Abstract
Desiccation tolerance was quantified in four cyanolichens (Lobaria hallii, Lobaria retigera, Lobaria scrobiculata, Pseudocyphellaria anomala), one cephalolichen (Lobaria pulmonaria) and one chlorolichen (Platismatia glauca) from xeric and mesic, open and closed North American boreal forests.
These sympatric epiphytes were exposed to 0%, 33%, 55% and 75% relative humidity with or without medium light (200 μmol m−2 s−1) for 7 d. Permanent and temporary photoinhibitory damage was recorded as viability measures.
All species tolerated well the drying in darkness, but L. hallii and L. retigera, associated with a very humid climate, showed minor damage at the hardest drying (silica gel). Simultaneous exposure to medium light severely aggravated the drying damage at all relative humidity levels. Combined drying–light exposure was particularly devastating for the widespread chloro- and cephalolichens, whereas cyanolichens, including rare old forest species, were fairly resistant.
The ability to recover after combined drying–light stress (this study) correlated positively with increasing species-specific water holding capacities (from the literature). Cyanolichens, depending on liquid water and large internal water storage, probably require strong drying–light resistance to handle long periods between hydration events, whereas chlorolichens can regularly maintain their photosynthetic apparatus during frequent and rapid activation by humid air on clear mornings.
Keywords: cephalolichens, chlorolichens, cyanolichens, desiccation tolerance, epiphytes, hydration regime, light tolerance, Nostoc
Introduction
Lichens are poikilohydric symbiotic associations between a mycobiont and one or more photobionts (green algae and/or cyanobacteria). Some lichens are extremely resistant to drying and extreme UV radiation, being alive after several days in outer space (Sancho et al., 2007; de la Torre et al., 2010). This tolerance is remarkable, as drying switches off dark reactions of photosynthesis, allowing the accumulation of harmful oxygen species (reactive oxygen species, ROS) when exposed to light (Kranner et al., 2008). However, drying activates special energy-dissipating mechanisms in lichens (Heber et al., 2006). Lichens with green algal photobionts using the energy-dissipating xanthophyll cycle, as well as cyanolichens lacking this cycle, both show strong drying-induced quenching of chlorophyll fluorescence (Heber et al., 2006). Thereby, both photobiont groups use a photosystem II (PSII) reaction center mechanism to dissipate energy when dry (Heber et al., 2006, 2010). The efficiency of protection mechanisms varies among lichens. Old forest cephalolichens (= chlorolichens with cyanobacteria in localized colonies, called cephalodia) are susceptible to high-light exposure in the dry state (Gauslaa & Solhaug, 1996).
Most members of the lichenized fungal order Peltigerales live in forests, particularly species in the two related genera Lobaria and Pseudocyphellaria treated in this article. They include cephalolichens as well as cyanolichens. Unlike other taxonomic groups, green algal and cyanobacterial members of Peltigerales consistently produce extracellular superoxide radicals at high rates after drying (Beckett et al., 2003). As the vast majority of these lichens are restricted to forests and/or humid climates (Marini et al., 2011), their distribution patterns may be shaped by intolerance to prolonged desiccation. At the same time, a number of cephalolichens in the Peltigerales are more adversely affected by high light when dry than are pure chlorolichens of other taxonomic groups (e.g. the Parmeliaceae) without cephalodia (Gauslaa & Solhaug, 1996, 1999, 2004). So far, photoinhibition in dry cyanolichens has not been studied in detail. The classical photoinhibition study of Demmig-Adams et al. (1990b) showed that cyanolichens had a lower recovery than chlorolichens after short-term high-light exposures whilst hydrated. They related the higher susceptibility of cyanolichens to the absence of xanthophyll cycle pigments in cyanobacteria (Demmig-Adams et al., 1990a). Cyanolichens that had dried in light before short-term high-light exposures exhibited greatly accelerated recovery (Demmig-Adams et al., 1990c). Similar responses after drying in light were seen in the cephalolichen Lobaria pulmonaria (Štepigováet al., 2008). However, during clear weather, high-light exposure sometimes lasts for many days without hydration, even at night. Lichens thus experience much longer periods of high light when dry than when hydrated. During light treatments in extended dry periods, irreversible damage accumulates with time (Gauslaa & Solhaug, 1996, 1999). As some cyanolichens tend to dominate the wettest forests and canopies in the spray zone of waterfalls (Goward & Spribille, 2005), their rareness and absence in drier sites are often ascribed to low drying tolerance, although experiments separating the effects of drying from those of simultaneous light exposure are lacking and their drying tolerance is unknown.
Water loss and uptake in lichens are physical processes. Their water potential reflects the water potential in the air. Lichens frequently experience water potentials too low for photosynthesis. Chloro- and cephalolichens have positive photosynthesis in the absence of liquid water at 97% relative humidity (RH) with a water potential (Ψair) of c. − 4 MPa, whereas cyanolichens require liquid water to activate photosynthesis (Lange et al., 1986). Positive CO2 exchange in two chlorolichens continued down to − 22 and − 38 MPa (Nash et al., 1990), whereas no further photosynthesis occurred in four other chlorolichens at − 40 MPa (Scheidegger et al., 1995). In drying-tolerant bryophytes, little or no photosynthesis occurred below – 15 to 20 MPa (Dilks & Proctor, 1979). However, metabolic processes other than photosynthesis take place at low rates down to at least 50% RH in cyano- and chlorolichens (Cowan et al., 1979). As our study aims to quantify drying damage, we study the effects of water potentials below the threshold for photosynthetic activity.
This study focuses on six sympatric foliose epiphytic lichens being locally dominant on conifer branches and stems of deciduous trees in old forests and/or humid boreal climates. By comparing photoinhibition in a mix of co-occurring cyano-, chloro- and cephalolichens in experiments using fixed RHs with and without light exposure, we aim to identify possible photobiont-specific responses to drying. Earlier studies of drying susceptibility have been conducted in darkness or light (e.g. Green et al., 1991; Kranner et al., 2003), but the light factor has rarely been considered to be relevant. We aim to discriminate between damage caused by drying alone and by combined light and drying exposure. Lichen anatomy, specific thallus mass and water holding capacity (WHC) have already been quantified in all of our specimens by Gauslaa & Coxson (2011). In order to understand lichen functioning, we intend to analyze whether inter- and/or intraspecific differences in these internal variables can explain parts of the variation in drying and/or light susceptibility assessed in this study. Finally, we wish to analyze possible implications of the results for lichen physiology, distribution patterns and rareness. As members of Lobaria and Pseudocyphellaria are species of concern, indicating ecological continuity in forest conservation management (Rose, 1976), knowledge on the basic responses to drought and high-light stress may facilitate ecologically viable management plans to secure declining and species-rich epiphytic communities in our forests.
Materials and Methods
Lichen material
We included chlorolichens (Platismatia glauca (L.) W.L.Culb. & C.F.Culb. with Trebouxia as the photobiont), cephalolichens (Lobaria pulmonaria (L.) Hoffm. with the green alga Dictyochloropsis as the main photobiont and the cyanobacterium Nostoc in cephalodia) and cyanolichens (with Nostoc only; Lobaria hallii (Tuck.) Zahlbr., L. retigera (Bory) Trevisan, L. scrobiculata (Scop.) DC., Pseudocyphellaria anomala Brodo & Ahti). The most widespread lichen, P. glauca, belongs to the Parmeliaceae, whereas the other species are members of the Lobariaceae in the Peltigerales. Lobaria hallii is mainly a species of spray zones of waterfalls in Europe (Jørgensen & Tønsberg, 2007) and humid forests west of the Rocky Mountains in North America (Goward et al., 1994), whereas L. retigera grows in very humid forests of western North America and eastern Asia. In subtropical–temperate moist forests in New Zealand, L. retigera is one of few lichens that, together with the highly drying-susceptible Pseudocyphellaria dissimilis (Green et al., 1991), successfully competes with bryophytes in very low light on damp forest floors (Galloway, 1988).
