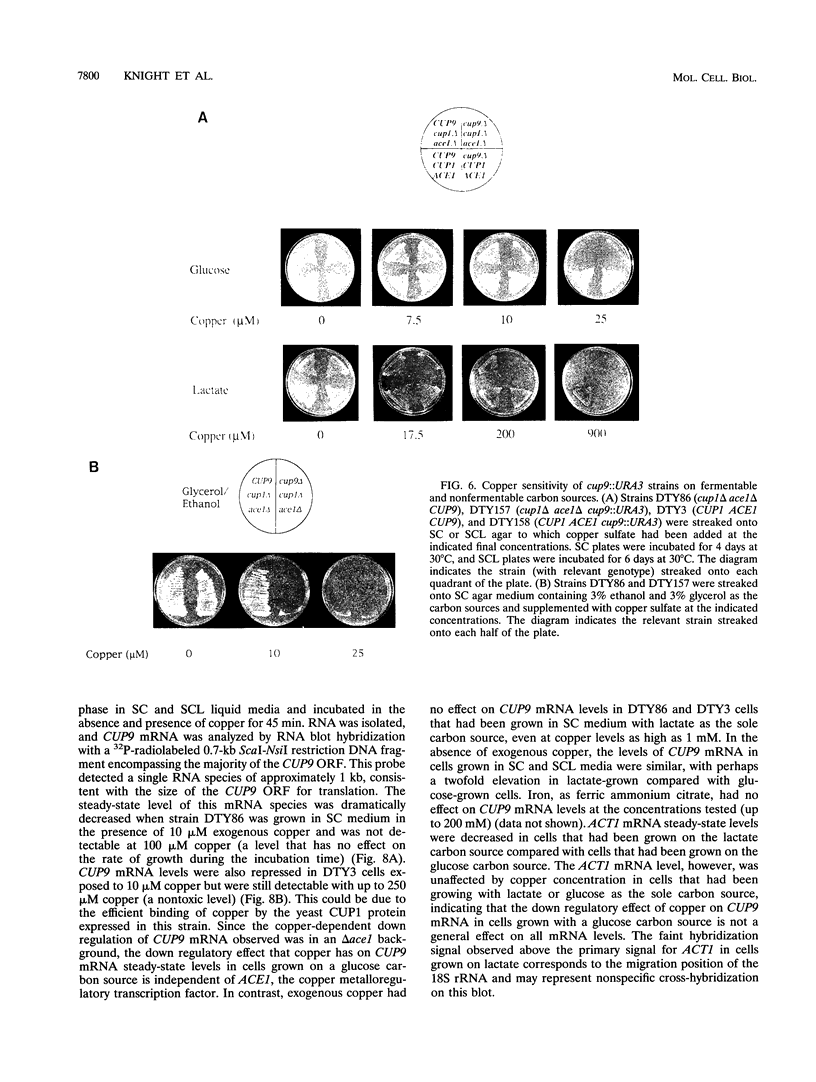
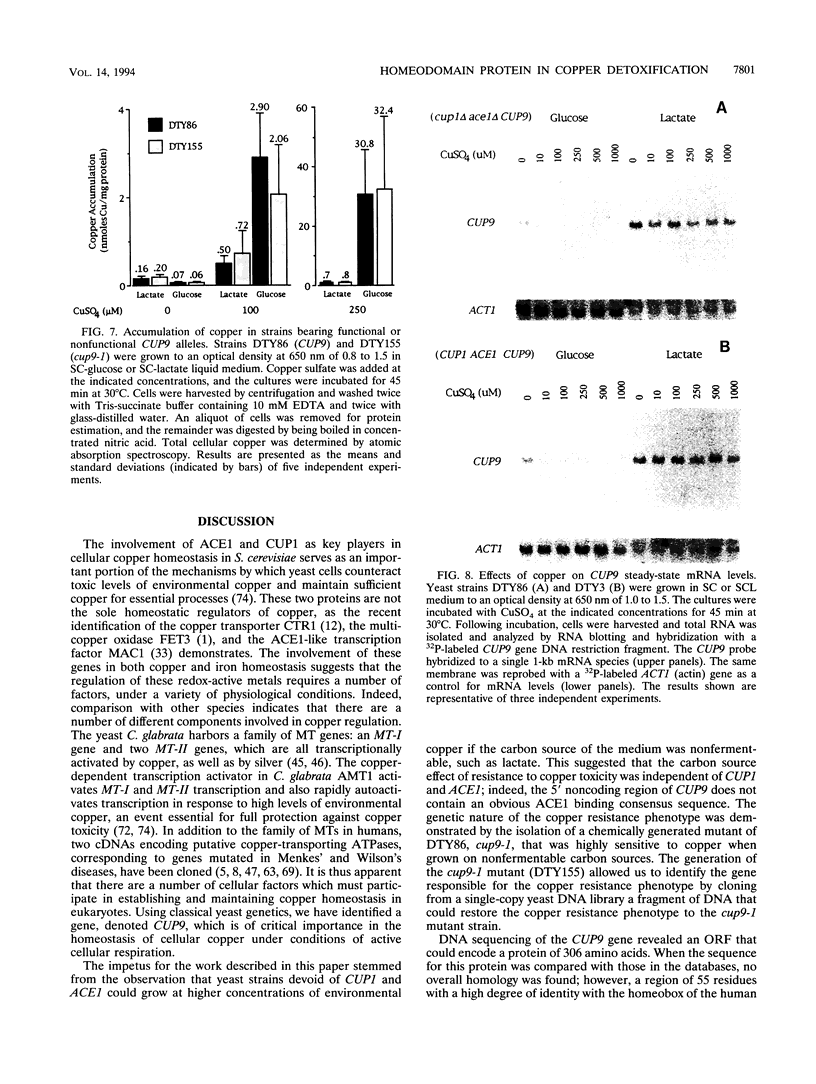
Abstract
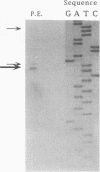
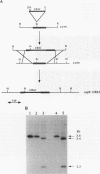
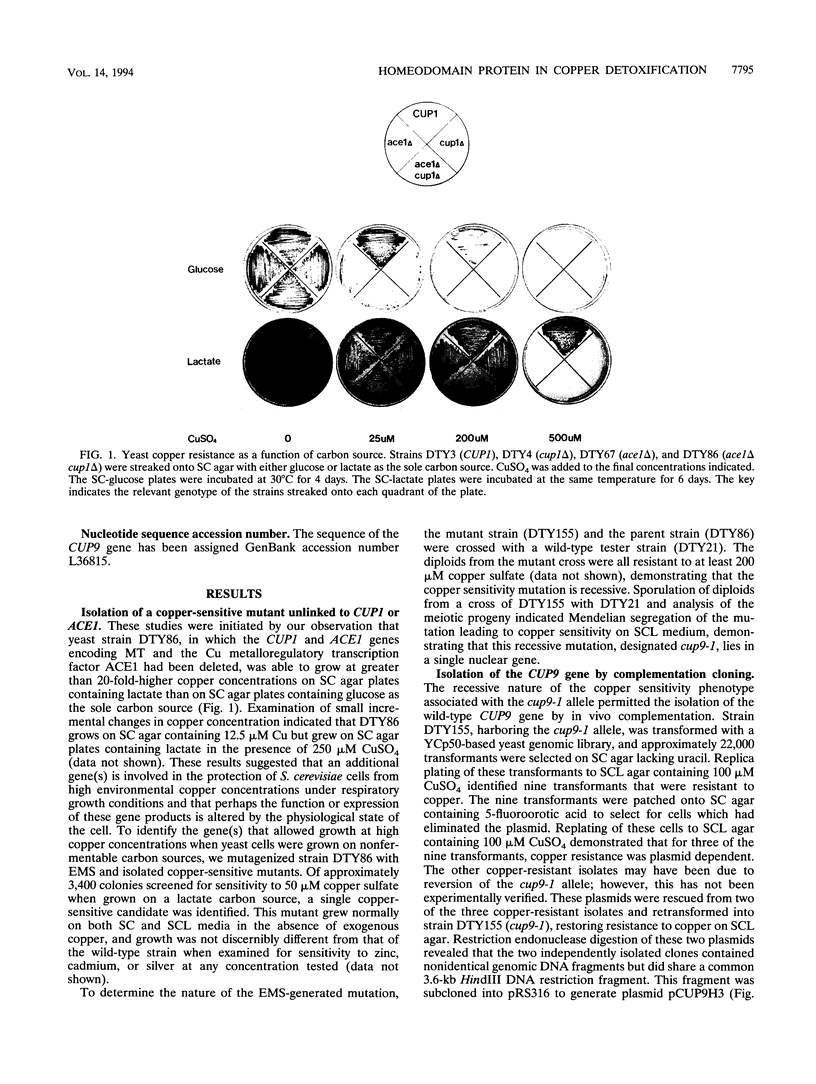
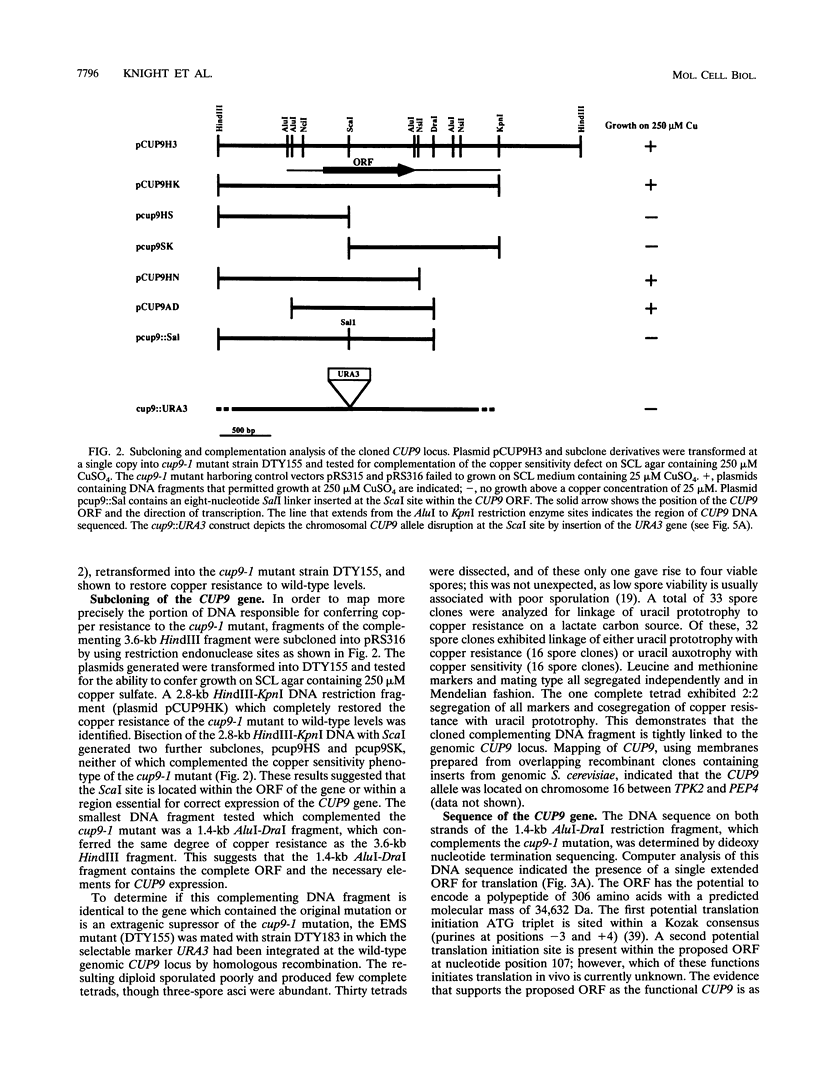
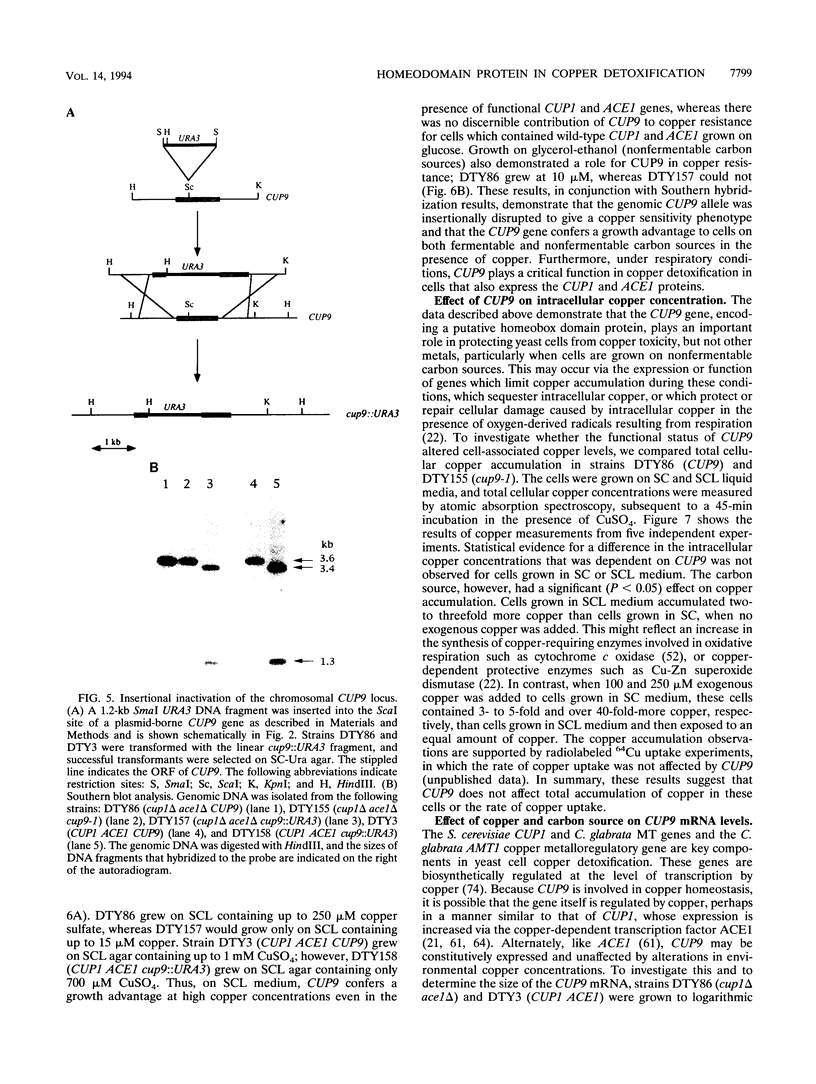
Yeast metallothionein, encoded by the CUP1 gene, and its copper-dependent transcriptional activator ACE1 play a key role in mediating copper resistance in Saccharomyces cerevisiae. Using an ethyl methanesulfonate mutant of a yeast strain in which CUP1 and ACE1 were deleted, we isolated a gene, designated CUP9, which permits yeast cells to grow at high concentrations of environmental copper, most notably when lactate is the sole carbon source. Disruption of CUP9, which is located on chromosome XVI, caused a loss of copper resistance in strains which possessed CUP1 and ACE1, as well as in the cup1 ace1 deletion strain. Measurement of intracellular copper levels of the wild-type and cup9-1 mutant demonstrated that total intracellular copper concentrations were unaffected by CUP9. CUP9 mRNA levels were, however, down regulated by copper when yeast cells were grown with glucose but not with lactate or glycerol-ethanol as the sole carbon source. This down regulation was independent of the copper metalloregulatory transcription factor ACE1. The DNA sequence of CUP9 predicts an open reading frame of 306 amino acids in which a 55-amino-acid sequence showed 47% identity with the homeobox domain of the human proto-oncogene PBX1, suggesting that CUP9 is a DNA-binding protein which regulates the expression of important copper homeostatic genes.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askwith C., Eide D., Van Ho A., Bernard P. S., Li L., Davis-Kaplan S., Sipe D. M., Kaplan J. The FET3 gene of S. cerevisiae encodes a multicopper oxidase required for ferrous iron uptake. Cell. 1994 Jan 28;76(2):403–410. doi: 10.1016/0092-8674(94)90346-8. [DOI] [PubMed] [Google Scholar]
