Abstract
Although the vast majority of research on the dopamine system has been performed in rodents, and it is assumed that this work will inform us about the human condition, there have been very few direct comparisons of presynaptic dopamine terminal function across multiple species. Because it is difficult to query rapid sub-second dopamine signaling in humans using voltammetric methods, we chose to compare dopamine signals across multiple striatal subregions in slices from C57BL/6J mice, Sprague–Dawley rats and rhesus macaques. We found a dorsal to ventral gradient of dopamine uptake rates with highest levels in the dorsal striatum and lowest levels in the nucleus accumbens shell, which is conserved across species. In addition to uptake rates, there was also a dorsal to ventral, high to low, gradient in the magnitude of stimulated DA release observed in monkeys, mice, and rats. These data demonstrate that there is considerable functional homology across striatal regions in non-human primates and rodents, lending support to the use of rodents as model systems to study dopamine-related circuitry and disorders that are clinically relevant to the human population.
Keywords: Voltammetry, Dopamine transporter, Striatum, Rhesus macaque, Rat, Mouse
1. Introduction
Because of the ethical limitations of conducting research in humans, model systems must be used to gather information about human disorders. Monkeys and rodents are both commonly used as model systems to study neurobiology, and there is a great deal of homology across various neural networks as well as the actions of drugs across species (Bradberry, 2008; Levey et al., 1993). Currently, monkeys are the closest models of human conditions, but rodents are commonly used to model processes such as neurodegenerative diseases, stress, anxiety and addiction, due to the ability to use more invasive and comprehensive techniques (Chesselet and Richter, 2011; Roberts et al., 2007; Silberman et al., 2010). Although it is obvious that humans and rats differ significantly, it is important to determine the translational capability of neurobiological studies in rodents by determining how well functional parameters in the rat and mouse brain are similar to non-human primates and humans.
The dopamine (DA) system is dysregulated in many human disorders, including addiction, schizophrenia, Parkinson’s disease, anxiety, attention deficit/hyperactivity disorder (ADHD) and many others (Carlsson, 1988; Jentsch et al., 2000; Goto et al., 2010). Thus, comparing the functionality of dopaminergic regions across species is vital for determining the translational validity of animal models of a multitude of human neurological conditions. There are some cross species comparisons using PET imaging and dopamine transporter (DAT) occupancy in humans, baboons and rats (Ding et al., 1997; Fowler et al., 1998), however, there is limited information on region specific DA kinetic parameters, such as release and uptake (Cragg et al., 2000, 2002). Anatomical distinctions within the striatum are generally similar in rodents and nonhuman primates; however, there are some important differences. Striatal organization differs in rodents and primates, most notably with a lack of anatomical separation of caudate and putamen structures in rodents, marked by the internal capsule in primates. In the rodent brain these structures are not anatomically distinct and are referred to as the caudate-putamen (CPu); however the striatum of both primates and rodents contains the nucleus accumbens (Mitchell et al., 1999). The lack of completely homologous structures across species complicates the translation of results of rodent studies to humans; however, additional knowledge of functional homology could lead to better and more accurate translational studies.
Although anatomically divergent, inputs into the striatum are largely conserved across species with the two major dopaminergic inputs being the mesolimbic and nigrostrial pathways from the A10 neurons of the ventral tegmental area (VTA) and the A9 neurons of the substantia nigra pars compacta, respectively (Zahm et al., 2011). These projections separate along a ventromedial-dorsolateral axis with ventromedial striatum predominately receiving A10 and dorsolateral striatum receiving A9 projections. Even more important is the functional homology across species with more dorsal regions of the striatum being associated with motor planning and execution, central regions with associative learning and directed attention, and ventral regions with emotional and reward-related processing (Lynd-Balta and Haber, 1994a,b; Reep et al., 2003; Künzle, 1975; Selemon and Goldman-Rakic, 1985; Haber and McFarland, 1999). Although neuroanatomical homology is convenient for comparisons across species, it is also important to determine functional homology, as changes in structure that do not result in changes in function are not as directly relevant to human behavior/disease processes.
This study was aimed at assessing functional homology of the DA system by measuring regional DA system parameters in the caudate and nucleus accumbens in three different species, monkeys (Rhesus Macaques), rats (Sprague–Dawley) and mice (C57BL/6J). Using fast-scan cyclic voltammetry, we measured release and uptake parameters for five specific regions in each species: dorsal CPu, medial CPu, ventral CPu, nucleus accumbens core, and nucleus accumbens shell. We found that there is a conserved gradient of both uptake and release with highest levels of both measures occurring in the dorsal caudate of all species, and lowest levels in the nucleus accumbens shell. This conservation of functionality across these species further validates the use of rodent models for translational studies of the DA system.
