Abstract
Antitumor functions of the host immune system are frequently compromised in patients with malignancies. In the current study, we evaluated the relationship between expression ratio of mRNAs for the antiapoptotic protein Bcl-2 and the proapoptotic protein Bax (the Bcl-2/Bax ratio) in peripheral blood mononuclear cells and clinical outcomes in patients with head and neck carcinomas. The overall survival (OS) time of patients with Bcl-2/Bax ratios ≥ 1.2 tended to be longer than that of patients with Bcl-2/Bax ratios < 1.2 but not significantly so (P = .084, n = 61). Disease-free survival (DFS) of patients with Bcl-2/Bax ratios ≥ 1.2 was statistically significantly longer than that of patients with Bcl-2/Bax ratios < 1.2 (P = .001, n = 76). All of the patients whose Bcl-2/Bax ratio is ≥ 2.0 were alive after 36 months and survived without any evidence of disease for 24 months (Bcl-2/Bax ≥ 2.0 versus Bcl-2/Bax < 2.0; P = .035, n = 61 in OS, P < .001, n = 76 in DFS, respectively). In 56 patients who received immunochemoradiotherapy using UFT and OK-432 in combination with radiotherapy, a statistically significant relationship between the Bcl-2/Bax ratio and the therapeutic effect estimated using Response Evaluation Criteria in Solid Tumors was observed, as well as a relation with interferon-γ (IFN-γ) induction in response to the therapy [P = .002 in complete response versus partial response + stable disease; P = .046 in IFN-γ(+) versus IFN-γ(-)]. In addition, there were significant correlations of the Bcl-2/Bax ratio with both the absolute number of CD4+ T cells and the rate of CD4+ T cell and natural killer cell activity. These findings strongly suggest that the balance of expression of Bcl-2 and Bax genes in circulating immune cells has a high prognostic value in head and neck cancer patients.
Introduction
Numerous investigators have reported that the decreased function of the immune system might be closely involved in the growth and metastasis of head and neck carcinoma (HNC) cells. Although a satisfactory immune status might be critical in the success of cancer treatments, such as surgery, radiotherapy, and chemotherapy, as well as immunotherapy, and in obtaining a favorable clinical outcome, antitumor functions of the host immune system are frequently compromised in patients with malignancies including HNC. It has been reported that HNC cells might escape from the immune surveillance by evading immune cell recognition and by inhibiting directly host immune function. Mechanisms of immune evasion by HNC cells include modulation of tumor antigen expression and downregulating expression of surface major histocompatibility complex class I molecules. In addition, HNC cells can directly inhibit the antitumor host responses through production of immune-suppressive soluble factors, such as transforming growth factor-β, prostaglandins, and Fas ligand, and through induction of immune inhibitory cells including regulatory T cells and myeloid-derived suppressor cells in the tumor micro-environment [1–4]. In vitro experiments involving co-incubation of activated T lymphocytes with tumor cells have shown that both receptor-mediated and mitochondrial pathways mediate tumor-induced apoptosis of T cells [5]. Kim et al. demonstrated that expression ratio of Bax and Bcl-2 proteins (Bax/Bcl-2 ratio), which was measured by quantitative flow cytometry, was elevated in circulating CD8+ T cells from patients with head and neck squamous cell carcinomas (HNSCCs) and that patient-derived CD8+ T cells appeared to be sensitive to apoptosis as compared with those from healthy donors [6]. In addition, it has been reported that Bcl-2 family proteins such as antiapoptotic proteins Bcl-2 and Bcl-xL, as well as the apoptotic protein Bax, play significant roles in survival and proliferation of many types of lymphocytes including CD4+ T cells [7], CD19+ B cells [8], natural killer (NK) cells [9], and γδT cells [10] and also showed that Bcl-2 protein is an important factor for survival of naive T cells as well as for development of memory T cells [7,11]. In mammalian cells, mitochondria have a central role in apoptosis that is regulated by members of the Bcl-2 family [12]. Many investigators have demonstrated that Bcl-2 family proteins play a significant role for survival of cancer cells and that expression of these proteins in cancer cells may be diagnostic and prognostic biomarker(s) in patients with many types of malignancies. In patients with HNSCC, Homma et al. reported that Bcl-2 positivity is associated with better locoregional control [13], while Gallo et al. reported that Bcl-2 expression is closely associated with a high risk of recurrence and poor survival in stage I and II HNSCC patients [14]; however, Bcl-2 expression in cancer cells has not yet been established as a prognostic biomarker. Bcl-2 family proteins also play a significant role for survival and functions of immune cells [7–11], and as described above, the host immune system plays a critical role in the success of cancer therapy. Therefore, various immune parameters such as serum cytokine levels, subset analysis of circulating lymphocytes, and profiles of infiltrating immune cells in the tumor microenvironment have been assessed as biomarkers, whereas no immunologic parameter has yet entered routine clinical reporting [15–17].
In the current study, we analyzed the expressions of Bcl-2 and Bax mRNAs in circulating immune cells derived from patients with HNC and assessed their prognostic values. This is the first report demonstrating that evaluation of mRNAs for proapoptotic and antiapoptotic proteins in circulating immune cells can play a role in prognosis.
Materials and Methods
Patients and Treatment Protocol
This study was carried out in accordance with the standards of our Institutional Committee for the Protection of Human Subjects. Informed written consent was obtained from all patients, and the collection of the samples was approved by the Institutional Review Board. From 1988 to 2006, 79 HNC patients (44 males and 35 females) who were treated at the Second Department of Oral and Maxillofacial Surgery, Tokushima University Hospital were enrolled in this study. The median patient age was 60.9 years (range, 22–90 years). On the basis of the tumor-node-metastasis (TNM) system for the classification of malignant diseases, there were 8 T1 cancers, 41 T2 cancers, 19 T3 cancers, 8 T4 cancers, 44 N0 cancers, 23 N1 cancers, 11 N2 cancers, and 1 N3 cancer. All cancers were M0. Seventy-five patients were histopathologically diagnosed with squamous cell carcinomas, two with adenoid cystic carcinomas and two with mucoepidermoid carcinomas. Primary sites of the tumors included the tongue (n = 30), lower gingiva (n = 21), upper gingiva (n =10), hard palate(n = 5), floor of the mouth (n = 4), buccal mucosa (n = 4), oropharynx (n = 2), submandibular gland (n = 2), and lip (n = 1).
