Abstract
Gene transfer of drug resistance (CTX-R) genes can be used to protect the hematopoietic system from the toxicity of anticancer chemotherapy and this concept recently has been proven by overexpression of a mutant O6-methylguaninemethyltransferase in the hematopoietic system of glioblastoma patients treated with temozolomide. Given its protection capacity against such relevant drugs as cytosine arabinoside (ara-C), gemcitabine, decitabine, or azacytidine and the highly hematopoiesis-specific toxicity profile of several of these agents, cytidine deaminase (CDD) represents another interesting candidate CTX-R gene and our group recently has established the myeloprotective capacity of CDD gene transfer in a number of murine transplant studies. Clinically, CDD overexpression appears particularly suited to optimize treatment strategies for acute leukemias and myelodysplasias given the efficacy of ara-C (and to a lesser degree decitabine and azacytidine) in these disease entities. This article will review the current state of the art with regard to CDD gene transfer and point out potential scenarios for a clinical application of this strategy. In addition, risks and potential side effects associated with this approach as well as strategies to overcome these problems will be highlighted.
Myeloprotective Gene Therapy by Overexpression of Drug Resistance Genes in Hematopoietic Stem Cells
Given their ability to self-renew, proliferate extensively, and differentiate into all the different mature cells of the lymphohematopoietic system, hematopoietic stem cells (HSCs) have been considered a particularly promising target population since the beginning of gene therapeutic activities in the mid-1980s. The attractivity of HSCs for gene therapy approaches is further supported by their easy accessibility by bone marrow biopsy or granulocyte-colony stimulating factor (G-CSF) mobilization and leukapheresis, as well as clearly defined procedures for their storage and transplantation. First proof of principle for HSC gene transfer in the murine system was established as early as the mid-1980s [1–3]; however, it took until the beginning of the current millennium that HSC gene therapy was transferred successfully to the clinical setting. So far, HSC gene therapy has been used in particular for the treatment of congenital diseases with manifestation in the hematopoietic system such as severe X-linked combined immunodeficiency, adenosine deaminase deficiency, chronic granulomatous disease, or more recently Wiskott-Aldrich syndrome and X-linked adrenoleukodystrophy [4,5].
Another potentially attractive strategy to put HSC gene therapy to clinical use is the protection of the lymphohematopoietic system from the side effects of anticancer chemotherapeutic drugs by (over)expression of drug resistance (CTX-R) genes. A number of such CTX-R genes have been identified, which according to their subcellular localization can roughly be classified into three groups: 1) membrane-associated pump or pore proteins, 2) cytoplasmic proteins involved in drug or prodrug metabolism, and 3) nuclear proteins associated with DNA repair. While some of these CTX-R genes primarily have been investigated in vitro, for others the potential to protect the lymphohematopoietic system from the associated cytotoxic agents has been firmly established in murine as well as large animal models (see Table 1 and for review [6–8]). Myeloprotective properties in particular have been demonstrated for mutants of the gene coding for the DNA repair protein O6-methylguanine DNA-methyltransferase (mutMGMTP140K), the multidrug resistance 1 (MDR1) gene coding for the cellular efflux pump p-glycoprotein, mutant forms of dihydrofolate reductase (mutDHFR), and cytidine deaminase (CDD). While the antileukemic properties of mutMGMT-associated drugs are limited, in particular MDR1 or CDD represents highly interesting CTX-R genes in the context of acute leukemias and myelodysplasias given their myeloprotective potential in the context of anthracyclines (and to a lesser extent etoposide) or cytidine analog therapy. Overexpression of mutDHFR, aldehyde dehydrogenase, or multidrug resistance-related protein and hypoxanthine-guanine phosphoribosyl transferase knockdown protecting from methotrexate, cyclophosphamide, or anthracyclines, and thioguanine, respectively, represent further applications of CTX-R genes with potential relevance in leukemia therapy.
Table 1.
Drug Resistance Genes and Their Potential Use in Chemotherapy.
| Gene and Localization | Resistance to | Mode of Action | Level of Evidence | References |
| Plasma membrane | ||||
| MDR1* (P-glycoprotein 1) | Anthracyclines, vinca alkaloids, taxoids, etoposide | Efflux of drugs | Human xenotransplant model (human clinical trial)† | [17,74] |
| Multidrug resistance-related protein‡ | Anthracyclines, vinca alkaloids, taxoids, etoposide | Efflux of drugs | Murine in vivo model | [75] |
| hENT2 nucleoside transporter | Trimetrexate, tomudex (plus NBMPR§) | Nucleoside transporter | Murine in vivo model | [76] |
| ABLG2¶ | Anthracyclines, taxoids | Efflux of drugs (side population) | Human cell lines | [77] |
| Cytoplasmic | ||||
| DHFR | Methotrexate, trimetrexate | Mutant form is unaffected by the drug | Large animal model | [78] |
| CDD | ara-C, gemcitabine | Detoxification of prodrug | Murine in vivo model | [39] |
| Aldehyde dehydrogenase | Cyclophosphamide | Detoxification of prodrug | Murine in vivo model | [79] |
| Glutathione S-transferase | Cyclophosphamide, anthracyclines | Detoxification of prodrug | Murine in vivo model | [80] |
| Thymidylate synthase | 5-Fluorouracil, tomudex | Mutant form is unaffected by the drug | Murine primary cells | [81] |
| Ribonucleotide reductase | Hydroxyurea | Mutant form is unaffected by the drug | Murine in vivo model | [82,83] |
| Nuclear | ||||
| MGMT# | Chloroethylnitrosoureas and decarbazine derivatives (plus BG) | Removal of O6 adducts from the DNA | Human clinical trial | [11] |
| APN1(yeast)/APE(human)** | Bleomycin and ionizing radiation | Repairs AP sites, oxidative damage, and alkylation damage in DNA | Human cell lines | [84,85] |
| 8-Oxoguanine glycosylase/Fapy-DNA glycosylase | ThioTEPA | Removal of alkylated bases in DNA | Murine primary cells/murine in vivo model | [86] |
| Superoxide dismutase | Anthracyclines and paraquat | Protection against oxidative damage | Human cell lines | [75,87] |
| Topoisomerase I | Camptothecin | DNA repair function | Human cell lines | [78] |
| Topoisomerase II | Anthracyclines and etoposide | DNA repair function | Human cell lines | [79] |
| HPRT†† | 6-Thioguanine | Ribosylation of purine analogs | Murine in vivo model | [88] |
Multidrug resistance gene.
Failed due to technical problems.
Multidrug resistance-related protein coding gene.
Nitrobenzylmercaptopurine riboside.
ATP-binding cassette subfamily G member 2 (also known as breast cancer resistance protein).
O6-Methylguanine DNA-methyltransferase.
Apurinic/apyrimidinic endonuclease.
Hypoxanthine-guanine phosphoribosyl transferase.
Currently, the clinically most advanced CTX-R gene transfer strategy for myeloprotection applies MGMT point mutants resistant to the specific wild-type MGMT inhibitor O6-benzylguanine (BG). MutMGMT gene transfer followed by combined BG/1,3-bis(2-chloroethyl)-1nitrosourea (BCNU) or BG/temozolomide chemotherapy has proven highly efficacious for myeloprotection as well as in vivo selection in murine and several large animal models [8–10]. Furthermore, a recent clinical trial has demonstrated efficient myeloprotection and in vivo enrichment of genetically modified cells following mutMGMT gene therapy in a cohort of glioblastoma patients demonstrating progression-free survival for more than 2 years in individual patients [11]. However, the clinical indications for mutMGMT-associated drugs such as temozolomide- or chloroethylnitrosourea-type agents are rather limited, and apart from brain tumors, these drugs are only considered standard therapy in malignant melanomas [12]. In addition, both drugs suffer from substantial nonhematopoietic toxicities and a considerable mutagenic potential [13].
MutDHFR has been investigated for myeloprotective gene transfer strategies since the late 1980s and significant protection has been shown in vitro as well as in animal models, and even effective in vivo selection of mutDHFR-transduced cells has been achieved in the murine model [14]. However, mutDHFR-induced myeloprotection is restricted to the small group of antifolate cytotoxic drugs such as methotrexate or trimetrexate. Although these agents induce considerable lymphotoxicity and are routinely administered for immunosuppression in a large number of diseases, generalized myelotoxicity rarely is encountered as the dose-limiting toxicity of folate antagonists, thus questioning the clinical relevance of mutDHFR for myeloprotection at least when used on its own.
