Abstract
The overexpression of AXL receptor tyrosine kinase is a frequent finding that has been associated with poor prognosis in esophageal adenocarcinoma (EAC). As the majority of EAC are intrinsically resistant to DNA-damaging therapies, an alternative therapeutic approach based on the activation of death receptors may be warranted. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) has been evaluated in clinical trials and found promising as anticancer agent with mild side effects; unfortunately, resistance to TRAIL remains a major clinical problem. Herein, we explored the role of AXL in TRAIL resistance and elucidated the underlying mechanism. Overexpression of AXL in OE33 and OE19 cells promoted cell survival and attenuated TRAIL-induced cellular and molecular markers of apoptosis. In contrast, knockdown of endogenous AXL sensitized FLO-1 cells to TRAIL. The mechanism by which AXL regulates TRAIL resistance was examined. Protein and mRNA expression of DR4 and DR5 death receptors was not downregulated by AXL. In addition, the possible involvement of FLICE-inhibitory protein (FLIP) in regulating the interaction of caspase-8 with Fas-associated death domain protein (FADD) was excluded, as AXL did not enhance FLIP expression or FLIP/FADD association. Alternatively, protein association of AXL with DR5, independent of TRAIL, was confirmed, suggesting that AXL could regulate DR5 receptor activity. The AXL/DR5 association had no negative effect on TRAIL-induced interaction with FADD. However, the AXL/DR5 interaction blocked the recruitment of caspase-8 to the death-inducing signal complex (DISC). Collectively, our findings uncover a novel mechanism of TRAIL resistance mediated by AXL through regulation of the DISC and provide strong evidence that AXL could be exploited as a therapeutic target to circumvent TRAIL resistance.
Introduction
Esophageal cancer, which includes squamous cell carcinoma and adenocarcinoma, is an aggressive neoplasm and a major cause of cancer-related deaths in the world [1]. Projections of approximately 14,000 new cases of esophageal cancer, most of which are esophageal adenocarcinoma (EAC), occur per year in the United States [2,3]. Since the majority of patients with EAC present with advanced disease, 5-year relative survival rates are estimated as low as 14% [4,5]. This clearly indicates the ineffectiveness of the current treatment regimens and highlights that the intrinsic resistance to therapy is a hallmark of EAC.
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) induces apoptosis upon binding to DR4 or DR5 death receptors [6]. TRAIL-induced activation of death receptors leads to the formation of death-inducing signaling complex (DISC), which consists of death receptor, Fas-associated death domain protein (FADD), and caspase-8. The autocatalytic activation of caspase-8 induced by DISC-mediated proximity leads to direct activation of caspase-3 and apoptosis (extrinsic pathway) in type I cells [7]. Additionally, caspase-8 cleaves Bid and activates the intrinsic mitochondrial apoptosis pathway in type II cells [8,9]. Because of the unique feature of TRAIL that selectively induces apoptosis in malignant cells and mostly sparing normal cells, several anticancer therapeutic strategies have been developed [10,11]. Recombinant proteins, such as TRAIL, or agonistic human monoclonal antibodies against DR4 or DR5 death receptors are frequently used to induce apoptosis of cancer cells [12,13]. Unfortunately, a significant proportion of cancer cells are refractory to TRAIL-induced cytotoxicity, even though they express functional death receptors. TRAIL resistance can be mediated by several mechanisms, such as expression of FLICE-inhibitory protein (FLIP), which is similar to caspase-8 but lacking the enzymatic activity. FLIP competes with caspase-8 for binding FADD, hence blocking TRAIL-induced apoptotic signaling cascade [14]. Moreover, TRAIL resistance can be modulated by expression of decoy death receptors, mutations in the caspase-8 gene, and activity of AKT and nuclear factor of kappa light polypeptide gene enhancer in B-cells (NF-kB; reviewed in [15]).
AXL, a member of the TAM family of receptor tyrosine kinases, was originally isolated from human leukemia cells and identified as a transforming gene [16,17]. Overexpression of AXL in the presence of its ligand Gas6 stimulation has been implicated in cell growth, migration, and survival through activation of AKT and mitogen-activated protein kinases (MAPK) pathways in solid tumors [17–19]. Findings from recent studies on non-small cell lung carcinoma indicated that increased activation of AXL-induced acquired resistance to epidermal growth factor receptor (EGFR)-targeted therapy [20], whereas inhibition of AXL promoted chemosensitivity and apoptosis [21]. A previous report indicated that AXL was upregulated in the multistep esophageal carcinogenesis and a marker of poor prognosis in EAC [22]. Recently, we have shown frequent overexpression of AXL in EAC and demonstrated that AXL promotes cisplatin resistance through regulation of c-ABL/p73 signaling [23].
The aim of this study was to investigate the role of AXL in TRAIL resistance in EAC and elucidate the molecular mechanism that controls this effect. We demonstrate that AXL interacts with the DR5/FADD complex, hence blocking TRAIL-induced activation of caspase-8 and apoptosis. These novel findings provide strong evidence that AXL may be exploited as a therapeutic target to sensitize cancer cells to TRAIL in EAC.
Materials and Methods
Cell Lines and Reagents
The human EAC cancer cell lines, OE33, OE19, and FLO-1, were a kind gift from Dr David Beer (University of Michigan, Ann Arbor, MI). The cells were examined weekly to ascertain conformity to the appropriate in vitro morphologic features [24]. Additionally, the tumorigenicity of the cells was tested by in vivo tumor growth in female athymic nude-Foxn 1 nu/nu mice (Harlan Laboratories, Indianapolis, IN). Human embryonic kidney (HEK-293) cells were obtained from ATCC (Manassas, VA). These cells were cultured in F12 (HAM) medium (Gibco, Carlsbad, CA) supplemented with 10% FBS (Invitrogen Life Technologies, Carlsbad, CA) and 1% penicillin/streptomycin (Gibco). Recombinant human TRAIL/Apo2 ligand was obtained from BioVision Research Products (Mountain View, CA). AXL, DR5, cleaved caspase-3 and caspase-9, poly (ADP-ribose) polymerase (PARP), FLIP, Bid, and β-actin antibodies were purchased from Cell Signaling Technology (Danvers, MA). FADD and DcR1 antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). DR4 and caspase-8 antibodies were purchased from Abcam (Cambridge, MA) and Epitomics (Burlingame, CA), respectively. The AXL pharmacological inhibitor (BMS 777607) was obtained from Selleck Chemicals LLC (Houston, TX).
