Abstract
Ellagitannins are the most abundant polyphenols in pomegranate (Punica granatum) husk and contribute greatly towards its biological properties. A pre-enriched pomegranate husk powder was extracted with water and then further purified by an Amberlite XAD-16 column. Punicalagin (PC) anomers were eluted using a gradient of methanol and water. Fractions eluted with 20% and 25% methanol yielded 1.08 g of light brown powder (purity > 97%) from a total of 40 g of extract. This fraction was identified as PC by HPLC-UV using reference compounds and confirmed by FTICR-MS analysis. PC (10–40 µM) was found to significantly inhibit oxidative DNA products, about 70% inhibition at 40 µM (p=0.0017), resulting from Cu2+-catalyzed redox cycling of 4-hydroxy-17β-estradiol as analyzed by 32P-postlabeling. Evidence of high antioxidant activity of PC was also obtained based on ORAC assay (1556±79 µmol of TE/g), as well as by 2,2′-azino-bis (3-ethylbenzthiazoline-6-sulphonic acid) (ABTS)-, 2,2-diphenyl-1-picrylhydrazyl (DPPH)-, hydrogen peroxide (H2O2) scavenging and ferrous ion-chelating activities (IC50=1.1, 17.1, 24 and 45.4 µg/ml, respectively). Further, PC exhibited strong anti-proliferative activity against the human lung, breast and cervical cancer cell lines. Together, these data suggest that PC can be isolated in its purified form by simple column chromatography, inhibits oxidative DNA damage and possesses high anti-proliferative activity.
Keywords: Punica granatum, Punicalagin, Antioxidant activity, Antiproliferative activity, Oxidative DNA damage, 32P-postlabeling
1. Introduction
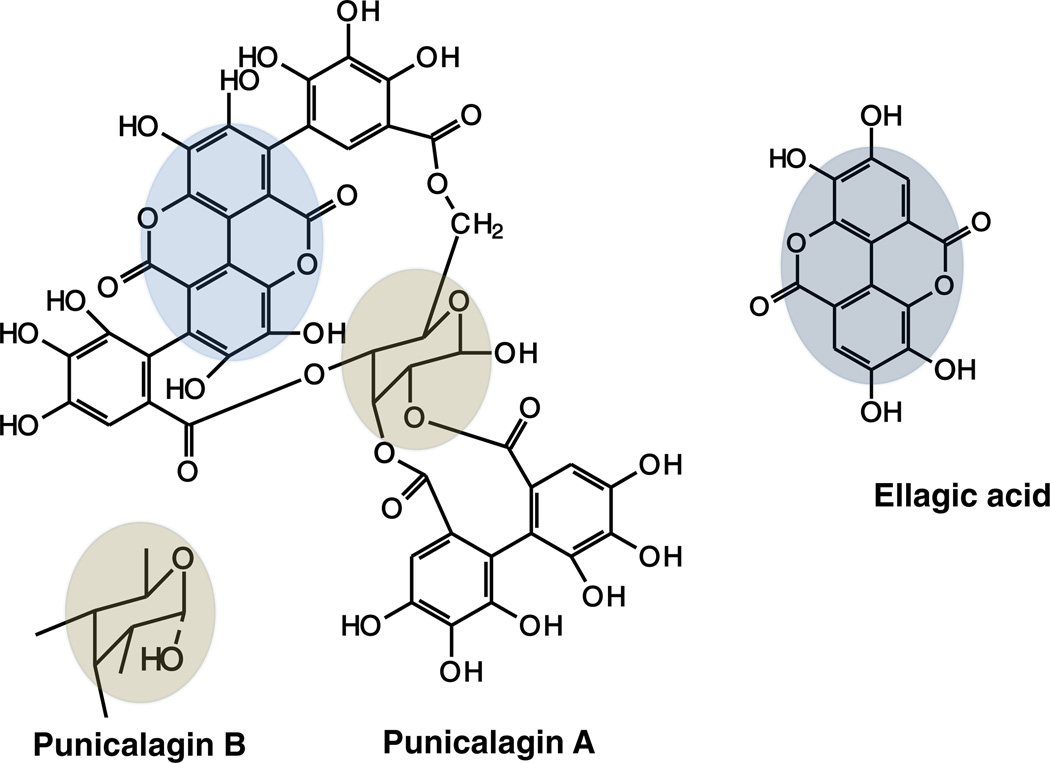
Pomegranate (Punica granatum L.) fruits are widely consumed fresh and in processed forms as juice, jams and wine. The husk of pomegranate is rich in ellagitannins (ETs) such as punicalagin (PC), punicalin, gallagic acid, ellagic acid (EA) and EA-glycosides (Seeram, Lee, Hardy, & Heber, 2005). PC (2,3-hexahydroxy-diphenoyl-4,6-gallagylglucose) is a high molecular weight (MW 1084 water-soluble compound isolated from pomegranate husk. PC is the predominant ellagitannin of Punica granatum and present in two anomers; punicalagin A and B (Fig. 1). PC belongs to the chemical class of hydrolyzable tannins and releases EA upon hydrolysis (Seeram et al., 2006). Anti-atherosclerotic and antioxidant properties of pomegranate have been attributed to its high polyphenol content especially PC (Adams et al., 2006). PC has also shown remarkable biological activities including anti-inflammatory (Lin, Hsu, & Lin, 1999), hepatoprotective (Lin, Hsu, Lin, & Hsu, 2001) and antigenotoxic activities (Chen, Li, Liu, & Lin, 2000).
Fig. 1.
Chemical structure of punicalagin and one of its hydrolyzed product ellagic acid. Highlighted portion shows the EA moiety in PC. Two anomers of PC are shown separately.
Pomegranate juice has recently been reported to have protective effects against prostate cancer in a pilot clinical study (Pantuck et al., 2006). However, there have been only limited studies on PC as to its biological efficacy (Aqil, Vadhanam, & Gupta, 2012; Kulkarni, Mahal, Kapoor, & Aradhya, 2007; Lee, Chen, Liang, & Wang, 2010; Seeram et al., 2005) largely due to limitations in availability of sufficiently large quantities. Available methods to purify the bioactive PC from pomegranate use multiple columns of different stationary phases (Seeram, Lee, Hardy, & Heber, 2005) and/or preparative HPLC (Lu, Ding, & Yuan, 2001; Lu, Wei, & Yuan, 2007). In this study we show isolation of PC from partially enriched Punica granatum husk powder. In addition to demonstrating its antioxidant potential by different methods, including an array of radicals, we also report inhibition of oxidative DNA adducts by PC as well as its anti-proliferative potential toward various human cancer cell lines.
2. Material and methods
2.1. Chemicals
XAD-16 (amberlite polymeric adsorbent of 20–50 mesh) was purchased from Supelco (Bellefonte, PA, USA). Salmon testes (st) DNA, ascorbic acid, 1,1-diphenyl-2-picryl hydrazyl (DPPH•), AAPH (2,2′-azobis 2-amidino-propane dihydrochloride), fluorescein, and 2,2′- azinobis (3-ethyl-benzothiazoline-6-sulfonic acid) (ABTS) were obtained from Sigma-Aldrich (St. Louis, MO). Trolox was purchased from Acros-Organic (Morris Plains, NJ). MTT (3-(5-dimethylthiazol-z-yl)-2, 5-diphenyl tetrazolium bromide) was obtained from Calbiochem (Gibbstown, NJ). PC standard was obtained from ChromaDex Inc. (Irvine, CA). 4-Hydroxy estradiol (4-OHE2) was purchased from Steraloids, Inc. (Newport, RI). st-DNA was purified from contaminating RNA and protein prior to use (Gupta, 1996). Micrococcal nucleases (MN), RNase A and RNase T1 were purchased from Sigma, nuclease P1 from Calbiochem (San Diego, CA) and proteinase K and spleen phosphodiesterase (SPD) were purchased from Boehringer-Mannhein Corp. (Indianapolis, IN). T4 polynucleotide kinase was purchased from New England Biolabs (Ipswich, MA). All other chemicals used in 32P-postlabeling were as described elsewhere (Gupta, 1985, 1996). All other chemicals were of analytical grade.
