Abstract
Tumor exosomes educate selected host tissues toward a prometastatic phenotype. We demonstrated this for exosomes of the metastatic rat adenocarcinoma BSp73ASML (ASML), which modulate draining lymph nodes and lung tissue to support settlement of poorly metastatic BSp73ASML-CD44v4-v7 knockdown (ASML-CD44vkd) cells. Now, we profiled mRNA and microRNA (miRNA) of ASMLwt and ASML-CD44vkd exosomes to define the pathway(s), whereby exosomes prepare the premetastatic niche. ASML exosomes, recovered in draining lymph nodes after subcutaneous injection, preferentially are taken up by lymph node stroma cells (LnStr) and lung fibroblasts (LuFb) that were chosen as exosome targets. ASMLwt and ASML-CD44vkd exosomes contain a restricted mRNA and miRNA repertoire that differs significantly between the two lines and exosomes thereof due to CD44v6 influencing gene and miRNA transcription/posttranscriptional regulation. Exosomal mRNA and miRNA are recovered in target cells, where transferred miRNA significantly affected mRNA translation. Besides others, this was exemplified for abundant ASMLwt-exosomal miR-494 and miR-542-3p, which target cadherin-17 (cdh17). Concomitantly, matrix metalloproteinase transcription, accompanying cdh17 down-regulation, was upregulated in LnStr transfected with miR-494 or miR-542-3p or co-cultured with tumor exosomes. Thus, tumor exosomes target non-transformed cells in premetastatic organs and modulate premetastatic organ cells predominantly through transferred miRNA, where miRNA from a metastasizing tumor prepares premetastatic organ stroma cells for tumor cell hosting. Fitting the demands of metastasizing tumor cells, transferred exosomal miRNA mostly affected proteases, adhesion molecules, chemokine ligands, cell cycle- and angiogenesis-promoting genes, and genes engaged in oxidative stress response. The demonstration of function-competent exosomal miRNA in host target cells encourages exploiting exosomes as a therapeutic gene delivery system.
Introduction
Metastasis formation accounts for the majority of cancer-induced deaths, where a given tumor type preferentially seeds in selected organs [1,2]. Premetastatic niche preparation supports the seed and soil hypothesis, as tumors prepare only defined organs for metastasizing cell settlement in advance of arrival [3–5]. Our suggestion that tumor-derived exosomes rather than individual molecules play an important role [6] was confirmed by several groups [7–16]. Exosomal microRNA (miRNA) in serum is also discussed as a potential marker for tumor diagnosis [17,18].
Exosomes, small vesicles delivered by many cells and abundantly by tumor cells [19], derive from early endosomes, which fuse to multi-vesicular bodies (MVBs), from where individual vesicles (exosomes) are released in the extracellular space [20–23]. Accordingly, exosomes are rich in proteins located in internalization-prone membrane domains and molecules engaged in fission, scission, and vesicular transport [14,22,24,25]. Exosomes also harbor selected mRNA and miRNA [26]. mRNA recruitment may be guided by a zip code in the 3′ untranslated region (3′UTR) [27]; miRNA recruitment is facilitated by physical and functional coupling of RNA-induced silencing complexes to components of the sorting complex, where GW182 containing GW bodies, sorted into MVB, promotes continuous assembly/disassembly of membrane-associated miRNA-loaded RNA-induced silencing complex [28,29].
Exosome binding and uptake by target cells are also selective processes that involve various endocytic pathways and proteins from exosome donor and target cells [30,31], where exosomal tetraspanin complexes bind to selected ligands, which are also located in internalization-prone microdomains [32,33]. Exosomal proteins, mRNA, and miRNA are functionally active [22,34,35] and exosome binding/uptake can severely alter target cells, as demonstrated for T cell activation, immunosuppression, and conversion to a malignant phenotype [14,36–38].
We showed for the metastasizing rat pancreatic adenocarcinoma BSp73ASML (ASML) [39] that exosomes contribute to premetastatic niche preparation. ASML cells highly express CD44 variant isoforms v4–v7 (CD44v) [6], where CD44v6 particularly promotes the metastatic phenotype [40]. First evidence for CD44v as a metastasis-promoting molecule deriving from metastasis formation of CD44v transfected non-metastasizing BSp73AS cells [41] was confirmed in numerous studies in human and animal models [ref. in 42]. The central role of CD44v in metastasis formation was confirmed by a knockdown of CD44v4-v7 (ASML-CD44vkd) in ASML cells that poorly metastasize [43]. As the metastatic process essentially depends on the cross talk between tumor cells and the host and exosomes being suggested to be the most important intracellular communicators, we speculated that ASMLwt exosomes might account for the metastatic spread. Controlling this hypothesis was facilitated by the peculiarity of ASML cells not to grow locally after subcutaneous injection and to form metastases selectively in lymph nodes and lung [39]. Thus, if ASML exosomes contribute to premetastatic organ preparation, ASML-CD44vkd cells that also do not grow locally should regain metastatic capacity after preparing the host with ASMLwt exosomes. Indeed, poorly metastasizing ASML-CD44vkd cells regain metastatic capacity, when rats are pretreated with conditioned medium (CM) of ASMLwt cells. The essential contribution of exosomes was supported by the finding that exosome-depleted CM (CM-exo) exerted no metastasis-promoting effect. Furthermore, compared to ASMLwt exosomes, ASML-CD44vkd exosomes exerted a weaker effect [6]. To obtain hints toward the weaker effect of ASML-CD44vkd versus ASMLwt exosomes, we explored the impact of metastasis-promoting CD44v6 on the exosomal mRNA, miRNA, and protein profiles and progressed toward elucidating how tumor exosomes modulate premetastatic organs using lymph node stroma cells (LnStr) and lung fibroblasts (LuFb) as targets.
Materials and Methods
Cell Lines
ASMLwt cells derive from the metastasizing variant of a spontaneously arisen rat pancreatic adenocarcinoma in the BDX rat strain. Subcutaneously implanted ASML cells do not grow locally and metastasize exclusively to lymph nodes and lung [39]. ASML-CD44vkd cells do not also grow locally. They metastasize with a strong delay to draining lymph nodes and do not settle in the lung [43]. A rat aortic endothelial cell line (EC) and a fibroblast line (LuFb) derived from the lung of BDX rats spontaneously immortalized. These lines as well as human embryo renal cortical cells (HEK293) and LnStr (B12) [44] were maintained in RPMI 1640/10% fetal calf serum, supplemented for ASML-CD44vkd cells with 750 µg/ml G418.
Antibodies
For antibodies, see Table W1.
Exosome Preparation
Cells were cultured (48 hours) in serum-free medium. Cleared supernatants (2 x 10 minutes, 500g; 1 x 20 minutes, 2000g; 1 x 30 minutes, 10,000g) were centrifuged (90 minutes, 100,000g) and washed [phosphate-buffered saline (PBS), 90 minutes, 100,000g]. The supernatant after the last centrifugation was collected as CM-exo. It was concentrated and, after protein determination, adjusted to 200 µg/25 µl for intrafootpad (ifp) injection. The pellet was resuspended (10 ml of PBS), layered on 10 ml of 40% sucrose, and centrifuged (90 minutes, 100,000g). The top layer was removed; the sucrose layer was diluted with PBS and centrifuged (90 minutes, 100,000g). Where indicated, exosomes were rhodamine-N-(lissamine rhodamine B sulfonyl) phosphatidyl ethanolamine (DHPE)- or SP-Dio18(3)-labeled (Invitrogen, Karlsruhe, Germany). Exosomes were directly labeled (30 minutes, 4°C) before sucrose gradient centrifugation and washed twice (90 minutes, 100,000g). Relative fluorescence intensity was adjusted to rhodamine-DHPE or SP-Dio18(3) standards.
mRNA and miRNA
After RNAse treatment, exosomal and cellular mRNA/miRNA were extracted using TRI reagent according to recommendation (Sigma, Munich, Germany).
Microarray mRNA Analysis
Expression levels of 22,523 rat transcripts of two independent preparations of ASMLwt and ASML-CD44vkd exosomes and cells and of untreated and ASMLwt- or ASML-CD44vkd exosome-treated LnStr cells were analyzed in duplicates or triplicates using Ilumina and SurePrintG3Rat-GE-8x60K microarray. Analyses, normalization, and statistics (Chipster analysis and Agilent annotation) were performed at the Core Facility, German Cancer Research Center. Cellular and exosomal samples were normalized independently. Transcripts with at least double signal intensity over background, bead standard error differences > 12, and P value < .05 were included (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE34739). RNA was analyzed according to function clustering (http://www.pantherdb.org).
Microarray miRNA Analysis
miRNA analysis of ASMLwt and ASML-CD44vkd exosomes and cells (Core Facility, European Molecular Biology Laboratories, Heidelberg, Germany) used the miRCURY LNA microRNA ver11.0-hsa,mmu, rno or the Agilent microRNA microarray evaluating quadruplicates of two independent preparation (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE34739). Mean values of normalized data (Agilent Feature Extraction Software) were compared. Differentially regulated miRNA were defined as those with more than two-fold changes in signal strength. The miRNA database (http://www.microrna.org) and the target scan database (http://www.targetscan.org) were used to predict potential miRNA targets and for correlating downregulated mRNA in exosome-treated LnStr with exosomal miRNA.
Reverse Transcription and Quantitative Reverse Transcription-Polymerase Chain Reaction
Reverse transcription (RT) reactions contained RNA samples including purified total RNA, cell lysate, or heat-treated cells, 50 nM stem loop reverse transcriptase primer (Applied Biosystems, Darmstadt, Germany), 1x reverse transcriptase buffer (Applied Biosystems), 0.25 mM each of deoxyribonucleoside triphosphates (dNTPs), 3.33 U/µl Multi-Scribe reverse transcriptase (Applied Biosystems), and 0.25 U/µl RNase inhibitor (Applied Biosystems). The 7.5-µl reactions were incubated in an Applied Biosystems 9700 Thermocycler in a 96- or 384-well plate for 30 minutes at 16°C, 30 minutes at 42°C, and 5 minutes at 85°C and then held at 4°C. All reverse transcriptase reactions, including no template controls and reverse transcriptase minus controls, were run in duplicate.
Real-time polymerase chain reaction (PCR) was performed using a standard TaqMan PCR kit protocol on an Applied Biosystems 7900HT Sequence Detection System (Applied Biosystems). The 10-µl PCR included 0.67 µl of reverse transcriptase product, 1x TaqMan Universal PCR Master Mix (Applied Biosystems), 0.2 µM TaqMan probe, 1.5 µM forward primer, and 0.7 µM reverse primer. The reactions were incubated in a 384-well plate at 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. All reactions were run in triplicate. Glyceraldehyde phosphate dehydrogenase (GAPDH) served as internal control for mRNA and 4.5SRNA was used as internal control for miRNA. The threshold cycle (CT) is defined as the fractional cycle number at which the fluorescence passes the fixed threshold. TaqMan CT values were converted into absolute copy numbers using a standard curve from synthetic lin-4 miRNA. Statistical analysis was done by the ΔCT method [ΔCT = CT test gene - CT endogenous control; ΔΔCT = ΔCT sample - ΔCT calibrator (untreated LnStr)], where relative quantification/fold change compared to the calibrator = 2-ΔΔCT.
miRNA Transfection
LnStr and HEK293 cells, seeded in antibiotic-free medium (24 hours), were transfected with 20 nM [quantitative RT-PCR (qRT-PCR)] or 10 nM (luciferase reporter assay) miRNA mimic (Qiagen, Hilden, Germany) using Lipofectamine 2000. After 48 hours, mRNA regulation was evaluated by qRT-PCR and/or was verified by flow cytometry (protein level) or used for luciferase reporter assay.
