Abstract
Prospective data are lacking concerning the effect of reduced mycophenolic acid (MPA) dosing on efficacy and the influence of concomitant tacrolimus exposure. The Mycophenolic Renal Transplant (MORE) Registry is a prospective, observational study of de novo kidney transplant patients receiving MPA therapy under routine management. The effect of MPA dose reduction, interruption, or discontinuation (dose changes) was assessed in 870 tacrolimus-treated patients: 375 (43.1%) reduced tacrolimus (≤7 ng/mL at baseline) and 495 (56.9%) standard tacrolimus (>7 ng/mL); enteric-coated mycophenolate sodium 589 (67.7%) and mycophenolate mofetil 281 (32.3%). During baseline to month 1, months 1–3, months 3–6, and months 6–12, 9.3% (78/838), 16.6% (132/794), 20.7% (145/701), and 13.1% (70/535) patients, respectively, required MPA dose changes. These patients experienced an increased risk of biopsy-proven acute rejection at one yr with tacrolimus exposure either included in the model (hazard ratio [HR] 2.60, 95% CI 1.28–5.29, p = 0.008) or excluded (HR 2.58, 95% CI 1.28–5.23, p = 0.008). MPA dose changes were significantly associated with one yr graft failure when tacrolimus exposure was included (HR 2.23; 95% CI 1.01–4.89, p = 0.047) but not when tacrolimus exposure was excluded (HR 2.16; 95% CI 0.99–4.79; p = 0.054). These results suggest that reducing or discontinuing MPA can adversely affect graft outcomes regardless of tacrolimus trough levels.
Keywords: cellcept, EC-MPS, kidney transplantation, mycophenolate mofetil, mycophenolate, myfortic, outcomes, tacrolimus
Mycophenolic acid (MPA) is a routine component of immunosuppression regimens following kidney transplantation (1). However, dose-dependent toxicity frequently necessitates reduction or interruption of MPA dosing (2–8). Retrospective data indicate that leukopenia is the most frequent trigger for MPA dose reduction in kidney transplantation, followed by gastrointestinal adverse events and infection (3). Such changes appear to have a marked effect on transplant outcomes. Mycophenolate mofetil (MMF) reduction or discontinuation is associated with a significant increase in acute rejection (3, 9) and graft loss (10) in kidney transplant patients. Large-scale retrospective analyses of registry data from the United States (5, 8) and Europe (11) have also demonstrated a significantly greater risk of kidney allograft loss following cessation of MMF (5, 8, 11) or a dose reduction of 50% or more (5, 8).
Current data, however, are subject to limitations. First, prospective data of reduced-dose MPA are limited to small single-center trials (12, 13). Second, little is known about the relationship between changes to MPA dosing and concomitant exposure to calcineurin inhibitors (CNIs). It has not been determined whether reduction or elimination of MPA treatment confers the same risk in patients receiving reduced-exposure or standard-exposure CNI therapy. Moreover, the available data are largely derived from studies in which either cyclosporine or mixed CNI therapy (cyclosporine or tacrolimus) was administered, whereas many centers now routinely use tacrolimus-based immunosuppression. Third, evidence is sparse concerning the comparative effect of the MMF and enteric-coated mycophenolate sodium (EC-MPS) formulations. While efficacy and safety of the two formulations were similar in pivotal trials (14, 15), subsequent trials using patient-reported outcomes have suggested that conversion from MMF to EC-MPS may improve the gastrointestinal symptom burden (16–18) with the potential to minimize dose reductions or discontinuations (19) and permit maintenance of higher MPA dosing (20–23).
The Mycophenolic Renal Transplant (MORE) Registry prospectively collects data on kidney transplant patients receiving MPA and tacrolimus at the time of transplantation, followed to five yr post-transplant. The purpose of the current analysis was to test the hypothesis that reduction, interruption, or discontinuation of MPA compromises immunosuppressive efficacy regardless of tacrolimus exposure. Specifically, data were analyzed to determine whether such changes to MPA dosing are associated with increased risks of biopsy-proven acute rejection (BPAR) and early graft failure and whether this association is influenced by the extent of exposure to tacrolimus.
Material and methods
Study design
The MORE Registry is a five-yr, international, prospective, observational study of de novo renal transplant patients receiving MPA therapy (either EC-MPS or MMF). Eligible sites were selected to meet geographic and size diversity. The study is performed under routine clinical conditions according to local practice. Recruitment started in June 2007 and closed in May 2010. Data collection is ongoing. The MORE Registry is conducted according to the Declaration of Helsinki, and informed consent is obtained for all enrolled patients.
Study population
Adult (≥18 yr) recipients of a deceased or living donor kidney transplant administered MPA (EC-MPS or MMF) prior to hospital discharge were eligible for enrollment. Patients were excluded if they had received or planned to receive a bone marrow or other solid organ transplant, if they were enrolled or were planned to enroll into an investigational clinical trial involving an immunosuppressive agent that is either blinded or unapproved by the Food and Drug Administration, or if they are unlikely to complete five yr of follow-up. To minimize the possibility of center-imposed bias, investigators at each site agreed to seek participation of all eligible de novo renal transplant recipients seen at the study site within two wk of transplantation. However, enrollment to the registry was capped at a ratio of EC-MPS /MMF recipients of approximately 2:1. The current analysis was restricted to patients who were receiving tacrolimus maintenance immunosuppression at baseline, as this group represented 95% of patients in the MORE Registry.
MPA therapy
The type of MPA utilized (EC-MPS or MMF) and any dose adjustments were determined by center-specific protocols.
Data collection and evaluation
Data are recorded at baseline (defined as within two wk of transplantation) and at specified intervals based on routine post-transplantation clinic visits, that is, months 1, 3, 6, and 12 and annually thereafter to five yr. Only data already available to the site clinical team are collected. Information is entered by designated investigator staff to a web-based electronic data capture system with real-time data validation checks to ensure data quality. Data undergo an automated data quality review followed by data management review and both electronic and on-site monitoring.