All six species were collected along a longitudinal gradient in British Columbia in April 2010 (Table 1). The three easternmost sites (Slim, Upper Fraser and Aleza) are located within or at the edge of mountain valleys, whereas the two westernmost sites fall within the drier interior plateau region of central-interior British Columbia. Although not all species were collected at each site (Table 1), these are co-occurring species throughout this region, although their abundance varies between sites (see Doering & Coxson, 2010). The exception to this pattern of co-occurrence was L. retigera, which was only found in the Slim Creek site (Table 1). These sites, from east to west, respectively, fall within the following biogeoclimatic subzones (from Meidinger & Pojar, 1991): Slim Creek – the very wet cool interior cedar–hemlock subzone; Upper Fraser and Aleza – the wet cool sub-boreal spruce climate subzone; and Salmon Valley and Prince George – the moist cool sub-boreal spruce climate subzone. The mean annual temperature in these sites varies from 2.6 to 3.5°C, with the two westernmost sites, Salmon River and Prince George, being markedly drier. Lichens collected (Table 1) from open-canopy sites (Upper Fraser and Salmon River) were from Salix discolor in mixed riparian woodlands with Alnus incana, Populus tremuloides and P. balsamifera. The mean canopy openness during the growing season in these riparian sites was 11%, with 980 stems ha−1 over 10 cm in diameter at breast height (Doering & Coxson, 2010). The Slim Creek closed-canopy site was dominated by Thuja plicata and Tsuga heterophylla. Radies et al. (2009) found that the mean canopy openness in wet interior (cedar–hemlock zone) coniferous stands in the Slim Creek area was 14.1%. Closed-canopy forests at both Aleza and Prince George were dominated by hybrid spruce, Picea engelmannii × glauca and Abies lasiocarpa. Campbell et al. (2010) found that the average light transmission at Aleza was 21.2% (direct plus diffuse light expressed as a percentage of the maximum).
Table 1.
Climate variables1 and location for lichen collection sites in British Columbia, Canada
| Site | Type | Location (latitude/longitude) | Elevation (m) | Mean annual precipitation (precipitation as snow; mm) | Mean annual temperature (mean July–August temperature, °C) | Collected lichens |
|---|---|---|---|---|---|---|
| Prince George | Xeric open (plateau) | 53°53′07.12′′N 122°49′13.69′′W | 785 | 570 (205) | 3.5 (14.2) | P. glauca |
| Salmon River | Xeric open (plateau) | 54°12′22.43′′N 122°41′48.83′′W | 743 | 668 (279) | 2.6 (13.4) | L. hallii, L. pulmonaria, L. scrobiculata, P. anomala |
| Aleza | Mesic closed (mountain) | 54°05′55.29′′N 122°05′02.84′′W | 660 | 888 (350) | 3.0 (14.3) | L. hallii, L. pulmonaria, L. scrobiculata, P. anomala, P. glauca |
| Upper Fraser | Mesic open (mountain) | 54°05′23.14′′N 121°52′07.78′′W | 608 | 816 (313) | 3.2 (14.4) | L. hallii, L. pulmonaria, L. scrobiculata |
| Slim Creek | Mesic closed (mountain) | 53°45′24.20′′N 121°12′05.25′′W | 778 | 838 (315) | 3.0 (13.4) | L. retigera |
Temperature and precipitation normals for 1961 to 1990 from Climate BC modelling (Wang et al., 2006); see http://genetics.forestry.ubc.ca/cfgc/ClimateBC/Default.aspx.
To develop experimental protocols for this study, we performed a number of pre-experiments with Norwegian material of L. pulmonaria sampled in Picea abies-dominated forests at Namsos, central western Norway (64°20–25′N, 11°16–30′E) from the same collection as used by Larsson et al. (2012). The Norwegian thalli responded exactly like those from British Columbia with respect to light and desiccation treatments.
With the large number of sites, species, treatments and replicates, we used thallus disks to obtain enough space under the experimental set-up. Lichens were hydrated by spraying de-ionized water on the upper surface in the laboratory. From each moistened thallus, one or two disks with an area of c. 1 cm2 each were taken at random positions by a cork borer. A similar number of disks (n = 40) were taken from each species in each of the collection sites (Table 1), amounting to 560 disks in total. The disks were randomly selected species-wise for each treatment. All disks were preconditioned to allow relaxation of the short-term down-regulation of PSII. This was performed by spraying the disks with de-ionized water on the upper surface and keeping them hydrated in low light (10 μmol photons m−2 s−1) at room temperature (20°C) for 48 h. Immediately after the preconditioning, Fv/Fm was recorded with a portable fluorimeter (Plant Efficiency Analyser; Hansatech, King’s Lynn, Norfolk, UK) after a 15-min period of dark adaptation. The disks were then dried until the next day between filter papers, using gentle pressure to keep them dry and flat before the drying and light treatments commenced. Treatments were conducted in clear 250-ml plastic boxes (10 × 8 × 4 cm3; length, width, height). The experiment was replicated three times. In each replication, disks for each species were randomly selected. Within each replicated experiment, disks were also randomized for each box. Both the measured Fv/Fm values and Fv/Fm as a percentage of initial preconditioning values are presented. This recognizes the generally lower Fv/Fm values in healthy cyanolichens relative to healthy cephalo- and chlorolichens. Only the last two groups with green algae have Fv/Fm values as high as those of vascular plants (for a further discussion of these differences, see Lüttge, 2011).
Drying treatments
Air-dry lichen disks were placed on a net fastened 5 mm below the top of each box. Relative humidities of 0%, 33%, 55% and 75% were obtained by placing silica gel and saturated solutions of MnCl2, Mn(NO3)2 and NaCl, respectively (Greenspan, 1977) in the bottom of each box. The boxes were tightly covered with cling film. Four boxes were used for each humidity level, two for each light treatment. Drying lasted for 7 d. Five additional disks were used for water content measurements at each humidity level for both light treatments. After 7 d of treatment at constant RH levels, these lichen disks were immediately weighed with the absolute dry mass subsequently being determined after drying at 70°C for 24 h. All studied lichen species had fairly equal water contents after equilibrium with each relative air humidity level, but P. glauca had slightly higher water contents than the other species (Table 2). The water content of lichens varied from 3.8% to 6% in 0% RH and from 13.7% to 16.3% in 75% RH. This range in RH and corresponding lichen water content spans more or less the entire humidity gradient below which photosynthetic activity in lichens can start. The water potential in the air (Ψair) was calculated using the equation:
Table 2.
Mean percentage water content in lichen thalli above silica gel and various saturated salt solutions after 7 d under 200 μmol m−2 s−1
| RH (%) | Silica or salt solution | Ψair, MPa | Lobaria hallii | Lobaria pulmonaria | Lobaria retigera | Lobaria scrobiculata | Platismatia glauca | Pseudocyphellaria anomala |
|---|---|---|---|---|---|---|---|---|
| 0 | Silica gel | −∞ | 4.2 ± 0.6 | 3.8 ± 0.6 | 5.1 ± 1.2 | 3.8 ± 0.7 | 6.1 ± 0.5 | 4.2 ± 0.6 |
| 35 | MgCl2 | −148 | 8.5 ± 0.1 | 8.6 ± 1.1 | 8.1 ± 0.1 | 7.8 ± 0.2 | 9.9 ± 0.2 | 9.5 ± 1.2 |
| 55 | Mg(NO3)2 | −80 | 11.4 ± 0.4 | 10.8 ± 0.2 | 12.1 ± 0.7 | 10.8 ± 0.1 | 12.3 ± 0.4 | 11.4 ± 0.4 |
| 75 | NaCl | −38 | 15.8 ± 0.1 | 14.3 ± 0.2 | 15.4 ± 0.1 | 14.4 ± 0.6 | 16.3 ± 1.31 | 13.7 ± 0.6 |
RH, relative humidity. Values are mean ± 1SE; n = 5.
| Eqn 1 |
(R, gas constant (8.31441 J mol K−1); T, air temperature assumed to be the same as the salt solution temperature; V, partial molar volume of water (18.0 × 10−6 m3 mol−1)) (Jonsson et al., 2008).
