Abstract
Objectives
Red blood cell (RBC) levels of eicosapentaenoic acid (EPA) plus docosahexaenoic acid (DHA, the omega-3 index, expressed as a percent of total fatty acids) are inversely related to risk for cardiovascular disease (CVD). Although several mechanisms underlying this relationship have been proposed, understanding the associations between the omega-3 index and markers of CVD in the community can shed additional light on this question. The objectives of this study were to define the relations between the omega-3 index and clinical factors and to determine the heritability of the omega-3 index.
Methods
RBC samples (n = 3196) drawn between 2005 and 2008 from participants in the Framingham Study [Examination 8 of the Offspring cohort plus Examination 3 of the Omni (minorities) cohort] were analyzed for fatty acid composition by gas chromatography.
Results
The mean (SD) omega-3 index was 5.6% (1.7%). In multivariable regression models, the factors significantly and directly associated with the omega-3 index were age, female sex, higher education, fish oil supplementation, dietary intake of EPA + DHA, aspirin use, lipid pharmacotherapy, and LDL-cholesterol. Factors inversely associated were Offspring cohort, heart rate, waist girth, triglycerides and smoking. The total explained variability in the omega-3 index for the fully adjusted model was 73%, which included major components due to heritability (24%), EPA + DHA intake (25%), and fish oil supplementation (15%).
Conclusion
The variability in the omega-3 index is determined primarily by dietary and genetic factors. An increased omega-3 index is associated with a generally cardioprotective risk factor milieu.
Keywords: Epidemiology, Cardiovascular disease, Risk factors, Omega-3 fatty acids, Erythrocytes, Heritability
1. Introduction
Red blood cell (RBC) levels of the long chain marine omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), which together constitute the omega-3 index [1], have been reported to be inversely related to risk for sudden cardiac death [2,3], acute coronary syndromes [4], depression [5], and total mortality [6] as summarized in recent reviews [7-9]. These may be secondary to effects of these fatty acids on heart rate [10], inflammation [11], endothelial function [12], serum lipid levels [13] and/or platelet function [14], which themselves may be mediated by changes in eicosanoid metabolism [15], membrane biophysics [16], and gene expression [17]. Although n-3 fatty acid supplementation has been successful in reducing risk for CVD events in several large randomized trials [18], more recent experience has been less uniform [19]. Indeed, the proposed anti-arrhythmic mechanism has recently been questioned [20].
An examination of the relations between RBC omega-3 content and markers of cardiovascular disease (CVD) in the community (where fish oil supplementation is uncommon) can help generate hypotheses regarding mediating mechanisms, whereas the associations of demographic and lifestyle factors with the omega-3 index can help define the clinical and behavioral variables that determine its levels. Among the latter are obviously fish intake and fish oil supplementation, but the effects of age, sex, smoking status, etc. and of genetic factors on the omega-3 index are unclear. The purpose of this study was to examine these cross-sectional relations in a well-characterized community, the Framingham Offspring cohort.
The red blood cell (RBC) membrane has been used by several investigators to quantify relatively long-term dietary exposure to trans and marine n-3 dietary fatty acids [3,21,22]. Indeed, fatty acid biomarkers (in plasma, plasma phospholipids, RBCs, etc) are better predictors of incident congestive heart failure [23], atrial fibrillation [24] and type 2 diabetes mellitus [25] than are questionnaire-based intake estimates. Hence n-3 fatty acid biomarkers are superior to n- 3 fatty acid intake estimates for predicting disease outcomes.
2. Methods
2.1. Participants
Children (and their spouses) of the original Framingham Heart Study cohort were recruited in 1971 and constitute the Framingham Offspring Study [26]. The detailed study designs and methods have been extensively described (http://www.nhlbi.nih.gov/about/framingham). In 1994, recruitment began for the Framingham Omni cohort comprising residents aged 40–74 who described themselves as members of a minority group, [27]. The Offspring Exam 8 and Omni Exam 3 were scheduled together from 2005 to 2008, and totaled 3319 participants. All study participants were comprehensively evaluated in an examination that included anthropometric measurements, biochemical assessment for traditional CVD risk factors, medical history and physical examination by a study physician. Written informed consent was provided by all participants, and the Institutional Review Board at the Boston University Medical Center approved the study protocol.
2.2. RBC fatty acid analysis
RBCs were isolated from blood drawn after a 10–12 h fast and frozen at −80 °C immediately after collection. RBC fatty acid composition was analyzed by gas chromatography (GC) with flame ionization detection as previously described [56]. Briefly, unwashed, packed RBCs were directly methylated with boron trifluoride and hexane at 100 °C for 10 min. The fatty acid methyl esters thus generated were analyzed using a GC2010 Gas Chromatograph (Shimadzu Corporation, Columbia, MD) equipped with an SP2560, fused silica capillary column (Supelco, Bellefonte, PA). Fatty acids were identified by comparison with a standard mixture of fatty acids characteristic of RBC (GLC 727, NuCheck Prep, Elysian, MN) which was also used to determine individual fatty acid response factors. The omega-3 index is defined as the sum of EPA and DHA expressed as a percent of total identified fatty acids. The coefficients of variation were 6.2% for EPA, 4.4% for DHA and 3.2% for the omega-3 index. All fatty acids present at >1% abundance had CVs of ≤7%.
2.3. Diet assessment
N-3 fatty acid intakes at Offspring Exam 8 were estimated by food frequency questionnaire, FFQ [28]. (No FFQ data were available from Omni Exam 3). Validity of this instrument for fatty acid intake has been documented by comparison with adipose tissue fatty acid composition [29,30] where the correlation for marine n-3 fatty acids was 0.43 (p < 0.0001). A nutrient database (released in 2002) was used to calculate the fatty acid composition of diets. Fish oil supplementation use was determined by self-report under Medications as “fish oil” or “omega-3” but not “flaxseed oil.”
2.4. Statistical analysis
There were 3196 participants from Offspring (n = 2899) and Omni (n = 297) with fatty acid data available for analyses. Differences in clinical factors, demographics and individual fatty acids between cohorts were tested using t-tests and chi-squared tests for continuous and categorical data, respectively.