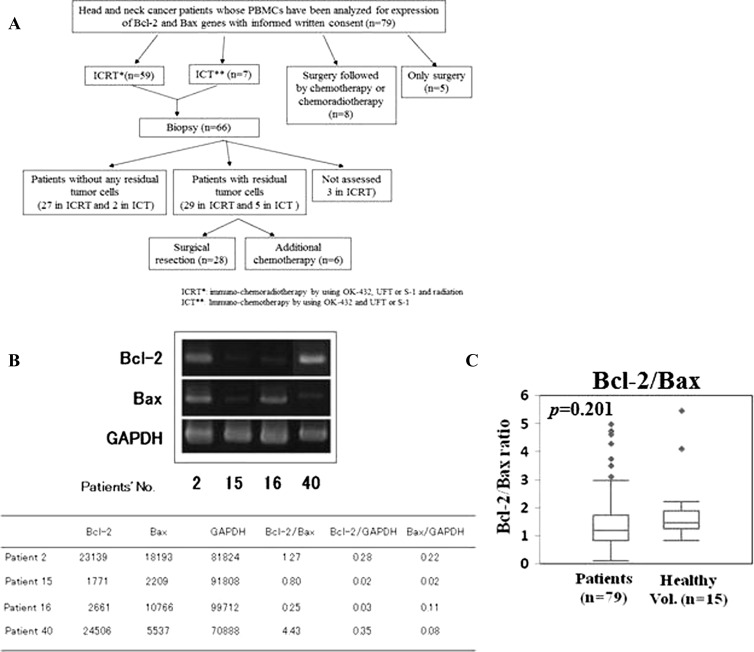
Of 79 patients, 59 received chemotherapy [UFT, an oral fluoropyrimidine formulation combining tegaful and uracil in a 1:4 ratio (Taiho Pharmaceutical Co, Tokyo, Japan), or S-1, an oral fluoropyrimidine formulation combining tegaful, gimeracil, and oteracil potassium in a 1:0.4:1 ratio (Taiho Pharmaceutical Co)] and immunotherapy (OK-432, Chugai Pharmaceutical Co, Ltd, Tokyo, Japan) in combination with radiation therapy (a total irradiation dose of 50 to 60 Gy) as a first-line treatment. OK-432, which is a penicillin-killed and lyophilized preparation of a low-virulence strain (Su) of Streptococcus pyogenes (group A), is being successfully used as an immunotherapeutic agent in many types of malignancies [18,19]. OK-432 was administered peritumorally or intradermally at a dose of 0.5 Klinische Einheit, i.e., 50 µg/week. When any severe adverse events were not shown, the dose was increased up to 5 Klinische Einheit/week, and OK-432 administration was continued until the 6th month after visible tumor(s) disappeared or surgically removed. Patients were monitored for general symptoms related to OK-432 therapy, such as increased fever and/or fatigue. The flowchart diagram describing the treatment of the patients is shown in Figure 1A. The tumor responses to the combination therapy were evaluated as complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) according to the Response Evaluation Criteria in Solid Tumors. Adverse events were evaluated by grading toxicity according to the National Cancer Institute Common Terminology Criteria for Adverse Events guidelines, version 3.0. Eight patients had surgery followed by chemotherapy or by chemoradiotherapy, and five patients received only surgery.
Figure 1.
(A) Flowchart diagram of the treatment of the patients. (B) Expression of Bcl-2 and Bax mRNAs in PBMCs derived from patients with head and neck cancer. Total RNAs were isolated from PBMCs from head and neck cancer patients and were assayed for expression of Bcl-2, Bax, and GAPDH mRNAs using semiquantitative RT-PCR analysis. Data are representative of at least three independent experiments. (C) Differences of Bcl-2/Bax ratio in head and neck cancer patients and healthy volunteers.
Sample Collection
Before any treatments, peripheral blood mononuclear cells (PBMCs) were prepared by the standard Ficoll-Hypaque gradient density centrifugation method [20]. Sera were also collected 5hours before and 18 hours after OK-432 administration and immediately frozen at -80°C until assayed for interferon-γ (IFN-γ). IFN-γ levels were analyzed by ELISA in SRL Inc (Tokyo, Japan), which is the company receiving the orders of cytokine assay in Tokyo, Japan. The ELISA system has a lower limit of sensitivity of 7.8 pg/ml for detecting human IFN-γ.
RNA Extraction and Semiquantitative Reverse Transcription-Polymerase Chain Reaction
Total RNAs were extracted from human PBMCs by a modified acid guanidinium thiocyanate-phenol-chloroform using ISOGEN RNA extracting mixture (Nippon Gene, Toyama, Japan) according to the manufacturer's recommendations. Expression of mRNAs for Bcl-2, Bax, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), a housekeeping gene that was used as an internal control, was detected by semiquantitative reverse transcription-polymerase chain reaction (RT-PCR). First, 1 µg of total RNA was reverse-transcribed using Moloney murine leukemia virus reverse transcriptase and random primers (Life Technologies Inc, Rockville, MD) in a volume of 20 µl at 42°C for 60 minutes by following the manufacturer's instructions. Next, 2 µl of the reverse-transcribed mixture was subjected to PCR [10 mM Tris-HCl (pH 8.3); 50 mM KCl; 1.5 mM MgCl2; 0.01% gelatin; dATP, dGTP, dTTP, and dCTP, each at 20 mM], 0.5 U of Taq polymerase (TaKaRa Bio Inc, Otsu, Japan), and 0.25 pmol of each primer in a total volume of 20 µl. This study used 5′-GTGGAGGAGCTCTTCAGGGA-3′ as an upstream primer and 5′-AGGCACCCAGGGTGAGCAA-3′ as a downstream primer for Bcl-2, which yielded a 304-bp fragment; 5′-GGCCCACCAGCTCTGAGCAGA-3′ as an upstream primer and 5′-GCCACGTGGGCGGTCCCAAAGT-3′ as a downstream primer for Bax, which yielded a 479-bp fragment [21]; and 5′-GAAATCCCAGCACCATCTTCCCAGG-3′ as an upstream primer and 5′-GTGGTGGACCTCATGGCCCACCATG-3′ as a downstream primer for GAPDH, which yielded a 781-bp fragment [22]. To amplify the fragments, 25, 28, or 30 PCR cycles were used, with each cycle consisting of 94°C for 60 seconds, 55°C for 90 seconds, and 72°C for 150 seconds, with an initial denaturation step of 94°C for 5 minutes and a final elongation step of 72°C for 5 minutes. PCR was carried out in a DNA Thermal Cycler (TaKaRa Bio Inc). The amplified PCR product was electrophoresed through 1.5% agarose gels containing 100 ng/ml ethidium bromide. After electrophoresis, gels were illuminated with UV light, viewed, and photographed (Polaroid type 667 film; Polaroid Corp, Cambridge, MA). Densitometric analysis for the RT-PCR band patterns was done using NIH Image 1.59 software (National Institutes of Health, Bethesda, MD). Expression values of Bcl-2 and Bax mRNAs were represented as ratios to the density of GAPDH. The identification of each amplified product was confirmed by automated DNA sequencing.