Light treatments
During the entire drying experiment, 50% of the boxes with lichen disks were exposed to medium light (200 μmol m−2 s−1) from a high-intensity light-emitting diode (LED) panel (LED light source, model SL3500; Photon Systems Instruments, Brno, Czech Republic). The light treatment was c. 10% of the maximal irradiance experienced under boreal field conditions (Gauslaa & Solhaug, 2000) and 200 μmol m−2 s−1, which is close to the light saturation point for photosynthesis in L. pulmonaria and L. hallii from our localities (K. A. Solhaug & X. Lie, unpublished), and is frequently encountered in lichen sites of the studied forest type in British Columbia (Coxson & Stevenson, 2007a). The light source had blue, green and red diodes which were individually regulated to give equal irradiance. The light exposure experiment was performed in a temperature-regulated chamber at 14°C. This gave a thallus temperature of 20°C (measured with a thin thermocouple) in closed boxes; the temperature in the shaded salt solutions beneath the lichens was c. 16°C. The boxes with the other 50% of lichen disks above the same salt solutions were kept in continuous darkness for the same period of time. However, they were kept at 20°C to reduce differences in temperature between the light and dark treatments. The light slightly reduced the water content at the three highest RH levels, but not in those kept over silica gel, as shown by an experiment on the Norwegian L. pulmonaria thalli (Fig. 1) as part of testing and developing the experimental set-up. On average, for all RH levels, the lichen water content was 1.5% higher in the dark controls.
Fig. 1.
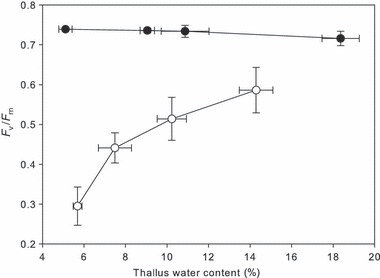
Maximum quantum yield of photosystem II (Fv/Fm; measured in hydrated thalli of Norwegian Lobaria pulmonaria after 48 h of recovery subsequent to drying treatment) as a function of the percentage water content in thalli experiencing 7 d of drying in darkness (closed circles) or at 200 μmol m−2 s−1 (open circles). Drying treatments were given in boxes in which thalli were in equilibrium with 0% (the lowest water content), 35%, 55% and 75% relative humidity. Error bars show standard errors.
After the treatments, disks were taken out of the boxes, placed on moist filter papers and given the same conditions during the 48-h recovery as during preconditioning. Fv/Fm (see earlier) was repeatedly measured during the recovery period.
Statistical analyses
One-way ANOVAs including the Holm–Sidak method (as given in Table 3) and linear regression models (Fig. 4) were computed in SigmaPlot 11.0 (Systat Software Inc., San Jose, CA). Two-way ANOVAs (Table 4) and repeated measures three-way ANOVAs (general linear model; as given in Table 5) were performed in Minitab 16 (Minitab Inc., State College, PA).
Table 3.
Habitat-specific mean Fv/Fm values before and after the start of the drying experiment for specimens selected for darkness and light treatment (200 μmol m−2 s−1)
| Fv/FmBefore start of experiment | Fv/FmAfter drying and 48 h recovery | Fv/FmPercentage of start values | ||||
|---|---|---|---|---|---|---|
| Darkness | Light | Darkness | Light | Darkness | Light | |
| Lobaria pulmonaria | ||||||
| Xeric open | 0.684 ± 0.010a | 0.680 ± 0.015a | 0.724 ± 0.007a | 0.528 ± 0.056a | 106.2 ± 1.2b | 77.5 ± 8.1b |
| Mesic open | 0.702 ± 0.011a | 0.700 ± 0.012a | 0.725 ± 0.004a | 0.533 ± 0.054a | 103.7 ± 1.5b | 77.2 ± 8.0b |
| Mesic closed | 0.736 ± 0.002b | 0.733 ± 0.003b | 0.733 ± 0.003a | 0.431 ± 0.045a | 99.6 ± 0.3a | 58.8 ± 6.1a |
| Platismatia glauca | ||||||
| Xeric open | 0.691 ± 0.013b | 0.686 ± 0.019a | 0.721 ± 0.012b | 0.538 ± 0.040b | 104.5 ± 1.1a | 79.3 ± 5.6b |
| Mesic closed | 0.612 ± 0.020a | 0.651 ± 0.017a | 0.655 ± 0.018a | 0.270 ± 0.053a | 108.0 ± 2.6a | 40.3 ± 7.7a |
| Lobaria hallii | ||||||
| Xeric open | 0.524 ± 0.007ab | 0.520 ± 0.009a | 0.488 ± 0.010a | 0.438 ± 0.011a | 93.2 ± 1.4a | 84.2 ± 1.1a |
| Mesic open | 0.510 ± 0.006a | 0.491 ± 0.009a | 0.474 ± 0.008a | 0.422 ± 0.008a | 92.9 ± 1.0a | 86.2 ± 1.8a |
| Mesic closed | 0.543 ± 0.008b | 0.546 ± 0.009b | 0.503 ± 0.010a | 0.457 ± 0.014a | 93.0 ± 1.0a | 83.6 ± 1.7a |
| Lobaria retigera | ||||||
| Mesic closed | 0.518 ± 0.006 | 0.520 ± 0.005 | 0.494 ± 0.007 | 0.429 ± 0.010 | 95.4 ± 1.3 | 82.5 ± 2.0 |
| Lobaria scrobiculata | ||||||
| Xeric open | 0.521 ± 0.012a | 0.530 ± 0.010a | 0.547 ± 0.007a | 0.521 ± 0.013a | 105.7 ± 1.9b | 98.7 ± 2.6a |
| Mesic open | 0.542 ± 0.007a | 0.551 ± 0.006a | 0.549 ± 0.008a | 0.506 ± 0.014a | 101.4 ± 1.2ab | 91.8 ± 2.2a |
| Mesic closed | 0.548 ± 0.007a | 0.527 ± 0.010a | 0.548 ± 0.007a | 0.492 ± 0.014a | 100.0 ± 0.7a | 93.6 ± 2.4a |
| Pseudocyphellaria anomala | ||||||
| Xeric open | 0.594 ± 0.007a | 0.586 ± 0.013a | 0.580 ± 0.005a | 0.538 ± 0.021a | 97.8 ± 0.9a | 91.0 ± 2.5a |
| Mesic closed | 0.581 ± 0.005a | 0.577 ± 0.004a | 0.572 ± 0.006a | 0.500 ± 0.014a | 98.5 ± 0.9a | 86.7 ± 2.4a |
Values are mean ± 1SE; n = 19–20.
Means combined measurements from all four relative humidity levels during the 7-d experiment. Within each species sampled at more than one site, an ANOVA was run to test whether the sampled sites differed significantly from each other (P < 0.05). Within each species, sites sharing the same letter for a given variable were not statistically different from each other (all pairwise multiple comparison procedures: Holm–Sidak method).
Fig. 4.
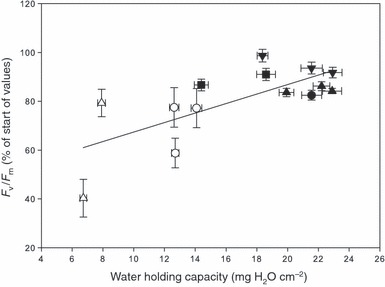
The relationship between thallus water holding capacity (WHC; from Gauslaa & Coxson, 2011) and permanent depression in Fv/Fm measured in this study as a percentage of the start values after a 7-d combined drying and light exposure (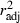 = 0.469; P = 0.005). Open symbols, the chlorolichens Platismatia glauca (triangles apex up) and Lobaria pulmonaria (circles). Closed symbols, Pseudocyphellaria anomala (squares), Lobaria scrobiculata (triangles apex down), L. hallii (triangles apex up) and L. retigera (circles). Each error bar (vertical as well as horizontal) represents ± 1SE (n = 39–40 for WHC and n = 19–20 for Fv/Fm) from each type of habitat (see Table 3).
= 0.469; P = 0.005). Open symbols, the chlorolichens Platismatia glauca (triangles apex up) and Lobaria pulmonaria (circles). Closed symbols, Pseudocyphellaria anomala (squares), Lobaria scrobiculata (triangles apex down), L. hallii (triangles apex up) and L. retigera (circles). Each error bar (vertical as well as horizontal) represents ± 1SE (n = 39–40 for WHC and n = 19–20 for Fv/Fm) from each type of habitat (see Table 3).
Table 4.