2. Materials and methods
2.1. Tissue preparation
Rats and mice were sacrificed by decapitation and brains were rapidly removed and transferred into ice-cold, pre-oxygenated (95% O2/5% CO2) artificial cerebral spinal fluid (aCSF) consisting of (in mM): NaCl (126), KCl (2.5), NaH2PO4 (1.2), CaCl2 (2.4), MgCl2 (1.2), NaHCO3 (25), glucose (11), L-ascorbic acid (0.4) and pH was adjusted to 7.4. Rhesus macaques were anesthetized with ketamine (10 mg/kg, i.m.) and maintained at a deep surgical plane of anesthesia with pentobarbital. Transcardial perfusion (90 s) with cold modified Kreb’s buffer (pH = 7.4) was performed and brains were rapidly removed and cooled in pre-oxygenated aCSF (Daunais et al., 2010). For all samples, the tissue was sectioned into 400 µm-thick coronal slices containing the striatum with a vibrating tissue slicer (Leica VT1000S, Vashaw Scientific, Norcross, GA). Brain slices were transferred to a submersion recording chamber, perfused at 1 ml/min at 32 °C with oxygenated aCSF.
2.2. Fast scan cyclic voltammetry
After a 30-min equilibration period, a carbon fiber microelectrode (≈150 µM length, 7 µM radius) and a bipolar stimulating electrode were placed in close proximity to each other (≈100 µM) into the dorsal, medial, or ventral striatum, or the nucleus accumbens core or shell. DA was evoked by a single, rectangular, electrical pulse (300 µA, 2 ms) applied every 5 min. Extracellular DA was recorded every 100 ms using fast-scan cyclic voltammetry (Kennedy et al., 1992) by applying a triangular waveform (−0.4 to +1.2 to −0.4 V vs Ag/AgCl, 400 V/s). One recording was obtained per subregion in each animal (rhesus n = 5, mouse n = 3, rat n = 4).Once the extracellular DA response was stable the electrode was moved to the next subregion for recording, where the process was repeated. Immediately following the completion of each experiment, recording electrodes were calibrated by recording their response (in current; nA) to 3 µM DA in aCSF using a flow-injection system.
To determine kinetic parameters, evoked levels of DA were modeled using Michaelis–Menten kinetics, as a balance between release and uptake (Wightman et al., 1988). Michaelis–Menten modeling of baseline DA signals provides parameters that describe the amount of DA released following stimulation (i.e., the peak height of the signal) and the maximal rate of DA uptake (Vmax). For baseline modeling, we followed standard voltammetric modeling procedures by setting the apparent Km value to 160 nM for each animal based on well-established research on the affinity of DA for the DAT (Wu et al., 2001), whereas baseline Vmax values were allowed to vary as the maximal rate of DA uptake. All voltammetry data were collected and modeled using Demon Voltammetry and Analysis Software (Yorgason et al., 2011).
2.3. Statistics