3′UTR Reporter Assay
miRNAs that could bind in the 3′UTR of MAL and cadherin-17 (cdh17) mRNAs were searched according to http://www.microrna.org. Those showing a good mirSVR score (lower than -0.1) and detected in exosomes were selected. The 3′UTR regions were cloned by PCR from genomic LnStr DNA (primers: Table W2). PCR products were cloned into the dual-luciferase pmiRGlo vector, downstream of firefly luciferase using Pme1 and Xba1 restriction sites. After ligation and bacterial transformation, positive clones (sequenced) were used for HEK293 transfection. HEK293 cells were transfected with cloned miRNA binding sites for miR-300-5p or of the 3′UTRs of MAL and cdh17 using HiPerFect for reverse transfection in 96-well plates. Briefly, miRNA mimics (10 nM; Qiagen) without or with 80 ng of the reporter plasmid was spotted in a well of 96-well flat-bottom plates. Thereafter, HiPerFect reagent (1 µl, diluted in 25 µl of serum-free medium) was added to the miRNA/DNA and mixed by pipetting. After an incubation time of 10 minutes, 4 x 105 cells in Iscoves/10% fetal calf serum were added. The cells were maintained under normal growth condition for 48 hours. The Dual-Luciferase Reporter Assay Kit (Promega, Mannheim, Germany) was performed following the manufacturer's instruction. Cells were lysed in 20 µl of Dual-Glo reagent (15 minutes, reverse transcriptase); the lysate was added to 100 µl of the same reagent in a white 96-well plate (Promega), and the firefly luciferase was measured using FLUOstar OPTIMA luminometer (BMG LABTECH, Offenburg, Germany), followed by measurement of Renilla luciferase, which was used for normalization of transfection efficiency.
In Vitro Translation
Exosomal RNA (10 µg) in water was mixed with 1.25 µl of 20x translation mix (Retic Lysate IVT Kit), 1 µl of 35S-methionine, and 17 µl of Retic lysate, adjusted to 25 µl with nuclease-free water. After vortexing and centrifugation, the reaction mix at the bottom of the tube was incubated for 90 minutes in a 30°C water bath and for 10 minutes with 2.5 µl of RNaseA; 10 µl of the complete reaction was mixed with an equal volume of 2x sodium dodecyl sulfate sample buffer and incubated for 5 minutes at 95°C. After centrifugation, samples were collected from the bottom and allowed to cool to room temperature. Samples were loaded on a 12% sodium dodecyl sulfate-gel. After electrophoresis, proteins were fixed (45% methanol and 10% acetic acid, 5 minutes, gentle agitation) and dried. Dried gels were exposed at -70°C for 48 hours to X-ray films using an intensifying screen and photographed.
Flow Cytometry
Flow cytometry for cells followed routine procedures. Where indicated, cells were fixed and permeabilized and/or stripped (two washes in PBS/HCl, pH 2.5). Exosomes (10–15 µg) were incubated with 1 µl of aldehyde-sulfate latex beads (4 µm) (Invitrogen) in PBS/1% BSA (90 minutes, 20°C, shaking). After centrifugation, free binding sites on the beads were blocked by incubation with 100 mM glycine in PBS (1 hour). After washing two times with PBS/1% BSA, exosome-coated beads (corresponding to 1 µl beads/well) are distributed in 96-well plates. Staining with primary and secondary dyelabeled antibodies follows the protocol for cell staining. For analyzing exosome uptake, cells were incubated with DHPE-labeled exosomes, washed, and stripped. Samples were analyzed in a FACSCalibur using the CellQuest program.
Zymography
CM of LnStr (1 x 106), starved for 24 hours, was centrifuged (15 minutes, 15,000g). Aliquots of supernatant were incubated with Laemmli buffer (15 minutes, 37°C) and separated in a 10% acrylamide gel containing 1 mg/ml gelatin. After washing (2.5% Triton), gels were incubated in developing buffer (37°C, 48 hours) and stained with Coomassie Blue.
In Vivo Assays
Rats (three per group) receiving 200 µg of SP-Dio18(3)-labeled exosomes in 25 µl of RPMI 1640, ifp, were sacrificed after 24 to 72 hours. Rats (three per group) receiving exosomes in RPMI 1640 or 20-fold concentrated CM-exo of ASMLwt or ASML-CD44vkd cells, ifp, were sacrificed after 48 hours. Popliteal lymph nodes were excised and dispersed to evaluate exosome uptake by flow cytometry. The experiment was government-approved (Baden-Wuerttemberg, Germany).
Statistical Analysis
All in vitro assays were run in triplicates and repeated at least three times. P values < .05 (two-tailed Student's t test, analysis of variance) were considered significant. The mRNA and miRNA microarray analyses were performed with two independent samples, each run in duplicate or triplicate (mRNA) or quadruplicate (miRNA) and contained 60 (mRNA) or >30 (miRNA) negative controls. As the duplicate/triplicate (mRNA) and quadruplicate (miRNA) samples frequently revealed P values < .05 with as low a variation in the signal strength as 1.2, mean values of the duplicates/quadruplicates of the two independent performed microarray analyses are presented throughout, where it should be noted that in the absolute ranking the individual mRNA/miRNA were very close and the few samples where this has not been the case were excluded. Nonetheless, as the total signal strength between the two microarrays differed, P values < .05 were rare and are only occasionally included. Instead, we indicate more than two-fold differences (which is five times the level to reach significant P values within the individual microarray analysis).
Results
ASML cells, not forming a local tumor, metastasize through the lymphatics to the lung [39], indicating an essential requirement of lymph node or lung environment for growth. This feature makes ASML cells ideal candidates for defining a tumor's impact on premetastatic niche preparation. In a previous work indicating that exosomes are essential [6], we here characterized ASMLwt exosomes and defined their impact on LnStr and LuFb. The comparison with ASML-CD44vkd exosomes aimed for hints toward their lower efficacy.
In Vivo and In Vitro Exosome Binding and Uptake
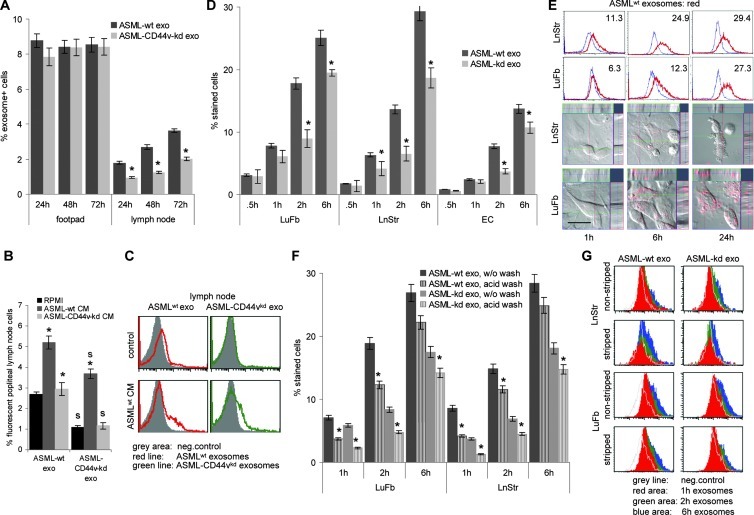
As a prerequisite for in vivo activity, we controlled that tumor exosomes reach the premetastatic organ from the distant site of the primary tumor. Ifp-injected ASMLwt exosomes were recovered in the popliteal node after 24 to 72 hours. ASMLwt exosome and, far more pronounced, ASML-CD44vkd exosome recovery was significantly increased, when supported by ASMLwt-CM-exo. The ASML-CD44vkd-CM-exo hardly supported exosome traffic toward the popliteal node (Figure 1, A–C).
Figure 1.
Exosome binding and uptake by non-transformed cells in vivo and in vitro. (A–C) Dye-labeled ASMLwt and ASML-CD44vkd exosomes in RPMI 1640, ASMLwt, or ASML-CD44vkd-CM-exo were injected ifp. Rats were sacrificed after 24 to 72 hours; the injection site and the popliteal node were excised and dispersed, and fluorescent exosome uptake was evaluated in single-cell suspensions by flow cytometry counting 100,000 cells for each organ in triplicate. (A) Mean values ± SD (triplicates, three rats) of fluorescent cells; significant differences between ASMLwt and ASML-CD44vkd exosomes: *. (B) Mean values ± SD (triplicates, three rats) of fluorescent cells; significant differences in the presence of CM-exo: *, and between ASMLwt- and ASML-CD44vkd exosomes: s. (C) representative example. (D–G) LuFb, LnStr, and EC were incubated with dye-labeled ASMLwt and ASML-CD44vkd exosomes (30 µg/ml) for the indicated times. (D) After washing, the percent fluorescent cells (mean ± SD, triplicates) were evaluated by flow cytometry; significant differences between the percentage of cells that bind/take up ASMLwt versus ASML-CD44vkd exosomes: *. (E) Representative example of exosome uptake as evaluated by flow cytometry and confocal microscopy. Overlays of light field and red fluorescence including sagittal sections are shown (scale bar, 5 µm). (F and G) After 1, 2, and 6 hours, bound exosomes were removed by two acid washes (stripping), evaluating remaining fluorescence as above. (F) The percentage of stained cells (mean ± SD of triplicates); significant differences between the percentage of cells that bound/took up versus the percentage of cells that took up ASMLwt or ASML-CD44vkd exosomes: *. (G) Representative examples are shown. Experiments in D to G were repeated at least three times revealing comparable results. ASMLwt and ASML-CD44vkd exosomes reach the draining node; the efficacy can be improved by ASMLwt-CM. ASML exosomes bind more readily to LuFb and LnStr than to EC. Binding and uptake of ASML-CD44vkd exosomes are less efficient and delayed compared to ASMLwt exosomes. It should be mentioned that the percentage of exosome uptake will be underestimated, as the signal strength of a single or few exosomes will be below the detection limit.
ASML exosomes are taken up by leukocytes [45] and stroma cells. As ASML cells metastasize exclusively through the lymphatic system, we chose LnStr and LuFb as targets to explore in vitro the impact of tumor exosomes. ASMLwt exosomes bind more rapidly than ASML-CD44vkd exosomes to LnStr and LuFb. Both exosomes bind less efficiently to ECs, included as control (Figure 1, D and E). Bound exosomes are taken up by their targets, as seen in the sagittal sections of exosome-treated LnStr and LuFb (Figure 1E) and confirmed by exposing LnStr and LuFb to two acid washes (pH 2.5; stripping), which remove bound without affecting integrated exosomes. ASMLwt exosome uptake proceeds more rapidly than ASML-CD44vkd exosome uptake (Figure 1, F and G).
