Abstract
The relationship between Helicobacter pylori (H. pylori) eradication therapy and the risk of developing gastroesophageal reflux disease (GERD) is controversial. We investigated the influence of H. pylori eradication on the risk of GERD by focusing on the quality of life (QOL) and evaluating reflux symptoms. Patients with H. pylori infection were administered triple therapy for H. pylori eradication. At 3 months and 1 year after the eradication therapy, surveys were conducted to determine the health-related QOL by quality of life in reflux and dyspepsia-Japanese version, (QOLRAD-J) and the severity of GERD symptoms by Carlsson-Dent questionnaire (CDQ). Forty patients were included in the analysis. Although no significant changes of these scores were apparent 3 months after H. pylori eradication, the QOLRAD-J and CDQ scores were significantly improved after 1 year. The degree of improvement was even more marked in cases with initially low scores. In conclusion, improved GERD-related QOL and reflux symptoms were noted 1 year after H. pylori eradication therapy. In addition, the degree of improvement was more marked in cases with severe reflux symptoms.
Keywords: Helicobacter pylori, eradication therapy, reflux symptoms, quality of life, questionnaire
Introduction
Helicobacter pylori (H. pylori) eradication therapy has been reported as an effective strategy in the treatment of peptic ulcers and gastric mucosa-associated lymphoid tissue lymphoma, in addition to the prevention of recurrence of gastric cancer after endoscopic resection.(1,2) On the other hand, the influence of H. pylori eradication in the management of gastroesophageal reflux disease (GERD) is controversial. Some researchers have suggested that H. pylori eradication leads to a more resilient GERD.(3–5) Decreased acid secretion in patients with H. pylori infection occurs as a result of progressive gastric mucosal atrophy.(6) Thus, reflux symptoms were thought to be exacerbated after H. pylori eradication therapy because of the recovery of acid secretion. Meanwhile, other researchers have reported that H. pylori eradication does not exacerbate GERD symptoms.(7–9) Sasaki et al.(10) reported that it was rare for reflux esophagitis that develops after H. pylori eradication therapy to become severe or cause long-term GERD symptoms.
Quality-of-life (QOL) is an important determinant of symptom generation in GERD patients. The endoscopic severity of GERD is not always correlated with heartburn severity.(11,12) Regardless of the endoscopic findings, QOL may be greatly reduced by the presence of strong symptoms. It has been reported that GERD-related QOL may be worse than that of mild heart failure or angina.(13) Laine et al.(14) reported no significant change in QOL 6 months after H. pylori eradication therapy, and concluded that H. pylori eradication did not worsen the GERD-related QOL. However, how the GERD-related QOL might change on long-term follow-up has not yet been explored.
Talking medical history is one of the most useful means of diagnosing GERD. The presence of GERD can be diagnosed only by history taking in many cases, although endoscopy, 24 h pH monitoring, etc., have been developed to assist in diagnosis. The heartburn version of QOLRAD(15) (quality of life in reflux and dyspepsia) is a self-administered questionnaire. QOLRAD was created with an emphasis on the GERD-related QOL. QOLRAD is a disease-specific instrument, including 25 items classified into 5 domains: emotional distress, sleep disturbance, food/drink problems, physical/social functioning, and vitality. The scores for each QOLRAD domain are expressed on a scale of +1 to +7: the lower the QOLRAD score, the more severe the effect on daily QOL. The QOLRAD has been extensively documented in international studies in patients with heartburn for its reliability, validity, and responsiveness, and in the assessment of GERD-related QOL.(15,16) The Japanese version of QOLRAD (QOLRAD-J) has demonstrated utility in the evaluation of GERD-related QOL in Japanese patients.(17)
The Carlsson-Dent questionnaire (CDQ) is a self-administered questionnaire designed for screening GERD.(18) CDQ contains 7 kinds of questions about regurgitation, stomach discomfort and chest discomfort. The response to each question is chosen from among 3 or 4 alternatives. A score ranging from −7 to +18 is calculated by adding the individual positive and negative scores for the items in the questionnaire: the higher the CDQ score, the stronger the reflux symptoms. Dent et al.(19) reported that the CDQ is useful for the diagnosis of GERD and a cut-off level of 4 points is frequently used for a clinical diagnosis of GERD. In addition, the Japanese version of the CDQ is useful as a diagnostic tool for GERD in Japan.(20)
The serum pepsinogen (PG) test is sensitive for atrophic gastritis.(21–23) Serum PG consists of 2 biochemically and immunologically distinct types, pepsinogen I (PGI) and pepsinogen II (PGII).(24) The levels of PGI and the PG I/II ratio are useful serological markers for chronic atrophic gastritis.(25–27) Kitahara et al.(28) reported that it is possible to detect gastric cancer by serum PG screening, using a PGI concentration of less than 70 ng/mL and a PG I/II ratio of less than 3.0 as the cut-off point for diagnosing severe atrophic gastritis.
This study was designed to identify the time course of changes in the GERD-related QOL and GERD symptoms using the QOLRAD-J and CDQ self-administered questionnaires after H. pylori eradication therapy.