There were 2964 subjects with a complete set of covariates (except for dietary data) for analyses. The n-3 fatty acids were transformed using natural logarithm to improve normality and homoscedasticity of the residuals in the adjusted linear models. The primary analysis examined the relations between ln (EPA + DHA) (dependent variable) with clinical factors (predictor variables). Age, sex, cohort, and highest level of education attained (the later as a marker of socioeconomic status) were forced into all models. Additionally PROC GLMSELECT was used with stepwise variable selection and Schwarz Bayesian Criterion [31] to select the ‘best’ model [32] from 21 candidate variables: systolic and diastolic blood pressure, heart rate, treatment for hypertension, waist girth at umbilicus, alcohol intake (heavy drinking defined as >14 drinks per week for men and >7 for women), physical activity index [33], current smoking, LDL- and HDL-cholesterol, triglycerides, fish oil supplementation, lipid lowering treatment, glucose, hemoglobin A1c, urinary albumin/creatinine, aspirin use, and prevalent diabetes, cardiovascular disease, congestive heart failure, and coronary heart disease. Triglycerides, physical activity index, and urinary albumin/creatinine were log-transformed to reduce leverage of extreme values. Then the selected model was refitted using generalized least squares in PROC MIXED which included a random coefficient to incorporate the familial clustering among siblings (Supplementary Table 1). The statistical parameters were estimated using restricted maximum likelihood with the ‘sandwich’ variance estimator; these standard errors are robust to misspecification of the covariance structure [34]. Covariates in the final model were collectively tested for interactions with age or sex using the partial F-test. In a secondary analysis, the individual RBC n-3 fatty acids (i.e., alpha-linolenic acid (ALA), docosapentaenoic acid (DPA), EPA and DHA) were modeled using the above method. For all results the critical level alpha was set to 0.05/25 = 0.0020 for statistical significance using Bonferroni correction.
To determine the extent to which the intake of EPA + DHA may have mediated the relations between the omega-3 index and the selected covariates, a subgroup analysis was conducted in the 2182 Offspring participants with dietary information. Bootstrapping was used with 1000 iterations to build nonparametric confidence intervals of the difference in the regression coefficients [35-37] between models that included or did not include dietary intake. Since 14 variables were tested for dietary mediation, the critical level was controlled using Bonferroni correction at 0.05/14 tests = 0.0036 (i.e., 99.64% confidence intervals). Clinical correlates analyses were performed using SAS software (version 9.2; SAS Institute Inc., Cary, NC).
The Offspring cohort with dietary intake (N = 2182) was used for heritability analyses of ln (EPA + DHA) since pedigree data were not collected in Omni. The same final multivariable model was used as above, which included – age, sex, highest education, heart rate, waist girth at umbilicus, current smoking, LDL and HDL cholesterol, ln[triglycerides], lipid lowering treatment, ln[n-3 dietary intake], fish oil supplementation and aspirin use. Unrelated participants were excluded, resulting in 2030 for analyses. Heritability estimates were calculated using the polygenic function in SOLAR applied to residuals from the adjusted linear models [38]. The initial model adjusted for age and sex; fish oil, ln[n-3 dietary intake], and smoking were added individually and the change in explained variance was determined. The fully adjusted model included the covariates listed above. We used the robust multivariate t-distribution, which includes a weighting parameter to reduce the potential effect of skewed residuals [39].
3. Results
3.1. Clinical correlates
There were several differences between the Offspring (white) and Omni (non-white) cohorts as seen in Table 1. The Offspring were on average 4 years older, less educated, and had a lower prevalence of diabetes. They also had higher levels of alcohol consumption, smoking, physical activity and fish oil supplementation. The overall mean (SD) omega-3 index was 5.62% (1.71%). It was 5.56% (1.69%) in the Offspring, but 6.16% (1.83%) in Omni (p < 0.0001) despite fewer Omni participants taking fish oil supplements. The difference was due primarily to a higher DHA level (Supplemental Table 2). A higher omega-3 index was found in non-Hispanic African American (n = 123) and Asian (n = 74) participants in whom the mean was 6.4%; in the Omni Hispanics (n = 80), the index (5.6%) was similar to that of the Offspring cohort. Whether nutritional factors were responsible for these differences could not be determined since dietary information was not available for Omni.
Table 1.
Offspring (Exam 8) and Omni (Exam 3) subjects’ characteristics.
| Variable [% missing]a | All N = 3196 | Offspring N = 2899 | Omni N = 297 | P-valueb |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 66(9)c | 66(9) | 62(9) | <0.0001 |
| Female | 1762(55%) | 1577(54%) | 185(62%) | 0.0092 |
| Highest education | <0.0001 | |||
| Grades <12 | 136(4%) | 103(4%) | 33(11%) | |
| Grade 12 | 1485(47%) | 1411(49%) | 74(26%) | |
| Associate/bachelor | 927(29%) | 840(29%) | 87(30%) | |
| Graduate or professional | 617(19%) | 522(18%) | 95(33%) | |
| Clinical factors | ||||
| Systolic (mm Hg) | 128(17) | 129(17) | 127(19) | 0.24 |
| Diastolic (mm Hg) | 74(10) | 73(10) | 74(9) | 0.18 |
| Heart rate (bpm) | 62(10) | 62(10) | 61(10) | 0.048 |
| Hypertension treatment | 1566(49%) | 1408(49%) | 158(53%) | 0.13 |
| Waist girth at umbilicus (inches) | 40.0(5.8) | 40.0(5.7) | 39.7(6.3) | 0.30 |
| BMI (kg/m2) | 28.4(5.5) | 28.3(5.4) | 29.0(6.4) | 0.029 |
| 1–2 drinks/dayd [1.3%] | 414(13%) | 404(14%) | 10(3%) | <0.0001 |
| Physical activity index [1.1%] | 35.2(5.3) | 35.3(5.4) | 34.2(4.6) | 0.0014 |
| Current smoker | 270(8%) | 259(9%) | 11(4%) | 0.0020 |
| LDL-cholesterol (mg/dL) | 105(32) | 105(31) | 106(34) | 0.54 |
| HDL cholesterol (mg/dL) | 57(18) | 57(18) | 57(17) | 0.91 |
| Triglycerides (mg/dL) | 118(71) | 118(69) | 115(83) | 0.48 |
| Lipid pharmacotherapy | 1373(43%) | 1246(43%) | 127(43%) | 0.92 |
| Fish oil supplementation | 322(10%) | 308(11%) | 14(5%) | 0.0013 |
| Aspirin (3+/week) | 1383(43%) | 1278(44%) | 105(35%) | 0.0035 |
| Glucose (mg/dL) | 107(24) | 107(24) | 107(27) | 0.78 |
| Hemoglobin A1c % | 5.8(0.7) | 5.7(0.7) | 6.0(0.8) | <0.0001 |
| Diabetes | 462(14%) | 398(14%) | 64(22%) | 0.0003 |
| Urine albumin/creatinine (mg/g) [1.7%] | 0.26(1.29) | 0.26(1.32) | 0.23(1.02) | 0.74 |
| Prevalent cardiovascular disease | 498(16%) | 464(16%) | 34(11%) | 0.039 |
| Prevalent congestive heart failure | 77(2%) | 75(3%) | 2(1%) | 0.041 |
| Prevalent coronary heart disease | 326(10%) | 307(11%) | 19(6%) | 0.023 |
All variables had <1% missing data unless noted otherwise.
