Abstract
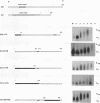
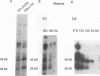
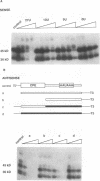
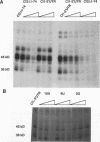
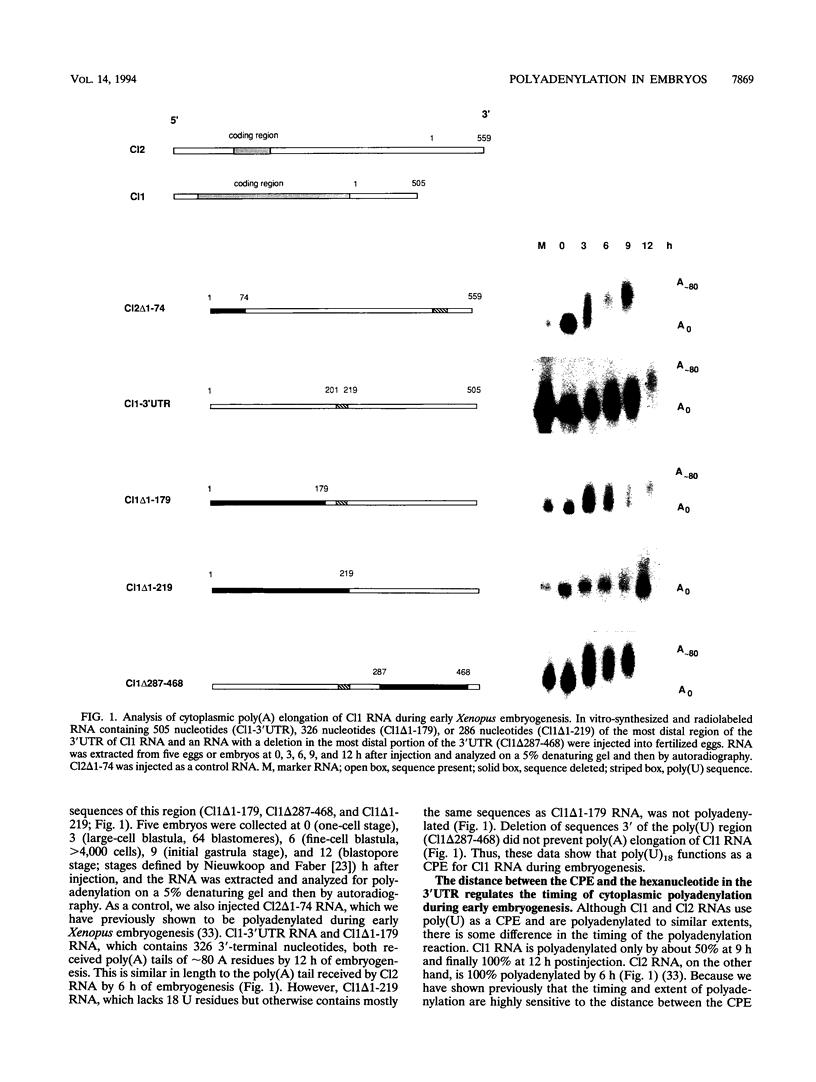
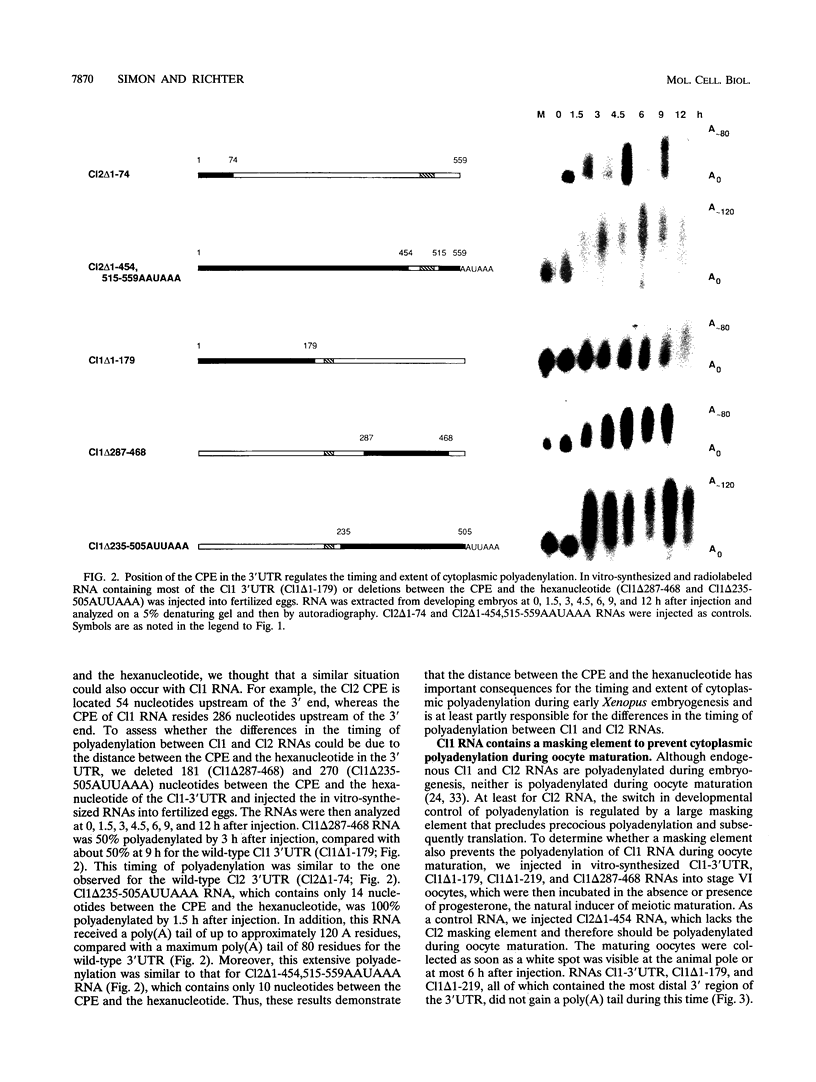
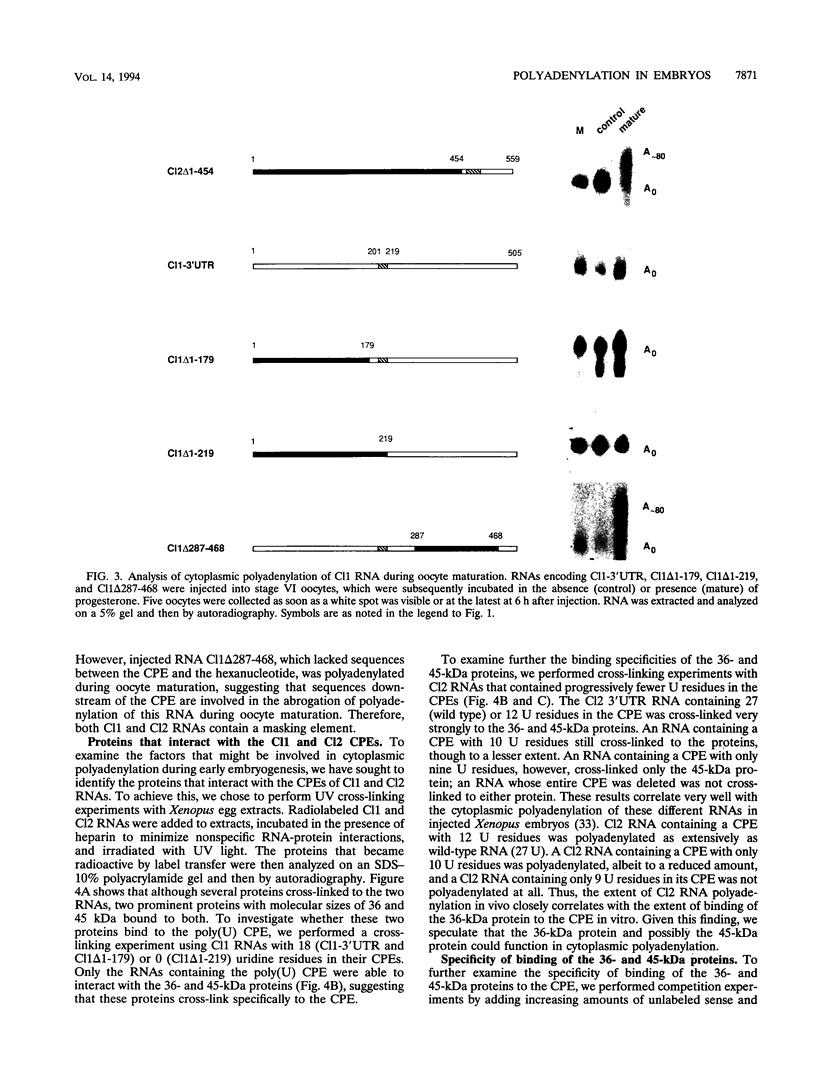
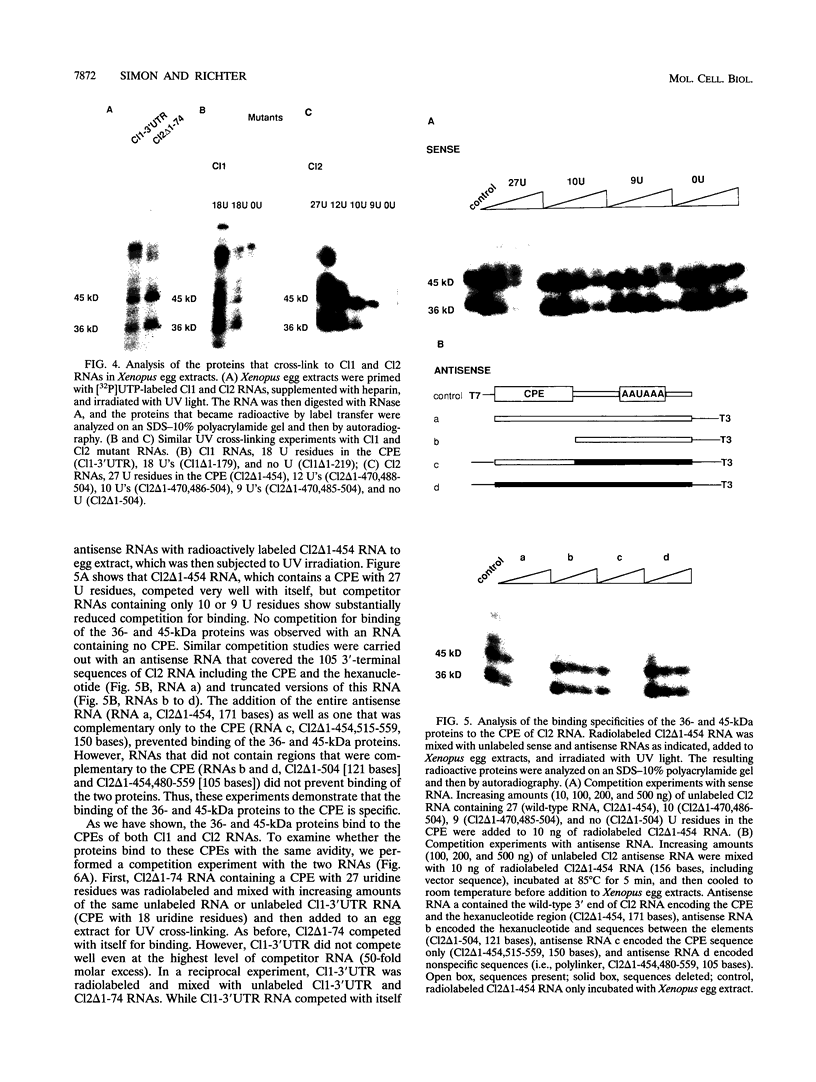
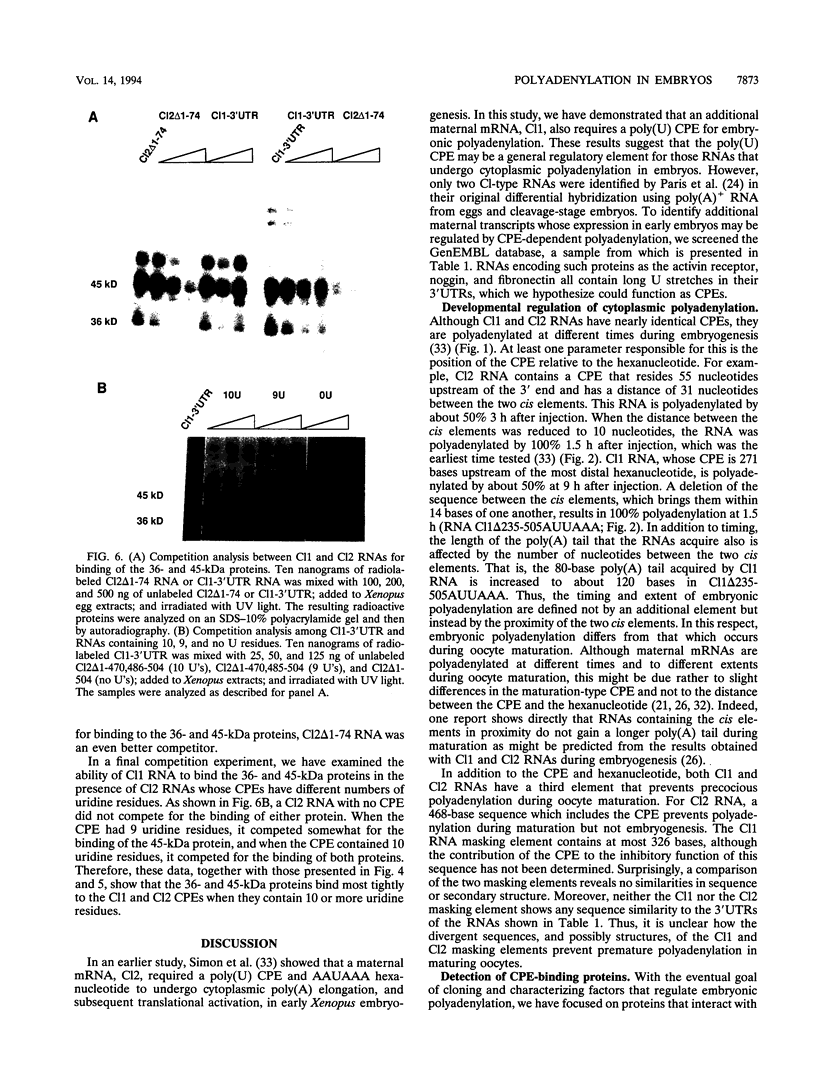
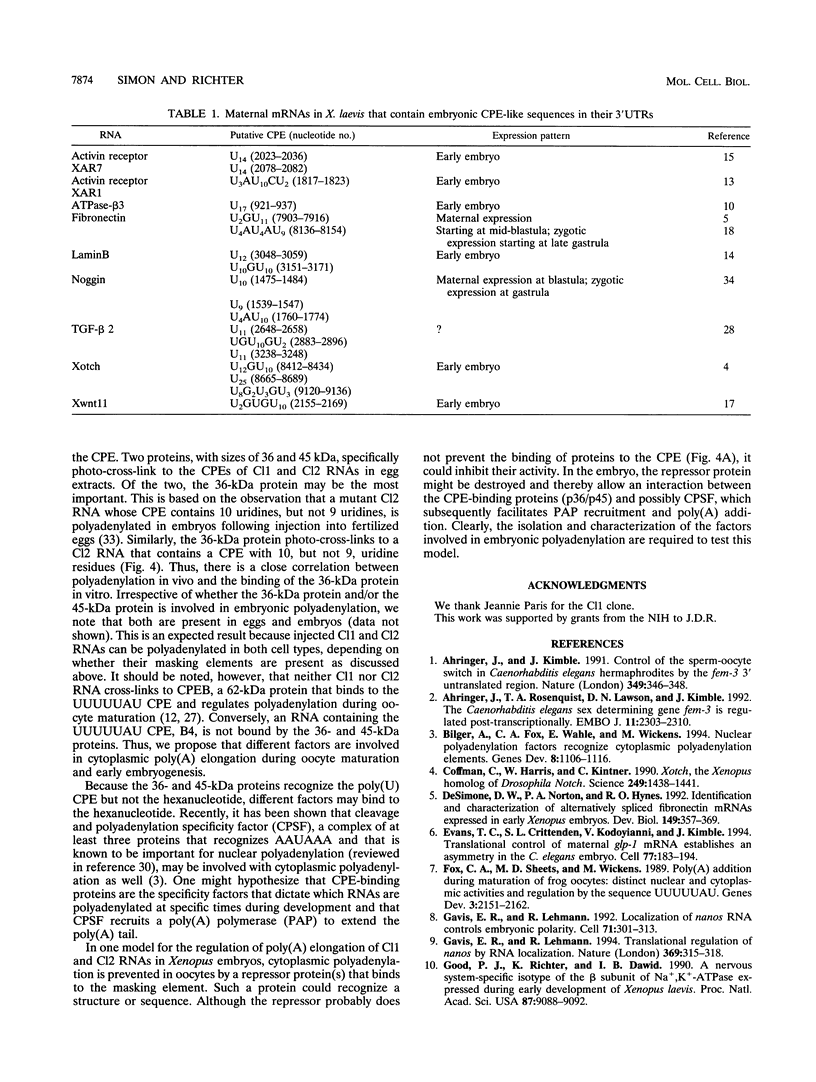
Early development in Xenopus laevis is programmed in part by maternally inherited mRNAs that are synthesized and stored in the growing oocyte. During oocyte maturation, several of these messages are translationally activated by poly(A) elongation, which in turn is regulated by two cis elements in the 3' untranslated region, the hexanucleotide AAUAAA and a cytoplasmic polyadenylation element (CPE) consisting of UUUUUAU or similar sequence. In the early embryo, a different set of maternal mRNAs is translationally activated. We have shown previously that one of these, C12, requires a CPE consisting of at least 12 uridine residues, in addition to the hexanucleotide, for its cytoplasmic polyadenylation and subsequent translation (R. Simon, J.-P. Tassan, and J.D. Richter, Genes Dev. 6:2580-2591, 1992). To assess whether this embryonic CPE functions in other maternal mRNAs, we have chosen Cl1 RNA, which is known to be polyadenylated during early embryogenesis (J. Paris, B. Osborne, A. Couturier, R. LeGuellec, and M. Philippe, Gene 72:169-176, 1988). Wild-type as well as mutated versions of Cl1 RNA were injected into fertilized eggs and were analyzed