Materials and Methods
Study design
This was a 2-center prospective cohort study. Outpatients with H. pylori infection at Keio University Hospital (Tokyo, Japan) and Eiju General Hospital (Tokyo, Japan) were enrolled from September 2008 to March 2009. H. pylori infection status was determined by the 13C urea breath test, microaerobic bacterial cultivation, or histopathological examination of endoscopic biopsy specimens. All patients were given eradication therapy for 1 week with omeprazole 20 mg b.i.d., clarithromycin 400 mg b.i.d., and amoxicillin 750 mg b.i.d. Eradication status was validated 3 months after eradication therapy. The QOLRAD-J and CDQ surveys were conducted and PG levels were measured before eradication (BE) therapy, and at 3 months (3M) and 1 y (1Y) after (Fig. 1). Using QOLRAD-J, the scores for the following 5 domains were determined: emotional distress, sleep disturbance, food/drink problems, physical/social functioning, and vitality. The questionnaires were mailed to the patients who did not come to the outpatient clinic. Patients who did not receive eradication therapy, dropped out during treatment because of side effects from the eradication therapy, did not undergo evaluation of the effect of eradication therapy, or showed eradication failure were excluded from the study. The study protocol was approved by the ethics committees of Keio University School of Medicine and Eiju General Hospital, and written informed consent was obtained prior to subject enrollment. The UMIN Clinical Trials Registry number for this study is UMIN000001399 [http://www.umin.ac.jp/ctr/]. The study was performed in accordance with the principles of the Declaration of Helsinki.
Fig. 1.
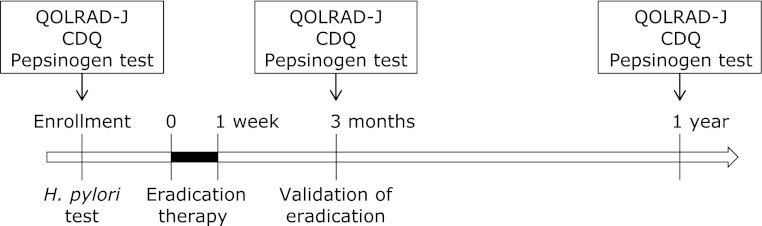
Study design. All patients were given H. pylori eradication therapy (omeprazole 20 mg b.i.d., clarithromycin 400 mg b.i.d., and amoxicillin 750 mg b.i.d.) for 1 week, and eradication status was validated 3 months later. The QOLRAD-J questionnaire survey, the CDQ questionnaire surveys, and serum pepsinogen test were performed before, and at 3 months and 1 year after eradication therapy.
Patient background
Height, weight, body mass index (BMI), alcohol consumption status, smoking status, presence/absence of dyspepsia, and previous history of peptic ulcer before eradication therapy were obtained from a review of medical records and medical interview sheets. Alcohol consumption status was defined as a positive/negative history of daily alcohol consumption. Smoking status was defined as a positive/negative history of smoking cigarettes. The presence/absence of dyspepsia was defined as a positive/negative history of epigastralgia, discomfort, or feeling of fullness in the epigastrium. Patients’ prescription histories of antisecretory agents (histamine type-2 receptor antagonist or proton pump inhibitor) at 1 week (1W), 3M, 6 months (6M), and 1Y after eradication therapy were also reviewed.
Statistical analysis
The average QOLRAD-J and CDQ scores and PG expression were compared using one-way repeated-measures analysis of variance and the Bonferroni post-hoc test. Associations between the clinical background factors—age, height, weight, and BMI—and changes in the QOLRAD-J and CDQ scores were compared using the Student’s t test, and the associations between clinical background factors—sex, the presence/absence of dyspepsia, previous history of peptic ulcer, alcohol consumption status, smoking status, and PG—and changes in the QOLRAD-J and CDQ scores were compared using the chi-square test. The correlation between changes in QOLRAD-J and CDQ scores were evaluated by a linear regression model. Statistical significance was defined as a p value of less than 0.05. All statistical analyses were performed using PASW Statistics (SPSS Inc., Chicago, IL).
Results
Patient characteristics
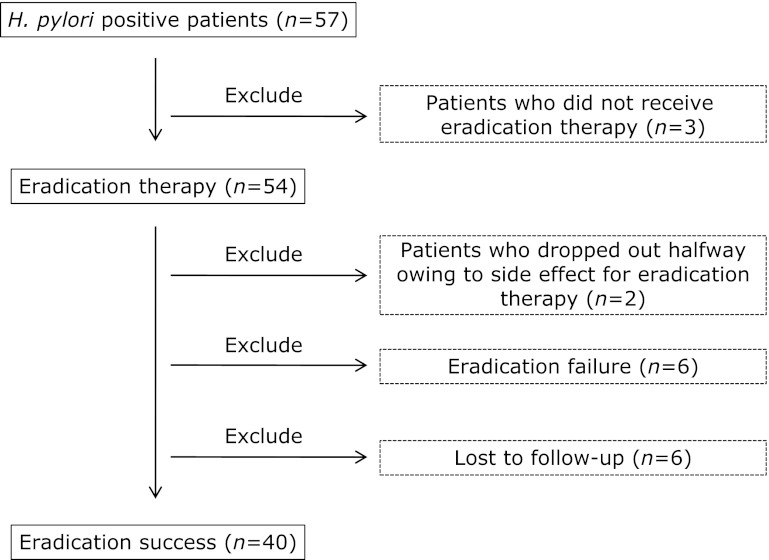
Fifty-seven patients were enrolled with informed consent. From these 57, 17 patients were excluded: 3 did not receive eradication therapy, 2 dropped out halfway owing to the appearance of side effects (nausea and hemorrhagic colitis), 6 did not undergo evaluation of the effect of eradication therapy, and 6 showed eradication failure (Fig. 2). Finally, data from 40 patients (55.7 ± 11.3 years old, range 22–76 y; 21 men and 19 women) were included. Patient characteristics are shown in Table 1. The number and percentage of subjects for whom data were collected was 40 (100.0%) at BE, 39 (97.5%) at 3M, and 35 (87.5%) at 1Y for estimation of the QOLRAD-J scores, and 37 (92.5%) at BE, 38 (95.0%) at 3M, and 34 (85.0%) at 1Y for the CDQ scores.