For testing differences between the two cohorts the critical level alpha was set to 0.05/25 factors = 0.0020 for statistical significance using Bonferroni correction (shown in bold).
Mean (SD).
Indicator variable for females > 7 drinks/week, males > 14 drinks/week.
Of 22 clinical variables examined individually, nine were significantly associated with the index (Table 2). Those directly related were the use of fish oil, aspirin, or lipid pharmacotherapy, whereas diastolic blood pressure, heart rate, waist girth, BMI, current smoking, and serum triglyceride levels were inversely related. Adjusted linear models were constructed to examine the relations between the omega-3 index with clinical and demographic factors (Table 3). The Pearson correlation between BMI and waist girth was very strong (r = 0.91), so BMI was excluded from multivariable models. In this analysis, factors directly associated with the omega-3 index were age, female sex, higher education, fish oil supplementation, aspirin use and/or lipid pharmacotherapy, and LDL-cholesterol. Factors inversely related included Offspring cohort, heart rate, waist girth, triglycerides and smoking. The relations between EPA + DHA and the variables in the adjusted model were not modified by gender (p = 0.27) or age (p = 0.19). In general, the same relations were observed between these factors and the individual fatty acids (EPA and DHA) as with the sum of the two (cf. Table 3 and Supplementary Table 3). The primary exception was for HDL-cholesterol which was not selected into the omega-3 index model (Table 3), because the significant relations with EPA (direct) and DHA (inverse) canceled each other (Supplementary Table 3). For the other two n-3 fatty acids, DPA and ALA, far fewer variables were selected into their models (Supplementary Table 4) than into the index model. Of note, DPA was lower in women than men, whereas DHA was higher. Also, compared to EPA and DHA (which had inverse relations with triglycerides), ALA was directly related to triglycerides.
Table 2.
Clinical correlates of RBC EPA + DHA in individual models.
| Variable (N = 2964) | Proportional changea | 95% CI
|
P-valueb | |
|---|---|---|---|---|
| Lower | Upper | |||
| Blood pressure | ||||
| Systolic (1 SD = 17 mm Hg) | 1.00 | 0.99 | 1.01 | 0.73 |
| Diastolic (1 SD = 10 mm Hg) | 0.98 | 0.97 | 0.99 | 0.0018 |
| Heart rate (1 SD = 10 bpm) | 0.96 | 0.95 | 0.97 | <0.0001 |
| Hypertension treatment | 1.03 | 1.01 | 1.06 | 0.0033 |
| Anthropometric & behavioral | ||||
| Waist girth (1 SD = 5.8 in) | 0.97 | 0.96 | 0.98 | <0.0001 |
| BMI (1 SD = 5.5 kg/m2) | 0.97 | 0.96 | 0.98 | <0.0001 |
| 1 – 2 Drinks/Dayc | 0.98 | 0.95 | 1.02 | 0.31 |
| ln[Physical activity index] (1 SD) | 1.00 | 0.99 | 1.01 | 0.97 |
| Current smoker | 0.84 | 0.81 | 0.87 | <0.0001 |
| Lipids & drugs | ||||
| LDL-cholesterol (1 SD = 32 mg/dL) | 0.99 | 0.98 | 1.00 | 0.14 |
| HDL cholesterol (1 SD = 18 mg/dL) | 1.01 | 1.00 | 1.02 | 0.051 |
| ln[Triglycerides] (1 SD) | 0.97 | 0.96 | 0.98 | <0.0001 |
| Lipid pharmacotherapy | 1.08 | 1.05 | 1.10 | <0.0001 |
| Fish oil supplementation | 1.38 | 1.33 | 1.43 | <0.0001 |
| Aspirin (3+/week) | 1.08 | 1.06 | 1.10 | <0.0001 |
| Glycemic control | ||||
| Glucose (1 SD = 23 mg/dL) | 0.99 | 0.98 | 1.00 | 0.18 |
| Hemoglobin A1c (1 SD = 0.7%) | 1.00 | 0.98 | 1.01 | 0.37 |
| Diabetes | 0.98 | 0.95 | 1.01 | 0.15 |
| ln[Urine albumin/creatinine] (1 SD) | 1.00 | 0.99 | 1.01 | 0.59 |
| Cardiovascular disease | ||||
| Prevalent cardiovascular disease | 1.03 | 1.00 | 1.06 | 0.074 |
| Prevalent congestive heart failure | 1.00 | 0.93 | 1.08 | 0.98 |
| Prevalent coronary heart disease | 1.05 | 1.01 | 1.09 | 0.0080 |
Represents the proportional change in EPA + DHA per 1 SD (continuous) or presence vs. absence (categorical) covariate.
The critical level alpha was set to 0.05/25 factors = 0.0020 for statistical significance using Bonferroni correction (shown in bold).
Indicator variable for females > 7 drinks/week, males > 14 drinks/week.
Table 3.
Clinical correlates of RBC EPA + DHA in multivariable model.
| Variable (N = 2964) | Proportional changea | 95% CI
|
P-valueb | |
|---|---|---|---|---|
| Lower | Upper | |||
| Demographics | ||||
| Age (10 years)c | 1.05 | 1.04 | 1.06 | <0.0001 |
| Sex (female)c | 1.04 | 1.02 | 1.07 | <0.0001 |
| Cohort (offspring)c | 0.89 | 0.85 | 0.92 | <0.0001 |
| Highest educationc | ||||
| Graduate or professional | 1.12 | 1.08 | 1.15 | <0.0001 |
| Associate/bachelor | 1.09 | 1.06 | 1.11 | <0.0001 |
| Grade 12 (reference) | 1.00 | – | – | – |
| Grades <12 | 0.93 | 0.88 | 0.97 | 0.0020 |
| Blood pressure | ||||
| Heart rate (1 SD = 10 bpm) | 0.98 | 0.97 | 0.99 | <0.0001 |
| Anthropometric & behavioral | ||||
| Waist girth (1 SD = 5.8 in) | 0.98 | 0.97 | 0.99 | 0.0003 |
| Current smoker | 0.90 | 0.87 | 0.93 | <0.0001 |
| Lipids & drugs | ||||
| LDL-cholesterol (1 SD = 32 mg/dL) | 1.02 | 1.01 | 1.03 | 0.0009 |
| Ln[Triglycerides] (1 SD) | 0.98 | 0.97 | 0.99 | <0.0001 |
| Lipid pharmacotherapy | 1.07 | 1.04 | 1.09 | <0.0001 |
| Fish oil supplementation | 1.35 | 1.31 | 1.39 | <0.0001 |
| Aspirin (3+/week) | 1.04 | 1.02 | 1.06 | 0.0005 |
Represents the proportional change in EPA + DHA per 1 SD (continuous) or presence vs. absence (categorical) covariate, e.g. a 1 SD increase in Waist girth is associated with 2% lower levels.
