Abstract
Antiphospholipid syndrome is a systemic autoimmune disease associated with thrombosis and recurrent fetal loss in the setting of detectable antiphospholipid (aPL) antibodies. The major antigenic target has been identifed as β2-glycoprotein I (β2GPI), which mediates binding of aPL antibodies to target cells including endothelial cells, monocytes, platelets and trophoblasts, leading to prothrombotic and proinfammatory changes that ultimately result in thrombosis and fetal loss. This article summarizes recent insights into the role of β2GPI in normal hemostasis, interactions between aPL antibodies, β2GPI and cell-surface molecules, molecular prothrombotic and proinfammatory changes induced by aPL antibodies and pathogenic changes leading to fetal loss in antiphospholipid syndrome. New directions in therapy using these insights are examined.
Keywords: annexin, anti-β2-glycoprotein I antibody, antiphospholipid antibodies, antiphospholipid syndrome, endothelial cell activation, pathogenesis, platelet activation, pregnancy loss, thrombosis, treatment
Antiphospholipid syndrome (APS) is a systemic autoimmune disease characterized by recurrent thrombosis and fetal loss in the presence of persistently positive antiphospholipid (aPL) antibodies (Abs) including lupus anticoagulant (LAC), IgG/IgM anticardiolipin (aCL) Abs and anti-β2-glycoprotein I (β2GPI) Abs [1–3]. aPL Abs are a heterogenous group of autoantibodies that react to phospholipids (PLs), PL-binding proteins and PL–protein complexes. aPL Abs mainly target the antigen β2GPI and along with Abs acting against prothrombin (PT) account for more than 90% of the Ab-binding activity in APS patients [4–6]. While many aPL Abs exhibit specifcity for a single antigen, purifed aPL Abs from some patients bind multiple proteins involved in coagulation, suggesting a single aPL Ab clone can induce multiple changes in coagulation and cell activity resulting in thrombosis or fetal loss [7].
Presence of aPL Abs per se does not guarantee a patient will develop APS as only 8.1% of patients with aPL Abs without a history of clinical thrombosis developed thrombosis during a 5-year follow-up period, suggesting that a patient needs an additional insult to develop the clinical disease [8]. Supporting this ‘two-hit’ hypothesis, comorbidities that have been identifed as significant risk factors for thrombosis in APS include hypertension, presence of an autoimmune disease, hypercholesterolemia, presence of anti-dsDNA Abs or medium-to-high titer aCL Abs [8]. Risk factors particularly for arterial thrombosis include hypertension, hyperhomocysteinemia and use of hormone-replacement therapy or oral contraceptives [9]. By contrast, venous thrombosis was associated with presence of hypertriglyceridemia, presence of a hereditary thrombophilia or aCL IgG more than 40 IU [9].
Antiphospholipid syndrome causes significant morbidity with positive LAC and/or aPL Abs conferring an increased risk of thrombosis with an odds ratio ranging from 3.1 to 9.4 [10,11]. The risk of recurrent thrombosis in APS over 5 years is 16.6% despite the use of anticoagulants and/or aspirin. APS is also associated with a 5-year mortality of 5.3% with most deaths occurring within the first year of diagnosis, with the leading causes of death being bacterial infections, myocardial infarction, stroke and cerebral hemorrhage [12]. Catastrophic APS – multiple simultaneous arterial or venous thromboses in the presence of aPL Abs – is a much-feared, albeit rare complication occurring in 0.9% of patients with APS. Despite aggressive treatment, mortality rates still range between 44 and 55.6% [12,13]. These data underscore the need for development of more effective therapies that target the pathologic processes involved in APS without the toxicities associated with chronic anticoagulation.
Structure & binding of β2GPI
Studies into the structure of β2GPI have revealed that the individual domains are important for interaction with aPL Abs as well as cell-surface molecules leading to the pathologic features of APS. Anti-β2GPI Abs associated with thrombosis have been shown to bind readily to domain I of recombinant β2GPI but interact only with plasma β2GPI attached to an anionic surface, implying that a conformational change is necessary for β2GPI binding [14–16]. This was recently confirmed and expanded upon by Agar et al. who demonstrated that β2GPI has a native circular structure in plasma maintained by interaction of domains V and I. Exposure to an anionic surface leads to the opening of the structure into a fish-hook shape via interaction between the anionic surface and domain V. This conformational change facilitates binding of anti-β2GPI to β2GPI by exposing an epitope in domain I that is hidden in the circular conformation [17]. The light chain fragments of anti-β2GPI Abs carry the antigen specifcity, establishing that the interaction is with β2GPI itself and not immunoglobulin in general [18]. Polymorphisms of β2GPI are also found in APS with the replacement of leucine by valine at position 247 being found more frequently in APS patients in certain populations [19–22]. This polymorphism, particularly in the homozygous state, has been shown to be associated with the production of anti-β2GPI Abs, which may be due to the presence of valine at this site, causing an increase in the antigenicity of β2GPI [20]. Providing clinical evidence of the importance of domain I binding in APS, de Laat et al. demonstrated that presence of domain I-specifc anti-β2GPI Abs was associated with increased risk of thrombosis, while there was no increased risk from anti-β2GPI Abs targeting other domains of β2GPI [15,23].
Recent studies have established that domain V is the primary site of interaction between β2GPI and the various cell-surface molecules with which it interacts. Rahgozar et al. demonstrated that β2GPI binds to thrombin and this binding prevents the inactivation of thrombin by heparin/heparin cofactor II [24,25]. Binding of anti-β2GPI Abs potentiates this interaction, leading to increased thrombin activity [24,25]. β2GPI also binds to ApoER2′, a splice variant of ApoE receptor 2 (ApoER2) found on platelets, and this interaction is dependent upon domain V [26,27]. Binding to ApoER2′ by dimerized β2GPI induces platelet activation and platelet aggregation. This effect was reproduced with anti-β2GPI Abs but not normal plasma β2GPI, indicating that anti-β2GPI Abs exert some of their effects by dimerization of β2GPI and interaction of dimerized β2GPI with cell-surface receptors [28]. In a recent study, Romay-Penabad et al. showed that ApoER2 mediates aPL-mediated pathogenic effects in vivo, in experiments that utilized ApoER2′-deficient mice and specifc inhibitors [29].
β2-glycoprotein I interacts with annexins, a family of phospholipid-binding proteins of which annexin (Ann) A2 and Ann A5 have been implicated in the pathogenesis of APS. Ann A2 acts as an endothelial cell-surface receptor for plasminogen and tissue plasminogen activator (tPA), and also mediates the binding of β2GPI/anti-β2GPI complexes to endothelial cells and monocytes, leading to cell activation and expression of a procoagulant phenotype [30–33]. Furthermore, aPL Ab-induced increases in endothelial cell adhesion molecules, ICAM and E-selectin have been shown to be abrogated by treatment with anti-Ann A2 Abs [34]. Also, knockout of Ann A2 in mice signifcantly blunted thrombus formation and diminished vascular tissue factor (TF) and VCAM expression induced by aPL Ab exposure [34]. Similarly, Zhou et al. have found that increased expression of ANX2, the gene coding for Ann A2, leads to increased anti-β2GPI Ab-induced TF expression while silencing of ANX2 partially blocks this increase in TF expression in HEK 293 T cells [31]. All this evidence highlights the essential role that Ann A2 plays as a cell-surface receptor for aPL in the induction of a procoagulant state in APS patients. However, it is important to note that although cell-surface Ann A2 lacks an intracellular tail and is unable to induce intracellular signal transduction by itself, it can act as a binding partner for intracellular surface molecules in lipid rafts [34,35]. This means that Ann A2 most likely utilizes a coreceptor for intracellular signal transduction and subsequent cell activation as a result of aPL action. The effects of anti-β2GPI Ab binding appear to be dependent upon Ann A2 crosslinking, suggesting anti-β2GPI Abs may exert their effects on cell signaling by inducing interaction between Ann A2-associated intracellular signaling molecules [36]. There is evidence for Tolllike receptors (TLRs), particularly TLR4 and TLR2, functioning as coreceptors for Ann A2 in aPL-mediated cell activation, both of these TLRs being present on endothelial cells and monocytes [37,38]. Abs directed against Ann A2 itself have also been detected in 14.8–40.4% of patients with APS and are associated with in vitro prothrombotic changes including increased expression of TF and inhibition of tPA-mediated plasmin activation suggesting an anti-β2GPI Ab-independent role in APS [30,39].
Annexin A5 is another member of the annexin family and is expressed by placental villous syncytiotrophoblasts and vascular endothelial cells. It exerts anticoagulant activity by binding to anionic PL blocking the activation of factor (F)IX and FX by TF and FVIIa and the activation of PT by FXa and FVa [40]. This interaction with anionic PL has made Ann A5 a promising possible target for aPL Abs. The anticoagulant effect of Ann A5 and aPL Ab-induced effects on Ann A5 can be monitored by evaluation of the Ann A5 anticoagulant ratio, a ratio of coagulation time in the presence of Ann A5 compared with the absence of Ann A5. Sera from patients with APS, particularly those with Abs targeting domain I of β2GPI, were found to decrease Ann A5 binding and decrease the Ann A5 anticoagulant ratio, indicating a prothrombotic state compared with control sera from healthy individuals and patients with syphilis [41–44]. This decrease in binding appears to be due to augmented β2GPI binding to anionic PL by aPL Abs and thus occupying the binding sites for Ann A5, preventing the anticoagulant effect of Ann A5 [44,45].