MDR1, however, confers resistance against a wide variety of clinically highly relevant chemotherapeutic agents such as anthracyclines, epipodophyllotoxins, taxoids, or vinca alkaloids, of which at least the first three groups are associated with profound and frequently dose-limiting myelosuppression. Significant protection from the toxicity of several of these agents upon MDR1 overexpression has been demonstrated for murine as well as human hematopoietic cells in vitro and in animal models [15,16], although effective transgenic MDR1 expression in clinical studies has been problematic [17,18]. These studies were performed more than a decade ago, however, and in the choice of γ-retroviral vectors as well as transduction protocols clearly do not represent the current state of the art of HSC gene transfer technology. Thus, at present the clinical potential of MDR1 in myeloprotective gene therapy strategies remains poorly defined.
CDD Gene Transfer and Protection from Myelosuppression Induced by Ara-C and Other Cytidine Analogs
CDD (EC 3.5.4.5) at the moment probably represents the most relevant CTX-R gene for myeloprotection in the context of acute leukemia or myelodysplasia therapy. CDD is an enzyme involved in the nucleotide salvage pathway and catalyzes the deamination of cytidine and deoxycytidine to uridine and deoxyuridine, respectively, and protects cells from the clinically highly relevant cytotoxic cytidine analogs cytosine arabinoside (1-β-d-arabinofuranosylcytosine, ara-C), gemcitabine (2′,2′-difluorodeoxycytidine), decitabine (5-aza-2-deoxycytidine), and azacytidine (5-azacytidine; see Figure 1). These nucleoside analogs constitute prodrugs that after entry into the cell and phosphorylation to the triphosphate state by the nucleotide salvage pathway exert their specific cytotoxic activities during or after incorporation into the DNA where they interfere with DNA strand elongation, replication, or repair processes [19] (see Figure 2). In particular, ara-C has been used clinically for extended time periods and has established itself as the most effective single agent in the treatment of acutemyeloid leukemias (AMLs). Importantly, ara-C has a predominantly hematopoietic toxicity profile and, when given at low to intermediate doses, hematotoxicity represents the most frequent side effect described. This toxicity profile may be related to the low CDD expression levels observed in hematopoietic progenitor/stem cells [20], while mature myeloid cells and in particular granulocytes represent the cells with the highest endogenous CDD expression within the hematopoietic system [21]. The notion of CDD as a resistance factor to ara-C goes back to 1971, when Steuart and colleagues described high levels of CDD in the leukemic blasts of AML patients relapsing after ara-C treatment [22]. This correlation between CDD activity in AML blasts and clinical response to ara-C therapy has been confirmed by several groups, and in particular, pretreatment CDD activity has been described to predict therapeutic outcome [21]. These data meanwhile have triggered a whole series of studies aiming at CDD overexpression for myeloprotection in the context of cytidine analog application (overview given in Table 2).
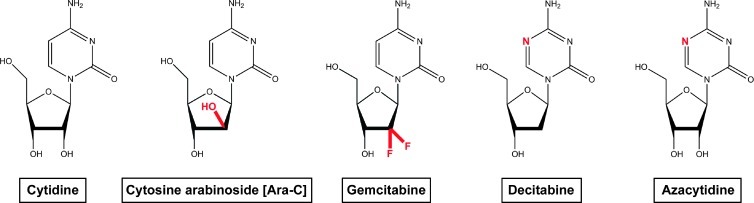
Figure 1.
Cytidine analog-type cytotoxic drugs for the treatment of AML. Chemical structure of cytidine and its cytotoxic drug analogs ara-C, gemcitabine, decitabine, and azacytidine.
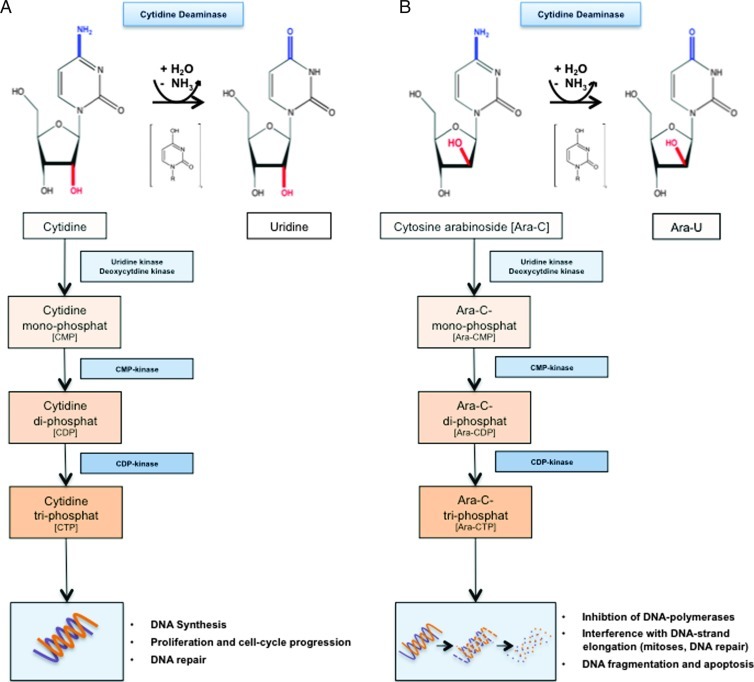
Figure 2.
Metabolism of cytidine and cytidine analog-type agents. (A) Deamination of cytidine to uridine by CDD as an alternative to phosphorylation of the molecule by uridine kinase and, more importantly, deoxycytidine kinase to CMP as a first step in the nucleotide salvage pathway. Subsequently, the CMP and cytidine di-phosphate (CDP) kinases phosphorylate CMP to its active counterpart cytidine triphosphate to be incorporated into the DNA. (B) Similarly, cytidine analogs such as ara-C either get inactivated by the CDD to Ara-U or are phosphorylated to their active triphosphate form, which upon integration into the DNA exert their various cytotoxic functions.
Table 2.
Overview of CDD in Myeloprotective Gene Therapy.
| Model | Cell Type | Gene Expression Systems | Year | References | ||
| In vitro | Cell lines | 3T3 fibroblast | Retroviral | Constitutive | 1996, 1998 | [24,25,28] |
| CCRF-CEM (hematopoietic) | Retroviral | Constitutive | 1996 | [24] | ||
| Various | Plasmid (cDNA) | Constitutive | 1996 | [20] | ||
| Fibroblasts | Plasmid (cDNA) | Constitutive | 1996 | [23] | ||
| NIH 3T3 fibroblast1,2 | Retroviral | Constitutive | 1998 to 2000 | [42,43,45] | ||
| WEHI-3 (hematopoietic) | Retroviral | Constitutive | 1999 | [29] | ||
| L1210 (hematopoietic) | Retroviral | Constitutive | 2001 | [26] | ||
| Human lung carcinoma cells | Retroviral | Constitutive | 2002 | [30] | ||
| 32D (hematopoietic) | Lentiviral (third generation) | Inducible | 2012 | [39] | ||
| Primary mouse | Bone marrow cells (BMCs) | Retroviral | Constitutive | 1996 to 2001 | [25,26,29] | |
| BMCs1 | Retroviral | Constitutive | 1998 to 1999 | [42,43] | ||
| Bone marrow stromal cells | Retroviral | Constitutive | 2002 | [30] | ||
| HSCs | Lentiviral (third generation) | Inducible | 2012 | [39] | ||
| Primary human | Cord blood- and peripheral blood-derived progenitor cells | Retroviral | Constitutive | 2005 | [36] | |
| In vivo | Primary mouse | Hematopoietic BMCs | Retroviral | Constitutive | 1998 | [27] |
| BMCs1 | Retroviral | Constitutive | 2004 | [44] | ||
| HSCs | Retroviral | Constitutive | 2006, 2012 | [37,38] | ||
| HSCs | Lentiviral (third generation) | Inducible | 2012 | [39] |
Combinations with other CTX-R genes:
mutDHFR,
glutathione S-transferase A3.
