Abstract
Recently, we administered fish oil containing eicosapentaenoic acid and docosahexaenoic acid (DHA) to senescence-accelerated mice P8 (SAMP8), in order to investigate the effects on lifespan. Surprisingly, the lifespan of SAMP8 that were fed fish oil was shortened significantly, through a mechanism that likely involved lipid peroxidation. In this study, we investigated this phenomenon in further detail. To examine whether this phenomenon occurs only in SAMP8, we investigated the effect of fish oil on the lifespan of another organism species, Caenorhabditis elegans (C. elegans). C. elegans fed fish oil were cultured and the lifespan monitored. As a consequence of the provision of large amounts of fish oil the lifespan of C. elegans was shortened significantly, whereas an appropriate amount of fish oil extended their lifespan significantly. Lipid peroxide levels in C. elegans that were fed fish oil increased significantly in a dose-dependent manner. However, lipid peroxide levels in C. elegans were inhibited by the addition of fish oil and an antioxidant, α-tocopherol, and completely abrogated the changes in the lifespan. To further confirm whether the oxidation of n-3 polyunsaturated fatty acid in fish oil would change the lifespan of C. elegans, the effect of oxidized DHA was examined. Large amounts of oxidized DHA were found to shorten their lifespan significantly. Thus, fish oil changes the lifespan of C. elegans through lipid peroxidation.
Keywords: C. elegans, fish oil, n-3 polyunsaturated fatty acid, lipid peroxidation, lifespan
Introduction
The life expectancy and health expectancy of the Japanese people are among the greatest in the world.(1) It is thought that diet is a particularly significant contributor to the Japanese longevity. Therefore, a Japanese food boom has occurred around the world. One of the traits of Japanese food is the consumption of a large proportion of seafood.(2) Seafood contains abundant levels of n-3 polyunsaturated fatty acids (n-3 PUFAs), eicosapentaenoic acid [EPA, 20:5 (n-3)] and docosahexaenoic acid [DHA, 22:6 (n-3)]. N-3 PUFAs have beneficial effects in the prevention of cardiovascular disease, cancer and metabolic syndrome.(3–6) Therefore, long-term ingestion of n-3 PUFAs may contribute to longevity. However, n-3 PUFAs are easily oxidized and can increase oxidative stress, both in vitro and in vivo,(7–11) which can in turn promote senescence.(12–14) Therefore, we questioned whether long-term ingestion of n-3 PUFAs would have positive health effects. We recently conducted a small study to examine the relationship between n-3 PUFA and lifespan.(15) In that study, we gave fish oil containing high levels of EPA and DHA to senescence-accelerated mice P8 (SAMP8) to investigate the effects on lifespan. Surprisingly, we found that the lifespan of SAMP8 that were fed fish oil was shortened significantly.(15) Furthermore, promotion of lipid peroxidation played a role in this phenomenon. Therefore, it was suggested that lipid peroxidation in mice that were fed fish oil promoted senescence and shortened their lifespan. Consequently, we began to question the safety of long-term ingestion of fish oil as a health supplement, and we investigated this phenomenon in more detail. In this study, to examine whether this phenomenon is unique to SAMP8, we also investigated the effect of fish oil on the lifespan of another organism species, Caenorhabditis elegans (C. elegans).
C. elegans is a popular model animal for studies of senescence and lifespan, because its lifespan is about three weeks and the culturing conditions are easy.(16) C. elegans is a small, round, transparent nematode (adult size about 1 mm length), that is a bacteriovorus vermiform.(17) C. elegans are predominantly self-fertilizing hermaphrodites, and each individual hermaphrodite produces approximately 300 clonal progeny during its adult life. C. elegans and humans have a lot of common features in the process of senescence. For instance, sarcopenia, the loss of muscle mass, increases with aging in both C. elegans and humans. Caloric restriction can also extend the lifespan of both C. elegans and mammals.(18–20) Furthermore, compounds that extend the lifespan of C. elegans have been shown to be useful for human health.(21–26) For instance, curcumin and catechin that extend the lifespan of C. elegans, also exhibit antitumor activity in mammals.(25,26)
In this study, the effect of fish oil on the lifespan of C. elegans was investigated. C. elegans were fed fish oil, cultured and the effects on lifespan and lipid peroxidation were assessed. To further elucidate the specificity of these effects, the effects of safflower oil and oxidized DHA on the lifespan of C. elegans were investigated.
Materials and Methods
Strain, maintenance and culture conditions
C. elegans strain used in this study was N2 (wild type) and was kindly provided by Dr. Shohei Mitani, Department of Physiology, Tokyo Women’s Medical University School of Medicine, Tokyo, Japan. All nematodes were cultivated at 20°C on nematode growth medium (NGM) agar plates seeded with live bacteria (E. coli, strain OP50) as a food source. Plates with a diameter of 60 mm were used for the lifespan assays, and 90 mm diameter plates were used for other experiments. In order to synchronize the nematode population, nematodes were treated with hypochlorite, which preserves the eggs while killing off bacteria and unprotected worms, and the resulting eggs were placed on NGM plates.(27,28)
Test oils and DHA preparation
The fatty acid composition of the test oils was measured using gas chromatography, as described previously.(29) The fish oil was comprised of 4.6% myristic acid (14:0), 26.1% palmitic acid (16:0), 5.7% stearic acid (18:0), 26.0% oleic acid (18:1 ω-9), 1.6% linoleic acid (18:2 ω-6), 0.5% α-linolenic acid (18:3 ω-3), 1.4% arachidonic acid (20:3 ω-6), 5.2% EPA (20:5 ω-3) and 12.7% DHA (22:6 ω-3). The safflower oil was comprised of 8.1% palmitic acid (16:0), 2.9% stearic acid (18:0), 13.5% oleic acid (18:1 ω-9), 73.6% linoleic acid (18:2 ω-6), 0.5% γ-linolenic acid (18:3 ω-6) and 0.5% α-linolenic acid (18:3 ω-3).
DHA was used as the ethyl ester type [Ethyl cis-4,7,10,13,16,19-Docosahexaenoate (Tokyo Chemical Industry Co., Tokyo, Japan)]. Oxidized DHA (oxDHA) was prepared as follows. DHA (50 mg) was oxidized in 60 mm plates for 10 days at 20°C in air. DHA was dissolved in dimethyl sulfoxide (DMSO) and transferred to a sample tube. The DHA was defined as ”oxDHA”. To confirm whether the oxDHA had been oxidized, the lipid hydroperoxide (LOOH) content in oxDHA was determined using the ferrous oxidation-xylenol orange assay.(30)
Lifespan assay
To assess the lifespan, nematodes were treated with hypochlorite to synchronize the population. Isolated eggs were placed on 60 mm NGM agar plates to hatch. Three-day-old synchronized nematodes were placed on NGM agar plates with OP50 and 5-fluorodeoxyuridine (FUDR, Sigma Chemical Co., Saint Louis, MO), which was used to block the development of progeny. On the next day, nematodes were transferred to test plates (10 nematodes/plate). One day prior to use, the test plates were spotted with the indicated doses of fish oil [0.5–2.0 mg/plate with or without α-tocopherol (αToc)] or safflower oil (0.5–2.0 mg/plate) dissolved in DMSO, or the same amount of DMSO alone, as a vehicle control. The doses of 0.5, 1.0, and 2.0 mg/plate were equal to 17.7, 35.4, and 70.8 µg/cm2, respectively. Nematodes were assessed every 2 days for survival. Nematodes that did not respond to repeated touching or dropping purified water were scored as dead, and were removed from the plates. Fifty to one hundred nematodes were scored for each analysis.(31,32) In addition, the lifespans of nematodes exposed to DHA (1.0 mg/plate) or oxDHA (1.0 mg/plate) were also measured by the above-mentioned method.