Two-way ANOVA for the level of photoinhibition after combined drying and light exposure in Lobaria pulmonaria and Platismatia glauca from different habitats, and exposed to four levels of desiccation (see Fig. 2)
| Species | Lobaria pulmonaria | Platismatia glauca | ||||||
|---|---|---|---|---|---|---|---|---|
| Source | df | F | P | df | F | P | ||
| Relative humidity (RH) | 3 | 35.95 | 0.000 | 3 | 12.25 | 0.000 | ||
| Habitat | 2 | 3.54 | 0.045 | 1 | 54.78 | 0.000 | ||
| RH × habitat | 6 | 3.17 | 0.020 | 3 | 7.32 | 0.003 | ||
| Error | 24 | 16 | ||||||
| Total | 35 | 23 | ||||||
|
77.5 | 82.2 | ||||||
Photoinhibition was expressed as Fv/Fm as a percentage of the start values. Factors were RH drying (0%, 35%, 55% and 75% RH) and habitat types specified in Table 3 for each species.
Table 5.
Repeated measures three-way ANOVA (general linear model) for Fv/Fm after drying at four levels with and without light exposure for six species nested in two photobiont types (green algae or cyanobacteria as main photobiont)
| Source | df | F | P |
|---|---|---|---|
| C1 (humidity light photobiont species) | 507 | 6.34 | 0.000 |
| Time (repeated during recovery process) | 4 | 1332.28 | 0.000 |
| Humidity | 3 | 71.03 | 0.000 x |
| Light | 1 | 1960.80 | 0.000 x |
| Species (nested in photobiont) | 4 | 10.39 | 0.000 x |
| Photobiont | 1 | 189.13 | 0.000 x |
| Time × humidity | 12 | 4.22 | 0.000 |
| Time × light | 4 | 233.32 | 0.000 |
| Time × species (photobiont) | 16 | 15.84 | 0.000 |
| Time × photobiont | 4 | 50.60 | 0.000 |
| Humidity × light | 3 | 55.36 | 0.000 x |
| Humidity × species (photobiont) | 12 | 1.31 | 0.208 x |
| Humidity × photobiont | 3 | 9.65 | 0.000 x |
| Light × species (photobiont) | 4 | 3.19 | 0.013 x |
| Light × photobiont | 1 | 456.97 | 0.000 x |
| Time × humidity × light | 12 | 8.90 | 0.000 |
| Time × humidity × species (photobiont) | 48 | 1.71 | 0.002 |
| Time × humidity × photobiont | 12 | 17.23 | 0.000 |
| Time × light × species (photobiont) | 16 | 4.78 | 0.000 |
| Time × light × photobiont | 4 | 16.18 | 0.028 x |
| Humidity × light × species (photobiont) | 12 | 1.94 | 0.001 x |
| Humidity × light × photobiont | 3 | 5.77 | 0.000 |
| Time × humidity × light × species (photobiont) | 48 | 1.14 | 0.234 |
| Time × humidity × light × photobiont | 12 | 16.05 | 0.000 |
| Error | 2011 | ||
| Total | 2757 |
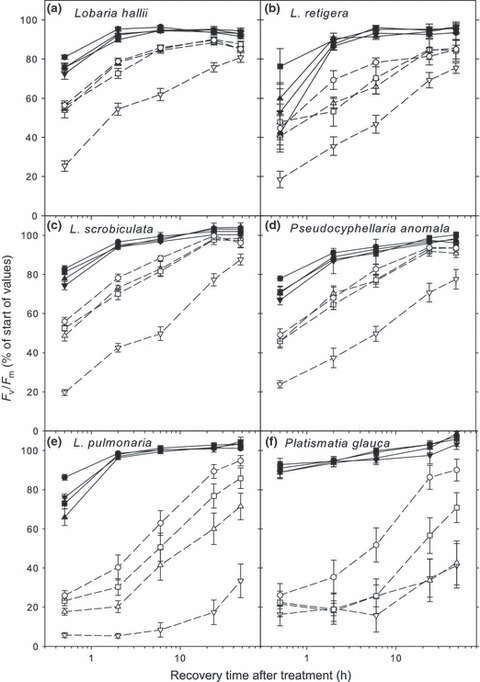
Fv/Fm was repeatedly measured during recovery at 0.5, 2, 6, 24 and 48 h after drying (0%, 35%, 55% and 75% relative humidity (RH) and light treatments (0 and 200 μmol m−2 s−1) lasting for 7 d. Data are shown in Fig. 3. 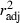 = 91.73%. x, not an exact F test.
= 91.73%. x, not an exact F test.
Results
In general, habitat type had a low impact on the measured variables in the studied lichens (see Table 3) in which Fv/Fm values after the drying experiment had been averaged across all four humidity treatments. Fv/Fm at the start was much more species specific than habitat specific, with the highest values in L. pulmonaria followed by P. glauca. The cyanolichen with the highest Fv/Fm values was P. anomala, whereas the three cyanobacterial Lobaria species had the lowest starting Fv/Fm values. In L. pulmonaria and L. scrobiculata, there was a slight tendency to the highest Fv/Fm value in the most shaded site (Table 3), even after 48 h of preconditioning of hydrated thalli at low light.
After 7 d of desiccation in darkness, followed by moistening and 48 h of recovery at low light, Fv/Fm was hardly affected. Indeed, Fv/Fm after drying in darkness, expressed as a percentage of Fv/Fm before the start, exceeded 100% in the three most widespread species (P. glauca, L. pulmonaria and L. scrobiculata). This showed that the PSII efficiency of these forest lichens was not adversely affected by 1 wk of continuous drying in darkness. Only the two rarest species (L. hallii and L. retigera), apparently restricted to very humid sites, showed slight, but significant, reductions: 7% and 5%, respectively (Table 3). As this reduction was measured after as much as 48 h of recovery at low light, the reduction can be considered to be permanent slight damage.
Using the same drying treatments in combination with medium light (200 μmol m−2 s−1), permanent photoinhibition occurred in all species (Table 3). The chloro- and cephalolichens were clearly the most severely affected, with visible bleached portions in many disks experiencing the hardest drying regimes. In these two functional groups, in which the habitat had the greatest influence, thalli from the closed forests showed the greatest photoinhibition after the combined drying and light exposure (Fig. 2, Table 4). In the cephalolichen (L. pulmonaria; Fig. 2a; Table 4), the effect of habitat was weak, but significant, whereas in the chorolichen (P. glauca; Fig. 2b; Table 4), the effect of habitat was very strong. Platismatia glauca from the open xeric forest was not affected by the level of drying, whereas thalli from the mesic closed forest experienced severely aggravated photoinhibition with decreasing RH (Fig. 2b; Table 4). None of the cyanolichens were as severely affected as those with green algal photobionts. Nevertheless, when drying occurred in combination with light, L. hallii and L. retigera were the most severely affected among the cyanolichens with > 15% reduction in Fv/Fm. The lowest level of permanent photoinhibition occurred in the most widespread cyanolichen (L. scrobiculata: Table 3).
Fig. 2.
Level of photoinhibition after combined drying (0%, 35%, 55% and 75% relative humidity) and light exposure (200 μmol m−2 s−1) in Lobaria pulmonaria and Platismatia glauca from different habitats (black circles, xeric open; gray circles, mesic open; white circles, mesic closed) as specified in Table 3. Each symbol represents the mean ± 1SE. See Table 4 for ANOVA results.
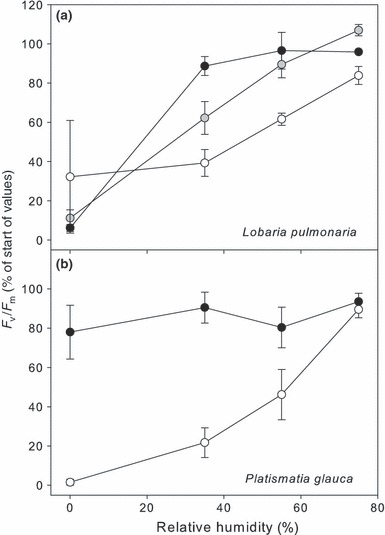
Details on the recovery kinetics in Fv/Fm after each of the four drying treatments with and without light are shown for all species in Fig. 3. As the influence of habitat was relatively low (Table 3), mean values across all habitats are given in Fig. 3. The dataset in Fig. 3 was analyzed by repeated measures ANOVA, with time as the repeated measurement during recovery, and species nested in the two photobiont groups: green alga and cyanobacteria (Table 5). This analysis suggested that up to 91.7% of the variation in the nearly 2800 recordings of Fv/Fm could be accounted for by the variables humidity level, light level, species and time. The ANOVA showed that photobiont type better predicted Fv/Fm than did the species (Table 5).