Thus, ASML exosomes reach the premetastatic organ in vivo and are in vitro taken up by LnStr and LuFb. The CD44vkd has some, not yet, explored impact on the efficacy of exosome binding and uptake.
ASML Exosomal mRNA and miRNA
Having demonstrated that ASMLwt and ASML-CD44vkd exosomes are taken up by host stroma cells, though with distinct efficacy, we focused on the potential contribution of CD44v to protein, mRNA, and miRNA recruitment into exosomes, where mRNA and miRNA are claimed to be selectively recruited into MVB/exosomes [20–23,26].
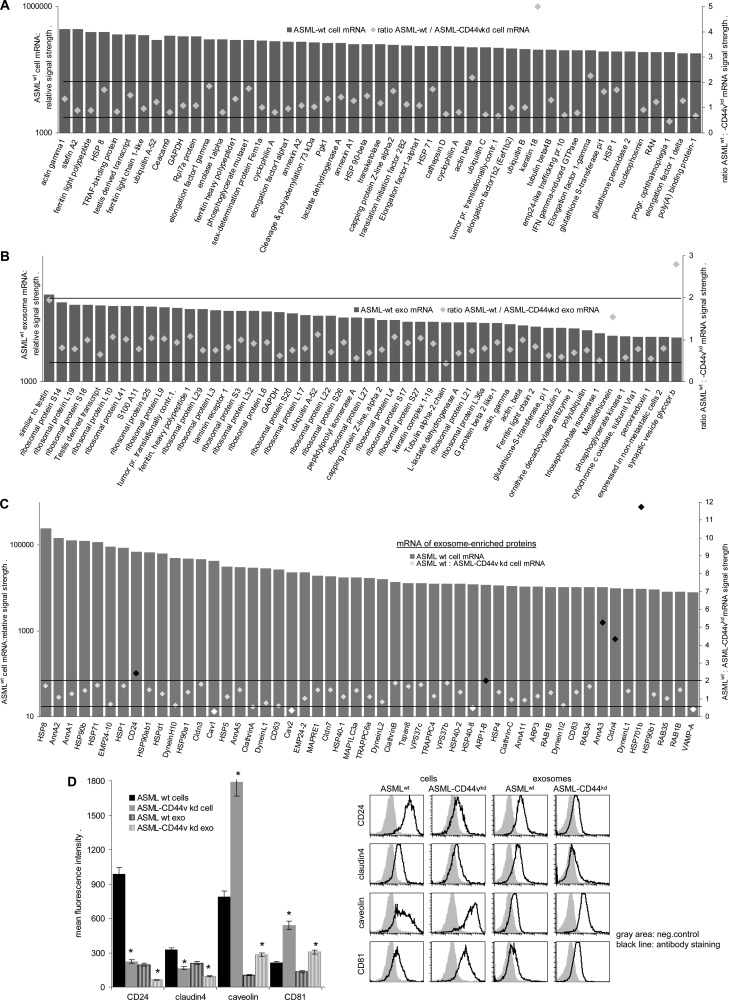
The RatRef-12 expression BeadChip array (23,365 transcripts) revealed <1500 mRNAs in ASMLwt and ASML-CD44vkd exosomes (Table W3) but >8000 mRNAs in ASML cells (Table W4), indicating a restricted mRNA uptake by exosomes. To strengthen the assumption, we compared the relative abundance of exosomal versus cellular mRNA. Although the overall distribution of function-assigned groups of mRNA was similar in ASMLwt and ASML-CD44vkd exosomes and cells and also LnStr (data not shown), the relative abundance of exosomal versus cellular mRNA differed. From 116 mRNAs highly recovered in ASMLwt exosomes, the relative recovery of >90 mRNAs differed more than two-fold in ASMLwt cells. Similar findings accounted for the comparison of ASML-CD44vkd exosomes versus cells (Table W5 and Figure W1). An elegant study by Eldh et al. [46] demonstrated that the mRNA isolation kit that was used provides a poor yield of exosomal mRNA. Nonetheless, several mRNAs were clearly enriched in exosomes. However, according to the above-mentioned study, we cannot exclude that an even higher number of mRNA is enriched in exosomes. As some mRNAs were enriched in ASMLwt as well as ASML-CD44vkd exosomes, we next asked whether CD44v has an impact on mRNA recruitment into MVB. To obtain a hint, we evaluated the mRNA in ASMLwt cells versus ASML-CD44vkd cells and compared these differences in cellular mRNA with those seen in exosomes. Should CD44v contribute to mRNA recruitment into exosomes, one would expect a significantly higher number of distinctly recovered mRNA in ASMLwt exosomes than in cells. This has not been the case. Taking the 50 mRNAs with the highest signal intensity, two exosomal versus three cellular mRNA differed more than two-fold depending on CD44v (Figure 2, A and B). Taking 2390 defined mRNAs in ASMLwt exosomes with a signal strength of >1000, only 74 (3.1%) differed more than two-fold in ASML-CD44vkd exosomes. Furthermore, when analyzing the cellular-to-exosomal mRNA ratio for 14 mRNAs (signal strength > 400), where the exosomal ASMLwt mRNA was at least two-fold higher than the ASML-CD44vkd mRNA, no correlation to a higher ASMLwt than ASML-CD44vkd cellular mRNA ratio was detected. Accordingly, no inverse correlation of the cellular mRNA was detected for ASML-CD44vkd exosomes containing a higher mRNA level than ASMLwt exosomes (Figure W2).
Figure 2.
ASMLwt and ASML-CD44vkd cellular and exosomal mRNAs. (A and B) Examples of 50 defined mRNAs with the highest signal strength in ASMLwt cells and exosomes and fold changes in ASML-CD44vkd cells and exosomes. (C) mRNA signals in ASMLwt cells and fold change in ASML-CD44vkd cells for 50 proteins known to be highly recovered in exosomes. (A–C) Mean values were derived from duplicates/triplicates in two independent microarray analyses, where it should be noted that in both arrays the absolute ranking of individual mRNA was very high, at least for those with a signal strength of >2000. However, whereas by calculating P values from the duplicates/triplicates of the individual array 1.2-fold differences mostly were significant, the absolute signal strength varied between the two arrays such that P values < .05 were mostly not reached. For these reasons, we indicate more than two-fold differences that are generally accepted as non-random. (D) For selected mRNA, protein recovery was evaluated in ASMLwt and ASML-CD44vkd cells and exosomes by flow cytometry. Representative examples and mean ± SD (triplicates) of staining intensity in ASMLwt and ASML-CD44vkd cells and exosomes are shown. Significant differences between ASMLwt versus ASML-CD44vkd cells and exosomes: *. (D) The experiment was repeated three times revealing comparable results. Exosomes contain a limited number of mRNA. However, more than two-fold differences in ASMLwt versus ASML-CD44vkd exosomal mRNA are rare and not selectively recovered in exosomes, suggesting that CD44v is unlikely to be directly involved in mRNA recruitment into MVB. Conversely, CD44v or associated molecules affect transcription of several genes, including transcription of genes/expression of proteins, which are constitutive exosome components. Thereby, CD44v contributes to the protein and mRNA profile of exosomes.
However, CD44v could still contribute to the protein composition of exosomes. To answer this question, we selected cellular mRNA of proteins abundantly expressed in exosomes (http://www.exocarta.org). From 164 selected proteins with a cellular mRNA signal > 1000 in ASMLwt cells, 25% showed a more than two-fold change in signal strength in ASML-CD44vkd cells, which is shown for the 50 mRNAs with the highest signals (Figure 2C) and as scatter for the first 100 mRNAs (Figure W3). The impact of CD44v on these mRNAs is reflected at the cellular and exosomal protein levels demonstrated by flow cytometry for CD24 and claudin-4 that expression is reduced in ASML-CD44vkd cells and exosomes, whereas caveolin-1 and CD81 expression are higher in ASML-CD44vkd than in ASMLwt cells and exosomes (Figure 2D).
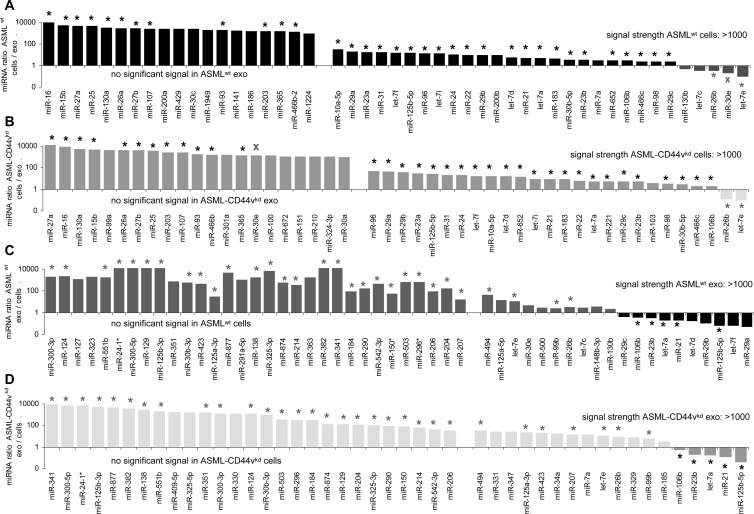
A rather low number (89–98) of miRNA were recovered in ASMLwt and ASML-CD44vkd exosomes and cells (Table W6 and Figure W4: most abundant miRNA). With a >1000 signal strength cutoff, 21 cellular and 50 exosomal miRNAs differed more than two-fold between ASMLwt and ASML-CD44vkd cells/exosomes (Figure W5). The distinct recovery in ASMLwt versus ASML-CD44vkd cells indicates a direct or indirect contribution of CD44v6 to miRNA transcription or posttranscriptional regulation. Should CD44v, in addition, actively contribute to miRNA recruitment into MVB/exosomes, one would expect that the ratio of cellular to exosomal miRNA differs in dependence on CD44v expression. This has not been the case. With very few exceptions, the ratio of ASMLwt to ASML-CD44vkd miRNA in cells did not differ significantly from that in exosomes and only one miRNA (miR-30e) was opposingly recruited into ASMLwt versus ASML-CD44vkd exosomes (Figure 3).
Figure 3.
miRNA ratio in ASML cells versus exosomes. (A–D) The miRNAs (signal strength > 1000, mean of quadruplicates of two microarray analyses) are depicted, where the ratio reveals a more than two-fold change in (A) ASMLwt and (B) ASML-CD44vkd cells to exosomes and in (C) ASMLwt and (D) ASML-CD44vkd exosomes to cells; alike regulation in ASMLwt and ASML-CD44vkd cells versus exosomes: *; opposing up-regulation or down-regulation: X. The miRNA profile of exosomes differs strikingly from that of cells, a considerable number of cellular miRNA being not detected in exosomes and vice versa. Instead, there are minor differences in ASMLwt versus ASML-CD44vkd cells and exosomes, indicating that CD44v might not be engaged in miRNA recruitment into MVB.