Lipid peroxidation
Nematodes were assayed for thiobarbituric acid reactive substances (TBARS), as an index of lipid peroxidation.(33,34) TBARS are products of the oxidative degradation of PUFAs, and are widely used to indicate the presence of oxidative stress.(35,36) In previous reports, TBARS have been used to assess lipid oxidation in fish oil-fed animals.(37–39) Furthermore, the measurement of TBARS has previously correlated well with other methods of assessing oxidative stress. Therefore, measurement of TBARS was used to investigate lipid oxidation in the fish oil-fed nematodes in this study.
Nematodes were treated with hypochlorite to synchronize the population. Isolated eggs were placed on 60 mm NGM agar plates to hatch. Three-day-old synchronized nematodes were placed on NGM agar plates with OP50 and FUDR. On the next day, nematodes were transferred to test plates (100 nematodes/plate). One day prior to use, the 90 mm NGM agar plates were spotted with the indicated doses of fish oil (0–4.5 mg/plate with or without αToc) or safflower oil (0–4.5 mg/plate) dissolved in DMSO, or a similar amount of DMSO alone, as a vehicle control. The doses of 1.125, 2.25, and 4.5 mg/plate were equal to 17.7, 35.4, and 70.8 µg/cm2, respectively, and these concentrations per area were the same as those used in the lifespan assay. Nematodes were incubated on test plates until day 10, and homogenized using the Micro SmashTM microhomogenizing system (Tomy Seiko Co. Ltd., Tokyo, Japan). The homogenized samples (25 µl) were then transferred to sample tubes, to which 1 ml of 1/24 M sulfuric acid and 0.125 ml of 10% phosphotungstic acid were added, and centrifuged at 875 g for 10 min. The resultant supernatants were removed and 0.1 ml of 0.67% thiobarbituric acid solution was added. Next, the tubes were reacted for 1 h at 95°C, after which, 0.5 ml of n-butanol was added. The tubes were then centrifuged at 875 g for 10 min and the fluorescence in the resultant supernatant was measured at 560 nm with excitation at 510 nm using a microplate reader (Infinit F200; Tecan Japan, Kawasaki, Japan). Fluorescence intensity was converted to nmol of malondialdehyde equivalents, based on a standard curve generated with 1,1,3,3-tetraethoxypropane. The amount of protein was determined using the bicinchoninic acid (BCA) protein assay (Thermo, Yokohama, Japan).
Fatty acid analysis
Nematodes were treated with hypochlorite to synchronize the population. Isolated eggs were placed on 60 mm NGM agar plates to hatch. Three-day-old synchronized nematodes were placed on NGM agar plates with OP50 and FUDR. On the next day, nematodes were transferred to test plates (100 nematodes/plate). One day before use, the 90 mm NGM agar plates were spotted with the indicated doses of fish oil (2.8 mg/plate) or safflower oil (2.8 mg/plate) dissolved in DMSO, or a similar amount of DMSO alone, as a vehicle control. Nematodes were incubated on test plates until day 10, and homogenized using the Micro SmashTM. Total lipids were extracted from the homogenized sample using the Bligh & Dyer procedure.(40) A 2 ml volume of methanol:chloroform (2:1, v/v, containing BHT) was added to 400 µl of homogenate and mixed by vortexing for 2 min. One ml of methanol:chloroform (1:1 v/v, containing BHT) was added, mixed by vortexing for 2 min, and centrifuged for 5 min at 4°C and 907 g. The lower layer was transferred to another tube and 1 ml of chloroform containing BHT was added to the upper layer, mixed by vortexing for 2 min, and centrifuged. This step was repeated three times. Total lipids were dried under a stream of nitrogen gas. A known amount of heptadecanoic acid (17:0) (Sigma, St Louis, MO) was added to each test fatty acid as an internal standard and the mixture was methylated with trimethylsilyldiazomethane for 30 min and sodium methoxide/methanol for 10 min at room temperature to prepare the fatty acid methylesters. The product was subjected to gas chromatography (GC-2014, Shimadzu, Kyoto, Japan) using a flame ionization detector and a Supelcowax®-10 fused silica capillary column (60 m × 0.32 mm, 0.25 µm film thickness, Supelco, Bellefonte, PA). The GC conditions were programmed, as described previously.(29) Helium was used as the carrier gas. The injector and detector temperatures were 220 and 250°C, respectively, and the column oven temperature was increased by 20°C/min from 50 to 220°C and then held constant for 27 min. Moreover, the temperature was increased from 220 to 250°C and then held constant for 18 min. Peak components were identified by comparing their retention times with those of a commercial fatty acid methylester (Funakoshi, Tokyo, Japan).
Statistical analysis
Survival data are all expressed relative to the non-treated control group. Survival curves were estimated using the Kaplan-Meier test and comparisons of the curves were performed using the log-rank test. For other experiments, one-way ANOVA was used with Dunnett’s post-hoc test. Results are expressed as the means ± SE. Differences were considered statistically significant at p<0.05 and p<0.01.
