Abstract
Background and aims
Biocompatibility of root-end filling materials is a matter of debate. The aim of this study was to compare the biocompatibility of a variety of commercial ProRoot WMTA cements and a resin-based cement (Geristore®) with different pH values of setting reaction and different aluminum contents, implanted into the subcutaneous connective tissue of rats at various time intervals.
Materials and methods
Fifty Sprague-Dawley rats were used in this study. Polyethylene tubes were filled with Angelus WMTA, ProRoot WMTA, Bioaggregate, and Geristore. Empty control tubes were implanted into subcutaneous tissues and harvested at 7-, 14-, 28- and 60-day intervals. Tissue sections of 5 μm were stained with hematoxylin and eosin and observed under a light microscope. Inflammatory reactions were categorized as 0, none (without inflammatory cells); 1, mild (inflammatory cells ≤25); 2, moderate (25–125 inflammatory cells); and 3, severe (>125 inflammatory cells). Statistical analysis was performed with Kruskal-Wallis and Mann Whitney U tests.
Results
ProRoot WMTA and Angelus elicited significantly less inflammation than other materials (P<0.05). After 7 days, however, all the materials induced significantly more inflammation than the controls (P<0.05). Angelus-MTA group exhi-bited no significant differences from the Bioaggregate group (P=0.15); however, ProRoot WMTA elicited significantly less inflammation than Bioaggregate (P=0.02). Geristore induced significantly more inflammation than other groups (P<0.05).
Conclusion
Geristore induced an inflammatory response higher than ProRoot WMTA; therefore, it is not recommended for clinical use.
Keywords: Bioaggregate, biocompatibility, endodontic cement, Geristore, mineral trioxide aggregate
Introduction
Mineral trioxide aggregate (MTA) is a hydraulic silicate cement (HSC), with several advantages including sealing, sterilizing, mineralizing, dentinogenic and osteogenic capacities.1 HSC is composed of tricalcium silicate, dicalcium silicate, tricalcium aluminate, tetracalcium aluminoferrite, and calcium sulfate.1 Almost all these cements contain some trace elements like aluminum.2 However, incorporation of aluminum is not suitable for biomedical purposes.3 Numerous studies have evaluated the biological and physical characteristics of MTA, such as setting time,1 acidic resistance,4 push-out bond strength,5 porosity,6 neurotoxicity,7 sealing ability,8 and the effect of environmental conditions on biocompatibility of MTA.9 MTA has been demonstrated to be non-toxic toward living tissues in many investigations in spite of aluminum as one of its components.10-12
Bioaggregate (BA) (Innovative Bioceramix, Vancouver, BC, Canada), a white nanoparticle-sized ceramic cement is composed of calcium silicate, calcium hydroxide, and hydroxyapatite and is used as a root-end filling material.13 BA has displayed cytocompatibility similar to MTA.13,14
Geristore (Den-Mat, Santa Maria, CA) is a hydrophilic Bis-GMA.15-17 Geristore is able to bond in the presence of moisture. Its histological biocompatibility, adherence to dentin and cementum, release of fluoride, lack of microleakage, low coefficient of thermal expansion and low polymerization shrinkage all combine to make it the restoration of choice for subgingival restorations when there is the possibility of trans-gingival contamination with the saliva.18,19
This study was designed to compare the biocompatibility of three types of hydraulic cement-based materials, including ProRoot WMTA, Angelus WMTA and BA with a resin-based cement, Geristore, in the subcutaneous connective tissue of rats at 7-, 14-, 28-, and 60-day intervals.