AXL Expression and Small Hairpin RNA
The plasmids of pcDNA4/AXL-myc-His and pcDNA4, kindly provided by Dr Rosa Marina Melillo (University of Naples, Naples, Italy), were used to a generate stable expression in EAC cells. Briefly, OE33 cells, with low endogenous AXL expression, were stably transfected using Lipofectamine 2000 (Invitrogen) and selected with 100 µg/ml Zeocin (Invitrogen) following previously described standard protocols [25]. The coding sequence of AXL from pcDNA3.1/AXL plasmid was subcloned into the shuttle vector (pACCMV). The recombinant AXL-expressing adenovirus was generated by co-transfecting HEK-293 cells with the shuttle and backbone (pJM17) adenoviral plasmids using the Calcium Phosphate Transfection Kit (Applied Biological Materials Inc, Richmond, BC). Lentivirus particles expressing control short hairpin RNA (shRNA) or a pool of five clones of AXL shRNA were generated and their specificity validated by Sigma-Aldrich (St Louis, MO). FLO-1 cells, with high endogenous AXL expression, were transduced with lentivirus particles and selected with 1 µg/ml puromycin (Invitrogen) for 10 days.
Cell Viability Assay
Cell viability was determined using the CellTiter-Glo Luminescent Cell Viability Assay Kit (Promega, Madison, WI). Briefly, cells (5 x 103 per well) were cultured onto a 96-well plate. Approximately 18 hours after seeding, cells were treated with vehicle or various concentrations of TRAIL for 5 hours. The luminescence was read on a Microplate Reader (FLUOstar OPTIMA). All experiments were performed in triplicates and repeated at least three times.
Apoptosis Assay
OE33 cells stably expressing AXL or pcDNA4 empty vector and FLO-1 cells transduced with lentivirus particles expressing AXL shRNA or control shRNA were cultured in 60-mm plates and treated with TRAIL (40 ng/ml) or vehicle overnight. Cells were harvested and stained with Annexin V-fluorescein isothiocyanate and propidium iodide (PI; R&D Systems, Minneapolis, MN). The cells were washed with phosphate-buffered saline (PBS) and resuspended in binding buffer and then subjected to fluorescence-activated cell sorting (FACS) analysis by a flow cytometer (Becton Dickinson, Franklin Lakes, NJ). Apoptotic cell death was determined by measuring the cell population that stained positive for Annexin V-fluorescein isothiocyanate and PI.
Quantitative Real-Time Reverse Transcription-Polymerase Chain Reaction
Total RNA was isolated from cells using an RNeasy Mini Kit (Qiagen, Valencia, CA). Total RNA (1 µg) was used to synthesize single-stranded cDNA by an iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). Quantitative real-time reverse transcription-polymerase chain reaction (RT-PCR) was performed in triplicate using an iCycler (Bio-Rad) with a threshold cycle number determined by use of iCycler software version 3.0. mRNA specific primers for DR5, DR4, and HPRT1 were designed, and the results were normalized to HPRT1 as a stable reference gene for quantitative real-time RT-PCR. The relative mRNA expression levels were calculated according to the formula 2(Et - Rt)/2(Rn - En), as described previously [26].
Western Blot Analysis
Cell lysates were prepared in RIPA buffer (50 mmol/l Tris-HCl buffer, pH 7.4, 150 mmol/l NaCl, 1% Triton X-100, 1% sodium deoxycholate, and 0.1% sodium dodecyl sulfate) supplemented with 1x Halt protease inhibitor cocktail and 1x Halt phosphatase inhibitor cocktail (Pierce, Rockford, IL). A Bio-Rad protein assay (Bio-Rad) was used to determine protein concentrations. Proteins were separated on 10–12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to Protran nitrocellulose membranes (Whatman, Boston, MA). Membranes were first hybridized with specific primary antibodies and then with HRP-conjugated secondary antibodies (Cell Signaling Technology). Protein bands were visualized using a commercial Immobilon Western Chemiluminescent HRP Substrate detection reagent (Millipore, Billerica, MA).
Immunoprecipitation
Cells were solubilized for 30 minutes with ice-cold RIPA buffer containing 1% Halt protease inhibitor cocktail (Pierce). The cell lysates were sonicated and spun down at 15,000 rpm for 10 minutes. Protein concentration in the supernatants was measured by the Bio-Rad Protein Assay. Using a primary antibody bound to Dynabeads Protein G (50 µl; Invitrogen), immunoprecipitations of equal total proteins (200 µg) were performed at room temperature for 1 hour. The beads were washed with ice-cold PBS and then heated to 100°C for 5 minutes in sample buffer. The denatured proteins were eluted by magnet and subjected to Western blot analysis.
Statistical Analysis
The results were expressed as means ± SD. The statistical significance of the studies was determined by the parametric unpaired Student's t test. Differences with P values ≤ .05 are considered significant.
Results
AXL Promotes Survival of EAC Cells
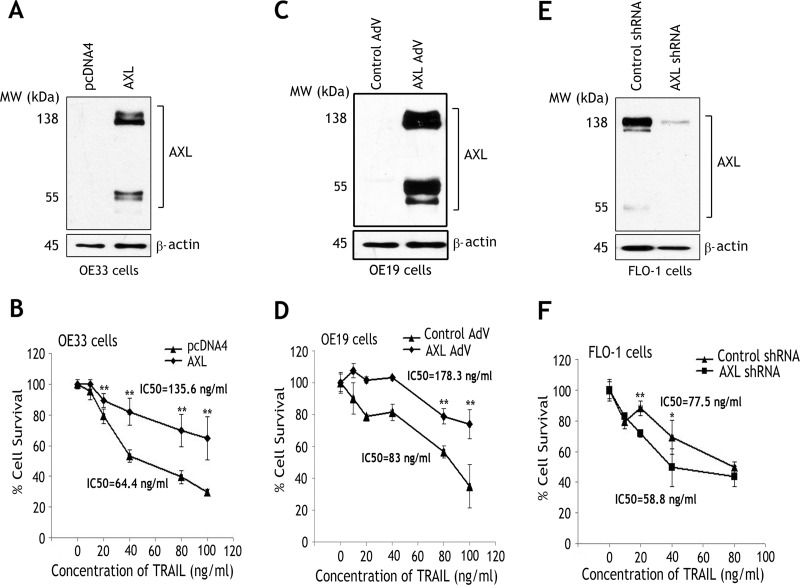
To investigate the role of AXL in cell survival, we generated two esophageal cell models. We employed OE33 and OE19 cells, with low endogenous AXL expression, to stably or transiently overexpress AXL, respectively (Figure 1, A and C), and FLO-1 cells, with high endogenous AXL protein level, to stably knockdown AXL expression (Figure 1E). Western blot analysis data confirmed the expression of exogenous and endogenous AXL proteins as indicated by the full-length protein (138 kDa) and the short fragment (55 kDa; Figure 1, A, C, and E). The cell viability assay results demonstrated that the reconstitution of AXL expression in OE33 and OE19 cells significantly enhanced cell survival relative to control cells in response to treatment with increasing concentrations of TRAIL for 5 hours (P < .01; Figure 1, B and D). Indeed, the TRAIL half maximal inhibitory concentration (IC50) in OE33 cells stably overexpressing AXL (135.6 ng/ml) was significantly higher than control cells (64.4 ng/ml; Figure 1B). Similarly, the TRAIL half maximal inhibitory concentration (IC50) in OE19 cells transiently overexpressing AXL (178.3 ng/ml) was also significantly higher than control cells (83 ng/ml; Figure 1D). To corroborate the role of AXL in regulating cell survival, we subjected FLO-1 cells stably expressing control shRNA or AXL shRNA to the cell viability assay after treatment with increasing concentrations of TRAIL for 5 hours. The data unequivocally indicated that knockdown of endogenous AXL significantly enhanced the sensitivity of cells to TRAIL (P < .05; Figure 1F). In fact, the TRAIL IC50 in FLO-1/AXL shRNA cells (58.8 ng/ml) was considerably lower than FLO-1/control shRNA cells (77.5 ng/ml; Figure 1F). Of note, we demonstrated that the pro-survival function of AXL was dependent on its kinase activity. The cell viability assay data indicated that inhibition of AXL with the pharmacological compound BMS-777607 significantly sensitized FLO-1 cells to TRAIL (P < .001; Figure W1).