- Astell C. R., Ahlstrom-Jonasson L., Smith M., Tatchell K., Nasmyth K. A., Hall B. D. The sequence of the DNAs coding for the mating-type loci of Saccharomyces cerevisiae. Cell. 1981 Nov;27(1 Pt 2):15–23. doi: 10.1016/0092-8674(81)90356-1. [DOI] [PubMed] [Google Scholar]
- Baxevanis A. D., Vinson C. R. Interactions of coiled coils in transcription factors: where is the specificity? Curr Opin Genet Dev. 1993 Apr;3(2):278–285. doi: 10.1016/0959-437x(93)90035-n. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull P. C., Thomas G. R., Rommens J. M., Forbes J. R., Cox D. W. The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat Genet. 1993 Dec;5(4):327–337. doi: 10.1038/ng1293-327. [DOI] [PubMed] [Google Scholar]
- Butler G., Thiele D. J. ACE2, an activator of yeast metallothionein expression which is homologous to SWI5. Mol Cell Biol. 1991 Jan;11(1):476–485. doi: 10.1128/mcb.11.1.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha J. S., Cooksey D. A. Copper resistance in Pseudomonas syringae mediated by periplasmic and outer membrane proteins. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):8915–8919. doi: 10.1073/pnas.88.20.8915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelly J., Tümer Z., Tønnesen T., Petterson A., Ishikawa-Brush Y., Tommerup N., Horn N., Monaco A. P. Isolation of a candidate gene for Menkes disease that encodes a potential heavy metal binding protein. Nat Genet. 1993 Jan;3(1):14–19. doi: 10.1038/ng0193-14. [DOI] [PubMed] [Google Scholar]
- Dancis A., Klausner R. D., Hinnebusch A. G., Barriocanal J. G. Genetic evidence that ferric reductase is required for iron uptake in Saccharomyces cerevisiae. Mol Cell Biol. 1990 May;10(5):2294–2301. doi: 10.1128/mcb.10.5.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancis A., Roman D. G., Anderson G. J., Hinnebusch A. G., Klausner R. D. Ferric reductase of Saccharomyces cerevisiae: molecular characterization, role in iron uptake, and transcriptional control by iron. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3869–3873. doi: 10.1073/pnas.89.9.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancis A., Yuan D. S., Haile D., Askwith C., Eide D., Moehle C., Kaplan J., Klausner R. D. Molecular characterization of a copper transport protein in S. cerevisiae: an unexpected role for copper in iron transport. Cell. 1994 Jan 28;76(2):393–402. doi: 10.1016/0092-8674(94)90345-x. [DOI] [PubMed] [Google Scholar]
- Danks D. M., Campbell P. E., Stevens B. J., Mayne V., Cartwright E. Menkes's kinky hair syndrome. An inherited defect in copper absorption with widespread effects. Pediatrics. 1972 Aug;50(2):188–201. [PubMed] [Google Scholar]
- Dolan J. W., Fields S. Cell-type-specific transcription in yeast. Biochim Biophys Acta. 1991 Feb 16;1088(2):155–169. doi: 10.1016/0167-4781(91)90051-m. [DOI] [PubMed] [Google Scholar]
- Flegel W. A., Singson A. W., Margolis J. S., Bang A. G., Posakony J. W., Murre C. Dpbx, a new homeobox gene closely related to the human proto-oncogene pbx1 molecular structure and developmental expression. Mech Dev. 1993 May;41(2-3):155–161. doi: 10.1016/0925-4773(93)90045-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. Oxygen: boon and bane. Am Sci. 1975 Jan-Feb;63(1):54–59. [PubMed] [Google Scholar]
- Fürst P., Hu S., Hackett R., Hamer D. Copper activates metallothionein gene transcription by altering the conformation of a specific DNA binding protein. Cell. 1988 Nov 18;55(4):705–717. doi: 10.1016/0092-8674(88)90229-2. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984 Apr 1;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer D. H., Thiele D. J., Lemontt J. E. Function and autoregulation of yeast copperthionein. Science. 1985 May 10;228(4700):685–690. doi: 10.1126/science.3887570. [DOI] [PubMed] [Google Scholar]