The critical level alpha was set to 0.05/25 factors = 0.0020 for statistical significance using Bonferroni correction (shown in bold).
Forced into model.
3.2. Effects of Diet
Dietary intake of EPA + DHA was available for 2182 Offspring cohort participants, so the question of the extent to which differences in dietary intake of n-3 fatty acids mediated the associations between the omega-3 index and the clinical/demographic factors was addressed in this subset (Supplementary Table 5). The EPA + DHA intake attenuated the associations between the omega-3 index and two factors: fish oil supplementation and education. Without EPA + DHA intake in the model, fish oil supplementation (yes/no) was associated with 36% [column 1; exp(0.311) = 1.36] higher levels of EPA + DHA. This, however, was an overestimate because those who took fish oil supplements also choose to eat more fish. A less biased estimate of the effect of fish oil supplementation alone, taking into account the coincidently higher EPA + DHA intake from the diet, was 19% [column 4; exp(0.178) = 1.19]. Likewise, the 12% higher omega-3 index level associated with having a graduate-level degree [column 1; exp(0.109) = 1.12] was more appropriately estimated as about 6% higher [column 4; exp(0.057) = 1.06] since highly educated participants also ate more fish. Importantly, relations between the omega-3 index and other demographic or clinical factors were not mediated by diet, so the entire sample was used to generate unbiased parameter estimates (Table 3). The correlations between the intake of EPA + DHA with RBC levels of these fatty acids in participants not taking fish oil supplements (n = 1949) was 0.59 (p < 0.0001); and was 0.19 (p = 0.003) in those taking supplements (n = 233).
3.3. Heritability
Five statistical models with increasing levels of complexity were used to partition the variance in the omega-3 index into that determined by the model covariates (those shown in Supplementary Table 5) and that determined by heritability, the later being based on pedigree information (Fig. 1). With age and sex as the only covariates, heritability explained 50% of the variability in the omega-3 index. When fish oil supplementation was added as a covariate to the model, the variance explained by the covariates increased from 4% to 19%, but there was little change in the heritability estimate (from 50% to 47%). When dietary ln(EPA + DHA) intake was next added to the model, the covariates’ variance estimate increased to 44%, and the heritability estimate decreased to 32%. Further covariate adjustment produced minimal changes. The total explained variability in the omega-3 index for the fully adjusted model was 73%, which included major components due to heritability (24%), dietary intake (25%), and fish oil supplementation (15%).
Fig. 1.
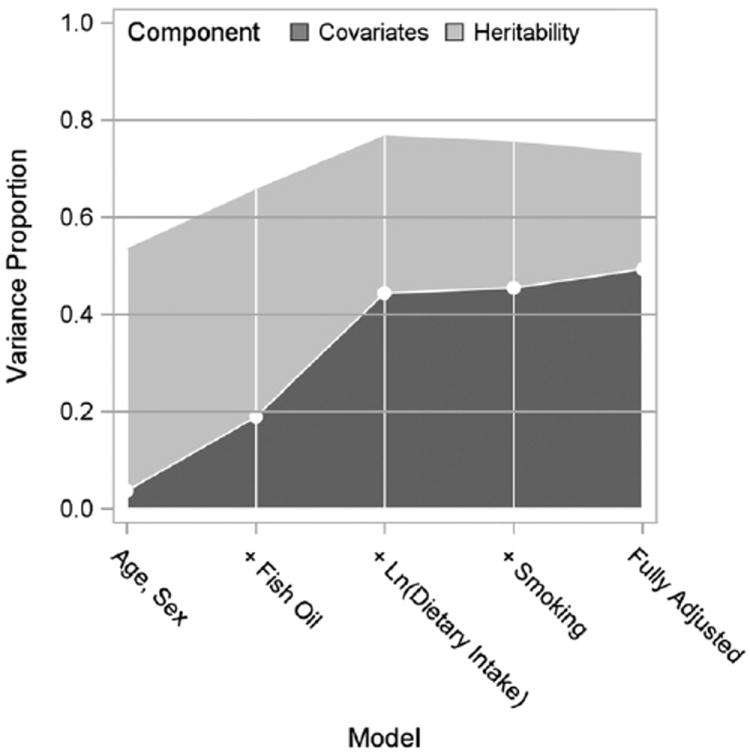
Total variance explained in the omega-3 index (RBC EPA + DHA) partitioned by heritability and covariates components for increasing model complexity. The fully adjusted model also included: HDL-C, LDL-C, ln(triglycerides), waist girth, aspirin (3+/wk), heart rate, lipid pharmacotherapy, and highest level of education.
4. Discussion
The purpose of this study was to explore the clinical, demographic, behavioral and genetic correlates of the omega-3 index in a well-defined cohort of subjects from the Framingham Heart Study. The cohort was comprised of 91% white participants (Offspring) and 9% minorities (Omni). The omega-3 index was lower in Offspring than Omni despite fish oil supplementation being more prevalent in the former; this effect remained after multivariable adjustment. Whether a higher dietary intake of n-3 fatty acids in Omni is responsible for this difference is unknown since no dietary information was available for this cohort. Higher levels of the omega-3 index in African Americans compared with Whites have been reported in acute myocardial infarction patients [40].
Overall the omega-3 index was directly associated with several factors in fully adjusted models. A significant relationship with age has been frequently reported [41-51] and could reflect increased fish intake in older individuals. However, we observed this association after controlling for n-3 fatty acid intake [as have some [42,44,46,52] but not all [53,54] other studies], and thus it may be a function of decreased n-3 fatty acid turnover in older subjects [55]. In an earlier study of 298 Offspring participants with RBCs tested 6.7 years apart [56], the mean omega-3 index did not change, suggesting that higher levels of the index in older people may not be due to aging per se, but perhaps to the “attrition of the susceptible.” Also female sex was associated with a higher omega-3 index. This too has been reported previously [48,49,53,57,58] [but inconsistently [42-44,50,51]], and could be due to enhanced synthesis of long chain n-3 fatty acids from alpha-linolenic acid mediated by estrogen [59,60]. This explanation is tentative, however, since the vast majority of women in this cohort were post menopausal, and only 10% were on hormone therapy. The reduced DPA (intermediate between EPA and DHA) and increased DHA levels in post menopausal women vs. men observed here are consistent with increased conversion of DPA to DHA seen in premenopausal [61] women. A higher omega-3 index was also directly associated with a greater use of aspirin and lipid lowering drugs, and with higher LDL-C levels. This confirms smaller crosssectional observations made in Canadian native Americans [50] and Inuits [49]. In the present study, the people taking aspirin and/or lipid drugs were more likely to be taking fish oil supplements (58% vs. 42%, p < 0.0001). In addition, they may have been taking higher n-3 fatty acid doses than the supplementers not on pharmacotherapy (dosage amounts were not available) which could explain the association of higher omega-3 index levels with medication use even after adjustment.