Thrombogenic mechanisms in APS
Effects of aPL Abs on monocytes, platelets & endothelial cells Monocytes
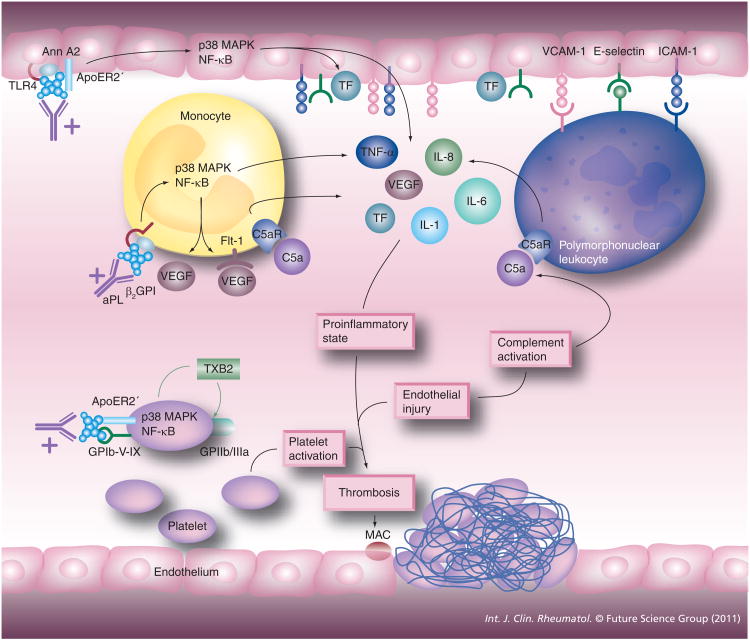
A role for monocyte activation in aPL-mediated thrombogenesis in APS patients has been described. Utilizing proteomics analysis, Lopez-Pedrera et al. demonstrated, in monocytes from APS patients with thrombosis, the differential expression of Ann I and Ann II, as well as Ras homolog gene family, member A (RhoA) and neural precursor cell expressed, developmentally downregulated 8 (Nedd8), proteins involved in cell signaling and heat shock protein 60 (Hsp60), an immune regulatory protein, when compared with patients with APS without thrombosis and those with thrombosis unrelated to APS (Figure 1) [46]. Titers of aCL correlated with the degree of dysregulation, and exposure of normal monocytes to APS sera caused a transformation of the protein expression profle to that of an APS patient [46]. The distinct pattern of protein expression in monocytes from these APS patients indicates that activation of these leukocytes occurs as a result of autoimmune related processes and leads to the induction of a procoagulant state. Multiple groups have found that aCL Abs induce increased TF expression by monocytes via activation of the p38 MAPK pathway, resulting in nuclear factor-κB (NF-κB) activation, as well as activation of the ERK1/2 pathway, which does not result in NF-κB activation [37,47–49]. Monocytes from patients with APS, particularly those with a history of thrombosis, exhibit increased TF mRNA expression and increased cell-surface TF. This TF upregulation may be due to stimulation of the Flt-1 tyrosine kinase receptor by VEGF since increased plasma levels of VEGF and surface expression of VEGF and Flt-1 on monocytes has been observed in APS patients when compared with controls [37,50]. Expression of protease-activated receptor (PAR)1 and PAR2 is also increased in monocytes from patients with APS and can be induced in normal monocytes with exposure to IgG purifed from APS patients, leading to a PAR2-dependent increase in TF expression [51]. This is of particular signifcance as PAR1 mediates many of the proinfammatory effects of thrombin, including induction of IL-6, IL-8, monocyte chemotactic protein-1 (MCP-1), PDGF, P-selectin and VEGF via ERK1/2 phosphorylation and ultimately NF-κB activation, while PAR2 mediates increased expression of IL-6, PDGF and MCP-1 by FXa, also through activation of the ERK1/2 pathway [51].
Figure 1. Cell activation in antiphospholipid syndrome.
aPL, including anti-β2GPI– β2GPI complexes, can activate platelets, endothelial cells and monocytes. Endothelial cell activation leads to the release of proinflammatory cytokines and increased leukocyte adhesion, making possible the activation of the polymorphonuclear leukocyte. Monocyte activation leads to the release of proinflammatory cytokines as well including TF, which can potentiate coagulation factor activation and ultimately fibrin production. Platelet activation leads to the release of thromboxane-B2, which potentiates the increased expression of GPIIb/IIIa, a major fibrinogen receptor. The net effect is the induction of a procoagulant state ultimately leading to thrombosis, occurring especially on the background of complement activation and endothelial injury due to infection and trauma.
β2GPI: β2-glycoprotein I; Ann: Annexin; aPL: Antiphospholipid; MAC: Membrane attack complex; NF-κB: Nuclear factor κB; TF: Tissue factor.
Platelets
Platelets are central to arterial thrombus formation in vivo and not surprisingly patients with APS have increased platelet activation, particularly patients with a history of a clinical thrombotic event [52]. aPL Abs enhance the expression of glycoprotein (GP)IIb/IIIa, a major fibrinogen receptor on platelets, as a result of p38 MAPK activation and thromboxane-β2 synthesis, and Pierangeli et al. have demonstrated the protective effect of deficiency or inhibition of this receptor on aPL-mediated thrombus formation [41]. It is also interesting that polymorphisms for platelet GPIa/IIa and GPIIb/IIIa are associated with an increased risk of arterial thrombosis as well as atherosclerosis in patients with APS [53]. ApoER2′ and the GPIbα subunit of the GPIb-V-IX receptor on the platelet membrane have been identifed as putative aPL receptors and Urbanus et al. demonstrated that signaling through both is required for platelet activation by anti-β2GPI/β2GPI complexes [54]. These two receptors are able to form a complex on the platelet membrane, suggesting an essential role for this complex in aPL-mediated platelet activation [54]. Platelet factor 4 (PF4), a member of the CXC chemokine family, is secreted by activated platelets and has multiple prothrombotic effects including inhibition of inactivation of thrombin by anti-thrombin, potentiation of platelet aggregation and accelerating cleavage of activated protein C (aPC). It is also an antigenic target in APS. Abs to PF4 are implicated in heparin-induced thrombocytopenia but have also been detected in patients with APS without a history of heparin-induced thrombocytopenia or recent exposure to heparin. Levels of anti-PF4 Abs also correlate with levels of IgM aPL and IgM anti-β2GPI Abs, but have activity independent of both [55,56]. These data suggest another potentially important mechanism in APS, but correlation with clinical features remains to be established. Recently, Sikara et al. demonstrated that PF4 may also facilitate the dimerization of β2GPI and subsequent binding to anti-β2GPI Abs and platelet cell-surface receptors including activation of the p38 MAPK pathway and NF-κB production [57]. This presents not only an attractive mechanistic model for platelet activation in APS but also for activation of endothelial cells and monocytes since PF4 is also expressed in these and other immune cells, albeit at lower concentrations than in platelets. PF4, like β2GPI, occurs in abundance in plasma and has many immunomodulatory effects. It is possible that PF4/β2GPI complexes may play a coordinating role in the activation of many immune-related cells in APS [58]. Furthermore, the fact that PF4 is released in abundance by dendritic cells following trauma may be one of the factors underlying the association of severe trauma with catastrophic APS [59]. However, further study is needed to fully characterize the role that PF4 plays in the pathophysiology of APS.
Endothelial cells
Endothelial cells play a key role in thrombosis by expression of integrins and TF upon activation and have also been implicated in APS. Patients with APS exhibit evidence of increased endothelial cell activity with impairment of endothelium-dependent vasodilatation and increased expression of von Willebrand factor (vWF), tPA, placental growth factor and soluble ICAM-1 [36,52,60–65]. Anti-β2GPI Abs induce a prothrombotic milieu in endothelial cells via increased production of FVIIa, PT-fragments I and II, and decreased levels of FXIIa and active urokinase-type plasminogen activator [66]. Microarray studies of endothelial cells have shown induction of apoptosis-related genes, BCL-2A1, TRAF1, CARD15 and BIRC3, multiple adhesion molecules including E-selectin, ICAM and VCAM, coagulation factors, cytokine/chemokine receptors and cytokines including IL-8, IL-6, IL-18 receptor, IL-1β and the TNF receptor superfamily [67]. This suggests that the procoagulant phenotype induced in endothelial cells in response to aPL Abs is characterized by increased apoptosis, leukocyte adhesion and release of proinflammatory cytokines. Similar to the cell signaling changes observed in monocytes, treatment of endothelial cells with aPL Abs induces activation of the p38 MAPK pathway and endothelial cell activation with increased expression of TF, VCAM, ICAM, P-selectin and E-selectin [48,61,68–70]. Mutations in the endothelial cell P-selectin ligand have also been identifed as a risk factor for thrombosis in APS [71].
Effects of aPL Abs on humoral regulators of thrombosis Regulators of coagulation
Protein C (PC) is activated by thrombomodulin-bound thrombin and acts as a key regulator of thrombosis by inactivating FVa and FVIIa and activating PAR1, resulting in inhibition of apoptosis (Figure 2). Modulation of aPC has been found in APS patients who have increased resistance to aPC, resulting in greater thrombin generation over time. The presence of in vitro aPC resistance is associated with the presence of LAC and a clinical history of thrombosis [72]. IgM Abs against aPC have been isolated from patients with APS and confer an increased risk of venous thromboembolism [73]. PC is released by membrane-bound endothelial PC receptor (EPCR) but soluble EPCR binds aPC, providing a counter-regulator mechanism. Anti-EPCR Abs have also been identified in APS sera and presence of these Abs is associated with a significant increase in risk of venous thrombosis and fetal loss, suggesting interference with this regulatory mechanism [74,75].
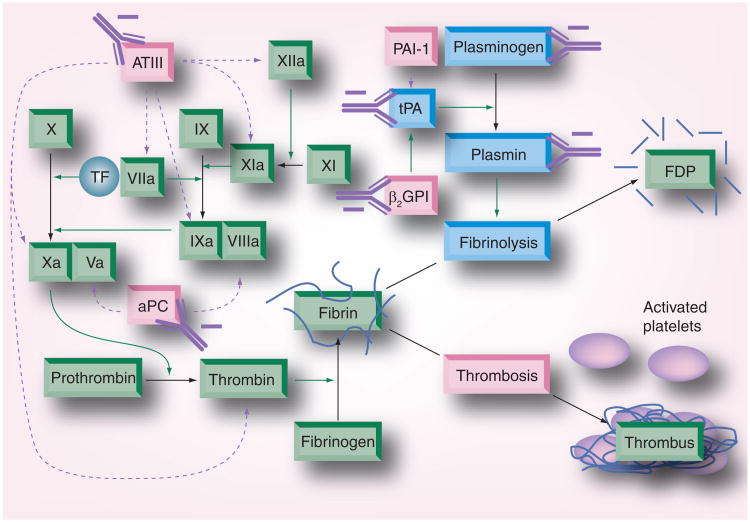
Figure 2. Dysregulation of anticoagulant and fibrinolytic systems in antiphospholipid syndrome.
aPC, an important anticoagulant, functions by inactivating activated forms of factors V (Va) and VIII (VIIIa). Antibodies acting against aPC inhibit its function. The function of anti-thrombin III, another major regulator of coagulation factors, is also inhibited by antibodies isolated from antiphospholipid syndrome patients. Ultimately this results in increased thrombin generation and subsequent fibrin formation. Dysregulation of the fibrinolytic system can also occur in antiphospholipid syndrome due to antibodies that inhibit tPA-mediated conversion of plasminogen to plasmin and those that directly inhibit plasmin function.
aPC: Activated protein C; tPA: Tissue plasminogen activator.
Antiphospholipid Abs have also been shown to interfere with the function of anti-thrombin III, another important regulator of coagulation, resulting in reduced inactivation of several coagulation factors including FXa and FIXa [76,77]. Anti-β2GPI Abs also induce increased thrombin generation by interference with TF pathway inhibitor-dependent inhibition of TF-induced thrombin generation [78]. β2GPI also exerts anti-thrombotic effects by interaction with activated vWF. β2GPI binds to vWF leading to a decrease in platelet binding by vWF and inhibition of platelet activation. Anti-β2GPI Abs exert some of their prothrombotic effects by interference with the binding of β2GPI to activated vWF, resulting in an increased concentration of circulating activated vWF and enhanced platelet aggregation [79].