Materials and Methods
The research protocol was approved by the Research Ethics Committee of Shiraz University of Medical Sciences. All the experiments were carried out in accordance to the rules of Institutional Animal Care and Use Committee (IACUC). The method used in this study was similar to those used previously.11,12Fifty 3-month-old male Sprague-Dawley rats weighting 220±20 g were randomly used in this study. The animals were kept in a restricted access room under controlled temperature (22°C) and light/dark cycles (12h/12h) and with free access to food and water (ad libitum); each cage housed three rats. All the animals were randomly divided into 5 groups (n=10) as follows:
ProRoot WMTA (Tooth-colored Formula; Dentsply, Tulsa Dental, Tulsa, OK, USA)
Angelus WMTA (Tooth-colored Formula Angelus, Londrina, Brazil)
Geristore (Den-Mat Corporation, Santa Maria, CA)
Bioaggregate (Innovative Bioceramix, Vancouver, BC, Canada)
Control group (Polyethylene tubes)
Each material was mixed according to manufacturers’ instructions under aseptic conditions. All the operations were performed under general anesthesia by intramuscular injection of 10% ketamine hydrochloride (90 mg/kg, IM, Alfasan Nederland BV, Woerden, The Netherlands) and 2% xylazine (8 mg, IM. Alfasan Nederland BV, Woerden, The Netherlands). Three separate 2-cm incisions were made on the back of the rats at least 2 cm away from each other. Freshly mixed cements were prepared and placed in sterile polyethylene tubes measuring 1.1 mm in inner diameter and 8 mm in length and were immediately implanted subcutaneously into two separate incisions. An empty polyethylene tube was implanted as a control. All the samples were harvested at 7-, 14-, 28- and 60-day intervals. The rats were euthanized by carbon dioxide inhalation with subsequent exsanguination.20
The tubes and surrounding tissues were removed in blocks and fixed in 10% buffered formalin solution for 2 weeks; 5-μm tissue sections were prepared longitudinally through the midline of the tubes and stained with hematoxylin and eosin. Evaluations of inflammatory cells (lymphocytes, plasmocytes, polymorphonuclear leukocytes, macrophages, and giant cells) were carried out in microscopic fields adjacent to the test materials at the end of the tubes under a light microscope (Carl Zeiss, Oberkochen, Germany) at ×400 magnification. An average value for each specimen was obtained from the sum of cells counted in 4 separate areas.21-23 The observer did not have any knowledge of the materials used in the specimens. The overall mean value for each material was determined in subjects at each time interval. The inflammatory reactions were categorized as:
0: none (without inflammatory cells)
1: mild (<25 inflammatory cells)
2: moderate (25–125 inflammatory cells)
3: severe (>125 inflammatory cells)
Statistics
Kruskal-Wallis and Mann-Whitney U tests were used for statistical analysis. Statistical significance was defined at P < 0.05.
Results
7 and 14 Days
The mean ± SD for Geristore, ProRoot WMTA, Angelus, Bioaggregate, and control groups were 2.90±0.31, 2.40±0.51, 2.50±0.52, 3.00±0.00, and 1.20±0.42, respectively. ProRoot WMTA and Angelus-MTA elicited significantly less inflammation than other materials (P < 0.05). However, all the materials induced significantly more inflammation than the control group (P < 0.05).
28 Days
The mean ± SD for Geristore, ProRoot WMTA, Angelus-MTA, BA, and control groups were 2.50 ± 0.52, 2.00 ± 0.00, 2.20 ± 0.42, 2.38 ± 0.51, and 1.20 ± 0.42, respectively. Geristore, and BA elicited significantly more inflammation than ProRoot WMTA and Angelus-MTA groups (P < 0.05). However, all the materials showed significantly more inflammation than the control group (P < 0.05).
60 Days
The mean ± SD for Geristore, ProRoot WMTA, Angelus-MTA, BA, and control groups were 2.10±0.56, 1.40±0.51, 1.70±0.48, 2.00±0.00, and 1.20±0.42, respectively. There were no significant differences between the ProRoot WMTA and Angelus-MTA groups (P=0.18). Moreover, Angelus-MTA group did not exhibit any significant difference from the BA group (P=0.15); however, ProRoot WMTA elicited significantly less inflammation than BA (P=0.02) (Figure 2). There were no significant differences between the control and WMTA or WMTA Angelus-MTA groups (P > 0.05). However, there were significant differences between either the Geristore group or the Bioaggregate group and the control group (P < 0.05). In other words, Geristore and bioaggregate induced more inflammation even after 60 days (Figure 1).
