Abstract
A new species of cyst nematode, Globodera ellingtonae, is described from soil collected from a field in Oregon. Second-stage juveniles (J2) of the species are characterized by body length of 365-515 μm, stylet length of 19-22.5 μm, basal knobs rounded posteriorly and pointed anteriorly, tail 39-55 μm, hyaline tail terminus 20-32.5 μm, and tail tapering uniformly but abruptly narrowing and constricted near the posterior third of the hyaline portion, ending with a peg-like, finely rounded to pointed terminus. Cysts are spherical to sub-spherical, dark to light brown and circumfenestrate and cyst wall pattern is ridge-like with heavy punctations. Males have a stylet length of 21-25 μm and spicule length of 30-37 μm with a pointed thorn-like tip. Females have a stylet length of 20-22.5 μm, one head annule and labial disc, heavy punctations on the cuticle, and short vulval slit 7.5-8 μm long. Morphologically this new, round-cyst species differs from the related species G. pallida, G. rostochiensis, G. tabacum complex and G. mexicana by its distinctive J2 tail, and by one or another of the following: shorter mean stylet length in J2, females and males; number of refractive bodies in the hyaline tail terminus of J2; cyst morphology including Granek’s ratio; number of cuticular ridges between the anus and vulva; and in the shape and length of spicules in males. Its relationship to these closely related species are discussed. Based upon analysis of ribosomal internal transcribed spacer (ITS) sequences, G. ellingtonae n. sp. is distinct from G. pallida, G. rostochiensis, G. tabacum and G. mexicana. Bayesian and Maximum Parsimony analysis of cloned ITS rRNA gene sequences indicated three clades, with intraspecific variability as high as 2.8%. In silico analysis revealed ITS restriction fragment length polymorphisms for enzymes Bsh 1236I, Hinf I, and Rsa I that overlap patterns for other Globodera species.
Keywords: Globodera ellingtonae, detection, diagnosis, morphology, new species, potato, RFLP, Solanum tuberosum, taxonomy
The taxonomy of Globodera has been advanced by numerous papers (Golden and Ellington, 1972; Miller and Gray, 1972; Mulvey, 1973; Stone, 1973; Miller, 1983; Mulvey and Golden, 1983; Stone, 1983; Sturhan, 1983; Wouts, 1984; Golden, 1986; Baldwin and Mundo-Ocampo, 1991; Mota and Eisenback, 1993a, 1993b, 1993c; Siddiqi, 2000; den Nijs and Karssen, 2004), and molecular diagnostic and/or phylogenetic studies (Baldwin and Schouest, 1990; Ferris et al., 1995; Szalanski et al., 1997; Thomas et al., 1997; Fleming and Powers, 1998; Wouts and Baldwin, 1998; Subbotin et al., 2000; Manduric et al., 2004; Madani et al., 2005; Skantar et al., 2007; Quader et al., 2008; Subbotin et al., 2010a, 2010b; Skantar et al., 2011). The most important members of the genus are the potato cyst nematodes (PCN, herein meaning Globodera species pathogenic on potato), which represent a significant threat to potato worldwide and to the U.S. potato industry, which produces annual crops valued at nearly $3.45 billion (Agricultural Statistics Board, 2011). In the United States, the first PCN species detected was the golden nematode, Globodera rostochiensis (Wollenweber, 1923) Skarbilovich, 1959, which was identified in New York in 1932 and has remained confined there as a result of regulatory and management practices (Brodie, 1998). More recently, the pale potato cyst nematode, G. pallida (Stone, 1973) Behrens, 1975, was discovered in Idaho (Hafez et al., 2007). Since then, an extensive survey of the U.S. potato growing acreage has been conducted jointly by the various state Departments of Agriculture and the USDA Animal and Plant Health Inspection Service to determine the extent of PCN distribution. Nematodes suspected of being a PCN are analyzed by morphological and molecular means at the USDA Nematology Laboratory in Beltsville, MD.