Assay for NK and Lymphokine-Activated Killer Cell Activities
The cytotoxic activities of human PBMCs were assayed against K-562, a markedly sensitive target for human NK cells, and Daudi, a sensitive target for human lymphokine-activated killer (LAK) cells but not destroyed by human NK cells, in a 51Cr release test. For measuring pre-LAK activity, PBMCs derived from patients were cultured with 1000 IU/ml recombinant interleukin-2 (Primmune Inc, Kobe, Japan) for 72 hours before the analysis as described by Aramaki et al. [23]. The 51Cr release was carried out as described previously [24]. For cell-mediated cytotoxicity assays, 4.0 x 105 effector cells were mixed in the wells of 96-well microtiter plates (Falcon; Becton Dickinson Labware, Lincoln Park, NJ) with 1.0 x 104 51Cr-labeled target cells (effector cell/target cell, 40:1) in a total volume of 200 µl of medium and incubated at 37°C for 4 hours. The percent specific 51Cr release was calculated according to the formula: [(E - S)/(M - S)] x 100, where E is the experimental 51Cr release, S is the spontaneous 51Cr release, and M is the maximum 51Cr release. Spontaneous 51Cr release was determined by incubation of target cells without effector cells and maximal 51Cr release was determined by adding 100 µl of NP-40 (Sigma, Deisenhofen, Germany) to labeled target cells. The percent specific 51Cr release was considered as the NK, LAK, or pre-LAK activity.
Flow Cytometric Analysis of Cell Surface Antigens
Cell surface staining was performed using the following mouse anti-human monoclonal antibodies. The antibodies to CD3 (clone SK7), CD4 (clone SK3), CD8 (clone SK1), CD16 (clone B73.1), and CD57 (clone HNK-1) were purchased from Pharmingen (San Diego, CA). Isotype-matched control mouse IgGs conjugated with fluorescein isothiocyanate were also purchased from Pharmingen. All monoclonal antibodies were directly labeled with either fluorescein isothiocyanate or phycoerythrin. The cells were resuspended in phosphate-buffered saline containing 0.1% sodium azide and 0.2% BSA and then were incubated for 30 minutes at 4°C with a saturating concentration of each monoclonal antibody according to the manufacturer's instructions. After the cells were washed twice, their fluorescence intensity was determined using a flow cytometer (EPICS XL-MCL; Beckman Coulter, Fullerton, CA).
Statistical Analysis
The relationships between Bcl-2/Bax, Bcl-2/GAPDH, and Bax/GAPDH ratios and sex, TNM classification, IFN-γ induction, prognosis, as well as therapeutic effect were performed by using Student's two-tailed t test. Correlations between Bcl-2/Bax, Bcl-2/GAPDH, and Bax/GAPDH ratios and immunologic parameters and age were quantified by Spearman's rank correlation coefficient and regression calculation. Kaplan-Meier curves and log-rank tests were used to assess differences in survival time between the groups. P < .05 were considered statistically significant.
Results
Expression of Bcl-2 and Bax mRNAs in PBMCs Derived from HNC Patients and from Healthy Donors
We examined the expression of Bcl-2 and Bax mRNAs by semi-quantitative RT-PCR analysis. Representative data of four patients are shown in Figure 1B. PBMCs from patient 2 highly expressed both Bcl-2 and Bax mRNAs; thus, the Bcl-2/Bax ratio was medium (1.27). Expression of both Bcl-2 and Bax mRNAs was only faint in those from patient 15, for whom the Bcl-2/Bax ratio was also medium (0.80). In patient 16, only slight expression of Bcl-2 mRNA and high expression of Bax mRNA were observed; hence, the Bcl-2/Bax ratio was low (0.25). In patient 40, Bcl-2 expression was high and Bax was low, so the Bcl-2/Bax ratio was high (4.43).
Next, we compared the Bcl-2/Bax ratio in the PBMCs derived from the head and neck cancer patients (n = 79) with that from healthy volunteers (n = 15). Although the Bcl-2/Bax ratios in the patients (1.475 ± 1.028; median value, 1.197) tended to be lower than those in healthy donors (1.864 ± 1.285; median value, 1.452), no statistically significant difference was shown (P = .201; Figure 1C).
No statistically significant relationship was observed between Bcl-2/Bax, Bcl-2/GAPDH, and Bax/GAPDH ratios and sex (P = .185, P = .350, and P = .985, respectively) or age (P = .866, P = .744, and P = .806, respectively). In addition, no statistical relationship between Bcl-2/Bax, Bcl-2/GAPDH, and Bax/GAPDH ratios and T stage [P = .233, P = .774, and P = .869, respectively in early state (T1 + T2) versus advanced state (T3 + T4)] or N stage [P = .324, P = .609, and P = .130, respectively in N(-) versus N(+)] was seen (Table W1).
Relationship between Expression of Bcl-2 and Bax mRNAs and Clinical Outcome
We assessed the prognostic significance of expression of Bcl-2 and Bax mRNAs in PBMCs derived from patients with HNC.
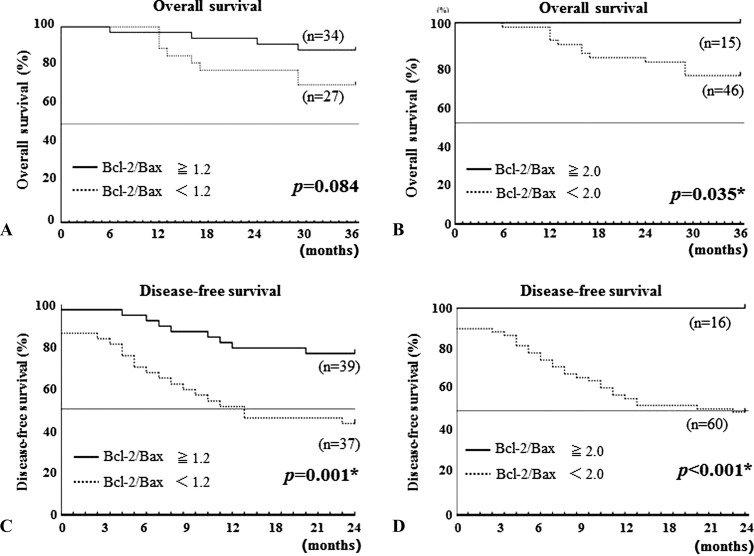
First, we evaluated the relationship between Bcl-2/Bax ratio and survival time. Overall survival (OS) was examined for the association with Bcl-2/Bax ratio in the 61 patients who could be followed up at least 36 months after the initial diagnosis (Figure 2). Because the median value of Bcl-2/Bax ratio in all 79 patients tested in the current study was 1.197, we have chosen 1.2 as a cutoff point. When the cutoff level was set to 1.2, patients who showed Bcl-2/Bax ratio ≥ 1.2 survived longer than the patients who showed a Bcl-2/Bax ratio < 1.2, but this difference between the two groups was not statistically significant (P = .0838; Figure 2A). When the cutoff level was set to 2.0, the survival time of the patients who showed high Bcl-2/Bax ratios was statistically longer than that of the patients with lower Bcl-2/Bax ratios (P = .035). All of the patients with Bcl-2/Bax ratio ≥ 2.0 have been alive over 36 months (Figure 2B). The relationship between Bcl-2/Bax ratio and disease-free survival (DFS) was also assessed in 76 patients whose long-term outcome (≥24 months after first treatment) was known. The DFS of the patients whose tumor(s) could not be completely cured by surgery, radiotherapy, chemotherapy, and/or immunotherapy was 0 month. When the cutoff level was set to 1.2, the DFS of patients with Bcl-2/Bax ratios ≥ 1.2 was significantly longer than that of patients with Bcl-2/Bax ratios < 1.2 (P = .001; Figure 2C). When the cutoff level was set to 2.0, the patients with Bcl-2/Bax ratios ≥ 2.0 survived much longer than the patients with Bcl-2/Bax ratios < 2.0 (P = .0005), and all patients with Bcl-2/Bax ratios ≥ 2.0 have been alive in disease-free condition for over 24 months (Figure 2D).