Taken together, exosomes collect a limited number of mRNA and miRNA. The abundant differences in the mRNA of ASMLwt versus ASML-CD44vkd cells argue for CD44v or associated molecules being engaged in gene transcription/regulation. This includes genes whose protein products are enriched in exosomes [6,45, Figure W3, and unpublished findings]. However, differences at the cellular mRNA level between ASMLwt and ASML-CD44vkd cells are closely reflected by differences in exosomes. Thus, CD44v seemingly does not actively contribute to MVB formation and has, if at all, only a minor impact on mRNA recruitment into MVB.
Having characterized ASMLwt and ASML-CD44vkd exosomal mRNA and miRNA, we asked whether they have any impact on exosome targets, where we focused on the general principle rather than on the differences between ASMLwt versus ASML-CD44vkd exosomes.
ASML Exosome mRNA and miRNA Are Transferred into LnStr
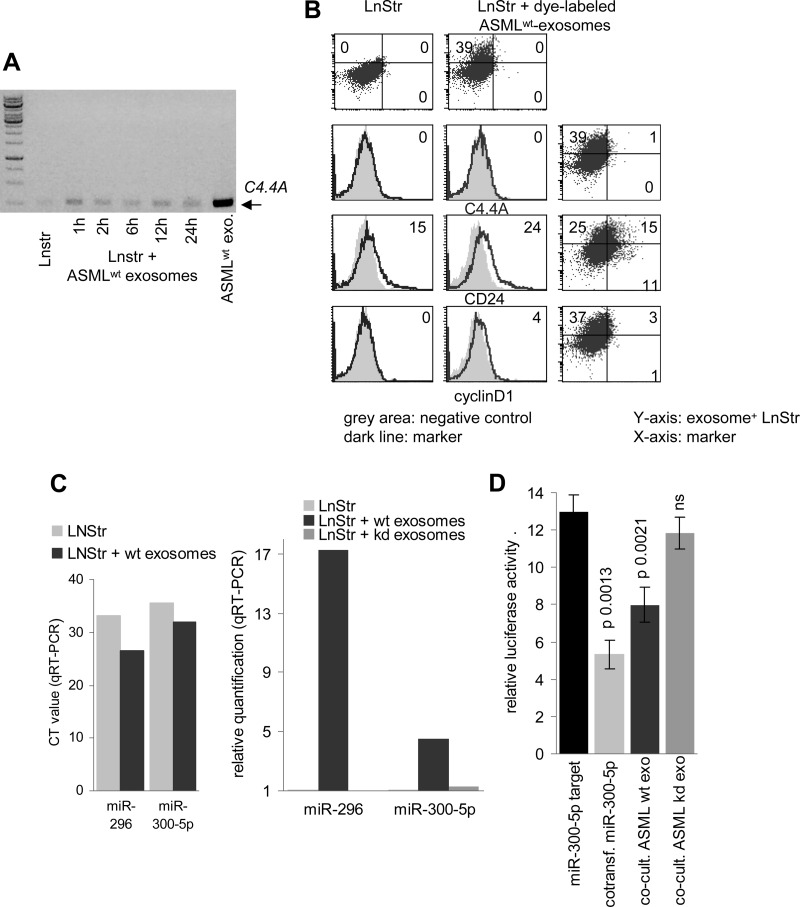
We first reaffirmed for selected mRNA and miRNA the transfer into target cells. For mRNA, C4.4A was chosen, as C4.4A is not detected in LnStr. Instead, it was recovered after co-culture with exosomes (Figure 4A). Though exosomal mRNA integrity was confirmed by in vitro translation (data not shown), in vivo translation of C4.4A was not detected. As revealed by double fluorescence analysis of LnStr co-cultured with dye-labeled exosomes, upregulated expression/translation of other more abundant exosomal mRNA, like CD24 and cyclin D1, was detected but was not restricted to LnStr that had taken up ASMLwt exosomes (Figure 4B).
Figure 4.
Recovery of exosomal mRNA and miRNA in target cells. (A) LnStr cells were co-cultured with ASMLwt exosomes. After 1 to 24 hours, mRNA was extracted, reverse transcribed, amplified with C4.4A-specific primers by RT-PCR, and separated by agarose gel. (B) LnStr were co-cultured (6 hours) with DHPE-labeled ASMLwt exosomes and were stained with anti-C4.4A (C4.4), anti-CD24, and anti-cyclin D1 after washing, fixation, and permeabilization. Flow cytometry of untreated and ASMLwt exosome-treated LnStr (overlay with negative control and double fluorescence: DHPE-labeled exosomes and marker) is shown. (C) After 48 hours of co-culture, LnStr RNA was extracted, reverse transcribed, and amplified using universal reverse primer and miR-specific forward primer with stem loop primers for miR-296 and miR-300-5p. CT and relative quantification values (mean of three replicate samples with SD < 0.25, indicating reliability according to the software program for ΔCT) in untreated versus ASMLwt and ASML-CD44vkd exosome-treated LnStr are shown. (D) HEK293 cells were transfected with the dual-luciferase pmiRGlo vector containing a miR-300-5p binding site and were co-transfected with miR-300-5p (10 nM) or co-cultured with ASMLwt or ASML-CD44vkd exosomes (20 µg). Firefly and, for normalization, Renilla luciferase activity was evaluated after 48 hours in a FLUOstar OPTIMA luminometer. Relative luciferase activity (mean ± SD, triplicates) and P values for miR-300-5p, ASMLwt and ASML-CD44vkd exosomes are shown. (C and D) Experiments were repeated three times revealing comparable results. Exosomal mRNA and miRNA are transferred. In vivo mRNA translation was hardly detectable and endogenous transcription/translation cannot be safely excluded. Transferred exosomal miRNA is active.
miRNA transfer was confirmed for miR-300-5p and miR-296, abundant in ASMLwt exosomes and found to be 4.5-fold and 17.3-fold increased in ASMLwt-exosome-treated compared to untreated LnStr. Notably, after co-culture with ASML-CD44vkd exosomes that express significantly less miR-300-5p, no significant increase in miR-300-5p was seen in LnStr (Figure 4C). Functionality of the transferred exosomal miRNA was controlled by a reporter assay with a dual-luciferase pmiRGlo vector with a binding site for miR-300-5p downstream of the firefly luciferase gene. Luciferase activity in HEK293 cells co-transfected with miR-300-5p mimic, but also and importantly, when co-incubated with ASMLwt exosomes was significantly decreased. A similar effect was not observed after co-culture with low-level miR-300-5p expressing ASML-CD44vkd exosomes (Figure 4D).
Taken together, exosomal mRNA and miRNA are transferred into target cells. Exosomal miRNA is function-competent. Exosomal mRNA becomes translated, but we could not unequivocally detect the exosomal mRNA translation product, which might be due to the low amount of exosomal mRNA.
The Impact of Exosomal mRNA on LnStr and LuFb
Having demonstrated exosomal mRNA and miRNA transfer, we searched for altered mRNA recovery in LnStr after co-culture with exosomes.
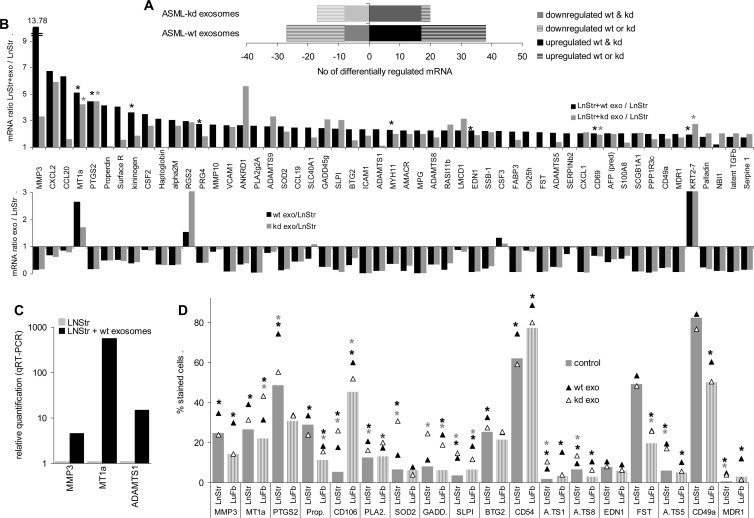
The signal strength of 38 mRNAs and 20 mRNAs with moderate to high expression in LnStr increased more than two-fold after co-culture with ASMLwt or ASML-CD44vkd exosomes, respectively (Figure 5A). Increased mRNA signals were unexpected, as with few exceptions the signal strength in exosomes was lower than in LnStr (Figure 5B), and for 36 of the 38 mRNAs upregulated in ASMLwt exosome-treated LnStr cells, the exosomal mRNA signal strength was low (<1000). Nonetheless, qRT-PCR confirmed matrix metalloproteinase 3 (MMP3), metallothionein (MT1a), and a disintegrin-like and metalloprotease with thrombospondin type 1 (ADAMTS1) up-regulation in LnStr after co-culture with ASML exosomes (Figure 5C). From 18 mRNAs, where expression was controlled at the protein level, 14 were significantly upregulated in LnStr and 13 in LuFb after co-culture with ASMLwt exosomes. Similar effects were observed after co-culture with ASML-CD44vkd exosomes (Figure 5D).
Figure 5.
Impact of ASMLwt and ASML-CD44vkd exosomes on mRNA and protein expression in LnStr and LuFb. (A–D) LnStr and LuFb were co-cultured for 48 hours with ASMLwt and ASML-CD44vkd exosomes. (A and B) Cells were harvested, washed, and lysed and mRNA was extracted and analyzed (Ilumina and SurePrintG3Rat-GE-8x60K microarray). (A) Number of LnStr mRNA with a more than two-fold change after co-culture with ASMLwt or ASML-CD44vkd exosomes. (B) mRNA in LnStr that are more than two-fold upregulated after co-culture with exosomes and comparison of the relative mRNA amount in exosomes versus LnStr. (A and B) Mean values of duplicates, respectively, triplicates, of two independent microarray analyses. (B) Significant differences between LnStr and LnStr co-cultured with ASML exosomes: *. (C) Upregulated gene expression (selected examples) in LnStr co-cultured with exosomes was confirmed by qRT-PCR (mean of three replicate samples with SD < 0.25, indicating reliability according to the software program for ΔCT) and (D) in LnStr and LuFb at the protein level by flow cytometry. The mean percentage of stained cells (triplicates) are shown; significant differences between untreated LnStr/LuFb and LnStr/LuFb co-cultured with ASMLwt exosomes: black *; significant differences between untreated LnStr/LuFb and LnStr/LuFb co-cultured with ASML-CD44vkd exosomes: gray *. (C and D) Experiments were repeated three times revealing comparable results. Abbreviations: MMP3, matrix metalloproteinase 3; CXCL2, chemokine ligand 2; CCL20, chemokine ligand 20; MT1a, metallothionein; PTGS2, prostaglandin-endoperoxide synthase 2; alpha2M, alpha-2-macroglobulin; RGS2, regulator of G-protein signaling 2; PRG4, proteoglycan 4; VCAM1, vascular cell adhesion molecule-1/CD106; ANKRD1, ankyrin repeat domain 1; PLA2g2A, phospholipase A2 group 2A; SOD2, superoxide dismutase 2; CCL19, chemokine ligand 19; SLC40A1, solute carrier family 39; GADD45g, growth arrest and DNA-damage-inducible 45γ; SLPI, secretory leukocyte peptidase inhibitor; BTG2, B-cell translocation gene 2; ICAM1, intercellular adhesion molecule 1/CD54; MYH11, myosin heavy chain 11; AMACR, α-methylacyl-CoA racemase; MPG, matrix Gla protein; ADAMTS8, a disintegrin-like and metalloprotease with thrombospondin type 1, motif 8; RASl11b, RAS-like family 11 member B; EDN1, endothelin 1; SSB-1, SPRY domain-containing SOCS box protein SSB-1; FABP3, fatty acid binding protein 3; FST, follistatin; ADAMTS5, ADAMTS, motif 5; CXCL1, chemokine ligand 1; SCBG1A1, secretoglobin, family 1A, member 1; PPP1R3c, protein phosphatase 1, regulatory subunit 3C; CD49a, integrin α1; MDR1, ATP-binding cassette, subfamily B, member 1; KRT2-7, keratin complex 2; NBI1, neuroblastoma suppression of tumorigenicity; latent TGFβ, latent TGFβ binding protein.