Fig. 3.
Kinetics during recovery (log-scale) for hydrated thalli of the studied species at low light after 7 d of drying treatment in darkness (closed symbols and solid lines) and in medium light (200 μmol m−2 s−1; open symbols and dotted lines). Each symbol represents the mean ± 1SE. Relative humidity during drying: triangles apex down, 0%; triangles apex up, 35%; squares, 55%; circles, 75%.
Recovery was rapid and complete, or nearly complete, in all lichen species subjected to drying in darkness (Fig. 3). For lichens experiencing drying in darkness, the activation time for restoring normal photosynthesis during hydration after drying exposure was shortest in the widespread P. glauca (Fig. 3), evidenced by high Fv/Fm values already after 30 min. The slowest activation of photosynthesis after drying in darkness occurred in the rare and humidity-dependent L. retigera. Schlensog et al. (2004) made the point that the time to recover is related to the length of active time; lichens that remain active for short times recover most rapidly. Light during drying was the most significant factor for Fv/Fm, with a close to 30 times higher F value than that of the humidity factor (Table 5). The light treatment substantially delayed the activation of photosynthetic responses after subsequent wetting, particularly in the two green algal lichens. Light also significantly aggravated the permanent (48 h) photoinhibition caused by drying (Fig. 3).
Fv/Fm declined with decreasing RH during drying (Fig. 3; Table 5). Furthermore, the standard errors tended to increase with decreasing values of Fv/Fm, particularly at prolonged recovery times. In general, humidity levels during drying did not have major effects on lichens in the absence of light, and the small humidity-dependent effects tended to decrease rapidly after 30 min of recovery. However, all RH treatments strongly impacted the lichens in the presence of light, as evidenced in the ANOVA by the highly significant light × humidity interaction (Table 5). The most severe drying treatment (0% RH) was particularly damaging in the presence of light. In all cyanolichens, the 35%, 55% and 75% RH levels gave clear, but relatively similar, levels of photoinhibition (Fig. 3); the largest contrast was between these humidity levels and the 0% RH treatment. Humidity-dependent differences in Fv/Fm after the drying–light treatment were relatively constant during recovery in cyanolichens, whereas humidity-dependent contrasts in Fv/Fm increased with recovery time in the two chlorolichens. This is one of the patterns that shaped the very strong interaction term between light and photobiont type (Table 5).
Discussion
The old forest lichens studied were highly drying resistant in darkness with slightly greater damage in the two rarest species associated with the most humid climate (Table 3, Fig. 3). Doubling the drying period in darkness from 7 to 14 d did not decrease permanent PSII efficiency in L. pulmonaria (K. A. Solhaug, unpublished). In addition, the CO2 exchange in L. pulmonaria and the cyanolichen Peltigera polydactyla was normal after a 2-wk desiccation period over silica gel at low light/darkness (Kranner et al., 2003). Light, however, strongly aggravated the damage caused by drying. The slight light-dependent differences in water content (Fig. 1) cannot explain the large contrast between damage after drying in light vs darkness (Fig. 3). As light, rather than drying alone, caused the recorded reduction in PSII efficiency, it is reasonable to believe that the susceptible partner is the photobiont. The reduction in PSII efficiency is probably a result of damage to PSII, as it is unlikely that a long-term nonphotochemical quenching caused by xanthophyll cycle pigments (Adams et al., 2002) occurs in our thalli preconditioned for 48 h at low light whilst hydrated. Furthermore, conversion to zeaxanthin is unlikely during light exposure in dry conditions (Štepigováet al., 2008). The cephalo- and chlorolichens with the strongest depression of PSII efficiency were also those in which damage in terms of bleaching occurred. Beyond reasonable doubt, we can conclude that light adversely affects the studied lichens during long dry periods (Table 3, Fig. 3). As studies in Swedish boreal forests have shown that L. pulmonaria can experience continuous periods in the dry state lasting for 700 h (Renhorn et al., 1997), our 7-d exposure is ecologically relevant.
Old forest lichens are caught between a need to maximize light absorbance in active periods and to reduce light absorbance at the photobiont level during dry periods. Insufficient light in hydration periods often limits lichen growth in forests (Gauslaa et al., 2006, 2007; Larsson et al., 2012), as the light received during hydration periods is a strong factor promoting forest lichen growth (Dahlman & Palmqvist, 2003; Gaio-Oliveira et al., 2004). Lichens use at least four strategies to reduce this conflict. Drying induces photoprotection by reducing light transmittance through the upper cortex to the underlying photobionts (Ertl, 1951; Büdel & Lange, 1994; Gauslaa & Solhaug, 2001), by curling lobes that shade the photobiont (Barták et al., 2006), by functional disconnection of components of the photochemical apparatus (Sigfridsson, 1980; Bilger et al., 1989) and by a mechanism that allows reaction centers of PSII to dissipate excess energy in the dry state (Heber et al., 2006, 2010, 2011). For lichens of sun-exposed environments, photoprotective mechanisms are more efficient than for old forest lichens (Gauslaa & Solhaug, 1996). For old forest lichens, however, protective mechanisms cannot always prevent irreversible photoinhibition under field conditions (Gauslaa & Solhaug, 2000; Gauslaa et al., 2001). The low level of intraspecific contrasts in light–drying tolerance between contrasting habitats for each of the studied Peltigerales species (Table 3) suggests that these lichens cannot easily escape damage by acclimation to local conditions. We believe that their association with old forests is at least partly shaped by their inability to cope with long-lasting light and drying in drier parts of their distribution areas. However, the widespread Parmeliaceae species P. glauca seems to be highly flexible with respect to habitat conditions (Fig. 2, Table 4).
The studied cyanolichens were much more resistant to extended dry and light periods than were experimental chloro- and cephalolichens. Based on the exceptionally high drying susceptibility of the cyanolichen P. dissimilis (Green et al., 1991) and the strong association of epiphytic cyanolichens with rainy habitats (e.g. Marini et al., 2011), the high resistance (Table 3, Fig. 3) was unexpected. Nostoc, the photobiont genus in our cyanolichens as well as in the drying-susceptible P. dissimilis, is known to be highly resistant to drying (Lüttge et al., 1995; Lüttge, 2011). Nostoc has extracellular polysaccharides that are important for drying tolerance and rapid recovery of photosynthetic O2 evolution when rewetted (Tamaru et al., 2005). These thick cyanobacterial sheaths store much water (Honegger et al., 1996), slow down desiccation and possibly reduce drying damage. Different drying protocols between our study and the P. dissimilis study (Green et al., 1991) may have caused the contrasting results. By placing hydrated thalli in a ventilated cuvette at very low RH, the drying rate was much faster in P. dissimilis than in our thalli that were first air dried at low light at 14°C and dried further in boxes with low constant RH. PSII of isolated green algal photobionts (Trebouxia ericii) recovers more slowly after rapid drying than after slower drying (Gasulla et al., 2009).