Figure 2.
Prognostic value of Bcl-2/Bax ratio. Kaplan-Meier estimates of (A and B) OS and (C and D) DFS. Differences (A and C) of Bcl-2/Bax ratio ≥ 1.2 (solid line) versus Bcl-2/Bax ratio < 1.2 (dotted line) and (B and D) of Bcl-2/Bax ratio ≥ 2.0 (solid line) versus Bcl-2/Bax ratio < 2.0 (dotted line) were calculated by log-rank test. Asterisk denotes P < .05, indicating statistical significance.
In the current cases, disease progression that is expressed with TNM classification was related to the duration of the survival (data not shown), similar to the results reported previously by numerous different investigators; however, disease progression and the Bcl-2/Bax ratio do not necessarily correlate with each other, as described above (Table W1). Namely, although the 15 patients with Bcl-2/Bax ratios ≥ 2 (Figure 2B) included six patients (40%) with advanced cancer, the 3-year survival was 100%. In DFS, 16 patients with Bcl-2/Bax ratios ≥ 2 (Figure 2D) included 7 (43.8%) with advanced cancer, while all of the 16 patients showed no evidence of disease (NED) at 24 months from completion of the first treatment (Figure 2 and data not shown). Furthermore, 76 patients who could be followed up for at least 24 months after the first treatment consisted of 39 patients (51.3%) with Bcl-2/Bax ratios ≥ 1.2 and 37 patients (48.7%) with Bcl-2/Bax ratios < 1.2 (Figure 2C). Of the 39 patients who showed Bcl-2/Bax ratios ≥ 1.2, 18 early-stage (T1 + T2; 46.2%) and 21 advanced-stage (T3 + T4) patients (53.8%) were included. The 37 patients with Bcl-2/Bax ratios < 1.2 consisted of 17 early-stage (45.9%) and 20 advanced-stage patients (54.1%). Although no significant difference was observed in the rates of early stage and advanced stage between Bcl-2/Bax ratio ≥ 1.2 and Bcl-2/Bax ratio < 1.2 patients (data not shown), the two-year DFS rate of Bcl-2/Bax ratio ≥ 1.2 group [76.9% (30 of 39 patients)] was statistically significantly higher than that of the Bcl-2/Bax ratio < 1.2 group [43.2% (16 of 37 patients)] (P = .001; Figure 2 and data not shown).
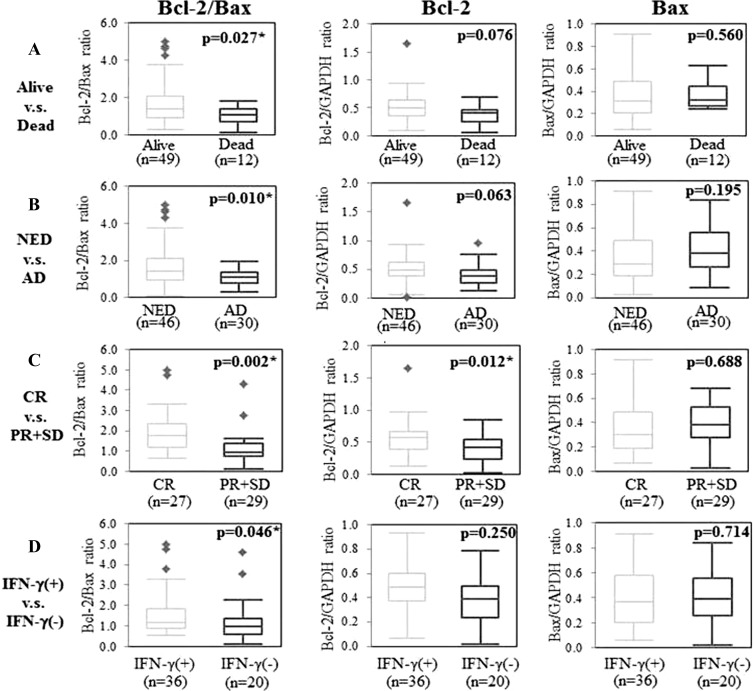
Next, we compared the Bcl-2/Bax ratio in PBMCs from patients who were alive and from patients who were dead at 36 months from initial diagnosis. A significant difference in the Bcl-2/Bax ratios of the two groups was observed (P = .027; Figure 3A). Furthermore, there was a statistically significant difference in the Bcl-2/Bax ratios of patients with NED at 24 months and in patients with active disease (AD) at 24 months from first treatments (P = .010; Figure 3B). The Bcl-2 and Bax expression values, as represented by Bcl-2/GAPDH and Bax/GAPDH ratios, showed no significant difference within these group pairings (alive vs dead, NED vs AD), but there was a tendency for higher Bcl-2 expression values in alive and NED groups than in the dead and AD groups (P = .076 and P = .063, respectively; Figure 3, A and B).
Figure 3.
Differences of Bcl-2/Bax ratio, Bcl-2 expression, and Bax expression in (A) alive and dead patients, (B) NED and AD patients, (C) CR and PR + SD patients, and (D) IFN-γ(+) and IFN-γ(-) patients were analyzed by Student's two-tailed t test. Asterisk denotes P < .05, indicating statistical significance.
We next examined the therapeutic effects in association with expression of Bcl-2 and Bax mRNAs in 56 patients who received immunochemoradiotherapy using OK-432 and UFT or S-1, in combination with radiation. The Bcl-2/Bax ratio as well as Bcl-2 expression value in patients who showed CR was significantly high compared with those in patients who showed PR and SD (P = .002 and P = .012, respectively; Figure 3C). The Bax expression value showed no significant association with the clinical response (P = .688).