2.2. Plant material and isolation of PC
Punica granatum (pomegranate) fruit husk extract (30% enriched for PC) was obtained from Pharmenza, Inc. (Gujarat, India), courtesy of Ajay Patel of Verdure Sciences. PC was isolated by a method of Seeram, Lee, Hardy, and Heber (2005) except that LH-20 column was not used. Briefly, the extract was prepared by dissolving 40 g of enriched husk extract in 5 vol (w/v; 200 ml) of water. Samples were centrifuged at 6000×g for 10 min and decanted. Residue was extracted two more times and the pooled supernatant was concentrated under vacuum at 40 °C using a rotary evaporator (Model R 215, Bucchi, Switzerland). PC was isolated by loading concentrated extract on an XAD-16 column (400 g capacity), followed by washing with a large volume of water (40–50 void vol) to remove free sugars. Phenolics were eluted with a step gradient of 15, 20, 25, 30, 35, 40, 50, 60 and 100% methanol in water (two void vol; 800 ml each). Fractions eluted with 20% and 25% methanol yielded 1.08 g of light brown powder from a total of 40 g of extract and was identified as PC. Purity of PC was confirmed by HPLC and its identity was established by co-chromatography with reference compound as well as confirmed by FTICR-MS analysis. Quantification was carried out using a standard curve of the reference PC. Fractions containing less pure material were reprocessed on the same column after regeneration.
2.3. HPLC analysis
HPLC analysis was carried out by the modified method of Seeram, Lee, Hardy, and Heber (2005) on a Shimadzu HPLC system (Kyoto, Japan) equipped with two LC-10ADvp pumps, an autosampler and diode array detectors (DAD) operated by Class VP (ver 7.4 SP3). Eluted fractions from the XAD-16 column that contained PC (20 µl injection volume; 1 mg/ml) were analyzed on a ShimPack reverse phase column (Shimadzu; 250×4.6 mm, 5 µm) using a gradient of water (solvent A) in methanol (solvent B), each containing 2% acetic acid. Solvent B was initially 1% for 5 min, increased linearly to 60% by 40 min; the later ratio was maintained for 41–50 min and finally reduced to 1% at 51 min at flow rate of 1 ml/min. Spectra were recorded between 200 and 400 nm and PC isomers were monitored at 378 nm. The PC concentrations were determined against a standard curve of commercial PC (ChromaDex, Irvine, CA).
2.4. Fourier transform ion cyclotron resonance (FTICR) mass spectrometry
Purity and identity of PC was confirmed on a hybrid linear ion trap FTICR mass spectrometer (Finnigan LTQ FT, Thermo Electron, Bremen, Germany) equipped with a TriVersa NanoMate ion source (Advion Bio-Sciences, Ithaca, NY) with an “A” electrospray chip (nozzle inner diameter 5.5 µm). The TriVersa NanoMate was operated in negative ion mode by applying 2.0 kV with no head pressure. MS runs were recorded over a mass range from 200 to 1200 Da. The LTQ-FT was tuned and calibrated according to the manufacturer's default standard recommendations, which achieved better than 1 ppm mass accuracy and capable of 400,000 resolution at m/z=400. FTICR mass spectra were exported as exact mass lists into a spreadsheet file using QualBrowser 2.0 (Thermo Electron), typically exporting all of the observed peaks. PC, punicalin, gallagic acid, EA and other compounds were assigned based on their accurate mass.
2.5. Induction of DNA damage by 4-OHE2/CuCl2
St-DNA (300 µg/ml) in 10 mM Tris–HCl (pH 7.4) was pre-incubated with either vehicle (DMSO) or PC at different concentrations (10–40 µM) for 1 h at 37 °C. After adding 4-OHE2 (30 µM) and CuCl2 (30 µM), the reaction mixture was incubated at 37 °C for 4 h, and the DNA was isolated by solvent extraction and ethanol precipitation as described (Gupta, 1996; Ravoori et al., 2006).
2.6. Analysis of DNA adducts by 32P-postlabeling/TLC
Analysis of oxidative DNA adducts was conducted as described previously (Aiyer et al., 2008). Briefly, 6 µg DNA was digested to 3′-monophosphates using a mixture of micrococcal nuclease and spleen phosphodiesterase (enzyme:DNA—1:5, 4 h, 37 °C). A 2 µg DNA digest was removed for normal nucleotide analysis, and 4 µg was enriched for oxidative adducts by treatment with nuclease P1 (E:S—1:2.5, 1 h, 37 °C). The 5′-32P-labeling of both enriched DNA adducts and normal nucleotides (2 ng) were done in parallel by T4-polynucleotide kinase and molar excess of [γ-32P] ATP (<3 µM; 550 Ci/mmol). Labeled adducts were separated by 2-D PEI-cellulose TLC using 30 mM sodium phosphate, pH 6.0 and 1 M formic acid in direction 1 (D1). Development in D2 was perpendicular to D1 using a solvent mixture of isopropanol:4 M ammonium hydroxide (0.9:1.0). Labeled normal nucleotides were separated by one-directional PEI-cellulose TLC in 1.2 M ammonium formate, pH 3.5.
Adducts and normal nucleotides were visualized using Packard InstantImager and were counted individually. Adduct levels were calculated as relative adduct labeling (RAL)
Adducts levels are expressed as adducts per 106 nucleotides.
2.7. Antioxidant assays
2.7.1. Total antioxidant potential by ORAC assay
ORAC capacity of PC and one of its hydrolyzed product, EA was determined at different concentrations by the method of Ou, Hampsch-Woodill, and Prior (2001) using fluorescein as the “fluorescent probe”. Briefly, 25 µl of phosphate buffer (75 mM, pH 7), trolox or PC at different concentrations were incubated with 150 µl of fluorescein (20 nM) at 37 °C for 1 h. The reaction was started by thermal decomposition of AAPH (19 mM in 75 mM phosphate buffer, pH 7.0) and performed at 37 °C in 96-well black plates. Fluorescence was measured immediately after the addition of AAPH at excitation of 485 nm and an emission wavelength of 535 nm for 2 h at 37 °C. The ORAC values, expressed as µmoles trolox equivalents per gram were calculated by applying the following formula
Relative ORAC value = (CTrolox * AUCSample−AUCBlank) * k)/(AUCTrolox−AUCBlank)
where CTrolox is the concentration of trolox, k is the sample dilution factor, and AUC is the area under the fluorescence decay curve of the sample, blank, and trolox, respectively, calculated by Spectramax M2 software.
2.7.2. DPPH radical-scavenging assay
Free radical-scavenging activity of PC and EA against stable DPPH was determined spectrophotometrically by the modified method of Gyamfi, Yonamine, and Aniya (1999). Briefly, 50 µl of PC and EA (6.25–100 µg/ml) were mixed with 0.5 ml of 0.1 mM DPPH in methanol and 450 µl of 50 mM Tris–HCl, pH 7.4. Control sample was incubated with vehicle alone. After 30 min of incubation at room temperature the reduction of the DPPH free radical was measured at 517 nm. Ascorbic acid and BHA were included as positive controls. Percent inhibition was calculated from the following equation:
% Inhibition = [(Absorbance of control−Absorbance of test sample)/Absorbance of control] × 100.