Figure 1.
AXL expression enhances survival of EAC cells. (A) OE33 cells were stably transfected with AXL or pcDNA4 control plasmids and subjected to Western blot analysis of AXL protein. (B) OE33/pcDNA4 and OE33/AXL stable cells were treated with vehicle or with the indicated concentrations of recombinant TRAIL for 5 hours. Cell viability was evaluated by CellTiter-Glo Luminescent Cell Viability Assay. Cell survival of AXL-expressing cells was significantly higher than control cells in response to TRAIL. (C) OE19 cells were infected with control (10 MOI, multiplicity of infection) or AXL (10 MOI) adenoviruses and subjected to Western blot analysis of AXL protein. (D) Cell viability of OE19 cells transiently expressing control empty vector or AXL in response to TRAIL was evaluated as in B. Survival of cells expressing AXL was significantly higher than control cells in response to TRAIL. (E) FLO-1 cells were transduced with lentivirus particles expressing AXL shRNA or control shRNA and subjected to Western blot analysis of AXL protein. (F) Cell viability of FLO-1 cells stably expressing AXL shRNA or control shRNA in response to TRAIL was assessed as in B. Knockdown of endogenous AXL in FLO-1 cells significantly decreased cell survival in response to TRAIL. Results are representative of at least three experiments and shown as means ± SD. *P < .05, **P < .01.
AXL Attenuates TRAIL-Induced Apoptosis and Activation of Caspases
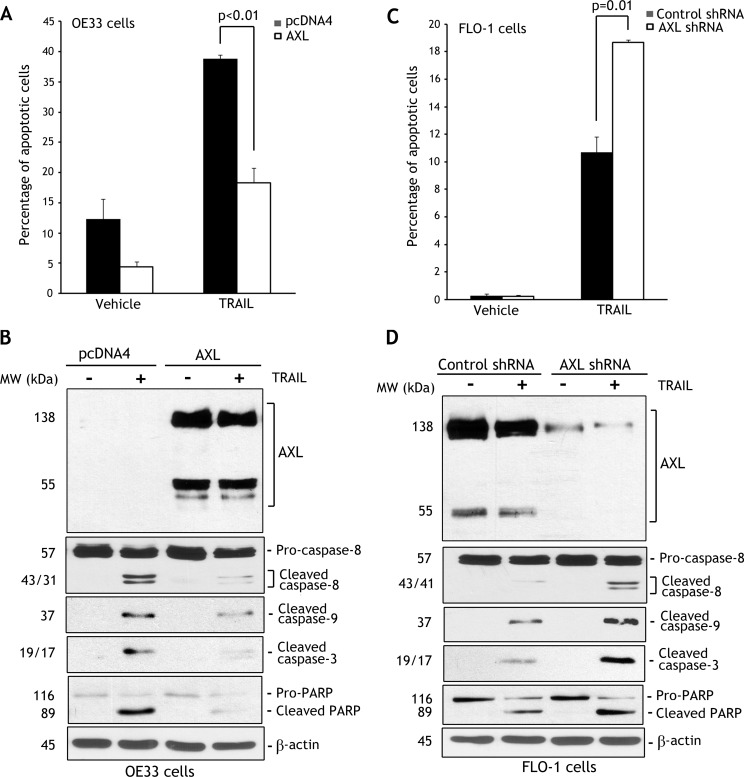
To assess the role of AXL in regulating TRAIL-induced apoptosis, we employed OE33 and FLO-1 cell models. On the basis of the survival data (Figure 1, B and F), we selected the TRAIL concentration 40 ng/ml to induce apoptosis in cells. The Annexin V/PI staining and FACS analysis data clearly demonstrated that the reconstitution of AXL expression in OE33 cells suppressed apoptosis events by 53% relative to control in response to TRAIL (P < .01; Figure 2A). In accordance with this finding, Western blot analysis showed considerably higher protein levels of cleaved caspase-8, caspase-9, and caspase-3 and cleaved PARP in control cells than AXL-expressing cells in response to TRAIL (Figure 2B). On the contrary, the data indicated that knockdown of highly expressed endogenous AXL in FLO-1 cells significantly increased apoptosis by 74% relative to control (P = .01) in response to TRAIL (Figure 2C). In line with this finding, Western blot analysis indicated that knockdown of endogenous AXL substantially increased protein levels of cleaved caspase-8, caspase-9, and caspase-3 and cleaved PARP relative to control in response to TRAIL (Figure 2D). To confirm that AXL regulates TRAIL-induced activation of the intrinsic mitochondrial apoptosis pathway, we evaluated cleavage of Bid in FLO-1 cells. Western blot data indicated that knockdown of endogenous AXL significantly enhanced cleavage of Bid in response to TRAIL (Figure W2).
Figure 2.