- Hannig E. M., Thiele D. J., Leibowitz M. J. Saccharomyces cerevisiae killer virus transcripts contain template-coded polyadenylate tracts. Mol Cell Biol. 1984 Jan;4(1):101–109. doi: 10.1128/mcb.4.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskowitz I., Jensen R. E. Putting the HO gene to work: practical uses for mating-type switching. Methods Enzymol. 1991;194:132–146. doi: 10.1016/0076-6879(91)94011-z. [DOI] [PubMed] [Google Scholar]
- Hoppler S., Bienz M. Specification of a single cell type by a Drosophila homeotic gene. Cell. 1994 Feb 25;76(4):689–702. doi: 10.1016/0092-8674(94)90508-8. [DOI] [PubMed] [Google Scholar]
- Hu S., Fürst P., Hamer D. The DNA and Cu binding functions of ACE1 are interdigitated within a single domain. New Biol. 1990 Jun;2(6):544–555. [PubMed] [Google Scholar]
- Hutchens T. W., Allen M. H., Li C. M., Yip T. T. Occupancy of a C2-C2 type 'zinc-finger' protein domain by copper. Direct observation by electrospray ionization mass spectrometry. FEBS Lett. 1992 Sep 7;309(2):170–174. doi: 10.1016/0014-5793(92)81088-4. [DOI] [PubMed] [Google Scholar]
- Huxley C., Green E. D., Dunham I. Rapid assessment of S. cerevisiae mating type by PCR. Trends Genet. 1990 Aug;6(8):236–236. doi: 10.1016/0168-9525(90)90190-h. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungmann J., Reins H. A., Lee J., Romeo A., Hassett R., Kosman D., Jentsch S. MAC1, a nuclear regulatory protein related to Cu-dependent transcription factors is involved in Cu/Fe utilization and stress resistance in yeast. EMBO J. 1993 Dec 15;12(13):5051–5056. doi: 10.1002/j.1460-2075.1993.tb06198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamps M. P., Baltimore D. E2A-Pbx1, the t(1;19) translocation protein of human pre-B-cell acute lymphocytic leukemia, causes acute myeloid leukemia in mice. Mol Cell Biol. 1993 Jan;13(1):351–357. doi: 10.1128/mcb.13.1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamps M. P., Murre C., Sun X. H., Baltimore D. A new homeobox gene contributes the DNA binding domain of the t(1;19) translocation protein in pre-B ALL. Cell. 1990 Feb 23;60(4):547–555. doi: 10.1016/0092-8674(90)90658-2. [DOI] [PubMed] [Google Scholar]