2.7.3. ABTS radical cation decolorization assay
The spectrophotometric analysis of ABTS•+ scavenging activity was determined according to the method of Re et al. (1999). ABTS•+ was produced by reacting 2 mM ABTS in H2O with 2.45 mM potassium persulfate, stored in the dark at room temperature overnight. The ABTS•+ solution was diluted in water to give an absorbance of 0.750±0.025 at 734 nm. Then, 250 µl of ABTS•+ solution was added to 0.75 ml of PC and EA (0.62–10 µg/ml). The absorbance was recorded 30 min after mixing and the percentage of radical scavenging was calculated against the blank. The scavenging capability of test compounds was calculated using the following equation:
% scavenging = [(Absorbance of control−Absorbance of sample)/Absorbance of control] × 100.
2.7.4. H2O2 scavenging activity
The hydrogen peroxide scavenging assay was carried out as described by Ruch, Cheng, and Klaunig (1989). PC (10–80 µg/ml) in 3.4 ml phosphate buffer was added to 0.6 ml of H2O2 solution (43 mM in 0.1 M phosphate buffer, pH 7.4) and absorbance of the reaction mixture was recorded at 230 nm. Percent scavenging of H2O2 by PC and standards was calculated using the following equation:
% H2O2 scavenging = [(Absorbance of control−Absorbance of test sample)/Absorbance of control] × 100.
2.7.5. FRAP assay (Fe3+ reducing antioxidant power assay)
The ferric reducing antioxidant power (FRAP) was determined by using the method of Oyaizu (1986), with minor modification as described (Aqil et al., 2012). Briefly, PC and EA (10–100 µg/ml) in 0.75 ml of water were mixed with 1.25 ml of 0.2 M sodium phosphate, pH 6.6 and 1.25 ml of 1% potassium ferricyanide. After 20 min incubation at 50 °C, the reaction mixture was acidified with 1.25 ml trichloroacetic acid (10%). Finally, 0.5 ml of FeCl3 (0.1%) was added to the reaction mixture, and the absorbance was measured at 700 nm. The increase in absorbance of mixture indicated the reduction capability. Percent reducing activity of test compounds was calculated using the following equation:
% Fe3+ reducing activity = [(Absorbance of control−Absorbance of test sample)/Absorbance of control] × 100.
2.8. Cell lines and culture medium
All cell lines were obtained from ATCC (Manassas, VA, USA). A-549 (human small cell lung carcinoma cell line), H1299 (human non-small cell lung carcinoma cell line) and MCF7 (human breast adenocarcinoma cell line) cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS and 1% penicillin/streptomycin. Cervical cancer cell lines (CaSki, HeLa and SiHa) obtained from sources described elsewhere (Munagala, Kausar, Munjal, & Gupta, 2011) were cultured in RPMI-1640 media (Invitrogen, Carlsbad, CA) containing supplements (10% FBS, 1% penicillin/streptomycin, 2 mM l-glutamine, 1 mM sodium pyruvate and 0.1 mM nonessential amino acids). All cell lines were maintained in humidified air containing 5% CO2 at 37 °C.
2.9. Cell proliferation assay
Inhibition of cell proliferation by different concentrations of PC was determined by the MTT assay (Plumb, 2004). In brief, the cells were seeded in triplicate at a density of 5000 cells/well in a 96-well plate and incubated overnight to allow cell attachment. The cells were then treated with vehicle alone (0.1% DMSO) or PC (12.5–200 µg/ml) for 72 h. The medium was then removed and incubated with 100 µl of fresh medium containing MTT (0.5 mg/ml) for 2 h. The number of viable cells was directly proportional to the production of formazan, which was then solubilized in 200 µl DMSO. The absorbance was measured with a spectrophotometer microplate reader at 570 nm wavelength.
% of cell viability = (OD of treated cells/OD of control cells) × 100.
The concentrations required for inhibition of 50% of cell viability (IC50) were calculated.
2.10. Statistical analysis
Analyses were performed with GraphPad Prism statistical software (version 4.03; La Jolla, CA). Data represent average ± standard deviation of three replicates. For cell proliferation assay, IC50 values were calculated using exponential growth with base-line fit by Microsoft Excel, XLFit version 5 software. Differences were considered a priori to be statistically significant if the p-value was less than 0.05.
3. Results
3.1. Isolation of PC isomers
Repeated extraction of pre-enriched husk extract of pomegranate with water provided almost 95–97% recovery of extracts. The pooled, concentrated extract (from 40 g of material), when applied onto the Amberlite XAD-16 column and eluted with a gradient of methanol yielded 1.08 g (2.7%) light brown powder with 20% and 25% methanol that was identified as PC (Table I).
Table I.
Isolation of punicalagin by column chromatography.
| Elute no. | Elute | Purity of PC (%) | PC yield (%) |
|---|---|---|---|
| 1 | 15% MeOH | 91.3 | 0.88 ± 0.18 |
| 2 | 20% MeoH | 97.6 | 0.67 ± 0.16 |
| 3 | 25% MeOH | 96.4 | 2.03 ± 0.59 |
| 4 | 30% MeOH | 86.4 | 5.01 ± 0.83 |
| 5 | 35% MeOH | 72.9 | 5.68 ± 0.56 |
| 6 | ≥40% MeOH | <50 | 5.95 ± 1.98 |
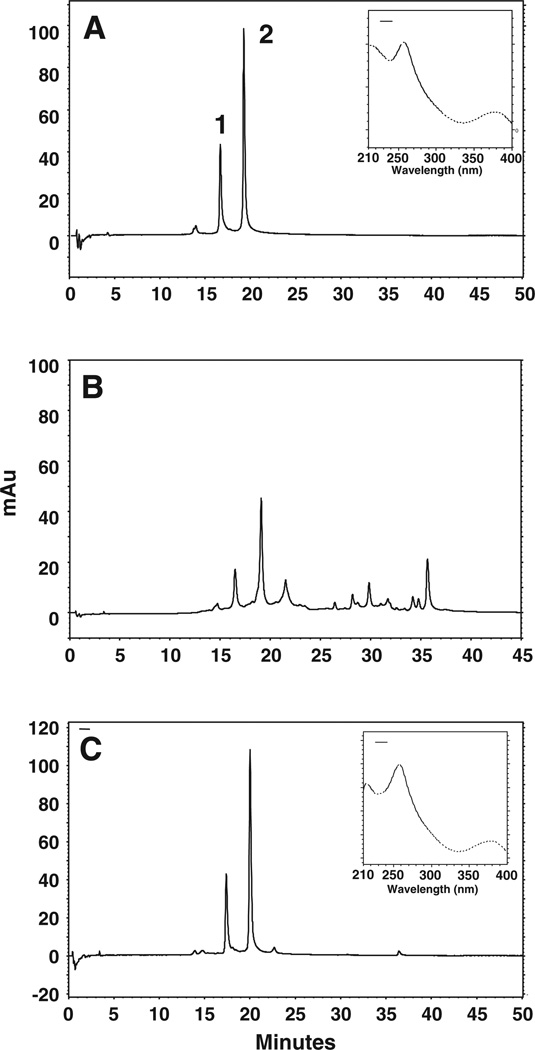
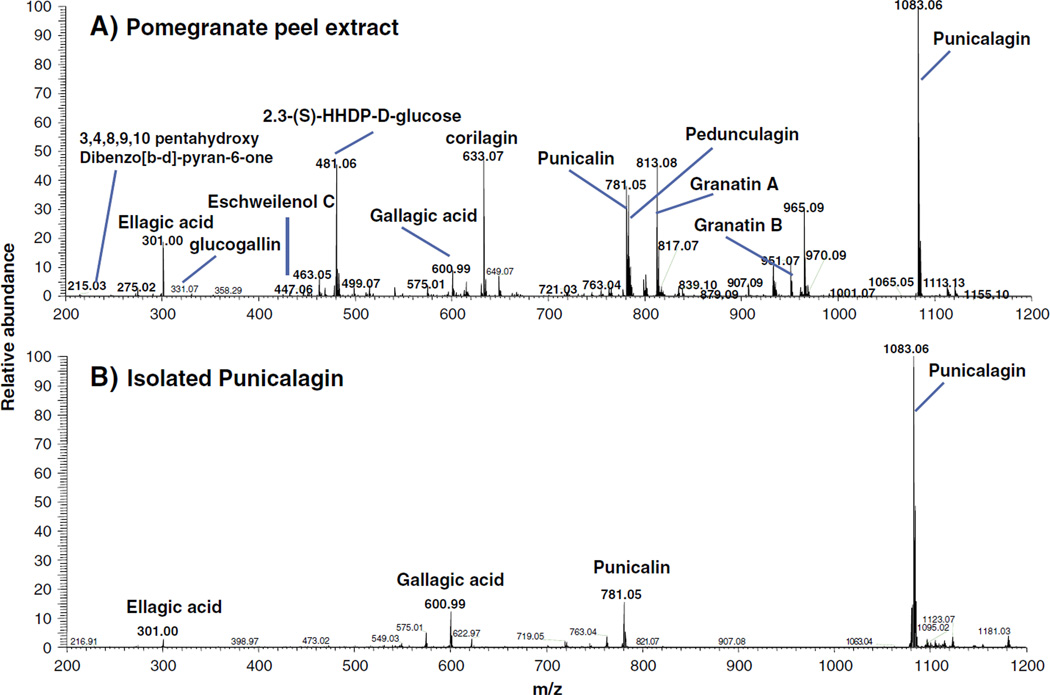
The purity of the isolated fractions for PC was determined by HPLC analysis, monitoring the chromatogram at 378 nm and co-chromatography with reference PC as shown in Fig. 2A–C. However, quantification was done against a standard curve of the reference compound. Methanol extract of pomegranate husk contained several compounds (Fig 2B), including PC (41%) as determined by HPLC. We could not identify the other peaks due to the lack of corresponding standards. However, FTICR-MS analysis by direct infusion of husk extract demonstrated the presence of more than 12 compounds and showed PC (M−H m/z 1083), punicalin (M−H m/z 781), corilagin (M−H m/z 633), gallagic acid (M−H m/z 601), 2,3-(S)-HHDP-d-glucose (M−H m/z 433) and EA (M−H m/z 301) as major components (Fig 3A).