In view of the low levels of exosomal mRNA and the inefficient translation in host cells, it becomes unlikely that altered mRNA/protein expression in exosome-treated targets derives from transferred exosomal mRNA translation. There are three possible explanations: exosome binding and/or uptake stimulates target cells to initiate gene transcription/silencing; transferred mRNA provides a trigger for master gene transcription; or miRNA allows for up-regulation of genes through silencing regulatory mRNA. We have not yet explored the first and second possibilities but searched for the impact of miRNA.
The Impact of Exosomal miRNA on LnStr and LuFb
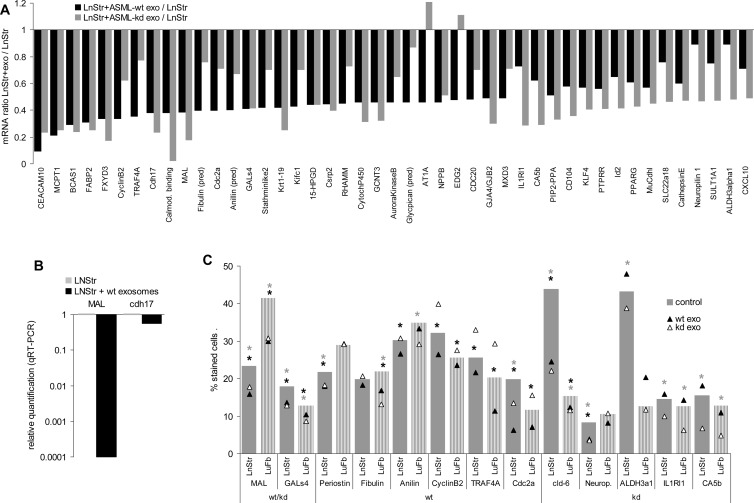
A direct impact of exosomal miRNA on target cell mRNA was supported by the finding that 11 mRNAs with high signal strength in LnStr become downregulated by ASMLwt and 18 mRNAs by ASML-CD44vkd exosomes (Figures 5A and 6A). Including mRNA with lower signal strength, 31 LnStr mRNAs were downregulated by ASMLwt and/or ASML-CD44vkd exosomes. Similar to upregulated mRNA, mRNA down-regulation was mostly seen after co-culture with ASMLwt and ASML-CD44vkd exosomes, although the degree of down-regulation differed. Down-regulation of MAL and cdh17 in LnStr co-cultured with ASMLwt exosomes was confirmed by qRT-PCR, and at the protein level for MAL and GALs4 (ASMLwt and ASML-CD44vkd exosomes), five of six genes were downregulated by ASMLwt exosomes [periostin, aniline, cyclin B2, TNF receptor-associated factor 4 (TRAF4), and Cdc2a] and five of five genes were downregulated by ASML-CD44vkd exosomes (claudin-6, neuropilin, ALDH3α1, IL1R1, and CA5b; Figure 6, B and C).
Figure 6.
Reduced mRNA recovery and protein expression in LnStr and LuFb after co-culture with ASMLwt and ASML-CD44vkd exosomes: mRNA analysis was performed as described in Figure 5. (A) mRNA whose expression was reduced in LnStr by more than two-fold after co-culture with exosomes. (B) Confirmation of mRNA down-regulation (selected examples) by qRT-PCR (mean of three replicate samples with SD < 0.25, indicating reliability according to the software program for ΔCT) and (C) at the protein level in LnStr and LuFb by flow cytometry (mean percentage of stained cells, triplicates); examples are grouped according to reduced mRNA recovery after co-culture with ASMLwt and ASML-CD44vkd or ASMLwt or ASML-CD44vkd exosomes; significant differences between untreated LnStr/LuFb and LnStr/LuFb co-cultured with ASMLwt exosomes: black *; significant differences between untreated LnStr/LuFb and LnStr/LuFb co-cultured with ASML-CD44vkd exosomes: gray *. (B and C) Experiments were repeated three times revealing comparable results. mRNA microarray analysis confirmed the strong impact of exosomes on mRNA recovery in target cells. Many effects were observed with ASMLwt and ASML-CD44vkd exosomes, but distinct regulations, e.g., of cyclin B2, TRAF4, IL1RI1, and Id2 by ASMLwt and ASML-CD44vkd exosomes were also observed. Abbreviations: CEACAM10, CEA-related cell adhesion molecule 10; MCPT1, mast cell protease; BCAS1, breast carcinoma amplified sequence; FABP2, fatty acid binding protein 2; FXYD3, FXYD domain-containing ion transporter regulator 3; TRAF4, TNF receptor-associated factor 4; cdh17, cadherin-17; MAL, myelin and lymphocyte protein; Cdc2a, cell division cycle 2 homolog A; GALs4, galactose binding soluble 4 lectin; Krt1-19, keratin complex 1, acidic, gene 19; Kifc1, kinesin family member C1; 15-HPGD, 15-hydroxyprostaglandin dehydrogenase; Csrp2, cysteine and glycine-rich protein 2; GCNT3, glucosaminyl (N-acetyl) transferase 3; AT1A, angiotensin II receptor 1; NPPb, natriuretic peptide precursor type B; EDG2, endothelial differentiation, lysophosphatidic acid G protein-coupled receptor 2; CDC20, cell division cycle 20 homolog; GJA4/GJB2, GAP junction membrane channel; MXD3, Max dimerization protein; IL1Rl1, interleukin-1 receptor-like 1; CA5b, carbonic anhydrase VB; CD104, integrin β4; KLF4, Kruppel-like factor 4; PTPRR, protein tyrosine phosphatase, receptor type; Id2, inhibitor of DNA binding 2; PPARG, peroxisome proliferator-activated receptor gamma; MuCdhl, mucin and cadherin like; SLC22a18, tumor-suppressing subtransferable candidate 5; SULT1A1, sulfotransferase family 1A; ALDH3α1, aldehyde dehydrogenase family 3, member A1.
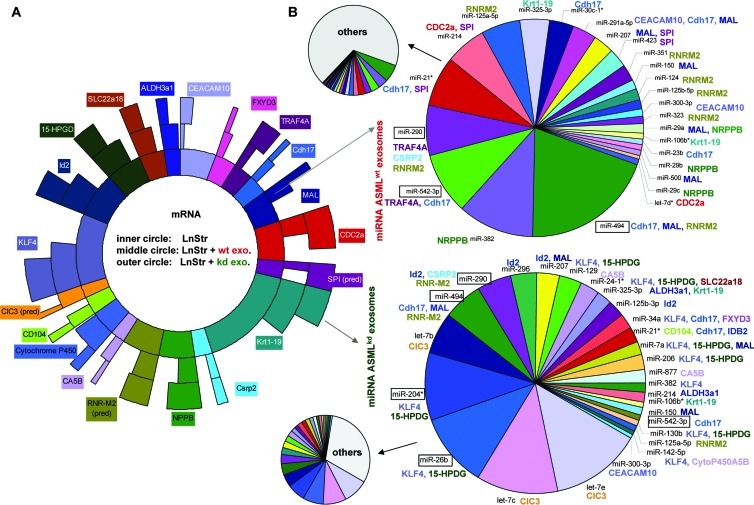
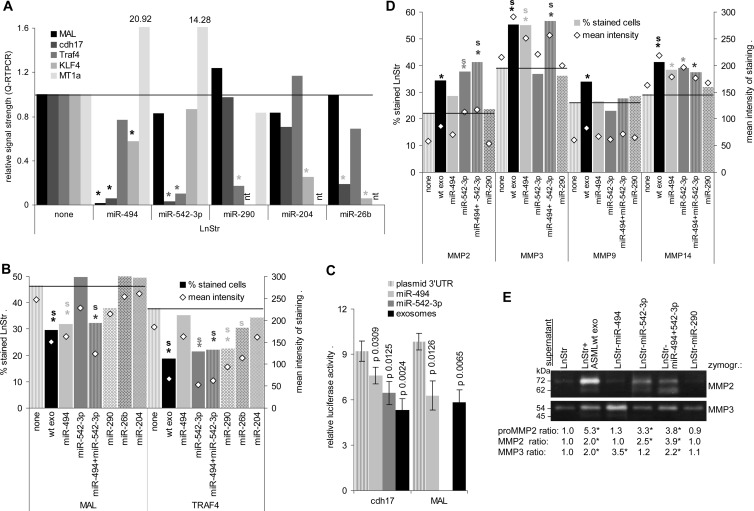
Curious, if exosomal miRNA might account for mRNA downregulation, we searched (targetscan.org) for exosomal miRNA for which the target mRNA was downregulated in exosome-treated LnStr. We selected 20 mRNAs with high signal strength that were significantly downregulated after co-culture with ASMLwt or ASML-CD44vkd exosomes. Arbitrarily taking these 20 mRNAs as 100% (inner circle), the relative reduction in co-cultures with ASMLwt (middle circle) and ASML-CD44vkd exosomes (outer circle) was calculated (Figure 7A). Expectedly, several exosomal miRNAs could potentially account for down-regulation of these 20 mRNAs in LnStr; only the miRNAs potentially targeting the selected LnStr mRNAs are presented. The contribution of these miRNAs to the total recovery of exosomal miRNA is shown in the arrowed small circles (Figure 7B). As MAL, cdh17, and TRAF4 mRNA were strongly downregulated and, potentially targeting miR-494, miR-542-3p and miR-290 were enriched in ASMLwt exosomes, we explored the effect of miRNA transfection on LnStr. miR-26b and miR-204, more abundant in ASML-CD44vkd exosomes and known to target Kruppel-like factor 4 (KLF4), whose expression was more strongly reduced in ASML-CD44vkd-treated LnStr, served as controls as well as MT1a, which is strongly upregulated in exosome-treated LnStr. qRT-PCR revealed down-regulation of MAL and cdh17 by miR-494, of cdh17 and TRAF4 by miR-542-3p, of TRAF4 by miR-290, and of KLF4 by miR-204 and miR-26b. The latter also affected cdh17, which is not a direct miR-26b target (Figure 8A). The findings were confirmed for MAL and TRAF4 protein expression in LnStr: miR-494 transfection downregulated MAL; miR-542-3p and miR-290 transfection downregulated TRAF4 expression, although with a lower efficacy than ASMLwt exosomes; miR-26b and miR-204 transfection did not affect MAL; only miR-26b transfection weakly affected TRAF4 expression (Figure 8B). Furthermore, a 3′UTR MAL and cdh17 luciferase reporter assay confirmed that MAL and cdh17 are targeted by miR-494 and cdh17 by miR-542-3p. Co-culture with ASMLwt exosomes exerted a stronger effect than a single miRNA, indicating that additional exosomal miRNA may regulate cdh17 and MAL expression (Figure 8C). Thus, functional exosomal miRNA is transferred in LnStr and affects target gene expression.