Fig. 2.
Exclusion criteria. Fifty-seven outpatients with H. pylori infection were enrolled after obtaining their written informed consent. Patients who did not receive the eradication therapy, dropped out halfway due to side effects, did not undergo evaluation of the effect of the eradication therapy, or showed eradication failure were excluded from the study.
Table 1.
Characteristics of subjects (n = 40)
| Sex, No. | |
| Male (%) | 21 (52.5) |
| Female (%) | 19 (47.5) |
| Age, years | |
| Mean ± SD (range) | 55.7 ± 11.3 (22–76) |
| Body Height, cm | |
| Mean ± SD (range) | 163.9 ± 10.3 (150.0–184.0) |
| Body Weight, kg | |
| Mean ± SD (range) | 58.7 ± 12.6 (41.0–110.0) |
| Body Mass Index, % | |
| Mean ± SD (range) | 21.9 ± 3.3 (17.1–32.5) |
| Dyspepsia, No. | |
| Presence (%) | 19 (47.5) |
| Absence (%) | 13 (32.5) |
| Unknown (%) | 8 (20.0) |
| Peptic ulcer, No. | |
| Presence (%) | 15 (37.5) |
| Absence (%) | 18 (45.0) |
| Unknown (%) | 7 (17.5) |
| Alcohol Habit, No. | |
| Everyday (%) | 13 (32.5) |
| Social drinker or nothing (%) | 22 (55.0) |
| Unknown (%) | 5 (12.5) |
| Smoking Habit, No. | |
| Presence (%) | 6 (15.0) |
| Absence (%) | 29 (72.5) |
| Unknown (%) | 5 (12.5) |
Changes in the QOLRAD-J and CDQ scores after H. pylori eradication therapy
Improvement in both the QOLRAD-J and CDQ scores was observed at 1Y, although no significant differences were noted between the levels at BE and at 3M. With regard to the sub-domains of QOLRAD-J, significant improvements were observed in emotional distress, sleep disturbance, and food/drink problems at 1Y. On the other hand, improvement in PG score was identified not only in 1Y but in 3M (Table 2.).
Table 2.
Alteration of QOLRAD-J and CDQ score after H. pylori eradication therapy
| BE | 3M† | 1Y† | |
|---|---|---|---|
| QOLRAD-J score | |||
| Overall average | 6.51 ± 0.15 | 6.71 ± 0.12 | 6.85 ± 0.06* |
| Emotional distress | 6.55 ± 0.13 | 6.75 ± 0.10 | 6.86 ± 0.06* |
| Sleep disturbance | 6.57 ± 0.16 | 6.78 ± 0.12 | 6.91 ± 0.05* |
| Food/Drink problems | 6.29 ± 0.20 | 6.54 ± 0.17 | 6.77 ± 0.08* |
| Physical/Social functioning | 6.72 ± 0.13 | 6.87 ± 0.66 | 6.97 ± 0.03 |
| Vitality | 6.58 ± 0.15 | 6.59 ± 0.16 | 6.72 ± 0.11 |
| CDQ score | 4.00 ± 0.69 | 4.43 ± 0.68 | 3.03 ± 0.72* |
| Serum pepsinogen level | |||
| Pepsinogen I (ng/ml) | 100.97 ± 22.91 | 51.94 ± 12.99* | 48.54 ± 3.62* |
| Pepsinogen II (ng/ml) | 32.97 ± 5.85 | 8.80 ± 1.70** | 8.72 ± 0.44** |
| Pepsinogen I/II ratio | 2.94 ± 0.36 | 5.55 ± 0.45** | 5.60 ± 0.38** |
Each value represents the mean ± SE. BE: before the eradication therapy, 3M: 3 months after the eradication therapy, 1Y: 1 year after the eradication therapy. †one-way repeated-measures analysis of variance and the Bonferroni post-hoc test compared to BE. *p<0.05, **p<0.01.
A positive history of gastric antisecretory agent prescription during the follow-up period was identified in 32 of 40 patients. The number of patients prescribed antisecretory agents at 1W was 9 (28.1%), at 3M was 4 (12.5%), at 6M was 3 (9.4%), and at 1Y was 3 (9.4%). Improvement in both the QOLRAD-J and CDQ scores was observed at 1Y, even when the 3 patients who were taking antisecretory agents from 6M to 1Y were excluded from the analysis.