Fig. 2.
HPLC profile of Punica granatum husk extract and isolated PC. Enriched extracts (20 µl; 1 mg/ml) were injected and analyzed using photo diode-array detector on a C18 (250×4.6 mm×5 µm) column in a gradient of 2% acetic acid in water and 2% acetic acid in methanol for 51 min. Chromatograms represent commercial punicalagin (A), enriched pomegranate extracts (B), and eluted punicalagin in 25% methanol (C) monitored at 378 nm. Two peaks represent two anomers of punicalagin; α- (peak 1) and β-punicalagin (peak 2), respectively. Insets in 2A and 2C show spectra of commercial and isolated β-punicalagin, respectively. mAu, milliabsorbance units.
Fig. 3.
FTICR-MS spectra of pomegranate husk enriched extract (A) and isolated punicalagin (B) analyzed by direct infusion. The spectra was recorded over amass range from 200 to 1200 Da. PC (M−H m/z 1083), punicalin (M−H m/z 781), corilagin (M−H m/z 633), gallagic acid (M−H m/z 601), 2,3-(S)-HHDP-d-glucose (M−H m/z 433), EA (M−H m/z 301) and other compounds shown were assigned based on their accurate mass.
The compound eluted in 20% and 25% methanol was characterized as PC with 97.6 and 96.4% purity, respectively as determined using a standard curve and further confirmed by co-chromatography with reference PC (Table I). MS analysis of PC fractions (20% and 25% elute) further confirmed its purity, with only trace amounts of punicalin and gallagic acid (Fig. 3B). Further MS–MS studies of PC at M−H m/z 1083 showed ions corresponding to punicalin at M−H m/z 781, gallagic acid at M−H m/z 601 and EA at M−H m/z 301 (data not shown).
3.2. PC modulates DNA adducts induced by 4-OHE2/CuCl2
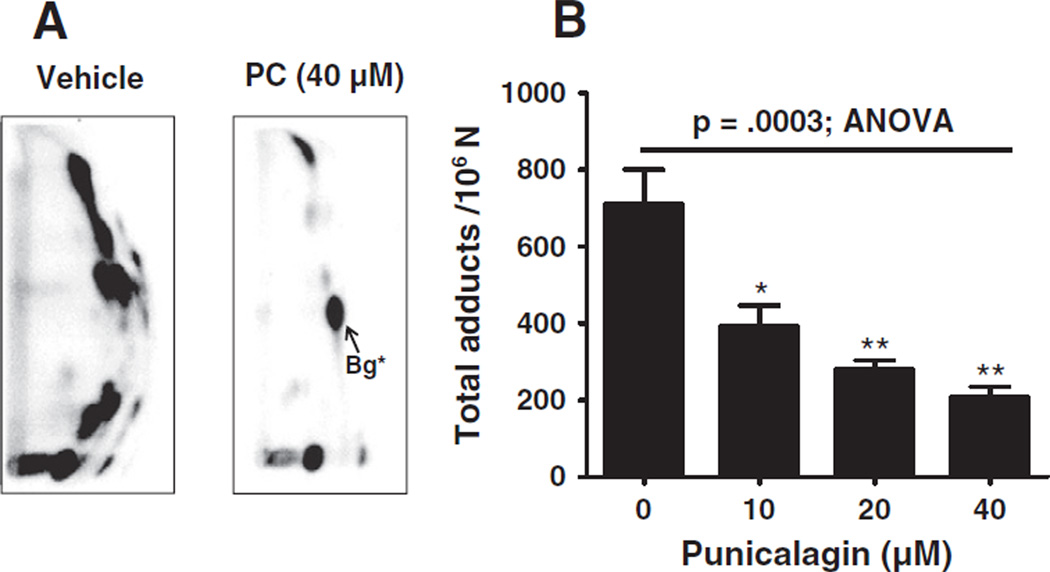
Analysis of oxidative DNA adducts, induced by the Cu2+ catalyzed redox cycling of 4-OHE2 revealed several oxidative adducts (Fig. 4A). These adducts have been assigned as unidentified dinucleotides formed by an attack of hydroxyl radicals (Spencer, Vadhanam, Jeyabalan, & Gupta, 2012). The formation of these adducts was significantly diminished in the presence of the highest concentration (40 µM) of PC (712±197 versus 210±51 per 106 normal nucleotides); the adduct inhibition occurred in a dose dependent manner (Fig. 4B).
Fig. 4.
A) Representative autoradiographs of 32P-postlabeling of DNA adducts generated from Cu2+ (30 µM) catalyzed redox cycling of 4 hydroxy estradiol (30 µM) in the presence of vehicle (1% DMSO) (left) and punicalagin (right). Labeled adducts were applied onto PEI-cellulose and separated by TLC as described in the Material and methods section. B) Graphical representation of the adduct levels at the indicated concentrations of PC. *Bg, back ground spot.
3.3. Antioxidant assays
3.3.1. ORAC capacity
PC exhibited concentration-dependent ORAC activity expressed as trolox equivalent. A progressive decrease of fluorescence was observed in the absence of either trolox or PC with almost total consumption after 30 min. The decay curve of the fluorescein was found to be concentration dependent with trolox and PC (data not shown). The ORAC of PC (1556±79 µmol of TE/g) was somewhat higher than EA (1333 ± 56 µmol of TE/g), its bioactive metabolite (Aqil, Vadhanam, & Gupta, 2012).