Coagulation factors
Dysregulation of thrombosis in APS also occurs due to interference with multiple coagulation factors by aPL Abs. Anti-FXa Abs are identifed in 13.2% of patients with APS and result in a prothrombotic state by interference with inactivation of FXa by anti-thrombin [76]. Mutation of FXIIIa with substitution of valine for leucine at position 34 appears to be protective with decreased risk of thrombosis in patients with aPL Abs [80]. Antiprothrombin (aPT) Abs have also been suggested as an antigenic target in APS. PT is the precursor form of thrombin, an integral protein in perpetuation and modulation of the thrombotic response. aPT Abs exhibit an anticoagulant effect in vitro but are associated with thrombosis and recurrent miscarriages in vivo and induce increased thrombin and fibrin generation and increased TF and E-selectin expression by endothelial cells [81–83]. These contradictory findings may be due to modulation of these effects by the local concentration of other coagulation factors, particularly FVa and FXa with low levels of FVa and high levels of FXa, being associated with increased thrombin generation [81]. However, it is important to note that there is currently no consensus regarding the role of aPT Abs in APS, at least partly due to a lack of standardization of laboratory assays leading to difficulty integrating results from groups using different assays.
Fibrinolytic system
Antiphospholipid syndrome patients also demonstrate defects in fibrinolysis in addition to the decreased expression of tPA by endothelial cells described above [66]. β2GPI regulates hemostasis by modulation of fibrinolysis as well. Plasma β2GPI binds to tPA, increasing its catalytic activity and inducing an increase in plasminogen resulting in an augmentation of fibrino lysis. Treatment with anti-β2GPI Abs blocks the stimulation of tPA-mediated plasminogen activation [84]. Antiplasminogen Abs are detectable in 25–46.9% of patients with APS and are associated with clinical thrombotic events. Patients with these autoantibodies had impaired fibrinolysis by inhibition of tPA-dependent plasminogen activation [84]. Fibrinolysis may also be impaired due to interaction with thrombin-activatable fibrinolysis inhibitor (TAFI), a proenzyme that upon activation by thrombin–thrombomodulin and multiple proteases potently inhibits fibrinolysis. While TAFI levels are elevated in patients with APS, actual activity of TAFI was lower in patients with APS, particularly in those with LAC [85]. In addition, aCL Abs inhibited the formation of TAFI [85]. However, modulation of TAFI activity was not correlated with clinical thrombosis and therefore its role in the pathogenesis of APS is still unclear.
Induction of proinfammatory changes by aPL Abs
In addition to activation of the canonical coagulation pathways, APS is marked by several proinfammatory changes. Family studies have suggested involvement of TLRs including TLR4 and infammatory signaling involving IL-1β, TNF-α, IL-6 and TGF- β. Recent genetic and protein expression studies have uncovered associations with single-nucleotide polymorphisms in genes coding for the cell signaling molecules STAT4 and BLK [86–88]. Patients with APS have been found to have elevated levels of TNF-α, which correlate with the presence of LAC and presence of both aCL and anti-β2GPI Abs [89]. In vitro studies have demonstrated that anti-β2GPI Abs induce TNF-α production by monoctyes [90]. In addition to endothelial cell and monocyte activation, and increased TF expression described above, aPL Ab-induced activation of endothelial cell p38 MAPK also leads to increased expression of the proinfammatory cytokines TNF-α, IL-1β, TGF-β, macrophage infammatory protein 3 and MCP-1 in monocytes and increased expression of IL-6 and IL-8 in endothelial cells [49,68].
There has been much interest over the past several years in TLRs in APS. Prothrombotic effects of sera from patients with APS were decreased in mice resistant to lipopolysaccharide, a potent activator of TLR4, implicating involvement of TLR4 signaling in APS [91]. This fnding was supported by further studies demon strating that β2GPI associates with lipid rafts and colocalizes with TLR2 and TLR4. β2GPI interacts with TLR2 as well as TLR4, leading to activation of cell signaling cascades including ERK and p38 MAPK, resulting in IRAK phosphorylation and activation of NF-κB [90,92]. The interaction with TLR2 is potentiated by dimerized β2GPI, suggesting the need for interactions with other cell-surface molecules such as Ann A2 to induce signaling [92]. In fbroblasts, this TLR2 activation leads to increased expression of IL-6, ICAM-1 and MCP-1 [38]. aPL Abs are also able to induce a infammatory milieu via other TLRs. TLR8 activation in monocytes and TLR 7 activation in dendritic cells by aPL Abs results in an increase in the proinfammatory cytokine IL-1β [93].
T cells play a key role in the regulation of the adaptive immune response and have also been implicated in the pathogenesis of APS. Patients with APS have increased circulating CD4+ T cells, decreased naive T cells (Th0) and cytotoxic CD8+ T-cell populations compared with patients with stable coronary artery disease with increased IFN-γ, IL-1β, IL-4 and IL-6 and decreased IL-8 and IL-10 production [63]. These findings suggest APS promotes an enhanced Th2 response promoting a humoral immune response and impairment of counter-regulatory cytokine production. T cells in peripheral blood cell cultures become activated when exposed to β2GPI/anti-β2GPI Ab complexes and also in the presence of oxidized low-density lipoprotein (oxLDL) and activated platelets [94]. This activation is dependent on the presence of macrophages and the FcγRI immuno globulin receptor suggesting T-cell activation is induced by presentation of PL antigens by macrophages [94].
Complement activation has also been implicated in APS. One study of a Japanese cohort of patients with primary APS found the majority had hypocomplementemia, which was associated with increased levels of TNF-α and the presence of LAC and anti-PT Abs [95]. Complement fixation occurs on the platelets of patients with APS and correlates with the presence of IgG aPL Abs, IgG anti-β2GPI Abs, platelet activation and also history of arterial thrombosis [96]. In vitro exposure to aPL Abs produced complement fixation on normal platelets and increased platelet activity correlated with complement fixation [96]. Additionally, patients with APS have been found to have lower levels of soluble CD21, the cell surface receptor for multiple components of activated complement protein 3 (C3). These lower levels of CD21 are independent of the presence of anti-β2GPI Abs, which supports the role of other aPL Abs in immune system activation in APS [97]. C5 has also been implicated as blockade of the C5a receptor. The use of specific inhibitors of C5a prevents aPL Ab-induced TF expression in neutrophils and knockout of C5a receptor protects mice from aPL Ab-induced thrombosis [62, 98,99].
Defective apoptosis is thought to be an integral part of the pathogenesis of systemic lupus erythematosus (SLE) and has also been implicated in APS [100]. Alessandri et al. demonstrated that aCL Abs bind to CL expressed on the surface of apoptotic endothelial cells in APS patients, including those that do not have SLE [101]. They speculated this may represent defective apoptosis leading to presentation of CL to the immune system and subsequent development of autoantibodies. Some patients with APS also exhibit antiendothelial cell Abs, which decrease the uptake of neighboring apoptotic debris by endothelial cells while healthy endothelial cells will readily uptake apoptotic cells [102]. These antiendothelial cell Abs also opsonized the apoptotic cells, increasing uptake by macrophages. Macrophages that took up these apoptotic cells exhibited increased TF production, resulting in a prothrombotic state.
Nonthrombotic mechanisms in APS
APS & atherosclerosis
Antiphospholipid syndrome is associated with markers of accelerated atherosclerosis including increased carotid intima medial thickness and impairment of flow-mediated dilatation, a measure of endothelial function [103]. APS sera were also able to suppress high-density lipoprotein induced nitric oxide release, VCAM-1 expression and superoxide production by endothelial cells as well as inhibiting monocyte adhesion to endothelial cells, indicating decreased endothelial cell activation [103]. β2GPI exerts anti-athero sclerotic effects in normal physiology by binding of β2GPI to oxLDL, forming complexes that inhibit the uptake of oxLDL by the macrophage scavenger receptor [104]. Anti-β2GPI Abs can bind to oxLDL–β2GPI complexes, facilitating uptake by the macrophage scavenger receptor and inducing transformation of macrophages into foam cells, thus promoting atherogenesis [105,106]. Some patients with APS express anti-oxLDL Abs, which can bind to β2GPI and are also associated with increased risk of arterial thrombosis [107].
Obstetric manifestations of APS
As described in aPL-mediated thrombosis, several pathogenic mechanisms have been suggested to play a role in APS-associated obstetrical manifestations, which is supported by the heterogeneity of the histological lesions found [108].
Intraplacental thrombosis with maternal–fetal blood exchange impairment was initially suggested to be the main pathogenic mechanism. Widespread thrombosis and infarction of placentas obtained from women with APS was reported both in first- and second-trimester abortions. However, such a histological finding is not specifc for APS, also being present in other conditions [108,109].
In favor of the pathogenic role of thrombotic events in aPL-associated pregnancy loss there is evidence from in vitro studies that aPL may induce a procoagulant state at the placental level [110]. A further thrombophilic mechanism mediated by aPL Abs involves the relationship between the autoantibodies and Ann A5. In physiological conditions, a crystal shield of Ann A5 is suggested to cover thrombogenic anionic surfaces and prevent the activation of the coagulation cascade by inhibiting the binding of activated FX and PT. In vitro studies demonstrated that aPL/anti-β2GPI might disrupt the anticoagulant Ann A5 crystal shield; such an effect was also reproduced on trophoblast and endothelial cell monolayers [43,111,112]. According to the hypothesis that a loss of the Ann A5 shield may play a pathogenic role, the same group reported a significantly lower distribution of Ann A5 covering the intervillous surfaces in the placenta of aPL-positive women [111]. A new mechanistic test that measures the decrease of the anticoagulant effect of Ann A5 by aPL Abs in plasma has recently been developed. Studies utilizing this test have shown that the assay correctly identifes aPL-mediated thrombosis and pregnancy losses and it is ‘positive’ in approximately 50% of patients with APS [113]. Pregnancy loss in APS is due to multiple causes including mechanisms independent of thrombosis. Trophoblast differentiation and invasion is a vital step in early fetal implantation and development, and is dependent on altered expression of the apoptosis regulatory proteins B-cell lymphoma 2 (Bcl-2) and Bcl-2-associated X protein (Bax). This change in expression is impaired by exposure to anti-β2GPI Abs [114]. Patients with APS without clinical thrombosis but with fetal loss were found to have lower expression of fibrinogen, which has been identifed as a risk factor for recurrent spontaneous miscarriages due to impaired trophoblast implantation and vascular rupture, suggesting another potential factor in the fetal loss observed in APS [46]. Anti-β2GPI Abs also cause trophoblasts to express a proinfammatory cytokine profle characterized by increased IL-8, IL-1β, MCP-1 and growth-regulated oncogene-α via activation of the TLR4/myeloid differentiation primary response protein 88 (MyD88) pathway [115]. Expression of these proinfammatory cytokines was associated with an increase in trophoblast apoptosis. Interestingly, heparin attenuated these increases in cytokine production and increased trophoblast survival, suggesting immunomodulatory effects in addition to its well-characterized anticoagulant properties [115]. Mulla et al. also demonstrated that anti-β2GPI Abs impaired first-trimester trophoblast migration by downregulating IL-6 expression and STAT3 activation, which are constitutively expressed by first-trimester trophoblasts [116]. This occurs in a TLR4/MyD88-independent manner, suggesting stimulation of other intracellular signaling pathways [116].