AXL expression attenuates TRAIL-induced apoptosis. (A) OE33/pcDNA4 and OE33/AXL stable cells were treated with vehicle or TRAIL (40 ng/ml) for 24 hours. Apoptosis was determined by Annexin V/PI staining and FACS analysis. Quantitative data indicated significantly less apoptosis in AXL-expressing cells than control cells (P < .01) in response to TRAIL. (B) Immunoblot analysis of AXL, caspase-8, cleaved caspase-9 and caspase-3, and PARP proteins in OE33/pcDNA4 and OE33/AXL cells after treatment with vehicle or TRAIL as described in A. (C) Apoptosis in FLO-1/control shRNA and FLO-1/AXL shRNA cells after treatment with TRAIL (40 ng/ml) for 24 hours was assessed as in A. Quantitative data showed that knocking down endogenous AXL induced significantly more apoptosis than control cells (P = .01) in response to TRAIL. (D) Western blot analysis of AXL, caspase-8, cleaved caspase-9, cleaved caspase-3, and PARP proteins in FLO-1/control shRNA and FLO-1/AXL shRNA cells after treatment with TRAIL as in C. Gel loading was normalized for equal β-actin. Results are representative of at least three experiments.
AXL Does Not Downregulate Differentially Expressed DR5 and DR4 Death Receptors in EAC Cells
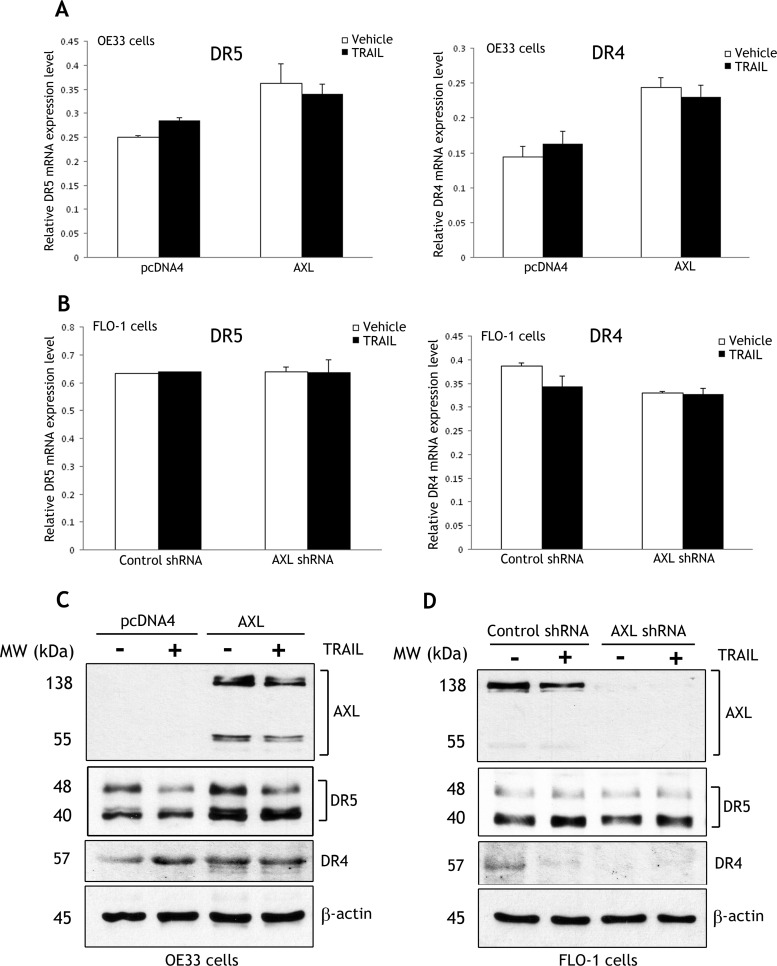
To investigate if AXL mediates TRAIL resistance through downregulation of the expression of death receptors, we evaluated mRNA and protein levels of DR5 and DR4 receptors in OE33 and FLO-1 cell models. Real-time RT-PCR data showed that the reconstitution of AXL expression and treatment with vehicle or TRAIL induced approximately a 40% increase in DR5 and DR4 mRNA levels in OE33 cells (Figure 3A). In addition, the results indicated that knocking down endogenous AXL and treatment with vehicle or TRAIL had no significant effect on DR5 and induced less than a 10% decrease in DR4 mRNA expression in FLO-1 cells (Figure 3B). Interestingly, the data revealed that DR5 and DR4 were consistently differentially expressed in both OE33 and FLO-1 cells, as mRNA expression of DR5 was approximately two-fold higher than DR4 relative to HPRT1 (Figure 3, A and B). Western blot data showed that the reconstitution of AXL expression in OE33 cells induced a slight increase in DR5 and DR4 protein levels relative to control cells after treatment with vehicle or TRAIL (Figure 3C). The data also indicated that knockdown of endogenous AXL in FLO-1 cells after treatment with vehicle or TRAIL had no significant effect on DR5 protein expression but slightly decreased the DR4 protein level relative to control (Figure 3D). Together, the data clearly indicated that AXL had no negative regulatory effect on mRNA and protein expression of DR5 and DR4 receptors in EAC cells. Moreover, we performed immunofluorescence assays to investigate the role of AXL in regulating DR5 and DR4 localization on the cell surface. The data indicated that AXL expression has no significant effect on the expression and localization of these receptors on the cell surface in OE33 cells (Figure W3A). We also explored the role of AXL in regulating the decoy death receptor DcR1 protein expression in OE33 cells. Western blot data showed that AXL expression has no effect on DcR1 protein level (Figure W3B).
Figure 3.
DR5 and DR4 death receptors are differentially expressed but not downregulated by AXL in EAC cells. (A) OE33/pcDNA4 and OE33/AXL cells were treated with vehicle or TRAIL (40 ng/ml) for 5 hours. Real-time RT-PCR analysis indicated that AXL expression alone or in combination with TRAIL induced a 40% increase in mRNA expression of DR5 and DR4 receptors. However, the mRNA expression of DR5 was approximately two-fold higher than DR4 relative to HPRT1 in OE33 cells. (B) FLO-1/control shRNA and FLO-1/AXL shRNA cells were treated as in A. Knocking down AXL alone or in combination with TRAIL had no significant effect on DR5 but induced a less than 10% decrease in DR4 mRNA expression. Similar to OE33 cells, FLO-1 cells expressed approximately two-fold higher mRNA level of DR5 than DR4 relative to HPRT1. (C) OE33/pcDNA4 and OE33/AXL cells were treated as in A. Western blot analysis indicated that AXL expression alone or in combination with TRAIL slightly increased protein levels of DR5 and DR4 in OE33 cells. (D) FLO-1/control shRNA and FLO-1/AXL shRNA cells were treated as in A. Western blot analysis showed that knocking down AXL alone or in combination with TRAIL had no significant effect on DR5 protein expression but slightly decreased DR4 protein level. Gel loading was normalized for equal β-actin.