3.3.2. DPPH, ABTS and H2O2 radical scavenging
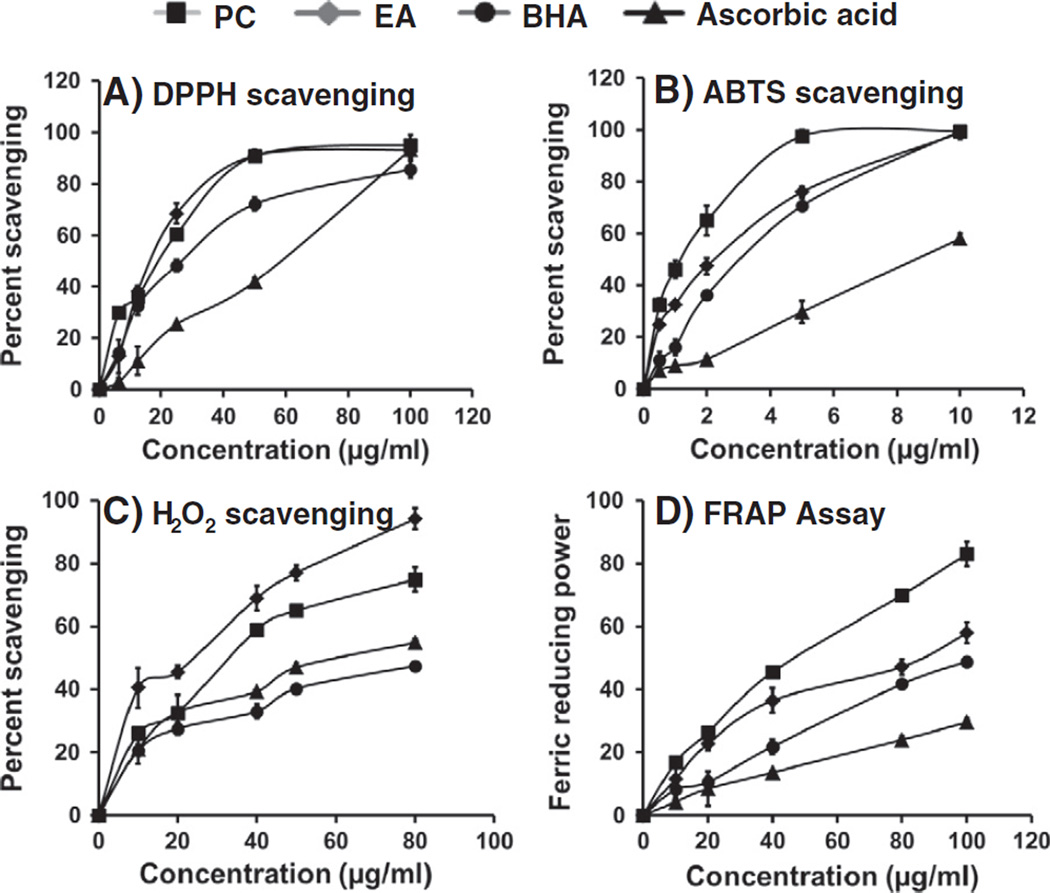
Scavenging activity of the PC was measured using DPPH and compared with EA, ascorbic acid and BHA (Fig. 5A). PC showed the inhibition of free radicals in a concentration-dependent manner in the range of 12.5–100 µg/ml. The IC50 (concentration that scavenged 50% of DPPH radicals) was almost similar for both PC and EA (~17 µg/ml) and almost completely (>90%) inhibited DPPH absorption at 50 µg/ml. PC was more effective than ascorbic acid and BHA.
Fig. 5.
Antioxidant potential of PC and EA in different assays viz; DPPH free radical scavenging (A), ABTS scavenging (B), H2O2 scavenging (C) and ferric ion reducing power (D). Each data point represents the mean of three experiments with standard deviation.
The scavenging of ABTS•+ by PC and EA is shown in Fig. 5B. Both PC and EA showed concentration-dependent scavenging of ABTS radicals. The IC50 for the various test compounds during the 30-minute incubation, obtained by interpolation of the data, were in the following ascending order: 1.1, 2.0, 3.0 and 8.6 µg/ml for PC, EA, BHA and ascorbic acid, respectively.
The abilities of PC compared with EA, BHA and ascorbic acid to scavenge hydrogen peroxide are shown in Fig. 5D. At 40 µg/ml, the percent scavenging of H2O2 radicals of test compounds was in the following descending order: EA (69 ± 3%), PC (59 ± 1%), BHA (39 ± 1%) and ascorbic acid (33 ± 1%).
3.3.3. FRAP assay
The ferric reducing antioxidant power of PC was found to be dose dependent (Fig. 5D). FRAP value for the PC was almost two-fold higher than that of EA. The IC50 for test compounds, obtained by interpolation of the data was in the following ascending order: PC (45.4 µg/ml), EA (75.6 µg/ml), BHA (>100 µg/ml) and ascorbic acid (>250 µg/ml).
3.4. Anti-proliferative activities against human cancer cells
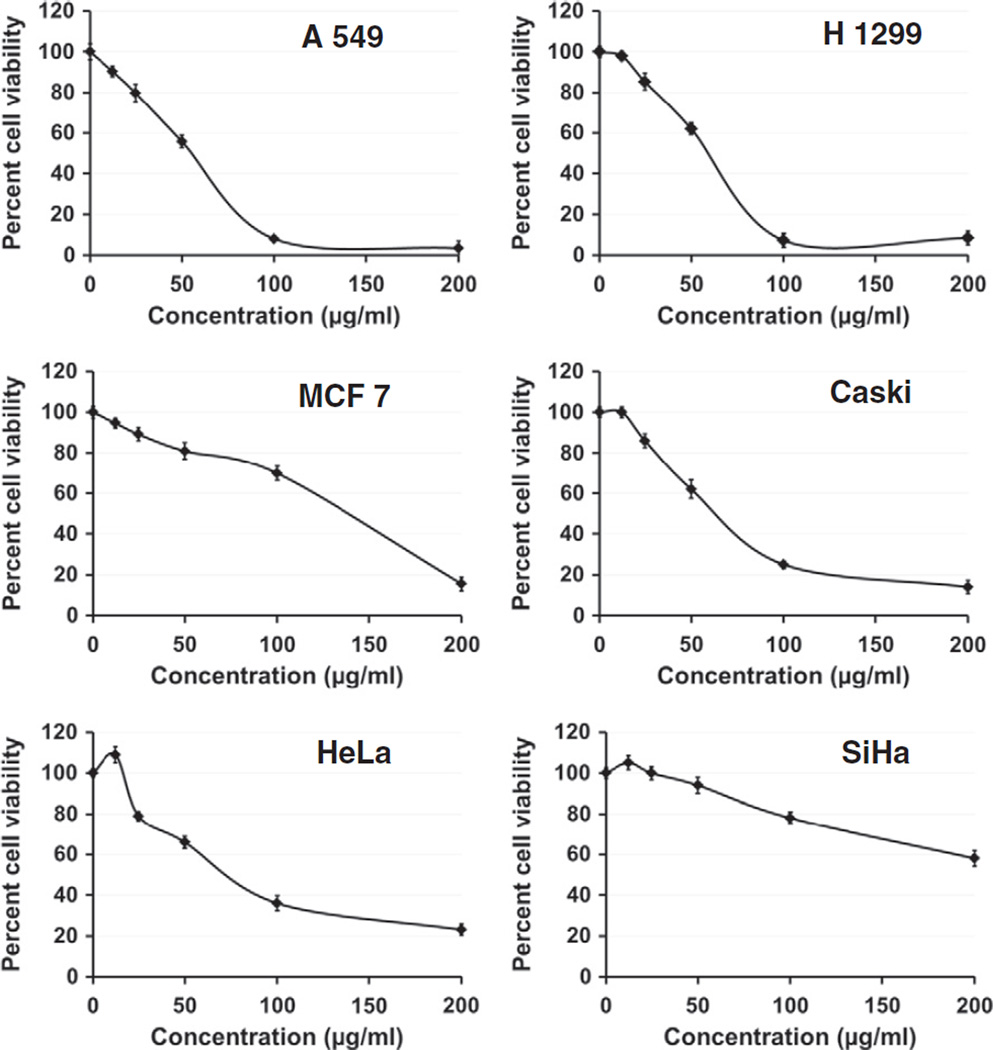
The anti-proliferative activity of PC was evaluated against a panel of cancer cell lines, i.e., lung cancer (A 549 and H 1299), breast cancer (MCF 7) and cervical cancer (CaSki, Hela and SiHa) cells (Fig. 6). PC demonstrated inhibitory effects against all the tested cell lines in a dose-dependent manner. The lung cancer cell lines were most sensitive to PC, whereas the cervical cancer cell line, SiHa was most resistant. The inhibitory concentrations (IC50) of PC against the various cell lines were in the following ascending order: A549 (50.1 µg/ml), H1299 (55.8 µg/ml), Caski (67 µg/ml), HeLa (77 µg/ml), MCF7 (125.6 µg/ml) and SiHa (>200 µg/ml) (Fig. 6). The results of MTT assay for several chemopreventive agents tested in our lab including PC were confirmed by CyQuant cell proliferation assay (Invitrogen, Carlsbad, CA), which is based on the measurement of cell number using DNA content and provides an accurate indication of relative cell number (not shown).