The immunotherapeutic effect was also evaluated in the association with Bcl-2/Bax expression. We analyzed serum IFN-γ levels in patients 5 hours before and 18 hours after OK-432 administration. Serum IFN-γ protein was not detectable in any of the patients before OK-432 treatment (data not shown). Eighteen hours after OK-432 injection, IFN-γ protein levels in the sera were detectable in 36 patients (64.3%) and were still not detectable in 20 patients (35.7%). Patients whose serum IFN-γ became detectable in response to the therapy were determined IFN-γ(+). The Bcl-2/Bax ratio but not the Bcl-2 or Bax expression value was significantly higher in IFN-γ(+) patients than IFN-γ(-) patients (P = .046; Figure 3D).
Differences between Primary Sites
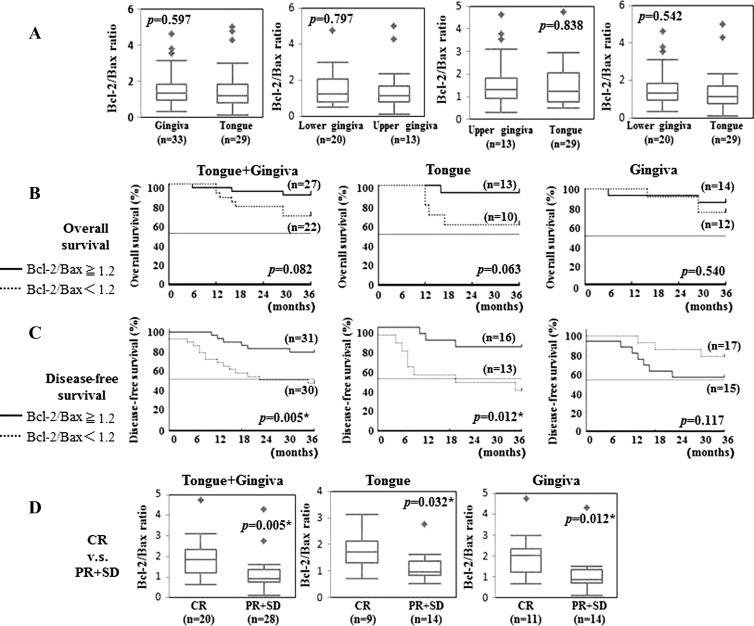
Next, we have examined the difference in Bcl-2/Bax ratio and in clinical outcome among the different primary sites such as tongue and gingiva that have enough number of cases for analysis. Squamous cell carcinoma of hard palate has been included in upper gingiva. A case of adenoid cystic carcinoma in tongue has been excluded from the analysis. There was no significant difference in Bcl-2/Bax ratio between tongue and upper gingiva, between tongue and lower gingiva, between upper and lower gingiva, as well as between tongue and gingiva (upper gingiva + lower gingiva; P = .597, 0.797, 0.838, and 0.542, respectively; Figure 4A). Interestingly, although tongue cancer patients who showed Bcl-2/Bax ratio ≥ 1.2 tended to survive longer than those who showed a Bcl-2/Bax ratio < 1.2 (P = .063), no tendency of the difference was observed between Bcl-2/Bax ratio high group and low group in the patients with gingival cancer (P = .540; Figure 4B). In DFS, a statistically significant difference was observed between high and low groups in Bcl-2/Bax ratio in the patients with tongue cancer (P = .012) but not in those with gingival cancer (P = .117; Figure 4C). However, in clinical response, the Bcl-2/Bax ratio in patients who showed CR were significantly high compared with those in patients who showed PR and SD both in tongue cancer patients and in gingival cancer patients (P = .032 and P = .012, respectively; Figure 4D).
Figure 4.
Differences of Bcl-2/Bax ratio among the different primary sites were analyzed by Student's two-tailed t test. Asterisk denotes P < .05, indicating statistical significance (A). Differences of relationship between Bcl-2/Bax ratio and (B) OS or (C) DFS among the different primary sites. Differences of Bcl-2/Bax ratio ≥ 1.2 (solid line) versus Bcl-2/Bax ratio < 1.2 (dotted line) were calculated by log-rank test. Asterisk denotes P < .05, indicating statistical significance. (D) Differences of relationship between Bcl-2/Bax ratio and clinical responses among the primary sites were analyzed by Student's two-tailed t test. Asterisk denotes P < .05, indicating statistical significance.
Correlation of Bcl-2 and Bax mRNA Expression with Immunologic Parameters
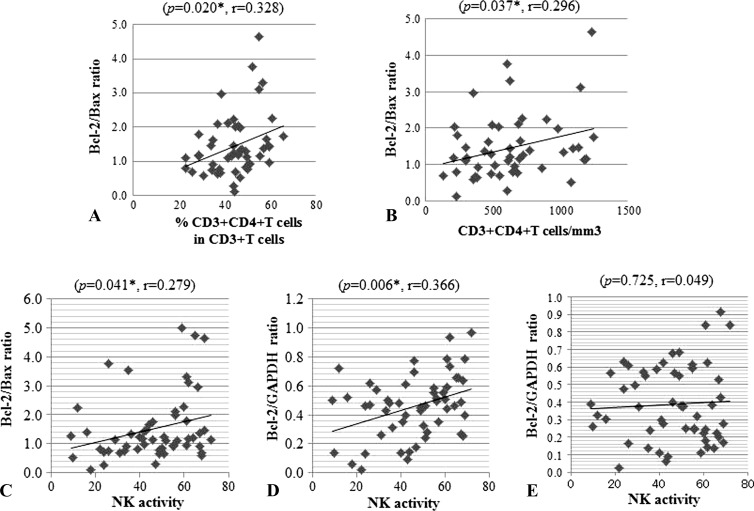
Finally, we analyzed the correlation between the expression of Bcl-2 and Bax mRNAs and the immunologic parameters of leucocytes derived from HNC patients before receiving any treatments. Data are shown in Table 1 and Figure 4. Both the percentage of CD3+CD4+ T cells in CD3+ T cells and the absolute number of CD3+CD4+ T cells were statistically significantly correlated with the Bcl-2/Bax ratio (P = .020 and P = .037, respectively). Additionally, statistically significant correlations between NK cell activity and the Bcl-2/Bax ratio as well as the Bcl-2 expression value (Bcl-2/GAPDH) were observed (P = .041 and P = .006, respectively).
Table 1.