Additional immune system abnormalities have been associated with recurrent fetal loss in APS. High levels of circulating natural killer cells have been found in APS patients with recurrent miscarriages [117,118]. Natural killer cell fractions of greater than 18% of circulating leukocytes were strongly associated with recurrent fetal loss [117]. Redecha et al. demonstrated that aPL Abs that activate complement induce TF expression on mouse decidua and led to fetal resorption while the inhibition of complement activation prevented fetal loss. Induction of TF production by complement occurred independently of membrane attack complex activation in this animal model while depletion of granuloctyes or selective knockout of TF in myeloid cells prevented aPL-induced fetal loss. This indicates that complement does not directly cause fetal injury but rather leads to fetal loss by recruitment of neutrophils and other cells associated with innate immunity [119]. Further studies by Redecha et al. showed that aPL Abs induce neutrophil recruitment and activation, with increased TF expression and ultimately fetal loss, by activation of PAR2 by TF–FVIIa complexes, supporting the role of neutrophils in obstetric manifestations of APS [120].
Di Simone et al. recently demonstrated that purifed aPL IgG from patients with APS impaired human endometrial endothelial cell angiogenesis in vitro, and this was at least partly due to suppression of VEGF and matrix metalloproteinase 2 expression [121]. Finally, a recent study by Praprotnik et al. found an intriguing association with hyperprolactinemia and recurrent fetal loss in APS without signs of a prolactinoma or major menstrual disturbances [122]. Elevated prolactin levels also seemed to be protective of thrombotic manifestations of APS, implying a nonthrombotic mechanism in fetal loss in APS [122]. Further studies will need to be carried out to clarify this relationship.
In vitro effects of novel therapies & new insights into old medications
Improved understanding of the interactions between β2GPI, aPL Abs and cell-surface molecules such as annexins has led to the development of a potential therapy by blocking binding of anti-β2GPI Abs to β2GPI. Ioannou et al. were able to demonstrate the use of a recombinant domain I peptide, which effectively inhibits binding of anti-β2GPI Abs to β2GPI in vitro. In a mouse model of thrombosis, the recombinant domain I peptide inhibited aPL Ab-induced thrombus formation and also abrogated aPL Ab-induced upregulation of VCAM-1 expression in endothelium and TF expression in macrophages [123].
Another new treatment for APS is the use of inhibitors of B-cell activating factor (BAFF), a cytokine that promotes B-cell expansion and its receptors BAFF-R, TACI and B-cell maturation antigen (BCMA), which have shown promise in treating SLE [124–126]. A recent study demonstrated that blockade of BAFF-receptor (BAFF-R) and TACI with BAFF-R-Ig or TACI-Ig blocked the expression of VCAM and P-selectin, resulted in fewer activated B and T cells, delayed disease onset and enhanced survival in a mouse model of APS. There were no changes in the development of aCL Abs and, interestingly, no prevention of thrombocyto penia in this study [127]. A tetramer of domain I peptide that interacts with B cells has also been developed with the goal of inducing tolerance to β2GPI and is currently in preclinical trials [128,129].
The use of older medications is also being evaluated in APS. Hydroxychloroquine has been associated with lower rates of thrombosis in cohorts of patients with APS as well as in mouse models of APS [87,130,131]. These protective effects may be mediated by interaction with Ann A5. Hydroxychloroquine treatment induces Ann A5 deposition over anti-β2GPI Ab–β2GPI complexes deposited on the PL bilayer but do not affect the immune complexes per se. Augmented deposition of Ann A5 results in restoration of the Ann A5 anticoagulant ratio, indicating normalization of the hypercoaguable state found in APS [44,45]. Hydroxymethylglutaryl coenzyme A reductase inhibitors, more commonly called statins, have also been investigated in APS with promising results. Simvastatin has decreased neutrophil activation and prevented aPL Ab-induced fetal loss in a mouse model of APS, and fuvastatin mitigates the increased proinfammatory and prothrombotic changes in APS with reductions in expression of ICAM, selectins, VEGF, TF, IL-6 and TNF-α and decreased thrombus formation and platelet aggregation in animal models of APS [60,120,132–134]. These effects may be due to inhibition of NF-κB binding to DNA [60]. These treatments offer the promise of improved outcomes in APS without the toxicities associated with chronic anticoagulation.
Conclusion & future perspective
While recent advances in our understanding of the pathogenesis of APS have revealed many interesting insights, they have also unveiled several areas of interest to be explored. The discovery of binding of β2GPI to cell-surface molecules such as Ann A2, which does not itself initiate intracellular signaling but rather is located in lipid rafts in association with intracellular proteins and cell-surface receptors capable of initiating cell signaling, reinforces the need for further study of these receptors and intracellular signaling molecules and possibly interactions induced by anti-β2GPI–β2GPI complexes. Studies are already ongoing to evaluate interactions between annexins and TLR4 and their effects on intracellular signaling and protein expression.
Recognition of the importance of domain I in binding for anti-β2GPI Abs opens additional avenues of study in APS. Blockade of binding of anti-β2GPI to β2GPI by decoy domain I and induction of tolerance to β2GPI are conceptually very promising therapies for APS but need to be further evaluated in animal studies and clinical trials. Additionally, development of clinical assays for domain I-specific anti-β2GPI Abs may be able to identify patients truly at increased risk for thrombosis and who would thus potentially beneft from primary prophylaxis against a thromboembolic event while sparing those without increased risk from the long-term complications of chronic anticoagulation.
Insights into the intracellular signaling involved in APS also opens the possibility of treatments targeting cell signaling pathways, particularly the p38 MAPK pathway and NF-κB, with early evidence of abrogation of the prothrombotic state induced by APS [50,135]. Inhibition of ERK signaling also has in vitro data supporting it as a possible therapeutic target with abrogation of anti-β2GPI-induced TNF-α and TF expression by monocytes [90]. Supporting the feasibility of modulating cell signaling in the treatment of autoimmune diseases, the use of signaling inhibitors has shown promising results in Phase II trials in rheumatoid arthritis [136–138]. Further studies in animal models of APS and clinical trials of cell signaling inhibitors should be carried out to determine the efficacy and safety of these medications in the prevention of thrombosis in APS. Well-designed clinical trials, some of which are ongoing, are also needed to evaluate the potential benefit of currently available medications with clearly defined and generally favorable risk profiles such as statins and hydroxychloroquine for the prevention of thrombosis.
Further studies are needed to clarify the effects of aPL on trophoblast differentiation and function as well as involvement of cells associated with the innate immune system in the pathogenesis of APS. Hopefully, these studies will reveal insights leading to the development of novel therapies resulting in improved obstetric outcomes. The potential immunomodulatory effects of heparin also warrant further investigation and may provide additional insights into the pathogenesis of APS.
Executive summary.
Structure & binding of β2-glycoprotein I
β2-glycoprotein I (β2GPI) is the primary antigenic target in antiphospholipid syndrome (APS) and acts in normal physiology by binding with exposed anionic phospholipids and associated proteins to produce overall procoagulant effects.
Interaction with exposed anionic phospholipids induces a conformational change, unfurling the circular conformation found in plasma to a fish-hook shape, thereby allowing binding to proteins involved in hemostasis by domain V and interaction with anti-β2GPI antibodies (Abs) by domain I.
Dimerization of β2GPI by anti-β2GPI Abs leads to close association and interaction of cell surface-associated signaling proteins associated in lipid rafts, particularly in association with annexin (Ann) A2, and induction of intracellular signaling cascades.
Disruption of the Ann A5 crystal shield by anti-β2GPI–β2GPI complex interaction with the exposed anionic phospholipid bilayer leads to prolonged exposure of the phospholipid bilayer and activation of coagulation factors.
Effects of antiphospholipid Abs on monocytes, platelets & endothelial cells
Antiphospholipid (aPL) Abs activate platelets, monocytes and endothelial cells by activation of p38 MAPK and ERK1/2 signaling pathways, resulting in increased nuclear factor κB activation and expression of prothrombotic proteins. Inhibition of these signaling cascades is a potential therapeutic target.
aPL Abs activate platelets by interaction with both GPIb-IX-V receptor and ApoER2'.
Platelet factor 4 (PF4) may be involved in APS by increased platelet activation by anti-β2GPI–β2GPI-PF4 complexes, resulting in activation of the p38 MAPK pathway.
Effects of aPL Abs on humoral regulators of thrombosis
aPL Abs induce increased thrombin generation by interfering with inactivation of factor (F)Xa and FIXa by anti-thrombin and inducing resistance to activated protein C.
Antiplasminogen Abs contribute to thrombosis in APS by inhibition of fbrinolysis. Induction of proinfammatory changes by aPL Abs
Induction of proinflammatory changes by aPL Abs
aPL Abs induce expression of proinfammatory cytokines via activation of p38 MAPK in endothelial cells and monocytes.
TLR2 and 4 are activated by anti-β2GPI–β2GPI complexes, resulting in activation of p38 MAPK and ERK pathways.
Complement activation occurs in APS and is associated with neutrophil activation.
APS & atherosclerosis
APS is associated with markers of accelerated atherosclerosis, and atherogenesis may be driven by interaction with oxididzed low-density lipoprotein.
Obstetric complications in APS
Fetal loss in APS is associated with a prothrombotic state at the level of the placenta, including disruption of the Ann A5 shield.
Fetal loss in APS is also associated with impairments with trophoblast differentiation, migration and induction of proinfammatory phenotype, resulting in trophoblast apoptosis.
Natural killer cells and neutrophils may be involved in the fetal loss in APS and further studies are necessary.
In vitro effects of novel therapies & new insights into old medications
Promising therapies targeting domain I of β2GPI by inhibiting binding of anti-β2GPI Abs to native β2GPI and induction of B-cell tolerance to β2GPI have emerged.
Hydroxychloroquine is associated with improved outcomes in APS. This is at least partially due to restoration of Ann A5 function.
B-cell activating factor blockade is a potential new therapy for prevention of thrombosis in APS but does not prevent the development of anticardiolipin autoantibodies.
Statin therapy is another potential therapy for APS by modulation of the proinfammatory changes in APS.