The proportions of patients in which no worsening of the QOLRAD-J scores was observed were 82.1% (32 of 39 patients) at 3M and 85.7% (30 of 35 patients) at 1Y, whereas those in which no worsening of the CDQ scores was observed were 73.0% (27 of 37 patients) at 3M and 88.2% (30 of 34 patients) at 1Y. In addition, worsening of the QOLRAD-J and CDQ scores was observed in 18.0% (7 of 39 patients) and 27.0% (10 of 37 patients) of the patients, respectively, at 3M, although score improvement was observed in 57.1% (4 of 7 patients) and 80.0% (8 of 10 patients) of the patients, respectively, at 1Y (Table 3).
Table 3.
The proportions of change in patients’ scores
| BE-3M | BE-1Y | |
|---|---|---|
| QOLRAD-J score | ||
| Improvement | 13/39 (33.3%) | 13/35 (37.1%) |
| No change | 19/39 (48.7%) | 17/35 (48.6%) |
| Aggravation | 7/39 (18.0%) | 5/35 (14.3%) |
| CDQ score | ||
| Improvement | 13/37 (35.1%) | 16/34 (47.1%) |
| No change | 14/37 (37.8%) | 14/34 (41.2%) |
| Aggravation | 10/37 (27.0%) | 4/34 (11.8%) |
Values are n (%). BE: before the eradication therapy, 3M: 3 months after the eradication therapy, 1Y: 1 year after the eradication therapy.
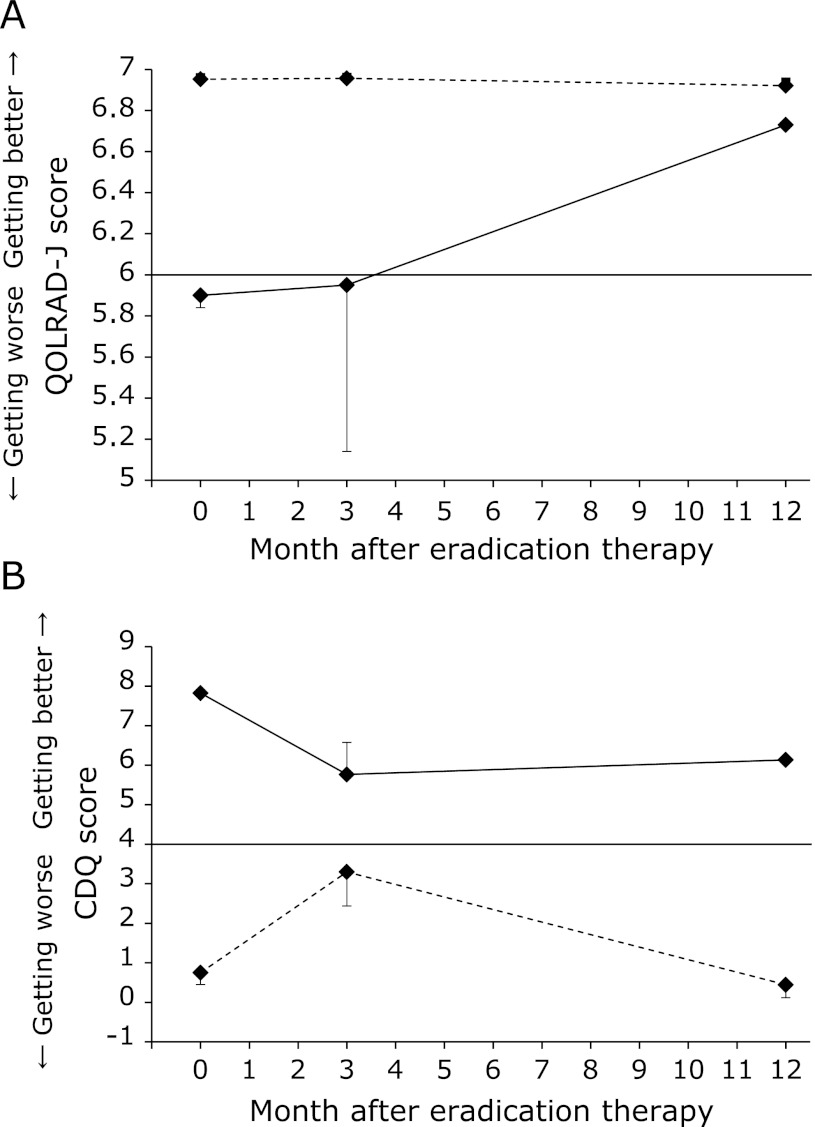
Changes from the initial scores
Fig. 3A shows the changes in the QOLRAD-J score and Fig. 3B shows the changes in the CDQ score relative to the initial score. The CDQ scores were divided into groups with initial scores of <4 and ≥4, which represents the clinical cutoff. QOLRAD-J does not have a cut-off level because of digitizing of the QOL, whereas CDQ was developed for screening GERD. In this study, the average QOLRAD-J score at BE was 6.51 ± 0.15. Therefore, for the QOLRAD-J score, we set a cut-off level of 6 points for descriptive purposes to analyze the group with lower QOL scores. In this study, 7 of the 40 patients had QOLRAD-J scores <6 points at BE and 17 of 37 patients had CDQ scores ≥4 points at BE. In the group of patients (n = 7) with QOLRAD-J scores of <6, 5 patients showed improvement, while 2 became worse at 3M. However, at 1Y, all 7 patients showed significant improvement. In addition, the group with QOLRAD-J scores ≥6 hovered around the same high scores throughout the study period. Meanwhile, the group of patients with CDQ scores <4 points at BE showed significant improvement even at 3M. In particular, 29.4% (5 of 17 patients) of the patients with scores <4 became completely asymptomatic within 3M. On the other hand, among the patients in whom the CDQ scores were <4 at BE, the scores became worse at 3M, even though the average score was better than 4 at that time. However, no significant changes were noted between BE and 1Y.