Early Cell Line and Murine In Vitro Studies on CDD-Mediated Drug Resistance
The first studies to describe a protective effect of CDD (over)expression on cytidine analog-induced cytotoxicity used murine fibroblast cell lines transfected with the cDNA of human (h)CDD and were reported in 1996 [20,23]. While these studies varied considerably with regard to the degree of CDD overexpression (50- to 2.5-fold) and ara-C resistance (100- to 3.0-fold), they clearly established a gene dose-dependent cytoprotective effect of transgenic CDD overexpression without obvious transgene-related toxicity. These observations were followed by studies employing γ-retroviral gene transfer technology to express hCDD in 3T3 fibroblasts, hematopoietic CCRF-CEM cells, as well as primary murine bone marrow cells [24,25]. While constitutive over-expression of hCDD resulted in a 2- to 10-fold increased ara-C resistance in CCRF-CEM or 3T3 cells and CDD was confirmed as the functional basis for the observed ara-C resistance by complete reversibility of the effect by the CDD-specific, competitive inhibitor tetrahydrouridine, an approximately 1.000-fold increased ara-C resistance was described in bone marrow-derived primary murine clonogenic progenitor cells [24,25]. CDD-mediated ara-C resistance was further confirmed by a number of in vitro studies all applying γ-retroviral gene transfer technology to transduce fibroblasts, hematopoietic cell lines, and primary murine hematopoietic cells. These studies also extended CDD-meditated drug resistance to other cytidine analog-type cytotoxic drugs, such as gemcitabine or azacytidine [26–29], and established in vitro selection of CDD-modified cells with up to 99% enrichment of transgene-positive cells by ara-C, gemcitabine, or azacytidine exposure [26,30]. However, protection conferred by CDD overexpression in these studies varied considerably, most likely due to substantial differences in endogenous CDD expression levels in different target tissues and/or suboptimal gene transfer rates.
CDD-Mediated Drug Resistance in Human In Vitro Models
For clinical application of CDD-mediated myeloprotection, stable CDD overexpression in human hematopoietic progenitor/stem cells clearly represents a crucial prerequisite. However, efficient gene transfer into primitive hematopoietic cells traditionally has been much harder to achieve in the human compared to the murine system and it took some time until the hurdles responsible for this discrepancy such as insufficient quality and purity of starting populations or low transduction efficiency due to insufficient transduction protocols for human HSCs could be overcome by meticulous studies performed in vitro as well as in murine, canine, and nonhuman primate models. Consequently, optimized transduction strategies nowadays apply specific cytokine combinations, fibronectin (retronectin) coculture, or vector particles pseudotyped with suitable envelope proteins [31–35]. Thus, it took until 2005 to establish clear evidence for the protection of primary human hematopoietic cells from cytidine analog-induced toxicity by CDD gene transfer. Transduction of umbilical cord- or peripheral blood-derived CD34+ stem/progenitor cells with a spleen focus-forming virus (SFFV)-based γ-retroviral vector harboring the hCDD-cDNA resulted in pronounced CDD overexpression and a significantly increased resistance of transduced progenitor cells to ara-C and gemcitabine as determined by the effect on the clonogenic growth of progenitor cells differentiated along the erythroid or myeloid lineage in methylcellulose-based assays. Furthermore, significant in vitro selection of CDD-transduced primary human clonogenic progenitor cells was observed upon culture of the cells in the presence of ara-C for 4 days [36].
In Vivo Efficacy of CDD Gene Transfer in Murine Bone Marrow Transplant Models
While experiments performed in the late 1980s only demonstrated moderately increased ara-C resistance in clonogenic progenitors harvested from the bone marrow of animals transplanted with CDD-transduced HSCs and no convincing evidence of in vivo myeloprotection was obtained [27], the technical shortcomings associated with these early studies meanwhile have been overcome. In a more recent study, not only stable and long-term expression of CDD in the hematopoietic system of recipients of CDD-transduced bone marrow cells has been achieved but constitutive overexpression of CDD in the murine lymphohematopoietic system was also demonstrated to confer significant myeloprotection in the context of ara-C therapy. Following short-term high-dose [500 mg/kg, days 1–4; intraperitoneal (i.p.)] ara-C application, granulocyte nadirs of 2.9 ± 0.6/nl versus 0.7 ± 0.1/nl and thrombocyte nadirs of 509 ± 147/nl versus 80 ± 9/nl for the CDD versus the control group were demonstrated [37], and similar differences were observed with prolonged low-dose (60 mg/kg, days 1–10; i.p.) treatment [38] (see Figure 3). Moreover, animals overexpressing CDD in their hematopoietic system were also protected from otherwise lethal gemcitabine doses and a 24- to 149-fold increased cytoplasmic CDD activity was observed in bone marrow and spleen cells of primary recipients [37]. While these data reflect the excellent expression levels achievable with SFFV/murine embryonic stem cell virus (MESV)-based vectors in hematopoietic cells, these very high levels may also have contributed to the transgene-related toxicities observed in these studies (see below). Significant in vivo myeloprotection was also demonstrated for tet-regulated overexpression of CDD [39], an approach that may be suited to circumvent CDD-induced transgene toxicity (see below).
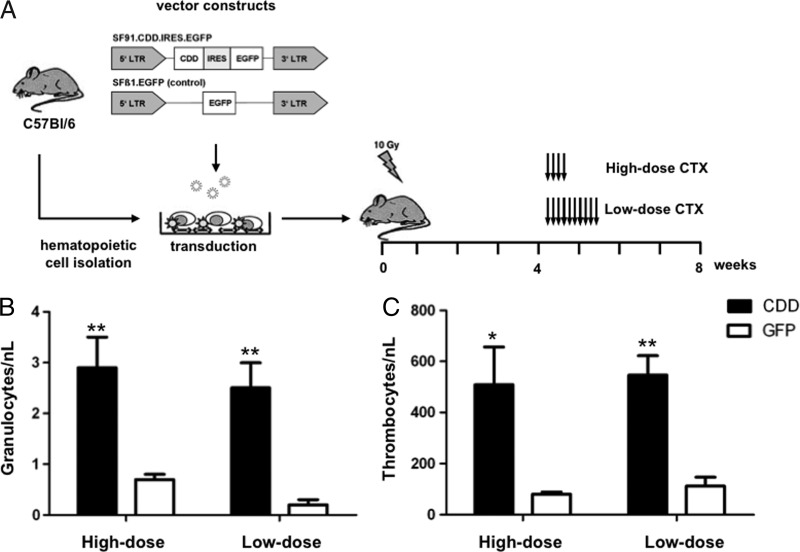
Figure 3.
Myeloprotection by CDD gene transfer in an in vivo murine transplant model. (A) Schematic overview of the gene transfer model, vector constructs, and chemotherapy application schedule used. Nadir levels of (B) peripheral blood granulocyte and (C) thrombocyte counts (mean ± SEM; n = 5) following high-dose (4 x 500 mg/kg, i.p., days 1–4) or prolonged low-dose (10 x 60 mg/kg, i.p., days 1–10) ara-C treatment are given. *P < .05 and **P < .01 denote significant differences by Student's t test (data compiled from Rattmann et al. [37] and Brennig et al. [38]; CDD: cytidine deaminase; IRES: internal ribosomal entry site; LTR: long terminal repeat; EGFP: enhanced green fluorescent protein).
Interestingly, despite the profound myeloprotective effects observed and in clear contrast to the data obtained from in vitro systems, no long-term in vivo selection of CDD-overexpressing cells was observed in these studies [37,38]. This suggests that the myeloprotective effect exerted by CDD is achieved primarily on the level of progenitor or more mature cells rather than stem cells. Indeed, relatively moderate stem cell toxicity of ara-C has been described [40], an observation readily explained by the relative quiescence of repopulating HSCs and the S-phase-specific activity of ara-C. Such a “stem cell-sparing” activity of ara-C is also suggested by its clinical toxicity profile. Here, after high-dose ara-C application (3 g/m2, six to eight doses), a profound and long-lasting myelosuppression nearly inevitably is followed by a complete hematopoietic reconstitution. However, repetitive low to intermediate-dose ara-C application has been reported to increase HSC cycling and thus ara-C susceptibility of HSCs [41]. Transfer of this strategy to a murine CDD gene transfer/HSC transplant model resulted in significant in vivo selection with an up to six-fold increase of CDD-transduced cells in the peripheral blood, when 30 to 60 mg/kg ara-C was administered repetitively for 10 to 20 days i.p. However, this effect was only transient, suggesting selection on the level of early progenitor cells rather than true HSCs [38].
Studies on Combined Gene Transfer of CDD and Other CTX-R Genes
Given the fact that modern anticancer chemotherapeutic regimen nearly inevitably combine a number of cytotoxic drugs to reduce agent-specific toxicities and delay the emergence of therapy-resistant tumor cells, simultaneous (over)expression of CTX-R genes appears as a suitable strategy to respond to this situation. With respect to CDD in particular, combinations with mutDHFR have been investigated using moloney murine leukemia virus (MMLV)-based γ-retroviral backbones to express a mutDHFR/CDD fusion protein. In these studies, combined mutDHFR and CDD expression resulted in significant protection against methotrexate as well as ara-C toxicity [42,43] and successful treatment of human lymphoma cells has been reported in a “humanized” murine xenotransplant model applying CDD/mutDHFR co-transduced murine bone marrow cells to protect the hematopoietic system from combined ara-C plus methotrexate chemotherapy [44]. CDD overexpression has also been combined with expression of glutathione S-transferase A3 to protect from combined nitrogen mustard/ara-C treatment, although this concept never exceeded the phase of in vitro cell line studies [45].