Figure 2.
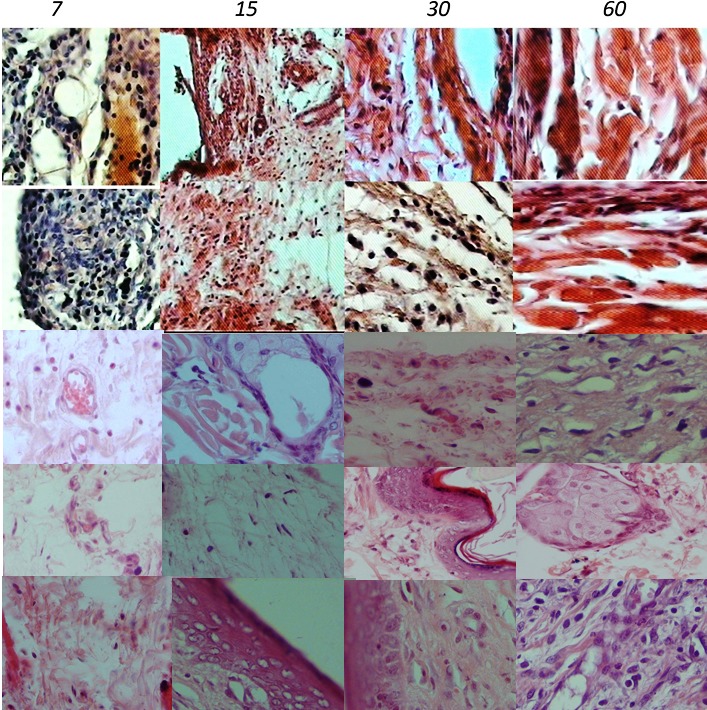
Histological images of inflammatory cell infiltration at the end of implanted tubes in both groups (First row = ProRoot WMTA; Second row = Angelus WMTA; Third row = Bioaggregate; Fourth row = Geristore® and Fifth row = Control Group) (hematoxylin/eosin staining; original magnification, ×400). Exposure time had inverse effects on the inflammatory response.
Figure 1.
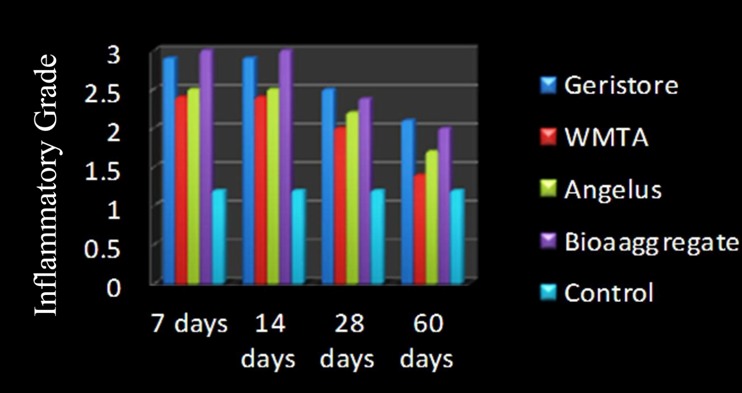
The means of inflammatory grades for Geristore, PROROOT WMTA, Angelus, BA, and control groups after dorsal implantation of test materials at different intervals in rats.
Discussion
Subcutaneous implantation was used to evaluate the biocompatibility of biomaterials. This technique was introduced by Torneck23 in 1966and confirmed by Olsson et al15 in 1981.
The differences between the experimental and the control groups at 7-day interval were significant, consistent with the results of other studies.11,12 At 7-day interval, both MTA-based cements evoked moderate to severe inflammatory reactions. These results are partially consistent with the findings of a previous study,12 showing moderate subcutaneous reaction to three types of MTA.