Baseline voltammetry data were compared across groups using one-way analysis of variance (ANOVA) using Graphpad statistical software (La Jolla, CA, USA). Data obtained from the five striatal areas was subjected to a two-way ANOVA, with species and brain region as the factors. When significant main effects were obtained (p < 0.05), differences between groups at each dose were tested using Bonferroni post hoc tests. Comparisons of release to Vmax ratios, as well as comparisons between species at specific brain regions were subjected to one-way ANOVA.
3. Results
3.1. Electrically stimulated DA release in the striatum follows a dorsal to ventral gradient
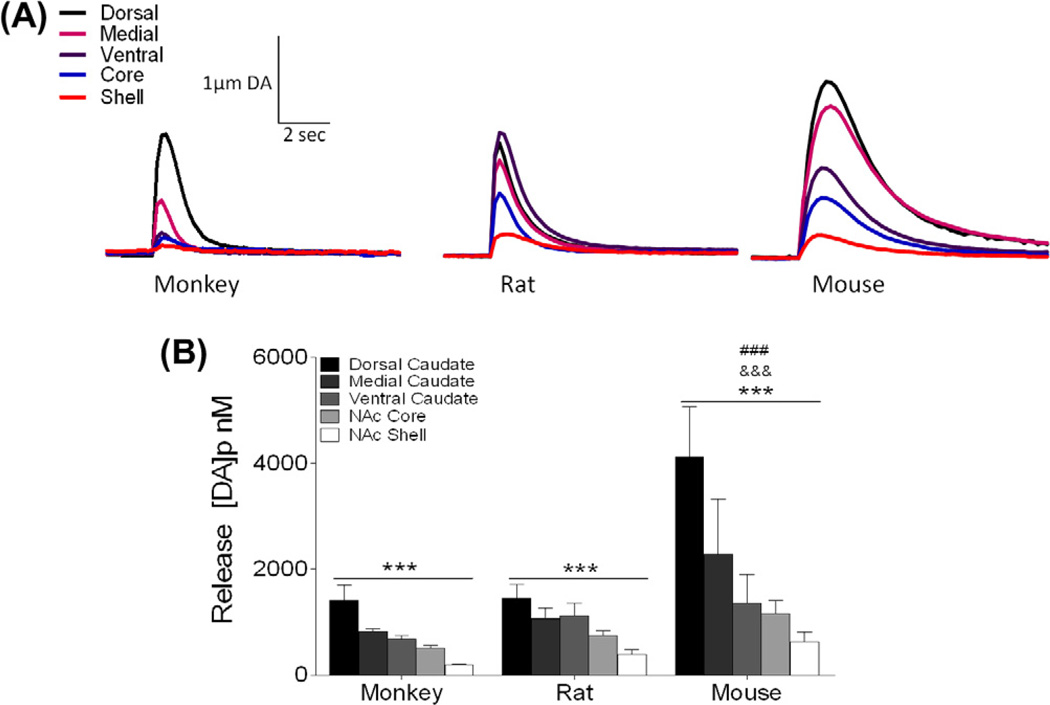
Fast scan cyclic voltammetry was used to assess DA system kinetics in five striatal subregions from C57BL/6J mice, Sprague-Dawley rats, and rhesus macaques (Fig. 1). Electrically stimulated DA release followed a similar pattern across the five measured regions in the three species. A two-way ANOVA revealed a significant difference by region (F2,45 = 6.906, p < 0.01) in all species (Fig. 2A and B). Bonferroni post hoc analysis determined that the dorsal caudate had the highest level of DA release and release was reduced in each region along a dorsal to ventral axis, with the nucleus accumbens shell displaying the lowest level of DA release (Fig. 2B). In addition, there were significant differences by species (F4,45 = 5.937, p < 0.01), with mouse release measurements being significantly higher overall than both monkey and rat (Fig. 2A and B).
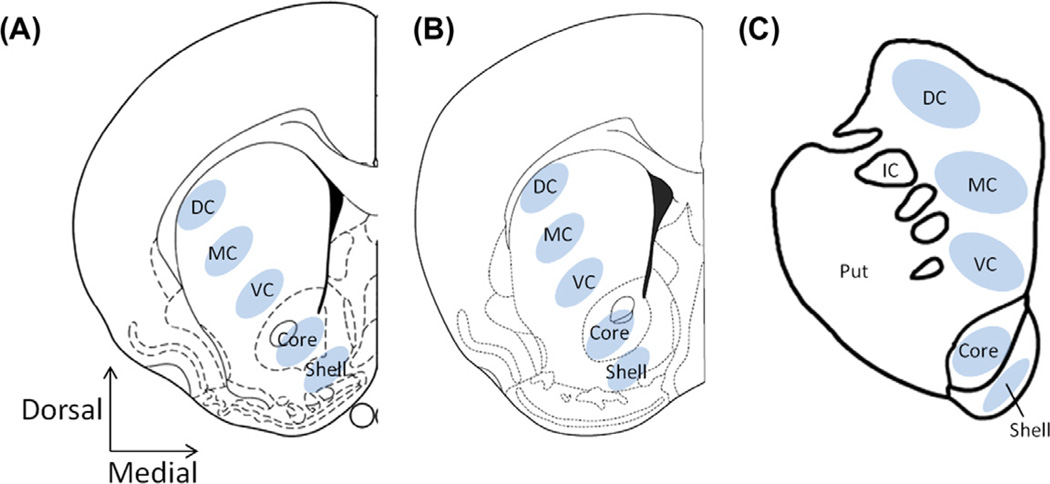
Fig. 1.
Location of voltammetric recordings in striatal slices from rat, mouse, and rhesus macaque. Five regions spanning the caudate putamen (CPu) and nucleus accumbens (NAc) were assessed across three species: Dorsolateral CPu (DC), medial CPu (MC), ventromedial CPu (VC), NAc core (core), and NAc shell (shell). (A) Diagram of a rat coronal section from Paxinos and Watson (2007) denoting the location where the recordings were made in each of the five regions, AP 1.32 (B) Diagram of a mouse coronal section adapted from Paxinos and Franklin (2007), AP 1.10 (C) Diagram of coronal section in rhesus macaque, AP 10.5. AP – anterior-posterior coordinates, Put – putamen, IC-internal capsule.
Fig. 2.
Dopamine (DA) release from five striatal regions in monkey, rat, and mouse. (A) Representative traces of the five striatal regions [dorsal caudate putamen (CPu), medial CPu, ventral CPu, nucleus accumbens core, nucleus accumbens shell] in monkey rat and mouse expressed as µM DA over time. (B) Mean peak release ± SEM evoked at five loci in each species showing an overall gradient of release from dorsal (highest) to ventral (lowest) that is conserved across species. ***p < 0.001 across region; ###p < 0.001 versus monkey; &&&p < 0.001 versus rat.