In 2008, three nematode samples distinct from the known PCN species were received at Beltsville. These atypical cyst nematodes had been isolated from an Oregon field in which potato and other crops had been grown, and from two agricultural fields within Idaho. In preliminary reports, this unnamed nematode was referred to as a new Globodera species (Fraley et al., 2009) or as atypical Globodera populations from Oregon and Idaho (Skantar et al., 2011). Due to the limited quantity of sample material then available, a full description of all major developmental stages was not possible at that time. Additional field sampling at the Oregon location and subsequent propagation of the nematode have permitted extensive morphometric characterization of second-stage juveniles (J2), females, males, and cysts and additional molecular characterization. These observations indicate that the Oregon isolate represents a new species, described here as Globodera ellingtonae. Morphological characteristics that define the species are described, as are features that overlap with other Globodera species. Intraspecific variation present within the ribosomal internal transcribed spacer (ITS) rRNA region is also described.
Materials and Methods
Cysts were collected during PCN surveys in May 2008 from a Powell Butte, Oregon, field with a cropping history of potato and other crops, and in August and September 2008 from two agricultural fields (#167, 347) in Idaho with unknown cropping histories. The Idaho isolate #167 cysts came from a field in Caribou County and the Idaho isolate #347 cysts were from a field in Teton County. Additional specimens used to prepare the description were obtained from greenhouse cultures on Solanum tuberosum established by Inga Zasada and Russell Ingham (USDA-ARS and Oregon State University, respectively, Corvallis, OR) from nematodes they collected at the Powell Butte site in 2009. Juveniles were hatched from cysts that had been sieved from fresh soil and kept in water in Syracuse watch glasses in the laboratory. Males, females and J2 were fixed in either warm (40°C) or hot (80°C) 4% formalin, processed to glycerin with a rapid method (Seinhorst, 1959), and mounted in anhydrous glycerin on microscope slides (Hooper, 1970; Golden, 1990). Cysts were removed from soil samples by sieving, fixed for 12 hr in 3% formaldehyde and similarly processed to glycerin. Photomicrographs of cyst vulval cones, females, males and J2 were taken with a Q-Imaging Retiga EXi Color Digital Camera (Q-Imaging, Austin, TX) attached to a Leica Wild MPS48 Leitz DMRB compound microscope (Leica Microsystems, Wetzlar, Germany), and measurements were made with an ocular micrometer on the same microscope. For head and tail variations, photomicrographs of live specimens were taken using an ocular micrometer on a Zeiss Ultraphot II with DIC (differential interference contrast) (Carl Zeiss, Jena, Germany) and a Q-I Micropublisher 5 color digital camera (Q-Imaging Inc., Austin, TX). Black and white tail profiles were created in Adobe Photoshop CS (Adobe Systems Inc., San Jose, CA, USA). Filter effects were applied to the images (Sketch → Photocopy: with low detail and high darkness levels) to create a sketch−like appearance. Brightness/contrast levels were modified to produce the background for the images, and brush tools were used to more clearly reveal stylets and lips. All original images are on file for comparison with the altered images.
Preparation of genomic DNA from juveniles, amplification of the ITS rRNA gene region, purification of amplicons, and cloning were carried out as described previously (Skantar et al., 2011). Amplification of the 28S rRNA region was as described in DeLey et al. (2005) using primers D2A and D3B (Ye et al., 2007). Sequencing was performed at the University of Maryland Center for Biosystems Research. Fourteen clones were sequenced from juveniles obtained in the initial sampling from Powell Butte (new GenBank accession numbers JF739926-JF39934 and previous sequences GQ896542, GQ896544, and GQ896545). Twenty-seven additional clones were sequenced from five morphologically variable juveniles from a single cyst (JF39899-JF39925). A total of 16 clones were sequenced from laboratory cultures of the Oregon population of G. ellingtonae n. sp. (JF739872-JF739887). Sequence obtained previously representing four juveniles from the single available cyst of Idaho sample #167 was GQ896546. Eleven clones were sequenced from Idaho sample #347 (new, JF739888-JF739898; previous, GQ896547). New 28S rDNA D2-D3 expansion segment sequences include accession numbers JN712217-JN712224.