for cytoplasmic polyadenylation at times up to the gastrula stage. This RNA also required a poly(U) CPE for cytoplasmic polyadenylation in embryos, but in this case the CPE consisted of 18 uridine residues. In addition, the timing and extent of cytoplasmic poly(A) elongation during early embryogenesis were dependent upon the distance between the CPE and the hexanucleotide. Further, as was the case with Cl2 RNA, Cl1 RNA contains a large masking element that prevents premature cytoplasmic polyadenylation during oocyte maturation. To examine the factors that may be involved in the cytoplasmic polyadenylation of both C12 and C11 RNAs, we performed UV cross-linking experiments in egg extracts. Two proteins with sizes of ~36 and ~45 kDa interacted specifically with the CPEs of both RNAs, although they bound preferentially to the C12 CPE. The role that these proteins might play in cytoplasmic polyadenylation is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahringer J., Kimble J. Control of the sperm-oocyte switch in Caenorhabditis elegans hermaphrodites by the fem-3 3' untranslated region. Nature. 1991 Jan 24;349(6307):346–348. doi: 10.1038/349346a0. [DOI] [PubMed] [Google Scholar]
- Ahringer J., Rosenquist T. A., Lawson D. N., Kimble J. The Caenorhabditis elegans sex determining gene fem-3 is regulated post-transcriptionally. EMBO J. 1992 Jun;11(6):2303–2310. doi: 10.1002/j.1460-2075.1992.tb05289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilger A., Fox C. A., Wahle E., Wickens M. Nuclear polyadenylation factors recognize cytoplasmic polyadenylation elements. Genes Dev. 1994 May 1;8(9):1106–1116. doi: 10.1101/gad.8.9.1106. [DOI] [PubMed] [Google Scholar]