Fig. 3.
Changes in QOLRAD-J and CDQ scores with low or high initial scores. The solid lines represent the low-score group and the dashed lines the high-score group. Changes in the QOLRAD-J score according to the initial score dichotomized at a cut-off of 6 [≥6 (n = 33); <6 (n = 7)] (A). Changes in the CDQ score according to initial score dichotomized at a cut-off of 4 [≥4 (n = 17); <4 (n = 20)] (B). *p<0.05 compared to BE using one-way repeated-measures analysis of variance and the Bonferroni post-hoc test.
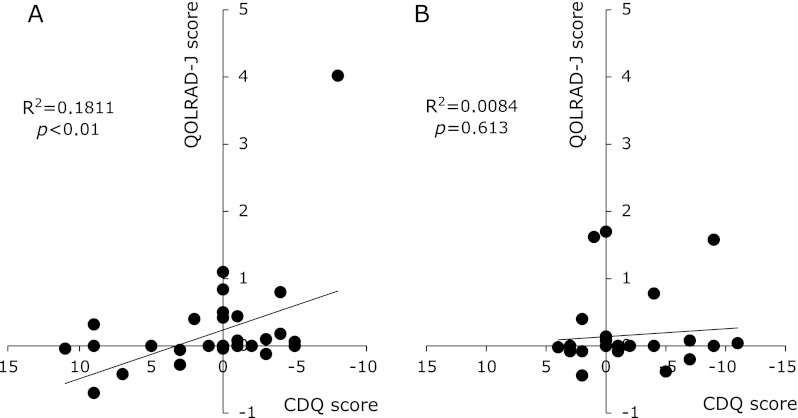
Correlations between the QOLRAD-J and CDQ scores
Fig. 4A shows the correlations between the changes in QOLRAD-J and CDQ scores at BE and 3M, and Fig. 4B shows the correlations between 3M and 1Y. Significant correlations were identified between the scores at 3M and 1Y, although no such correlation was identified between the scores at BE and 3M.
Fig. 4.
Correlations between the changes in the QOLRAD-J and CDQ scores. The changes in the QOLRAD-J score are plotted on the y axis, and those in the CDQ score are plotted on the x axis. Correlation between the changes in the 2 scores from BE to 3M. (A) Correlation between the changes in the 2 scores from 3M to 1Y. (B) Significant correlation was identified between the changes in QOLRAD-J and CDQ scores from BE to 3M; however, no such significant correlation was identified from 3M to 1Y.
Association between clinical background factors and the QOLRAD-J and CDQ scores
Table 4 shows the association between clinical background factors and changes in the QOLRAD-J and CDQ scores. In order to identify the difference between the groups in which the scores worsened or did not worsen, the associations with clinical background factors were analyzed; however, no significant correlation was identified. When the association between clinical background factors and the baseline QOLRAD-J and CDQ scores were analyzed, no significant correlations were identified.
Table 4.
Association between clinical background factors and change of QOLRAD-J and CDQ score
| Clinical background factor | p value | |||
|---|---|---|---|---|
| QOLRAD-J score | CDQ score | |||
| BE-3M | BE-1Y | BE-3M | BE-1Y | |
| Sex1) | 0.671 | 0.682 | 0.863 | 0.052 |
| Age2) | 0.342 | 0.424 | 0.435 | 0.859 |
| Body height2) | 0.873 | 0.914 | 0.729 | 0.419 |
| Body weight2) | 0.511 | 0.501 | 0.872 | 0.814 |
| Body Mass Index2) | 0.748 | 0.551 | 0.693 | 0.223 |
| Dyspepsia1) | 0.132 | 0.971 | 0.976 | 0.606 |
| Peptic ulcer1) | 0.732 | 0.286 | 0.400 | 0.128 |
| Alcohol habit1) | 0.192 | 0.686 | 0.491 | 0.901 |
| Smoking habit1) | 0.218 | 0.424 | 0.882 | 0.464 |
BE: before the eradication therapy, 3M: 3 months after the eradication therapy, 1Y: 1 year after the eradication therapy. 1)chi-square test, 2)Student’s t test.