- Kelly M., Burke J., Smith M., Klar A., Beach D. Four mating-type genes control sexual differentiation in the fission yeast. EMBO J. 1988 May;7(5):1537–1547. doi: 10.1002/j.1460-2075.1988.tb02973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyhani E. Morphological changes in yeast cell mitochondria grown at various copper concentrations. Exp Cell Res. 1973 Sep;81(1):73–78. doi: 10.1016/0014-4827(73)90112-2. [DOI] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kägi J. H., Kojima Y. Chemistry and biochemistry of metallothionein. Experientia Suppl. 1987;52:25–61. doi: 10.1007/978-3-0348-6784-9_3. [DOI] [PubMed] [Google Scholar]
- Laughon A. DNA binding specificity of homeodomains. Biochemistry. 1991 Dec 3;30(48):11357–11367. doi: 10.1021/bi00112a001. [DOI] [PubMed] [Google Scholar]
- Lin C. M., Crawford B. F., Kosman D. J. Distribution of 64Cu in Saccharomyces cerevisiae: cellular locale and metabolism. J Gen Microbiol. 1993 Jul;139(7):1605–1615. doi: 10.1099/00221287-139-7-1605. [DOI] [PubMed] [Google Scholar]
- Link A. J., Olson M. V. Physical map of the Saccharomyces cerevisiae genome at 110-kilobase resolution. Genetics. 1991 Apr;127(4):681–698. doi: 10.1093/genetics/127.4.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra R. K., Garey J. R., Butt T. R., Gray W. R., Winge D. R. Candida glabrata metallothioneins. Cloning and sequence of the genes and characterization of proteins. J Biol Chem. 1989 Nov 25;264(33):19747–19753. [PubMed] [Google Scholar]
- Mehra R. K., Thorvaldsen J. L., Macreadie I. G., Winge D. R. Disruption analysis of metallothionein-encoding genes in Candida glabrata. Gene. 1992 May 1;114(1):75–80. doi: 10.1016/0378-1119(92)90709-x. [DOI] [PubMed] [Google Scholar]