DNA sequences were assembled with Sequencher 5.0 (Genecodes, Ann Arbor, MI). The program MUSCLE (Edgar, 2004) was used for DNA sequence alignments, with minor corrections made in GeneDoc (Nicholas et al., 1997). Globodera rostochiensis sequence FJ212167 was specified as the outgroup. JModelTest (Guindon and Gascuel, 2003; Posada, 2008; Posada and Crandall, 2001) was used to determine the model of DNA evolution that best fit the data. The Akaike Information Criterion (AIC) values from JModelTest were used to run the Bayesian Interference (BI) analysis with the program MrBayes 3.1.2 (Huelsenbeck and Ronquist, 2001) under the model GTR + I + G, with a random seed, and four Metropolis-coupled Markov chain Monte Carlo (MCMC) for 1,000,000 generations with subsampling frequency 1,000. The log-likelihood values stabilized after approximately 5,000 generations; sample points obtained prior to convergence were discarded as burn-in. Convergence was evaluated through the potential scale reduction factor (PSRF) and further confirmed through the use of AWTY, a web-based interface for graphical visualization of MCMC diagnostics (Nylander et al., 2008). Tree topologies were used to generate a 50% majority rule consensus tree in PAUP* version 4.0b10 (Swofford, 2003). Maximum Parsimony (MP) procedures employed a heuristic search, random taxon addition, MaxTrees = 500, and tree bisection-reconnection (TBR) branch-swapping. Gaps were treated as missing data. Support for each node was estimated by bootstrap searches with 1000 replicates. BI and MP trees were drawn with FigTree 1.3.1 (Rambaut, 2009) and CorelDraw X4 (Corel, Mountain View, CA).
Results and Discussion
Globodera ellingtonae n. sp.
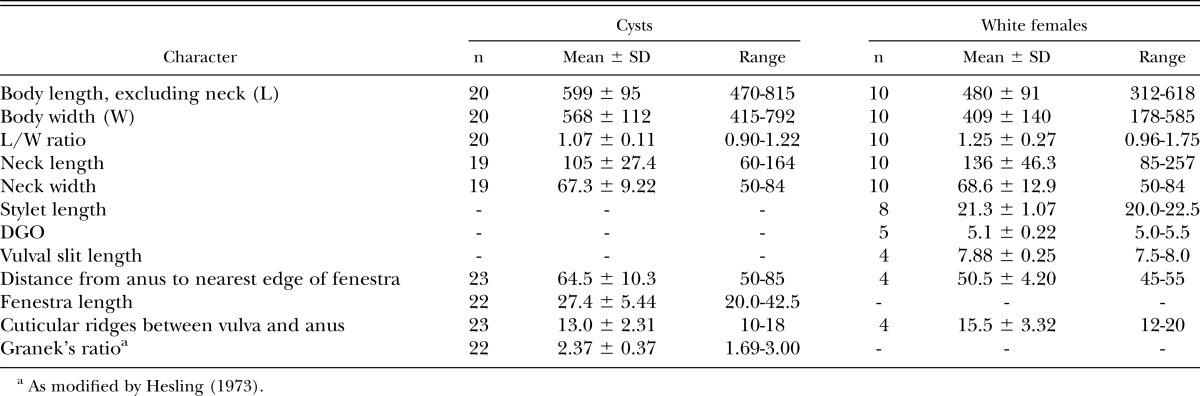
Table 1.
Morphometrics (in μm) of cysts and white females of Globodera ellingtonae from Oregon.
Oregon population: Holotype (female): Length (including neck) 545 μm; width 386 μm; L/W ratio 1.4; stylet 22.5 μm; dorsal esophageal gland orifice 5.0 μm from the stylet base; neck length 129 μm; neck width 75 μm.