Predictions from Preclinical Studies for Clinical Application Scenarios
In general, in vitro studies employing cell lines or murine clonogenic progenitor growth have demonstrated CDD-mediated ara-C resistance at concentrations of 10 to 500 nM [29]. The only exception was an early study reporting resistance to ara-C doses of up to 50 µM [25], but also these authors later describe resistance only in the aforementioned dose range [27]. CDD-mediated resistance to ara-C doses ranging from 60 to 500 nM was also observed for primary human clonogenic cells [36]. These similarities come to no surprise, as for both human and murine progenitor/stem cells low CDD activity has been demonstrated [20], and in both species, the hematopoietic system represents a critical target organ for ara-C and cytidine analog toxicity. These data strongly implies that in the clinical situation protection of hematopoietic cells from conventionally dosed ara-C should be possible. Ara-C conventionally is administered at doses of 100 to 200 mg/m2 given consecutively for 3 to 7 days, and when delivered as continuous infusion, this result in steady-state plasma concentrations of 100 to 1200 nM. Currently, the most reliable parameter to predict ara-C cytotoxicity, within certain limits, is the product of exposure time and drug concentration. This product was clearly exceeded in studies demonstrating myeloprotection in clonogenic assays applying ara-C doses of up to 500 nM (and in our recent studies with advanced lentiviral vectors even up to 600 nM; own unpublished data) for 10 to 14 days. Furthermore, the data from in vivo transplant studies [37] suggests that protection in the clinical situation should also be possible from high-dose ara-C application (3 g/m2 i.v., six to eight doses at 12-hour intervals). For the standard patient (75 kg, 1.75-m2 surface area), this schedule results in a total dose of approximately 0.6 g ara-C/kg body weight administered over a 4-day period, while in the murine transplant studies protection from up to 2 g/kg ara-C was demonstrated. These calculations should be addressed with some caution, however, as different application schedules (1x daily i.p. in the murine model versus 2x daily i.v. in the clinical situation) have to be taken into account.
Clinical Application of CDD Gene Therapy
Application of CDD Gene Transfer in the Treatment of Acute Leukemias and Myelodysplasias
Clearly, treatment of high-risk disease states in a post-autologous/ allogeneic HSC transplantation setting represents the most suitable clinical scenario for the initial application of CDD gene therapy in these disease entities. In this situation, effective therapies to decrease post-HSC relapse are clearly needed [46]; however, dose-intensive consolidation or maintenance therapy with ara-C, azacytidine, or decitabine frequently is associated with severe myelosuppression leading to dose adjustments and consecutively inferior therapeutic results. This may be prevented by overexpression of CDD either alone or in combination with other CTX-R genes before cytidine analog therapy. As especially for patients with complex aberrant karyotypes, early allogeneic HSC appears to be beneficial [47]. CTX-R gene transfer could be incorporated into relatively early phases of treatment for these high-risk patients to decrease side effects and thereby potentially increase dose intensity and outcome of subsequent cytidine analog chemotherapy. In addition to ara-C, there currently is an increasing interest to evaluate also azacytidine in the post-allo HSC transplantation situation for relapsed disease. While azacytidine exerts considerable activity in this situation, again cytopenias giving rise to bleeding and infections represent the main side effects [47–49]. Furthermore, CDD gene transfer in the context of HSC transplantation following reduced intensity conditioning (RIC) may be an option for elderly or less fit patients to receive adequate chemotherapy doses. In this situation, the reduction of hematotoxic side effects may lead to increased tolerability and subsequently better treatment compliance, which otherwise is a significant problem in this patient population [50]. Refractory or relapsing disease in acute leukemias has been associated with the survival of leukemic stem cells (LSCs) [51,52] as defined by the xenotransplantation model [53,54]. However, the activity of ara-C in leukemia stem cells has been questioned [55]. As this may be related to the relative quiescent state of LSCs in the “LSC-niche” [56,57], cell cycle-promoting application schedules of ara-C such as prolonged low-dose treatment or combinations with cycle-inducing cytokines such as G-CSF or interferons [58,59] may be preferred in this context.
CTX-R Gene Combinations
As stated above, modern CTX regimen usually combine cytotoxic agents to improve efficacy and delay therapy resistance. This may be counteracted by the combined use of CTX-R genes, an approach clearly feasible with current gene transfer vector technology [60]. Considering the dominant role of cytidine analogs in AML and high-risk myelodysplasia therapy and the fact that ara-C/anthracycline combinations such as the classical “3 + 7” combinations, TAD9, HAM, or Flac-Ida [61] still represent a cornerstone of first- and second-line anti-AML chemotherapies and are associated with a profound and long-lasting myelosuppression considerably contributing to therapy-related morbidity and occasionally even mortality, MDR1 appears as a natural combination partner for CDD gene therapy in these disease entities.
In addition, given the success in a recent phase I study in glioblastoma patients [11], mutMGMT represents another potential combination partner for CDD gene transfer. This will allow not only for myeloprotection from O6-alkylating agents but also for effective in vivo selection of drug-resistant HSCs. Though diseases responsive to ara-C as well as chloroethylnitrosoureas or triazene derivatives are quite rare, one context to exploit the CDD/MGMT combination (with a potential add-on of MDR1) may be salvage therapy of aggressive lymphomas based on the dexamethasone, BCNU, etoposide, ara-C, melphalan regimen. Combinations with mutDHFR (see above) may be considered for the treatment of highly aggressive lymphoid malignancies such as B cell acute lymphocytic leukemias or B-lymphoblastic Non-Hodgkin lymphomas, which are routinely treated with combination regimen including high-dose methotrexate application.
Potential Side Effects of CDD Gene Therapy and Strategies to Overcome These
Insertional Mutagenesis
Insertional mutagenesis has been described in several HSC gene therapy studies and clearly represents a major concern for all approaches using integrating vector systems. This carries a particular relevance for CDD gene transfer applications as in this setting insertional mutagenesis may give rise to drug-resistant leukemic cells. In clinical studies, insertional mutagenesis has been observed specifically for γ-retroviral constructs expressing the therapeutic gene directly from the strong viral promoter/enhancer sequences situated in the U3 region of the long terminal repeat (LTR). In this context, insertion of the vector upstream of a cellular gene has been observed to lead to an up-regulation of cellular (onco)genes such as LMO2 or EVI1 mediated by the viral enhancer [62]. Significant reduction of the mutagenic risk can be achieved by the use of improved γ-retroviral or lentiviral vector design such as self-inactivating constructs harboring inactivating deletions in their 3′LTR U3 promoter/enhancer region and the use of internal promoters derived from housekeeping genes [63]. Our group currently investigates the potential of such safety-improved constructs in the context of CDD gene transfer. However, given the risk of generating CDD-overexpressing leukemic cells, incorporation of inducible suicide genes such as herpes simplex virus thymidine kinase [64] or a genetically engineered variant of caspase 9 (i-caspase) [65] into the gene transfer vectors should be considered as a fail-safe mechanism.
Inadvertent Transduction of Leukemic Cells
As CDD-associated drug resistance primarily is observed for agents that exert their therapeutic activity in acute leukemias and other hematological malignancies, inadvertent transduction of leukemic cells present in the cell preparation used for the ex vivo genetic modification procedure constitutes another potential problem of CDD gene transfer. Thus, at present, it appears preferable to use this strategy in combination with an allogeneic bone marrow transplant approach, as it is state of the art for high-risk acute myeloid or lymphoid leukemias and myelodysplasias [66]. If CDD gene transfer is applied in an autologous setting, again suicide genes may be considered as a fail-safe mechanism.