- Mercer J. F., Livingston J., Hall B., Paynter J. A., Begy C., Chandrasekharappa S., Lockhart P., Grimes A., Bhave M., Siemieniak D. Isolation of a partial candidate gene for Menkes disease by positional cloning. Nat Genet. 1993 Jan;3(1):20–25. doi: 10.1038/ng0193-20. [DOI] [PubMed] [Google Scholar]
- Monica K., Galili N., Nourse J., Saltman D., Cleary M. L. PBX2 and PBX3, new homeobox genes with extensive homology to the human proto-oncogene PBX1. Mol Cell Biol. 1991 Dec;11(12):6149–6157. doi: 10.1128/mcb.11.12.6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman S. B., Wunsch C. D. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970 Mar;48(3):443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- Nourse J., Mellentin J. D., Galili N., Wilkinson J., Stanbridge E., Smith S. D., Cleary M. L. Chromosomal translocation t(1;19) results in synthesis of a homeobox fusion mRNA that codes for a potential chimeric transcription factor. Cell. 1990 Feb 23;60(4):535–545. doi: 10.1016/0092-8674(90)90657-z. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman P. S., Mahler H. R. Derepression of mitochondria and their enzymes in yeast: regulatory aspects. Arch Biochem Biophys. 1974 May;162(1):248–271. doi: 10.1016/0003-9861(74)90125-8. [DOI] [PubMed] [Google Scholar]
- Petrukhin K., Fischer S. G., Pirastu M., Tanzi R. E., Chernov I., Devoto M., Brzustowicz L. M., Cayanis E., Vitale E., Russo J. J. Mapping, cloning and genetic characterization of the region containing the Wilson disease gene. Nat Genet. 1993 Dec;5(4):338–343. doi: 10.1038/ng1293-338. [DOI] [PubMed] [Google Scholar]
- Predki P. F., Sarkar B. Effect of replacement of "zinc finger" zinc on estrogen receptor DNA interactions. J Biol Chem. 1992 Mar 25;267(9):5842–5846. [PubMed] [Google Scholar]
- Robbins J., Dilworth S. M., Laskey R. A., Dingwall C. Two interdependent basic domains in nucleoplasmin nuclear targeting sequence: identification of a class of bipartite nuclear targeting sequence. Cell. 1991 Feb 8;64(3):615–623. doi: 10.1016/0092-8674(91)90245-t. [DOI] [PubMed] [Google Scholar]