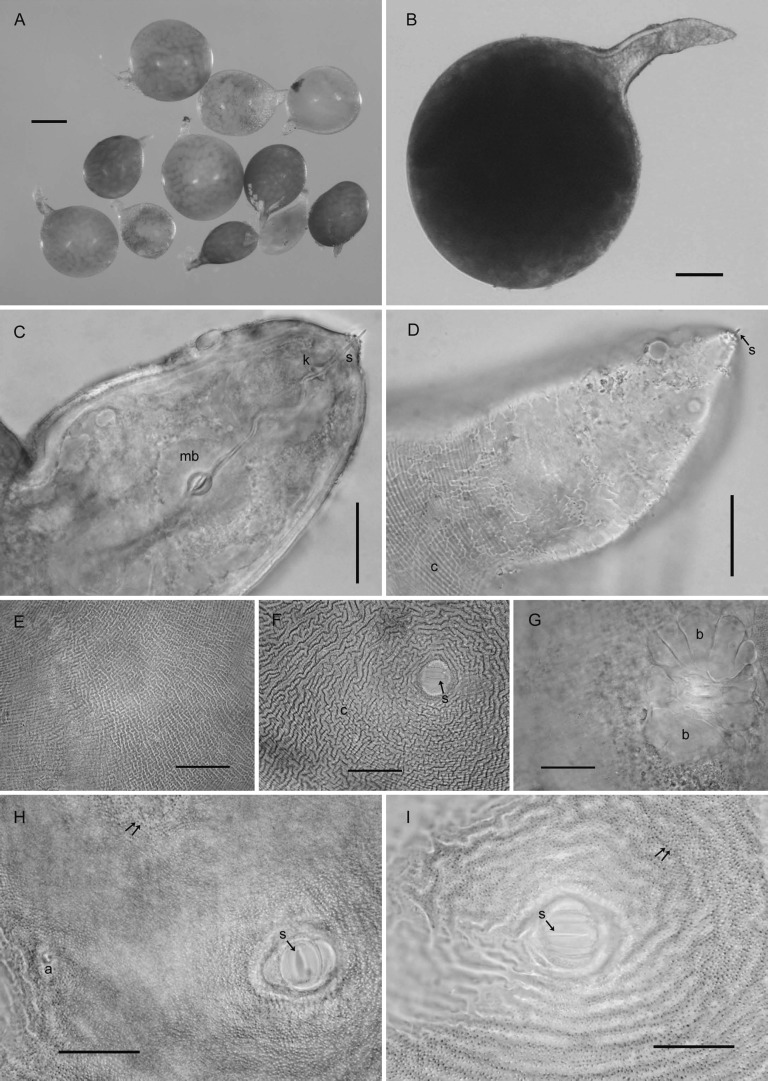
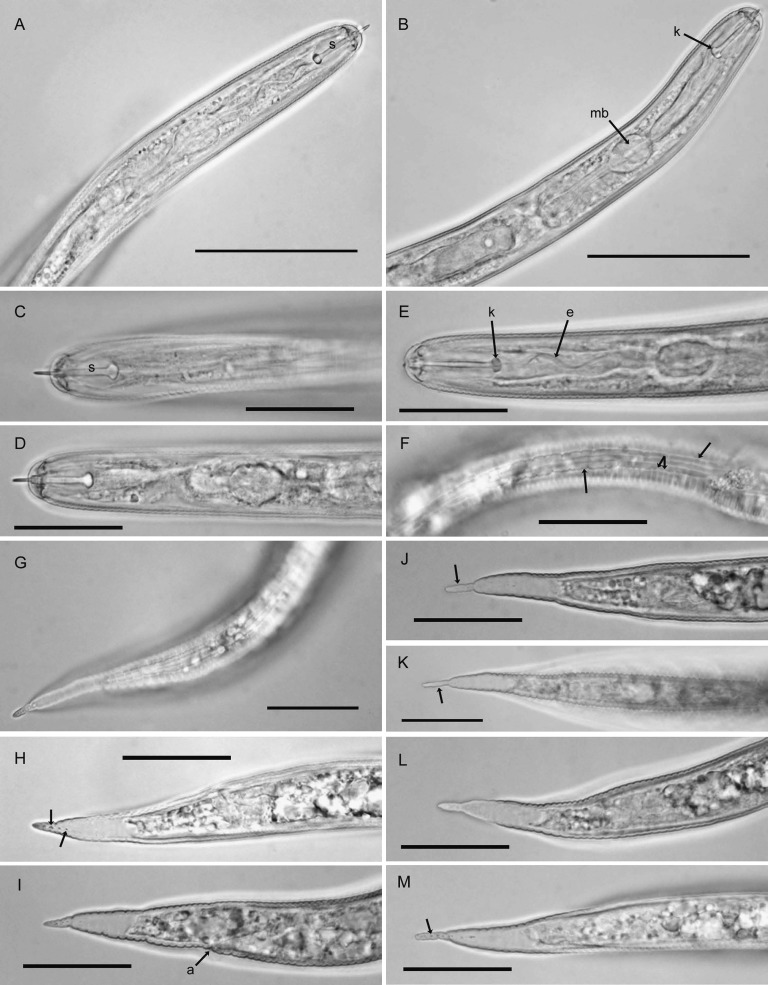
Females (n = 10): Body white becoming yellow to pale brown as eggs mature, ovate to rounded or subspherical in shape with elongate, protruding neck, rounded posteriorly (Fig. 1A-D); dorsal esophageal gland orifice ≥ 5 μm distance from base of stylet; outer cuticular layer marked by rugose pattern and the inner layer by heavy punctations (Fig. 1E,F,H,I); subcrystalline layer absent. Head slightly set off from neck, bearing one prominent annule and labial disc. Cephalic framework weakly developed. Stylet with well-developed, posteriorly inclined knobs (Fig. 1C,D). Median bulb large, nearly spherical with well-developed valve (Fig. 1C). Esophageal glands mostly obscured, contained in a single lobe. Excretory pore about two annules posterior to hemizonid and located at or near base of neck. Vulva slit-like, small, measuring 7-8 μm (Fig. 1F,H,I). Vulval bodies, highly variable in size, shape and generally clustered, usually discernible underneath the vulva (Fig. 1G). Anus prominent, often with clear circular area around it (Fig. 1H).
Fig. 1.
Photomicrographs of females of Globodera ellingtonae n. sp. from Oregon: A, B, Whole females. C, Anterior region with s = stylet, k = knobs, mb = median bulb. D, Anterior region with s = stylet, c = cuticular ridges on neck. E, Cuticular pattern at mid body. F-I, Anal-vulval regions with arrows in F, H, I showing vulval slit (s) and c = cuticular ridges in F, double arrows = punctations on body wall in H, I, a = anal area in H, and b = clustered vulval denticles/bullae-like structures beneath vulval area in G. (Scale bars: A = 250 μm; B = 100 μm; C-I = 25 μm).