Footnotes
Financial & competing interests disclosure: Silvia S Pierangeli is funded by an Arthritis Foundation (Texas chapter), an Arthritis Research Campaign and two National Institutes of Health RO-1 grants. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Harris E. Syndrome of the black swan. Br J Rheumatol. 1987;26(5):324–326. doi: 10.1093/rheumatology/26.5.324. [DOI] [PubMed] [Google Scholar]
- 2.Wilson W, Gharavi A, Koike T, et al. International consensus statement on preliminary classification criteria for definite antiphospholipid syndrome. Arthritis Rheum. 1999;42(7):1309–1311. doi: 10.1002/1529-0131(199907)42:7<1309::AID-ANR1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 3.Miyakis S, Lockshin M, Atusmi T, et al. International consensus statement on an update of the classification criteria for defnite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 4.Fleck R, Rapaport S, Rao L. Anti-prothrombin antibodies and the lupus anticoagulant. Blood. 1988;72(2):512–519. [PubMed] [Google Scholar]
- 5.McNeil H, Simpson R, Chesterman C, Krilis S. Antiphospholipid antibodies are directed against a complex antigen that includes a lipid-binding inhibitor of coagulation:β2-glycoprotein I (apolipoprotein H) Proc Natl Acad Sci USA. 1990;87:4120–4124. doi: 10.1073/pnas.87.11.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danowski A, Kickler T, Petri M. Anti-β2-glycoprotein I: prevalence, clinical correlations, and importance of persistent positivity in patients with antiphospholipid syndrome and systemic lupus erythematosus. J Rheumatol. 2006;33(9):1775–1779. [PubMed] [Google Scholar]
- 7.Lin WS, Chen PC, Yang CD, et al. Some antiphospholipid antibodies recognize conformational epitopes shared by β2-glycoprotein I and the homologous catalytic domains of several serine proteases. Arthritis Rheum. 2007;56(5):1638–1647. doi: 10.1002/art.22522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruffatti A, Del Ross T, Ciprian M, et al. Risk factors for a first thrombotic event in antiphospholipid antibody carriers. A multicentre, retrospective follow-up study. Ann Rheum Dis. 2009;68:397–399. doi: 10.1136/ard.2008.096669. [DOI] [PubMed] [Google Scholar]
- 9.Danowski A, de Azevedo M, de Souza Papi J, Petri M. Determinants of risk for venous and arterial thrombosis in primary antiphospholipid syndrome and in antiphospholipid syndrome with systemic lupus erythematosus. J Rheumatol. 2009;36:1195–1199. doi: 10.3899/jrheum.081194. [DOI] [PubMed] [Google Scholar]
- 10.Galli M, Luciani D, Bertolini G, Barbui T. Lupus anticoagulants are stronger risk factors for thrombosis than anticardiolipin antibodies in the antiphospholipid syndrome: a systematic review of the literature. Blood. 2003;101(5):1827–1832. doi: 10.1182/blood-2002-02-0441. [DOI] [PubMed] [Google Scholar]
- 11.de Groot P, Lutters B, Derksen R, Lisman T, Maijers J, Rosendaal F. Lupus anticoagulants and the risk of a first episode of deep venous thrombosis. J Thromb Haemost. 2005;3:1993–1997. doi: 10.1111/j.1538-7836.2005.01485.x. [DOI] [PubMed] [Google Scholar]
- 12.Cervera R, Khamashta M, Shoenfeld Y, et al. Morbidity and mortality in the antiphospholipid syndrome during a 5-year period: a multicentre prospective study of 1000 patients. Ann Rheum Dis. 2009;68:1428–1432. doi: 10.1136/ard.2008.093179. [DOI] [PubMed] [Google Scholar]
- 13.Bucciarelli S, Espinosa G, Cervera R, et al. Mortality in the catastrophic antiphospholipid syndrome: causes of death and prognostic factors in a series of 250 patients. Arthritis Rheum. 2006;54(8):2568–2576. doi: 10.1002/art.22018. [DOI] [PubMed] [Google Scholar]
- 14.de Laat B, Derksen R, Lummel M, Pennings M, de Groot P. Pathogenic anti-β2-glycoprotein I antibodies recognize domain I of the β2-glycoprotein I only after a conformational change. Blood. 2006;107:1916–1924. doi: 10.1182/blood-2005-05-1943. [DOI] [PubMed] [Google Scholar]
- 15.de Laat B, Derksen R, Urbanus R, de Groot P. IgG antibodies that recognize epitope Gly40-Arg43 in domain I of β2-glycoprotein I cause LAC, and their presence correlates strongly with thrombosis. Blood. 2005;105:1540–1545. doi: 10.1182/blood-2004-09-3387. [DOI] [PubMed] [Google Scholar]
- 16.Ioannou Y, Pericleous C, Giles I, Latchman D, Isenberg D, Rahman A. Binding of antiphospholipid antibodies to discontinuous epitopes on domain I of human β2-glycoprotein I. Arthritis Rheum. 2007;56(1):280–290. doi: 10.1002/art.22306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17••.Agar C, van Os G, Morgelin M, et al. β2-glycoprotein I can exist in 2 conformations: implications for our understanding of the antiphospholipid syndrome. Blood. 2010;116:1336–1343. doi: 10.1182/blood-2009-12-260976. Confirms the important role of conformational changes of β2-glycoprotein I (β2GPI) in the pathogenesis of antiphospholipid syndrome (APS) and prompts interpretation of previous and future studies with consideration of what conformation of either plasma-purifed or recombinant β2GPI was used. [DOI] [PubMed] [Google Scholar]
- 18.Kumar S, Nagl S, Kalsi J, et al. β-2-glycoprotein specificity of human anti-phospholipid antibody resides on the light chain: a novel mechanism for acquisition of cross-reactivity by an autoantibody. Mol Immunol. 2004;42:39–48. doi: 10.1016/j.molimm.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 19.Atsumi T, Tsutsumi A, Amengual O, et al. Correlation between β2-glycoprotein I valine/leucine 247 polymorphism and anti-β2-glycoprotein I antibodies in patients with primary antiphospholipid syndrome. Rheumatology. 1999;38(8):721–723. doi: 10.1093/rheumatology/38.8.721. [DOI] [PubMed] [Google Scholar]
- 20.Yasuda S, Atusmi T, Matsuura E, et al. Significance of valine/leucine 247 polymorphism of β2-glycoprotein I in antiphospholipid syndrome: increased reactivity of anti-β2-glycoprotein I autoantibodies to the valine 247 β2-glycoprotein I variant. Arthritis Rheum. 2005;52(1):212–218. doi: 10.1002/art.20741. [DOI] [PubMed] [Google Scholar]
- 21.Hirose N, Williams R, Alberts A, et al. A role for the polymorphism at position 247 of the β2-glycoprotein I gene in the generation of anti-β2-glycoprotein I antibodies in the antiphospholipid syndrome. Arthritis Rheum. 1999;42(8):1655–1661. doi: 10.1002/1529-0131(199908)42:8<1655::AID-ANR14>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 22.Pernambuco-Climaco J, Brochado M, Frietas M, Roselino A, Louzado-Junior P. Val/Leu247 polymorphism of β2-glycoprotein I in Brazilian patients with antiphospholipid syndrome – a genetic risk factor? Ann NY Acad Sci. 2009;1173:509–514. doi: 10.1111/j.1749-6632.2009.04655.x. [DOI] [PubMed] [Google Scholar]
- 23.de Laat B, Pengo V, Pabinger I, et al. The association between circulating antibodies against domain I of β2-glycoprotein I and thrombosis: an international multicenter study. J Thromb Haemost. 2009;7:1767–1773. doi: 10.1111/j.1538-7836.2009.03588.x. [DOI] [PubMed] [Google Scholar]
- 24.Rahgozar S, Yang Q, Giannakopoulos B, Yan X, Miyakis S, Krilis S. β2-glycoprotein I binds thrombin via exosite I and exosite II: anti-β2-glycoprotein I antibodies potentiate the inhibitory effect of β2-glycoprotein I on thrombin-mediated factor XIa generation. Arthritis Rheum. 2007;56(2):605–613. doi: 10.1002/art.22367. [DOI] [PubMed] [Google Scholar]
- 25.Rahgozar S, Giannakopoulos B, Yan X, et al. β2-glycoprotein I protects thrombin from inhibition by heparin cofactor II: potentiation of this effect in the presence of anti-β2-glycoprotein I autoantibodies. Arthritis Rheum. 2008;58:1146–1155. doi: 10.1002/art.23387. [DOI] [PubMed] [Google Scholar]
- 26.van Lummel M, Pennings M, Derksen R, et al. The binding site in β2-glycoprotein I for ApoER2' on platelets is located in domain. V J Biol Chem. 2005;280(44):36729–36736. doi: 10.1074/jbc.M504172200. [DOI] [PubMed] [Google Scholar]
- 27.Pennings M, Derksen R, Urbanus R, Tekelenburg W, Hemrika W, de Groot P. Platelets express three different splice variants of ApoER2 that are all involved in signaling. J Thromb Haemost. 2007;5:1538–1544. doi: 10.1111/j.1538-7836.2007.02605.x. [DOI] [PubMed] [Google Scholar]
- 28.Lutters B, Derksen R, Tekelenburg W, Lenting P, Arnout J, de Groot P. Dimers of β2-glycoprotein I increase platelet deposition to collagen via interaction with phospholipids and the apolipoprotein E receptor 2′. J Biol Chem. 2003;278(36):33831–33838. doi: 10.1074/jbc.M212655200. [DOI] [PubMed] [Google Scholar]
- 29•.Romay-Penabad Z, Aguilar-Valenzuela R, Urbanus R, et al. Apolipoprotein E receptor 2′ is involved in the thrombotic complications in a murine model of the antiphospholipid syndrome. Blood. 2011;117(4):1408–1414. doi: 10.1182/blood-2010-07-299099. This study demonstrated the importance of the interaction of ApoER2' and β2GPI in an in vivo model of APS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salle V, Maziere J, Smail A, et al. Anti-annexin II antibodies in systemic autoimmune disease and antiphospholipid syndrome. J Clin Immunol. 2008;28:291–297. doi: 10.1007/s10875-008-9188-1. [DOI] [PubMed] [Google Scholar]
- 31.Zhou H, Wang H, Li N, et al. Annexin A2 mediates anti-β2GPI/β2GPI-induced tissue factor expression on monocytes. Int J Mol Med. 2009;(24):557–562. doi: 10.3892/ijmm_00000265. [DOI] [PubMed] [Google Scholar]
- 32.Cesarman G, Guevara C, Hajjar K. An endothelial cell receptor for plasminogen/tissue plasminogen activator (t-PA) J Biol Chem. 1994;269(33):21198–21203. [PubMed] [Google Scholar]
- 33.Ma K, Simantov R, Zhang JC, Silverstein R, Hajjar K, McCrae K. High affinity binding of β2-glycoprotein I to human endothelial cells is mediated by annexin II. J Biol Chem. 2000;275(20):15541–15548. doi: 10.1074/jbc.275.20.15541. [DOI] [PubMed] [Google Scholar]