As regards the direct association between plasma LDL-C and the omega-3 index, n-3 fatty acid supplementation has been reported to increase LDL-C, particularly in hypertriglyceridemic patients [62], but this may be due more to a change in particle composition than in particle number [63]. Whether nutritional variations in n-3 fatty acid intakes affect LDL-C is not known, but the relation between the omega-3 index and LDL-C became non-significant when dietary EPA + DHA was included in the model (Supplementary Table 5).
There were several inverse associations with the omega-3 index in the fully adjusted model. That with smoking status, which has been noted before [40-43,48-50,53,64], was driven by the DHA (not the EPA) component (Supplementary Table 3). This relationship may be due to the oxidative destruction of most highly unsaturated fatty acids [65,66], and/or from altered metabolism of EPA and DHA in smokers. The fact that smoking was associated with lower omega-3 levels independent of EPA + DHA intake and fish oil supplementation (Supplementary Table 5) argues against intake differences.
Waist circumference was inversely associated with the omega-3 index (and both of its fatty acid components) in univariate and multivariable models. Studies in three Canadian cohorts reported contradictory findings in this regard: inverse associations between waist girth and RBC n-3 fatty acids in Cree Indians [50] but direct relations in Inuits [49] and Quebecers [48]. Therewas no univariate relation with girth in a Spanish cohort [53]. In a previous study, lower levels of EPA + DHA were associated with higher levels of pro-oxidants in RBCs from obese subjects [67], and with higher levels of inflammatory markers [43,68,69] suggesting that increased adiposity, oxidative stress, and inflammation and lower membrane n-3 fatty acids appear to coexist.
The triglyceride-lowering effects of supplemental n-3 fatty acids are well-known [13]. However, relations between triglycerides and n-3 fatty acid biomarkers in (largely) non-supplementing populations have received less attention. Inverse associations were, however, observed in the Heart and Soul study [70], Canadian Inuits [49], Spanish patients at high CHD risk [53] and outpatients in Kansas City [42]. These are likely to be causal since n-3 fatty acids lower TG production in the liver [71]. Finally, a higher omega-3 index was associated with a lower heart rate. This too has been reported in intervention studies [72,73] and observed crosssectionally [10,74]. N-3 fatty acids appear to improve autonomic tone as evidenced by their ability to increase heart rate variability in some but not all studies [75].
Taking advantage of the pedigree information from the Framingham study, we were able to estimate that 24% of the variability in the omega-3 index was due to polygenic factors (i.e., heritability). This increased the total explained variability to 73%, which was greater than previously reported [42,76]. The largest shift in amount of explained variance between heritability (15% decrease) and covariates (25% increase) was observed after dietary EPA + DHA intake was included in the model. That is to say, that related participants apparently maintained similar eating habits, and that the variance in the omega-3 index attributed to genes was overestimated when EPA + DHA consumption was not taken into account. Genome-wide association studies are needed to identify genetic loci that may determine the observed heritability [77].
In the Offspring cohort (where dietary data were available), the correlation between EPA + DHA intake and the omega-3 index (0.59) was stronger than that observed previously using the same FFQ but with adipose tissue EPA + DHA as a biomarker (r = 0.43) [30]. As RBC membranes are more enriched in these long chain n-3 fatty acids than is adipose tissue [78], this finding provides stronger confirmation of the validity of the FFQ for these specific fatty acids.
4.1. Strengths and limitations
Among the strengths of this study were the large, well-characterized, and racially-diverse (via the inclusion of Omni) cohort; and the use of validated analytical methods for clinical CVD risk markers (including the omega-3 index). Also this study included a formal analysis of heritability. There were also limitations. Causal relations cannot be deduced from a cross-sectional analysis, so the associations observed here – unless previously observed in n-3 fatty acid intervention studies – must be considered only hypothesis-generating. For those individuals reporting fish oil supplementation we had no information on the dosage, potency or intake frequency of the capsules. As noted earlier, we did not have data on the dietary habits nor pedigree information of the Omni participants.
In summary, a higher omega-3 index was independently associated with some factors known to be associated with reduced risk for cardiovascular disease (female sex, smaller waist girth, lower triglyceride levels, less smoking, lower heart rate, and a greater use of aspirin and lipid pharmacotherapy). On the other hand, a higher index was also associated with a slightly higher LDL-cholesterol level. After adjusting for n-3 fatty acid intake and other relevant covariates, 24% of the variability in the omega-3 index was explained by Mendelian inheritance. Overall, an increased omega-3 index appears to coexist with a healthier cardiovascular risk profile.
Supplementary Material
Acknowledgments
Sources of support
This study was supported by National Heart Lung and Blood Institute (NHLBI; R01 HL089590) and by Contract N01-HC-25195, the Framingham Heart Study (NHLBI) and Boston University School of Medicine.
Roles of authors
WSH, SJR, RSV, and MGL designed research (project conception, development of overall research plan, and study oversight); WSH conducted research (oversaw the hands-on lab analyses); JVP and SML (with oversight from MGL) analyzed data or performed statistical analysis; WSH and JVP wrote paper with major editorial input from SJR, MGL and RSV; and WSH had primary for final content. All authors read and approved the final manuscript.
Footnotes
Potential conflicts of interest
WSH is a scientific advisor to companies with interests in fatty acids including Monsanto, Aker Biomarine, Omthera, Amarin and GlaxoSmithKline, and was a speaker for the latter. In addition, he is the owner of OmegaQuant Analytics, LLC and an employee of Health Diagnostics Laboratory, Inc., both of which offer blood fatty acid testing commercially. None of the other authors have any potential conflicts to disclose. All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Appendix A. Supplementary data
Supplementary data related to this article can be found online at http://dx.doi.org/10.1016/j.atherosclerosis.2012.05.030.