At 14-day interval, ProRoot WMTA provoked moderate to severe inflammatory reactions. In a similar manner, the results of some studies have shown moderate to severe reactions to ProRoot WMTA.11,12 The main major phase of MTA cements, C3S (3CaO, SiO2), C2S (2CaO, SiO2), and C3A (3CaO, Al2O3), might be influenced by the pH of surrounding materials or their ingredients, impeding or improving the biocompatibility of the cement.16,17 Some studies have shown that ProRoot WMTA actively promotes hard tissue formation by inducing osteogenesis and cementogenesis. MTA cement interacts with the surrounding tissues and forms a shell on the surface, which improves osteogenesis.17,24
At 28- and 60-day intervals, all the groups, experimental and control, exhibited mild to moderate infiltration of inflammatory cells. Similarly, an investigation revealed mild to moderate inflammatory reactions to 30-day- and 60-day-old subcutaneous ProRoot WMTA and GMTA.12 When MTA powder is mixed with water, calcium phosphate and calcium oxide are released. When ProRoot WMTA comes in contact with tissue fluids, it produces calcium hydroxide.25 A high pH and release of calcium and phosphorus ions are required for a material to stimulate mineralization during the process of hard tissue healing.26
Geristore has a low pH compared to Bioaggregate, which has a higher pH after the setting reaction. Previous studies have confirmed that both acidic and alkaline conditions influence inflammatory cells through different routes and the present study illu illustrated the effect of both alkaline and acidic materials on inflammation severity.5-12 The excellent biocompatibility of a hydraulic cement-based material and other calcium-containing materials might be attributed to their ability to release calcium ions which react with phosphate ions of tissue fluids, resulting in hard tissue formation.27 Furthermore, high pH levels contributes to the antibacterial activity which is a critical factor in the formation of a mineralized tissue barrier.28
According to manufacturer’s claims, the hybrid ionomer-composite materials, such as Geristore, with the same indications as MTA-based cements, are biocompatible. Geristore was selected due to its different setting pH value compared to other cements. Geristore has a low pH after setting reaction, which might explain the induction of significantly more inflammation than other groups even after 60 days.18,19 Geristore has some advantages such as insolubility in oral fluids, increased adhesion to tooth structure, dual-curing capabilities, low polymerization shrinkage, low coefficient of thermal expansion, radiopacity, fluoride release, and biocompatibility.18 Geristore has been reported to be less biocompatible than gray MTA,29 consistent with the results of the present study. Geristore releases five monomers of Bis-GMA, Bis-DMA, TEGDMA, UDMA and Bisphenol A (Table 1). Furthermore, Geristore releases calcium, and aluminum ions, and fluoride.30 Resin monomers are reported to show cytotoxic effects 31,32 and might be capable of tumor initiation at relatively low concentrations.33
Table 1. Main composition of the test materials in each group .
| Test materials | Composition |
| ProRoot WMTA | tricalcium silicate, dicalcium silicate, tricalcium aluminate and tetracalcium aluminoferrite |
| Angelus WMTA | tricalcium silicate, dicalcium silicate, tricalcium aluminate and tetracalcium aluminoferrite |
| Bioaggregate® | tricalcium silicate, dicalcium silicate, tantalum pentoxide, and calcium phosphate monobasic.Tantalum pentoxide |
| Geristore® | Bis-GMA, TEGDMA, UDMA, Bis-DMA, and Bisphenol A |
Formation of calcium hydroxide is the cause of high alkalinity of MTA after hydration,34 which is considered an initial tissue irritant when ProRoot WMTA comes into contact with the tissue.35 This would explain the inflammatory reactions subsequent to the subcutaneous implantation of ProRoot WMTA.36