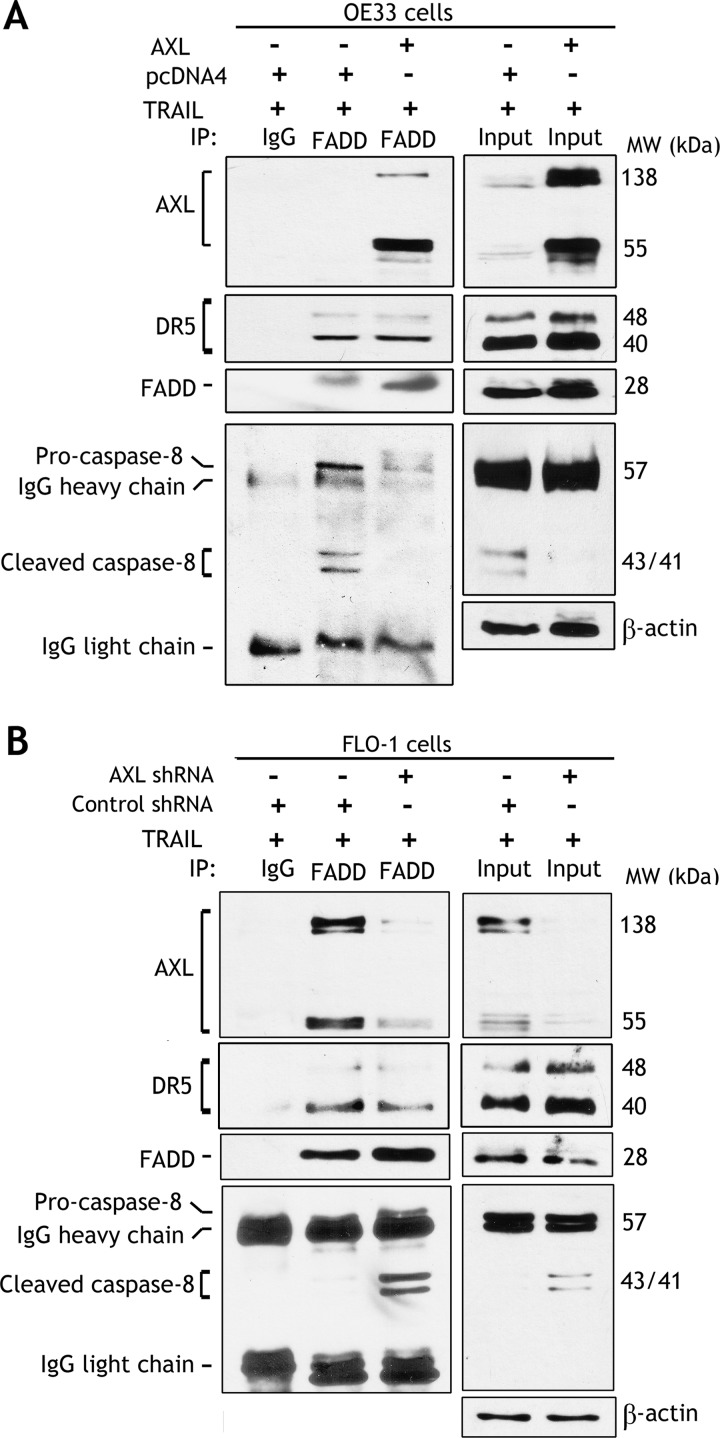
AXL Associates with DISC and Prevents TRAIL-Induced Caspase-8 Interaction
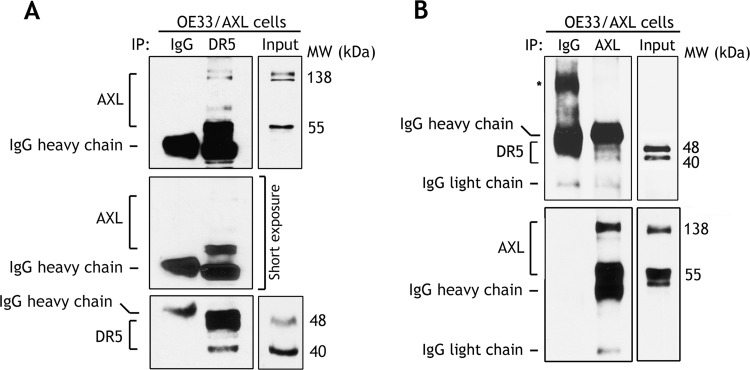
On the basis of our finding that DR5 mRNA expression was considerably higher than DR4 in EAC cells (Figure 3), we decided to focus on DR5 death receptor in our study to investigate the molecular mechanism by which AXL promotes TRAIL resistance. After excluding down regulation of the expression of death receptors (Figure 3) as a mechanism of AXL-mediated TRAIL resistance, we next postulated that AXL regulates DISC, thereby blocking TRAIL-induced apoptosis signaling in EAC cells. Indeed, reciprocal immunoprecipitation and Western blot data indicated that AXL associated with DR5, a component of DISC, independent of TRAIL treatment in OE33 cells (Figure 4, A and B). Of note, the short fragment of AXL (55 kDa) appeared to interact relatively more efficiently than the full-length AXL (138 kDa) with DR5 (Figure 4A). Furthermore, we investigated whether AXL/DR5 protein association could interfere with the recruitment of FADD in response to TRAIL in OE33 cells. Western blot analysis of FADD immunoprecipitates clearly showed that AXL/DR5 association had no effect on FADD interaction with DR5 in response to TRAIL (Figure 5A). This finding suggested that AXL-mediated TRAIL resistance mechanism could be downstream of the DR5/FADD protein complex. Accordingly, we next examined if AXL/DR5/FADD protein interaction could block the recruitment of the pro-form of caspase-8 in response to TRAIL. This step is critical for activation of caspase-8 and initiation of TRAIL-induced extrinsic apoptotic signaling cascade. The immunoprecipitation and Western blot data clearly indicated significantly less interaction of pro-caspase-8 with the DR5/FADD complex in response to TRAIL in AXL-expressing OE33 cells than control cells (Figure 5A). Interestingly, in addition to pro-caspase-8, cleaved forms of caspase-8 also interacted with the protein complex (Figure 5A). We validated these data in a FLO-1 cell model by knocking down endogenous AXL and examining the formation of DISC in response to TRAIL. Western blot analysis of FADD immunoprecipitates showed that knocking down AXL had no effect on DR5/FADD association but significantly increased the recruitment of pro-caspase-8 by FADD in response to TRAIL (Figure 5B). Similar to OE33 cells, in addition to pro-caspase-8, cleaved forms of caspase-8 also associated with the DISC protein complex in FLO-1 cells (Figure 5B). Together, these data demonstrated a novel mechanism of TRAIL resistance by which AXL regulates the DISC through interaction with DR5 and FADD, thereby blocking the recruitment and activation of caspase-8, a key molecular event in TRAIL-induced apoptosis.