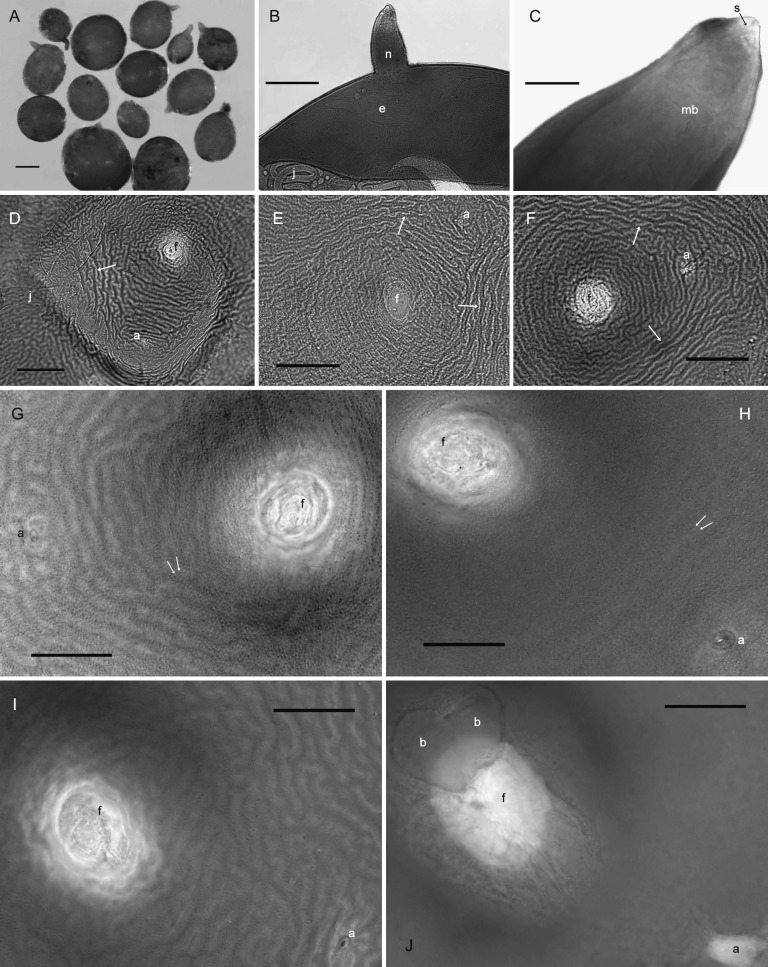
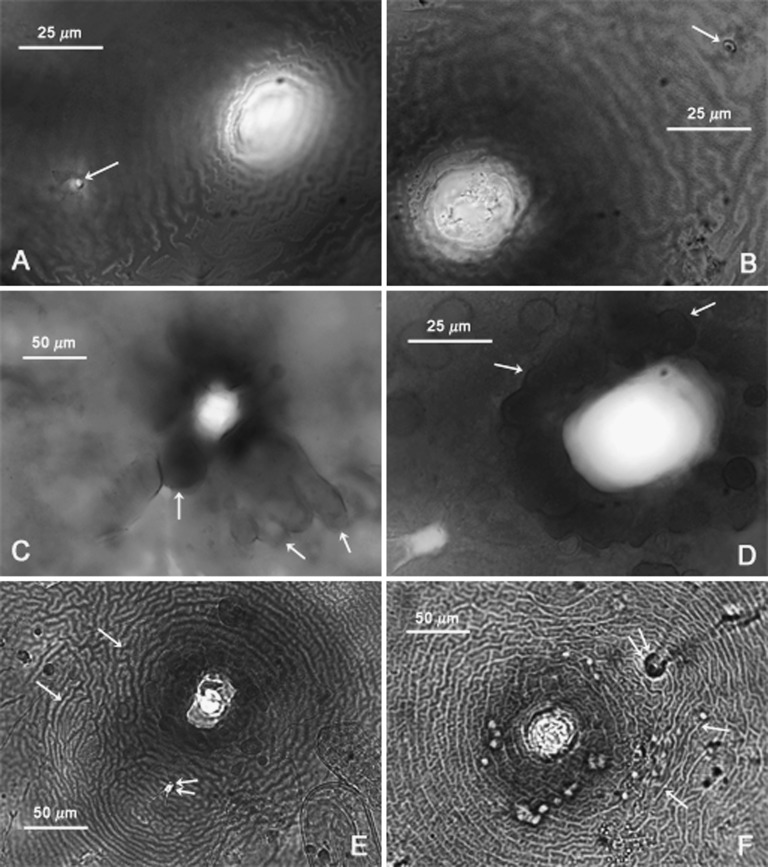
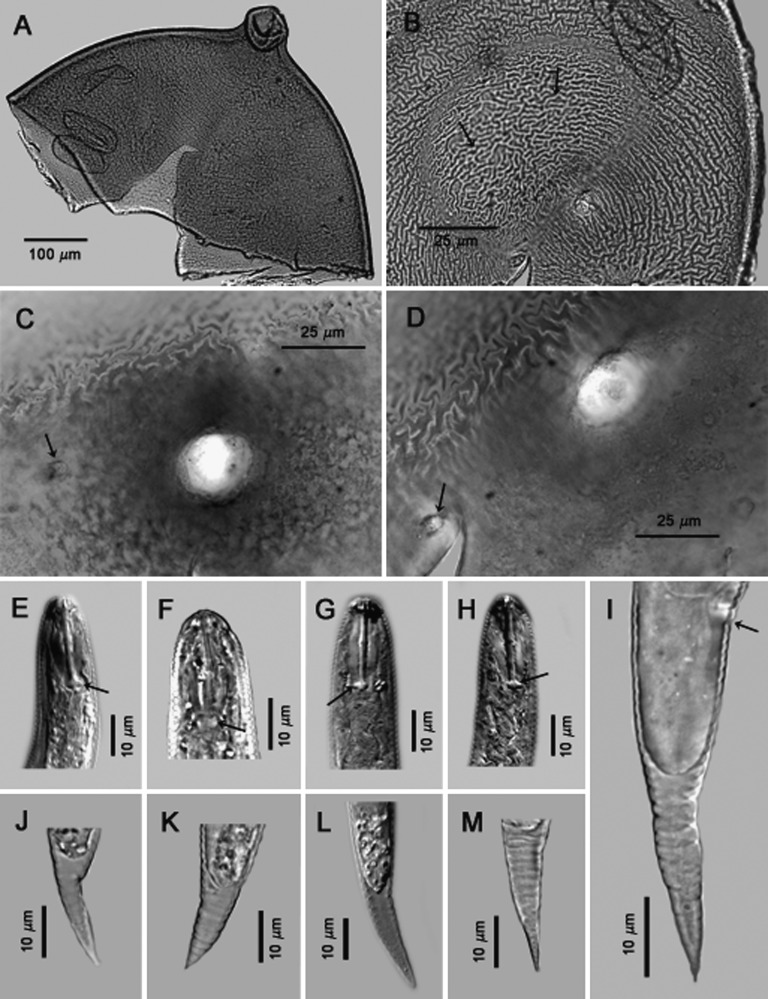
Cysts (n = 20): Light brown to brown in color, spherical to subspherical with a protruding neck (Fig. 2A); young cysts occasionally with clustered vulval bodies of variable size and shape similar to bullae (Figs. 3C-D; 2J). Anus prominent and visible in all specimens, often with clear circular surrounding region (Figs. 3A,B,D; 2G-J). Subcrystalline layer absent. Vulval region intact or fenestrated with a single, circumfenestrate opening occupying all or part of vulval basin. Vulval bridge, underbridge and true bullae absent. Cyst wall pattern ridge-like to irregular, wavy or whorled (Figs. 3A,B,E,F), with heavy punctations (Fig. 2D-I).
Fig. 2.
Photomicrographs of whole, anterior, and terminal areas of cysts of Globodera ellingtonae n. sp. from Oregon: A, whole cysts. B, C, anterior region showing e = egg, j = juvenile, and n = neck in B, and s = stylet and mb = median bulb in C. D-J, Anal-vulval regions with f = vulval fenestra and a = anal area in D-J, j = juvenile, arrow = ridges on cyst wall in D-F; double arrows in G, H = punctations on cyst wall, and b = vulval bodies/bullae-like structures in J. (Scale bars: A = 250 μm; B = 100 μm; C, G-J = 25 μm); D-F = 50 μm).