At each post-baseline study visit, data collection includes occurrence of BPAR, graft and patient survival status, type of immunosuppression, laboratory results, and adverse events. Any reduction, interruption, or discontinuation of MPA dose since the previous visit was recorded and included under the general term “MPA dose change.” The reason for MPA dose change was recorded. EC-MPS dose was converted to the MMF equivalent by multiplying the EC-MPS dose by 1.3889 (24). Tacrolimus trough level was recorded at each clinic visit, using immunoassay-based methods in >89% of cases at each time point. Patients were categorized as receiving reduced (≤7 ng/mL) or standard (>7 ng/mL) tacrolimus exposure (25). Serum creatinine at baseline and at months 1, 3, 6, and 12 was used to calculate estimated glomerular filtration rate (eGFR) using the abbreviated Modification of Diet in Renal Disease four-variable formula (26).
Statistical methods
Data recorded to June 2010 are presented for the first year post-transplant. All analyses were performed using data pooled across centers and are exploratory. Between-group comparisons were performed based on analysis of variance (ANOVA) for continuous variables and Pearson's chi-square test for categorical variables. Cox proportional hazards modeling was used to analyze adjusted risk of first episode of BPAR and graft failure, with MPA dose change included in the models as a time-varying covariate. To evaluate the impact of tacrolimus exposure on the effect of MPA dose change, two multivariable regression models were fit to the data: one included tacrolimus exposure as a time-dependent covariate, and one did not include tacrolimus exposure. A third model, in which only tacrolimus exposure was included, was also fit to the data. All regression models included recipient age and donor type (living vs. deceased donor). Regression models for BPAR and eGFR also included baseline MPA dose (per mg), recipient race and gender, primary indication for renal transplantation, baseline serum creatinine (per mg/dL), and donor age. Because of low event rates, these factors were not included in the multivariable Cox proportional hazards model for graft failure. Missing data were not imputed in any of the analyses. Analyses were performed using SAS statistical software (SAS Institute, Cary, NC, USA). p < 0.05 was considered statistically significant.
Results
In total, 870 patients from 40 centers received tacrolimus at the time of transplant, provided baseline tacrolimus trough concentration data, and formed the analysis population. The mean (SD) duration of follow-up was 429 (280) d (median 370, range 10–1107 d). The majority of patients were men (63.8%, 555/870), with a mean age of approximately 52 yr, and fewer than 9.0% of patients (78/870) had undergone a previous kidney transplant.
Tacrolimus immunosuppression
At baseline, 375 patients (43.1%) were receiving reduced tacrolimus exposure (≤7 ng/mL), and 495 (56.9%) were receiving standard exposure (>7 ng/mL). There was a slightly older recipient age, more white donors, a lower rate of pre-transplant dialysis, and more living donors with fewer donations after cardiac death in the standard-exposure group (Table 1). As would be expected, the proportion of patients with tacrolimus trough concentration >7 ng/mL peaked at months 1 and 3 post-transplant (Fig. 1).
Table 1.
Recipient and donor characteristics by baseline tacrolimus exposure and type of MPA treatment
| Baseline tacrolimus exposurea | Baseline MPA | |||||
|---|---|---|---|---|---|---|
| Reduced tacrolimus N = 375 | Standard tacrolimus N = 495 | p Valueb | EC-MPS N = 589 | MMF N = 281 | p Valueb | |
| Follow-up (d) | 0.034 | |||||
| Mean (SD) | 410 (266) | 443 (289) | 415 (271) | 459 (296) | ||
| Median | 365 | 386 | 367 | 388 | ||
| Range | 10–1107 | 11–1094 | 10–1107 | 14–1094 | ||
| Recipient | ||||||
| Age (yr), mean (SD) | 50.6 (13.5) | 52.5 (13.4) | 0.039 | 52.4 (13.4) | 50.2 (13.5) | 0.025 |
| Male gender, n (%) | 246 (65.6) | 309 (62.4) | 370 (62.8) | 185 (65.8) | ||
| Race/ethnicity, n (%)c | <0.001d | |||||
| White | 236 (62.9) | 355 (71.7) | 400 (67.9) | 191 (68.0) | ||
| African American | 116 (30.9) | 92 (18.6) | 143 (24.3) | 65 (23.1) | ||
| Other | 28 (7.5) | 50 (10.1) | 50 (8.5) | 28 (10.0) | ||
| Previous renal transplant, n (%) | 34 (9.1) | 44 (8.9) | 54 (9.2) | 24 (8.5) | ||
| Pre-transplant dialysis, n (%) | 317 (84.5) | 391 (79.0) | 0.038 | 484 (82.2) | 224 (79.7) | |
| Reason for transplantation, n (%) | ||||||
| Hypertension/nephrosclerosis | 89 (23.7) | 110 (22.2) | 140 (23.8) | 59 (21.0) | ||
| Diabetes mellitus | 83 (22.1) | 119 (24.0) | 133 (22.6) | 69 (24.6) | ||
| Polycystic disease | 46 (12.3) | 54 (10.9) | 71 (12.1) | 29 (10.3) | ||
| Glomerulonephritis/glomerular disease | 52 (13.9) | 73 (14.8) | 93 (15.8) | 32 (11.4) | ||
| Other | 90 (24.0) | 114 (23.0) | 122 (20.7) | 82 (29.2) | ||
| Unknown | 15 (4.0) | 25 (5.1) | 30 (5.1) | 10 (3.6) | ||
| Peak panel-reactive antibody <30%, n/N,% | 274/329 (83.3) | 368/445 (82.7) | 433/523 (82.8) | 209/251 (83.3) | ||
| Delayed graft function, n/N (%) | 53/360 (14.7) | 78/485 (16.1) | 85/574 (14.8) | 46/271 (17.0) | ||
| Donor | ||||||
| Age (yr), mean (SD) | 42.2 (14.7) | 40.7 (14.6) | 41.3 (14.8) | 41.5 (12.3) | ||
| Age ≥60 yr, n (%) | 33 (8.9) | 47 (9.6) | 56 (9.6) | 24 (8.6) | ||
| Male gender, n (%) | 197 (52.5) | 244 (49.5) | 316 (53.7) | 125 (44.6) | 0.012 | |
| Type of donor, n/N (%) | 0.017d | |||||
| Deceased (heart beating) | 160/374 (42.8) | 223/494 (45.1) | 272/587 (46.3) | 111/281 (39.5) | ||
| Donation after cardiac death | 66/374 (17.6) | 51/494 (10.3) | 84/587 (14.3) | 33/281 (11.7) | ||
| Living related | 82/374 (21.9) | 117/494 (23.7) | 127/587 (21.6) | 72/281 (25.6) | ||
| Living unrelated | 66/374 (17.6) | 103/494 (20.9) | 104/587 (17.7) | 65/281 (23.1) | ||
| Expanded criteria donor, n/N (%) | 49/374 (13.1) | 56/492 (11.4) | 73/587 (12.4) | 32/279 (11.5) | ||
| Cold ischemia time (h), mean (SD) | 11.0 (9.9) | 10.3 (10.1) | 11.0 (9.9) | 9.8 (10.3) | ||
MPA, mycophenolic acid; EC-MPS, enteric-coated mycophenolate sodium; MMF, mycophenolate mofetil; SD, standard deviation.