Figure 7.
Assignment of exosomal miRNA toward downregulated mRNA in LnStr. (A) Semiquantitative presentation of 20 abundant LnStr mRNAs that were strongly downregulated in exosome-treated LnStr. These 20 mRNAs have arbitrarily been taken as 100% (inner circle). The relative reduction by co-culture with ASMLwt exosomes is shown in themiddle circle and that by ASML-CD44vkd exosomes in the outer circle. (B) Exosomal miRNA potentially targeting these 20 downregulated LnStr mRNAs again were arbitrarily taken as 100%, their actual contribution to the total exosomal miRNA being indicated by the arrowed small circles. miRNA are assigned only for more than 50% reduced mRNA. Text box colors in A corresponds to text colors in B to facilitated coordination of potential effector miRNA in ASMLwt (upper right) and ASML-CD44vkd exosomes (lower right) to the downregulated mRNA (abbreviations correspond to Figure 6).
Figure 8.
Functional activity of exosomal miRNA. (A and B) Impact of miR-494, miR-542-3p, miR-290, miR-204, and miR-26b transfection on MAL, cdh17, KLF4, and TRAF4 and, for comparison, MT1a expression in LnStr as revealed 48 hours after transfection or co-culture with exosomes by (A) qRT-PCR (mean of three replicate samples with SD < 0.25, indicating reliability according to the software program for ΔCT) and (B) flow cytometry. (A) Significant differences in mRNA recovery between untreated and miRNA-transfected LnStr: *; (B) significant differences between untreated and miRNA-transfected LnStr in the mean percentage of stained cells (triplicates, two experiments): *; significant differences in the mean intensity of staining (triplicates, two experiments): s. (C) HEK293 cells were transfected with the dual-luciferase pmiRGlo vector containing the 3′UTR of MAL or cdh17 and were co-transfected with miR-494 or miR-542-3p (10 nM) or were co-cultured with ASMLwt exosomes (20 µg). Firefly and, for normalization, Renilla luciferase activity was evaluated after 48 hours in a FLUOstar OPTIMA luminometer. The relative luciferase activity and P values for miR co-transfection or ASMLwt exosome co-culture are shown. Significant differences are indicated. (D and E) Impact of cdh17 down-regulation in LnStr by miR-494 and/or miR-542-3p or, as control, miR-290 transfection on MMP2, MMP3, MMP9, and MMP14 expression as revealed by (D) flow cytometry and (E) representative example of zymography of LnStr culture supernatant, which confirmed MMP2 and MMP3 up-regulation. (D) Significant differences between untreated and miRNA-transfected LnStr in the mean percentage of stained cells (triplicates, two experiments): *; significant differences in the mean intensity of staining (triplicates): s. (E) The MMP ratio (mean of three experiments) compared to untreated LnStr is shown; significant differences: *. Transferred exosomal miRNA affects selective RNA expression in LnStr. As demonstrated for cdh17, exosomal miRNA repression of target mRNA can be accompanied by release of suppression for genes regulated by the primary miRNA target.
MT1a mRNA up-regulation in LnStr upon co-culture with exosomes, but also by transfection with miR-494 and miR-542-3p, supported miRNA-mediated down-regulation of target mRNA to affect expression of genes regulated by the primary miRNA target. With the expression of several proteases being upregulated after co-culture with ASML exosomes despite low exosomal mRNA expression, we finally asked whether miRNA silencing of cdh17 might be accompanied by protease up-regulation, MMP2 and MMP9 up-regulation being described to accompany cdh17 down-regulation [47]. Though expression of MMP9 was unaffected, MMP2 and MMP3 were strongly and MMP14 was weakly upregulated in miR-494, miR-542-3p, and miR-494 plus miR542-3p but not in control miR-290-transfected LnStr. miR-542-3p exerted a strong effect on MMP2 and miR-494 on MMP3 expression. MMP14 expression was equally affected by both miRNAs. Up-regulation of MMP2 and MMP3 in miRNA-transfected LnStr was confirmed by zymography (Figure 8, D and E).
Thus, tumor exosome miRNA strongly affects non-transformed target cells through silencing mRNA including mRNA up-regulation by release from repression by directly targeted mRNA.
Overview of Exosome-Modulated Gene Expression in Target Cells
Finally, we searched for functional activities of transcripts significantly upregulated by ASMLwt exosomes in LnStr. For six genes, no or very preliminary data on functional activity were found (not settled). There has been no hint for up-regulation of oncogenes. Instead, increased protease activity, pronounced adhesion molecule and chemokine ligand expression, and up-regulation of cell cycle- and angiogenesis-promoting genes and of genes engaged in oxidative stress response all fit the demands of metastasizing tumor cells for settlement and growth (Figure W6).
Taken together, the transfer of exosomal miRNA has severe consequences on target cell gene expression, where miRNA-induced changes could facilitate metastasizing tumor cell settlement in pre-metastatic organs.
Discussion
Tumor cells can establish a niche for metastasizing cells preceding their arrival [3–5]. Tumor exosomes, carrying growth factors, cytokines/receptors, and matrix degrading enzymes and transferring tumor mRNA and miRNA [8,12,48], could well provide the essential trigger [6–8,10–12,15,16,49]. We showed [6] that poorly metastatic ASML-CD44vkd cells regain metastatic potential after conditioning rats with ASMLwt or ASML-CD44vkd exosomes together with ASMLwt-CM-exo. We here demonstrate that ASML exosomes are taken up in vivo and that exosomal miRNA strongly affects favorite targets shown for LnStr and LuFb. We particularly want to discuss two points: 1) Though exosomes are characterized by a protein profile that is rich in molecules located in internalization-prone membrane domains and molecules engaged in fission, scission, and vesicular transport [14,22,24,25], a single protein that is involved in gene transcription/posttranscriptional regulation, like CD44v, can have significant bearing on the composition of exosomal proteins, mRNA, and miRNA, even if not involved in directly guiding proteins or harboring mRNA/miRNA into MVB; 2) though we did not yet explore the impact of exosome binding- or uptake-induced signal transduction, our data support exosomal miRNA strongly affecting target cells.
Exosome Proteins, mRNA, and miRNA
Our findings confirm that recruitment of mRNA into MVB is a selective process such that the exosomal mRNA profile does not reflect that of the donor cell [18,22]. Only 1500 mRNAs were recovered in ASML exosomes compared to >8000 in ASML cells. This difference might be an overestimate as the mRNA isolation kit that was used meanwhile was demonstrated to unproportionally enrich for small RNA [46]. Nonetheless, the relative abundance of mRNA in exosomes and cells differed and there has been a significant number of mRNA that was enriched in exosomes compared to cells, strengthening the selective recruitment of mRNA into MVB. However, with few exceptions, the ratio of exosomal ASMLwt versus ASML-CD44vkd mRNA did not differ significantly from that in cells. From there, we conclude that CD44v or associated molecules or molecules whose expression is regulated by CD44v may not be engaged in mRNA recruitment into MVB/exosomes. As already demonstrated [6,45], this also accounts for exosomal proteins. Protein expression differs between ASMLwt and ASML-CD44vkd exosomes. However, these changes are also seen at the cellular level. However, CD44v/asssociated molecules clearly contribute to transcription of several genes recovered in exosomes, as the mRNA profile of ASMLwt versus ASML-CD44vkd cells showed strong differences.
The cellular ASMLwt and ASML-CD44vkd miRNA profiles also differ, suggesting engagement of CD44v/associated molecules in miRNA transcription or posttranscriptional regulation. Thus, the tumor suppressors let-7b, let-7d, let-7e, and miR-101 were increased in ASML-CD44kd exosomes and cells. It has been suggested that tumor cells get rid of let-7 through exosomes [50]. Alternatively, our findings point toward CD44v to be engaged in downregulating let-7 and miR-101. Irrespective of the underlying mechanism, the higher level of let-7 in ASML-CD44vkd exosomes is in line with the reduced metastatic capacity of these cells [43]. Notably, too, miR-34a, which suppresses tumor growth by CD44 down-regulation [51], was very low in ASMLwt exosomes and cells but abundant in ASML-CD44vkd exosomes, which argues, in turn, for CD44v or associated molecules to be engaged in miR-34a silencing. Metastasis-promoting miR-494 and miR-21 and apoptosis-regulating miR-24-1 [52–54] are also abundant only in ASMLwt exosomes. miRNA transcription and/or posttranscriptional regulation appear also to be affected by CD44v-associated c-Met [6], which supports miR-103 transcription [55] that is more than two-fold increased in ASMLwt exosomes. CD44v-related changes are mostly reflected in the exosomal miRNA profile such that miRNA reduced in ASML-CD44vkd cells are also lower in ASML-CD44vkd than ASMLwt exosomes. Irrespective of a possible additional involvement of CD44v in MVB recruitment, CD44v clearly is engaged in miRNA transcription/posttranscriptional regulation.
Taken together, proteins and mRNA of genes, whose expression is regulated by CD44v (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE34739) [6,43,45], are recovered at a reduced level in ASML-CD44vkd exosomes. Yet, there is no evidence for CD44v contributing to protein or mRNA recruitment into MVB. Our data also indicate an engagement of CD44v in miRNA transcription/silencing/posttranscriptional regulation. Exosomal miRNA having a significant impact on target cells, the finding that a molecule not actively engaged in exosome assembly/transport can strongly affect the exosome composition requires detailed exploration that may provide key answers to metastasis-promoting CD44v activities.