To understand the strong association of epiphytic cyanolichens with humid climates, we may search for explanations other than low drying tolerance. Cyanolichens, unlike chloro- and cephalolichens, need liquid water to restore photosynthesis after drying (Lange et al., 1986). In equilibrium with 90% RH, the cephalolichen L. pulmonaria assimilates carbon in the absence of liquid water, whereas the cyanolichen L. scrobiculata releases carbon, indicating some respiration, but no photosynthesis (Máguas et al., 1995). Every specimen used in our study has known WHC (Gauslaa & Coxson, 2011). If we compare the mean Fv/Fm values (expressed as a percentage of the start values) for all species–habitat combinations after the combined light–drying treatment (Table 2) with the corresponding WHC values reported in Gauslaa & Coxson (2011), there is a significant and positive interspecific regression (Fig. 4; 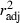 = 0.469; P = 0.005), but no significant intraspecific relationships (data not shown), between these two parameters. There is no overlap between the low WHC and Fv/Fm values in green algal lichens with high values of both parameters in cyanolichens (Fig. 4). The significantly higher WHC in cyanolichens than in cephalo- and chlorolichens, driven by the higher specific thallus mass of these cyanolichens (Gauslaa & Coxson, 2011), is presumably a compensatory mechanism for their inability, as reported by Lange et al. (1986), to use humid air to activate photosynthesis. The studied chloro- and cephalolichens have thinner thalli than cyanolichens (Gauslaa & Coxson, 2011). Thin lichens take up humidity more rapidly than thick and compact lichens (Larson & Kershaw, 1976; Larson, 1981). Water-conserving cyanolichens often replace opportunistic chlorolichens with rapid water uptake and loss along a forested gradient from continental to oceanic climates. The frequent hydration–drying cycles in chlorolichens in continental sites with cool and humid nights allow regular photosynthetic activation and maintenance, which is not the case for cyanolichens. Cyanolichens depend on temporal rain and/or heavy dew fall and a large capacity to store water to maximize the length of rarer photosynthetic events. For example, an increase in WHC from 71 to 357 mg H2O cm−2 in Degelia plumbea prolongs its photosynthetic active period from 3 to 41 h (Gauslaa & Solhaug, 1998). The cyanolichen water economy requires a strong high-light resistance to handle the sometimes long periods between hydration periods, whereas chlorolichens may experience hydration every night in dry periods as a result of nocturnal cooling and the concurring rise in RH. In such a perspective, cyanolichens share a stress-tolerant strategy, whereas chlorolichens occupy the space between a competitive (K) strategy (best represented by the broad-lobed shading and shade-adapted cephalolichen L. pulmonaria) and a ruderal (r) strategy (various chlorolichens, such as the widespread P. glauca) in Grime’s (1977) triangle model of plant life history strategies (later applied for lichens by, for example, Rogers, 1990). At optimal hydration, the cyanolichen L. scrobiculata has rates of CO2 assimilation that are more than twice as high as those of the cephalolichen L. pulmonaria, but substantially lower relative growth rates under field conditions (Larsson et al., 2012). The lower growth rate in L. scrobiculata is consistent with a stress-tolerant strategy.
= 0.469; P = 0.005), but no significant intraspecific relationships (data not shown), between these two parameters. There is no overlap between the low WHC and Fv/Fm values in green algal lichens with high values of both parameters in cyanolichens (Fig. 4). The significantly higher WHC in cyanolichens than in cephalo- and chlorolichens, driven by the higher specific thallus mass of these cyanolichens (Gauslaa & Coxson, 2011), is presumably a compensatory mechanism for their inability, as reported by Lange et al. (1986), to use humid air to activate photosynthesis. The studied chloro- and cephalolichens have thinner thalli than cyanolichens (Gauslaa & Coxson, 2011). Thin lichens take up humidity more rapidly than thick and compact lichens (Larson & Kershaw, 1976; Larson, 1981). Water-conserving cyanolichens often replace opportunistic chlorolichens with rapid water uptake and loss along a forested gradient from continental to oceanic climates. The frequent hydration–drying cycles in chlorolichens in continental sites with cool and humid nights allow regular photosynthetic activation and maintenance, which is not the case for cyanolichens. Cyanolichens depend on temporal rain and/or heavy dew fall and a large capacity to store water to maximize the length of rarer photosynthetic events. For example, an increase in WHC from 71 to 357 mg H2O cm−2 in Degelia plumbea prolongs its photosynthetic active period from 3 to 41 h (Gauslaa & Solhaug, 1998). The cyanolichen water economy requires a strong high-light resistance to handle the sometimes long periods between hydration periods, whereas chlorolichens may experience hydration every night in dry periods as a result of nocturnal cooling and the concurring rise in RH. In such a perspective, cyanolichens share a stress-tolerant strategy, whereas chlorolichens occupy the space between a competitive (K) strategy (best represented by the broad-lobed shading and shade-adapted cephalolichen L. pulmonaria) and a ruderal (r) strategy (various chlorolichens, such as the widespread P. glauca) in Grime’s (1977) triangle model of plant life history strategies (later applied for lichens by, for example, Rogers, 1990). At optimal hydration, the cyanolichen L. scrobiculata has rates of CO2 assimilation that are more than twice as high as those of the cephalolichen L. pulmonaria, but substantially lower relative growth rates under field conditions (Larsson et al., 2012). The lower growth rate in L. scrobiculata is consistent with a stress-tolerant strategy.
Globally, cyanolichens tend to dominate the lower canopy of rain forests, whereas chlorolichens dominate the drier upper canopy (McCune, 1993; McCune et al., 1997). A number of old forest cyanolichens, however, such as L. scrobiculata, can frequently occur in low biomass in open sites from continental inland areas. Such areas often experience a > 15°C drop in air temperature at nights during clear weather. When the temperature drops below the dew point, condensation replenishes the large WHC in cyanolichens, allowing early morning photosynthesis also in clear weather. This occurs in open stands from inland British Columbia (Y. Gauslaa, pers. obs. over a 2-wk period in early August 2011 showing hydration every clear morning), and may explain the strong growth response of L. pulmonaria to canopy gaps in old forests (Coxson & Stevenson, 2007a) and to forest edges (Coxson & Stevenson, 2007b), as well as the increasing growth response of L. retigera to increasing light (openness) at soft forest edges (Stevenson & Coxson, 2008). Cyanolichens of lichenized orders other than Peltigerales often grow on rocks or as soil crusts in arid climates (Büdel et al., 1997; Belnap & Lange, 2005), where hydration by dew in clear nights is the main source of moisture and growth (Lange, 2001). The very high light–drying resistance of cyanolichens may be an ancient trait from before the land became shaded by plants. The epiphytic cyanolichen association with humid forests is probably not formed by a drying-susceptible Nostoc photobiont, but by the need for liquid water for the activation of photosynthesis and/or by a mycobiont being more susceptible than photobionts to drought-induced oxidative stress (Beckett et al., 2003). The extreme sensitivity of L. retigera to edge effects (Stevenson & Coxson, 2008) may be shaped by its lower drying tolerance (Table 3).
The functional group cyanolichens includes cephalolichens (McCune, 1993). Most recent lichen studies applying the functional group concept have adopted this view. However, our study, Gauslaa & Coxson (2011) and photosynthetic studies all show that cephalolichens differ from cyanolichens in a number of functions and share important traits with chlorolichens. Field evidence shows that cephalolichens and cyanolichens tend to have only partly overlapping ecological niches. We thus follow Goward’s (2012) recommendations and distinguish between cephalo- and cyanolichens.
In conclusion, the old forest lichens studied were resistant to drying, whereas light exposure during drying could be highly detrimental. Cyanolichens, even rare old forest species associated with very humid climates, were substantially more resistant to combined drying–light treatments than were widespread chloro- and cephalolichens. The results lead to the following interpretation: cyanolichens depending on liquid water and large internal water storage require strong drying–light resistance to handle long periods between hydration events, whereas chlorolichens, which additionally and regularly activate photosynthesis by nocturnal humid air, do not need such high resistance.