All differences were non-significant unless stated otherwise. Significant p values (<0.05) are shown.
Within two wk post-transplant.
p Value is based on analysis of variance for continuous variables and Pearson's chi-square test for categorical variables.
Patients could be entered in more than one category.
p Value refers to a comparison across all categories.
Fig. 1.
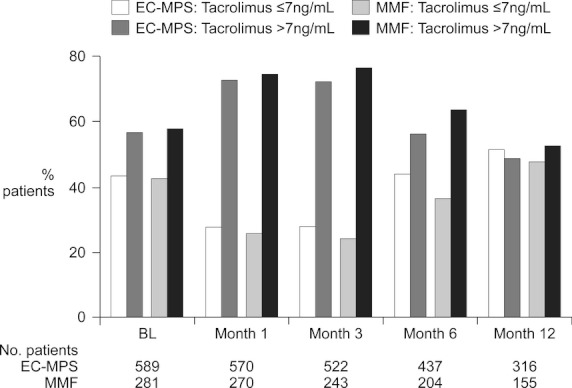
Proportion of patients receiving reduced tacrolimus (trough concentration ≤7 ng/mL) and standard tacrolimus (trough concentration >7 ng/mL) at baseline (i.e., within two wk of transplantation) and months 1, 3, 6, and 12.
Patients receiving reduced tacrolimus exposure received a significantly lower dose of MPA at baseline, were less likely to receive corticosteroid induction therapy, were less likely to receive rabbit antithymocyte antibody induction, and were more likely to receive alemtuzumab induction, compared with those given a standard tacrolimus regimen (Table 2).
Table 2.
Immunosuppression at baseline according to tacrolimus exposure and type of MPA treatment at baselinea
| Baseline tacrolimus exposurea | Baseline MPA treatmenta | |||||
|---|---|---|---|---|---|---|
| Reduced tacrolimus N = 375 | Standard tacrolimus N = 495 | p Value | EC-MPS N = 589 | MMF N = 281 | p Value | |
| Tacrolimus | ||||||
| Dose (mg/d) | 6.4 (3.9) | 6.6 (3.8) | 0.479 | 6.3 (3.9) | 6.9 (3.6) | 0.018 |
| Trough concentration (ng/mL) | 4.4 (1.7) | 11.9 (4.1) | <0.001 | 8.6 (4.8) | 8.9 (5.2) | 0.303 |
| EC-MPSb | ||||||
| n | 256 (68.3) | 333 (67.3) | 589 (100.0) | – | ||
| Dose (MMF equivalent)b (mg/d) | 1806 (471) | 1909 (309) | 0.002 | 1864 (391) | – | 0.599 |
| <2 g/d, n (%) | 65 (25.4) | 42 (12.7) | 107 (18.2) | – | ||
| 2 g/d, n (%) | 183 (71.5) | 287 (86.5) | 470 (79.9) | – | ||
| >2 g/d, n (%) | 8 (3.1) | 3 (0.9) | 11 (1.9) | – | ||
| MMF | ||||||
| n | 119 (31.7) | 162 (32.7) | – | 281 (100.0) | ||
| Dose (mg/d) | 1771 (470) | 1906 (382) | 0.009 | – | 1849 (426) | 0.599 |
| <2 g/d, n (%) | 39 (32.8) | 26 (16.1) | – | 65 (23.1) | ||
| 2 g/d, n (%) | 76 (63.9) | 130 (80.3) | – | 206 (73.3) | ||
| >2 g/d, n%) | 4 (3.4) | 6 (3.7) | – | 10 (3.6) | ||
| Corticosteroids | ||||||
| Induction, n (%) | 315 (84.0) | 440 (88.9) | 0.0350 | 508 (86.3) | 247 (87.9) | 0.501 |
| Maintenance, n (%) | 214 (57.1) | 286 (57.8) | 0.834 | 349 (59.3) | 151 (53.7) | 0.124 |
| Dose (mg/d) | 33.8 (50.4) | 36.7 (51.8) | 0.532 | 35.6 (53.8) | 35.1 (44.6) | 0.923 |
| Induction therapy, n (%) | ||||||
| IL-2 receptor antibody | 100 (26.7) | 119 (24.0) | 0.377 | 144 (24.5) | 75 (26.7) | 0.476 |
| Antithymocyte antibody (rabbit) | 204 (54.4) | 329 (66.5) | <0.001 | 376 (63.8) | 157 (55.9) | 0.024 |
| Alemtuzumab | 59 (15.7) | 38 (7.7) | <0.001 | 56 (9.5) | 41 (14.6) | 0.026 |
EC-MPS, enteric-coated mycophenolate sodium; MMF, mycophenolate mofetil; MPA, mycophenolic acid; SD, standard deviation.
Continuous variables are shown as mean (SD).
Within two wk post-transplantation. Two outliers (tacrolimus dose recorded as 1080 and 360 mg/d were excluded).
EC-MPS dose was converted to the MMF equivalent by multiplying the EC-MPS dose by 1.3889 (24).
MtPA immunosuppression
Approximately two-thirds of patients (n = 589, 67.7%) were receiving EC-MPS at baseline; the remaining 281 patients (32.3%) received MMF. Compared to the MMF-treated cohort, patients receiving EC-MPS had a significantly shorter follow-up period (reflecting more recent adoption of the newer formulation), were slightly older, and were more likely to receive a graft from a donor aged 60 yr or older (Table 1). The proportion of patients with a baseline dose <2 g/d (in MMF equivalent doses) was 18.2% (107/589) in the EC-MPS group and 23.1% (65/281) in the MMF group (n.s.); mean baseline dose was similar with either formulation. Concomitant immunosuppression, including tacrolimus exposure (Fig. 1), was similar in the MMF and EC-MPS groups other than a greater incidence of rabbit antibody induction in the EC-MPS cohort and a greater incidence of alemtuzumab induction in the MMF cohort (Table 2).