Exosomal mRNA, miRNA, and Target Cell Fate
LnStr mRNA and protein recovery was altered by co-culture with ASML exosomes. Besides mRNA/miRNA, exosomal proteins can affect target cells as demonstrated for dendritic cell exosome-promoted T cell activation, which proceeds through exosome binding-initiated signal transduction and gene transcription [20]. Exosome binding-initiated signal transduction will be facilitated by exosomal ligands being located in internalization-prone membrane domains [33,56], which are enriched in receptor tyrosine kinases, phosphatases, proteases, and, at the inner membrane, linker and signal transduction molecules [57]. Without question, target cell stimulation by tumor exosome binding and uptake requires to be evaluated. Nonetheless, we did not observe a measurable impact of exosomal proteins taken up by target cells [58] and high protein expression in ASMLwt exosomes is hardly reflected by increased recovery in target cells. Therefore, we proceeded to ask for the impact of uptaken mRNA and miRNA. Though there are discrete differences in the mRNA and, more markedly, the miRNA profile of ASMLwt and ASML-CD44vkd exosomes, it should be mentioned that we were mostly concerned about the impact of exosomal mRNA and miRNA in general and controlled at the functional level only ASMLwt exosomes.
Similar to our findings on exosomal proteins, our data argue against transferred exosomal mRNA to account for increased target cell mRNA: 1) With few exceptions, mRNA levels are significantly higher in LnStr than exosomes; 2) mRNA levels become unproportionally upregulated, e.g., the MMP3 mRNA level in LnStr increased 13-fold upon exosome uptake, but the mRNA level in exosomes was about 10% that in LnStr, excluding the effect to be due to transferred exosomal mRNA; 3) mRNA levels in LnStr remained increased for 48 hours. RNA recovery in LnStr unlikely deriving from transferred exosomal mRNA points toward exosome-initiated transcription, which remains to be explored, or toward miRNA contributing to mRNA upregulation by silencing repressive mRNA.
In LnStr co-cultured with ASML exosomes, several mRNA became strongly downregulated. Though it is not possible to differentiate between direct and indirect effects, as most miRNAs have multiple targets, there is evidence for a direct impact of exosomal miRNA. ASMLwt and ASML-CD44vkd exosomes show distinct miRNA profiles and reduced mRNA levels in LnStr co-cultured with ASMLwt versus ASML-CD44vkd exosomes overlap only partially. In addition, miRNA targeting mRNA selectively downregulated by ASMLwt exosomes were more abundant in ASMLwt than ASML-CD44vkd exosomes and the ASML-CD44vkd exosome miRNA that could potentially target the 18 mRNAs downregulated in LnStr accounts for more than 60% of the total miRNA.
We focused on abundant miR-494, potentially targeting MAL and cdh17, and miR-542-3p, targeting cdh17 and TRAF4. MAL can contribute to differentiation and apical sorting [59,60] and cdh17 to tumor growth/Wnt signaling [61]; TRAF4 exerts morphogenetic functions [62]. LnStr transfection with these miRNAs was accompanied by down-regulation of the predicted target(s), which also accounted for miR-204 and miR-26b transfection that downregulated KLF4, which can be opposingly affected by miRNA in normal versus malignant cells [63,64]. We confirmed by miRNA transfection and by luciferase reporter assay in co-culture with exosomes or transfection with miRNA that the transfer of exosomal miRNA can directly affect target cell mRNA.
Finally, significant up-regulation of mRNA in exosome-treated LnStr pointed toward mRNA up-regulation through miRNA silencing regulatory mRNA. Altered protease expression being a dominating feature in ASML-exosome-treated LnStr and in vivo in draining lymph nodes after ASMLwt-CM application [6], and cdh17 down-regulation being known to promote MMP2 and MMP9 up-regulation [47], the finding that cdh17 down-regulation in miR-494 and miR-542-3p transfected LnStr was accompanied by MMP2, MMP3, and MMP14 up-regulation strengthens a direct impact of transferred exosomal miRNA on target cells.
Conclusion
Tumor exosomes being of central importance in premetastatic niche preparation [6,16], we characterized exosomes from a metastatic tumor line and evaluated their mode of action. CD44v contributing to the cross talk between tumor exosomes and host stroma, we additionally defined the impact of CD44v on the exosome composition.
As summarized in Figure W7, tumor exosomes contain a restricted mRNA and miRNA panel and there is evidence that CD44v contributes to shaping the exosomal protein, mRNA, and miRNA profiles by regulating gene and miRNA transcription/posttranscriptional regulation without a direct impact on recruitment into MVB/exosomes (Figure W7A). Exosomes reach premetastatic organs in vivo, bind, and are taken up by selected targets (Figure W7B). Exosome binding/uptake severely alters target cells. This can be due to exosome binding-initiated target modulation or target cell activation, which has not been explored in the present manuscript (Figure W7C), and to transferred exosomal miRNA, where we provide for the first time evidence that not only the direct miRNA target but also release from repression by the primary target significantly contributes to target cell reprogramming by tumor exosomes (Figure W7D). Finally, supporting the concept of a central role of tumor exosomes in metastasis, exosomal miRNA from a metastasizing tumor line, though not being oncogenic, preferentially regulates mRNA, which contributes to establishing a premetastatic niche (Figure W6).
Exosomes are discussed as a most potent gene delivery system. Our findings support this hypothesis and suggest that competing tumor exosomes could well be a promising therapeutic option by preventing establishing a premetastatic niche. Beyond this, tailored exosomes might allow to rescind tumor exosome-induced host cell modulation.
Supplementary Material
Acknowledgments
We thank Shijing Yue and Florian Thuma for help with the luciferase reporter and in vitro mRNA translation assays.
Abbreviations
- ASMLwt
BSp73ASML
- ASML-CD44vkd
BSp73ASML-CD44v4-v7 knockdown
- cdh17
cadherin-17
- CM
conditioned medium
- CM-exo
exosome-depleted CM
- ECs
endothelial cells
- ifp
intrafootpad
- KLF4
Kruppel-like factor 4
- LnStr
lymph node stroma cells
- LuFb
lung fibroblasts
- MVBs
multivesicular bodies
- CT
threshold cycle
Footnotes
This work was supported by the Deutsche Krebshilfe (M.Z.), the Wilhelm Sander Foundation (M.Z.), and NCT Interdisciplinary Research Program (M.Z.). The authors declare no conflict of interest. Array data are deposited at http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE34739.
This article refers to supplementary materials, which are designated by Tables W1 to W6 and Figures W1 to W7 and are available online at www.neoplasia.com.
References
- 1.Kraljevic Pavelic S, Sedic M, Bosnjak H, Spaventi S, Pavelic K. Metastasis: new perspectives on an old problem. Mol Cancer. 2011;10:22. doi: 10.1186/1476-4598-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Talmadge JE, Fidler IJ. AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res. 2010;70:5649–5669. doi: 10.1158/0008-5472.CAN-10-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geiger TR, Peeper DS. Metastasis mechanisms. Biochim Biophys Acta. 2006;1796:293–308. doi: 10.1016/j.bbcan.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan RN, Rafii S, Lyden D. Preparing the “soil”: the pre-metastatic niche. Cancer Res. 2006;66:11089–11093. doi: 10.1158/0008-5472.CAN-06-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGovern M, Voutev R, Maciejowski J, Corsi AK, Hubbard EJ. A “latent niche” mechanism for tumor initiation. Proc Natl Acad Sci USA. 2009;106:11617–11622. doi: 10.1073/pnas.0903768106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jung T, Castellana D, Klingbeil P, Cuesta Hernández I, Vitacolonna M, Orlicky DJ, Roffler SR, Brodt P, Zöller M. CD44v6 dependence of premetastatic niche preparation by exosomes. Neoplasia. 2009;11:1093–1105. doi: 10.1593/neo.09822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chairoungdua A, Smith DL, Pochard P, Hull M, Caplan MJ. Exosome release of β-catenin: a novel mechanism that antagonizes Wnt signaling. J Cell Biol. 2010;190:1079–1091. doi: 10.1083/jcb.201002049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hood JL, San Roman S, Wickline SA. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 2011;71:3792–3801. doi: 10.1158/0008-5472.CAN-10-4455. [DOI] [PubMed] [Google Scholar]
- 9.Lee TH, D'Asti E, Magnus N, Al-Nedawi K, Meehan B, Rak J. Microvesicles as mediators of intercellular communication in cancer—the emerging science of cellular ‘debris’. Semin Immunopathol. 2011;33:455–467. doi: 10.1007/s00281-011-0250-3. [DOI] [PubMed] [Google Scholar]
- 10.Lima LG, Chammas R, Monteiro RQ, Moreira ME, Barcinski MA, Tumor-derived microvesicles modulate the establishment of metastatic melanoma in a phosphatidylserine-dependent manner. Cancer Lett. 2009;283:168–175. doi: 10.1016/j.canlet.2009.03.041. [DOI] [PubMed] [Google Scholar]
- 11.McCready J, Sims JD, Chan D, Jay DG. Secretion of extracellular hsp90 via exosomes increases cancer cell motility: a role for plasminogen activation. BMC Cancer. 2010;10:294. doi: 10.1186/1471-2407-10-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park JE, Tan HS, Datta A, Lai RC, Zhang H, Meng W, Lim SK, Sze SK. Hypoxic tumor cell modulates its microenvironment to enhance angiogenic and metastatic potential by secretion of proteins and exosomes. Mol Cell Proteomics. 2010;9:1085–1099. doi: 10.1074/mcp.M900381-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peinado H, Lavotshkin S, Lyden D. The secreted factors responsible for pre-metastatic niche formation: old sayings and new thoughts. Semin Cancer Biol. 2011;21:139–146. doi: 10.1016/j.semcancer.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Rak J. Microparticles in cancer. Semin Thromb Hemost. 2010;36:888–906. doi: 10.1055/s-0030-1267043. [DOI] [PubMed] [Google Scholar]
- 15.Ronquist KG, Ronquist G, Larsson A, Carlsson L. Proteomic analysis of prostate cancer metastasis-derived prostasomes. Anticancer Res. 2010;30:285–290. [PubMed] [Google Scholar]