- Rose M. D., Novick P., Thomas J. H., Botstein D., Fink G. R. A Saccharomyces cerevisiae genomic plasmid bank based on a centromere-containing shuttle vector. Gene. 1987;60(2-3):237–243. doi: 10.1016/0378-1119(87)90232-0. [DOI] [PubMed] [Google Scholar]
- Schreiber V., Molinete M., Boeuf H., de Murcia G., Ménissier-de Murcia J. The human poly(ADP-ribose) polymerase nuclear localization signal is a bipartite element functionally separate from DNA binding and catalytic activity. EMBO J. 1992 Sep;11(9):3263–3269. doi: 10.1002/j.1460-2075.1992.tb05404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989 May;122(1):19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczypka M. S., Thiele D. J. A cysteine-rich nuclear protein activates yeast metallothionein gene transcription. Mol Cell Biol. 1989 Feb;9(2):421–429. doi: 10.1128/mcb.9.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamai K. T., Gralla E. B., Ellerby L. M., Valentine J. S., Thiele D. J. Yeast and mammalian metallothioneins functionally substitute for yeast copper-zinc superoxide dismutase. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8013–8017. doi: 10.1073/pnas.90.17.8013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzi R. E., Petrukhin K., Chernov I., Pellequer J. L., Wasco W., Ross B., Romano D. M., Parano E., Pavone L., Brzustowicz L. M. The Wilson disease gene is a copper transporting ATPase with homology to the Menkes disease gene. Nat Genet. 1993 Dec;5(4):344–350. doi: 10.1038/ng1293-344. [DOI] [PubMed] [Google Scholar]
- Thiele D. J. ACE1 regulates expression of the Saccharomyces cerevisiae metallothionein gene. Mol Cell Biol. 1988 Jul;8(7):2745–2752. doi: 10.1128/mcb.8.7.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele D. J. Metal-regulated transcription in eukaryotes. Nucleic Acids Res. 1992 Mar 25;20(6):1183–1191. doi: 10.1093/nar/20.6.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele D. J., Walling M. J., Hamer D. H. Mammalian metallothionein is functional in yeast. Science. 1986 Feb 21;231(4740):854–856. doi: 10.1126/science.3080806. [DOI] [PubMed] [Google Scholar]