References
- 1.Harris WS, von Schacky C. The omega-3 index: a new risk factor for death from coronary heart disease? Prev Med. 2004;39:212–20. doi: 10.1016/j.ypmed.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 2.Albert CM, Campos H, Stampfer MJ, Ridker PM, Manson JE, Willett WC, et al. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N Engl J Med. 2002;346:1113–8. doi: 10.1056/NEJMoa012918. [DOI] [PubMed] [Google Scholar]
- 3.Siscovick DS, Raghunathan TE, King I, et al. Dietary intake and cell membrane levels of long-chain n-3 poly-unsaturated fatty acids and the risk of primary cardiac arrest. J Am Med Assoc. 1995;274:1363–7. doi: 10.1001/jama.1995.03530170043030. [DOI] [PubMed] [Google Scholar]
- 4.Block RC, Harris WS, Reid KJ, Sands SA, Spertus JA. EPA and DHA in blood cell membranes from acute coronary syndrome patients and controls. Atherosclerosis. 2007;197:821–8. doi: 10.1016/j.atherosclerosis.2007.07.042. [DOI] [PubMed] [Google Scholar]
- 5.Baghai TC, Varallo-Bedarida G, Born C, et al. Major depressive disorder is associated with cardiovascular risk factors and low omega-3 index. J Clin Psychiatry. 2010 doi: 10.4088/JCP.09m05895blu. [DOI] [PubMed] [Google Scholar]
- 6.Pottala JV, Garg S, Cohen BE, Whooley MA, Harris WS. Blood eicosapentaenoic and docosahexaenoic acids predict all-cause mortality in patients with stable coronary heart disease: The Heart and Soul Study. Circ Cardiovasc Qual Outcomes. 2010;3:406–12. doi: 10.1161/CIRCOUTCOMES.109.896159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Caterina R. n-3 fatty acids in cardiovascular disease. N Engl J Med. 2011;364:2439–50. doi: 10.1056/NEJMra1008153. [DOI] [PubMed] [Google Scholar]
- 8.Lavie CJ, Milani RV, Mehra MR, Ventura HO. Omega-3 polyunsaturated fatty acids and cardiovascular diseases. J Am Coll Cardiol. 2009;54:585–94. doi: 10.1016/j.jacc.2009.02.084. [DOI] [PubMed] [Google Scholar]
- 9.Saravanan P, Davidson NC, Schmidt EB, Calder PC. Cardiovascular effects of marine omega-3 fatty acids. Lancet. 2010;376:540–50. doi: 10.1016/S0140-6736(10)60445-X. [DOI] [PubMed] [Google Scholar]
- 10.Mozaffarian D, Geelen A, Brouwer IA, Geleijnse JM, Zock PL, Katan MB. Effect of fish oil on heart rate in humans: a meta-analysis of randomized controlled trials. Circulation. 2005;112:1945–52. doi: 10.1161/CIRCULATIONAHA.105.556886. [DOI] [PubMed] [Google Scholar]
- 11.de Roos B, Mavrommatis Y, Brouwer IA. Long-chain n-3 polyunsaturated fatty acids: new insights into mechanisms relating to inflammation and coronary heart disease. Br J Pharmacol. 2009;158:413–28. doi: 10.1111/j.1476-5381.2009.00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egert S, Stehle P. Impact of n-3 fatty acids on endothelial function: results from human interventions studies. Curr Opin Clin Nutr Metab Care. 2011;14:121–31. doi: 10.1097/MCO.0b013e3283439622. [DOI] [PubMed] [Google Scholar]
- 13.Balk EM, Lichtenstein AH, Chung M, Kupelnick B, Chew P, Lau J. Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: a systematic review. Atherosclerosis. 2006;189:19–30. doi: 10.1016/j.atherosclerosis.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Violi F, Pignatelli P, Basili S. Nutrition, supplements, and vitamins in platelet function and bleeding. Circulation. 2010;121:1033–44. doi: 10.1161/CIRCULATIONAHA.109.880211. [DOI] [PubMed] [Google Scholar]
- 15.Shearer GC, Harris WS, Pedersen TL, Newman JW. Detection of omega-3 oxylipins in human plasma and response to treatment with omega-3 acid ethyl esters. J Lipid Res. 2009 doi: 10.1194/jlr.M900193-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yaqoob P, Shaikh SR. The nutritional and clinical significance of lipid rafts. Curr Opin Clin Nutr Metab Care. 2010;13:156–66. doi: 10.1097/MCO.0b013e328335725b. [DOI] [PubMed] [Google Scholar]
- 17.Jump DB. N-3 polyunsaturated fatty acid regulation of hepatic gene transcription. Curr Opin Lipidol. 2008;19:242–7. doi: 10.1097/MOL.0b013e3282ffaf6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58:2047–67. doi: 10.1016/j.jacc.2011.06.063. [DOI] [PubMed] [Google Scholar]
- 19.Kwak SM, Myung SK, Lee YJ, Seo HG. Efficacy of omega-3 fatty acid supplements (eicosapentaenoic acid and docosahexaenoic acid) in the secondary prevention of cardiovascular disease: a meta-analysis of randomized, doubleblind, placebo-controlled trials. Arch Intern Med. 2012 doi: 10.1001/archinternmed.2012.262. [DOI] [PubMed] [Google Scholar]
- 20.Rauch B, Senges J. The effects of supplementation with omega-3 polyunsaturated fatty acids on cardiac rhythm: anti-arrhythmic, pro-arrhythmic, both or neither? It depends. Front Physiol. 2012;3:57. doi: 10.3389/fphys.2012.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lemaitre RN, King IB, Raghunathan TE, Pearce RM, Weinmann S, Knopp RH, et al. Cell membrane trans-fatty acids and the risk of primary cardiac arrest. Circulation. 2002;105:697–701. doi: 10.1161/hc0602.103583. [DOI] [PubMed] [Google Scholar]
- 22.Harris WS. The omega-3 index: from biomarker to risk marker to risk factor. Curr Atheroscler Rep. 2009;11:411–7. doi: 10.1007/s11883-009-0062-2. [DOI] [PubMed] [Google Scholar]
- 23.Mozaffarian D, Lemaitre RN, King IB, et al. Circulating long-chain omega-3 fatty acids and incidence of congestive heart failure in older adults: the cardiovascular health study: a cohort study. Ann Intern Med. 2011;155:160–70. doi: 10.1059/0003-4819-155-3-201108020-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu JH, Lemaitre RN, King IB, et al. Association of plasma phospholipid longchain omega-3 fatty acids with incident atrial fibrillation in older adults: the cardiovascular health study. Circulation. 2012;125:1084–93. doi: 10.1161/CIRCULATIONAHA.111.062653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Djousse L, Biggs ML, Lemaitre RN, et al. Plasma omega-3 fatty acids and incident diabetes in older adults. Am J Clin Nutr. 2011;94:527–33. doi: 10.3945/ajcn.111.013334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–90. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 27.Quan SF, Howard BV, Iber C, et al. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20:1077–85. [PubMed] [Google Scholar]