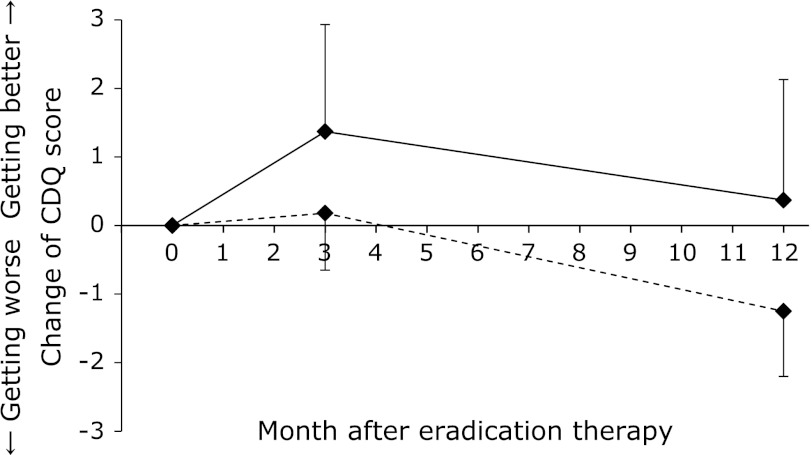
Changes of CDQ scores in initial positive/negative PG test group
In addition, we evaluated the degree of atrophic gastritis by performing the PG test at BE. The number and percentage of subjects for whom data were collected at BE was 31 of 40 (77.5%). Eight patients were positive for the PG test (PGI >70 and PG I/II ratio <3), and 23 were negative. Changes of CDQ scores in positive/negative PG test groups were shown in Fig. 5. The CDQ score varied from 2.88 ± 1.55 to 3.25 ± 1.76 (from BE to 1Y) in the positive-PG test group. On the other hand, the CDQ score varied from 4.65 ± 0.85 to 3.40 ± 0.95 (from BE to 1Y) in the negative-PG test group. Therefore, CDQ score tended to improve in the negative-PG test group than in the positive-PG test group (p = 0.065). No significant differences were observed in the QOLRAD-J score.
Fig. 5.
Changes in CDQ scores with initial PG positive/negative test group. The solid lines represent the PG positive test group (n = 8) and the dashed lines the PG negative test group (n = 23). CDQ score tended to improve in the PG-negative test group than in the PG-positive test group (p = 0.065).
Discussion
This study showed significant improvement in both the QOLRAD-J and CDQ scores at 1Y after H. pylori eradication therapy, even though no significant changes were observed at 3M after therapy. In a previous study, no significant change in QOL was identified at 6M after H. pylori eradication therapy.(14) However, our data showed improvement in the GERD-related QOL at 1Y after H. pylori eradication therapy, the period of follow-up being longer in this than in the previous report. On the other hand, no change in the GERD-related QOL was observed at 3M after H. pylori eradication therapy.
The degree of improvement was even more marked in cases with low initial scores. On the other hand, in patients with high initial scores, the scores remained high even after eradication therapy. On the basis of these results, we conclude that H. pylori eradication therapy may be more effective in patients with severe reflux symptoms or low QOL scores. Furthermore, strong reflux symptoms or reduction in the QOL may not occur after H. pylori eradication therapy.
Antisecretory agents, such as histamine type-2 receptor antagonist or proton pump inhibitor, are thought to primarily protect gastric mucosa by inhibiting gastric acid secretion.(29) Therefore, the effect of antisecretory agents should be considered as a confounding factor, because antisecretory agents might improve the QOL and/or the symptoms of reflux. Yoshida et al.(30) reported that omeprazole improved symptoms and QOL in patients with reflux esophagitis. In this study, significant improvement in the QOLRAD-J and CDQ scores were noted at 1Y, even among patients who did not take antisecretory agents from 6M to 1Y. Furthermore, the number of patients who were prescribed antisecretory agents at their own request decreased in a time-dependent fashion after the eradication therapy. This result also suggests that the symptoms of reflux and GERD-related QOL improved gradually after H. pylori eradication therapy.
This study also showed that the changes in the QOLRAD-J and CDQ scores were not correlated during the period from 3M to 1Y, whereas a significant correlation was identified during the period from BE to 3M. This result suggests that exacerbation of symptoms might not always imply decreased QOL after 3M. GERD-related QOL might improve following recovery from H. pylori infection, even if the reflux symptoms worsen after 3M. The announcement of eradication success at 3M might possibly influence the improvement in the QOL.
In this study, no significant correlation was identified between clinical background factors and the QOLRAD-J and CDQ scores. Hunt et al.(31) reported that there was no association between H. pylori eradication and the risk of GERD in a population of dyspeptic patients, while a 2-fold higher risk of erosive GERD was observed in patients with peptic ulcer disease. No significant association was identified between dyspepsia and the development of reflux symptoms or GERD-related QOL in our study as well; however, no association between the presence of peptic ulcer disease and the risk of developing reflux symptoms or GERD-related QOL was observed, although our study population included 15 patients with a previous history of peptic ulcer and 18 patients without a history of peptic ulcer. Patients with peptic ulcer might develop erosive GERD following H. pylori eradication therapy; however, the GERD-related QOL and severity of reflux symptoms may remain unchanged.