Fig. 6.
Anti-proliferative activity of PC against indicated human cancer cells by MTT assay. Cells were treated with PC at 12.5–200 εg/ml concentrations for 72 h. Data are expressed as percentage of untreated cells, mean ± SD (n=4).
4. Discussion
Polyphenols have been reported to have many beneficial health effects. Pomegranate is an excellent source of polyphenolics especially ETs, and the most abundant polyphenols in the fruit husk of pomegranate are hydrolyzable tannins called PC. We have isolated PC by simple column chromatography using Amberlite XAD-16 as shown previously by Seeram, Lee, Hardy, and Heber (2005). The enrichment of total pomegranate tannins has been shown previously (Seeram, Lee, Hardy, & Heber, 2005), but isolation of purified PC by means of simple gradient elution is presented here for the first time. Multiple peaks at 280 and 370 nm were observed representing various phenolics including PC, punicalin, and EA. Identification of all the individual constituents was not possible by HPLC-UV due to the lack of authentic commercial standards. However, FTICR-MS analysis identified more than 12 compounds in the enriched extract, consistent with the literature (Seeram, Zhang, Reed, Krueger, & Vaya, 2006).
PC isolated in this study was essentially free of EA. Seeram, Lee, Hardy, and Heber (2005) demonstrated the enrichment of total pomegranate tannins that contain 85% PC. Our results show the isolation of PC in its purified form using essentially the same Amberlite, XAD-16 consistent with the published literature (Seeram, Lee, Hardy, & Heber, 2005). We also provided identification of some minor contaminant phenolics and confirmed purity of PC by the FTICR-MS analysis. MS analysis of the isolated PC further indicated its purity. The presence of trace amounts of punicalin and gallagic acid in the spectra might be the product of PC degradation due to the high energy used in the analysis. This is consistent with our unpublished work and other published reports (Seeram, Lee, Hardy, & Heber, 2005) where MS–MS analysis of PC resulted in partial conversion into punicalin (M−H m/z 781), gallagic acid (M−H m/z 601) and EA (M−H m/z 301).
PC exhibits potent antioxidant (Lin et al., 2001) and anti-inflammatory properties (Seeram, Adams, et al., 2005). PC has been shown to work as potential antioxidant via different mechanisms, including direct scavenging of free radicals, reactive nitrogen species, and ROS, including hydroxyl radicals, peroxyl radicals and NO2 radicals (Kulkarni et al., 2007; Lin et al., 2001; Seeram, Adams, et al., 2005). We previously demonstrated by co-chromatography that the profile of polar oxidative adducts generated in vitro using either 4-OHE2/CuCl2 or H2O2/CuCl2 was similar (Spencer et al., 2012), indicating that adducts in 4-OHE2 and CuCl2 reaction originated from oxidative mechanisms. The potential of PC to inhibit 4-OHE2/Cu(II)-induced oxidative DNA adducts was evaluated for its efficacy in this model system. Although PC does not interact with DNA directly, its hydrolyzed product EA has been shown to protect DNA from oxidative damage by covalent binding (Thulstrup, Thormann, Spanget-Larsen, & Bisgaard, 1999). The other protective mechanisms include inhibition of ROS production, and chelation of metal ions such as copper and iron (Kulkarni et al., 2007). Results from this study correlate well with previous studies from this laboratory in which aqueous and organic extracts of berries and EA significantly reduced unidentified polar adduct burden induced by 4-OHE2/CuCl2 (Aiyer, Vadhanam, et al., 2008).
Antioxidants have attracted much interest for their protective effect against free radical damage associated with many diseases including cancer. The ORAC determination is regarded as a direct means of measuring the ability to trap free radicals. ORAC measures the capacity of antioxidants to scavenge peroxyl radicals which occur during oxidation of lipids in oxidative stress. This method was claimed to have high specificity with an excellent response to a wide range of antioxidants (Cao & Prior, 1998). Our results revealed that PC had a higher antioxidant capacity relative to EA, and was much higher than trolox, used as a reference compound. Trolox is a water-soluble vitamin E analogue, therefore the ORAC value of trolox is equivalent to α-tocopherol. The relative ORAC values for PC and EA in our study are consistent with values reported previously (Pfundstein et al., 2010).
Assays based on the use of DPPH and ABTS radicals are also among the most popular spectrophotometric methods for determination of the antioxidant capacity of compounds, foods, beverages and vegetable extracts (Gulcin, 2009). PC showed potent DPPH and ABTS radical scavenging activity consistent with the published literature (Kulkarni, Aradhya, & Divakar, 2004; Kulkarni et al., 2007). ABTS•+ radicals are more reactive than DPPH radicals and unlike the reactions with DPPH radical, which involve H-atom transfer, the reactions with ABTS•+ radicals involve an electron-transfer process (Ak & Gulcin, 2008). It is well-known that phenolic groups stabilize radicals formed on phenolic carbons with their resonance structure. PC has many phenolic rings and, an abstraction of a hydrogen atom from a phenolic hydroxyl group may occur easily (Gulcin, 2010). Previous studies have reported a high antioxidant potential of total pomegranate tannins that contain almost 85% PC (Seeram, Adams, et al., 2005). This study coupled with some other studies (Aqil, Vadhanam, & Gupta, 2012) on PC, EA and on pomegranate husk extracts support that PC is protective against the free radicals generated by oxidative stress.
Hydrogen peroxide is an oxidant that is being formed continuously in living tissues as a result of several metabolic processes, but its detoxification is very crucial in preventing it from reacting in deleterious Fenton-type reactions, which generate extremely reactive oxygen species, including hydroxyl free radical (Graf, Mahoney, Bryant, & Eaton, 1984; Henle & Linn, 1997). In this study, EA showed somewhat higher free radical scavenging compared with PC, ascorbic acid and BHA indicating that lipophilicity of the molecule does not correlate with its activity, but the order of efficacy appeared to be dictated by structural properties of the compound. EA has two catechol (ortho-dihydroxyl) groups while PC has additional four pyrogallol (vicinal trihydroxyl) groups contributing 16 dissociable hydroxyl groups. These structural features are likely to induce a significant radical scavenging capacity of these compounds (Bors, Heller, Michel, & Saran, 1990; Rice-Evans, Miller, & Paganga, 1996).