3.2. Maximal rate of DA uptake follows a dorsal to ventral gradient
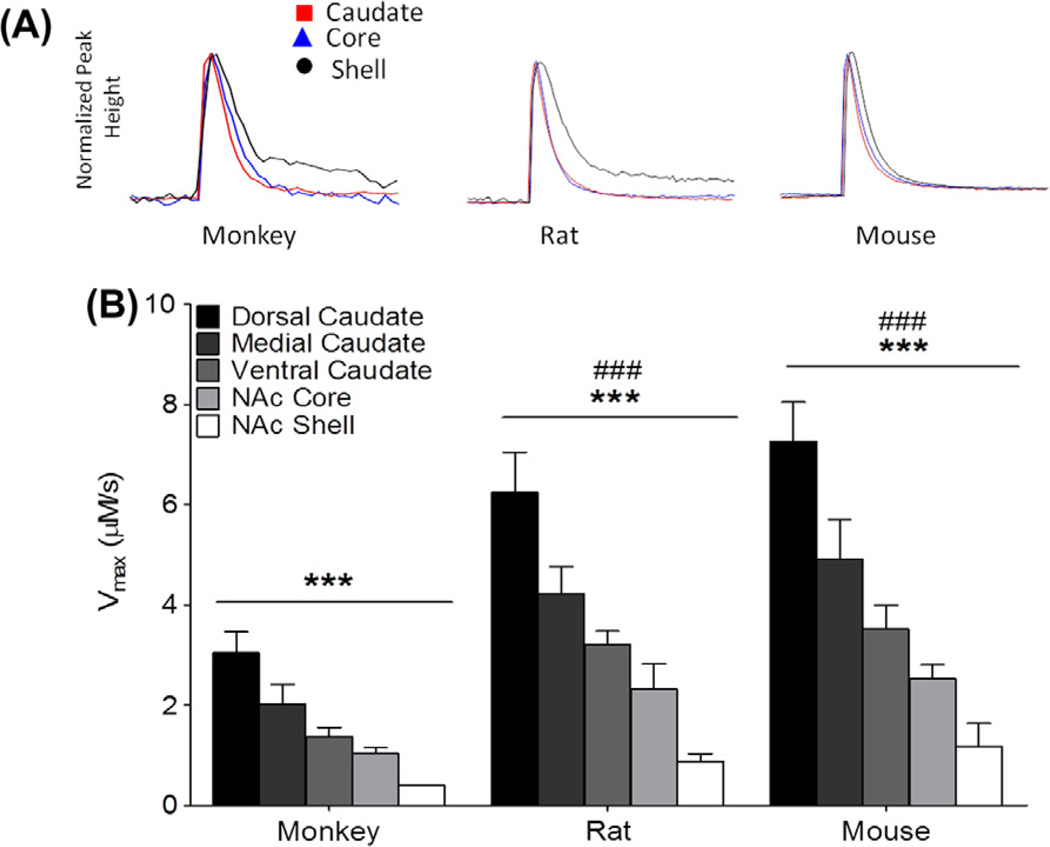
This study also assessed the rate of DA uptake following electrical stimulation and revealed a gradient of uptake across the striatal regions. There were significant differences by region (F2,45 = 32.47, p < 0.001) which were present in all species (Fig. 3A and B). Bonferroni post hoc analysis revealed the highest levels of uptake in the dorsal striatum and lower levels in a gradient from more dorsal to more ventral regions with the lowest level of uptake being present in the nucleus accumbens shell (Fig. 3B).
Fig. 3.
Maximal dopamine (DA) uptake rate (Vmax) as measured by fast scan cyclic voltammetry from dorsal striatum through ventral striatum in each species. (A) Representative traces of DA over time normalized for peak height. (B) Vmax across five regions (dorsal caudate-putamen (CPu), medial CPu, ventral CPu, nucleus accumbens core, nucleus accumbens shell) showing a gradient of uptake rate in three species. Vmax was determined using Michalis Menten modeling to determine kinetic parameters. Mean peak Vmax (µM/s) ± SEM evoked at the five listed loci. ***p < 0.001 across region; ###p < 0.001 versus monkey.
In addition to a conserved gradient of uptake across species, there were differences in overall uptake rates between rat, mouse, and monkey (F4,45 = 42.24, p < 0.001). Post hoc analysis revealed that rat and mouse rates of uptake were significantly higher than the uptake rates recorded for the monkeys (Fig. 3B).