- 34••.Romay-Penabad Z, Montiel-Manzano M, Shilagard T, et al. Annexin A2 is involved in antiphospholipid antibody-mediated pathogenic effects in vitro and in vivo. Blood. 2009;114:3074–3083. doi: 10.1182/blood-2008-11-188698. Demonstrated that the prothrombotic effects of anti-β2GPI antibodies (Abs) are mediated in part by binding to annexin (Ann) A2 both in vitro and in a murine model of APS. The discussion provides a clear overview of the suspected role of Ann A2 in APS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He K, Deora A, Xiong H, et al. Endothelial cell annexin A2 regulates polyubiquitination and degradation of its binding partner S100A10/p11. J Biol Chem. 2008;283(28):19192–19200. doi: 10.1074/jbc.M800100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang J, McCrae K. Annexin A2 mediates endothelial cell activation by antiphospholipid/anti-{β}-2 glycoprotein I antibodies. Blood. 2005;105:1964–1969. doi: 10.1182/blood-2004-05-1708. [DOI] [PubMed] [Google Scholar]
- 37.Lambrianides A, Carroll C, Pierangeli S, et al. Effects of polyclonal IgG derived from patients with different clinical types of the antiphospholipid syndrome on monocyte signalling pathways. J Immunol. 2010;184:6622–6628. doi: 10.4049/jimmunol.0902765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Satta N, Dunoyer-Geindre S, Reber G, et al. The role of TLR2 in the infammatory activation of mouse fibroblasts by human antiphospholipid antibodies. Blood. 2007;109:1507–1514. doi: 10.1182/blood-2005-03-024463. [DOI] [PubMed] [Google Scholar]
- 39.Cesarman-Maus G, Rios-Luna N, Deora A, et al. Autoantibodies against the fibrinolytic receptor, annexin 2, in antiphospholipid syndrome. Blood. 2006;107:4375–4382. doi: 10.1182/blood-2005-07-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frank M, Sodin-Semri S, Irman S, Bozic B, Rozman B. β2-glycoprotein I and annexin A5 phospholipid interactions: artificial and cell membranes. Autoimmun Rev. 2009;9:5–10. doi: 10.1016/j.autrev.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 41.Pierangeli S, Vega-Ostertag M, Harris E. Intracellular signaling triggerd by antiphospholipid antibodies in platelets and endothelial cells: a pathway to targeted therapies. Thromb Res. 2004;114:467–476. doi: 10.1016/j.thromres.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 42.Wu X, Pierangeli S, Rand J. Resistance to annexin A5 binding and anticoagulant activity in plasmas from patients with the antiphospholipid syndrome but not with syphilis. J Thromb Haemost. 2006;4:271–273. doi: 10.1111/j.1538-7836.2005.01700.x. [DOI] [PubMed] [Google Scholar]
- 43•.de Laat B, Wu X, van Lummel M, Derksen R, de Groot P, Rand J. Correlation between antiphospholipid antibodies that recognize domain I of β2-glycoprotein I and a reduction in the anticoagulant activity of annexin A5. Blood. 2007;109:1490–1494. doi: 10.1182/blood-2006-07-030148. Demonstrates the interference of β2GPI with Ann A5 with in vitro evidence of induction of a procoagulant state and establishes that this is specifically mediated through domain I of β2GPI. [DOI] [PubMed] [Google Scholar]
- 44••.Rand J, Wu X, Quinn A, et al. Hydroxychloroquine protects the annexin A5 anticoagulant shield from disruption by antiphospholipid antibodies: evidence for a novel effect for an old anitmalarial drug. Blood. 2010;115:2292–2299. doi: 10.1182/blood-2009-04-213520. Showed both the disruption of the Ann A5 shield by anti-β2GPI Abs and restoration of this barrier with hydroxychloroquine, providing a mechanistic rationale for the use of hydroxychloroquine in APS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rand J, Wu X, Quinn A, Chen P, Hathcock J, Taatjes D. Hydroxychloroquine directly reduces the binding of antiphospholipid antibody-β2-glycoprotein I complexes to phospholipid bilayers. Blood. 2008;112:1687–1695. doi: 10.1182/blood-2008-03-144204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lopez-Pedrera C, Cuadrado M, Hernandez V, et al. Proteomic analysis in monocytes of antiphospholipid syndrome patients: deregulation of proteins related to the development of thrombosis. Arthritis Rheum. 2006;58(9):2835–2844. doi: 10.1002/art.23756. [DOI] [PubMed] [Google Scholar]
- 47•.Lopez-Pedrera C, Buendia P, Cuadrado M, et al. Antiphospholipid antibodies from patients with the antiphospholipid syndrome induce monocyte tissue factor expression through the simultaneous activation of NF-κB/Rel proteins via the p38 mitogen-activated protein kinase pathway, and of the MEK-1/ERK pathway. Arthritis Rheum. 2006;54(1):301–311. doi: 10.1002/art.21549. Established the involvement of cell signaling pathways resulting in induction of a prothrombotic state in monocytes. [DOI] [PubMed] [Google Scholar]
- 48.Vega-Ostertag M, Ferrara D, Romay-Penabad Z, et al. Role of p38 mitogen-activated protein kinase in antiphospholipid antibody-mediated thrombosis and endothelial cell activation. J Thromb Haemost. 2007;5:1828–1834. doi: 10.1111/j.1538-7836.2007.02680.x. [DOI] [PubMed] [Google Scholar]
- 49.Bohgaki M, Atsumi T, Yamashita Y, et al. The p38 mitogen-activated protein kinase (MAPK) pathway mediates induction of the tissue factor gene in monocytes stimulated with human monoclonal anti-β2glycoprotein I antibodies. Int Immunol. 2004;16(1):1633–1641. doi: 10.1093/intimm/dxh166. [DOI] [PubMed] [Google Scholar]
- 50.Cuadrado M, Buendia P, Velasco F, et al. Vascular endothelial growth factor expression in monocytes from patients with primary antiphospholipid syndrome. J Thromb Haemost. 2006;4:2461–2469. doi: 10.1111/j.1538-7836.2006.02193.x. [DOI] [PubMed] [Google Scholar]
- 51.Lopez-Pedrera C, Aguirre M, Buendia P, et al. Differential expression of protease-activated receptors in monocytes from patients with primary antiphospholipid syndrome. Arthritis Rheum. 2010;62:869–877. doi: 10.1002/art.27299. [DOI] [PubMed] [Google Scholar]
- 52.Jy W, Tiede M, Bidot C, et al. Platelet activation rather than endothelial injury identifies risk of thrombosis in subjects positive for antiphospholipid antibodies. Thromb Res. 2007;121:319–325. doi: 10.1016/j.thromres.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 53.Jimenez S, Tassies D, Espinosa G, et al. Double heterozygosity polymorphisms for platelet glycoproteins Ia/IIa and IIb/IIIa increases arterial thrombosis and arteriosclerosis in patients with antiphospholipid syndrome or with systemic lupus erythematosus. Ann Rheum Dis. 2008;67:835–840. doi: 10.1136/ard.2007.077321. [DOI] [PubMed] [Google Scholar]
- 54.Urbanus R, Pennings M, Derksen R, de Groot P. Platelet activation by dimeric β2-glycoprotein I requires signaling via both glycoprotein Ibα and apolipoprotein E receptor 2′. J Thromb Haemost. 2008;6:1405–1412. doi: 10.1111/j.1538-7836.2008.03021.x. [DOI] [PubMed] [Google Scholar]
- 55.Alpert D, Mandl L, Erkan D, Yin W, Peerschke W, Salmon J. Anti-heparin platelet factor 4 antibodies in systemic lupus erythaematosus are associated with IgM antiphospholipid antibodies and the antiphospholipid syndrome. Ann Rheum Dis. 2008;67:395–401. doi: 10.1136/ard.2007.074476. [DOI] [PubMed] [Google Scholar]
- 56.Martin-Toutain I, Piette J, Diemert M, Faucher C, Jobic L, Ankri A. High prevalence of antibodies to platelet factor 4 heparin in patients with antiphospholipid syndrome in absence of heparin-induced thrombocytopenia. Lupus. 2007;16:79–83. doi: 10.1177/0961203306075562. [DOI] [PubMed] [Google Scholar]
- 57••.Sikara M, Routsias J, Samiotaki M, Panayotou G, Moutsopoulos H, Vlachoyiannopoulos P. β2 glycoprotein I (β2GPI) binds platelet factor 4 (PF4): implications for the pathogenesis of antiphospholipid syndrome. Blood. 2010;115:713–723. doi: 10.1182/blood-2009-03-206367. Establishes formation of anti-β2GPI–β2GPI–platelet factor 4 complexes as playing a key role in platelet activation and subsequent thrombosis in APS. [DOI] [PubMed] [Google Scholar]
- 58.Slungaard A. Platelet factor 4: a chemokine enigma. Int J Biochem Cell Biol. 2008;37:1162–1167. doi: 10.1016/j.biocel.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 59.Maier M, Wutzler S, Bauer M, Trendaflov P, Henrich D, Marzi I. Altered gene expression patterns in dendritic cells after severe trauma: implications for systemic inflammation and organ injury. Shock. 2008;30:344–351. doi: 10.1097/SHK.0b013e3181673eb4. [DOI] [PubMed] [Google Scholar]
- 60.Meroni P, Raschi E, Testoni C, et al. Statins prevent endothelial cell activation induced by antiphospholipid (anti-β2-glycoprotein I) antibodies: effect on the proadhesive and proinfammatory phenotype. Arthritis Rheum. 2001;44(12):2870–2878. doi: 10.1002/1529-0131(200112)44:12<2870::aid-art475>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 61.Pierangeli S, Colden-Stanfeld M, Liu X, Barker J, Anderson G, Harris E. Antiphospholipid antibodies from antiphospholipid syndrome patients activate endothelial cells in vitro and in vivo. Circulation. 1999;99:1997–2002. doi: 10.1161/01.cir.99.15.1997. [DOI] [PubMed] [Google Scholar]
- 62.Romay-Penabad Z, Liu X, Montiel-Manzano G, de Martinez E, Pierangeli S. C5a receptor-deficient mice are protected from thrombophilia and endothelial cell activation induced by some antiphospholipid antibodies. Ann NY Acad Sci. 2007;1108:554–566. doi: 10.1196/annals.1422.058. [DOI] [PubMed] [Google Scholar]
- 63.Soltesz P, Der H, Veres K, et al. Immunological features of primary anti-phospholipid syndrome in connection with endothelial dysfunction. Rheumatology. 2008;47:1628–1634. doi: 10.1093/rheumatology/ken349. [DOI] [PubMed] [Google Scholar]
- 64.Cugno M, Borghi M, Lonati L, et al. Patients with antiphospholipid syndrome display endothelial perturbation. J Autoimmunity. 2010;34:105–110. doi: 10.1016/j.jaut.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 65.Smadja D, Gaussem P, Roncal C, Fischer AM, Emmerich J, Darnige L. Arterial and venous thrombosis is associated with different angiogenic cytokine patterns in patients with antiphospholid syndrome. Lupus. 2010;19(7):837–843. doi: 10.1177/0961203309360985. [DOI] [PubMed] [Google Scholar]