- 16.Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, García-Santos G, Ghajar C, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitchell PJ, Welton J, Staffurth J, Court J, Mason MD, Tabi Z, Clayton A. Can urinary exosomes act as treatment response markers in prostate cancer? J Transl Med. 2009;7:4. doi: 10.1186/1479-5876-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wittmann J, Jäck HM. Serum microRNAs as powerful cancer biomarkers. Biochim Biophys Acta. 2010;1806:200–207. doi: 10.1016/j.bbcan.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Pap E, Pállinger E, Pásztói M, Falus A. Highlights of a new type of intercellular communication:microvesicle-based information transfer. Inflamm Res. 2009;58:1–8. doi: 10.1007/s00011-008-8210-7. [DOI] [PubMed] [Google Scholar]
- 20.Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci. 2000;113(pt 19):3365–3374. doi: 10.1242/jcs.113.19.3365. [DOI] [PubMed] [Google Scholar]
- 21.Lakkaraju A, Rodriguez-Boulan E. Itinerant exosomes: emerging roles in cell and tissue polarity. Trends Cell Biol. 2008;18:199–209. doi: 10.1016/j.tcb.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73:1907–1920. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 23.van Niel G, Porto-Carreiro I, Simoes S, Raposo G. Exosomes: a common pathway for a specialized function. J Biochem. 2006;140:13–21. doi: 10.1093/jb/mvj128. [DOI] [PubMed] [Google Scholar]
- 24.Raimondo F, Morosi L, Chinello C, Magni F, Pitto M. Advances in membranous vesicle and exosome proteomics improving biological understanding and biomarker discovery. Proteomics. 2010;11:709–720. doi: 10.1002/pmic.201000422. [DOI] [PubMed] [Google Scholar]
- 25.Zöller M. Tetraspanins: push and pull in suppressing and promoting metastasis. Nat Rev Cancer. 2009;9:40–55. doi: 10.1038/nrc2543. [DOI] [PubMed] [Google Scholar]
- 26.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 27.Pant S, Hilton H, Burczynski ME. The multifaceted exosome: biogenesis, role in normal and aberrant cellular function, and frontiers for pharmacological and biomarker opportunities. Biochem Pharmacol. 2012;83:1484–1494. doi: 10.1016/j.bcp.2011.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gibbings DJ, Ciaudo C, Erhardt M, Voinnet O. Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat Cell Biol. 2009;11:1143–1149. doi: 10.1038/ncb1929. [DOI] [PubMed] [Google Scholar]
- 29.Zomer A, Vendrig T, Hopmans ES, van Eijndhoven M, Middeldorp JM, Pegtel DM. Exosomes: fit to deliver small RNA. Commun Integr Biol. 2010;3:447–450. doi: 10.4161/cib.3.5.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atay S, Gercel-Taylor C, Taylor DD. Human trophoblast-derived exosomal fibronectin induces pro-inflammatory Il-1β production by macrophages. Am J Reprod Immunol. 2011;66:259–269. doi: 10.1111/j.1600-0897.2011.00995.x. [DOI] [PubMed] [Google Scholar]
- 31.Escrevente C, Keller S, Altevogt P, Costa J. Interaction and uptake of exosomes by ovarian cancer cells. BMC Cancer. 2011;11:108. doi: 10.1186/1471-2407-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rana S, Claas C, Kretz CC, Nazarenko I, Zöller M. Activation-induced internalization differs for the tetraspanins CD9 and Tspan8: impact on tumor cell motility. Int J Biochem Cell Biol. 2011;43:106–119. doi: 10.1016/j.biocel.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Rana S, Shijing Y, Stadel D, Zöller M. Toward tailored exosomes: the exosomal tetraspanin web contributes to target cell selection. Int J Biochem Cell Biol. 2012;44:1574–1584. doi: 10.1016/j.biocel.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 34.Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010;285:17442–17452. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lykke-Andersen S, Brodersen DE, Jensen TH. Origins and activities of the eukaryotic exosome. J Cell Sci. 2009;122:1487–1494. doi: 10.1242/jcs.047399. [DOI] [PubMed] [Google Scholar]
- 36.Hwang I, Ki D. Receptor-mediated T cell absorption of antigen presenting cell-derived molecules. Front Biosci. 2011;16:411–421. doi: 10.2741/3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor DD, Gercel-Taylor C. Exosomes/microvesicles: mediators of cancer-associated immunosuppressive microenvironments. Semin Immunopathol. 2011;33:441–454. doi: 10.1007/s00281-010-0234-8. [DOI] [PubMed] [Google Scholar]
- 38.Whiteside TL, Mandapathil M, Szczepanski M, Szajnik M. Mechanisms of tumor escape from the immune system: adenosine-producing Treg, exosomes and tumor-associated TLRs. Bull Cancer. 2011;98:25–31. doi: 10.1684/bdc.2010.1294. [DOI] [PubMed] [Google Scholar]
- 39.Matzku S, Komitowski D, Mildenberger M, Zöller M. Characterization of BSp73, a spontaneous rat tumor and its in vivo selected variants showing different metastasizing capacities. Invasion Metastasis. 1983;3:109–123. [PubMed] [Google Scholar]
- 40.Matzku S, Wenzel A, Liu S, Zöller M. Antigenic differences between metastatic and nonmetastatic BSp73 rat tumor variants characterized by monoclonal antibodies. Cancer Res. 1989;49:1294–1299. [PubMed] [Google Scholar]
- 41.Günthert U, Hofmann M, Rudy W, Reber S, Zöller M, Haussmann I, Matzku S, Wenzel A, Ponta H, Herrlich P. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell. 1991;65:13–24. doi: 10.1016/0092-8674(91)90403-l. [DOI] [PubMed] [Google Scholar]
- 42.Zöller M. CD44: can a cancer-initiating cell profit from an abundantly expressed molecule? Nat Rev Cancer. 2011;11:254–267. doi: 10.1038/nrc3023. [DOI] [PubMed] [Google Scholar]
- 43.Klingbeil P, Marhaba R, Jung T, Kirmse R, Ludwig T, Zöller M. CD44 variant isoforms promote metastasis formation by a tumor cell-matrix cross-talk that supports adhesion and apoptosis resistance. Mol Cancer Res. 2009;7:168–179. doi: 10.1158/1541-7786.MCR-08-0207. [DOI] [PubMed] [Google Scholar]
- 44.LeBedis C, Chen K, Fallavollita L, Boutros T, Brodt P. Peripheral lymph node stromal cells can promote growth and tumorigenicity of breast carcinoma cells through the release of IGF-I and EGF. Int J Cancer. 2002;100:2–8. doi: 10.1002/ijc.10481. [DOI] [PubMed] [Google Scholar]
- 45.Zech D, Rana S, Büchler MW, Zöller M. Tumor-exosomes and leukocyte activation: an ambivalent crosstalk. Cell Commun Signal. 2012;10:37. doi: 10.1186/1478-811X-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eldh M, Lötvall J, Malmhäll C, Ekström K. Importance of RNA isolation methods for analysis of exosomal RNA: evaluation of different methods. Mol Immunol. 2012;50:278–286. doi: 10.1016/j.molimm.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 47.Liu QS, Zhang J, Liu M, Dong WG. Lentiviral-mediated miRNA against liver-intestine cadherin suppresses tumor growth and invasiveness of human gastric cancer. Cancer Sci. 2010;101:1807–1812. doi: 10.1111/j.1349-7006.2010.01600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bussolati B, Grange C, Camussi G. Tumor exploits alternative strategies to achieve vascularization. FASEB J. 2011;25:2874–2882. doi: 10.1096/fj.10-180323. [DOI] [PubMed] [Google Scholar]
- 49.Bobrie A, Krumeich S, Reyal F, Recchi C, Moita LF, Seabra MC, Ostrowski M, Théry C. Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res. 2012;72:4920–4930. doi: 10.1158/0008-5472.CAN-12-0925. [DOI] [PubMed] [Google Scholar]
- 50.Ohshima K, Inoue K, Fujiwara A, Hatakeyama K, Kanto K, Watanabe Y, Muramatsu K, Fukuda Y, Ogura S, Yamaguchi K, et al. Let-7 microRNA family is selectively secreted into the extracellular environment via exosomes in a metastatic gastric cancer cell line. PLoS One. 2010;5:e13247. doi: 10.1371/journal.pone.0013247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu C, Kelnar K, Liu B, Chen X, Calhoun-Davis T, Li H, Patrawala L, Yan H, Jeter C, Honorio S, et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med. 2011;17:211–215. doi: 10.1038/nm.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ali S, Almhanna K, Chen W, Philip PA, Sarkar FH. Differentially expressed miRNAs in the plasma may provide a molecular signature for aggressive pancreatic cancer. Am J Transl Res. 2010;3:28–47. [PMC free article] [PubMed] [Google Scholar]
- 53.Liu L, Jiang Y, Zhang H, Greenlee AR, Han Z. Overexpressed miR-494 down-regulates PTEN gene expression in cells transformed by antibenzo(a)pyrene-trans-7,8-dihydrodiol-9,10-epoxide. Life Sci. 2010;86:192–198. doi: 10.1016/j.lfs.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 54.Qin W, Shi Y, Zhao B, Yao C, Jin L, Ma J, Jin Y. miR-24 regulates apoptosis by targeting the open reading frame (ORF) region of FAF1 in cancer cells. PLoS One. 2010;5:e9429. doi: 10.1371/journal.pone.0009429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garofalo M, Romano G, Di Leva G, Nuovo G, Jeon YJ, Ngankeu A, Sun J, Lovat F, Alder H, Condorelli G, et al. EGFR and MET receptor tyrosine kinase-altered microRNA expression induces tumorigenesis and gefitinib resistance in lung cancers. Nat Med. 2011;18:74–82. doi: 10.1038/nm.2577. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 56.Rana S, Zöller M. Exosome target cell selection and the importance of exosomal tetraspanins: a hypothesis. Biochem Soc Trans. 2011;39:559–562. doi: 10.1042/BST0390559. [DOI] [PubMed] [Google Scholar]
- 57.Simons K, Gerl MJ. Revitalizing membrane rafts: new tools and insights. Nat Rev Mol Cell Biol. 2010;11:688–699. doi: 10.1038/nrm2977. [DOI] [PubMed] [Google Scholar]
- 58.Nazarenko I, Rana S, Baumann A, McAlear J, Hellwig A, Trendelenburg M, Lochnit G, Preissner KT, Zöller M. Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation. Cancer Res. 2010;70:1668–1678. doi: 10.1158/0008-5472.CAN-09-2470. [DOI] [PubMed] [Google Scholar]
- 59.Brandt DT, Xu J, Steinbeisser H, Grosse R. Regulation of myocardin-related transcriptional coactivators through cofactor interactions in differentiation and cancer. Cell Cycle. 2009;8:2523–2527. doi: 10.4161/cc.8.16.9398. [DOI] [PubMed] [Google Scholar]
- 60.Frank M. MAL, a proteolipid in glycosphingolipid enriched domains: functional implications in myelin and beyond. Prog Neurobiol. 2000;60:531–544. doi: 10.1016/s0301-0082(99)00039-8. [DOI] [PubMed] [Google Scholar]
- 61.Lee NP, Poon RT, Shek FH, Ng IO, Luk JM. Role of cadherin-17 in oncogenesis and potential therapeutic implications in hepatocellular carcinoma. Biochim Biophys Acta. 2010;1806:138–145. doi: 10.1016/j.bbcan.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 62.Mathew SJ, Haubert D, Kronke M, Leptin M. Looking beyond death: a morphogenetic role for the TNF signalling pathway. J Cell Sci. 2009;122:1939–1946. doi: 10.1242/jcs.044487. [DOI] [PubMed] [Google Scholar]
- 63.Lin CC, Liu LZ, Addison JB, Wonderlin WF, Ivanov AV, Ruppert JM. A KLF4-miRNA-206 autoregulatory feedback loop can promote or inhibit protein translation depending upon cell context. Mol Cell Biol. 2011;31:2513–2527. doi: 10.1128/MCB.01189-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Papp B, Plath K. Reprogramming to pluripotency: stepwise resetting of the epigenetic landscape. Cell Res. 2011;21:486–501. doi: 10.1038/cr.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.