Results
Effect of fish oil on C. elegans lifespan
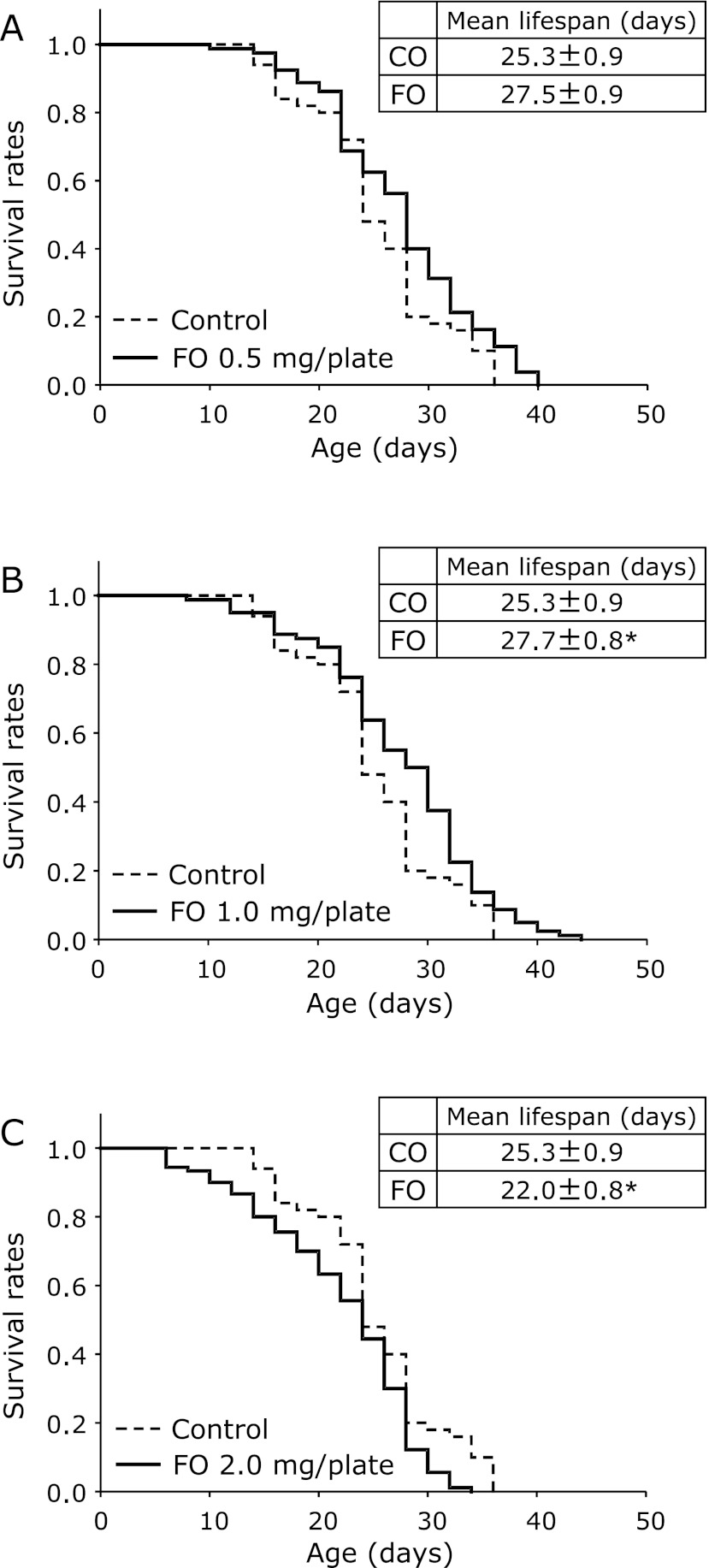
The effect of fish oil on the lifespan of C. elegans was examined. The lifespan of the nematodes was affected by fish oil administration (Fig. 1). The mean lifespan in the 1.0 mg fish oil/plate group was 109.6% of that in the control group; with a significant increase. However, the mean lifespan in the 2.0 mg/plate fish oil group decreased significantly to 87.2% of that in the control group. On the other hand, no significant difference in the mean lifespan was found between the 0.5 mg/plate fish oil group and the control group. Thus, low levels of fish oil increased the lifespan of the nematodes, whereas high levels of fish oil reduced the lifespan.
Fig. 1.
Effects of fish oil on the lifespan of C. elegans. The survival curves of C. elegans fed fish oil [0.5 mg/plate (A), 1.0 mg/plate (B), and 2.0 mg/plate (C)] were compared with that of the control group. Values are expressed as the means ± SE, n = 50–100 for each group. CO, control group. FO, fish oil group. p<0.05 vs CO.
Effect of safflower oil on C. elegans lifespan
To investigate whether the effects on lifespan extension and reduction are specific to fish oil, as opposed to a common effect of oils (i.e., due to the triacylglycerol structure), the effect of safflower oil on the lifespan of C. elegans was examined. The mean lifespans in the 0.5, 1.0, and 2.0 mg/plate safflower oil groups were 101.2%, 104.3%, and 97.2% of that in the control group, respectively (Fig. 2). There were no significant differences in the lifespan among the four groups. Thus, safflower oil did not influence the lifespan of the nematodes.
Fig. 2.
Effects of safflower oil on the lifespan of C. elegans. The lifespan curves of C. elegans fed safflower oil [0.5 mg/plate (A), 1.0 mg/plate (B), and 2.0 mg/plate (C)] were compared with that of the control group. Values are expressed as the means ± SE, n = 50–100 for each group. CO, control group. SO, safflower oil group. p<0.05 vs CO.
Effects of fish oil or safflower oil on lipid peroxidation in C. elegans
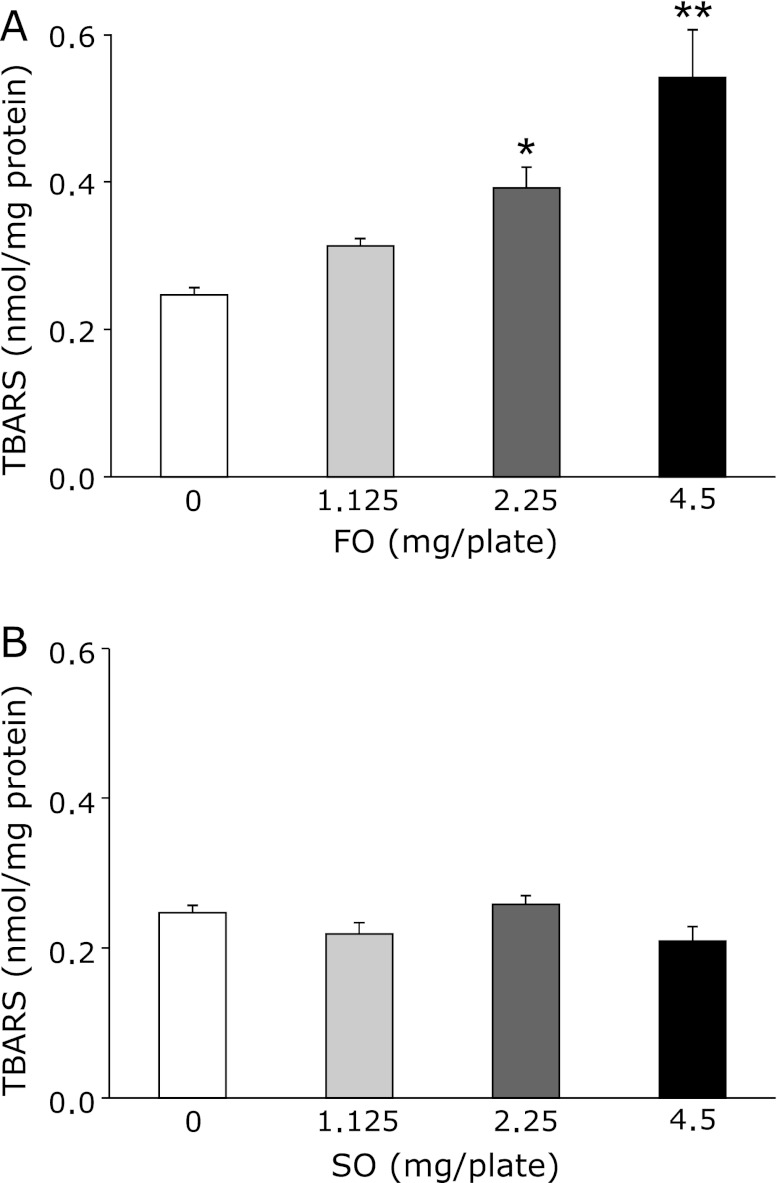
In our previous study, we investigated the effects of fish oil on the lifespan of senescence-accelerated mice, and showed that the lifespan of mice that were fed fish oil was significantly shorter than that of mice that were fed safflower oil.(15) It was also suggested that the mechanism of this phenomenon was through increases in the lipid peroxide levels in mice that were fed fish oil.(15) Therefore, in this study, it was hypothesized that lipid peroxidation would be increased in nematodes that were fed fish oil. To investigate lipid peroxidation in nematodes that were fed fish or safflower oil, 4-day-old nematodes were cultured on plates containing fish oil or safflower oil and 10-day-old nematodes were harvested and utilized for the TBARS assay. TBARS levels in the fish oil groups increased significantly in a dose-dependent manner (Fig. 3). On the other hand, TBARS levels in the safflower oil fed groups were not altered significantly. Thus, we found that fish oil promoted lipid peroxidation in nematodes.