3.3. Ratio of DA release to Vmax across species
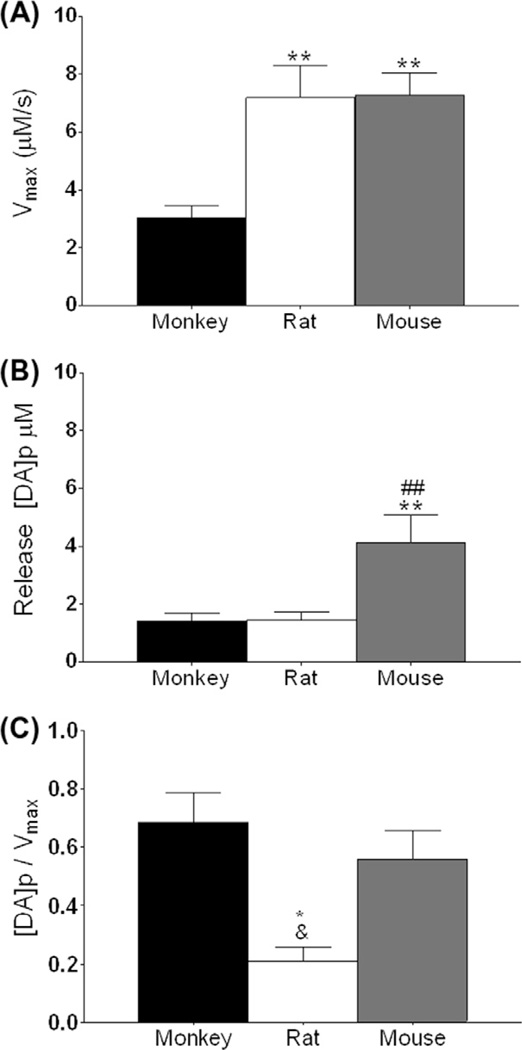
The ratio of DA release to Vmax was used to determine if there were differences in the regulation of presynaptic DA kinetics, across regions or species. There was a significantly lower ratio of release to uptake in rats in the dorsal caudate (F2,6 = 10.60, p < 0.05) (Fig. 4C) as compared to both monkey and mice. This difference between species was maintained across all regions. In addition, there were no regional differences in the ratio of DA release to Vmax in any of the species tested, suggesting that this measure is inherent to the type of animal and not regionally variable (Fig. 4).
Fig. 4.
Ratio of dopamine (DA) release to uptake differs across species. (A) Maximal rate of DA uptake (Vmax) in the dorsal striatum across monkey, rat, and mouse. (B) Stimulated DA release in the dorsal striatum across species, represented as DA per pulse. (C) Ratio of DA release to Vmax in the dorsal striatum across species. *p < 0.05 vs monkey; #p < 0.05 vs rat; and p < 0.05 vs mouse.
4. Discussion
We found that DA system organization in the striatum is conserved across three common model systems: rats, mice, and monkeys. The dorsal to ventral gradient in stimulated DA release across regions has been shown previously in marmosets by Cragg et al. (2002), however, the present study is the first to show that this gradient is present in rhesus macaques. This gradient of release suggests that the distinctions between striatal subregions are not absolute, but rather, they lie along a functional continuum. Because this striatal organization is conserved across species it suggests that the organization of DA inputs into these regions are also conserved and that studies in these regions are translatable across species. In addition, maximal uptake rates, Vmax, also exhibited a gradient, with greater uptake in dorsal regions of the striatum and the lowest uptake in the nucleus accumbens shell. It is likely that differences in uptake rates are associated with differences in transporter levels along a similar gradient. The fact that the gradient in uptake is similar to the gradient in release suggests that the two aspects of DA signaling are linked, and this may be related to the innervation density of DA terminals.
It is not surprising that DA system functionality is conserved across these species. Although there are considerable differences in cortical organization between rodents and primates, there have been many studies outlining the anatomical homology between species within sub-cortical structures. DA plays similar major roles in a number of essential functions such as movement, goal-directed behaviors, associative learning, and reward and reinforcement, across species (Bradberry, 2008; Levey et al., 1993l; Lynd-Balta and Haber, 1994a,b; Reep et al., 2003; Künzle, 1975; Selemon and Goldman-Rakic, 1985; Haber and McFarland, 1999). In addition, It has been determined previously that the DAT is highly conserved, with 92% homology between rat and human DAT transporters (Giros and Caron, 1993), supporting the idea of DA system conservation across species. Conservation in not only function, but also the proteins involved in the regulation of DA in these regions, suggests that interventions in rodents are likely to result in changes similar to those in higher primates under similar conditions, although studies in rodents allow more mechanistic and invasive studies on the DA system. Although the proteins are conserved across species, the levels of presynaptic DA regulatory proteins including D2 autoreceptors and DAT differ across brain regions (Shimada et al., 1992; Hurd et al., 1994; Haber et al., 1995), suggesting regional differences in DA function/regulation.