The mean dose of EC-MPS (in MMF equivalent doses) and MMF was 1850 ± 381 and 1789 ± 443 mg/d at month 1 (p = 0.044), 1752 ± 469 and 1657 ± 493 mg/d at month 3 (p = 0.011), 1594 ± 518 and 1506 ± 533 mg/d at month 6 (p = 0.051), and 1545 ± 507 and 1448 ± 540 mg/d at month 12 (p = 0.057). Post-baseline, 10 patients switched from EC-MPS to MMF: the reasons were economic (2), acute rejection (2), optimization of therapy (1), administrative (1), and other (4). Thirty-three patients switched from MMF to EC-MPS owing to gastrointestinal adverse events (23), viral adverse event (1), hematologic event (1), or economic reasons (1); the reason for switch was missing for seven patients.
During the periods baseline to month 1, months 1–3, months 3–6, and months 6–12, 9.3% (78/838), 16.6% (132/794), 20.7% (145/701), and 13.1% (70/535) patients, respectively, required one or more MPA dose change. The most frequent modification was MPA dose reduction (69–85% of cases [Table 3]). There were no significant differences in the proportion of EC-MPS- or MMF-treated patients requiring a MPA dose change at any period during the first 12 months post-transplant, or any marked variation in the causes (Table 3). Patients who required a MPA dose change had a lower mean tacrolimus trough concentration at all time points, which was significant at month 1 (p = 0.047) and month 12 (p = 0.014), and received a slightly lower dose of corticosteroids than those in whom the initial MPA dose remained unchanged (Table 4).
Table 3.
Incidence and causes of MPA dose changes by time post-transplantation according to type of MPA treatment at baseline, n (%)
| EC-MPS | MMF | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline to month 1 N = 569 | Months 1–3 N = 537 | Months 3–6 N = 474 | Months 6–12 N = 358 | Baseline to Month 1 N = 269 | Months 1–3 N = 257 | Months 3–6 N = 227 | Months 6–12 N = 177 | |
| MPA dose change | 58 (10.2) | 85 (15.8) | 103 (21.7) | 47 (13.1) | 20 (7.4) | 47 (18.3) | 42 (18.5) | 23 (13.0) |
| MPA reduced | 49 (84.5) | 66 (77.7) | 77 (74.8) | 34 (72.3) | 17 (85.0) | 38 (80.9) | 29 (69.1) | 18 (78.3) |
| MPA interrupted | 3 (5.2) | 6 (7.1) | 8 (7.8) | 4 (8.5) | 2 (10.0) | 0 | 5 (11.9) | 0 |
| MPA discontinued | 6 (10.3) | 13 (15.3) | 18 (17.5) | 9 (19.2) | 1 (5.0) | 9 (19.2) | 8 (19.1) | 5 (21.7) |
| p Value for MPA dose change EC-MPS vs. MMF | 0.200 | 0.384 | 0.324 | 0.966 | ||||
| Reason(s)a | ||||||||
| Hematologicb | 20 (3.5) | 44 (8.2) | 54 (11.4) | 14 (3.9) | 8 (3.0) | 29 (11.3) | 27 (11.9) | 9 (5.1) |
| Gastrointestinal adverse eventb | 35 (6.2) | 30 (5.6) | 24 (5.1) | 15 (4.2) | 11 (4.1) | 17 (6.6) | 5 (2.2) | 3 (1.7) |
| Viral adverse eventb | 4 (1.7) | 15 (2.8) | 28 (5.9) | 16 (4.5) | 1 (0.4) | 4 (1.6) | 13 (5.7) | 11 (6.2) |
| CMV infectionb | 1 (0.2) | 4 (0.7) | 7 (1.5) | 7 (2.0) | 0 | 1 (0.4) | 3 (1.3) | 4 (2.3) |
| BKV infectionb | 3 (0.5) | 12 (2.2) | 22 (4.6) | 9 (2.5) | 1 (0.4) | 3 (1.2) | 11 (4.8) | 8 (4.5) |
| Acute rejection | 1 (0.2) | 0 | 2 (0.4) | 2 (0.6) | 1 (0.4) | 0 | 2 (0.9) | 2 (1.1) |
| Chronic allograft nephropathy | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bone-related adverse event | 0 | 1 (0.2) | 0 | 1 (0.3) | 0 | 1 (0.4) | 0 | 0 |
| Nephrotoxicity | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Diabetes mellitus | 0 | 0 | 0 | 1 (0.3) | 0 | 0 | 0 | 0 |
| Compliance | 0 | 0 | 1 (0.2) | 0 | 0 | 0 | 0 | 0 |
EC-MPS, enteric-coated mycophenolate sodium; MMF, mycophenolate mofetil; MPA, mycophenolic acid; BKV, BK polyoma virus; CMV, cytomegalovirus.
Month 1 = reporting period between baseline and month 1; month 3 = reporting period between month 1 and month 3; month 6 = reporting period between month 3 and month 6; month 12 = reporting period between month 6 and month 12.
More than one reason could be indicated as the reason for a MPA dose change.
All differences between EC-MPS and MMF were non-significant at all time points. p Values were not calculated for less-frequent reasons.
Table 4.