- Coffman C., Harris W., Kintner C. Xotch, the Xenopus homolog of Drosophila notch. Science. 1990 Sep 21;249(4975):1438–1441. doi: 10.1126/science.2402639. [DOI] [PubMed] [Google Scholar]
- DeSimone D. W., Norton P. A., Hynes R. O. Identification and characterization of alternatively spliced fibronectin mRNAs expressed in early Xenopus embryos. Dev Biol. 1992 Feb;149(2):357–369. doi: 10.1016/0012-1606(92)90291-n. [DOI] [PubMed] [Google Scholar]
- Evans T. C., Crittenden S. L., Kodoyianni V., Kimble J. Translational control of maternal glp-1 mRNA establishes an asymmetry in the C. elegans embryo. Cell. 1994 Apr 22;77(2):183–194. doi: 10.1016/0092-8674(94)90311-5. [DOI] [PubMed] [Google Scholar]
- Fox C. A., Sheets M. D., Wickens M. P. Poly(A) addition during maturation of frog oocytes: distinct nuclear and cytoplasmic activities and regulation by the sequence UUUUUAU. Genes Dev. 1989 Dec;3(12B):2151–2162. doi: 10.1101/gad.3.12b.2151. [DOI] [PubMed] [Google Scholar]
- Gavis E. R., Lehmann R. Localization of nanos RNA controls embryonic polarity. Cell. 1992 Oct 16;71(2):301–313. doi: 10.1016/0092-8674(92)90358-j. [DOI] [PubMed] [Google Scholar]
- Gavis E. R., Lehmann R. Translational regulation of nanos by RNA localization. Nature. 1994 May 26;369(6478):315–318. doi: 10.1038/369315a0. [DOI] [PubMed] [Google Scholar]