Independent of determining functional homology between model systems, it is also important to determine the functional differences across brain regions, specifically in the striatum, where the DA pathways that innervate this region have divergent actions. In both rodents and non-human primates the striatum is divided into functional domains that control different behavioral outputs, and receive inputs from different dopaminergic nuclei. The A9 pathway, which is degenerated in Parkinson’s disease, projects to the dorsolateral striatum and is involved in motor function, while the A10 projections, which synapse in the ventromedial striatum, are implicated in reward and reinforcement, and are essential for the reinforcing effects of drugs of abuse (Kish et al., 1988; Gibb and Lees, 1991). Consistent with functional differences in behavioral outputs, here we show that there are differences in regional DA release, characterized by a dorsal to ventral pattern of DA release across five regions spanning the striatum in rodents as well as non-human primates. This gradient in release could be due to a similar gradient that is seen with the rate limiting enzyme for the production of DA synthesis, tyrosine hydroxylase (Salvatore and Pruett, 2012). In addition, there are regional differences between the dorsal striatum and NAc in the levels of vesicular monoamine transporter 2 expression, which may also contribute to the differences in repackaging of DA and release in these areas (Salvatore et al., 2005). This functional organization has been shown in marmosets whereCragg et al. (2002) demonstrated that there is a dorsal to ventral gradient in DA release with more dorsal regions of the striatum exhibiting higher levels of stimulated DA release. Because we did not have access to putamen tissue, we were unable to assess if the same trend was present in that region; however,Cragg et al. (2002) previously showed that in marmosets the same trend is present in both the caudate and putamen. Even though marmosets and rhesus macaques are very different, the conservation of a gradient between macaques and rodents, which are different species of a different genus, makes it likely that there is similar functional homology between two animals of the same genus. It is likely that this regional conservation in uptake and release across regions in all species is due to the conservation of inputs from dopaminergic cell bodies in the VTA and substantia nigra, suggesting that rodents are good model systems to study the disease processes that affect these regions. In addition, Montague and Phillips have done voltammetric recordings in humans that are also similar to the kinetic parameters we find here, giving further support to the idea that rodents are acceptable translational models of dopaminergic organization (Kishida et al., in press).
Extracellular levels of DA are controlled by a delicate balance between vesicular release and reuptake of DA by the dopamine transporter (DAT) (Jones et al., 1998; Kuhar, 1973; Horn, 1990; Amara and Kuhar, 1993). Thus, determining the contribution of each measure to evoked efflux profiles can give us some insight as to what is driving extracellular DA signaling in the behaving animal. The data here show that there is a smaller ratio of DA release to DA uptake in the rat compared to mouse and monkey, suggesting that Vmax plays more of a role in controlling DA kinetics in the rat than the other species, thus the rat has uptake dominated DA signals. The ratio of release to uptake does not differ across regions, suggesting that even if there are overall differences in each measure, the relationship between these two measures remains constant. In other words, the gradient in Vmax and release are proportional to each other across regions, even if there is a difference in overall Vmax or release as compared to other species. It is important to note that absolute values of DA parameters differ across species; however, because of the conserved relationship between the regions, it is logical to postulate that one can obtain translatable results in a range of neurological models. This is important because it suggests that not only is there a functional gradient, but the species differences in Vmax and release values do not alter the functional relationship across regions.
5. Conclusions
Overall, this study demonstrates that extracellular DA is dynamically controlled in a similar fashion across the three species studied here (Rhesus macaque monkey, Sprague–Dawley rat and C57BL/6J mouse) and that DA dynamics are different in limbic and motor associated functional regions. These findings allow studies of DA dynamics in rodents to be more broadly generalized to the functioning of primate striatal regions. Although it is too simplistic to say the DA systems are identical, results here reveal an impressive conservation of function across the striatum. This suggests that vulnerabilities found in the DA system in rodents, either to drug or chemical insult, are likely to have similar responses and results in the primate brain. In an era of constraints on science with both budgetary and ethical concerns, this finding confirms a long-held assumption that DA function is similar across species.
Acknowledgements
This work was funded by NIH grants R01DA021325, RO1DA030161, U01AA014091, P50DA06634, P01AA017056 (SRJ), T32DA007246 and F31DA031533 (ESC).