Correlation between Expression of Bcl-2 and Bax mRNAs and Immunologic Parameters.
| Bcl-2/Bax | Bcl-2/GAPDH | Bax/GAPDH | N | ||||
| P1 | r2 | P | r | P | r | ||
| WBC (per mm3) | .662 | 0.054 | .265 | 0.137 | .131 | 0.185 | 76 |
| Lymphocyte (%) | .691 | 0.049 | .057 | 0.232 | .110 | 0.196 | 76 |
| Lymphocyte (per mm3) | .681 | 0.051 | .250 | 0.142 | .682 | 0.051 | 76 |
| Monocyte (per mm3) | .630 | 0.059 | .357 | 0.113 | .091 | 0.207 | 76 |
| CD3+ (%) | .098 | 0.236 | .240 | 0.169 | .417 | 0.117 | 51 |
| CD3+ (per mm3) | .181 | 0.192 | .142 | 0.211 | .473 | 0.104 | 51 |
| CD3+CD4+/CD3+ (%) | .020*3 | 0.328 | .131 | 0.216 | .386 | 0.125 | 51 |
| CD3+CD4+ (per mm3) | .037*3 | 0.296 | .053 | 0.275 | .410 | 0.119 | 51 |
| CD3+CD8+/CD3+ (%) | .325 | 0.142 | .233 | 0.172 | .843 | 0.029 | 51 |
| CD3+CD8+ (per mm3) | .764 | 0.044 | .731 | 0.050 | .664 | 0.063 | 51 |
| CD4+/CD8+ ratio | .299 | 0.150 | .383 | 0.126 | .675 | 0.061 | 51 |
| CD16+ (%) | .392 | 0.131 | .978 | 0.004 | .406 | 0.127 | 51 |
| CD16+ (per mm3) | .526 | 0.097 | .807 | 0.037 | .624 | 0.075 | 51 |
| CD57+ (%) | .586 | 0.083 | .692 | 0.061 | .353 | 0.142 | 51 |
| CD57+ (per mm3) | .698 | 0.059 | .595 | 0.081 | .590 | 0.083 | 51 |
| NK activity (%) | .041*3 | 0.279 | .006*3 | 0.366 | .725 | 0.049 | 54 |
| LAK activity (%) | .500 | 0.094 | .297 | 0.145 | .351 | 0.130 | 54 |
| Pre-LAK activity (%) | .337 | 0.133 | .870 | 0.023 | .323 | 0.137 | 54 |
P values were arrived at using regression calculation.
r, Spearmans rank correlation coefficient.
Asterisk denotes P < .05, indicating statistical significance.
Discussion
We have previously reported that the combination therapy using UFT and OK-432 together with radiation has a marked therapeutic effect in patients with HNC and that OK-432-induced immunity plays a significant role in the antitumor effect of this combination therapy through Toll-like receptor 4 (TLR4)/MD-2 [25–27]. It has been reported that OK-432 elicits antitumor effects by stimulating immunocompetent cells such as macrophages, T cells, and NK cells [28,29] and that OK-432 induces interleukin-12 and polarizes the T cell response to a T helper cell 1 (Th1)-dominant state [30]. Recently, we have demonstrated that OK-432 induces the maturation of dendritic cells, which are dedicated antigen-presenting cells through TLR4 signaling, and that OK-432-stimulated dendritic cells can induce tumor antigen-specific cytotoxic T lymphocytes in vitro as well as in vivo [26,31,32]. Therefore, we have applied the combination therapy by using radiation, UFT or S-1, and OK-432 to the patients who entered the study. Furthermore, in our previous study, we observed a statistically significant relationship between increased IFN-γ protein levels in the sera as well as clinical responses in HNC patients administered OK-432 and expression of TLR4 and MD-2 genes in circulating immune cells from the patients [26]. Although it was expected that expression of both TLR4 and MD-2 genes in patient-derived PBMCs may be a useful biomarker to discriminate between likely responders and nonresponders to OK-432 as well as to predict the therapeutic effect of OK-432-based immunotherapy, its utility is so far limited to patients undergoing OK-432-based immunotherapy. While expression of TLR4 and MD-2 genes in PBMCs from the patients was not a prognostic biomarker for numerous cancer patients except for the patients undergoing OK-432 therapy, these previous data suggested at least that evaluation of the characteristics of circulating immune cells derived from cancer patients might have prognostic value.
The findings from the current study suggested that expression ratio of the genes for antiapoptotic protein Bcl-2 and proapoptotic protein Bax in circulating immune cells derived from patients with HNC might have a marked prognostic impact. Enhancing antitumor host response in cancer patients is critical for improvement of therapeutic effects and clinical outcomes; thus, it may be a useful prognostic strategy to assess expression of proapoptotic and antiapoptotic genes in circulating immune cells.
In addition, the comparison of Bcl-2/Bax ratio with TNM classification in prognostic value for head and neck cancer patients indicates that expression of these genes might detect the prognostic risks and advantages that could not be detected by TNM classification; thus, using both expression ratio of Bcl-2/Bax mRNAs and TNM classification should contribute more strongly to prediction of outcomes, selection of cases, and establishment of personalized therapy for individual patients. Furthermore, the results from the study evaluating the difference among the different primary sites in Bcl-2/Bax ratio and in clinical outcome suggested that Bcl-2/Bax ratio may be a better prognostic biomarker in patients with tongue cancer than in those with gingival cancer.
IFN-γ, a representative Th1 cytokine, plays an important role in anticancer immunity. It has been reported that IFN-γ production is associated with a favorable clinical outcome in patients with several types of malignancies [33] and that OK-432 augments anticancer host responses by increasing production of Th1 cytokines, especially IFN-γ [26]. Actually, the findings in the current experiments have clearly indicated that the Bcl-2/Bax ratio in the circulating immune cells was significantly higher in IFN-γ(+) patients than in IFN-γ(-) patients and also higher in the patients who showed favorable therapeutic effect and clinical outcome. It was strongly suggested that the increase of the apoptosis-associated gene expression might inhibit IFN-γ production in response to OK-432 and then decreased anticancer effect of the therapy.
The cellular and molecular mechanisms for regulating expression of Bcl-2 and Bax genes should be elucidated for these genes to be a more valuable biomarker. Kim et al. reported that expression of the proapoptotic protein Bax was elevated in peripheral blood CD8+ T cells from the patient with HNSCC [6], while our data showed that both the percentage and absolute number of CD4+ T cells but not CD8+ in peripheral blood were statistically significantly correlated with the Bcl-2/Bax ratio (Figure 5). In addition, the number of CD4+ T cells was relatively high in PBMCs, which showed high Bcl-2 expression values (P = .053, Table 1); thus, we considered that the Bcl-2/Bax balance was affected by the change of Bcl-2 gene expression level but not Bax expression in CD4+ T cells. Most of the patients in the present study received radiation and oral 5-fluorouracil in combination with OK-432-based immunotherapy. Different results between our study and the study of Kim et al. may be due to the difference of the therapies. Further, in the current cases, NK cell activity was significantly correlated both with the Bcl-2/Bax ratio and with Bcl-2 expression, whereas the number of NK cells estimated by CD16+ and CD57+ was not correlated with expression of these genes. The findings suggest that the balance of expression of Bcl-2 and Bax genes may have an effect on NK cell activity but not on cell number.