Our data show that the CDQ score tended to improve in the negative-PG group than in the positive-PG group. This means that milder atrophic gastritis may indicate improval of reflux symptoms by H. pylori eradication therapy. Atrophic gastritis spreads from an antral-predominant phenotype to a pangastritis phenotype as it progresses. Because of H. pylori eradication therapy, recovery of acid secretion may strongly affect reflux symptoms in patients with severe atrophic gastritis.
The limitation of this study is that we could not analyze the groups with H. pylori eradication failure. There were only 6 patients with eradication failure in our study. We performed a second eradication as soon as eradication failure was identified. Therefore, we did not follow the eradication failure group.
In summary, improvement in GERD-related QOL and reflux symptoms was observed at 1Y after H. pylori eradication therapy. While some patients showed worsening of GERD-related QOL and reflux symptoms 3M after eradication therapy, all patients showed improvement at 1Y. In addition, the degree of improvement was even more pronounced in cases with severe symptoms. Thus, H. pylori eradication therapy may be a valid therapeutic option for improving the GERD-related QOL and reflux symptoms.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research (B) from the Japan Society for the Promotion of Science (No. 22300169, to H.S.), a grant of the Adaptable and Seamless Technology Transfer Program through target-driven R&D (A-STEP) (AS231Z00132G to H.S.) from the Japan Science and Technology Agency (JST), a grant from the Smoking Research Foundation (to H.S.), the Keio Gijuku Academic Development Fund (to H.S.), a Research Fund of Mitsukoshi Health and Welfare Foundation (to H.S.) and a Nateglinide Memorial Toyoshima Research and Education Fund (to H.S.).
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Suzuki H, Iwasaki E, Hibi T. Helicobacter pylori and gastric cancer. Gastric Cancer. 2009;12:79–87. doi: 10.1007/s10120-009-0507-x. [DOI] [PubMed] [Google Scholar]
- 2.Nishizawa T, Suzuki H, Suzuki M, Takahashi M, Hibi T. Proton pump inhibitor-amoxicillin-clarithromycin versus proton pump inhibitor-amoxicillin-metronidazole as first-line Helicobacter pylori eradication therapy. J Clin Biochem Nutr. 2012;51:114–116. doi: 10.3164/jcbn.D-11-00029R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu JC, Chan FK, Ching JY, et al. Effect of Helicobacter pylori eradication on treatment of gastro-oesophageal reflux disease: a double blind, placebo controlled, randomised trial. Gut. 2004;53:174–179. doi: 10.1136/gut.2003.012641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Labenz J, Blum AL, Bayerdörffer E, et al. Curing Helicobacter pylori infection in patients with duodenal ulcer may provoke reflux esophagitis. Gastroenterology. 1997;112:1442–1447. doi: 10.1016/s0016-5085(97)70024-6. [DOI] [PubMed] [Google Scholar]
- 5.Koike T, Ohara S, Sekine H, et al. Increased gastric acid secretion after Helicobacter pylori eradication may be a factor for developing reflux oesophagitis. Aliment Pharmacol Ther. 2001;15:813–820. doi: 10.1046/j.1365-2036.2001.00988.x. [DOI] [PubMed] [Google Scholar]
- 6.El-Omar EM, Oien K, El-Nujumi A, et al. Helicobacter pylori infection and chronic gastric acid hyposecretion. Gastroenterology. 1997;113:15–24. doi: 10.1016/s0016-5085(97)70075-1. [DOI] [PubMed] [Google Scholar]
- 7.Malfertheiner P, Megraud F, O’Morain C, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772–781. doi: 10.1136/gut.2006.101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moayyedi P, Bardhan C, Young L, Dixon MF, Brown L, Axon AT. Helicobacter pylori eradication does not exacerbate reflux symptoms in gastroesophageal reflux disease. Gastroenterology. 2001;121:1120–1126. doi: 10.1053/gast.2001.29332. [DOI] [PubMed] [Google Scholar]
- 9.Lundell L, Miettinen P, Myrvold HE, et al. Lack of effect of acid suppression therapy on gastric atrophy. Nordic Gerd Study Group. Gastroenterology. 1999;117:319–326. doi: 10.1053/gast.1999.0029900319. [DOI] [PubMed] [Google Scholar]
- 10.Sasaki A, Haruma K, Manabe N, Tanaka S, Yoshihara M, Chayama K. Long-term observation of reflux oesophagitis developing after Helicobacter pylori eradication therapy. Aliment Pharmacol Ther. 2003;17:1529–1534. doi: 10.1046/j.1365-2036.2003.01643.x. [DOI] [PubMed] [Google Scholar]
- 11.Green JRB. Is there such an entity as mild oesophagitis? Eur J Clin Res. 1993;4:29–34. [Google Scholar]