References
- Amara SG, Kuhar MJ. Neurotransmitter transporters: recent progress. Annu. Rev. Neurosci. 1993;16:73–93. doi: 10.1146/annurev.ne.16.030193.000445. [DOI] [PubMed] [Google Scholar]
- Bradberry CW. Comparison of acute and chronic neurochemical effects of cocaine and cocaine cues in rhesus monkeys and rodents: focus on striatal and cortical dopamine systems. Rev. Neurosci. 2008;19(2–3):113–128. doi: 10.1515/revneuro.2008.19.2-3.113. [DOI] [PubMed] [Google Scholar]
- Carlsson A. The current status of the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1988;1(3):179–186. doi: 10.1016/0893-133x(88)90012-7. [DOI] [PubMed] [Google Scholar]
- Chesselet MF, Richter F. Modelling of Parkinson’s disease in mice. Lancet Neurol. 2011;10(12):1108–1118. doi: 10.1016/S1474-4422(11)70227-7. [DOI] [PubMed] [Google Scholar]
- Cragg SJ, Hille CJ, Greenfield SA. Dopamine release and uptake dynamics within nonhuman primate striatum in vitro. J. Neurosci. 2000;20(21):8209–8217. doi: 10.1523/JNEUROSCI.20-21-08209.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg SJ, Hille CJ, Greenfield SA. Functional domains in dorsal striatum of the nonhuman primate are defined by the dynamic behavior of dopamine. J. Neurosci. 2002;22(13):5705–5712. doi: 10.1523/JNEUROSCI.22-13-05705.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daunais JB, Kraft RA, Davenport AT, Burnett EJ, Maxey VM, Szeliga KT, Rau AR, Flory GS, Hemby SE, Kroenke CD, Grant KA, Friedman DP. MRI-guided dissection of the nonhuman primate brain: a case study. Methods. 2010;50(3):199–204. doi: 10.1016/j.ymeth.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding YS, Fowler JS, Volkow ND, Dewey SL, Wang GJ, Logan J, Gatley SJ, Pappas N. Chiral drugs: comparison of the pharmacokinetics of [11C]d-threo and l-threo-methylphenidate in the human and baboon brain. Psychopharmacology (Berl) 1997;131(1):71–78. doi: 10.1007/s002130050267. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Logan J, Gatley SJ, Pappas N, King P, Ding YS, Wang GJ. Measuring dopamine transporter occupancy by cocaine in vivo: radiotracer considerations. Synapse. 1998;28(2):111–116. doi: 10.1002/(SICI)1098-2396(199802)28:2<111::AID-SYN1>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Gibb WR, Lees AJ. Anatomy, pigmentation, ventral and dorsal subpopulations of the substantia nigra, and differential cell death in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry. 1991;54(5):388–396. doi: 10.1136/jnnp.54.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giros B, Caron MG. Molecular characterization of the dopamine transporter. Trends Pharmacol. Sci. 1993;14(2):43–49. doi: 10.1016/0165-6147(93)90029-j. [DOI] [PubMed] [Google Scholar]
- Goto Y, Yang CR, Otani S. Functional and dysfunctional synaptic plasticity in prefrontal cortex: roles in psychiatric disorders. Biol Psychiatry. 2010;67(3):199–207. doi: 10.1016/j.biopsych.2009.08.026. [DOI] [PubMed] [Google Scholar]
- Haber SN, McFarland NR. The concept of the ventral striatum in nonhuman primates. Ann N Y Acad Sci. 1999;877:33–48. doi: 10.1111/j.1749-6632.1999.tb09259.x. [DOI] [PubMed] [Google Scholar]
- Haber SN, Ryoo H, Cox C, Lu W. Subsets of midbrain dopaminergic neurons in monkeys are distinguished by different levels of mRNA for the dopamine transporter: comparison with the mRNA for the D2 receptor, tyrosine hydroxylase and calbindin immunoreactivity. J. Comp. Neurol. 1995;362(3):400–410. doi: 10.1002/cne.903620308. [DOI] [PubMed] [Google Scholar]
- Horn AS. Dopamine uptake: a review of progress in the last decade. Prog. Neurobiol. 1990;34(5):387–400. doi: 10.1016/0301-0082(90)90033-d. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Pristupa ZB, Herman MM, Niznik HB, Kleinman JE. The dopamine transporter and dopamine D2 receptor messenger RNAs are differentially expressed in limbic- and motor-related subpopulations of human mesencephalic neurons. Neuroscience. 1994;63(2):357–362. doi: 10.1016/0306-4522(94)90535-5. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Roth RH, Taylor JR. Role for dopamine in the behavioral functions of the prefrontal corticostriatal system: implications for mental disorders and psychotropic drug action. Prog. Brain Res. 2000;126:433–453. doi: 10.1016/S0079-6123(00)26028-7. [DOI] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Jaber M, Giros B, Wightman RM, Caron MG. Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc Natl Acad Sci USA. 1998;95(7):4029–4034. doi: 10.1073/pnas.95.7.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy RT, Jones SR, Wightman RM. Dynamic observation of dopamine autoreceptor effects in rat striatal slices. J. Neurochem. 1992;59:449–455. doi: 10.1111/j.1471-4159.1992.tb09391.x. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease. Pathophysiologic and clinical implications. N. Engl. J. Med. 1988;318(14):876–880. doi: 10.1056/NEJM198804073181402. [DOI] [PubMed] [Google Scholar]