Figure 4.
AXL associates with DR5 in a protein complex. (A) Western blot analysis of immunoprecipitated proteins with control IgG or DR5 antibodies in OE33 cells stably expressing AXL without treatment with TRAIL. (B) Western blot analysis of control IgG or AXL immunoprecipitates in OE33 cells stably expressing AXL without TRAIL treatment. The reciprocal co-immunoprecipitation results demonstrated protein association of AXL with DR5 death receptor independent of TRAIL-induced apoptosis; *, non-specific protein band.
Figure 5.
AXL modulates caspase-8/FADD protein interaction in response to TRAIL. Western blot analysis of immunoprecipitated proteins with control IgG or FADD antibodies in OE33 cells stably expressing pcDNA4 or AXL (A) and FLO-1 cells stably expressing control shRNA or AXL shRNA (B), after treatment with TRAIL (40 ng/ml) for 5 hours. The data indicated that the reconstitution of AXL expression in OE33 cells had no effect on DR5/FADD interaction but attenuated the recruitment of pro-caspase-8 by FADD in response to TRAIL. Conversely, knockdown of endogenous AXL in FLO-1 cells significantly promoted the interaction between FADD and caspase-8 without affecting DR5/FADD association in response to TRAIL.
An alternative mechanism of TRAIL resistance could involve regulation of FLIP, which resembles caspase-8 but lacks the enzymatic activity and competes with caspase-8 to bind to FADD, hence inhibiting TRAIL-induced apoptosis. Western blot analysis data showed that the reconstitution of AXL expression in OE33 cells and treatment with vehicle or TRAIL had no significant effect on FLIP protein levels (Figure W4A). In addition, we investigated whether AXL could regulate the interaction of FLIP with FADD in response to TRAIL in OE33 cells. The immunoprecipitation and Western blot data showed that AXL did not enhance the recruitment of FLIP by FADD but rather reduced FLIP/FADD association in response to TRAIL (Figure W4B). These findings clearly demonstrated that AXL-mediated TRAIL resistance did not involve regulation of FLIP.
Discussion
The therapeutic strategies for advanced EAC involve surgery, platinum-based therapy, and radiotherapy, which are ineffective and frequently associated with poor outcome [5]. The fact that the majority of EAC are deficient or mutant in p53, which is critical for mediating the apoptotic response to DNA-damaging therapies [27,28], underscores the need for alternative therapeutic approaches. Better knowledge of the molecular basis of cell growth and apoptosis of cancer cells is paramount for the development of novel targeted anticancer therapies. Accordingly, direct induction of death receptor-mediated apoptosis by recombinant proteins, such as TRAIL or DR4/DR5 agonistic monoclonal antibodies, has been evaluated in clinical trials. Overall, the clinical data indicated promising antitumor growth activities with mild side effects (reviewed by Kruyt [29]). However, it has become evident that intrinsic and acquired resistance to TRAIL remains a major clinical challenge [15]. Elucidation of the molecular mechanisms that control TRAIL resistance is critical to predicting response to therapy and to developing new therapeutic approaches that can overcome resistance. On the basis of the reported findings of frequent overexpression of AXL in EAC and its association with the multistep esophageal carcinogenesis [22,23] and the implication of AXL in promoting cell survival in various malignancies [17,18,23], we examined the role of AXL in TRAIL resistance in EAC.
Our findings demonstrated that AXL enhanced cell survival and inhibited apoptosis in response to TRAIL, confirming an important role of AXL in TRAIL resistance in EAC. Of note, we showed that the pro-survival function of AXL was dependent on its kinase activity. To examine the effects of AXL on the molecular signaling of apoptosis, we clearly showed that the reconstitution of AXL expression blocked TRAIL-induced activation of caspase-8, caspase-9, and caspase-3 and cleavage of PARP. Conversely, knockdown of endogenous AXL considerably sensitized cells to TRAIL as indicated by increased activation of caspase-8, caspase-9, and caspase-3 and cleavage of PARP. Upon binding to DR4 or DR5 death receptors, TRAIL induces the formation of DISC through interaction with FADD and the recruitment and activation of caspase-8, thereby inducing the extrinsic apoptosis pathway [6,7]. The activated caspase-8 can directly cleave and activate caspase-3 leading to apoptosis in type I cells. Additionally, it can induce the intrinsic mitochondrial apoptosis pathway through cleavage of Bid and activation of caspase-9 and caspase-3 in type II cells [8,9]. In fact, we confirmed that AXL regulated TRAIL-induced activation of the intrinsic apoptosis pathway in FLO-1 cells. We demonstrated that knockdown of endogenous AXL significantly enhanced cleavage of Bid in response to TRAIL. Our data indicated that OE33 and FLO-1 cell models used in this study correspond to type II cells.
In an attempt to identify the molecular mechanism by which AXL mediates TRAIL resistance, we investigated whether AXL downregulates the expression of death receptors in EAC cells. Loss of expression or elevated endocytic down regulation of death receptors has been shown to induce TRAIL resistance in cancer cells [30,31]. Our data indicated that the AXL-mediated TRAIL resistance mechanism does not involve down regulation of death receptors. Therefore, we postulated that AXL associates with DR5 and blocks its activation in response to TRAIL in EAC cells. Indeed, our results demonstrated AXL/DR5 protein interaction independent of TRAIL treatment. Interestingly, the full-length AXL (138 kDa) associated significantly less than the short form of AXL (55 kDa) with the DR5 receptor, although the protein expression level of AXL short form was considerably lower than the full-length. Together with the full-length, the AXL short form was consistently expressed endogenously and ectopically in EAC cells. Moreover, it is likely that the AXL short form was generated by proteolytic degradation of the full-length protein through cleavage and shedding of the extracellular domain. Our data confirmed that the AXL short form protein consists of the cytosolic domain as it was detected by immunoblot analysis using an antibody directed against the intracellular portion of AXL. Consistent with our finding, a previous study indicated that AXL is proteolytically cleaved at the cell surface immediately NH2-terminal to the transmembrane domain producing a soluble extracellular domain (80 kDa) and an intracellular membrane-bound kinase domain (55 kDa) [32]. The increased affinity of AXL short form to bind DR5 relative to the full-length suggests that the stoichiometry and composition of this protein interaction could play a major role in TRAIL resistance. Additional studies will be required to test this hypothesis. Although we focused our investigation on DR5, we also checked if AXL could similarly interact with DR4. The immunoprecipitation and Western blot data showed that AXL associated with DR4 receptor (Figure W5).