- 12.Johnson DA, Fennerty MB. Heartburn severity underestimates erosive esophagitis severity in elderly patients with gastroesophageal reflux disease. Gastroenterology. 2004;126:660–664. doi: 10.1053/j.gastro.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Glise H, Hallerback B, Wiklund I. Quality of life: a reflection of symptoms and concerns. Scand J Gastroenterol Suppl. 1996;221:14–17. doi: 10.3109/00365529609095545. [DOI] [PubMed] [Google Scholar]
- 14.Laine L, Dhir V. Helicobacter pylori eradication does not worsen quality of life related to reflux symptoms: a prospective trial. Aliment Pharmacol Ther. 2002;16:1143–1148. doi: 10.1046/j.1365-2036.2002.01267.x. [DOI] [PubMed] [Google Scholar]
- 15.Wiklund IK, Junghard O, Grace E, et al. Quality of life in reflux and dyspepsia patients. Psychometric documentation of a new disease-specific questionnaire (QOLRAD) Eur J Surg Suppl. 1998:41–49. [PubMed] [Google Scholar]
- 16.Talley NJ, Fullerton S, Junghard O, Wiklund I. Quality of life in patients with endoscopy-negative heartburn: reliability and sensitivity of disease-specific instruments. Am J Gastroenterol. 2001;96:1998–2004. doi: 10.1111/j.1572-0241.2001.03932.x. [DOI] [PubMed] [Google Scholar]
- 17.Hongo M, Kinoshita Y, Shimozuma K, Kumagai Y, Sawada M, Nii M. Psychometric validation of the Japanese translation of the quality of life in reflux and dyspepsia questionnaire in patients with heartburn. J Gastroenterol. 2007;42:807–815. doi: 10.1007/s00535-007-2098-9. [DOI] [PubMed] [Google Scholar]
- 18.Carlsson R, Dent J, Glise H, Riley S, et al. Evaluation of a questionnaire for the diagnosis of symptomatic gastroesophageal reflux disease (GERD) Gastroenterology. 1996;110:A76. [Google Scholar]
- 19.Carlsson R, Dent J, Bolling-Sternevald E, et al. The usefulness of a structured questionnaire in the assessment of symptomatic gastroesophageal reflux disease. Scand J Gastroenterol. 1998;33:1023–1029. doi: 10.1080/003655298750026697. [DOI] [PubMed] [Google Scholar]
- 20.Danjo A, Yamaguchi K, Fujimoto K, et al. Comparison of endoscopic findings with symptom assessment systems (FSSG and QUEST) for gastroesophageal reflux disease in Japanese centres. J Gastroenterol Hepatol. 2009;24:633–638. doi: 10.1111/j.1440-1746.2008.05747.x. [DOI] [PubMed] [Google Scholar]
- 21.Borch K, Axelsson CK, Halgreen H, Damkjaer Nielsen MD, Ledin T, Szesci PB. The ratio of pepsinogen A to pepsinogen C: a sensitive test for atrophic gastritis. Scand J Gastroenterol. 1989;24:870–876. doi: 10.3109/00365528909089228. [DOI] [PubMed] [Google Scholar]
- 22.Senmaru T, Fukui M, Tanaka M, et al. Atrophic gastritis is associated with coronary artery disease. J Clin Biochem Nutr. 2012;51:39–41. doi: 10.3164/jcbn.11-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuhisa T, Tsukui T. Relation between reflux of bile acids into the stomach and gastric mucosal atrophy, intestinal metaplasia in biopsy specimens. J Clin Biochem Nutr. 2012;50:217–221. doi: 10.3164/jcbn.11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miki K. Gastric cancer screening using the serum pepsinogen test method. Gastric Cancer. 2006;9:245–253. doi: 10.1007/s10120-006-0397-0. [DOI] [PubMed] [Google Scholar]
- 25.Rugge M, Correa P, Dixon MF, et al. Gastric mucosal atrophy: interobserver consistency using new criteria for classification and grading. Aliment Pharmacol Ther. 2002;16:1249–1259. doi: 10.1046/j.1365-2036.2002.01301.x. [DOI] [PubMed] [Google Scholar]
- 26.Broutet N, Plebani M, Sakarovitch C, et al. Pepsinogen A, pepsinogen C, and gastrin as markers of atrophic chronic gastritis in European dyspeptics. Br J Cancer. 2003;88:1239–1247. doi: 10.1038/sj.bjc.6600877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki H, Matsuzaki J, Hibi T. Ghrelin and oxidative stress in gastrointestinal tract. J Clin Biochem Nutr. 2011;48:122–125. doi: 10.3164/jcbn.10-16GFR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kitahara F, Kobayashi K, Sato T, Kojima Y, Araki T, Fujino MA. Accuracy of screening for gastric cancer using serum pepsinogen concentrations. Gut. 1999;44:693–697. doi: 10.1136/gut.44.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki H, Nishizawa T, Tsugawa H, Mogami S, Hibi T. Roles of oxidative stress in stomach disorders. J Clin Biochem Nutr. 2012;50:35–39. doi: 10.3164/jcbn.11-115SR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshida S, Nii M, Date M. Effects of omeprazole on symptoms and quality of life in Japanese patients with reflux esophagitis: final results of OMAREE, a large-scale clinical experience investigation. BMC Gastroenterol. 2011;11:15. doi: 10.1186/1471-230X-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yaghoobi M, Farrokhyar F, Yuan Y, Hunt RH. Is there an increased risk of GERD after Helicobacter pylori eradication?: a meta-analysis. Am J Gastroenterol. 2010;105:1007–1013. doi: 10.1038/ajg.2009.734. [DOI] [PubMed] [Google Scholar]