Cell Type-Specific Toxicities with High CDD Expression Levels
Another problem encountered in some studies on CDD gene transfer [37] was a lymphotoxic effect associated with high constitutive CDD expression levels. A potential mechanism for this lymphotoxicity has been suggested by a recent analysis of mice devoid of deoxycytidine kinase (dCK) activity, which in the nucleotide salvage pathway represents the physiologic “counter-enzyme” of CDD, as both, increased CDD but also reduced dCK activity, directly result in decreased cytidine monophosphate (CMP) levels. Mice deficient in dCK have a block in intrathymic T as well as early B cell development, indicating a critical role of the nucleotide salvage pathway during early T and B cell development when T cell receptor or VDJ recombination is followed by massive cellular proliferation and clonal expansion [67]. Furthermore, a mild to moderate myelotoxicity of CDD overexpression has been described as most probably related to the negative feedback loop instituted by the release of high levels of CDD by mature human granulocytes, which in turn inhibits the differentiation and proliferation of granulocyte-macrophage colony-forming cells and thereby regulates late steps of myeloid differentiation [21,37]. While cell type-specific toxicities may be ameliorated by CDD expression from suitable internal promoters such as the truncated human elongation factor 1a or the SFFV promoter (personal unpublished observation), advanced regulated gene expression systems allowing for inducible (doxycyclineregulated) or cell type-specific [microRNA (miRNA)-regulated] expression of the CDD transgene represent another approach to address this problem. Powerful transcriptionally regulated systems controlled by the application of doxycycline (also known as tetracycline, Dox) and allowing for the rapid induction and switch-off of transgene expression in a tightly controlled fashion have been developed over the last decade (Figure 4A) [68,69]. An area that appears particularly suited for the use of this system is the transfer of CTX-R genes for myeloprotection, as here transgene expression only is required for the relatively short periods of cytotoxic drug administration. Thus, toxic effects associated with prolonged constitutive transgene expression may be prevented and doxycycline-regulated expression systems already have been demonstrated to allow for significant CDD overexpression in the absence of lymphotoxic side effects [39]. In addition, endogenous miRNAs can be used to target transgenic mRNAs and achieve cell type-specific transgene expression [70,71]. With this approach, miRNA target sites corresponding to a specific miRNA are added to the transgene cDNA rendering the respective mRNA susceptible to miRNA-mediated degradation selectively in cells that express this miRNA (Figure 4B). In the context of CDD-induced lymphotoxicity, miRNA-150, which is predominantly expressed during the late stages of T and B lymphoid development, may be targeted [72]. However, as the studies in dCK-deficient mice indicate relatively early stages of T and B lymphopoiesis as a major target of CDD toxicity, also alternative miRNAs, specifically expressed at these earlier differentiation stages such as miRNA-181, may be exploited [73].
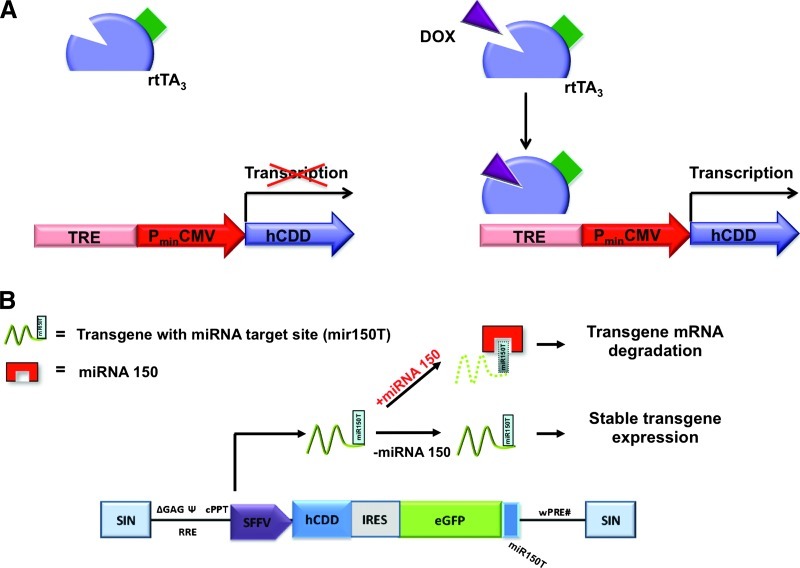
Figure 4.
Regulation of transgene expression by doxycycline-induced (Tet-on) and miRNA-mediated expression systems. (A) The doxycycline (dox; also known as tetracycline, Tet)-controlled reverse transactivator protein (rTA) represents a Tet-repressor protein fused to three minimal VP16 activation domains of the herpes simplex virus. In the absence of dox, transgene expression (as of the hCDD) is inhibited, whereas in the presence of dox, rTA binds to the Tet-responsive element (TRE) and activates transgene transcription from the cytomegalovirus (CMV) minimal promoter (PminCMV). (B) Optimized self-inactivating (SIN) lentiviral vector backbone for cell type-specific transgene (hCDD) expression. The vector carries specific miRNA target sites (here for miRNA-150) fused to the transgenic cDNA. Transgene expression is suppressed in cells expressing the corresponding miRNA, as the miRNA will bind to the respective target site (here mir150T) and lead to transgene mRNA degradation. In cells not expressing the miRNA, stable transgene expression is maintained.
Abbreviations
- AML
acute myeloid leukemia
- Ara-C
cytosine arabinoside
- BG
O6-benzylguanine
- CDD
cytidine deaminase
- CTX-R gene
drug resistance gene
- dCK
deoxycytidine kinase
- DHFR
dihydrofolate reductase
- HSCs
hematopoietic stem cells
- LSCs
leukemic stem cells
- MDR1
multidrug resistance 1
- MGMT
O6-methylguanine DNA-methyltransferase
- SFFV
spleen focus-forming virus
Footnotes
This work was supported by grants from the Deutsche Forschungsgemeinschaft: Cluster of Excellence REBIRTH (Exc 62/1), SPP1230: grant MO 886/3 (T.M.), and individual grant MO 886/4 (T.M.).
References
- 1.Dick JE, Magli MC, Huszar D, Phillips RA, Bernstein A. Introduction of a selectable gene into primitive stem cells capable of long-term reconstitution of the hemopoietic system of W/Wv mice. Cell. 1985;42:71–79. doi: 10.1016/s0092-8674(85)80102-1. [DOI] [PubMed] [Google Scholar]
- 2.Keller G, Paige C, Gilboa E, Wagner EF. Expression of a foreign gene in myeloid and lymphoid cells derived from multipotent haematopoietic precursors. Nature. 1985;318:149–154. doi: 10.1038/318149a0. [DOI] [PubMed] [Google Scholar]
- 3.Williams DA, Lemischka IR, Nathan DG, Mulligan RC. Introduction of new genetic material into pluripotent haematopoietic stem cells of the mouse. Nature. 1984;310:476–480. doi: 10.1038/310476a0. [DOI] [PubMed] [Google Scholar]
- 4.Fischer A, Hacein-Bey-Abina S, Cavazzana-Calvo M. Gene therapy for primary immunodeficiencies. Hematol Oncol Clin North Am. 2011;25:89–100. doi: 10.1016/j.hoc.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Rivat C, Santilli G, Gaspar HB, Thrasher AJ. Gene therapy for primary immunodeficiencies. Hum Gene Ther. 2012;23:668–675. doi: 10.1089/hum.2012.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flasshove M, Moritz T, Bardenheuer W, Seeber S. Hematoprotection by transfer of drug-resistance genes. Acta Haematol. 2003;110:93–106. doi: 10.1159/000072458. [DOI] [PubMed] [Google Scholar]
- 7.Moritz T, Williams DA. Marrow protection—transduction of hematopoietic cells with drug resistance genes. Cytotherapy. 2001;3:67–84. doi: 10.1080/14653240152584640. [DOI] [PubMed] [Google Scholar]
- 8.Trobridge GD, Kiem HP. Large animal models of hematopoietic stem cell gene therapy. Gene Ther. 2010;17:939–948. doi: 10.1038/gt.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu L, Gerson SL. Targeted modulation of MGMT: clinical implications. Clin Cancer Res. 2006;12:328–331. doi: 10.1158/1078-0432.CCR-05-2543. [DOI] [PubMed] [Google Scholar]
- 10.Milsom MD, Williams DA. Live and let die: in vivo selection of gene-modified hematopoietic stem cells via MGMT-mediated chemoprotection. DNA Repair (Amst) 2007;6:1210–1221. doi: 10.1016/j.dnarep.2007.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adair JE, Beard BC, Trobridge GD, Neff T, Rockhill JK, Silbergeld DL, Mrugala MM, Kiem HP. Extended survival of glioblastoma patients after chemoprotective HSC gene therapy. Sci Transl Med. 2012;4:133ra157. doi: 10.1126/scitranslmed.3003425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, McClay EF. Systemic chemotherapy for the treatment of metastatic melanoma. Semin Oncol. 2002;29:413–426. doi: 10.1053/sonc.2002.35237. [DOI] [PubMed] [Google Scholar]