Tacrolimus dose and levels and corticosteroid dose by MPA dose changes at 1, 3, 6, and 12 months post-transplant
| Tacrolimusb | ||||
|---|---|---|---|---|
| MPA dose since last visita | Dose (mg/d) | Trough concentration (ng/mL) | Corticosteroids Dose (mg/d) | |
| Month 1 | No dose change | 7.0 (4.7) n = 751 | 9.7 (4.7) n = 746 | 15.6 (24.3) n = 475 |
| Dose change | 7.1 (4.6) n = 77 | 8.6 (3.2) n = 77 | 15.2 (6.7) n = 29 | |
| p Valuec | 0.861 | 0.047 | 0.928 | |
| Month 3 | No dose change | 6.2 (4.1) n = 634 | 9.3 (3.9) n = 624 | 10.1 (25.2) n = 393 |
| Dose change | 6.7 (4.2) n = 129 | 9.0 (3.7) n = 127 | 8.7 (8.5) n = 56 | |
| p Valuec | 0.225 | 0.310 | 0.684 | |
| Month 6 | No dose change | 6.0 (3.6) n = 511 | 8.1 (3.2) n = 498 | 6.6 (4.6) n = 325 |
| Dose change | 5.6 (3.5) n = 138 | 7.7 (3.2) n = 133 | 6.6 (3.7) n = 87 | |
| p Valuec | 0.174 | 0.213 | 0.956 | |
| Month 12 | No dose change | 5.6 (3.6) n = 413 | 7.7 (3.2) n = 402 | 6.5 (6.9) n = 263 |
| Dose change | 5.3 (2.9) n = 63 | 6.7 (2.1) n = 61 | 6.2 (7.7) n = 52 | |
| p Valuec | 0.570 | 0.014 | 0.751 | |
MPA, mycophenolic acid.
Doses are shown as mean (SD). MPA dose change included dose reduction, interruption, or discontinuation.
For patients in whom tacrolimus exposure was known at each time point.
Outliers excluded at months 1, 6 and 12.
For MPA dose vs. no MPA dose change.
Efficacy
The unadjusted incidence of BPAR from baseline to month 1, months 1–3, months 3–6, and months 6–12 was 4.7% (39/823), 1.3% (10/751), 1.3% (8/631), and 2.4% (11/463), respectively, among patients for whom tacrolimus exposure was known at each time point (Table 5). Twelve-month risk of BPAR was 9.3% (95% CI 7.1–11.4%) based on Kaplan–Meier estimates. A further three episodes of BPAR were reported, which were not confirmed by biopsy. Multivariate analysis showed a significantly higher risk of BPAR in patients with a MPA dose change that was unaffected by the inclusion of tacrolimus exposure as a covariate (Table 6) that is, patients with a MPA dose change were at a similarly increased risk of BPAR regardless of concomitant tacrolimus trough level. Consistent with this, the unadjusted incidence of BPAR in patients with reduced or standard tacrolimus exposure was similar for patients who did or did not require a MPA dose change (Table 5), and there was no significant association between tacrolimus exposure and risk of BPAR after adjustment for confounding variables (hazard ratio [HR] 0.83, 95% CI 0.45–1.53, p = 0.545 [Table 6]).
Table 5.
Unadjusted incidence of BPAR according to MPA dose changes and baseline tacrolimus exposure, n/N (%)a MPA dose change included dose reduction, interruption, or discontinuation
| No MPA dose change | MPA dose change | |||||
|---|---|---|---|---|---|---|
| Reduced tacrolimus | Standard tacrolimus | All | Reduced tacrolimus | Standard tacrolimus | All | |
| Baseline to Month 1 | 22/554 (4.0) | 11/192 (5.7) | 33/746 (4.4) | 3/46 (6.5) | 3/31 (9.7) | 6/77 (7.8) |
| Months 1–3 | 3/460 (0.7) | 3/164 (1.8) | 6/624 (1.0) | 3/90 (3.3) | 1/37 (2.7) | 4/127 (3.2) |
| Months 1–6 | 1/292 (0.3) | 5/206 (2.4) | 6/498 (1.2) | 1/77 (1.3) | 1/56 (1.8) | 2/133 (1.5) |
| Months 6–12 | 3/207 (1.5) | 5/195 (2.6) | 8/402 (2.0) | 0/24 (0) | 3/37 (8.1) | 3/61 (4.9) |
MPA, mycophenolic acid; BPAR, biopsy-proven acute rejection.
For patients in whom tacrolimus exposure was known at each time point.
Table 6.
Risk of BPAR at one yr and graft loss at one yr after adjustment for confounding variables (Cox regression analysis)
| HR | 95% CI | p Value | |
|---|---|---|---|
| BPAR at one yr | |||
| MPA dose change | |||
| Tacrolimus exposure included as a covariable | 2.60 | 1.28–5.29 | 0.008 |
| Tacrolimus exposure not included as a covariable | 2.58 | 1.28–5.23 | 0.008 |
| Reduced tacrolimus exposure | 0.83 | 0.45–1.53 | 0.545 |
| Graft loss at one yr | |||
| MPA dose change | |||
| Tacrolimus exposure included as a covariable | 2.23 | 1.01–4.89 | 0.047 |
| Tacrolimus exposure not included as a covariable | 2.16 | 0.99–4.79 | 0.054 |
| Reduced tacrolimus exposure | 1.17 | 0.56–2.44 | 0.684 |
HR, hazard ratio; CI, confidence interval; MPA, mycophenolic acid; BPAR, biopsy-proven acute rejection.
MPA dose change included dose reduction, interruption, or discontinuation.
Twelve-month graft survival was 95.6% (95% CI 94.0–97.2%) based on Kaplan–Meier estimates. Cox regression modeling showed MPA dose change to be associated with more than a twofold increase in risk of graft failure at one yr, an association that was significant when tacrolimus exposure was included as a covariable in the model (Table 6). There was no evidence of a statistically significant association between tacrolimus exposure and risk of graft failure at one yr (HR for reduced exposure vs. standard exposure 1.17; 95% CI 0.56–2.44; p = 0.684).
Renal function
eGFR was significantly higher in patients with no change in MPA dose at month 1: mean 54.8 (19.0) mL/min/1.73 m2 vs. 45.1 (17.6) mL/min/1.73 m2 in patients requiring a MPA dose change (p < 0.001), but similar between groups thereafter. Baseline eGFR was significantly higher in the standard-tacrolimus group (43.7 [25.5] mL/min/1.73 m2) than in the reduced-tacrolimus group (37.2 [43.7] mL/min/1.73 m2, p = 0.0004). However, eGFR was subsequently similar between groups: mean (SD) values at 1, 3, 6, and 12 months post-transplantation were 53.2 (18.7), 56.8 (18.4), 59.0 (20.2), and 59.5 (20.3) mL/min/1.73 m2 in the reduced-exposure group, respectively, vs. 52.2 (19.6), 56.2 (18.1), 57.5 (18.7), and 56.4 (19.6) mL/min/1.73 m2 for the standard-exposure group (p = n.s. at each time point).