We next investigated if AXL could interfere with the interaction of DR5 with the adaptor protein FADD in response to TRAIL. Our results showed that AXL association with DR5 had no effect on TRAIL-induced interaction of FADD with DR5, suggesting that DR5 ligand binding and DR5 clustering were not hindered by AXL. On the contrary, previous reports indicated that Met, a growth factor receptor tyrosine kinase (RTK), directly binds and sequesters the death receptor Fas, preventing its self-aggregation, Fas ligation, and apoptosis in hepatocytes and endothelial cells [33,34]. On the basis of our findings, we hypothesized that AXL could regulate DISC formation through blocking the recruitment of pro-caspase-8 by FADD, hence preventing activation of caspase-8 and apoptosis in response to TRAIL. Indeed, we confirmed that AXL association with DR5 and FADD significantly reduced the recruitment of pro-caspase-8 to the DISC. Conversely, we demonstrated that knocking down endogenous AXL enhanced the recruitment of pro-caspase-8 to the DISC and increased TRAIL-induced apoptosis. Up-regulation of the endogenous FLIP is reported to function by competing with pro-caspase-8 for binding to DISC, thereby inhibiting apoptosis in response to TRAIL [14].We demonstrated that AXL did not significantly enhance FLIP expression but rather decreased FLIP/FADD interaction in EAC cells, indicating that AXL-dependent attenuation of the recruitment of pro-caspase-8 to the DISC was independent of FLIP.
The fact that AXL/DR5 association was independent of TRAIL suggested that this protein interaction could potentiate AXL-expressing cells for resistance against TRAIL. Although AXL had no effect on the binding of activated DR5 to FADD through death domain, it significantly reduced caspase-8/FADD protein association through death effector domain. It is likely that the presence of AXL in the DISC could affect the protein conformation of the complex such that the death effector domain of FADD is not accessible for pro-caspase-8. Additional studies will be required to fully characterize the exact mechanism by which AXL regulates these protein interactions. This is the first instance, to the best of our knowledge, where an RTK association with DR5 death receptor is reported to mediate TRAIL resistance in cancer cells. In conclusion, our findings demonstrate that AXL promotes TRAIL resistance through binding to DR5 and regulation of the DISC in EAC cells. Together, our data provide strong evidence that AXL could be exploited as a therapeutic target to sensitize tumors to recombinant TRAIL in EAC.
Supplementary Material
Supplemental Materials and Methods
Immunofluorescence
Cells (5 x 103 per chamber) were seeded on eight-chamber culture slides (BD Falcon, Franklin Lakes, NJ) 1 day before the experiment. Cells were fixed with 4% formaldehyde in PBS for 20 minutes at room temperature followed by permeabilization with 0.1% sodium citrate plus 0.1% Triton X-100. Immunofluorescence was performed with both DR5 (1:500) and DR4 (1:500) antibodies. The cells were then washed with cold PBS three times for 3 minutes each and incubated with Alexa 488-labeled anti-rabbit antibody (1:800; Invitrogen) at room temperature for 1 hour. The cells were counterstained with 4′,6-diamidino-2-phenylindole and examined by fluorescence microscopy (Olympus America Inc, Center Valley, PA).
Abbreviations
- EAC
esophageal adenocarcinoma
- TRAIL
tumor necrosis factor-related apoptosis-inducing ligand
- FLIP
FLICE-inhibitory protein
- DISC
death-inducing signaling complex
- FADD
Fas-associated death domain protein
Footnotes
This study was supported by grants from Vanderbilt SPORE in Gastrointestinal Cancer (P50 CA95103) and the Vanderbilt Digestive Disease Research Center (DK058404). The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of Vanderbilt University. No potential conflicts of interest were disclosed.
This article refers to supplementary materials, which are designated by Figures W1 to W5 and are available online at www.neoplasia.com.
References
- 1.Parkin DM. International variation. Oncogene. 2004;23:6329–6340. doi: 10.1038/sj.onc.1207726. [DOI] [PubMed] [Google Scholar]
- 2.el-Serag HB. The epidemic of esophageal adenocarcinoma. Gastroenterol Clin North Am. 2002;31:421–440. viii. doi: 10.1016/s0889-8553(02)00016-x. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, Feuer EJ, Thun MJ. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 4.Tew WP, Kelsen DP, Ilson DH. Targeted therapies for esophageal cancer. Oncologist. 2005;10:590–601. doi: 10.1634/theoncologist.10-8-590. [DOI] [PubMed] [Google Scholar]
- 5.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241–2252. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 6.Wu GS, Burns TF, McDonald ER, III, Jiang W, Meng R, Krantz ID, Kao G, Gan DD, Zhou JY, Muschel R, et al. KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor gene. Nat Genet. 1997;17:141–143. doi: 10.1038/ng1097-141. [DOI] [PubMed] [Google Scholar]
- 7.Muzio M, Stockwell BR, Stennicke HR, Salvesen GS, Dixit VM. An induced proximity model for caspase-8 activation. J Biol Chem. 1998;273:2926–2930. doi: 10.1074/jbc.273.5.2926. [DOI] [PubMed] [Google Scholar]
- 8.Scaffidi C, Schmitz I, Zha J, Korsmeyer SJ, Krammer PH, Peter ME. Differential modulation of apoptosis sensitivity in CD95 type I and type II cells. J Biol Chem. 1999;274:22532–22538. doi: 10.1074/jbc.274.32.22532. [DOI] [PubMed] [Google Scholar]
- 9.Yamada H, Tada-Oikawa S, Uchida A, Kawanishi S. TRAIL causes cleavage of bid by caspase-8 and loss of mitochondrial membrane potential resulting in apoptosis in BJAB cells. Biochem Biophys Res Commun. 1999;265:130–133. doi: 10.1006/bbrc.1999.1641. [DOI] [PubMed] [Google Scholar]
- 10.Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5:157–163. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 11.Almasan A, Ashkenazi A. Apo2L/TRAIL: apoptosis signaling, biology, and potential for cancer therapy. Cytokine Growth Factor Rev. 2003;14:337–348. doi: 10.1016/s1359-6101(03)00029-7. [DOI] [PubMed] [Google Scholar]