- 13.Geiger H, Schleimer D, Nattamai KJ, Dannenmann SR, Davies SM, Weiss BD. Mutagenic potential of temozolomide in bone marrow cells in vivo. Blood. 2006;107:3010–3011. doi: 10.1182/blood-2005-09-3649. [DOI] [PubMed] [Google Scholar]
- 14.Banerjee D, Mayer-Kuckuk P, Capiaux G, Budak-Alpdogan T, Gorlick R, Bertino JR. Novel aspects of resistance to drugs targeted to dihydrofolate reductase and thymidylate synthase. Biochim Biophys Acta. 2002;1587:164–173. doi: 10.1016/s0925-4439(02)00079-0. [DOI] [PubMed] [Google Scholar]
- 15.Schiedlmeier B, Kuhlcke K, Eckert HG, Baum C, Zeller WJ, Fruehauf S. Quantitative assessment of retroviral transfer of the human multidrug resistance 1 gene to human mobilized peripheral blood progenitor cells engrafted in nonobese diabetic/severe combined immunodeficient mice. Blood. 2000;95:1237–1248. [PubMed] [Google Scholar]
- 16.Sorrentino BP, Brandt SJ, Bodine D, Gottesman M, Pastan I, Cline A, Nienhuis AW. Selection of drug-resistant bone marrow cells in vivo after retroviral transfer of human MDR1. Science. 1992;257:99–103. doi: 10.1126/science.1352414. [DOI] [PubMed] [Google Scholar]
- 17.Abonour R, Williams DA, Einhorn L, Hall KM, Chen J, Coffman J, Traycoff CM, Bank A, Kato I, Ward M, et al. Efficient retrovirus-mediated transfer of the multidrug resistance 1 gene into autologous human long-term repopulating hematopoietic stem cells. Nat Med. 2000;6:652–658. doi: 10.1038/76225. [DOI] [PubMed] [Google Scholar]
- 18.Cowan KH, Moscow JA, Huang H, Zujewski JA, O'Shaughnessy J, Sorrentino B, Hines K, Carter C, Schneider E, Cusack G, et al. Paclitaxel chemotherapy after autologous stem-cell transplantation and engraftment of hematopoietic cells transduced with a retrovirus containing the multidrug resistance complementary DNA (MDR1) in metastatic breast cancer patients. Clin Cancer Res. 1999;5:1619–1628. [PubMed] [Google Scholar]
- 19.Iwasaki H, Huang P, Keating MJ, Plunkett W. Differential incorporation of ara-C, gemcitabine, and fludarabine into replicating and repairing DNA in proliferating human leukemia cells. Blood. 1997;90:270–278. [PubMed] [Google Scholar]
- 20.Schroder JK, Kirch C, Flasshove M, Kalweit H, Seidelmann M, Hilger R, Seeber S, Schutte J. Constitutive overexpression of the cytidine deaminase gene confers resistance to cytosine arabinoside in vitro. Leukemia. 1996;10:1919–1924. [PubMed] [Google Scholar]
- 21.Gran C, Boyum A, Johansen RF, Lovhaug D, Seeberg EC. Growth inhibition of granulocyte-macrophage colony-forming cells by human cytidine deaminase requires the catalytic function of the protein. Blood. 1998;91:4127–4135. [PubMed] [Google Scholar]
- 22.Steuart CD, Burke PJ. Cytidine deaminase and the development of resistance to arabinosyl cytosine. Nat New Biol. 1971;233:109–110. doi: 10.1038/newbio233109a0. [DOI] [PubMed] [Google Scholar]
- 23.Momparler RL, Laliberte J, Eliopoulos N, Beausejour C, Cournoyer D. Transfection of murine fibroblast cells with human cytidine deaminase cDNA confers resistance to cytosine arabinoside. Anticancer Drugs. 1996;7:266–274. doi: 10.1097/00001813-199605000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Neff T, Blau CA. Forced expression of cytidine deaminase confers resistance to cytosine arabinoside and gemcitabine. Exp Hematol. 1996;24:1340–1346. [PubMed] [Google Scholar]
- 25.Momparler RL, Eliopoulos N, Bovenzi V, Letourneau S, Greenbaum M, Cournoyer D. Resistance to cytosine arabinoside by retrovirally mediated gene transfer of human cytidine deaminase into murine fibroblast and hematopoietic cells. Cancer Gene Ther. 1996;3:331–338. [PubMed] [Google Scholar]
- 26.Beausejour CM, Eliopoulos N, Momparler L, Le NL, Momparler RL. Selection of drug-resistant transduced cells with cytosine nucleoside analogs using the human cytidine deaminase gene. Cancer Gene Ther. 2001;8:669–676. doi: 10.1038/sj.cgt.7700358. [DOI] [PubMed] [Google Scholar]
- 27.Eliopoulos N, Bovenzi V, Le NL, Momparler LF, Greenbaum M, Letourneau S, Cournoyer D, Momparler RL. Retroviral transfer and long-term expression of human cytidine deaminase cDNA in hematopoietic cells following transplantation in mice. Gene Ther. 1998;5:1545–1551. doi: 10.1038/sj.gt.3300767. [DOI] [PubMed] [Google Scholar]
- 28.Eliopoulos N, Cournoyer D, Momparler RL. Drug resistance to 5-aza-2′-deoxycytidine, 2′,2′-difluorodeoxycytidine, and cytosine arabinoside conferred by retroviral-mediated transfer of human cytidine deaminase cDNA into murine cells. Cancer Chemother Pharmacol. 1998;42:373–378. doi: 10.1007/s002800050832. [DOI] [PubMed] [Google Scholar]
- 29.Flasshove M, Frings W, Schroder JK, Moritz T, Schutte J, Seeber S. Transfer of the cytidine deaminase cDNA into hematopoietic cells. Leuk Res. 1999;23:1047–1053. doi: 10.1016/s0145-2126(99)00128-9. [DOI] [PubMed] [Google Scholar]
- 30.Eliopoulos N, Al-Khaldi A, Beausejour CM, Momparler RL, Momparler LF, Galipeau J. Human cytidine deaminase as an ex vivo drug selectable marker in gene-modified primary bone marrow stromal cells. Gene Ther. 2002;9:452–462. doi: 10.1038/sj.gt.3301675. [DOI] [PubMed] [Google Scholar]
- 31.Bauer TR, Jr, Miller AD, Hickstein DD. Improved transfer of the leukocyte integrin CD18 subunit into hematopoietic cell lines by using retroviral vectors having a gibbon ape leukemia virus envelope. Blood. 1995;86:2379–2387. [PubMed] [Google Scholar]
- 32.Burns JC, Friedmann T, Driever W, Burrascano M, Yee JK. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and non-mammalian cells. Proc Natl Acad Sci USA. 1993;90:8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanenberg H, Xiao XL, Dilloo D, Hashino K, Kato I, Williams DA. Colocalization of retrovirus and target cells on specific fibronectin fragments increases genetic transduction of mammalian cells. Nat Med. 1996;2:876–882. doi: 10.1038/nm0896-876. [DOI] [PubMed] [Google Scholar]
- 34.Moritz T, Dutt P, Xiao X, Carstanjen D, Vik T, Hanenberg H, Williams DA. Fibronectin improves transduction of reconstituting hematopoietic stem cells by retroviral vectors: evidence of direct viral binding to chymotryptic carboxy-terminal fragments. Blood. 1996;88:855–862. [PubMed] [Google Scholar]
- 35.Zhang CC, Lodish HF. Murine hematopoietic stem cells change their surface phenotype during ex vivo expansion. Blood. 2005;105:4314–4320. doi: 10.1182/blood-2004-11-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bardenheuer W, Lehmberg K, Rattmann I, Brueckner A, Schneider A, Sorg UR, Seeber S, Moritz T, Flasshove M. Resistance to cytarabine and gemcitabine and in vitro selection of transduced cells after retroviral expression of cytidine deaminase in human hematopoietic progenitor cells. Leukemia. 2005;19:2281–2288. doi: 10.1038/sj.leu.2403977. [DOI] [PubMed] [Google Scholar]
- 37.Rattmann I, Kleff V, Sorg UR, Bardenheuer W, Brueckner A, Hilger RA, Opalka B, Seeber S, Flasshove M, Moritz T. Gene transfer of cytidine deaminase protects myelopoiesis from cytidine analogs in an in vivo murine transplant model. Blood. 2006;108:2965–2971. doi: 10.1182/blood-2006-03-011734. [DOI] [PubMed] [Google Scholar]