Fig. 3.
Effects of fish oil or safflower oil on lipid peroxidation in C. elegans. TBARS levels of C. elegans fed fish oil (A) or safflower oil (B) were compared with that of the 0 mg/mL Control group. Values are expressed as the means ± SE, n = 6 for each group. TBARS, thiobarbituric acid reactive substances. FO, fish oil group. SO, safflower oil group. *p<0.05, **p<0.01 vs 0 mg/plate.
Effects of fish oil and αToc on the lifespan and lipid peroxidation in C. elegans
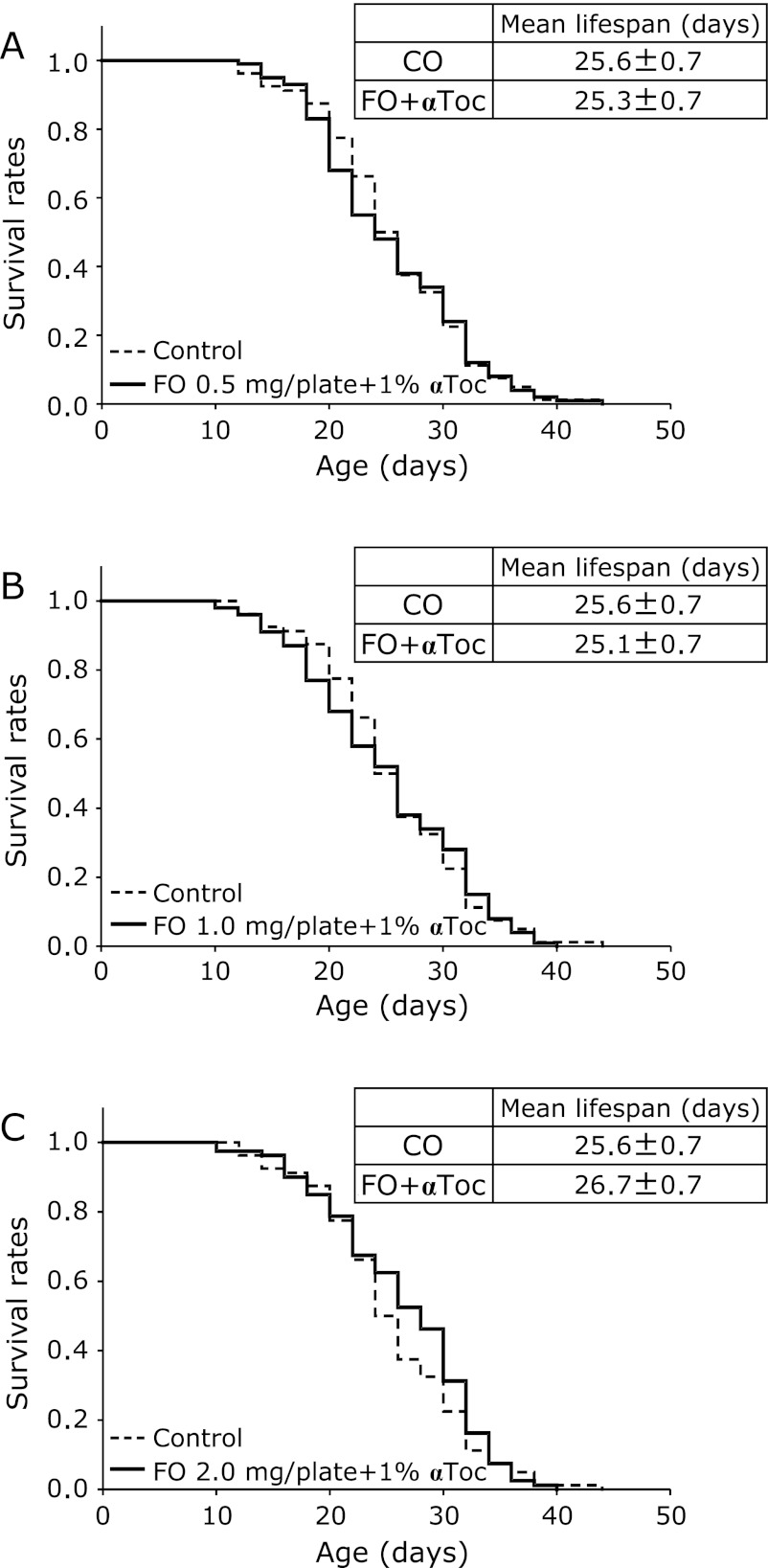
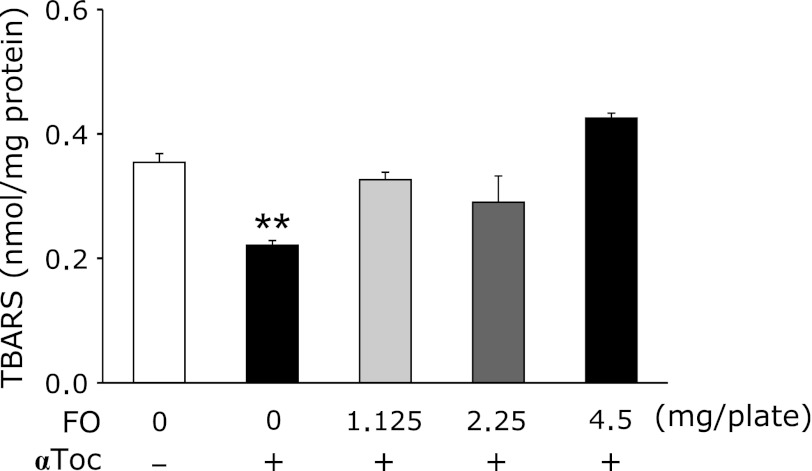
To determine whether lipid peroxidation was involved with the extension and reduction of the lifespan, nematodes were cultured on plates containing fish oil and an antioxidant, αToc. The mean lifespans in 0.5, 1.0, and 2.0 mg/plate fish oil groups with 1% αToc were 98.8%, 98.1%, and 104.3% of that in the control group, respectively (Fig. 4). There were no significant differences in the lifespan between the four groups. The changes in the lifespan of nematodes that were fed fish oil alone were completely abrogated by the addition of αToc. Moreover, the increases in TBARS levels observed in nematodes that were fed fish oil alone were also completely prevented by the addition of αToc (Fig. 5). Thus, lipid peroxidation in the nematodes was inhibited by the addition of the antioxidant and prevented the fish oil-induced changes in the lifespan.