- 28.Rumawas ME, Dwyer JT, McKeown NM, Meigs JB, Rogers G, Jacques PF. The development of the Mediterranean-style dietary pattern score and its application to the American diet in the Framingham Offspring Cohort. J Nutr. 2009;139:1150–6. doi: 10.3945/jn.109.103424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garland M, Sacks FM, Colditz GA, et al. The relation between dietary intake and adipose tissue composition of selected fatty acids in US women. Am J Clin Nutr. 1998;67:25–30. doi: 10.1093/ajcn/67.1.25. [DOI] [PubMed] [Google Scholar]
- 30.London SJ, Sacks FM, Caesar J, Stampfer MJ, Siguel E, Willett WC. Fatty acid composition of subcutaneous adipose tissue and diet in postmenopausal US women. Am J Clin Nutr. 1991;54:340–5. doi: 10.1093/ajcn/54.2.340. [DOI] [PubMed] [Google Scholar]
- 31.Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;6:461–4. [Google Scholar]
- 32.Yang Y. Can the strengths of AIC and BIC be shared? A conflict between model identification and regression estimation. Biometrika. 2005;92:937–50. [Google Scholar]
- 33.Kannel WB, Sorlie P. Some health benefits of physical activity. The Framingham Study Arch Intern Med. 1979;139:857–61. [PubMed] [Google Scholar]
- 34.Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis. Hoboken, NJ: John Wiley and Sons; 2004. [Google Scholar]
- 35.Efron B. Bootstrap methods: another look at the jackknife. Ann Stat. 1979;7:1–26. [Google Scholar]
- 36.Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychol Meth. 2002;7:422–45. [PubMed] [Google Scholar]
- 37.Krause MR, Serlin RC, Ward SE, Rony RY, Ezenwa MO, Naab F. Testing mediation in nursing research: beyond Baron and Kenny. Nurs Res. 2010;59:288–94. doi: 10.1097/NNR.0b013e3181dd26b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blangero J, Williams JT, Almasy L. Variance component methods for detecting complex trait loci. Adv Genet. 2001;42:151–81. doi: 10.1016/s0065-2660(01)42021-9. [DOI] [PubMed] [Google Scholar]
- 39.Lange KL, Little RJ, Taylor JM. Robust statistical modeling using the t distribution. J Am Stat Assoc. 1989;84:881–96. [Google Scholar]
- 40.Salisbury AC, Amin AP, Harris WS, et al. Predictors of omega-3 index in patients with acute myocardial infarction. Mayo Clin Proc. 2011;86:626–32. doi: 10.4065/mcp.2011.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ogura T, Takada H, Okuno M, et al. Fatty acid composition of plasma, erythrocytes and adipose: their correlations and effects of age and sex. Lipids. 2010;45:137–44. doi: 10.1007/s11745-010-3386-3. [DOI] [PubMed] [Google Scholar]
- 42.Block RC, Harris WS, Pottala JV. Determinants of blood cell omega-3 fatty acid content. Open Biomarkers J. 2008;1:1–6. doi: 10.2174/1875318300801010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farzaneh-Far R, Harris WS, Garg S, Na B, Whooley MA. Inverse association of erythrocyte n-3 fatty acid levels with inflammatory biomarkers in patients with stable coronary artery disease: The Heart and Soul Study. Atherosclerosis. 2009;205:538–43. doi: 10.1016/j.atherosclerosis.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Itomura M, Fujioka S, Hamazaki K, et al. Factors influencing EPA+DHA levels in red blood cells in Japan. In Vivo. 2008;22:131–5. [PubMed] [Google Scholar]
- 45.Thorlaksdottir AY, Skuladottir GV, Petursdottir AL, et al. Positive association between plasma antioxidant capacity and n-3 PUFA in red blood cells from women. Lipids. 2006;41:119–25. doi: 10.1007/s11745-006-5079-5. [DOI] [PubMed] [Google Scholar]
- 46.Sands SA, Reid KJ, Windsor SL, Harris WS. The impact of age, body mass index, and fish intake on the EPA and DHA content of human erythrocytes. Lipids. 2005;40:343–7. doi: 10.1007/s11745-006-1392-2. [DOI] [PubMed] [Google Scholar]
- 47.Cohen BE, Garg SK, Ali S, Harris WS, Whooley MA. Red blood cell docosahexaenoic acid and eicosapentaenoic acid concentrations are positively associated with socioeconomic status in patients with established coronary artery disease: data from the heart and soul study. J Nutr. 2008;138:1135–40. doi: 10.1093/jn/138.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dewailly E, Blanchet C, Gingras S, et al. Relations between n-3 fatty acid status and cardiovascular disease risk factors among Quebecers. Am J Clin Nutr. 2001;74:603–11. doi: 10.1093/ajcn/74.5.603. [DOI] [PubMed] [Google Scholar]
- 49.Dewailly E, Blanchet C, Lemieux S, et al. n-3 Fatty acids and cardiovascular disease risk factors among the Inuit of Nunavik. AmJ Clin Nutr. 2001;74:464–73. doi: 10.1093/ajcn/74.4.464. [DOI] [PubMed] [Google Scholar]
- 50.Dewailly E, Blanchet C, Gingras S, Lemieux S, Holub BJ. Cardiovascular disease risk factors and n-3 fatty acid status in the adult population of James Bay Cree. Am J Clin Nutr. 2002;76:85–92. doi: 10.1093/ajcn/76.1.85. [DOI] [PubMed] [Google Scholar]
- 51.Yanagisawa N, Shimada K, Miyazaki T, et al. Polyunsaturated fatty acid levels of serum and red blood cells in apparently healthy Japanese subjects living in an urban area. J Atheroscler Thromb. 2010;17:285–94. doi: 10.5551/jat.2618. [DOI] [PubMed] [Google Scholar]
- 52.de Groot RH, van Boxtel MP, Schiepers OJ, Hornstra G, Jolles J. Age dependence of plasma phospholipid fatty acid levels: potential role of linoleic acid in the age-associated increase in docosahexaenoic acid and eicosapentaenoic acid concentrations. Br J Nutr. 2009;102:1058–64. doi: 10.1017/S0007114509359103. [DOI] [PubMed] [Google Scholar]
- 53.Sala-Vila A, Harris WS, Cofan M, et al. Determinants of the omega-3 index in a Mediterranean population at increased risk for CHD. Br J Nutr. 2011:1–7. doi: 10.1017/S0007114511000171. [DOI] [PubMed] [Google Scholar]
- 54.Babin F, Abderrazik M, Favier F, et al. Differences between polyunsaturated fatty acid status of non-institutionalised elderly women and younger controls: a bioconversion defect can be suspected. Eur J Clin Nutr. 1999;53:591–6. doi: 10.1038/sj.ejcn.1600792. [DOI] [PubMed] [Google Scholar]