- 66.Lazaro I, Carmona F, Reverter J, Cervera R, Tassies D, Balasch J. Antiphospholipid antibodies may impair factor-XIIa-dependent activation of fibrinolysis in pregnancy: in vitro evidence with human endothelial cells in culture and monoclonal anticardiolipin antibodies. Am J Obstet Gynecol. 2009;201(87):e1–e6. doi: 10.1016/j.ajog.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 67.Hamid C, Norgate K, D'Cruz D, et al. Anti-β2GPI-antibody-induced endothelial cell gene expression profling reveals induction of novel pro-infammatory genes potentially involved in primary antiphospholipid syndrome. Ann Rheum Dis. 2007;66:1000–1007. doi: 10.1136/ard.2006.063909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vega-Ostertag M, Casper K, Swerlick R, Ferrara D, Harris E, Pierangeli S. Involvement of p38 MAPK in the upregulation of tissue factor on endothelial cells by antiphospholipid antibodies. Arthritis Rheum. 2005;52(5):1545–1554. doi: 10.1002/art.21009. [DOI] [PubMed] [Google Scholar]
- 69.Espinola R, Liu X, Colden-Stanfeld M, Hall J, Harris E, Pierangeli S. E-Selectin mediates pathogenic effects of antiphospholipid antibodies. J Thromb Haemost. 2003;1(4):843–848. doi: 10.1046/j.1538-7836.2003.00119.x. [DOI] [PubMed] [Google Scholar]
- 70.Pierangeli S, Espinola R, Liu X, Harris E. Thrombogenic effects of antiphospholipid syndrome antibodies are mediated by intercellular cell adhesion molecule-1, vascular cell adhesion molecule-1, and P-selectin. Circ Res. 2001;88:245–250. doi: 10.1161/01.res.88.2.245. [DOI] [PubMed] [Google Scholar]
- 71.Diz-Kucukkaya R, Inanc M, Afshar-Kharghan V, Zhang Q, Lopez J, Pekcelen Y. P-selectin glycoprotein ligand-1 VNTR polymorphisms and risk of thrombosis in the antiphospholipid syndrome. Ann Rheum Dis. 2007;66:1378–1380. doi: 10.1136/ard.2007.075945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liestol S, Sandset P, Mowinckel MC, Wisloff F. Activated protein C resistance determined with a thrombin generation-based test is associated with thrombotic events in patients with lupus anticoagulants. J Thromb Haemost. 2007;5:2204–2210. doi: 10.1111/j.1538-7836.2007.02734.x. [DOI] [PubMed] [Google Scholar]
- 73.Rossetto V, Spiezia L, Franz F, et al. The role of antiphospholipid antibodies toward the protein C/protein S system in venous thromboembolic disease. Am J Hematol. 2009;84(9):594–596. doi: 10.1002/ajh.21466. [DOI] [PubMed] [Google Scholar]
- 74.van Hylckama Vlieg A, Montes R, Rosendaal F, Hermida J. Autoantibodies against endothelial protein C receptor and the risk of a frst deep vein thrombosis. J Thromb Haemost. 2007;5:1449–1454. doi: 10.1111/j.1538-7836.2007.02582.x. [DOI] [PubMed] [Google Scholar]
- 75.Hurtado V, Montes R, Gris J, et al. Autoantibodies against EPCR are found in antiphospholipid syndrome and are a risk factor for fetal death. Blood. 2004;104:1369–1374. doi: 10.1182/blood-2004-03-0793. [DOI] [PubMed] [Google Scholar]
- 76.Yang YH, Hwang KK, FitzGerald J, et al. Antibodies against the activated coagulation factor X (FXa) in the antiphospholipid syndrome that interfere with the FXa inactivation by antithrombin. J Immunol. 2006;177(8219):8225. doi: 10.4049/jimmunol.177.11.8219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang YH, Chien D, Wu M, et al. Novel autoantibodies against the activated coagulation factor IX (FIXa) in the antiphospholipid syndrome that interpose the FIXa regulation by antithrombin. J Immunol. 2009;182:1674–1680. doi: 10.4049/jimmunol.182.3.1674. [DOI] [PubMed] [Google Scholar]
- 78.Lean S, Ellery P, Ivey L, et al. The effects of tissue factor pathway inhibitor and anti-β-2-glycoprotein-I IgG on thrombin generation. Haematologica. 2006;91:1360–1366. [PubMed] [Google Scholar]
- 79.Hulstein J, Lenting P, de Laat B, Derksen R, Fijnheer R, de Groot P. β2-glycoprotein I inhibits von Willebrand factor dependent platelet adhesion and aggregation. Blood. 2007;110:1483–1491. doi: 10.1182/blood-2006-10-053199. [DOI] [PubMed] [Google Scholar]
- 80.de la Red G, Tassies D, Espinosa G, et al. Factor XIII-A subunit Val34Leu polymorphism is associated with the risk of thrombosis in patients with antiphospholipid antibodies and high fibrinogen levels. Thromb Haemost. 2009;101:312–316. [PubMed] [Google Scholar]
- 81.Sakai Y, Atsumi T, Ieko M, et al. The effects of phosphatidylserine-dependent antiprothrombin antibody on thrombin generation. Arthritis Rheum. 2009;60(8):2457–2467. doi: 10.1002/art.24708. [DOI] [PubMed] [Google Scholar]
- 82.Sabatini L, Torricelli M, Scaccia V, et al. Increased plasma concentration of antiprothrombin antibodies in women with recurrent spontaneous abortions. Clin Chem. 2007;53:228–232. doi: 10.1373/clinchem.2006.073098. [DOI] [PubMed] [Google Scholar]
- 83.Vega-Ostertag M, Liu X, Kwank-Ki H, Chen P, Pierangeli S. A human monoclonal antiprothrombin antibody is thrombogenic in vivo and upregulates expression of tissue factor and E-selectin on endothelial cells. Br J Haematol. 2006;135(2):214–219. doi: 10.1111/j.1365-2141.2006.06283.x. [DOI] [PubMed] [Google Scholar]
- 84.Bu C, Gao L, Xie W, et al. β2-glycoprotein I is a cofactor for tissue plasminogen activator-mediated plasminogen activation. Arthritis Rheum. 2009;60(2):559–568. doi: 10.1002/art.24262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ieko M, Yoshida M, Naito S, et al. Increase in plasma thrombin-activatable fibrinolysis inhibitor may not contribute to thrombotic tendency in antiphospholipid syndrome because of inhibitory potential of antiphospholipid antibodies toward TAFI activation. Int J Haematol. 2010;91:776–783. doi: 10.1007/s12185-010-0590-0. [DOI] [PubMed] [Google Scholar]
- 86.Horita T, Atusmi T, Yoshida N, et al. STAT4 single nucleotide polymorphism, rs7574865 G/T, as a risk for antiphospholipid syndrome. Ann Rheum Dis. 2009;68:1366–1367. doi: 10.1136/ard.2008.094367. [DOI] [PubMed] [Google Scholar]
- 87.De Angelis V, Scurati S, Raschi E, et al. Pro-infammatory genotype as a risk factor for aPL-associated thrombosis: report of a family with multiple anti-phospholipid positive members. J Autoimmunity. 2009;32:60–63. doi: 10.1016/j.jaut.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 88.Yin H, Borghi M, Delgado-Vega A, Tincani A, Meroni P, Alarcon-Riquelme M. Association of STAT4 and BLK, but not BANK1 or IRF5 with primary antiphospholipid syndrome. Arthritis Rheum. 2009;60(8):2468–2471. doi: 10.1002/art.24701. [DOI] [PubMed] [Google Scholar]
- 89.Swadzba J, Iwaniec T, Musial J. Increased level of tumor necrosis factor-α in patients with antiphospholipid syndrome: marker not only of infammation but also of the prothrombotic state. Rheumatol Int. 2009;31(3):307–313. doi: 10.1007/s00296-009-1314-8. [DOI] [PubMed] [Google Scholar]
- 90••.Sorice M, Longo A, Capozzi A, et al. Anti-β2-glycoprotein I antibodies induce monocyte release of tumor necrosis factor a and tissue factor by signal transduction pathways involving lipid rafts. Arthritis Rheum. 2007;56:2687–2697. doi: 10.1002/art.22802. Provides support for the theory that interaction of anti-β2GPI Abs and cell surface signaling molecules is mediated by Toll-like receptors and associated cell surface molecules, including Ann A2 in lipid rafts, with induction of a prothrombotic and proinfammatory state being dependent on these lipid rafts in monocytes. [DOI] [PubMed] [Google Scholar]
- 91.Pierangeli S, Vega-Ostertag M, Raschi E, et al. Toll-like receptor and antiphospholipid mediated thrombosis: in vivo studies. Ann Rheum Dis. 2007;66:1327–1333. doi: 10.1136/ard.2006.065037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Alard J, Gaillard F, Daridon C, Shoenfeld Y, Jamin C, Youinou P. TLR2 is one of the endothelial receptors for β2-glycoprotein I. J Immunol. 2010;185:1550–1557. doi: 10.4049/jimmunol.1000526. [DOI] [PubMed] [Google Scholar]
- 93.Hurst J, Prinz N, Lorenz M, et al. TLR7 and TLR8 ligands and antiphospholipid antibodies show synergistic effects on the induction of the IL-1β and caspase-1 in monocytes and dendritic cells. Immunobiology. 2009;214:683–691. doi: 10.1016/j.imbio.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 94.Yamaguchi Y, Seta N, Kaburaki J, Kobayashi K, Matsuura E, Matsuura E. Excessive exposure to anionic surfaces maintains autoantibody response to β2-glycoprotein I in patients with antiphospholipid syndrome. Blood. 2007;110:4312–4318. doi: 10.1182/blood-2007-07-100008. [DOI] [PubMed] [Google Scholar]
- 95.Oku K, Atsumi T, Bohgaki M, et al. Complement activation in patients with primary antiphospholipid syndrome. Ann Rheum Dis. 2009;68:1030–1035. doi: 10.1136/ard.2008.090670. [DOI] [PubMed] [Google Scholar]
- 96.Peerschke E, Yin W, Alpert D, Roubey R, Salmon J, Ghebrehiwet B. Serum complement activation on heterologous platelets is associated with arterial thrombosis in patients with systemic lupus erythematosus and antiphospholipid syndrome. Lupus. 2009;18:530–538. doi: 10.1177/0961203308099974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Singh A, Blank M, Shoenfeld Y, Illges H. Antiphospholipid syndrome patients display reduced titers of soluble CD21 in their sera irrespective of circulating anti-β2-glycoprotein-I autoantibodies. Rheumatol Int. 2008;28:661–665. doi: 10.1007/s00296-007-0503-6. [DOI] [PubMed] [Google Scholar]
- 98.Ritis K, Doumas M, Mastellos D, et al. A novel C5a receptor-tissue factor cross-talk in neutrophils links innate immunity to coagulation pathways. J Immunol. 2006;177:4794–4802. doi: 10.4049/jimmunol.177.7.4794. [DOI] [PubMed] [Google Scholar]
- 99.Pierangeli S, Girardi G, Vega-Ostertag M, Liu X, Espinola R, Salmon J. Requirement of activation of complement C3 and C5 for antiphospholipid antibody-mediated thrombophilia. Arthritis Rheum. 2005;52(7):2120–2124. doi: 10.1002/art.21157. [DOI] [PubMed] [Google Scholar]
- 100.Munoz L, Lauber K, Schiller M, Manfredi A, Hermann M. The role of defective clearance of apoptotic cells in systemic autoimmunity. Nat Rev Rheumatol. 2010;6(5):280–289. doi: 10.1038/nrrheum.2010.46. [DOI] [PubMed] [Google Scholar]
- 101.Alessandri C, Sorice M, Bombardieri M, et al. Antiphospholipid reactivity against cardiolipin metabolites occurring during endothelial cell apoptosis. Arthritis Res Ther. 2006;8:R180. doi: 10.1186/ar2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Graham A, Ford I, Morrison R, Barker R, Greaves M, Erwig LP. Anti-endothelial antibodies interfere in apoptotic cell clearance and promote thrombosis in patients with antiphospholipid syndrome. J Immunol. 2009;182:1756–1762. doi: 10.4049/jimmunol.182.3.1756. [DOI] [PubMed] [Google Scholar]