Figure 5.
Correlations between (A) Bcl-2/Bax ratio and percentage of CD4+ T cells, (B) Bcl-2/Bax ratio and absolute number of CD4+ T cells, (C) Bcl-2/Bax ratio and NK activity, (D) Bcl-2 expression value and NK activity, and (E) Bax expression value and NK activity were quantified by Spearman's rank correlation coefficient and regression calculation. Asterisk denotes P < .05, which was considered statistically significant.
The markers may be also useful as therapeutic targets. If a cytokine that can increase the Bcl-2/Bax ratio can be elucidated, a therapy using the cytokine could be established for patients who show low levels of that cytokine. Further, anticytokine therapy using a neutralizing antibody may have a therapeutic ability for patients with an elevated level of the serum cytokine that can decrease the Bcl-2/Bax ratio. Moreover, it has been reported that certain immunotherapeutic agents increase antiapoptotic molecules and decrease proapoptotic molecules in immune cells [34,35], and combination therapy using the immune adjuvant may have a therapeutic value for patients with malignancies.
We expect that the ongoing prospective study will elucidate the expression mechanism for these genes in circulating immune cells from cancer patients and will establish a biomarker.
Supplementary Material
Footnotes
This work was supported in part by a grant-in-aid for Scientific Research from the Ministry of Education, Science and Culture of Japan. All authors agreed to the submission of this article, and there is no conflict of interest to disclose.
This article refers to supplementary material, which is designated by Table W1 and is available online at www.neoplasia.com.
References
- 1.Walsh JE, Lathers DM, Chi AC, Gillespie MB, Day TA, Young MR. Mechanisms of tumor growth and metastasis in head and neck squamous cell carcinoma. Curr Treat Options Oncol. 2007;8:227–238. doi: 10.1007/s11864-007-0032-2. [DOI] [PubMed] [Google Scholar]
- 2.Whiteside TL. Inhibiting the inhibitors: evaluating agents targeting cancer immunosuppression. Expert Opin Biol Ther. 2010;10:1019–1035. doi: 10.1517/14712598.2010.482207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen CT, Judd NP, Bui JD, Uppaluri R. The clinical implications of antitumor immunity in head and neck cancer. Laryngoscope. 2012;122:144–157. doi: 10.1002/lary.21913. [DOI] [PubMed] [Google Scholar]
- 4.Ostrand-Rosenberg S. Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer Immunol Immunother. 2010;59:1593–1600. doi: 10.1007/s00262-010-0855-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gastman BR, Atarashi Y, Reichert TE, Saito T, Balkir L, Rabinowich H, Whiteside TL. Fas ligand is expressed on human squamous cell carcinomas of the head and neck, and it promotes apoptosis of T lymphocytes. Cancer Res. 1999;59:5356–5364. [PubMed] [Google Scholar]
- 6.Kim JW, Tsukishiro T, Johnson JT, Whiteside TL. Expression of pro- and antiapoptotic proteins in circulating CD8+ T cells of patients with squamous cell carcinoma of the head and neck. Clin Cancer Res. 2004;10:5101–5110. doi: 10.1158/1078-0432.CCR-04-0309. [DOI] [PubMed] [Google Scholar]
- 7.Manicassamy S, Gupta S, Huang Z, Sun Z. Protein kinase C-θ-mediated signals enhance CD4+ T cell survival by up-regulating Bcl-xL. J Immunol. 2006;176:6709–6716. doi: 10.4049/jimmunol.176.11.6709. [DOI] [PubMed] [Google Scholar]
- 8.Liphaus BL, Kiss MH, Carrasco S, Goldenstein-Schainberg C. Increased Fas and Bcl-2 expression on peripheral mononuclear cells from patients with active juvenile-onset systemic lupus erythematosus. J Rheumatol. 2007;34:1580–1584. [PubMed] [Google Scholar]
- 9.Pillet AH, Thèze J, Rose T. Interleukin (IL)-2 and IL-15 have different effects on human natural killer lymphocytes. Hum Immunol. 2011;72:1013–1017. doi: 10.1016/j.humimm.2011.07.311. [DOI] [PubMed] [Google Scholar]
- 10.DeBarros A, Chaves-Ferreira M, d'Orey F, Ribot JC, Silva-Santos B. CD70-CD27 interactions provide survival and proliferative signals that regulate T cell receptor-driven activation of human γδ peripheral blood lymphocytes. Eur J Immunol. 2011;41:195–201. doi: 10.1002/eji.201040905. [DOI] [PubMed] [Google Scholar]
- 11.Oh S, Perera LP, Terabe M, Ni L, Waldmann TA, Berzofsky JA. IL-15 as a mediator of CD4+ help for CD8+ T cell longevity and avoidance of TRAIL-mediated apoptosis. Proc Natl Acad Sci USA. 2008;105:5201–5206. doi: 10.1073/pnas.0801003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim H, Rafiuddin-Shah M, Tu HC, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nat Cell Biol. 2006;8:1348–1358. doi: 10.1038/ncb1499. [DOI] [PubMed] [Google Scholar]
- 13.Homma A, Furuta Y, Oridate N, Nakano Y, Kohashi G, Yagi K, Nagahashi T, Yagi K, Nagahashi T, Fukuda S, et al. Prognostic significance of clinical parameters and biological markers in patients with squamous cell carcinoma of the head and neck treated with concurrent chemoradiotherapy. Clin Cancer Res. 1999;5:801–806. [PubMed] [Google Scholar]
- 14.Gallo O, Boddi V, Calzolari A, Simonett L, Trovati M, Bianchi S. Bcl-2 protein expression correlates with recurrence and survival in early stage head and neck cancer treated by radiotherapy. Clin Cancer Res. 1996;2:261–267. [PubMed] [Google Scholar]
- 15.Green VL, Irune E, Prasai A, Alhamarneh O, Greenman J, Stafford ND. Serum IL10, IL12 and circulating CD4+CD25high T regulatory cells in relation to long-term clinical outcome in head and neck squamous cell carcinoma patients. Int J Oncol. 2012;40:833–839. doi: 10.3892/ijo.2011.1259. [DOI] [PubMed] [Google Scholar]
- 16.Chang KP, Chang YT, Wu CC, Liu YL, Chen MC, Tsang NM, Hsu CL, Chang YS, Yu JS. Multiplexed immunobead-based profiling of cytokine markers for detection of nasopharyngeal carcinoma and prognosis of patient survival. Head Neck. 2011;33:8868–8897. doi: 10.1002/hed.21557. [DOI] [PubMed] [Google Scholar]