- 12.Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104:155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pukac L, Kanakaraj P, Humphreys R, Alderson R, Bloom M, Sung C, Riccobene T, Johnson R, Fiscella M, Mahoney A, et al. HGS-ETR1, a fully human TRAIL-receptor 1 monoclonal antibody, induces cell death in multiple tumour types in vitro and in vivo. Br J Cancer. 2005;92:1430–1441. doi: 10.1038/sj.bjc.6602487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tschopp J, Irmler M, Thome M. Inhibition of Fas death signals by FLIPs. Curr Opin Immunol. 1998;10:552–558. doi: 10.1016/s0952-7915(98)80223-9. [DOI] [PubMed] [Google Scholar]
- 15.Shankar S, Srivastava RK. Enhancement of therapeutic potential of TRAIL by cancer chemotherapy and irradiation: mechanisms and clinical implications. Drug Resist Updat. 2004;7:139–156. doi: 10.1016/j.drup.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 16.O'Bryan JP, Frye RA, Cogswell PC, Neubauer A, Kitch B, Prokop C, Espinosa R, III, Le Beau MM, Earp HS, Liu ET. Axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol Cell Biol. 1991;11:5016–5031. doi: 10.1128/mcb.11.10.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Linger RM, Keating AK, Earp HS, Graham DK. TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv Cancer Res. 2008;100:35–83. doi: 10.1016/S0065-230X(08)00002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hafizi S, Dahlback B. Signalling and functional diversity within the Axl subfamily of receptor tyrosine kinases. Cytokine Growth Factor Rev. 2006;17:295–304. doi: 10.1016/j.cytogfr.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Sainaghi PP, Castello L, Bergamasco L, Galletti M, Bellosta P, Avanzi GC. Gas6 induces proliferation in prostate carcinoma cell lines expressing the Axl receptor. J Cell Physiol. 2005;204:36–44. doi: 10.1002/jcp.20265. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Z, Lee JC, Lin L, Olivas V, Au V, LaFramboise T, Abdel-Rahman M, Wang X, Levine AD, Rho JK, et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat Genet. 2012;44:852–860. doi: 10.1038/ng.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Linger RM, Cohen RA, Cummings CT, Sather S, Migdall-Wilson J, Middleton DH, Lu X, Baron AE, Franklin WA, Merrick DT, et al. Mer or Axl receptor tyrosine kinase inhibition promotes apoptosis, blocks growth and enhances chemosensitivity of human non-small cell lung cancer. Oncogene. 2012;31:4171–4181. doi: 10.1038/onc.2012.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hector A, Montgomery EA, Karikari C, Canto M, Dunbar KB, Wang JS, Feldmann G, Hong SM, Haffner MC, Meeker AK, et al. The Axl receptor tyrosine kinase is an adverse prognostic factor and a therapeutic target in esophageal adenocarcinoma. Cancer Biol Ther. 2010;10:1009–1018. doi: 10.4161/cbt.10.10.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hong J, Peng D, Chen Z, Sehdev V, Belkhiri A. ABL regulation by AXL promotes cisplatin resistance in esophageal cancer. Cancer Res. 2013;73:331–340. doi: 10.1158/0008-5472.CAN-12-3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boonstra JJ, van Marion R, Beer DG, Lin L, Chaves P, Ribeiro C, Pereira AD, Roque L, Darnton SJ, Altorki NK, et al. Verification and unmasking of widely used human esophageal adenocarcinoma cell lines. J Natl Cancer Inst. 2010;102:271–274. doi: 10.1093/jnci/djp499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belkhiri A, Zaika A, Pidkovka N, Knuutila S, Moskaluk C, El-Rifai W. Darpp-32: a novel antiapoptotic gene in upper gastrointestinal carcinomas. Cancer Res. 2005;65:6583–6592. doi: 10.1158/0008-5472.CAN-05-1433. [DOI] [PubMed] [Google Scholar]
- 26.El-Rifai W, Moskaluk CA, Abdrabbo MK, Harper J, Yoshida C, Riggins GJ, Frierson HF, Jr, Powell SM. Gastric cancers overexpress S100A calcium-binding proteins. Cancer Res. 2002;62:6823–6826. [PubMed] [Google Scholar]
- 27.Schneider PM, Stoeltzing O, Roth JA, Hoelscher AH, Wegerer S, Mizumoto S, Becker K, Dittler HJ, Fink U, Siewert JR. P53 mutational status improves estimation of prognosis in patients with curatively resected adenocarcinoma in Barrett's esophagus. Clin Cancer Res. 2000;6:3153–3158. [PubMed] [Google Scholar]
- 28.Hamelin R, Flejou JF, Muzeau F, Potet F, Laurent-Puig P, Fekete F, Thomas G. TP53 gene mutations and p53 protein immunoreactivity in malignant and premalignant Barrett's esophagus. Gastroenterology. 1994;107:1012–1018. doi: 10.1016/0016-5085(94)90225-9. [DOI] [PubMed] [Google Scholar]
- 29.Kruyt FA. TRAIL and cancer therapy. Cancer Lett. 2008;263:14–25. doi: 10.1016/j.canlet.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Cheng J, Hylander BL, Baer MR, Chen X, Repasky EA. Multiple mechanisms underlie resistance of leukemia cells to Apo2 ligand/TRAIL. Mol Cancer Ther. 2006;5:1844–1853. doi: 10.1158/1535-7163.MCT-06-0050. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Zhang B. TRAIL resistance of breast cancer cells is associated with constitutive endocytosis of death receptors 4 and 5. Mol Cancer Res. 2008;6:1861–1871. doi: 10.1158/1541-7786.MCR-08-0313. [DOI] [PubMed] [Google Scholar]
- 32.O'Bryan JP, Fridell YW, Koski R, Varnum B, Liu ET. The transforming receptor tyrosine kinase, Axl, is post-translationally regulated by proteolytic cleavage. J Biol Chem. 1995;270:551–557. doi: 10.1074/jbc.270.2.551. [DOI] [PubMed] [Google Scholar]
- 33.Wang X, DeFrances MC, Dai Y, Pediaditakis P, Johnson C, Bell A, Michalopoulos GK, Zarnegar R. A mechanism of cell survival: sequestration of Fas by the HGF receptor Met. Mol Cell. 2002;9:411–421. doi: 10.1016/s1097-2765(02)00439-2. [DOI] [PubMed] [Google Scholar]
- 34.Smyth LA, Brady HJ. cMet and Fas receptor interaction inhibits death-inducing signaling complex formation in endothelial cells. Hypertension. 2005;46:100–106. doi: 10.1161/01.HYP.0000167991.82153.16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.