Iron can stimulate lipid peroxidation by the Fenton reaction (H2O2+Fe2+=Fe3++OH−+OH•) and can also accelerate lipid peroxidation by decomposing lipid hydroperoxides into peroxyl and alkoxyl radicals that can perpetuate the chain reaction (Halliwell, 1991). Metal chelating capacity is significant since it reduces the concentration of the transition metal that catalyzes lipid peroxidation (Duh, Tu, & Yen, 1999). Metal ion chelation is an important antioxidant property and few compounds (e.g. resveratrol) have an ability to compete with ferrozine for ferrous ion in the solution (Gulcin, 2010). PC showed very strong metal chelating activity (70% at 50 µg/ml), almost twice higher than EA, BHA and ascorbic acid at the same concentration. These differences in activity can be attributed to phenolic −OH groups, which have been shown to be capable of chelating ferrous ion (Kulkarni et al., 2007). PC with 16 dissociable −OH groups, from gallagyl and ellagyl units, had maximum ion-chelating activity as expected.
The high antiproliferative activity of PC against different cancer cell lines was demonstrated in this study. The lung cancer cell lines A549 and H1299 were most sensitive followed by cervical (CaSki and HeLa) and breast cancer (MCF7) cell lines. However, another cervical cancer cell line SiHa was less sensitive to PC. The anti-proliferative activity of PC and EA against oral, colon and prostate cancer cell lines have been previously demonstrated (Seeram, Adams, et al., 2005). In another study, both PC and EA were found to induce apoptosis via mitochondrial pathway in colon cancer Caco-2 cells but not in normal colon cells. The mechanism of action included down-regulation of cyclins A and B1 and upregulation of cyclin E, cell-cycle arrest in S phase, induction of apoptosis via an intrinsic pathway (FAS-independent, caspase 8-independent) through Bcl-XL down-regulation with mitochondrial release of cytochrome c into the cytosol, and activation of initiator caspase 9 and effector caspase 3 (Larrosa, Tomas-Barberan, & Espin, 2006). Thus, our data and other literature reports (Seeram, Adams, et al., 2005) clearly demonstrate the anti-proliferative potential of PC and EA and therefore suggest its health benefits against various diseases in prophylactic mode.
PC is a major constituent of pomegranate-based food supplements available over the counter in the U.S. These supplements have shown strong potential as a powerful antioxidant. In addition, we also reported its major bioactive metabolite released in vivo, ellagic acid, demonstrated antioxidant activity which is on par with, if not better than punicalagin and has also shown protective effects in various in vitro and in vivo studies (Aiyer, Srinivasan, & Gupta, 2008; Aqil, Vadhanam, & Gupta, 2012; Seeram, Adams, et al., 2005).
5. Conclusion
Punica granatum husk powder has very high ellagitannin content. XAD-16 resins provide an effective means to isolate the PC in bulk quantities. This chromatographic method is cost effective, reproducible and can be easily adopted for analytical as well as industrial applications. PC was found to be very effective in inhibiting oxidative DNA adducts generated by oxidation of 4-hydroxy estradiol catalyzed by Cu (II). PC and EA were found to be more potent antioxidants compared with the classical antioxidants, ascorbic acid, butylated hydroxyanisole and trolox. The antiproliferative activity of PC against various human cancer cell types further supports its evaluation for the cancer chemoprevention studies in vivo.
Acknowledgements
This work was supported by the Agnes Brown Duggan Endowment and in part by USPHS CA-118114. Dr. Ramesh Gupta holds the Agnes Brown Duggan Chair in Oncological Research. Ajay Patel of Verdure Sciences is gratefully acknowledged for generously providing enriched pomegranate husk powder and Dr. Richard M. Higashi, University of Louisville for helping in FTICR-MS analysis.
Abbreviations
- DPPH
2,2-diphenyl-1-picrylhydrazyl
- ABTS
2,2′-azino-bis (3-ethylbenzthiazoline-6-sulphonic acid)
- ROS
reactive oxygen species
- ORAC
oxygen radical absorbance capacity
- Rt
retention time
- AuC
area under curve
References
- 1.Adams LS, Seeram NP, Aggarwal BB, Takada Y, Sand D, Heber D. Pomegranate juice, total pomegranate ellagitannins, and punicalagin suppress inflammatory cell signaling in colon cancer cells. Journal of Agricultural and Food Chemistry. 2006;54(3):980–985. doi: 10.1021/jf052005r. [DOI] [PubMed] [Google Scholar]
- 2.Aiyer HS, Srinivasan C, Gupta RC. Dietary berries and ellagic acid diminish estrogen-mediated mammary tumorigenesis in ACI rats. Nutrition and Cancer. 2008;60(2):227–234. doi: 10.1080/01635580701624712. [DOI] [PubMed] [Google Scholar]
- 3.Aiyer HS, Vadhanam MV, Stoyanova R, Caprio GD, Clapper ML, Gupta RC. Dietary berries and ellagic acid prevent oxidative DNA damage and modulate expression of DNA repair genes. International Journal of Molecular Sciences. 2008;9(3):327–341. doi: 10.3390/ijms9030327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ak T, Gulcin I. Antioxidant and radical scavenging properties of curcumin. Chemico-Biological Interactions. 2008;174(1):27–37. doi: 10.1016/j.cbi.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Aqil F, Gupta A, Munagala R, Jeyabalan J, Kausar H, Sharma RJ, et al. Antioxidant and antiproliferative activities of anthocyanin/ellagitannin-enriched extracts from Syzygium cumini L. (Jamun, the Indian blackberry) Nutrition and Cancer. 2012;64(3):428–438. doi: 10.1080/01635581.2012.657766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aqil F, Vadhanam MV, Gupta RC. Enhanced activity of punicalagin delivered via polymeric implants against benzo[a]pyrene-induced DNA adducts. Mutation Research. 2012;743(1–2):59–66. doi: 10.1016/j.mrgentox.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bors W, Heller W, Michel C, Saran M. Flavonoids as antioxidants: Determination of radical-scavenging efficiencies. Methods in Enzymology. 1990;186:343–355. doi: 10.1016/0076-6879(90)86128-i. [DOI] [PubMed] [Google Scholar]
- 8.Cao G, Prior RL. Comparison of different analytical methods for assessing total antioxidant capacity of human serum. Clinical Chemistry. 1998;44(6 Pt 1):1309–1315. [PubMed] [Google Scholar]
- 9.Chen PS, Li JH, Liu TY, Lin TC. Folk medicine Terminalia catappa and its major tannin component, punicalagin, are effective against bleomycin-induced genotoxicity in Chinese hamster ovary cells. Cancer Letters. 2000;152(2):115–122. doi: 10.1016/s0304-3835(99)00395-x. [DOI] [PubMed] [Google Scholar]
- 10.Duh PD, Tu YY, Yen GC. Antioxidant activity of water extract of Harng Jyur (Chrysanthemum morifolium Ramat) Food Science and Technology-Lebensmittel-Wissenschaft & Technologie. 1999;32(5):269–277. [Google Scholar]
- 11.Graf E, Mahoney JR, Bryant RG, Eaton JW. Iron-catalyzed hydroxyl radical formation. Stringent requirement for free iron coordination site. The Journal of Biological Chemistry. 1984;259(6):3620–3624. [PubMed] [Google Scholar]