- 55.Plourde M, Chouinard-Watkins R, Vandal M, et al. Plasma incorporation, apparent retroconversion and beta-oxidation of 13C-docosahexaenoic acid in the elderly. Nutr Metab (Lond) 2011;8:5. doi: 10.1186/1743-7075-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harris WS, Pottala JV, Vasan RS, Larson MG, Robins SJ. Changes in erythrocyte membrane trans and marine fatty acids between 1999 and 2006 in older Americans. J Nutr. doi: 10.3945/jn.112.158295. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Crowe FL, Skeaff CM, Green TJ, Gray AR. Serum n-3 long-chain PUFA differ by sex and age in a population-based survey of New Zealand adolescents and adults. Br J Nutr. 2008;99:168–74. doi: 10.1017/S000711450779387X. [DOI] [PubMed] [Google Scholar]
- 58.Tavendale R, Lee AJ, Smith WC, Tunstall-Pedoe H. Adipose tissue fatty acids in Scottish men and women: results from the Scottish Heart Health Study. Atherosclerosis. 1992;94:161–9. doi: 10.1016/0021-9150(92)90241-8. [DOI] [PubMed] [Google Scholar]
- 59.Burdge GC, Wootton SA. Conversion of alpha-linolenic acid to palmitic, palmitoleic, stearic and oleic acids in men and women. Prostaglandins Leukot Essent Fatty Acids. 2003;69:283–90. doi: 10.1016/s0952-3278(03)00111-x. JID – 8802730. [DOI] [PubMed] [Google Scholar]
- 60.Giltay EJ, Gooren LJ, Toorians AW, Katan MB, Zock PL. Docosahexaenoic acid concentrations are higher in women than in men because of estrogenic effects. Am J Clin Nutr. 2004;80:1167–74. doi: 10.1093/ajcn/80.5.1167. [DOI] [PubMed] [Google Scholar]
- 61.Burdge GC, Wootton SA. Conversion of alpha-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br J Nutr. 2002;88:411–20. doi: 10.1079/BJN2002689. [DOI] [PubMed] [Google Scholar]
- 62.Wei MY, Jacobson TA. Effects of eicosapentaenoic acid versus docosahexaenoic acid on serum lipids: a systematic review and meta-analysis. Curr Atheroscler Rep. 2011;13:474–83. doi: 10.1007/s11883-011-0210-3. [DOI] [PubMed] [Google Scholar]
- 63.Maki KC, Bays HE, Dicklin MR, Johnson SL, Shabbout M. Effects of prescription omega-3-acid ethyl esters, coadministered with atorvastatin, on circulating levels of lipoprotein particles, apolipoprotein CIII, and lipoprotein-associated phospholipase A2 mass in men and women with mixed dyslipidemia. J Clin Lipidol. 2011;5:483–92. doi: 10.1016/j.jacl.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 64.Hibbeln JR, Makino KK, Martin CE, Dickerson F, Boronow J, Fenton WS. Smoking, gender, and dietary influences on erythrocyte essential fatty acid composition among patients with schizophrenia or schizoaffective disorder. Biol Psychiatry. 2003;53:431–41. doi: 10.1016/s0006-3223(02)01549-4. [DOI] [PubMed] [Google Scholar]
- 65.Polidori MC, Mecocci P, Stahl W, Sies H. Cigarette smoking cessation increases plasma levels of several antioxidant micronutrients and improves resistance towards oxidative challenge. Br J Nutr. 2003 Jul;90(1):147–50. doi: 10.1079/bjn2003890. [DOI] [PubMed] [Google Scholar]
- 66.Morrow JD, Frei B, Longmire AW, et al. Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. Smoking as a cause of oxidative damage. N Engl J Med. 1995;332:1198–203. doi: 10.1056/NEJM199505043321804. [DOI] [PubMed] [Google Scholar]
- 67.Cazzola R, Rondanelli M, Russo-Volpe S, Ferrari E, Cestaro B. Decreasedmembrane fluidity and altered susceptibility to peroxidation and lipid composition in overweight and obese female erythrocytes. J Lipid Res. 2004;45:1846–51. doi: 10.1194/jlr.M300509-JLR200. [DOI] [PubMed] [Google Scholar]
- 68.Makhoul Z, Kristal AR, Gulati R, et al. Associations of obesity with triglycerides and C-reactive protein are attenuated in adults with high red blood cell eicosapentaenoic and docosahexaenoic acids. Eur J Clin Nutr. 2011;65:808–17. doi: 10.1038/ejcn.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reinders I, Virtanen JK, Brouwer IA, Tuomainen TP. Association of serum n-3 polyunsaturated fatty acids with C-reactive protein in men. Eur J Clin Nutr. 2011 doi: 10.1038/ejcn.2011.195. [DOI] [PubMed] [Google Scholar]
- 70.Farzaneh-Far R, Lin J, Epel ES, Harris WS, Blackburn EH, Whooley MA. Association of marine omega-3 fatty acid levels with telomeric aging in patients with coronary heart disease. JAMA. 2010;303:250–7. doi: 10.1001/jama.2009.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shearer GC, Savinova OV, Harris WS. Fish oil – how does it reduce plasma triglycerides? Biochim Biophys Acta. 2011 doi: 10.1016/j.bbalip.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Harris WS, Gonzales M, Laney N, Sastre A, Borkon AM. Effects of omega-3 fatty acids onheart rate in cardiac transplant recipients. Am J Cardiol. 2006;98:1393–5. doi: 10.1016/j.amjcard.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 73.O’Keefe JH, Abuissa H, Sastre A, Steinhaus DM, Harris WS. Effects of omega-3 fatty acids on resting heart rate, heart rate recovery after exercise, and heart rate variability in men with healed myocardial infarctions and depressed ejection fractions. Am J Cardiol. 2006;97:1127–30. doi: 10.1016/j.amjcard.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 74.Moyers B, Farzaneh-Far R, Harris WS, Garg S, Na B, Whooley MA. Relation of whole blood n-3 fatty acid levels to exercise parameters in patients with stable coronary artery disease (from the Heart and Soul Study) Am J Cardiol. 2011 doi: 10.1016/j.amjcard.2010.11.040. [DOI] [PubMed] [Google Scholar]
- 75.Christensen JH. Omega-3 polyunsaturated fatty acids and heart rate variability. Front Physiol. 2011;2:84. doi: 10.3389/fphys.2011.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ladesich JB, Pottala JV, Romaker A, Harris WS. Membrane level of omega-3 docosahexaenoic acid is associated with severity of obstructive sleep apnea. J Clin Sleep Med. 2011;7:391–6. doi: 10.5664/JCSM.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lemaitre RN, Tanaka T, Tang W, et al. Genetic loci associated with plasma phospholipid n-3 fatty acids: a meta-analysis of genome-wide association studies from the CHARGE consortium. PLoS Genet. 2011;7:e1002193. doi: 10.1371/journal.pgen.1002193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Katan MB, Deslypere JP, van Birgelen AP, Penders M, Zegwaard M. Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: an 18-month controlled study. J Lipid Res. 1997;38:2012–22. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