- Van Dijk M. A., Voorhoeve P. M., Murre C. Pbx1 is converted into a transcriptional activator upon acquiring the N-terminal region of E2A in pre-B-cell acute lymphoblastoid leukemia. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6061–6065. doi: 10.1073/pnas.90.13.6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulpe C., Levinson B., Whitney S., Packman S., Gitschier J. Isolation of a candidate gene for Menkes disease and evidence that it encodes a copper-transporting ATPase. Nat Genet. 1993 Jan;3(1):7–13. doi: 10.1038/ng0193-7. [DOI] [PubMed] [Google Scholar]
- Watson T. G. Effect of carbon source on lysine-mediated inhibition of postexponential growth of Saccharomyces cerevisiae. J Bacteriol. 1983 May;154(2):1013–1014. doi: 10.1128/jb.154.2.1013-1014.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winge D. R., Nielson K. B., Gray W. R., Hamer D. H. Yeast metallothionein. Sequence and metal-binding properties. J Biol Chem. 1985 Nov 25;260(27):14464–14470. [PubMed] [Google Scholar]
- Zhou P. B., Thiele D. J. Isolation of a metal-activated transcription factor gene from Candida glabrata by complementation in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6112–6116. doi: 10.1073/pnas.88.14.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Szczypka M. S., Sosinowski T., Thiele D. J. Expression of a yeast metallothionein gene family is activated by a single metalloregulatory transcription factor. Mol Cell Biol. 1992 Sep;12(9):3766–3775. doi: 10.1128/mcb.12.9.3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
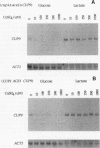
- Zhou P., Thiele D. J. Copper and gene regulation in yeast. Biofactors. 1993 May;4(2):105–115. [PubMed] [Google Scholar]