- Kishida KT, Sandberg SG, Lohrenz T, Comair YG, Sáez I, Phillips PE, Montague PR. Sub-second dopamine detection in human striatum. PLoS One. 2011 doi: 10.1371/journal.pone.0023291. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhar MJ. Neurotransmitter uptake: a tool in identifying neurotransmitter-specific pathways. Life Sci. 1973;13(12):1623–1634. doi: 10.1016/0024-3205(73)90110-0. [DOI] [PubMed] [Google Scholar]
- Künzle H. Autoradiographic tracing of the cerebellar projections from the lateral reticular nucleus in the cat. Exp. Brain Res. 1975;22(3):255–266. doi: 10.1007/BF00234768. [DOI] [PubMed] [Google Scholar]
- Levey AI, Hersch SM, Rye DB, Sunahara RK, Niznik HB, Kitt CA, Price DL, Maggio R, Brann MR, Ciliax BJ. Localization of D1 and D2 dopamine receptors in brain with subtype-specific antibodies. Proc. Natl. Acad. Sci. 1993;90:8861–8865. doi: 10.1073/pnas.90.19.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynd-Balta E, Haber SN. The organization of midbrain projections to the ventral striatum in the primate. Neuroscience. 1994a;59(3):609–623. doi: 10.1016/0306-4522(94)90181-3. [DOI] [PubMed] [Google Scholar]
- Lynd-Balta E, Haber SN. The organization of midbrain projections to the striatum in the primate: sensorimotor-related striatum versus ventral striatum. Neuroscience. 1994b;59(3):625–640. doi: 10.1016/0306-4522(94)90182-1. [DOI] [PubMed] [Google Scholar]
- Mitchell IJ, Cooper AJ, Griffiths MR. The selective vulnerability of striatopallidal neurons. Prog. Neurobiol. 1999;59(6):691–719. doi: 10.1016/s0301-0082(99)00019-2. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic Press; 2007. [Google Scholar]
- Paxinos G, Watson C. The rat brain in Stereotaxic Coordinates. Amsterdam: Elsevier; 2007. [DOI] [PubMed] [Google Scholar]
- Reep RL, Cheatwood JL, Corwin JV. The associative striatum: organization of cortical projections to the dorsocentral striatum in rats. J. Comp. Neurol. 2003;467:271–292. doi: 10.1002/cne.10868. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Morgan D, Liu Y. How to make a rat addicted to cocaine. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2007;31(8):1614–1624. doi: 10.1016/j.pnpbp.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore MF, Fisher B, Surgener SP, Gerhardt GA, Rouault T. Neurochemical investigations of dopamine neuronal systems in ironregulatory protein 2 (IRP-2) knockout mice. Brain Res. Mol. Brain Res. 2005;139(2):341–347. doi: 10.1016/j.molbrainres.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Salvatore MF, Pruett BS. Dichotomy of tyrosine hydroxylase and dopamine regulation between somatodendritic and terminal field areas of nigrostriatal and mesoaccumbens pathways. PLoS One. 2012;7(1):e29867. doi: 10.1371/journal.pone.0029867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. J. Neurosci. 1985;5(3):776–794. doi: 10.1523/JNEUROSCI.05-03-00776.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada S, Kitayama S, Walther D, Uhl G. Dopamine transporter mRNA: dense expression in ventral midbrain neurons. Brain Res Mol Brain Res. 1992;13(4):359–362. doi: 10.1016/0169-328x(92)90220-6. [DOI] [PubMed] [Google Scholar]
- Silberman Y, Ariwodola OJ, Chappell AM, Yorgason JT, Weiner JL. Lateral paracapsular GABAergic synapses in the basolateral amygdala contribute to the anxiolytic effects of beta 3 adrenoceptor activation. Neuropsychopharmacology. 2010;35(9):1886–1896. doi: 10.1038/npp.2010.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman RM, Amatore C, Engstrom RC, Hale PD, Kristensen EW, Kuhr WG, May LJ. Real-time characterization of dopamine overflow and uptake in the rat striatum. Neuroscience. 1988;25:513–523. doi: 10.1016/0306-4522(88)90255-2. [DOI] [PubMed] [Google Scholar]
- Wu Q, Reith MEA, Wightman RM, Kawagoe KT, Garris PA. Determination of release and uptake parameters from electrically evoked dopamine dynamics measured by real-time voltammetry. J. Neurosci. Methods. 2001;112:119–133. doi: 10.1016/s0165-0270(01)00459-9. [DOI] [PubMed] [Google Scholar]
- Yorgason JT, Espana RA, Jones SR. Demon voltammetry and analysis software: analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J. Neurosci. Methods. 2011;202:158–164. doi: 10.1016/j.jneumeth.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS, Cheng AY, Lee TJ, Ghobadi CW, Schwartz ZM, Geisler S, Parsely KP, Gruber C, Veh RW. Inputs to the midbrain dopaminergic complex in the rat with emphasis on extended amygdala-recipient sectors. J. Comp. Neurol. 2011;519(16):3159–3188. doi: 10.1002/cne.22670. [DOI] [PMC free article] [PubMed] [Google Scholar]