Discussion
In this first prospective analysis of the impact of MPA dosing on graft outcomes following kidney transplantation, reduction, interruption, or discontinuation of MPA treatment was associated with more than a twofold increase in the risk of both BPAR and graft failure. Notably, the risk that patients would require a MPA dose change was unaffected by concomitant tacrolimus exposure.
To our knowledge, the current analysis is the first to show that the increased risk of BPAR in patients with MPA dose reduction, interruption, or discontinuation is not influenced by whether the patient is receiving standard or reduced tacrolimus exposure. Patients who did not continue to receive their initial MPA dose experienced more than a 2.5-fold increase in adjusted risk of BPAR vs. those in whom MPA dose remained unchanged, a difference that was virtually unaffected by the inclusion of tacrolimus exposure in the model. In an early dose-finding study of MMF, in which kidney transplant patients were given doses of between 1000 mg/d and 3.5 g/d, there was a clear correlation between MMF dose and incidence of rejection (27), and previous retrospective studies have confirmed that MPA dose reductions or discontinuation incur a higher risk of rejection (3, 9), but concomitant CNI concentrations were not described. Evidence from clinical trials (25, 28, 29) indicates that reduced CNI exposure does not influence rejection rates in MPA-treated patients but, conversely, changes to MPA dosing have not been reported. The recent Symphony study observed no difference in the one-yr (25) or three-yr (28) incidence of BPAR in kidney transplant patients receiving reduced or standard cyclosporine exposure in combination with MMF. Similarly, a meta-analysis of 19 randomized studies undertaken in kidney transplant patients receiving MPA found that acute rejection rates were not affected by CNI exposure reduction (29), consistent with data from retrospective series (30, 31).
The risk of graft failure was also significantly increased by more than twofold in patients who required a MPA dose change, regardless of tacrolimus level. Adequate MPA dosing appears essential for optimal graft outcomes. Retrospective registry analyses have previously shown significantly higher rates of graft loss following cessation of MMF (an increase of between 1.5- and 3.2-fold [5, 8, 11]) or a dose reduction of 50% or more (1.3–2.4-fold) (5, 8, 11), consistent with our results. Here, the absolute rate of BPAR was relatively low (7.8% in patients for whom the MPA dose was changed by month 1), and although data on the severity of BPAR were not consistently captured and cannot be analyzed reliably, mild episodes are likely to have accounted for at least some of the reported rejection events and may have had a relatively minor effect on subsequent graft survival (32, 33). Nevertheless, the differences in BPAR between groups appear to account for the variation in graft survival rates.
Hematologic adverse events were the most frequent cause of MPA dose change during months 1–6, declining thereafter. MPA dose changes in response to BK polyoma virus infection (34) peaked during months 3–6. Gastrointestinal events as a cause of MPA dose changes became less frequent after month 6, possibly due to a lessening of tacrolimus-related gastrointestinal effects (35) as tacrolimus exposure decreased over time. The pattern of MPA dose changes must, however, be interpreted with caution as patients who were most susceptible to complications withdrew from the study throughout follow-up, leaving patients who were progressively less likely to require a MPA dose change remaining in the analysis. No consistent differences between EC-MPS and MMF were observed in terms of either the rate or causes of MPA dose changes, including gastrointestinal events. The pivotal comparative trial of EC-MPS vs. MMF in de novo kidney transplant recipients showed no difference in the frequency of dose changes owing to gastrointestinal intolerance (14), but more sensitive patient-reported outcomes were not used (16–18). Here, the dose of MPA was maintained at a significantly higher level in the EC-MPS group vs. the MMF group during the first three months post-transplant, with a trend to significance thereafter. This is consistent with recent findings from a randomized trial of kidney transplants with gastrointestinal symptoms in which significantly more patients tolerated a dose increase with EC-MPS than with MMF (20). In the current analysis, 23 patients converted from MMF to EC-MPS for gastrointestinal symptoms, but there were no switches from EC-MPS to MMF for this cause.
The strengths of this study include the prospective data collection, the correct use of a time-dependent variable for MPA dose changes and tacrolimus exposure, and a relatively large patient population representative of routine clinical practice. Drug dosage changes and rejection episodes were captured in detail during routine monitoring, although timing of data collection was not necessarily consistent across centers. However, as an observational study, the analysis inevitably has limitations. First, a causal relationship between MPA dosing and efficacy outcomes cannot be established. An interventional trial in which the MPA dose is administered below the recommended level without a medical indication would be considered unethical. Second, transplant centers were free to select immunosuppression according to local protocols, and the potential for bias in patient selection cannot be ruled out. Preferential use of EC-MPS or MMF in particular patients seems unlikely as centers typically have only one MPA treatment on their inpatient hospital formulary. Perhaps more pertinently, indications for MPA dose adjustment and tacrolimus exposure were not protocol-mandated, and reasons for dosing choices are not known. The observation that tacrolimus exposure was lower in patients who required a MPA dose change might reflect an attempt to reduce all drugs known to exert gastrointestinal effects in symptomatic patients, lowering the exposure of all immunosuppressant agents in patients with BK infection, or a more aggressive immunosuppression minimization strategy at certain transplant centers. Similarly, steroid dosing or withdrawal was not protocol-specified. Third, MPA concentrations were not measured, so the effect of MPA exposure on efficacy cannot be assessed, although data regarding the benefit of measuring MPA levels are inconclusive (36–39).
Despite these limitations, the findings of this prospective analysis of patients managed under routine clinical conditions suggest that caution should be exercised when the MPA dose is reduced or discontinued in patients receiving tacrolimus, regardless of the extent of tacrolimus exposure.
Acknowledgments
The authors would like to thank the MORE Study Principal Investigators for their invaluable contribution and Jianmin Wang of RTI Health Solutions, Durham, NC, and William Irish of CTI for statistical and writing support. The MORE Registry is funded by Novartis Pharmaceuticals Corporation.
Author's contributions
All authors recruited patients to the MORE Study and provided data, reviewed and approved the manuscript, and gave approval for submission.