- 38.Brennig S, Rattmann I, Lachmann N, Schambach A, Williams DA, Moritz T. In vivo enrichment of cytidine deaminase gene-modified hematopoietic cells by prolonged cytosine-arabinoside application. Cytotherapy. 2012;14:451–460. doi: 10.3109/14653249.2011.646043. [DOI] [PubMed] [Google Scholar]
- 39.Lachmann N, Brennig S, Pfaff N, Schermeier H, Dahlmann J, Phaltane R, Gruh I, Modlich U, Schambach A, Baum C, et al. Efficient in vivo regulation of cytidine deaminase expression in the haematopoietic system using a doxycycline-inducible lentiviral vector system. Gene Ther. doi: 10.1038/gt.2012.40. (in press) [DOI] [PubMed] [Google Scholar]
- 40.Neben S, Hellman S, Montgomery M, Ferrara J, Mauch P. Hematopoietic stem cell deficit of transplanted bone marrow previously exposed to cytotoxic agents. Exp Hematol. 1993;21:156–162. [PubMed] [Google Scholar]
- 41.Leach WB, Laster WR, Jr, Mayo JG, Griswold DP, Jr, Schabel FM., Jr Toxicity studies in mice treated with 1-β-d-arabinofuranosylcytosine (ara-C) Cancer Res. 1969;29:529–535. [PubMed] [Google Scholar]
- 42.Beausejour CM, Le NL, Letourneau S, Cournoyer D, Momparler RL. Coexpression of cytidine deaminase and mutant dihydrofolate reductase by a bicistronic retroviral vector confers resistance to cytosine arabinoside and methotrexate. Hum Gene Ther. 1998;9:2537–2544. doi: 10.1089/hum.1998.9.17-2537. [DOI] [PubMed] [Google Scholar]
- 43.Sauerbrey A, McPherson JP, Zhao SC, Banerjee D, Bertino JR. Expression of a novel double-mutant dihydrofolate reductase-cytidine deaminase fusion gene confers resistance to both methotrexate and cytosine arabinoside. Hum Gene Ther. 1999;10:2495–2504. doi: 10.1089/10430349950016834. [DOI] [PubMed] [Google Scholar]
- 44.Budak-Alpdogan T, Alpdogan O, Banerjee D, Wang E, Moore MA, Bertino JR. Methotrexate and cytarabine inhibit progression of human lymphoma in NOD/SCID mice carrying a mutant dihydrofolate reductase and cytidine deaminase fusion gene. Mol Ther. 2004;10:574–584. doi: 10.1016/j.ymthe.2004.06.115. [DOI] [PubMed] [Google Scholar]
- 45.Letourneau S, Palerme JS, Delisle JS, Beausejour CM, Momparler RL, Cournoyer D. Coexpression of rat glutathione S -transferase A3 and human cytidine deaminase by a bicistronic retroviral vector confers in vitro resistance to nitrogen mustards and cytosine arabinoside in murine fibroblasts. Cancer Gene Ther. 2000;7:757–765. doi: 10.1038/sj.cgt.7700169. [DOI] [PubMed] [Google Scholar]
- 46.Burnett AK. New induction and postinduction strategies in acute myeloid leukemia. Curr Opin Hematol. 2012;19:76–81. doi: 10.1097/MOH.0b013e3283500a92. [DOI] [PubMed] [Google Scholar]
- 47.Schroeder TC, Czibere A, Kroeger N, Platzbecker U, Bug G, Uharek L, Luft T, Wolschke C, Bruns I, Zohren F, et al. Phase II study of Azacitidine (Vidaza®, Aza) and donor lymphocyte infusion (DLI) as first salvage therapy in patients with acute myeloid leukemia (AML) or myelodysplastic syndromes (MDS) relapsing after allogeneic hematopoietic stem cell transplantation (all-SCT): final results from the AZARELA-trial (NCT-00795548) Blood. 2011;118:300. [Google Scholar]
- 48.Bolanos-Meade J, Smith BD, Gore SD, McDevitt MA, Luznik L, Fuchs EJ, Jones RJ. 5-Azacytidine as salvage treatment in relapsed myeloid tumors after allogeneic bone marrow transplantation. Biol Blood Marrow Transplant. 2011;17:754–758. doi: 10.1016/j.bbmt.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Platzbecker U, Wermke M, Radke J, Oelschlaegel U, Seltmann F, Kiani A, Klut IM, Knoth H, Rollig C, Schetelig J, et al. Azacitidine for treatment of imminent relapse in MDS or AML patients after allogeneic HSCT: results of the RELAZA trial. Leukemia. 2012;26:381–389. doi: 10.1038/leu.2011.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roboz GJ. Novel approaches to the treatment of acute myeloid leukemia. Hematology Am Soc Hematol Educ Program. 2011;2011:43–50. doi: 10.1182/asheducation-2011.1.43. [DOI] [PubMed] [Google Scholar]
- 51.Eppert K, Takenaka K, Lechman ER, Waldron L, Nilsson B, van Galen P, Metzeler KH, Poeppl A, Ling V, Beyene J, et al. Stem cell gene expression programs influence clinical outcome in human leukemia. Nat Med. 2011;17:1086–1093. doi: 10.1038/nm.2415. [DOI] [PubMed] [Google Scholar]
- 52.Moshaver B, van Rhenen A, Kelder A, van der Pol M, Terwijn M, Bachas C, Westra AH, Ossenkoppele GJ, Zweegman S, Schuurhuis GJ. Identification of a small subpopulation of candidate leukemia-initiating cells in the side population of patients with acute myeloid leukemia. Stem Cells. 2008;26:3059–3067. doi: 10.1634/stemcells.2007-0861. [DOI] [PubMed] [Google Scholar]
- 53.Dick JE. Acute myeloid leukemia stem cells. Ann N Y Acad Sci. 2005;1044:1–5. doi: 10.1196/annals.1349.001. [DOI] [PubMed] [Google Scholar]
- 54.Lapidot T, Pflumio F, Doedens M, Murdoch B, Williams DE, Dick JE. Cytokine stimulation of multilineage hematopoiesis from immature human cells engrafted in SCID mice. Science. 1992;255:1137–1141. doi: 10.1126/science.1372131. [DOI] [PubMed] [Google Scholar]
- 55.Guzman ML, Neering SJ, Upchurch D, Grimes B, Howard DS, Rizzieri DA, Luger SM, Jordan CT. Nuclear factor-κB is constitutively activated in primitive human acute myelogenous leukemia cells. Blood. 2001;98:2301–2307. doi: 10.1182/blood.v98.8.2301. [DOI] [PubMed] [Google Scholar]
- 56.Roboz GJ, Guzman M. Acute myeloid leukemia stem cells: seek and destroy. Expert Rev Hematol. 2009;2:663–672. doi: 10.1586/ehm.09.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saito Y, Kitamura H, Hijikata A, Tomizawa-Murasawa M, Tanaka S, Takagi S, Uchida N, Suzuki N, Sone A, Najima Y, et al. Identification of therapeutic targets for quiescent, chemotherapy-resistant human leukemia stem cells. Sci Transl Med. 2010;2:17ra19. doi: 10.1126/scitranslmed.3000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Essers MA, Offner S, Blanco-Bose WE, Waibler Z, Kalinke U, Duchosal MA, Trumpp A. IFNα activates dormant haematopoietic stem cells in vivo. Nature. 2009;458:904–908. doi: 10.1038/nature07815. [DOI] [PubMed] [Google Scholar]
- 59.Morrison SJ, Wright DE, Weissman IL. Cyclophosphamide/granulocyte colony-stimulating factor induces hematopoietic stem cells to proliferate prior to mobilization. Proc Natl Acad Sci USA. 1997;94:1908–1913. doi: 10.1073/pnas.94.5.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schambach A, Baum C. Clinical application of lentiviral vectors—concepts and practice. Curr Gene Ther. 2008;8:474–482. doi: 10.2174/156652308786848049. [DOI] [PubMed] [Google Scholar]
- 61.Estey EH. Acute myeloid leukemia: 2012 update on diagnosis, risk stratification, and management. Am J Hematol. 2012;87:89–99. doi: 10.1002/ajh.22246. [DOI] [PubMed] [Google Scholar]