- Good P. J., Richter K., Dawid I. B. A nervous system-specific isotype of the beta subunit of Na+,K(+)-ATPase expressed during early development of Xenopus laevis. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9088–9092. doi: 10.1073/pnas.87.23.9088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin E. B., Okkema P. G., Evans T. C., Kimble J. Translational regulation of tra-2 by its 3' untranslated region controls sexual identity in C. elegans. Cell. 1993 Oct 22;75(2):329–339. doi: 10.1016/0092-8674(93)80074-o. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A., Wright D. A., Melton D. A. Embryonic expression and functional analysis of a Xenopus activin receptor. Dev Dyn. 1992 May;194(1):1–11. doi: 10.1002/aja.1001940102. [DOI] [PubMed] [Google Scholar]
- Höger T. H., Zatloukal K., Waizenegger I., Krohne G. Characterization of a second highly conserved B-type lamin present in cells previously thought to contain only a single B-type lamin. Chromosoma. 1990 Oct;99(6):379–390. doi: 10.1007/BF01726689. [DOI] [PubMed] [Google Scholar]
- Kondo M., Tashiro K., Fujii G., Asano M., Miyoshi R., Yamada R., Muramatsu M., Shiokawa K. Activin receptor mRNA is expressed early in Xenopus embryogenesis and the level of the expression affects the body axis formation. Biochem Biophys Res Commun. 1991 Dec 16;181(2):684–690. doi: 10.1016/0006-291x(91)91245-8. [DOI] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984 Sep 25;12(18):7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku M., Melton D. A. Xwnt-11: a maternally expressed Xenopus wnt gene. Development. 1993 Dec;119(4):1161–1173. doi: 10.1242/dev.119.4.1161. [DOI] [PubMed] [Google Scholar]
