005;17:1578–92. doi: 10.1016/j.cellsig.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 27.Jung Y, Wang J, Song J, Shiozawa Y, Havens A, Wang Z, et al. Annexin II expressed by osteoblasts and endothelial cells regulates stem cell adhesion, homing, and engraftment following transplantation. Blood. 2007;110:82–90. doi: 10.1182/blood-2006-05-021352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–61. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Yin T, Li L. The stem cell niches in bone. J Clin Invest. 2006;116:1195–201. doi: 10.1172/JCI28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takubo K, Goda N, Yamada W, Iriuchishima H, Ikeda E, Kubota Y, et al. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell. 2010;7:391–402. doi: 10.1016/j.stem.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 31.Simsek T, Kocabas F, Zheng J, Deberardinis RJ, Mahmoud AI, Olson EN, et al. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell. 2010;7:380–90. doi: 10.1016/j.stem.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li XF, O’Donoghue JA. Hypoxia in microscopic tumors. Cancer Lett. 2008;264:172–80. doi: 10.1016/j.canlet.2008.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer. 2007;7:834–46. doi: 10.1038/nrc2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Townson JL, Chambers AF. Dormancy of solitary metastatic cells. Cell Cycle. 2006;5:1744–50. doi: 10.4161/cc.5.16.2864. [DOI] [PubMed] [Google Scholar]
- 35.Ramasamy R, Lam EW, Soeiro I, Tisato V, Bonnet D, Dazzi F. Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: impact on in vivo tumor growth. Leukemia. 2007;21:304–10. doi: 10.1038/sj.leu.2404489. [DOI] [PubMed] [Google Scholar]
- 36.Bewick MA, Lafrenie RM. Adhesion dependent signalling in the tumour microenvironment: the future of drug targetting. Curr Pharm Des. 2006;12:2833–48. doi: 10.2174/138161206777947704. [DOI] [PubMed] [Google Scholar]
- 37.Shain KH, Yarde DN, Meads MB, Huang M, Jove R, Hazlehurst LA, et al. Beta1 integrin adhesion enhances IL-6-mediated STAT3 signaling in myeloma cells: implications for microenvironment influence on tumor survival and proliferation. Cancer Res. 2009;69:1009–15. doi: 10.1158/0008-5472.CAN-08-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Havens AM, Pedersen EA, Shiozawa Y, Ying C, Jung Y, Sun Y, et al. An in vivo mouse model for human prostate cancer metastasis. Neoplasia. 2008;10:371–80. doi: 10.1593/neo.08154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shiozawa Y, Pedersen EA, Havens AM, Jung Y, Mishra A, Joseph J, et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest. 2011 doi: 10.1172/JCI43414. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Visnjic D, Kalajzic Z, Rowe DW, Katavic V, Lorenzo J, Aguila HL. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004;103:3258–64. doi: 10.1182/blood-2003-11-4011. [DOI] [PubMed] [Google Scholar]
- 41.Nervi B, Link DC, DiPersio JF. Cytokines and hematopoietic stem cell mobilization. J Cell Biochem. 2006;99:690–705. doi: 10.1002/jcb.21043. [DOI] [PubMed] [Google Scholar]
- 42.Kollet O, Dar A, Shivtiel S, Kalinkovich A, Lapid K, Sztainberg Y, et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med. 2006;12:657–64. doi: 10.1038/nm1417. [DOI] [PubMed] [Google Scholar]
- 43.Christopher MJ, Liu F, Hilton MJ, Long F, Link DC. Suppression of CXCL12 production by bone marrow osteoblasts is a common and critical pathway for cytokine-induced mobilization. Blood. 2009;114:1331–9. doi: 10.1182/blood-2008-10-184754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Semerad CL, Christopher MJ, Liu F, Short B, Simmons PJ, Winkler I, et al. G-CSF potently inhibits osteoblast activity and CXCL12 mRNA expression in the bone marrow. Blood. 2005;106:3020–7. doi: 10.1182/blood-2004-01-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lucas D, Battista M, Shi PA, Isola L, Frenette PS. Mobilized hematopoietic stem cell yield depends on species-specific circadian timing. Cell Stem Cell. 2008;3:364–6. doi: 10.1016/j.stem.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mendez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–7. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 47.Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–21. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 48.Bonig H, Watts KL, Chang KH, Kiem HP, Papayannopoulou T. Concurrent blockade of alpha4-integrin and CXCR4 in hematopoietic stem/progenitor cell mobilization. Stem Cells. 2009;27:836–7. doi: 10.1002/stem.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramirez P, Rettig MP, Uy GL, Deych E, Holt MS, Ritchey JK, et al. BIO5192, a small molecule inhibitor of VLA-4, mobilizes hematopoietic stem and progenitor cells. Blood. 2009;114:1340–3. doi: 10.1182/blood-2008-10-184721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nervi B, Ramirez P, Rettig MP, Uy GL, Holt MS, Ritchey JK, et al. Chemosensitization of acute myeloid leukemia (AML) following mobilization by the CXCR4 antagonist AMD3100. Blood. 2009;113:6206–14. doi: 10.1182/blood-2008-06-162123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Azab AK, Runnels JM, Pitsillides C, Moreau AS, Azab F, Leleu X, et al. CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood. 2009;113:4341–51. doi: 10.1182/blood-2008-10-186668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matsunaga T, Takemoto N, Sato T, Takimoto R, Tanaka I, Fujimi A, et al. Interaction between leukemic-cell VLA-4 and stromal fibronectin is a decisive factor for minimal residual disease of acute myelogenous leukemia. Nat Med. 2003;9:1158–65. doi: 10.1038/nm909. [DOI] [PubMed] [Google Scholar]
- 53.Zhao Y, Bachelier R, Treilleux I, Pujuguet P, Peyruchaud O, Baron R, et al. Tumor alphavbeta3 integrin is a therapeutic target for breast cancer bone metastases. Cancer Res. 2007;67:5821–30. doi: 10.1158/0008-5472.CAN-06-4499. [DOI] [PubMed] [Google Scholar]
- 54.Adachi Y, Yoshio-Hoshino N, Nishimoto N. The blockade of IL-6 signaling in rational drug design. Curr Pharm Des. 2008;14:1217–24. doi: 10.2174/138161208784246072. [DOI] [PubMed] [Google Scholar]
- 55.Wallner L, Dai J, Escara-Wilke J, Zhang J, Yao Z, Lu Y, et al. Inhibition of interleukin-6 with CNTO328, an anti-interleukin-6 monoclonal antibody, inhibits conversion of androgen-dependent prostate cancer to an androgen-independent phenotype in orchiectomized mice. Cancer Res. 2006;66:3087–95. doi: 10.1158/0008-5472.CAN-05-3447. [DOI] [PubMed] [Google Scholar]
- 56.Gallagher JC. Advances in bone biology and new treatments for bone loss. Maturitas. 2008;60:65–9. doi: 10.1016/j.maturitas.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 57.Biswas S, Guix M, Rinehart C, Dugger TC, Chytil A, Moses HL, et al. Inhibition of TGF-beta with neutralizing antibodies prevents radiation-induced acceleration of metastatic cancer progression. J Clin Invest. 2007;117:1305–13. doi: 10.1172/JCI30740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stover DG, Bierie B, Moses HL. A delicate balance: TGF-beta and the tumor microenvironment. J Cell Biochem. 2007;101:851–61. doi: 10.1002/jcb.21149. [DOI] [PubMed] [Google Scholar]