Fig. 4.
Effects of fish oil with α-tocopherol on the lifespan of C. elegans. The survival curves of C. elegans fed safflower oil [0.5 mg/plate (A), 1.0 mg/plate (B), and 2.0 mg/plate (C)] were compared with that of the control group. Values are expressed as the means ± SE, n = 80–100 for each group. CO, control group. FO, fish oil group. αToc; α-tocopherol.
Fig. 5.
Effects of fish oil with α-tocopherol on lipid peroxidation in C. elegans. TBARS levels of C. elegans fed fish oil were compared with that of the 0 mg/mL Control group. Values are expressed as the means ± SE, n = 6 for each group. TBARS, thiobarbituric acid reactive substances. FO, fish oil group. aToc; α-tocopherol. **p<0.01 vs 0 mg/plate without α-tocopherol.
Effects of fish oil on fatty acid composition in C. elegans
To confirm whether nematodes ingest the test oils, we examined the fatty acid composition in nematodes that were fed test oils. As a result, DHA, which is a component of fish oil, was increased in the fish oil group and linoleic acid, which is a component of safflower oil, was increased in the safflower oil group, compared with the respective control groups (Table 1). In the fish oil and safflower oil groups, while oleic acid levels were increased significantly, 13-methyltetradecanoic acid (C15iso), 15-methylhexadecanoic acid (C17iso) and 9,10-methylene hexadecanoic acid (17Δ) levels were decreased significantly. Therefore, because the fatty acid composition in nematodes was greatly altered by the addition of the test oils, we confirmed that the nematodes ingested the test oils.
Table 1.
Fatty acid composition in C. elegans fed test oils
| CO (%) | FO (%) | SO (%) | |
|---|---|---|---|
| 14:0 | 2.2 ± 0.2 | 2.9 ± 0.5 | 1.8 ± 0.2 |
| C15 iso | 5.4 ± 0.2 | 3.0 ± 0.3** | 4.1 ± 0.1** |
| 16:0 | 9.2 ± 2.1 | 14.0 ± 2.5 | 9.6 ± 0.9 |
| 16:1 n-7 | 4.2 ± 0.2 | 5.0 ± 0.1* | 3.3 ± 0.3** |
| C17 iso | 7.4 ± 0.4 | 3.7 ± 0.3** | 4.5 ± 0.4** |
| 17 Δ | 19.2 ± 1.0 | 11.3 ± 1.4** | 13.8 ± 0.7** |
| 18:0 | 7.1 ± 0.6 | 8.1 ± 1.1 | 8.3 ± 0.4 |
| 18:1 n-9 | 2.7 ± 0.6 | 10.9 ± 1.6** | 7.0 ± 1.1* |
| 18:1 n-7 | 19.5 ± 1.5 | 15.2 ± 0.8* | 13.1 ± 0.9** |
| 18:2 n-6 | 6.0 ± 0.2 | 7.9 ± 1.2 | 18.6 ± 0.9** |
| 18:3 n-3 | 3.0 ± 1.6 | 2.3 ± 0.9 | 3.7 ± 1.3 |
| 19 Δ | 5.7 ± 0.9 | 3.1 ± 0.8 | 4.3 ± 1.1 |
| 20:3 n-6 | 1.1 ± 0.2 | 0.5 ± 0.0* | 0.9 ± 0.2 |
| 20:4 n-3 | 1.0 ± 0.1 | 1.8 ± 0.4* | 1.6 ± 0.1 |
| 20:5 n-3 | 6.1 ± 0.2 | 8.0 ± 1.2 | 5.5 ± 0.5 |
| 22:6 n-3 | nd | 2.3 ± 1.0 | nd |
Mean ± SE, *p<0.05, **p<0.01 (vs CO), nd; not detected.
Effect of DHA on C. elegans lifespan
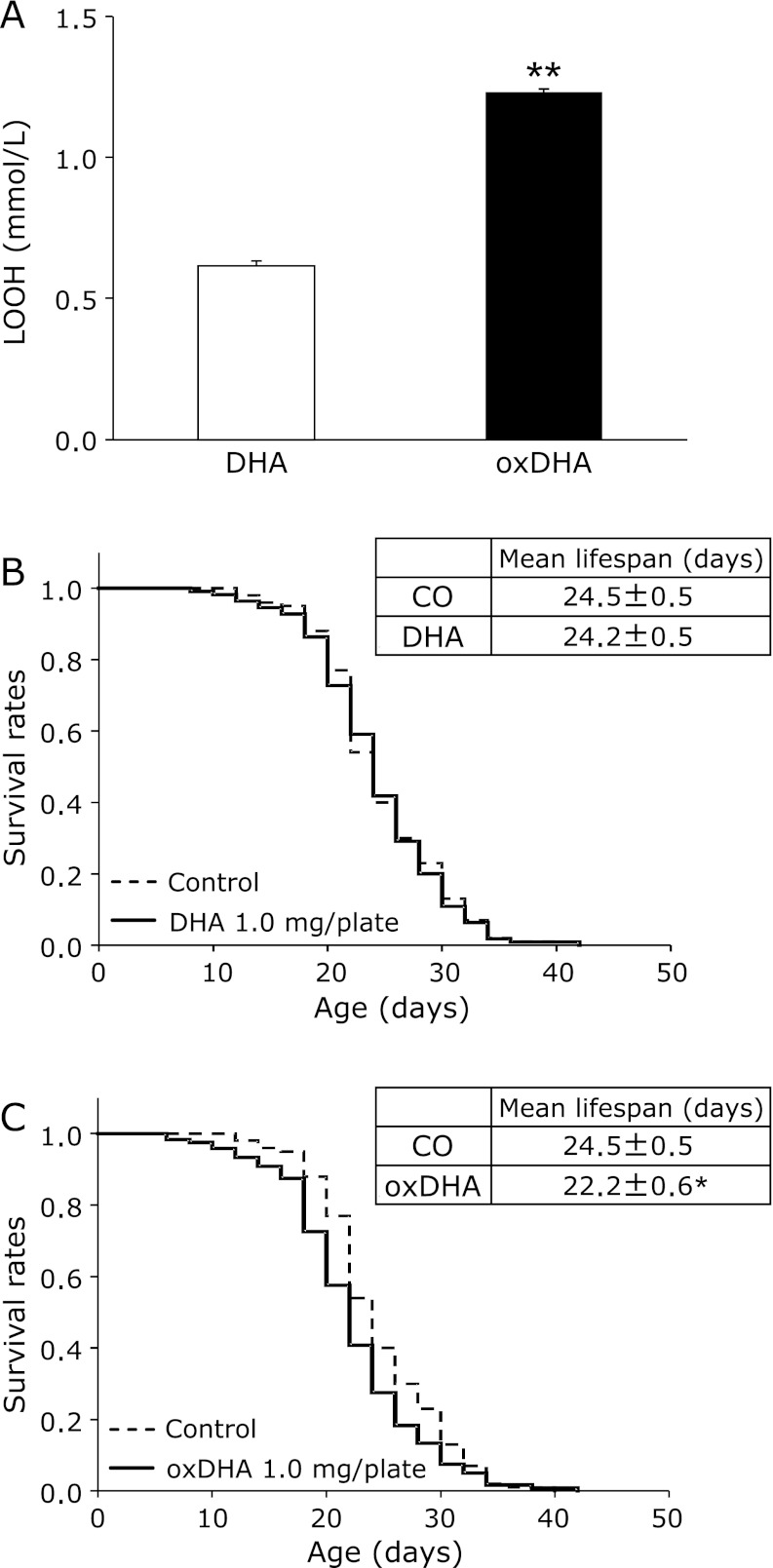
To confirm whether the oxidation of n-3 PUFA in fish oil changes the lifespan of nematodes, the effect of oxidized DHA was examined. First, to confirm whether the oxDHA was oxidized, the LOOH content in oxDHA was determined, and showed that oxDHA had been oxidized significantly (Fig. 6A). Next, nematodes were cultured on plates containing DHA or oxDHA, and the lifespan of nematodes was investigated. No significant difference in the mean lifespan was observed between the DHA group and the control group (Fig. 6B). On the other hand, the mean lifespan in the oxDHA group was decreased significantly to 90.7% of that in the control group (Fig. 6C). Thus, oxidized DHA shortened the lifespan of the nematodes.