References
- Adams WW, Demmig-Adams B, Rosenstiel TN, Brightwell AK, Ebbert V. Photosynthesis and photoprotection in overwintering plants. Plant Biology. 2002;4:545–557. [Google Scholar]
- Barták M, Solhaug KA, Vrábliková H, Gauslaa Y. Curling during desiccation protects the foliose lichen Lobaria pulmonaria against photoinhibition. Oecologia. 2006;149:553–560. doi: 10.1007/s00442-006-0476-2. [DOI] [PubMed] [Google Scholar]
- Beckett RP, Minibayeva FV, Vylegzhanina NN, Tolpysheva T. High rates of extracellular superoxide production by lichens in the suborder Peltigerineae correlate with indices of high metabolic activity. Plant, Cell & Environment. 2003;26:1827–1837. [Google Scholar]
- Belnap J, Lange OL. Lichens and microfungi in biological soil crusts: community structure, physiology, and ecological functions. In: Dighton J, White JF, Oudemans P, editors. The fungal community. Its organization and role in the ecosystem. Boca Raton, FL, USA: CRC Press; 2005. pp. 117–138. [Google Scholar]
- Bilger W, Rimke S, Schreiber U, Lange OL. Inhibition of energy-transfer to photosystem II in lichens by dehydration: different properties of reversibility with green and blue–green phycobionts. Journal of Plant Physiology. 1989;134:261–268. [Google Scholar]
- Büdel B, Karsten U, Garcia-Pichel F. Ultraviolet-absorbing scytonemin and mycosporine-like amino acid derivates in exposed, rock-inhabiting cyanobacterial lichens. Oecologia. 1997;112:165–172. doi: 10.1007/s004420050296. [DOI] [PubMed] [Google Scholar]
- Büdel B, Lange OL. The role of cortical and epinecral layers in the lichen genus Peltula. Cryptogamic Botany. 1994;4:262–269. [Google Scholar]
- Campbell J, Bradfield GE, Prescott CE, Fredeen AL. The influence of overstorey Populus on epiphytic lichens in subboreal spruce forests of British Columbia. Canadian Journal of Forest Research. 2010;40:143–154. [Google Scholar]
- Cowan DA, Green TGA, Wilson AT. Lichen metabolism.1. The use of tritium labeled water in studies of anhydrobiotic metabolism in Ramalina celastri and Peltigera polydactyla. New Phytologist. 1979;82:489–503. [Google Scholar]
- Coxson DS, Stevenson SK. Growth rate responses of Lobaria pulmonaria to canopy structure in even-aged and old-growth cedar–hemlock forests of central-interior British Columbia, Canada. Forest Ecology and Management. 2007a;242:5–16. [Google Scholar]
- Coxson DS, Stevenson SK. Influence of high-contrast and low-contrast forest edges on growth rates of Lobaria pulmonaria in the inland rainforest, British Columbia. Forest Ecology and Management. 2007b;253:103–111. [Google Scholar]
- Dahlman L, Palmqvist K. Growth in two foliose tripartite lichens, Nephroma arcticum and Peltigera aphthosa: empirical modelling of external vs internal factors. Functional Ecology. 2003;17:821–831. [Google Scholar]
- Demmig-Adams B, Adams WW, III, Czygan FC, Schreiber U, Lange OL. Differences in the capacity for radiationless energy dissipation in the photochemical apparatus of green and blue–green algal lichens associated with differences in carotenoid composition. Planta. 1990a;180:582–589. doi: 10.1007/BF02411457. [DOI] [PubMed] [Google Scholar]
- Demmig-Adams B, Adams WW, III, Green TGA, Czygan FC, Lange OL. Differences in the susceptibility to light stress in two lichens forming a phycosymbiodeme, one partner possessing and one lacking the xanthophyll cycle. Oecologia. 1990b;84:451–456. doi: 10.1007/BF00328159. [DOI] [PubMed] [Google Scholar]
- Demmig-Adams B, Maguas C, Adams WW, III, Meyer A, Kilian E, Lange OL. Effect of high light on the efficiency of photochemical energy conversion in a variety of lichen species with green and blue–green phycobionts. Planta. 1990c;180:400–409. doi: 10.1007/BF00198792. [DOI] [PubMed] [Google Scholar]
- Dilks TJK, Proctor MCF. Photosynthesis, respiration and water-content in bryophytes. New Phytologist. 1979;82:97–114. [Google Scholar]
- Doering M, Coxson D. Riparian alder ecosystems as epiphytic lichen refugia in sub-boreal spruce forests of British Columbia. Botany. 2010;88:144–157. [Google Scholar]
- Ertl L. Über die Lichtverhältnisse in Laubflechten. Planta. 1951;39:245–270. [Google Scholar]
- Gaio-Oliveira G, Dahlman L, Maguas C, Palmqvist K. Growth in relation to microclimatic conditions and physiological characteristics of four Lobaria pulmonaria populations in two contrasting habitats. Ecography. 2004;27:13–28. [Google Scholar]
- Galloway DJ. Studies in Pseudocyphellaria (lichens). I. The New Zealand species. Bulletin of the British Museum (Natural History), Botany Series. 1988;17:1–267. [Google Scholar]
- Gasulla F, de Nova PG, Esteban-Carrasco A, Zapata JM, Barreno E, Guera A. Dehydration rate and time of desiccation affect recovery of the lichenic algae Trebouxia erici: alternative and classical protective mechanisms. Planta. 2009;231:195–208. doi: 10.1007/s00425-009-1019-y. [DOI] [PubMed] [Google Scholar]
- Gauslaa Y, Coxson D. Interspecific and intraspecific variations in water storage in epiphytic old forest foliose lichens. Botany. 2011;89:787–798. [Google Scholar]
- Gauslaa Y, Lie M, Solhaug KA, Ohlson M. Growth and ecophysiological acclimation of the foliose lichen Lobaria pulmonaria in forests with contrasting light climates. Oecologia. 2006;147:406–416. doi: 10.1007/s00442-005-0283-1. [DOI] [PubMed] [Google Scholar]
- Gauslaa Y, Ohlson M, Solhaug KA, Bilger W, Nybakken L. Aspect-dependent high-irradiance damage in two transplanted foliose forest lichens, Lobaria pulmonaria and Parmelia sulcata. Canadian Journal of Forest Research. 2001;31:1639–1649. [Google Scholar]
- Gauslaa Y, Palmqvist K, Solhaug KA, Holien H, Hilmo O, Nybakken L, Myhre LC, Ohlson M. Growth of epiphytic old forest lichens across climatic and successional gradients. Canadian Journal of Forest Research. 2007;37:1832–1845. [Google Scholar]
- Gauslaa Y, Solhaug KA. Differences in the susceptibility to light stress between epiphytic lichens of ancient and young boreal forest stands. Functional Ecology. 1996;10:344–354. [Google Scholar]
- Gauslaa Y, Solhaug KA. The significance of thallus size for the water economy of the cyanobacterial old forest lichen Degelia plumbea. Oecologia. 1998;116:76–84. doi: 10.1007/s004420050565. [DOI] [PubMed] [Google Scholar]
- Gauslaa Y, Solhaug KA. High-light damage in air-dry thalli of the old forest lichen Lobaria pulmonaria– interactions of irradiance, exposure duration and high temperature. Journal of Experimental Botany. 1999;50:697–705. [Google Scholar]
- Gauslaa Y, Solhaug KA. High-light-intensity damage to the foliose lichen Lobaria pulmonaria within a natural forest: the applicability of chlorophyll fluorescence methods. Lichenologist. 2000;32:271–289. [Google Scholar]
- Gauslaa Y, Solhaug KA. Fungal melanins as a sun screen for symbiotic green algae in the lichen Lobaria pulmonaria. Oecologia. 2001;126:462–471. doi: 10.1007/s004420000541. [DOI] [PubMed] [Google Scholar]
- Gauslaa Y, Solhaug KA. Photoinhibition in lichens depends on cortical characteristics and hydration. Lichenologist. 2004;36:133–143. [Google Scholar]
- Goward T. Twelve readings on the lichen thallus: XII. Formal propositions. Evansia. 2012;29:28–49. [Google Scholar]
- Goward T, McCune B, Meidinger DV. The lichens of British Columbia illustrated keys, part I: foliose and squamulose species. Victoria, B.C.: British Colombia Ministry of Forest, Research Branch; 1994. Special Report Series. [Google Scholar]
- Goward T, Spribille T. Lichenological evidence for the recognition of inland rain forests in western North America. Journal of Biogeography. 2005;32:1209–1219. [Google Scholar]
- Green TGA, Kilian E, Lange OL. Pseudocypellaria dissimilis: a desiccation-sensitive, highly shade-adapted lichen from New Zealand. Oecologia. 1991;85:498–503. doi: 10.1007/BF00323761. [DOI] [PubMed] [Google Scholar]