- Lee G., Hynes R., Kirschner M. Temporal and spatial regulation of fibronectin in early Xenopus development. Cell. 1984 Mar;36(3):729–740. doi: 10.1016/0092-8674(84)90353-2. [DOI] [PubMed] [Google Scholar]
- McGrew L. L., Dworkin-Rastl E., Dworkin M. B., Richter J. D. Poly(A) elongation during Xenopus oocyte maturation is required for translational recruitment and is mediated by a short sequence element. Genes Dev. 1989 Jun;3(6):803–815. doi: 10.1101/gad.3.6.803. [DOI] [PubMed] [Google Scholar]
- McGrew L. L., Richter J. D. Translational control by cytoplasmic polyadenylation during Xenopus oocyte maturation: characterization of cis and trans elements and regulation by cyclin/MPF. EMBO J. 1990 Nov;9(11):3743–3751. doi: 10.1002/j.1460-2075.1990.tb07587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A. W., Kirschner M. W. Cyclin synthesis drives the early embryonic cell cycle. Nature. 1989 May 25;339(6222):275–280. doi: 10.1038/339275a0. [DOI] [PubMed] [Google Scholar]
- Paris J., Osborne H. B., Couturier A., Le Guellec R., Philippe M. Changes in the polyadenylation of specific stable RNA during the early development of Xenopus laevis. Gene. 1988 Dec 10;72(1-2):169–176. doi: 10.1016/0378-1119(88)90139-4. [DOI] [PubMed] [Google Scholar]
- Paris J., Philippe M. Poly(A) metabolism and polysomal recruitment of maternal mRNAs during early Xenopus development. Dev Biol. 1990 Jul;140(1):221–224. doi: 10.1016/0012-1606(90)90070-y. [DOI] [PubMed] [Google Scholar]