Fig. 6.
Effects of oxidized DHA on the lifespan of C. elegans. LOOH content in oxDHA (A). Values are expressed as the means ± SE, n = 6 for each group. **p<0.01 vs DHA. The survival curves of C. elegans fed DHA (1.0 mg/plate) (B) and oxDHA (1.0 mg/plate) (C) were compared with that of the control group. Values are expressed as the means ± SE, n = 100–120 for each group. CO, control group. DHA, DHA group. oxDHA, oxDHA group. *p<0.05 vs CO.
Discussion
In a previous report, we administered fish oil to SAMP8 and measured the effects on the lifespan of these mice. Surprisingly, the lifespan of SAMP8 that were fed fish oil was shortened significantly.(15) In this study, to investigate whether this phenomenon is unique to SAMP8, we investigated the effect of fish oil on the lifespan of another organism species, C. elegans. We found that large amounts of fish oil significantly reduced the lifespan of C. elegans (Fig. 1). To investigate whether this phenomenon is an effect specific to fish oil vs an effect that is common to oils (i.e., due to the triacylglycerol structure), the effect of safflower oil on the lifespan of C. elegans was examined and was found to not influence the lifespan (Fig. 2). Hence, we suggested that the change in the lifespan of nematodes that were fed fish oil was due to the constituent fatty acids of the fish oil. In our previous study, the lifespan reduction in SAMP8 that were fed fish oil was considered to be due to the promotion of lipid peroxidation.(15) Therefore, we investigated lipid peroxidation in nematodes that were fed fish oil and showed that lipid peroxide levels in nematodes increased significantly in a dose-dependent manner (Fig. 3). To determine whether lipid peroxidation was involved in the reduction of the lifespan, nematodes were cultured on plates containing fish oil and an antioxidant, αToc. The changes in lifespan and lipid peroxide levels in nematodes that were fed fish oil alone were abrogated by the addition of αToc (Fig. 4 and 5). It has been reported previously that the ingestion of fish oil can promote lipid peroxidation in human and model animals.(7–11) Furthermore, it has also been reported that oxidative stress promotes senescence,(12–14) and that the nematode lifespan was shortened by oxidative stress;(17,41) similar results were also observed in mice.(15) Overall, it was suggested that the reduction of the lifespan of nematodes that were fed fish oil is dependent on lipid peroxidation. Fish oil is easily oxidized compared with the plant oils, such as safflower oil.(42–45) The increased susceptibility to oxidation of fish oil is due to the content of EPA and DHA, which both have many double bonds. In this study, large amounts of EPA and DHA accumulated in those nematodes that were fed fish oil (Table 1). Therefore, it was suggested that lipid peroxidation in nematodes was promoted by EPA and DHA. In fact, the lifespan of nematodes that were fed oxidized DHA were shortened (Fig. 6).
In this study, the low dose of fish oil significantly prolonged the lifespan of nematodes (Fig. 2). It was believed that the reason for this phenomenon was that lipid peroxide levels were minimal in nematodes that ingested a low dose of fish oil. Moderate oxidative stress increases the stress resistance of C. elegans by increasing antioxidant enzymes, and extends their lifespan.(17,41,46) It has also been reported that n-3 PUFA enhances superoxide dismutase, catalase, and glutathione activity.(47–49) Therefore, in this study, it was suggested that low dose of fish oil induced a moderate oxidative stress that extended the lifespan of the nematodes.
It has been reported that low doses of physiologically-active substances, such as quercetin, curcumin, and plant adaptogen, which have been confirmed to be effective in both human and animal studies, can extend the lifespan of nematodes, whereas high doses of these mediators can shorten the lifespan.(21,50,51) In this study, a similar tendency was observed. It was reported that fish oil has the beneficial effects of preventing cardiovascular disease, cancer and metabolic syndrome.(3–6) On the other hand, the adverse effects of fish oil, including those reported in this study, have also been reported.(17,52–54) Accordingly, it will be necessary to further examine the ingestion level and the duration of fish oil, and to establish methods to effectively use fish oil in the future.
Acknowledgments
This study was supported by a grant-in-aid from the Toyo Institute of Food Technology, Japan.