- Greenspan L. Humidity fixed points of binary saturated aqueous solutions. Journal of Research of the National Bureau of Standards. Section A: Physics and Chemistry. 1977;81:89–96. [Google Scholar]
- Grime JP. Evidence for existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. American Naturalist. 1977;111:1169–1194. [Google Scholar]
- Heber U, Bilger W, Türk R, Lange OL. Photoprotection of reaction centres in photosynthetic organisms: mechanisms of thermal energy dissipation in desiccated thalli of the lichen Lobaria pulmonaria. New Phytologist. 2010;185:459–470. doi: 10.1111/j.1469-8137.2009.03064.x. [DOI] [PubMed] [Google Scholar]
- Heber U, Lange OL, Shuvalov VA. Conservation and dissipation of light energy as complementary processes: homoiohydric and poikilohydric autotrophs. Journal of Experimental Botany. 2006;57:1211–1223. doi: 10.1093/jxb/erj104. [DOI] [PubMed] [Google Scholar]
- Heber U, Soni V, Strasser RJ. Photoprotection of reaction centers: thermal dissipation of absorbed light energy vs charge separation in lichens. Physiologia Plantarum. 2011;142:65–78. doi: 10.1111/j.1399-3054.2010.01417.x. [DOI] [PubMed] [Google Scholar]
- Honegger R, Peter M, Scherrer S. Drought-induced structural alterations at the mycobiont–photobiont interface in a range of foliose macrolichens. Protoplasma. 1996;190:221–232. [Google Scholar]
- Jonsson AV, Moen J, Palmqvist K. Predicting lichen hydration using biophysical models. Oecologia. 2008;156:259–273. doi: 10.1007/s00442-008-0990-5. [DOI] [PubMed] [Google Scholar]
- Jørgensen PM, Tønsberg T. Lobariaceae. In: Jørgensen PM, Tønsberg T, Vitikainen O, editors. Nordic lichen flora. Vol. 3. Uppsala, Sweden: Nordic Lichen Society; 2007. pp. 77–86. [Google Scholar]
- Kranner I, Beckett RP, Hochman A, Nash TH., III Desiccation-tolerance in lichens: a review. Bryologist. 2008;111:576–593. [Google Scholar]
- Kranner I, Zorn M, Turk B, Wornik S, Beckett RR, Batic F. Biochemical traits of lichens differing in relative desiccation tolerance. New Phytologist. 2003;160:167–176. doi: 10.1046/j.1469-8137.2003.00852.x. [DOI] [PubMed] [Google Scholar]
- Lange OL. Photosynthesis of soil-crust biota as dependent on environmental factors. In: Belnap J, Lange OL, editors. Biological soil crusts: structure, function, and management. Berlin, Germany: Springer Verlag; 2001. pp. 217–240. [Google Scholar]
- Lange OL, Kilian E, Ziegler H. Water vapor uptake and photosynthesis in lichens: performance differences in species with green and blue–green algae as phycobionts. Oecologia. 1986;71:104–110. doi: 10.1007/BF00377327. [DOI] [PubMed] [Google Scholar]
- Larson DW. Differential wetting in some lichens and mosses: the role of morphology. Bryologist. 1981;84:1–15. [Google Scholar]
- Larson DW, Kershaw KA. Studies on lichen-dominated systems. XVIII. Morphological control of evaporation in lichens. Canadian Journal of Botany. 1976;54:2061–2073. [Google Scholar]
- Larsson P, Solhaug KA, Gauslaa Y. Seasonal partitioning of growth into biomass and area expansion in a cephalolichen and a cyanolichen of the old forest genus Lobaria. New Phytologist. 2012;194:991–1000. doi: 10.1111/j.1469-8137.2012.04130.x. [DOI] [PubMed] [Google Scholar]
- Lüttge U. Cyanobacteria: multiple stresses, desiccation-tolerant photosynthesis and di-nitrogen fixation. Ecological Studies. 2011;215:23–43. [Google Scholar]
- Lüttge U, Büdel B, Ball E, Strube F, Weber P. Photosynthesis of terrestrial cyanobacteria under light and desiccation stress as expressed by chlorophyll fluorescence and gas exchange. Journal of Experimental Botany. 1995;46:309–319. [Google Scholar]
- Máguas C, Griffiths H, Broadmeadow MSJ. Gas-exchange and carbon-isotope discrimination in lichens – evidence for interactions between CO2-concentrating mechanisms and diffusion limitation. Planta. 1995;196:95–102. [Google Scholar]
- Marini L, Nascimbene J, Nimis PL. Large-scale patterns of epiphytic lichen species richness: photobiont-dependent response to climate and forest structure. Science of the Total Environment. 2011;409:4381–4386. doi: 10.1016/j.scitotenv.2011.07.010. [DOI] [PubMed] [Google Scholar]
- McCune B. Gradients in epiphyte biomass in three Pseudotsuga–Tsuga forests of different ages in western Oregon and Washington. Bryologist. 1993;96:405–411. [Google Scholar]
- McCune B, Amsberry KA, Camacho FJ, Clery S, Cole C, Emerson C, Felder G, French P, Greene D, Harris R, et al. Vertical profile of epiphytes in a Pacific Northwest old-growth forest. Northwest Science. 1997;71:145–152. [Google Scholar]
- Meidinger D, Pojar J. Ecosystems of British Columbia. Victoria, BC, Canada: Research Brand, Ministry of Forests; 1991. Special Report Series. [Google Scholar]
- Nash TH, III, Reiner A, Demmig-Adams B, Kilian E, Kaiser WM, Lange OL. The effect of atmospheric dessication and osmotic water stress on photosynthesis and dark respiration of lichens. New Phytologist. 1990;116:269–276. [Google Scholar]
- Radies D, Coxson D, Johnson C, Konwicki K. Predicting canopy macrolichen diversity and abundance within old-growth inland temperate rainforests. Forest Ecology and Management. 2009;259:86–97. [Google Scholar]
- Renhorn KE, Esseen PA, Palmqvist K, Sundberg B. Growth and vitality of epiphytic lichens I. Responses to microclimate along a forest edge–interior gradient. Oecologia. 1997;109:1–9. doi: 10.1007/s004420050051. [DOI] [PubMed] [Google Scholar]
- Rogers RW. Ecological strategies of lichens. Lichenologist. 1990;22:149–162. [Google Scholar]
- Rose F. Lichenological indicators of age and environmental continuity in woodlands. In: Brown DH, editor. Lichenology: progress and problems. London, UK: Academic Press; 1976. pp. 279–307. [Google Scholar]
- Sancho LG, de la Torre R, Horneck G, Ascaso C, de los Rios A, Pintado A, Wierzchos J, Schuster M. Lichens survive in space: results from the 2005 LICHENS experiment. Astrobiology. 2007;7:443–454. doi: 10.1089/ast.2006.0046. [DOI] [PubMed] [Google Scholar]
- Scheidegger C, Schroeter B, Frey B. Structural and functional processes during water vapour uptake and desiccation in selected lichens with green algal photobionts. Planta. 1995;197:399–409. [Google Scholar]
- Schlensog M, Pannewitz S, Green TGA, Schroeter B. Metabolic recovery of continental Antarctic cryptogams after winter. Polar Biology. 2004;27:399–408. [Google Scholar]
- Sigfridsson B. Some effects of humidity on the light reaction of photosynthesis in the lichens Cladonia implexa and Collema flaccidum. Physiologia Plantarum. 1980;49:320–326. [Google Scholar]
- Štepigová J, Gauslaa Y, Cempírková-Vrábliková H, Solhaug KA. Irradiance prior to and during desiccation improves the tolerance to excess irradiance in the desiccated state of the old forest lichen Lobaria pulmonaria. Photosynthetica. 2008;46:286–290. [Google Scholar]
- Stevenson SK, Coxson DS. Growth responses of Lobaria retigera to forest edge and canopy structure in the inland temperate rainforest, British Columbia. Forest Ecology and Management. 2008;256:618–623. [Google Scholar]
- Tamaru Y, Takani Y, Yoshida T, Sakamoto T. Crucial role of extracellular polysaccharides in desiccation and freezing tolerance in the terrestrial cyanobacterium Nostoc commune. Applied and Environmental Microbiology. 2005;71:7327–7333. doi: 10.1128/AEM.71.11.7327-7333.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre R, Sancho LG, Horneck G, de los Rios A, Wierzchos J, Olsson-Francis K, Cockell CS, Rettberg P, Berger T, de Vera JPP, et al. Survival of lichens and bacteria exposed to outer space conditions – results of the Lithopanspermia experiments. Icarus. 2010;208:735–748. [Google Scholar]
- Wang T, Hamann A, Aitken SN, Spittlehouse DL. 2006. Climate BC, V. 2. A program to generate climate normal data for genecology and climate change. Studies in British Columbia. [WWW document] URL http://genetics.forestry.ubs.ca/cfgc/climate-models.html [accessed 1 March 2011]