- Paris J., Richter J. D. Maturation-specific polyadenylation and translational control: diversity of cytoplasmic polyadenylation elements, influence of poly(A) tail size, and formation of stable polyadenylation complexes. Mol Cell Biol. 1990 Nov;10(11):5634–5645. doi: 10.1128/mcb.10.11.5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris J., Swenson K., Piwnica-Worms H., Richter J. D. Maturation-specific polyadenylation: in vitro activation by p34cdc2 and phosphorylation of a 58-kD CPE-binding protein. Genes Dev. 1991 Sep;5(9):1697–1708. doi: 10.1101/gad.5.9.1697. [DOI] [PubMed] [Google Scholar]
- Rebbert M. L., Bhatia-Dey N., Dawid I. B. The sequence of TGF-beta 2 from Xenopus laevis. Nucleic Acids Res. 1990 Apr 25;18(8):2185–2185. doi: 10.1093/nar/18.8.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter J. D. Translational control during early development. Bioessays. 1991 Apr;13(4):179–183. doi: 10.1002/bies.950130406. [DOI] [PubMed] [Google Scholar]
- Sachs A., Wahle E. Poly(A) tail metabolism and function in eucaryotes. J Biol Chem. 1993 Nov 5;268(31):22955–22958. [PubMed] [Google Scholar]
- Sallés F. J., Darrow A. L., O'Connell M. L., Strickland S. Isolation of novel murine maternal mRNAs regulated by cytoplasmic polyadenylation. Genes Dev. 1992 Jul;6(7):1202–1212. doi: 10.1101/gad.6.7.1202. [DOI] [PubMed] [Google Scholar]
- Sheets M. D., Fox C. A., Hunt T., Vande Woude G., Wickens M. The 3'-untranslated regions of c-mos and cyclin mRNAs stimulate translation by regulating cytoplasmic polyadenylation. Genes Dev. 1994 Apr 15;8(8):926–938. doi: 10.1101/gad.8.8.926. [DOI] [PubMed] [Google Scholar]