References
- 1.Meier-Kriesche HU, Li S, Gruessner RW, et al. Immunosuppression: evolution in practice and trends, 1994–2004. Am J Transplant. 2006;6:1111. doi: 10.1111/j.1600-6143.2006.01270.x. [DOI] [PubMed] [Google Scholar]
- 2.Bunnapradist S, Ambühl PM. Impact of gastrointestinal-related side effects on mycophenolate mofetil dosing and potential therapeutic strategies. Clin Transplant. 2008;22:815. doi: 10.1111/j.1399-0012.2008.00892.x. [DOI] [PubMed] [Google Scholar]
- 3.Knoll GA, MacDonald I, Khan A, van Walraven C. Mycophenolate mofetil dose reduction and the risk of acute rejection after renal transplantation. J Am Soc Nephrol. 2003;14:2381. doi: 10.1097/01.asn.0000079616.71891.f5. [DOI] [PubMed] [Google Scholar]
- 4.Pelletier RP, Akin B, Henry ML, Elkhammas EA, Rajab A, Ferguson RM. The impact of mycophenolate mofetil dosing patterns on clinical outcome after renal transplantation. Clin Transplant. 2003;17:200. doi: 10.1034/j.1399-0012.2003.00026.x. [DOI] [PubMed] [Google Scholar]
- 5.Machnicki G, Ricci JF, Brennan DC, Schnitzler MA. Economic impact and long-term graft outcomes of mycophenolate mofetil dosage modifications following gastrointestinal complications in renal transplant recipients. Pharmacoeconomics. 2008;26:951. doi: 10.2165/00019053-200826110-00007. [DOI] [PubMed] [Google Scholar]
- 6.Hardinger KL, Brennan DC, Lowell J, Schnitzler MA. Long-term outcome of gastrointestinal complications in renal transplant patients treated with mycophenolate mofetil. Transplant Int. 2004;17:609. doi: 10.1007/s00147-004-0768-6. [DOI] [PubMed] [Google Scholar]
- 7.Cooper M, Deering KL, Slakey DP, et al. Comparing outcomes associated with dose manipulations of enteric-coated mycophenolate sodium versus mycophenolate mofetil in renal transplant recipients. Transplantation. 2009;88:514. doi: 10.1097/TP.0b013e3181b0e65e. [DOI] [PubMed] [Google Scholar]
- 8.Bunnapradist S, Lentine KL, Burroughs TE, et al. Mycophenolate mofetil dose reductions and discontinuations after gastrointestinal complications are associated with renal transplant graft failure. Transplantation. 2006;82:102. doi: 10.1097/01.tp.0000225760.09969.1f. [DOI] [PubMed] [Google Scholar]
- 9.Tierce JC, Porterfield-Baxa J, Petrilla AA, Kilburg A, Ferguson R. Impact of mycophenolate mofetil (MMF)-related gastrointestinal complications and MMF dose alterations on transplant outcomes and healthcare costs in renal recipients. Clin Transplant. 2005;19:779. doi: 10.1111/j.1399-0012.2005.00421.x. [DOI] [PubMed] [Google Scholar]
- 10.Shah S, Collett D, Johnson R, Raftery M, Rudge C, Yaqoob MM. The effect of mycophenolate mofetil and azathioprine dose on renal allograft outcome in the United Kingdom. Transplantation. 2008;86:1035. doi: 10.1097/TP.0b013e318186dc81. [DOI] [PubMed] [Google Scholar]
- 11.Opelz G, Döhler B. Effect on kidney graft survival of reducing or discontinuing maintenance immunosuppression after the first year posttransplant. Transplantation. 2008;86:371. doi: 10.1097/TP.0b013e31817fdddb. [DOI] [PubMed] [Google Scholar]
- 12.Khosroshahi HT, Shoja MM, Peyrovifar A, Hashemi SR, Amjadi M. Mycophenolate mofetil dose reduction in renal transplant recipients: a 5-year follow-up study. Transplant Proc. 2009;41:2797. doi: 10.1016/j.transproceed.2009.07.036. [DOI] [PubMed] [Google Scholar]
- 13.Yeung S, Tsang WK, Tong KL, et al. Primary immunosuppression with tacrolimus and low-dose mycophenolate mofetil in renal transplant recipients. Transplant Proc. 2004;36:2084. doi: 10.1016/j.transproceed.2004.08.110. [DOI] [PubMed] [Google Scholar]
- 14.Salvadori M, Holzer H, de Mattos A, et al. ERL B301 Study Groups Enteric-coated mycophenolate sodium is therapeutically equivalent to mycophenolate mofetil in de novo renal transplant patients. Am J Transplant. 2004;4:231. doi: 10.1046/j.1600-6143.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- 15.Budde K, Curtis J, Knoll G, et al. ERL B302 Study Group Enteric-coated mycophenolate sodium can be administered in maintenance renal transplant patients: results of a 1-year study. Am J Transplant. 2004;4:237. doi: 10.1046/j.1600-6143.2003.00321.x. [DOI] [PubMed] [Google Scholar]
- 16.Langone AJ, Chan L, Bolin P, Cooper M. Enteric-coated mycophenolate sodium versus mycophenolate mofetil in renal transplant recipients experiencing gastrointestinal intolerance: a multicenter, double-blind, randomized study. Transplantation. 2011;91:470. doi: 10.1097/TP.0b013e318205568c. [DOI] [PubMed] [Google Scholar]
- 17.Chan L, Mulgaonkar S, Walker R, Arns W, Ambühl P, Schiavelli R. Patient-reported gastrointestinal symptom burden and health-related quality of life following conversion from mycophenolate mofetil to enteric-coated mycophenolate sodium. Transplantation. 2006;81:1290. doi: 10.1097/01.tp.0000209411.66790.b3. [DOI] [PubMed] [Google Scholar]
- 18.Bolin P, Tanriover B, Zibari GB, et al. Improvement in 3-month patient-reported gastrointestinal symptoms after conversion from mycophenolate mofetil to enteric-coated mycophenolate sodium in renal transplant patients. Transplantation. 2007;84:1443. doi: 10.1097/01.tp.0000290678.06523.95. [DOI] [PubMed] [Google Scholar]
- 19.Sollinger HW, Sundberg AK, Leverson G, Voss BJ, Pirsch JD. Mycophenolate mofetil versus enteric-coated mycophenolate sodium: a large, single-center comparison of dose adjustments and outcomes in kidney transplant recipients. Transplantation. 2010;89:446. doi: 10.1097/TP.0b013e3181ca860d. [DOI] [PubMed] [Google Scholar]