- 17.Uppaluri R, Dunn GP, Lewis JS., Jr Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in head and neck cancers. Cancer Immun. 2008;8:16. [PMC free article] [PubMed] [Google Scholar]
- 18.Kikkawa F, Kawai M, Oguchi H, Kojima M, Ishikawa H, Iwata M, Maeda O, Tomoda Y, Arii Y, Kuzuya K. Randomised study of immunotherapy with OK-432 in uterine cervical carcinoma. Eur J Cancer. 1993;29:1542–1546. doi: 10.1016/0959-8049(93)90291-m. [DOI] [PubMed] [Google Scholar]
- 19.Maehara Y, Okuyama T, Kakeji Y, Baba H, Furusawa M, Sugimachi K. Postoperative immunochemotherapy including streptococcal lysate OK-432 is effective for patients with gastric cancer and serosal invasion. Am J Surg. 1994;168:36–40. doi: 10.1016/s0002-9610(05)80068-6. [DOI] [PubMed] [Google Scholar]
- 20.Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of mononuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest. 1968;97(suppl):77–89. [PubMed] [Google Scholar]
- 21.Messina E, Gazzaniga P, Micheli V, Guaglianone MR, Barbato S, Morrone S, Frati L, Aglianò AM, Giacomello A. Guanine nucleotide depletion triggers cell cycle arrest and apoptosis in human neuroblastoma cell lines. Int J Cancer. 2004;108:812–817. doi: 10.1002/ijc.11642. [DOI] [PubMed] [Google Scholar]
- 22.Nakashiro K, Kawamata H, Hino S, Uchida D, Miwa Y, Hamano H, Omotehara F, Yoshida H, Sato M. Down-regulation of TSC-22 (transforming growth factor β-stimulated clone 22) markedly enhances the growth of a human salivary gland cancer cell line in vitro and in vivo. Cancer Res. 1998;86:549–555. [PubMed] [Google Scholar]
- 23.Aramaki M, Kim YI, Shimoda K, Nakashima K, Okada K, Kobayashi M. Induction by OK-432 of lymphokine activated killer precursor cells in hepatocellular carcinoma. Hepatogastroenterology. 1994;4:363–366. [PubMed] [Google Scholar]
- 24.Timonen T, Saksela E. A simplified isotope release assay for cell-mediated cytotoxicity against anchorage dependent target cells. J Immunol Methods. 1977;18:123–132. doi: 10.1016/0022-1759(77)90163-6. [DOI] [PubMed] [Google Scholar]
- 25.Sato M, Harada K, Yoshida H, Yura Y, Azuma M, Iga H, Bando T, Kawamata H, Takegawa Y. Therapy for oral squamous cell carcinoma by tegafur and streptococcal agent OK-432 in combination with radiotherapy: association of the therapeutic effect with differentiation and apoptosis in the cancer cells. Apoptosis. 1997;2:227–238. doi: 10.1023/a:1026428918301. [DOI] [PubMed] [Google Scholar]
- 26.Okamoto M, Oshikawa T, Tano T, Ohe G, Furuichi S, Nishikawa H, Ahmed SU, Akashi S, Miyake K, Takeuchi O, et al. Involvement of Toll-like receptor 4 signaling in interferon-γ production and antitumor effect by streptococcal agent OK-432. J Natl Cancer Inst. 2003;95:316–326. doi: 10.1093/jnci/95.4.316. [DOI] [PubMed] [Google Scholar]
- 27.Okamoto M, Furuichi S, Nishioka Y, Oshikawa T, Tano T, Ahmed SU, Takeda K, Akira S, Ryoma Y, Moriya Y, et al. Expression of Toll-like receptor 4 on dendritic cells is significant for anticancer effect of dendritic cell-based immunotherapy in combination with an active component of OK-432, a streptococcal preparation. Cancer Res. 2004;64:5461–5470. doi: 10.1158/0008-5472.CAN-03-4005. [DOI] [PubMed] [Google Scholar]
- 28.Oshimi K, Kano S, Takaku F, Okumura K. Augmentation of mouse natural killer cell activity by a streptococcal preparation, OK-432. J Natl Cancer Inst. 1980;65:1265–1269. [PubMed] [Google Scholar]
- 29.Misaki T, Watanabe Y, Iida Y, Hidaka A, Kasagi K, Fukushima H, Konishi J. Recruitment of T lymphocytes and induction of tumor necrosis factor in thyroid cancer by a local immunotherapy. Cancer Immunol Immunother. 1992;35:92–96. doi: 10.1007/BF01741855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujimoto T, Duda RB, Azilvasi A, Chen X, Mai M, O'Donnell MA. Streptococcal preparation OK-432 is a potent inducer of IL-12 and a T helper cell 1 dominant state. J Immunol. 1997;158:5619–5626. [PubMed] [Google Scholar]
- 31.Ahmed SU, Okamoto M, Oshikawa T, Tano T, Sasai A, Kan S, Hiroshima T, Ohue H, Moriya Y, Ryoma Y, et al. Anti-tumor effect of an intratumoral administration of dendritic cells in combination with TS-1, an oral fluoropyrimidine anti-cancer drug, and OK-432, a streptococcal immunopotentiator: involvement of Toll-like receptor 4. J Immunother. 2004;27:432–441. doi: 10.1097/00002371-200411000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Okamoto M, Oshikawa T, Tano T, Ahmed SU, Kan S, Sasai A, Akashi S, Miyake K, Moriya Y, Ryoma Y, et al. Mechanism of anticancer host response induced by OK-432, a streptococcal preparation, mediated by phagocytosis and Toll-like receptor 4 signaling. J Immunother. 2006;29:78–86. doi: 10.1097/01.cji.0000192106.32206.30. [DOI] [PubMed] [Google Scholar]
- 33.Matsushita K, Takenouchi T, Kobayashi S, Hayashi H, Okuyama K, Ochiai T. HLA-DR antigen expression on colorectal carcinomas: influence of expression by IFN-γ in situ and its association with tumour progression. Br J Cancer. 1996;73:644–648. doi: 10.1038/bjc.1996.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Czystowska M, Szczepanski MJ, Szajnik M, Quadrini K, Brandwein H, Hadden JW, Whiteside TL. Mechanisms of T-cell protection from death by IRX-2: a new immunotherapeutic. Cancer Immunol Immunother. 2011;60:495–506. doi: 10.1007/s00262-010-0951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Czystowska M, Han J, Szczepanski MJ, Szajnik M, Quadrini K, Brandwein H, Hadden JW, Signorelli K, Whiteside TL. IRX-2, a novel immunotherapeutic, protects human T cells from tumor-induced cell death. Cell Death Differ. 2009;16:708–718. doi: 10.1038/cdd.2008.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.