Although a previous study has shown that hydraulic silicate-based cement has good market in endodontics, the present study confirmed that inflammation in the BioAggregate group was more severe or equal to the Geristore group, especially at 7-, 14-, and 28-day intervals; therefore, it should be noted that although Bioaggregate is a silicate-based cement, lack of trace elements, such as aluminum, might accelerate setting and/or hydration reaction of this kind of cement, with an important role in its hydration.1
The presence of aluminum is a major disadvantage of the materials derived from Portland cement (such as MTA) when used for biomedical and dental applications.37 Aluminum ions are released into human biological systems during hydration and setting reactions of such cements.38 Moreover, in the case of permanent and long-term applications, such as root-end filling and direct pulp capping, tricalcium aluminate in the cements continually releases aluminum ions into the human biological systems.39 Aluminum ions are toxic to the human biological systems 37 and to osteoblasts,40 inhibiting mineralization of bone.41
Accumulation of aluminum in the body tends to occur when the gastrointestinal barrier is circumvented, as is the case with implants or dental procedures.42-44 Metal oxides, such as aluminum and iron oxides, have been known to cause abnormal tissue reactions equivalent to a chemical insult.45
The three above-mentioned cements are based on (or derived from) Portland cement, and as such rely on aluminum compounds to achieve early strength during setting.46 Aluminum might improve the strength and solubility of MTA cement. If aluminum was to be removed from such compositions, the increase in strength would be much slower, rendering the cement useless for its intended applications.47
Angelus-MTA was selected due to its difference in its aluminum content from ProRoot. Although Angelus-MTA has more aluminum content than ProRoot MTA,2 there were no significant differences in tissue reactions at any time interval. ProRoot MTA has a chemical composition similar to that of Angelus-MTA; however, ProRoot MTA is reported to have slightly higher percentages of bismuth oxide than the other one.2,48 BA is composed of tricalcium silicate, dicalcium silicate, tantalum pentoxide and monobasic calcium phosphate. Tantalum pentoxide in BA provides radiopacity instead of bismuth oxide in MTA, and monobasic calcium phosphate in BA adjusts its hydrate setting.13 BA was selected due to the absence of aluminum in its chemical composition; BA induced more inflammation at all time intervals. The higher inflammation in the BA group, compared to the MTA group, might be attributed to the effect of aluminum compounds on the insolubility of MTA cement.49 In addition, the use of small amounts of MTA for clinical applications limits the release of aluminum into tissue fluids, with a potentially toxic effect.
Conclusion
The three types of HSC-based cements exhibited biocompatibility; minute amounts of aluminum compounds have less negative effects on the inflammatory cell response. Geristore elicited significantly more inflammation, demonstrating that it is not the material of choice for clinical use.
References
- 1.Darvell B, Wu R. “MTA”—An Hydraulic Silicate Cement: Review update and setting reaction. Dent Mater . 2011;27:407–22. doi: 10.1016/j.dental.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 2.de OLIVEIRA MG, Xavier CB, Demarco F, AL ALP, Costa A, Pozza D. Comparative chemical study of MTA and Portland cements. Braz Dent J . 2007;18:1. doi: 10.1590/s0103-64402007000100002. [DOI] [PubMed] [Google Scholar]