- 20.Shehata M, Bhandari S, Venkat-Raman G, et al. Effect of conversion from mycophenolate mofetil to enteric-coated mycophenolate sodium on maximum tolerated dose and gastrointestinal symptoms following kidney transplantation. Transpl Int. 2009;22:821. doi: 10.1111/j.1432-2277.2009.00877.x. [DOI] [PubMed] [Google Scholar]
- 21.Bilodeau JF, Montambault P, Wolff JL, Lemire J, Masse M. Evaluation of tolerability and ability to increase immunosuppression in renal transplant patients converted from mycophenolate mofetil to enteric-coated mycophenolate sodium. Transplant Proc. 2009;41:3683. doi: 10.1016/j.transproceed.2009.06.183. [DOI] [PubMed] [Google Scholar]
- 22.Massari P, Duro-Garcia V, Girón F, et al. myPROMS LatAm Study Group Safety assessment of the conversion from mycophenolate mofetil to enteric-coated mycophenolate sodium in stable renal transplant recipients. Transplant Proc. 2005;37:916. doi: 10.1016/j.transproceed.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 23.Sabbatini M, Capone D, Gallo R, et al. EC-MPS permits lower gastrointestinal symptom burden despite higher MPA exposure in patients with severe MMF-related gastrointestinal side-effects. Fundam Clin Pharmacol. 2009;23:617. doi: 10.1111/j.1472-8206.2009.00711.x. [DOI] [PubMed] [Google Scholar]
- 24.Arns W, Breuer S, Choudhury S, et al. Enteric-coated mycophenolate sodium delivers bioequivalent MPA exposure compared with mycophenolate mofetil. Clin Transplant. 2005;19:199. doi: 10.1111/j.1399-0012.2004.00318.x. [DOI] [PubMed] [Google Scholar]
- 25.Ekberg H, Tedesco-Silva H, Demirbas A, et al. ELITE-Symphony Study Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 2007;357:2562. doi: 10.1056/NEJMoa067411. [DOI] [PubMed] [Google Scholar]
- 26.Poggio ED, Wang X, Weinstein DM, et al. Assessing glomerular filtration rate by estimation equations in kidney transplant recipients. Am J Transplant. 2006;6:100. doi: 10.1111/j.1600-6143.2005.01140.x. [DOI] [PubMed] [Google Scholar]
- 27.Sollinger HW, Deierhoi MH, Belzer FO, Diethelm AG, Kauffman RS. RS-61443–a phase I clinical trial and pilot rescue study. Transplantation. 1992;53:428. doi: 10.1097/00007890-199202010-00031. [DOI] [PubMed] [Google Scholar]
- 28.Ekberg H, Bernasconi C, Tedesco-Silva H, et al. Calcineurin inhibitor minimization in the Symphony study: observational results 3 years after transplantation. Am J Transplant. 2009;9:1876. doi: 10.1111/j.1600-6143.2009.02726.x. [DOI] [PubMed] [Google Scholar]
- 29.Moore J, Middleton L, Cockwell P, et al. Calcineurin inhibitor sparing with mycophenolate in kidney transplantation: a systemic review and meta-analysis. Transplantation. 2009;87:591. doi: 10.1097/TP.0b013e318195a421. [DOI] [PubMed] [Google Scholar]
- 30.Cosio FG, Amer H, Grande JP, Larson TS, Stegall MD, Griffin MD. Comparison of low versus high tacrolimus levels in kidney transplantation: assessment of efficacy by protocol biopsies. Transplantation. 2007;83:411. doi: 10.1097/01.tp.0000251807.72246.7d. [DOI] [PubMed] [Google Scholar]
- 31.Sánchez-Fructuoso AI, de la Higuera MA, Giorgi M, et al. Inadequate mycophenolic acid exposure and acute rejection in kidney transplantation. Transplant Proc. 2009;41:2104. doi: 10.1016/j.transproceed.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 32.Meier-Kriesche H-U, Schold JD, Srinivas TR, Kaplan B. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant. 2004;4:378. doi: 10.1111/j.1600-6143.2004.00332.x. [DOI] [PubMed] [Google Scholar]
- 33.McDonald S, Russ G, Campbell S, Chadban S, On behalf of ANZDATA Kidney transplant rejection in Australia and New Zealand: relationships between rejection and graft outcome. Am J Transplant. 2007;7:1201. doi: 10.1111/j.1600-6143.2007.01759.x. [DOI] [PubMed] [Google Scholar]
- 34.Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO Clinical Practice Guideline for the Care of Kidney Transplant Recipients. Am J Transplant. 2009;9(Suppl 3):S1. doi: 10.1111/j.1600-6143.2009.02834.x. [DOI] [PubMed] [Google Scholar]
- 35.Webster AC, Woodroffe RC, Taylor RS, Chapman JR, Craig JC. Tacrolimus versus cyclosporin as primary immunosuppression for kidney transplant recipients: meta-analysis and meta-regression of randomised trial data. Br Med J. 2005;331:810. doi: 10.1136/bmj.38569.471007.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuypers DR, Meur YL, Cantarovich M, et al. Transplantation Society (TTS) Consensus Group on TDM of MPA Consensus report on therapeutic drug monitoring of mycophenolic acid in solid organ transplantation. Clin J Am Soc Nephrol. 2010;5:341. doi: 10.2215/CJN.07111009. [DOI] [PubMed] [Google Scholar]
- 37.Le Meur Y, Büchler M, Thierry A, et al. Individualized mycophenolate mofetil dosing based on drug exposure significantly improves patient outcomes after renal transplantation. Am J Transplant. 2007;7:2496. doi: 10.1111/j.1600-6143.2007.01983.x. [DOI] [PubMed] [Google Scholar]
- 38.Gaston RS, Kaplan B, Shah T, et al. Fixed- or controlled-dose mycophenolate mofetil with standard- or reduced-dose calcineurin inhibitors: the opticept trial. Am J Transplant. 2009;9:1607. doi: 10.1111/j.1600-6143.2009.02668.x. [DOI] [PubMed] [Google Scholar]
- 39.van Gelder T, Silva HT, de Fijter JW, et al. Comparing mycophenolate mofetil regimens for de novo renal transplant recipients: the Fixed-Dose Concentration-Controlled Trial. Transplantation. 2008;86:1043. doi: 10.1097/TP.0b013e318186f98a. [DOI] [PubMed] [Google Scholar]