- Simon R., Tassan J. P., Richter J. D. Translational control by poly(A) elongation during Xenopus development: differential repression and enhancement by a novel cytoplasmic polyadenylation element. Genes Dev. 1992 Dec;6(12B):2580–2591. doi: 10.1101/gad.6.12b.2580. [DOI] [PubMed] [Google Scholar]
- Smith W. C., Harland R. M. Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell. 1992 Sep 4;70(5):829–840. doi: 10.1016/0092-8674(92)90316-5. [DOI] [PubMed] [Google Scholar]
- St Johnston D., Nüsslein-Volhard C. The origin of pattern and polarity in the Drosophila embryo. Cell. 1992 Jan 24;68(2):201–219. doi: 10.1016/0092-8674(92)90466-p. [DOI] [PubMed] [Google Scholar]
- Stebbins-Boaz B., Richter J. D. Multiple sequence elements and a maternal mRNA product control cdk2 RNA polyadenylation and translation during early Xenopus development. Mol Cell Biol. 1994 Sep;14(9):5870–5880. doi: 10.1128/mcb.14.9.5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassan J. P., Le Guellec K., Kress M., Faure M., Camonis J., Jacquet M., Philippe M. In Xenopus laevis, the product of a developmentally regulated mRNA is structurally and functionally homologous to a Saccharomyces cerevisiae protein involved in translation fidelity. Mol Cell Biol. 1993 May;13(5):2815–2821. doi: 10.1128/mcb.13.5.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalli J. D., Huarte J., Belin D., Gubler P., Vassalli A., O'Connell M. L., Parton L. A., Rickles R. J., Strickland S. Regulated polyadenylation controls mRNA translation during meiotic maturation of mouse oocytes. Genes Dev. 1989 Dec;3(12B):2163–2171. doi: 10.1101/gad.3.12b.2163. [DOI] [PubMed] [Google Scholar]