- 3.Keegan G, Learmonth I, Case C. Orthopaedic metals and their potential toxicity in the arthroplasty patient: a review of current knowledge and future strategies. J Bone Joint Surg-Br . 2007;89:567–73. doi: 10.1302/0301-620X.89B5.18903. [DOI] [PubMed] [Google Scholar]
- 4.Saghiri MA, Lotfi M, Saghiri AM, Vosoughhosseini S, Fatemi A, Shiezadeh V. et al. Effect of pH on sealing ability of white mineral trioxide aggregate as a root-end filling material. J Endod . 2008;34:1226–9. doi: 10.1016/j.joen.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 5.Saghiri MA, Shokouhinejad N, Lotfi M, Aminsobhani M, Saghiri AM. Push-out Bond Strength of Mineral Trioxide Aggregate in the Presence of Alkaline pH. J Endod . 2010;36:1856–9. doi: 10.1016/j.joen.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 6.Saghiri MA, Asgar K, Lotfi M, Karamifar K, Neelakantan P, Ricci JL. Application of mercury intrusion porosimetry for studying the porosity of mineral trioxide aggregate at two different pH. Acta Odontol Scan 2012:1-5. [DOI] [PubMed] [Google Scholar]
- 7.Saghiri MA, Asgar K, Daliri M, Lotfi M, Delvarani A, Mehrvarzfar P. et al. Morphological behavior and attachment of p19 neural cells to root-end filling materials. Scanning . 2010;32:369–74. doi: 10.1002/sca.20209. [DOI] [PubMed] [Google Scholar]
- 8.Mehrvarzfar P, Dahi-Taleghani A, Saghiri MA, Karamifar K, Shababi B, Behnia A. The comparison of MTA, Geristore® and Amalgam with or without Bioglass as a matrix in sealing the furcal perforations. Saudi Dent J . 2010;22:119–24. doi: 10.1016/j.sdentj.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saghiri MA, Lotfi M, Joupari MD, Aeinehchi M, Saghiri AM. Effects of storage temperature on surface hardness, microstructure, and phase formation of white mineral trioxide aggregate. J Endod . 2010;36:1414–8. doi: 10.1016/j.joen.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 10.De Deus G, Ximenes R, Gurgel-Filho E, Plotkowski M, Coutinho-Filho T. Cytotoxicity of MTA and Portland cement on human ECV 304 endothelial cells. Int Endod J . 2005;38:604–9. doi: 10.1111/j.1365-2591.2005.00987.x. [DOI] [PubMed] [Google Scholar]
- 11.Lotfi M, Vosoughhosseini S, Saghiri MA, Mesgariabbasi M, Ranjkesh B. Effect of white mineral trioxide aggregate mixed with disodium hydrogen phosphate on inflammatory cells. J Endod . 2009;35:703–5. doi: 10.1016/j.joen.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 12.Vosoughhosseini S, Lotfi M, Shahi S, Baloo H, Mesgariabbasi M, Saghiri MA. et al. Influence of white versus gray mineral trioxide aggregate on inflammatory cells. J Endod . 2008;34:715–7. doi: 10.1016/j.joen.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Olsson B, Sliwkowski A, Langeland K. Subcutaneous implantation for the biological evaluation of endodontic materials. J Endod . 1981;7:355–69. doi: 10.1016/S0099-2399(81)80057-X. [DOI] [PubMed] [Google Scholar]
- 14.Lee YL, Lee BS, Lin FH, Yun Lin A, Lan WH, Lin CP. Effects of physiological environments on the hydration behavior of mineral trioxide aggregate. Biomaterials . 2004;25:787–93. doi: 10.1016/s0142-9612(03)00591-x. [DOI] [PubMed] [Google Scholar]
- 15.Danesh F, Tootian Z, Jahanbani J, Rabiee M, Fazelipour S, Taghva O. et al. Biocompatibility and mineralization activity of fresh or set white mineral trioxide aggregate, biomimetic carbonated apatite, and synthetic hydroxyapatite. J Endod . 2010;36:1036–41. doi: 10.1016/j.joen.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Dragoo M. Dragoo MResin-ionomer and hybrid-ionomer cements: part IComparison of three materials for the treatment of subgingival root lesions. Int J Periodont Rest Dent . 1996;16:594. [PubMed] [Google Scholar]
- 17.Al-Sabek F, Shostad S, Kirkwood KL. Preferential attachment of human gingival fibroblasts to the resin ionomer Geristore. J Endod . 2005;31:205–8. doi: 10.1097/01.don.0000137650.61607.25. [DOI] [PubMed] [Google Scholar]