Abbreviations
- αToc
α-tocopherol
- BCA
bicinchoninic acid
- BHT
butylated hydroxytoluene
- CO
control
- DHA
docosahexaenoic acid
- DMSO
dimethyl sulfoxide
- EPA
eicosapentaenoic acid
- FUDR
5-fluorodeoxyuridine
- FO
fish oil
- GC
gas chromatography
- LOOH
lipid hydroperoxide
- NGM
nematode growth medium
- oxDHA
oxidized DHA
- PUFA
polyunsaturated fatty acids
- SAMP8
senescence-accelerated mouse P8
- SO
safflower oil
- TBARS
thiobarbituric acid reactive substances
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.World Health Organization World Health Statistics 2012. (http://www.who.int/gho/publications/world_health_statistics/EN_WHS2012_Full.pdf). [Google Scholar]
- 2.Food and Agriculture Organization Fishery and Aquaculture Statistics 2009. (http://www.fao.org/docrep/015/ba0058t/ba0058t.pdf). [Google Scholar]
- 3.Spencer L, Mann C, Metcalfe M, et al. The effect of omega-3 FAs on tumour angiogenesis and their therapeutic potential. Eur J Cancer. 2009;45:2077–2086. doi: 10.1016/j.ejca.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 4.Mori TA, Beilin LJ. Long-chain ω 3 fatty acids, blood lipids and cardiovascular risk reduction. Curr Opin Lipidol. 2001;12:11–17. doi: 10.1097/00041433-200102000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Lim K, Han C, Dai Y, Shen M, Wu T. ω-3 polyunsaturated fatty acids inhibit hepatocellular carcinoma cell growth through blocking β-catenin and cyclooxygenase-2. Mol Cancer Ther. 2009;8:3046–3055. doi: 10.1158/1535-7163.MCT-09-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poudyal H, Panchal SK, Diwan V, Brown L. ω-3 fatty acids and metabolic syndrome: effects and emerging mechanisms of action. Prog Lipid Res. 2011;50:372–387. doi: 10.1016/j.plipres.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Kaasgaard SG, Hølmer G, Høy CE, Behrens WA, Beare-Rogers JL. Effects of dietary linseed oil and marine oil on lipid peroxidation in monkey liver in vivo and in vitro. Lipids. 1992;27:740–745. doi: 10.1007/BF02535843. [DOI] [PubMed] [Google Scholar]
- 8.Meydani M, Natiello F, Goldin B, et al. Effect of long-term fish oil supplementation on vitamin E status and lipid peroxidation in women. J Nutr. 1991;121:484–491. doi: 10.1093/jn/121.4.484. [DOI] [PubMed] [Google Scholar]
- 9.Harats D, Dabach Y, Hollander G, et al. Fish oil ingestion in smokers and nonsmokers enhances peroxidation of plasma lipoproteins. Atherosclerosis. 1991;90:127–139. doi: 10.1016/0021-9150(91)90107-e. [DOI] [PubMed] [Google Scholar]
- 10.Miret S, Sáiz MP, Mitjavila MT. Effects of fish oil- and olive oil-rich diets on iron metabolism and oxidative stress in the rat. Br J Nutr. 2003;89:11–18. doi: 10.1079/BJN2002737. [DOI] [PubMed] [Google Scholar]
- 11.Ji H, Li J, Liu P. Regulation of growth performance and lipid metabolism by dietary n-3 highly unsaturated fatty acids in juvenile grass carp, Ctenopharyngodon idellus. Comp Biochem Physiol B Biochem Mol Biol. 2011;159:49–56. doi: 10.1016/j.cbpb.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yanaka M, Honma T, Sato K, et al. Increased monocytic adhesion by senescence in human umbilical vein endothelial cells. Biosci Biotechnol Biochem. 2011;75:1098–1103. doi: 10.1271/bbb.100909. [DOI] [PubMed] [Google Scholar]
- 14.Honma T, Shinohara N, Ito J, et al. High-fat diet intake accelerates aging, increases expression of Hsd11b1, and promotes lipid accumulation in liver of SAMP10 mouse. Biogerontology. 2012;13:93–103. doi: 10.1007/s10522-011-9363-2. [DOI] [PubMed] [Google Scholar]
- 15.Tsuduki T, Honma T, Nakagawa K, Ikeda I, Miyazawa T. Long-term intake of fish oil increases oxidative stress and decreases lifespan in senescence-accelerated mice. Nutrition. 2011;27:334–337. doi: 10.1016/j.nut.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 16.Klass MR. Aging in the nematode Caenorhabditis elegans: major biological and environmental factors influencing life span. Mech Ageing Dev. 1977;6:413–429. doi: 10.1016/0047-6374(77)90043-4. [DOI] [PubMed] [Google Scholar]
- 17.Van Raamsdonk JM, Hekimi S. Reactive oxygen species and aging in Caenorhabditis elegans: causal or casual relationship? Antioxid Redox Signal. 2010;13:1911–1953. doi: 10.1089/ars.2010.3215. [DOI] [PubMed] [Google Scholar]
- 18.Johnson TE, Mitchell DH, Kline S, Kemal R, Foy J. Arresting development arrests aging in the nematode Caenorhabditis elegans. Mech Ageing Dev. 1984;28:23–40. doi: 10.1016/0047-6374(84)90150-7. [DOI] [PubMed] [Google Scholar]
- 19.Houthoofd K, Johnson TE, Vanfleteren JR. Dietary restriction in the nematode Caenorhabditis elegans. J Gerontol A Biol Sci Med Sci. 2005;60:1125–1131. doi: 10.1093/gerona/60.9.1125. [DOI] [PubMed] [Google Scholar]
- 20.Trepanowski JF, Canale RE, Marshall KE, Kabir MM, Bloomer RJ. Impact of caloric and dietary restriction regimens on markers of health and longevity in humans and animals: a summary of available findings. Nutr J. 2011;10:107. doi: 10.1186/1475-2891-10-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liao VH, Yu CW, Chu YJ, Li WH, Hsieh YC, Wang TT. Curcumin-mediated lifespan extension in Caenorhabditis elegans. Mech Ageing Dev. 2011;132:480–487. doi: 10.1016/j.mad.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 22.Saul N, Pietsch K, Menzel R, Stürzenbaum SR, Steinberg CE. Catechin induced longevity in C. elegans: from key regulator genes to disposable soma. Mech Ageing Dev. 2009;130:477–486. doi: 10.1016/j.mad.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Bass TM, Weinkove D, Houthoofd K, Gems D, Partridge L. Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans. Mech Ageing Dev. 2007;128:546–552. doi: 10.1016/j.mad.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Collins JJ, Evason K, Kornfeld K. Pharmacology of delayed aging and extended lifespan of Caenorhabditis elegans. Exp Gerontol. 2006;41:1032–1039. doi: 10.1016/j.exger.2006.06.038. [DOI] [PubMed] [Google Scholar]
- 25.Sebastià N, Soriano JM, Barquinero JF, et al. In vitro cytogenetic and genotoxic effects of curcumin on human peripheral blood lymphocytes. Food Chem Toxicol. 2012;50:3229–3233. doi: 10.1016/j.fct.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 26.Frei B, Higdon JV. Antioxidant activity of tea polyphenols in vivo: evidence from animal studies. J Nutr. 2003;133:3275S–3284S. doi: 10.1093/jn/133.10.3275S. [DOI] [PubMed] [Google Scholar]
- 27.Emmons SW, Klass MR, Hirsh D. Analysis of the constancy of DNA sequences during development and evolution of the nematode Caenorhabditis elegans. Proc Natl Acad Sci USA. 1979;76:1333–1337. doi: 10.1073/pnas.76.3.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steiernagle T. Maintenance of C. elegans. In: Hope IA, editor. C. elegans; a practical approach. Oxford University Press; 1999. pp. 51–67. [Google Scholar]