- 103.Charakida M, Besler C, Batuca J, et al. Vascular abnormalities, paraoxonase activity, and dysfunctional HDL in primary antiphospholipid syndrome. JAMA. 2009;302(11):1210–1217. doi: 10.1001/jama.2009.1346. [DOI] [PubMed] [Google Scholar]
- 104.Lin K, Pan J, Yang D, et al. Evidence for inhibition of low density lipoprotein oxidation and cholesterol accumulation by apolipoprotein H (β2-glycoprotein I) Life Sci. 2001;69(6):707–719. doi: 10.1016/s0024-3205(01)01164-x. [DOI] [PubMed] [Google Scholar]
- 105.Kobayashi K, Tada K, Ueno T, et al. Distinguished effects of antiphospholipid antibodies and anti-oxidized LDL antibodies on oxidized LDL uptake by macrophages. Lupus. 2007;16:929–938. doi: 10.1177/0961203307084170. [DOI] [PubMed] [Google Scholar]
- 106.Kajiwara T, Yasuda T, Matsuura E. Intracellular trafficking of β2-glycoprotein I complexes with lipid vesicles in macrophages: implications on the development of antiphospholipid syndrome. J Autoimmunity. 2007;29:164–173. doi: 10.1016/j.jaut.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 107.Pengo V, Bison E, Ruffatti A, Iliceto S. Antibodies to oxidized LDL/β2-glycoprotein I in antiphospholipid syndrome patients with venous and arterial thromboembolism. Thromb Res. 2008;122:556–559. doi: 10.1016/j.thromres.2007.12.028. [DOI] [PubMed] [Google Scholar]
- 108.Van Horn J, Craven C, Wark K, Branch D, Silver R. Histologic features of placentas and abortion specimens from women with antiphospholipid and antiphospholipid-like syndromes. Placenta. 2004;25:642–648. doi: 10.1016/j.placenta.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 109.Nayar R, Lage J. Placental changes in a first trimester missed abortion in maternal systemic lupus erythematosus with antiphospholipid syndrome: a case report and review of the literature. Hum Pathol. 1996;27:201–206. doi: 10.1016/s0046-8177(96)90377-9. [DOI] [PubMed] [Google Scholar]
- 110.Peaceman A, Rehnberg K. The effect of immunoglobulin G fractions from patients with lupus anticoagulant on placental prostacyclin and thromboxane production. Am J Obstet Gynecol. 1993;169:1403–1406. doi: 10.1016/0002-9378(93)90408-b. [DOI] [PubMed] [Google Scholar]
- 111.Rand J, Wu X, Guller S, et al. Reduction of annexin-V (placental anticoagulant protein-I) on placental villi of women with antiphospholipid antibodies and recurrent spontaneous abortion. Am J Obstet Gynecol. 1994;171(6):1566–1572. doi: 10.1016/0002-9378(94)90403-0. [DOI] [PubMed] [Google Scholar]
- 112.Rand J. Molecular pathogenesis of the antiphospholipid syndrome. Circ Res. 2002;11:29–37. doi: 10.1161/hh0102.102795. [DOI] [PubMed] [Google Scholar]
- 113.Rand J, Wu X, Quinn A, Taatjes D. The annexin A5-mediated pathogenic mechanism in the antiphospholipid syndrome: role in pregnancy losses and thrombosis. Lupus. 2010;19:460–469. doi: 10.1177/0961203310361485. [DOI] [PubMed] [Google Scholar]
- 114.Di Simone N, Castellani R, Raschi E, Borghi M, Meroni P, Caruso A. Anti-β-2 glycoprotein i antibodies affect Bcl-2 and Bax trophoblast expression without evidence of apoptosis. Ann NY Acad Sci. 2006;1069:364–376. doi: 10.1196/annals.1351.034. [DOI] [PubMed] [Google Scholar]
- 115.Mulla M, Brosens J, Chamley L, et al. Antiphospholipid antibodies induce a pro-infammatory response in first trimester trophoblast via the TLR4/MyD88 pathway. Am J Reprod Immunol. 2009;62:96–R108. doi: 10.1111/j.1600-0897.2009.00717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116••.Mulla M, Myrtolli K, Brosens J, et al. antiphospholipid antibodies limit trophoblast migration by reducing IL-6 production and STAT3 activity. Am J Reprod Immunol. 2010;63:339–348. doi: 10.1111/j.1600-0897.2009.00805.x. Establishes the possible role of nonthrombotic mechanisms, including induction of a proinfammatory state and impairment of trophoblast function, in fetal loss in APS. [DOI] [PubMed] [Google Scholar]
- 117.King K, Smith S, Chapman M, Sacks G. Detailed analysis of peripheral Blood natural killer (NK) cells in women with recurrent miscarriage. Hum Reprod. 2010;25(1):52–58. doi: 10.1093/humrep/dep349. [DOI] [PubMed] [Google Scholar]
- 118.Perricone C, De Carolis C, Giacomelli R, et al. High levels of NK cells in the peripheral Blood of patients affected with anti-phospholipid syndrome and recurrent sponatenous abortion: a potential new hypothesis. Rheumatology. 2007;46:1574–1578. doi: 10.1093/rheumatology/kem197. [DOI] [PubMed] [Google Scholar]
- 119.Redecha P, Tilley R, Tencati M, et al. Tissue factor: a link between C5a and neutrophil activation in antiphospholipid antibody induced fetal injury. Blood. 2007;110:2423–2431. doi: 10.1182/blood-2007-01-070631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Redecha P, Franzke CW, Ruf W, Mackman N, Girardi G. Neutrophil activation by the tissue factor/factor VIIa/PAR2 axis mediates fetal death in a mouse model of antiphospholipid syndrome. J Clin Immunol. 2008;118(10):3453–3454. doi: 10.1172/JCI36089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Di Simone N, Di Nicuolo F, D'Ippolito S, et al. Antiphospholipid antibodies affect human endometrial angiogenesis. Biol Reprod. 2010;83:212–219. doi: 10.1095/biolreprod.110.083410. [DOI] [PubMed] [Google Scholar]
- 122.Praprotnik S, Agmon-Levin N, Porat-Katz B, et al. Prolactin's role in the pathogenesis of the antiphospholipid syndrome. Lupus. 2010;19(13):1515–1519. doi: 10.1177/0961203310373781. [DOI] [PubMed] [Google Scholar]
- 123.Ioannou Y, Romay-Penabad Z, Pericleous C, et al. In vivo inhibition of antiphospholipid antibody-induced pathogenecity utilizing the antigenic target peptide domain of β2-glycoprotein I: proof of concept. J Thromb Haemost. 2009;7:833–842. doi: 10.1111/j.1538-7836.2009.03316.x. [DOI] [PubMed] [Google Scholar]
- 124.Jacobi A, Huand W, Wang T, et al. Effect of long-term belimumab treatment on B cells in systemic lupus erythematosus: extension of a Phase II, double-blind, placebo-controlled, dose-ranging study. Arthritis Rheum. 2010;62(1):201–210. doi: 10.1002/art.27189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wallace D, Stohl W, Furie R, et al. A Phase II, randomized, double-blind, placebo-controlled, dose-ranging study of belimumab in patients with active systemic lupus erythematosus. Arthritis Rheum. 2009;61(9):1168–1178. doi: 10.1002/art.24699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dillon S, Harder B, Lewis K, et al. B-lymphocyte stimulator/a proliferation-inducing ligand heterotrimers are elevated in the sera of patients with autoimmune disease and are neutralized by atacicept and B-cell maturation antigen-immunoglobulin. Arthritis Res Ther. 2010;12(2):R45. doi: 10.1186/ar2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kahn P, Ramanujam M, Bethunaickan R, et al. Prevention of murine antiphospholipid syndrome by BAFF blockade. Arthritis Rheum. 2008;58(9):2824–2834. doi: 10.1002/art.23764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jia L, Gu Y, Zeng E, Linnik M, Jones D. Pharmacokinetics of LJP 993, a tetrameric conjugate of domain 1 of β2-glycoprotein I for antiphospholipid syndrome. Lupus. 2010;19(2):130–137. doi: 10.1177/0961203309348982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jones D, Cockerill K, Gamino C, et al. Synthesis of LJP 993, a multivalent conjugate of the N-terminal domain of β2GPI and suppression of an anti-β2GPI immune response. Bioconjugate Chem. 2001;12(6):1012–1020. doi: 10.1021/bc015512x. [DOI] [PubMed] [Google Scholar]
- 130.Petri M. Hydroxychloroquine use in the Baltimore Lupus Cohort: effects on lipid, glucose and thrombosis. Lupus. 1996;5(Suppl. 1):S16–S22. [PubMed] [Google Scholar]
- 131.Edwards M, Pierangeli S, Liu X, Barker J, Anderson G, Harris E. Hydroxochloroquine reverses thrombogenic properties of antiphospholipid antibodies in mice. Circulation. 1997;96(12):4380–4384. doi: 10.1161/01.cir.96.12.4380. [DOI] [PubMed] [Google Scholar]
- 132.Ferrara D, Swerlick R, Casper K, et al. Fluvastatin inhibits up-regulation of tissue factor expression by antiphospholipid antibodies on endothelial cells. J Thromb Haemost. 2004;2(9):1558–1563. doi: 10.1111/j.1538-7836.2004.00896.x. [DOI] [PubMed] [Google Scholar]
- 133.Ferrara D, Liu X, Espinola R, et al. Inhibition of the thrombogenic and infammatory properties of antiphospholipid antibodies by fuvastatin in an in vivo animal model. Arthritis Rheum. 2003;48(11):3272–3279. doi: 10.1002/art.11449. [DOI] [PubMed] [Google Scholar]
- 134.Jajoria P, Murthy V, Papalardo E, Romay-Penabad Z, Gleason C, Pierangeli S. Statins for the treatment of antiphospholipid syndrome? Ann NY Acad Sci. 2009;1173:736–745. doi: 10.1111/j.1749-6632.2009.04815.x. [DOI] [PubMed] [Google Scholar]
- 135.Montiel-Manzano G, Romay-Penabad Z, de Martinez E, et al. In vivo effects of an inhibitor of nuclear factor-κB on thrombogenic properties of antiphospholipid antibodies. Ann NY Acad Sci. 2007;1108:540–553. doi: 10.1196/annals.1422.057. [DOI] [PubMed] [Google Scholar]
- 136.Coombs J, Bloom B, Breedveld F, et al. Improved pain, physical functioning and health status in patients with rheumatoid arthritis treated with CP-690,550, an orally active Janus kinase (JAK) inhibitor: results from a randomized, double-blind placebo controlled trial. Ann Rheum Dis. 2010;69(2):413–416. doi: 10.1136/ard.2009.108159. [DOI] [PubMed] [Google Scholar]
- 137.Kremer J, Bloom B, Breedveld F, et al. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: Results of a double-blind, placebo-controlled Phase IIa trial of three dosage levels of CP-690,550 versus placebo. Arthritis Rheum. 2009;60(7):1895–1905. doi: 10.1002/art.24567. [DOI] [PubMed] [Google Scholar]
- 138.Weinblatt M, Kavanaugh A, Genovese M, Musser T, Grossbard E, Magilavy D. An oral spleen tyrosine kinase (Syk) inhibitor for rheumatoid arthritis. N Engl J Med. 2010;363(14):1303–1312. doi: 10.1056/NEJMoa1000500. [DOI] [PubMed] [Google Scholar]