- 18.Zhang H, Pappen FG, Haapasalo M. Dentin enhances the antibacterial effect of mineral trioxide aggregate and bioaggregate. J Endod . 2009;35:221–4. doi: 10.1016/j.joen.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Yuan Z, Peng B, Jiang H, Bian Z, Yan P. Effect of bioaggregate on mineral-associated gene expression in osteoblast cells. J Endod . 2010;36:1145–8. doi: 10.1016/j.joen.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 20.Loch D, Hoey A, Morisseau C, Hammock BO, Brown L. Prevention of hypertension in DOCA-salt rats by an inhibitor of soluble epoxide hydrolase. Cell Biochem Biophys . 2007;47:87–98. doi: 10.1385/cbb:47:1:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zmener O, Guglielmotti MB, Cabrini RL. Biocompatibility of two calcium hydroxide-based endodontic sealers: a quantitative study in the subcutaneous connective tissue of the rat. J Endod . 1988;14:229–35. doi: 10.1016/S0099-2399(88)80175-4. [DOI] [PubMed] [Google Scholar]
- 22.Zmener O, Guglielmotti MB, Cabrini RL. Tissue response to an experimental calcium hydroxide-based endodontic sealer: a quantitative study in subcutaneous connective tissue of the rat. Dent Traumatol . 1990;6:66–71. doi: 10.1111/j.1600-9657.1990.tb00393.x. [DOI] [PubMed] [Google Scholar]
- 23.Torneck CD. Torneck CDReaction of rat connective tissue to polyethylene tube implantsI. Oral Surg Oral Med Oral Pathol . 1966;21:379. doi: 10.1016/0030-4220(66)90077-6. [DOI] [PubMed] [Google Scholar]
- 24.Tay FR, Pashley DH, Rueggeberg FA, Loushine RJ, Weller RN. Calcium phosphate phase transformation produced by the interaction of the Portland cement component of white mineral trioxide aggregate with a phosphate-containing fluid. J Endod . 2007;33:1347–51. doi: 10.1016/j.joen.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Koh ET, McDonald F, Pitt Ford TR, Torabinejad M. Cellular response to mineral trioxide aggregate. J Endod . 1998;24:543–7. doi: 10.1016/S0099-2399(98)80074-5. [DOI] [PubMed] [Google Scholar]
- 26.Ulibarri RP, Gonzalez WL, Torres J. Hydraulic cement with accelerated high strength development. Google Patents; 2000.
- 27.Antunes Bortoluzzi E, Juárez Broon N, Antonio Hungaro Duarte, de Oliveira Demarchi, Monteiro Bramante C. The use of a setting accelerator and its effect on pH and calcium ion release of mineral trioxide aggregate and white Portland cement. J Endod . 2006;32:1194–7. doi: 10.1016/j.joen.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 28.Eldeniz AU, Hadimli HH, Ataoglu H, Ørstavik D. Antibacterial effect of selected root-end filling materials. J Endod . 2006;32:345–9. doi: 10.1016/j.joen.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 29.Hammad HM, Hamadah MA, Al-Omari WM. Histological evaluation of rat tissue response to GMTA, Retroplast, and Geristore retrograde filling materials. Aus Endod J . 2011;37:18–25. doi: 10.1111/j.1747-4477.2009.00195.x. [DOI] [PubMed] [Google Scholar]
- 30.Al-Sa'eed OR, Al-Hiyasat AS, Darmani H. The effects of six root-end filling materials and their leachable components on cell viability. J Endod . 2008;34:1410–4. doi: 10.1016/j.joen.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Geurtsen W, Lehmann F, Spahl W, Leyhausen G. Cytotoxicity of 35 dental resin composite monomers/additives in permanent 3T3 and three human primary fibroblast cultures. J Biomed Mater Res . 1998;41:474–80. doi: 10.1002/(sici)1097-4636(19980905)41:3<474::aid-jbm18>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 32.Al-Hiyasat A, Darmani H, Milhem M. Cytotoxicity evaluation of dental resin composites and their flowable derivatives. Clin Oral Invest . 2005;9:21–5. doi: 10.1007/s00784-004-0293-0. [DOI] [PubMed] [Google Scholar]