- 29.Shinohara N, Tsuduki T, Ito J, et al. Jacaric acid, a linolenic acid isomer with a conjugated triene system, has a strong antitumor effect in vitro and in vivo. Biochim Biophys Acta. 2012;1821:980–988. doi: 10.1016/j.bbalip.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Tsuzuki T, Igarashi M, Iwata T, et al. Oxidation rate of conjugated linoleic acid and conjugated linolenic acid is slowed by triacylglycerol esterification and α-tocopherol. Lipids. 2004;39:475–480. doi: 10.1007/s11745-004-1253-z. [DOI] [PubMed] [Google Scholar]
- 31.Powolny AA, Singh SV, Melov S, Hubbard A, Fisher AL. The garlic constituent diallyl trisulfide increases the lifespan of C. elegans via skn-1 activation. Exp Gerontol. 2011;46:441–452. doi: 10.1016/j.exger.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiegant FA, Surinova S, Ytsma E, Langelaar-Makkinje M, Wikman G, Post JA. Plant adaptogens increase lifespan and stress resistance in C. elegans. Biogerontology. 2009;10:27–42. doi: 10.1007/s10522-008-9151-9. [DOI] [PubMed] [Google Scholar]
- 33.Gavino VC, Miller JS, Ikharebha SO, Milo GE, Cornwell DG. Effect of polyunsaturated fatty acids and antioxidants on lipid peroxidation in tissue cultures. J Lipid Res. 1981;22:763–769. [PubMed] [Google Scholar]
- 34.Tsuzuki T, Tokuyama Y, Igarashi M, Miyazawa T. Tumor growth suppression by α-eleostearic acid, a linolenic acid isomer with a conjugated triene system, via lipid peroxidation. Carcinogenesis. 2004;25:1417–1425. doi: 10.1093/carcin/bgh109. [DOI] [PubMed] [Google Scholar]
- 35.Vlková B, Stanko P, Minárik G, et al. Salivary markers of oxidative stress in patients with oral premalignant lesions. Arch Oral Biol. 2012;57:1651–1656. doi: 10.1016/j.archoralbio.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 36.Celec P, Hodosy J, Celecová V, et al. Salivary thiobarbituric acid reacting substances and malondialdehyde—their relationship to reported smoking and to parodontal status described by the papillary bleeding index. Dis Markers. 2005;21:133–137. doi: 10.1155/2005/693437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ebeid TA. The impact of incorporation of n-3 fatty acids into eggs on ovarian follicular development, immune response, antioxidative status and tibial bone characteristics in aged laying hens. Animal. 2011;5:1554–1562. doi: 10.1017/S1751731111000619. [DOI] [PubMed] [Google Scholar]
- 38.Tou JC, Altman SN, Gigliotti JC, Benedito VA, Cordonier EL. Different sources of ω-3 polyunsaturated fatty acids affects apparent digestibility, tissue deposition, and tissue oxidative stability in growing female rats. Lipids Health Dis. 2011;10:179. doi: 10.1186/1476-511X-10-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richard MJ, Portal B, Meo J, Coudray C, Hadjian A, Favier A. Malondialdehyde kit evaluated for determining plasma and lipoprotein fractions that react with thiobarbituric acid. Clin Chem. 1992;38:704–709. [PubMed] [Google Scholar]
- 40.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 41.Zhou KI, Pincus Z, Slack FJ. Longevity and stress in Caenorhabditis elegans. Aging (Albany NY) 2011;3:733–753. doi: 10.18632/aging.100367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boran G, Karacam H, Boran M. Changes in the quality of fish oils due to storage temperature and time. Food Chemistry. 2006;98:693–698. [Google Scholar]
- 43.Cho S-Y, Miyashita K, Miyazawa T, Fujimoto K, Kaneda T. Autoxidation of ethyl eicosapentaenoate and docosahexaenoate. J Am Oil Chem Soc. 1987;64:876–879. [Google Scholar]
- 44.Song JH, Inoue Y, Miyazawa T. Oxidative stability of docosahexaenoic acid-containing oils in the form of phospholipids, triacylglycerols, and ethyl esters. Biosci Biotechnol Biochem. 1997;61:2085–2088. doi: 10.1271/bbb.61.2085. [DOI] [PubMed] [Google Scholar]
- 45.Fritsche KL, Johnston PV. Rapid autoxidation of fish oil in diets without added antioxidants. J Nutr. 1988;118:425–426. doi: 10.1093/jn/118.4.425. [DOI] [PubMed] [Google Scholar]
- 46.Cypser JR, Johnson TE. Multiple stressors in Caenorhabditis elegans induce stress hormesis and extended longevity. J Gerontol A Biol Sci Med Sci. 2002;57:B109–B114. doi: 10.1093/gerona/57.3.b109. [DOI] [PubMed] [Google Scholar]
- 47.Garrel C, Alessandri JM, Guesnet P, Al-Gubory KH. ω-3 fatty acids enhance mitochondrial superoxide dismutase activity in rat organs during post-natal development. Int J Biochem Cell Biol. 2012;44:123–131. doi: 10.1016/j.biocel.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 48.Bhattacharya A, Lawrence RA, Krishnan A, Zaman K, Sun D, Fernandes G. Effect of dietary n-3 and n-6 oils with and without food restriction on activity of antioxidant enzymes and lipid peroxidation in livers of cyclophosphamide treated autoimmune-prone NZB/W female mice. J Am Coll Nutr. 2003;22:388–399. doi: 10.1080/07315724.2003.10719322. [DOI] [PubMed] [Google Scholar]
- 49.Chen HW, Tsai CW, Yang JJ, Liu CT, Kuo WW, Lii CK. The combined effects of garlic oil and fish oil on the hepatic antioxidant and drug-metabolizing enzymes of rats. Br J Nutr. 2003;89:189–200. doi: 10.1079/BJN2002766. [DOI] [PubMed] [Google Scholar]
- 50.Wiegant FA, Surinova S, Ytsma E, Langelaar-Makkinje M, Wikman G, Post JA. Plant adaptogens increase lifespan and stress resistance in C. elegans. Biogerontology. 2009;10:27–42. doi: 10.1007/s10522-008-9151-9. [DOI] [PubMed] [Google Scholar]
- 51.Pietsch K, Saul N, Chakrabarti S, Stürzenbaum SR, Menzel R, Steinberg CE. Hormetins, antioxidants and prooxidants: defining quercetin-, caffeic acid- and rosmarinic acid-mediated life extension in C. elegans. Biogerontology. 2011;12:329–347. doi: 10.1007/s10522-011-9334-7. [DOI] [PubMed] [Google Scholar]
- 52.de Meijer VE, Kalish BT, Meisel JA, Le HD, Puder M.Dietary fish oil aggravates paracetamol-induced liver injury in mice JPEN J Parenter Enteral Nutr 2012. in press. [DOI] [PubMed] [Google Scholar]
- 53.Yi D, Zeng S, Guo Y. A diet rich in n-3 polyunsaturated fatty acids reduced prostaglandin biosynthesis, ovulation rate, and litter size in mice. Theriogenology. 2012;78:28–38. doi: 10.1016/j.theriogenology.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 54.Church MW, Jen KL, Anumba JI, Jackson DA, Adams BR, Hotra JW. Excess ω-3 fatty acid consumption by mothers during pregnancy and lactation caused shorter life span and abnormal ABRs in old adult offspring. Neurotoxicol Teratol. 2010;32:171–181. doi: 10.1016/j.ntt.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]