- 12.Gulcin I. Antioxidant activity of L-adrenaline: A structure–activity insight. Chemico-Biological Interactions. 2009;179(2–3):71–80. doi: 10.1016/j.cbi.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 13.Gulcin I. Antioxidant properties of resveratrol: A structure–activity insight. Innovative Food Science & Emerging Technologies. 2010;11(1):210–218. [Google Scholar]
- 14.Gupta RC. Enhanced sensitivity of 32P-postlabeling analysis of aromatic carcinogen: DNA adducts. Cancer Research. 1985;45(11 Pt 2):5656–5662. [PubMed] [Google Scholar]
- 15.Gupta RC. 32P-postlabeling for detection of DNA adducts. In: Pfeifer P, editor. Technologies for detection of DNA damage and mutations. New York: Plenum Press; 1996. pp. 45–61. [Google Scholar]
- 16.Gyamfi MA, Yonamine M, Aniya Y. Free-radical scavenging action of medicinal herbs from Ghana: Thonningia sanguinea on experimentally-induced liver injuries. General Pharmacology. 1999;32(6):661–667. doi: 10.1016/s0306-3623(98)00238-9. [DOI] [PubMed] [Google Scholar]
- 17.Halliwell B. Reactive oxygen species in living systems: Source, biochemistry, and role in human disease. The American Journal of Medicine. 1991;91(3C):14S–22S. doi: 10.1016/0002-9343(91)90279-7. [DOI] [PubMed] [Google Scholar]
- 18.Henle ES, Linn S. Formation, prevention, and repair of DNA damage by iron/hydrogen peroxide. The Journal of Biological Chemistry. 1997;272(31):19095–19098. doi: 10.1074/jbc.272.31.19095. [DOI] [PubMed] [Google Scholar]
- 19.Kulkarni AP, Aradhya SM, Divakar S. Isolation and identification of a radical scavenging antioxidant — Punicalagin from pith and carpellary membrane of pomegranate fruit. Food Chemistry. 2004;87(4):551–557. [Google Scholar]
- 20.Kulkarni AP, Mahal HS, Kapoor S, Aradhya SM. In vitro studies on the binding, antioxidant, and cytotoxic actions of punicalagin. Journal of Agricultural and Food Chemistry. 2007;55(4):1491–1500. doi: 10.1021/jf0626720. [DOI] [PubMed] [Google Scholar]
- 21.Larrosa M, Tomas-Barberan FA, Espin JC. The dietary hydrolysable tannin punicalagin releases ellagic acid that induces apoptosis in human colon adenocarcinoma Caco-2 cells by using the mitochondrial pathway. The Journal of Nutritional Biochemistry. 2006;17(9):611–625. doi: 10.1016/j.jnutbio.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Lee CJ, Chen LG, Liang WL, Wang CC. Anti-inflammatory effects of Punica granatum Linne in vitro and in vivo. Food Chemistry. 2010;118(2):315–322. [Google Scholar]
- 23.Lin CC, Hsu YF, Lin TC. Effects of punicalagin and punicalin on carrageenan-induced inflammation in rats. The American Journal of Chinese Medicine. 1999;27(3–4):371–376. doi: 10.1142/S0192415X99000422. [DOI] [PubMed] [Google Scholar]
- 24.Lin CC, Hsu YF, Lin TC, Hsu HY. Antioxidant and hepatoprotective effects of punicalagin and punicalin on acetaminophen-induced liver damage in rats. Phytotherapy Research. 2001;15(3):206–212. doi: 10.1002/ptr.816. [DOI] [PubMed] [Google Scholar]
- 25.Lu J, Ding K, Yuan Q. One-step purification of punicalagin by preparative HPLC and stability study on punicalagin. Separation Science and Technology. 2001;46(1):147. [Google Scholar]
- 26.Lu J, Wei Y, Yuan Q. Preparative separation of punicalagin from pomegranate husk by high-speed countercurrent chromatography. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences. 2007;857(1):175–179. doi: 10.1016/j.jchromb.2007.06.038. [DOI] [PubMed] [Google Scholar]
- 27.Munagala R, Kausar H, Munjal C, Gupta RC. Withaferin A induces p53-dependent apoptosis by repression of HPV oncogenes and upregulation of tumor suppressor proteins in human cervical cancer cells. Carcinogenesis. 2011;32(11):1697–1705. doi: 10.1093/carcin/bgr192. [DOI] [PubMed] [Google Scholar]
- 28.Ou B, Hampsch-Woodill M, Prior RL. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. Journal of Agricultural and Food Chemistry. 2001;49(10):4619–4626. doi: 10.1021/jf010586o. [DOI] [PubMed] [Google Scholar]
- 29.Oyaizu M. Studies on product of browning reaction prepared from glucose amine. Japanese Journal of Nutrition. 1986;44:307–315. [Google Scholar]
- 30.Pantuck AJ, Leppert JT, Zomorodian N, Aronson W, Hong J, Barnard RJ, et al. Phase II study of pomegranate juice for men with rising prostate-specific antigen following surgery or radiation for prostate cancer. Clinical Cancer Research. 2006;12(13):4018–4026. doi: 10.1158/1078-0432.CCR-05-2290. [DOI] [PubMed] [Google Scholar]
- 31.Pfundstein B, El Desouky SK, Hull WE, Haubner R, Erben G, Owen RW. Polyphenolic compounds in the fruits of Egyptian medicinal plants (Terminalia bellerica, Terminalia chebula and Terminalia horrida): Characterization, quantitation and determination of antioxidant capacities. Phytochemistry. 2010;71(10):1132–1148. doi: 10.1016/j.phytochem.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 32.Plumb JA. Cell sensitivity assays: The MTT assay. Methods in Molecular Medicine. 2004;88:165–169. doi: 10.1385/1-59259-406-9:165. [DOI] [PubMed] [Google Scholar]
- 33.Ravoori S, Vadhanam MV, Davey DD, Srinivasan C, Nagarajan B, Gupta RC. Modulation of novel DNA adducts during human uterine cervix cancer progression. International Journal of Oncology. 2006;29(6):1437–1443. [PubMed] [Google Scholar]
- 34.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology & Medicine. 1999;26(9–10):1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 35.Rice-Evans CA, Miller NJ, Paganga G. Structure–antioxidant activity relationships of flavonoids and phenolic acids. Free Radical Biology & Medicine. 1996;20(7):933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 36.Ruch RJ, Cheng SJ, Klaunig JE. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis. 1989;10(6):1003–1008. doi: 10.1093/carcin/10.6.1003. [DOI] [PubMed] [Google Scholar]
- 37.Seeram NP, Adams LS, Henning SM, Niu Y, Zhang Y, Nair MG, et al. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. The Journal of Nutritional Biochemistry. 2005;16(6):360–367. doi: 10.1016/j.jnutbio.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 38.Seeram NP, Henning SM, Zhang Y, Suchard M, Li Z, Heber D. Pomegranate juice ellagitannin metabolites are present in human plasma and some persist in urine for up to 48 hours. The Journal of Nutrition. 2006;136(10):2481–2485. doi: 10.1093/jn/136.10.2481. [DOI] [PubMed] [Google Scholar]
- 39.Seeram N, Lee R, Hardy M, Heber D. Rapid large scale purification of ellagitannins from pomegranate husk, a by-product of the commercial juice industry. Separation and Purification Technology. 2005;41(1):49–55. [Google Scholar]
- 40.Seeram NP, Zhang Y, Reed JD, Krueger CG, Vaya J. Pomegranate phytochemicals. In: Seeram NP, Schulman RN, Heber D, editors. Pomegranates: Ancient roots to modern medicine. New York: CRC Press; 2006. [Google Scholar]
- 41.Spencer WA, Vadhanam MV, Jeyabalan J, Gupta RC. Oxidative DNA damage following microsome/Cu(II)-mediated activation of the estrogens, 17β-estradiol, equilenin, and equilin: Role of reactive oxygen species. Chemical Research in Toxicology. 2012;25:305–314. doi: 10.1021/tx200356v. [DOI] [PubMed] [Google Scholar]
- 42.Thulstrup PW, Thormann T, Spanget-Larsen J, Bisgaard HC. Interaction between ellagic acid and calf thymus DNA studied with flow linear dichroism UV–VIS spectroscopy. Biochemical and Biophysical Research Communications. 1999;265(2):416–421. doi: 10.1006/bbrc.1999.1694. [DOI] [PubMed] [Google Scholar]