- 33.Kleinsasser NH, Schmid K, Sassen AW, Harréus UA, Staudenmaier R, Folwaczny M. et al. Cytotoxic and genotoxic effects of resin monomers in human salivary gland tissue and lymphocytes as assessed by the single cell microgel electrophoresis (Comet) assay. Biomaterials . 2006;27:1762–70. doi: 10.1016/j.biomaterials.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 34.Camilleri J. Characterization of hydration products of mineral trioxide aggregate. Int Endod J . 2008;41:408–17. doi: 10.1111/j.1365-2591.2007.01370.x. [DOI] [PubMed] [Google Scholar]
- 35.Yaltirik M, Ozbas H, Bilgic B, Issever H. Reactions of connective tissue to mineral trioxide aggregate and amalgam. J Endod . 2004;30:95–9. doi: 10.1097/00004770-200402000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Islam I, Kheng Chng H, Jin Yap AU. Comparison of the physical and mechanical properties of MTA and Portland cement. J Endod . 2006;32:193–7. doi: 10.1016/j.joen.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 37.Nicholson JW, Czarnecka B. Review paper: role of aluminum in glass-ionomer dental cements and its biological effects. J Biomater App . 2009;24:293–308. doi: 10.1177/0885328209344441. [DOI] [PubMed] [Google Scholar]
- 38.Camilleri J. The biocompatibility of modified experimental Portland cements with potential for use in dentistry. Int Endod J . 2008;41:1107–14. doi: 10.1111/j.1365-2591.2008.01483.x. [DOI] [PubMed] [Google Scholar]
- 39.Fridland M, Rosado R. MTA solubility: a long term study. J Endod . 2005;31:376–9. doi: 10.1097/01.don.0000140566.97319.3e. [DOI] [PubMed] [Google Scholar]
- 40.Bellows C, Aubin J, Heersche J. Aluminum inhibits both initiation and progression of mineralization of osteoid nodules formed in differentiating rat calvaria cell cultures. J Bone Min Res . 1995;10:2011–6. doi: 10.1002/jbmr.5650101222. [DOI] [PubMed] [Google Scholar]
- 41.Sprague SM, Krieger NS, Bushinsky DA. Aluminum inhibits bone nodule formation and calcification in vitro. Amer J Physiol-Renal Physiol . 1993;264:F882–F90. doi: 10.1152/ajprenal.1993.264.5.F882. [DOI] [PubMed] [Google Scholar]
- 42.Savarino L, Cervellati M, Stea S, Cavedagna D, Donati M, Pizzoferrato A. et al. In vitro investigation of aluminum and fluoride release from compomers, conventional and resin-modified glass-ionomer cements: A standardized approach. J Biomater Sci, Polymer Edition . 2000;11:289–300. doi: 10.1163/156856200743706. [DOI] [PubMed] [Google Scholar]
- 43.Rodriguez M, Felsenfeld AJ, Llach F. Aluminum administration in the rat separately affects the osteoblast and bone mineralization. J Bone Min Res . 1990;5:59–67. doi: 10.1002/jbmr.5650050110. [DOI] [PubMed] [Google Scholar]
- 44.Monteagudo F, Cassidy M, Folb P. Recent developments in aluminum toxicology. Med Toxicol Adver drug Exp . 1989;4:1. doi: 10.1007/BF03259899. [DOI] [PubMed] [Google Scholar]
- 45.Steinemann SG. Titanium—the material of choice? . Periodontol 2000 . 1998;17:7–21. doi: 10.1111/j.1600-0757.1998.tb00119.x. [DOI] [PubMed] [Google Scholar]
- 46.Asgary S, Shahabi S, Jafarzadeh T, Amini S, Kheirieh S. The properties of a new endodontic material. J Endod . 2008;34:990–3. doi: 10.1016/j.joen.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 47.Andersen MD, Jakobsen HJ, Skibsted J. Incorporation of aluminum in the calcium silicate hydrate (CSH) of hydrated Portland cements: A high-field 27Al and 29Si MAS NMR investigation. Inorg Chemist . 2003;42:2280–7. doi: 10.1021/ic020607b. [DOI] [PubMed] [Google Scholar]
- 48.Song JS, Mante FK, Romanow WJ, Kim S. Chemical analysis of powder and set forms of Portland cement, gray ProRoot MTA, white ProRoot MTA, and gray MTA-Angelus. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol . 2006;102:809–15. doi: 10.1016/j.tripleo.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 49.Poggio C, Lombardini M, Alessandro C, Simonetta R. Solubility of root-end-filling materials: a comparative study. J Endod . 2007;33:1094–7. doi: 10.1016/j.joen.2007.05.021. [DOI] [PubMed] [Google Scholar]