- 62.Baum C, von Kalle C, Staal FJ, Li Z, Fehse B, Schmidt M, Weerkamp F, Karlsson S, Wagemaker G, Williams DA. Chance or necessity? Insertional mutagenesis in gene therapy and its consequences. Mol Ther. 2004;9:5–13. doi: 10.1016/j.ymthe.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 63.Maetzig T, Galla M, Baum C, Schambach A. Gammaretroviral vectors: biology, technology and application. Viruses. 2011;3:677–713. doi: 10.3390/v3060677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barese CN, Krouse AE, Metzger ME, King CA, Traversari C, Marini FC, Donahue RE, Dunbar CE. Thymidine kinase suicide gene-mediated ganciclovir ablation of autologous gene-modified rhesus hematopoiesis. Mol Ther. 2012;20:1932–1943. doi: 10.1038/mt.2012.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ramos CA, Asgari Z, Liu E, Yvon E, Heslop HE, Rooney CM, Brenner MK, Dotti G. An inducible caspase 9 suicide gene to improve the safety of mesenchymal stromal cell therapies. Stem Cells. 2010;28:1107–1115. doi: 10.1002/stem.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gyurkocza B, Rezvani A, Storb RF. Allogeneic hematopoietic cell transplantation: the state of the art. Expert Rev Hematol. 2010;3:285–299. doi: 10.1586/ehm.10.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Toy G, Austin WR, Liao HI, Cheng D, Singh A, Campbell DO, Ishikawa TO, Lehmann LW, Satyamurthy N, Phelps ME, et al. Requirement for deoxycytidine kinase in T and B lymphocyte development. Proc Natl Acad Sci USA. 2010;107:5551–5556. doi: 10.1073/pnas.0913900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ting DT, Kyba M, Daley GQ. Inducible transgene expression in mouse stem cells. Methods Mol Med. 2005;105:23–46. doi: 10.1385/1-59259-826-9:023. [DOI] [PubMed] [Google Scholar]
- 70.Brown BD, Venneri MA, Zingale A, Sergi Sergi L, Naldini L. Endogenous microRNA regulation suppresses transgene expression in hematopoietic lineages and enables stable gene transfer. Nat Med. 2006;12:585–591. doi: 10.1038/nm1398. [DOI] [PubMed] [Google Scholar]
- 71.Gentner B, Visigalli I, Hiramatsu H, Lechman E, Ungari S, Giustacchini A, Schira G, Amendola M, Quattrini A, Martino S, et al. Identification of hematopoietic stem cell-specific miRNAs enables gene therapy of globoid cell leukodystrophy. Sci Transl Med. 2010;2:58ra84. doi: 10.1126/scitranslmed.3001522. [DOI] [PubMed] [Google Scholar]
- 72.Lachmann N, Jagielska J, Heckl D, Brennig S, Pfaff N, Maetzig T, Modlich U, Cantz T, Gentner B, Schambach A, et al. MicroRNA-150-regulated vectors allow lymphocyte-sparing transgene expression in hematopoietic gene therapy. Gene Ther. 2012;19:915–924. doi: 10.1038/gt.2011.148. [DOI] [PubMed] [Google Scholar]
- 73.Papapetrou EP, Kovalovsky D, Beloeil L, Sant'angelo D, Sadelain M. Harnessing endogenous miR-181a to segregate transgenic antigen receptor expression in developing versus post-thymic T cells in murine hematopoietic chimeras. J Clin Invest. 2009;119:157–168. doi: 10.1172/JCI37216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moscow JA, Huang H, Carter C, Hines K, Zujewski J, Cusack G, Chow C, Venzon D, Sorrentino B, Chiang Y, et al. Engraftment of MDR1 and NeoR gene-transduced hematopoietic cells after breast cancer chemotherapy. Blood. 1999;94:52–61. [PubMed] [Google Scholar]
- 75.Zhou J, Du Y. Acquisition of resistance of pancreatic cancer cells to 2-methoxyestradiol is associated with the upregulation of manganese superoxide dismutase. Mol Cancer Res. 2012;10:768–777. doi: 10.1158/1541-7786.MCR-11-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Patel DH, Allay JA, Belt JA, Sorrentino BP. Retroviral transfer of the hENT2 nucleoside transporter cDNA confers broad-spectrum antifolate resistance in murine bone marrow cells. Blood. 2000;95:2356–2363. [PubMed] [Google Scholar]
- 77.Balabanov S, Gontarewicz A, Keller G, Raddrizzani L, Braig M, Bosotti R, Moll J, Jost E, Barett C, Rohe I, et al. Abcg2 overexpression represents a novel mechanism for acquired resistance to the multi-kinase inhibitor Danusertib in BCR-ABL-positive cells in vitro. PLoS One. 2011;6:e19164. doi: 10.1371/journal.pone.0019164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Munster PN, Daud AI. Preclinical and clinical activity of the topoisomerase I inhibitor, karenitecin, in melanoma. Expert Opin Investig Drugs. 2011;20:1565–1574. doi: 10.1517/13543784.2011.617740. [DOI] [PubMed] [Google Scholar]
- 79.Tomicic MT, Kaina B. Topoisomerase degradation, DSB repair, p53 and IAPs in cancer cell resistance to camptothecin-like topoisomerase I inhibitors. Biochim Biophys Acta. 2013;1835:11–27. doi: 10.1016/j.bbcan.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 80.Matsunaga T, Sakamaki S, Kuga T, Kuroda H, Kusakabe T, Akiyama T, Konuma Y, Hirayama Y, Kobune M, Kato J, et al. GST-π genetransduced hematopoietic progenitor cell transplantation overcomes the bone marrow toxicity of cyclophosphamide in mice. Hum Gene Ther. 2000;11:1671–1681. doi: 10.1089/10430340050111322. [DOI] [PubMed] [Google Scholar]
- 81.Capiaux GM, Budak-Alpdogan T, Alpdogan O, Bornmann W, Takebe N, Banerjee D, Maley F, Bertino JR. Protection of hematopoietic stem cells from pemetrexed toxicity by retroviral gene transfer with a mutant dihydrofolate reductase-mutant thymidylate synthase fusion gene. Cancer Gene Ther. 2004;11:767–773. doi: 10.1038/sj.cgt.7700683. [DOI] [PubMed] [Google Scholar]
- 82.Mayhew CN, Mampuru LJ, Chendil D, Ahmed MM, Phillips JD, Greenberg RN, Elford HL, Gallicchio VS. Suppression of retrovirus-induced immunodeficiency disease (murine AIDS) by trimidox and didox: novel ribonucleotide reductase inhibitors with less bone marrow toxicity than hydroxyurea. Antiviral Res. 2002;56:167–181. doi: 10.1016/s0166-3542(02)00108-0. [DOI] [PubMed] [Google Scholar]
- 83.Sumpter LR, Inayat MS, Yost EE, Duvall W, Hagan E, Mayhew CN, Elford HL, Gallicchio VS. In vivo examination of hydroxyurea and the novel ribonucleotide reductase inhibitors trimidox and didox in combination with the reverse transcriptase inhibitor abacavir: suppression of retrovirus-induced immunodeficiency disease. Antiviral Res. 2004;62:111–120. doi: 10.1016/j.antiviral.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 84.Fung H, Demple B. Distinct roles of Ape1 protein in the repair of DNA damage induced by ionizing radiation or bleomycin. J Biol Chem. 2011;286:4968–4977. doi: 10.1074/jbc.M110.146498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morris LP, Degtyareva N, Sheppard C, Heyburn L, Ivanov AA, Kow YW, Doetsch PW. Saccharomyces cerevisiae Apn1 mutation affecting stable protein expression mimics catalytic activity impairment: implications for assessing DNA repair capacity in humans. DNA Repair (Amst) 2012;11:753–765. doi: 10.1016/j.dnarep.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kobune M, Xu Y, Baum C, Kelley MR, Williams DA. Retrovirus-mediated expression of the base excision repair proteins, formamidopyrimidine DNA glycosylase or human oxoguanine DNA glycosylase, protects hematopoietic cells from N,N′,N″-triethylenethiophosphoramide (thioTEPA)-induced toxicity in vitro and in vivo. Cancer Res. 2001;61:5116–5125. [PubMed] [Google Scholar]
- 87.Dhar SK, Tangpong J, Chaiswing L, Oberley TD, St Clair DK. Manganese superoxide dismutase is a p53-regulated gene that switches cancers between early and advanced stages. Cancer Res. 2011;71:6684–6695. doi: 10.1158/0008-5472.CAN-11-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hacke K, Szakmary A, Cuddihy AR, Rozengurt N, Lemp NA, Aubrecht J, Lawson GW, Rao NP, Crooks GM, Schiestl RH, et al. Combined preconditioning and in vivo chemoselection with 6-thioguanine alone achieves highly efficient reconstitution of normal hematopoiesis with HPRT-deficient bone marrow. Exp Hematol. 2012;40:3–13. doi: 10.1016/j.exphem.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]