Fig. 3.
Photomicrographs of terminal areas of cysts of Globodera ellingtonae n. sp. from Oregon: A-F, Anal-vulval regions with single arrows in A-B, C-D, and E-F showing anal areas, clustered vulval bodies/bullae-like structures, and cyst wall patterns, respectively, and the double arrows in E-F showing anal areas. (Modified from Skantar et al., Phytopathology, 2011).
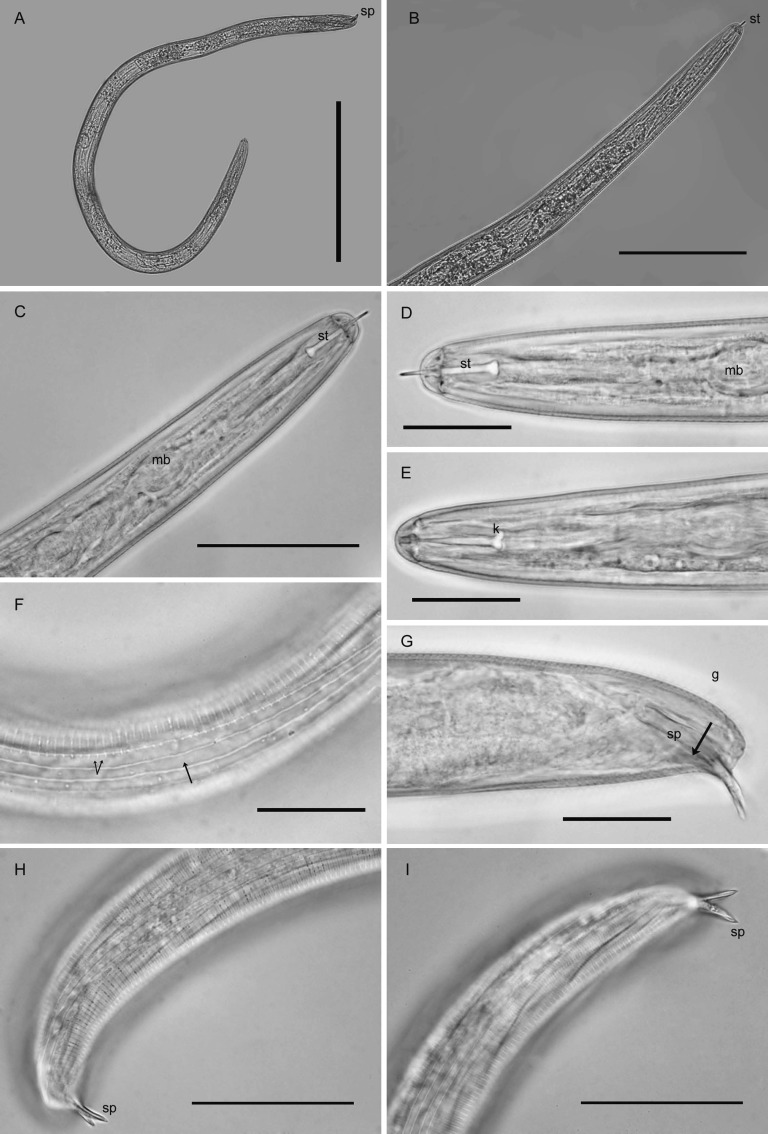
Males (n = 20): Length highly variable (Table 2, Fig. 4A). Body slender, vermiform, tapering slightly at both extremities. Cuticle with distinct annulations. Lip region rounded to hemispherical, continuous or slightly set-off, with five indistinct annules (Fig. 4B-E). Cephalic framework heavily sclerotized (Fig. 4E). Lateral field with four incisures, outer bands with scattered, incomplete areolation (Fig. 4F). Tail tip rounded or bluntly conoid (Fig. 4G-I). Stylet well-developed with prominent rounded to posteriorly directed knobs (Fig. 4C-E), dorsal gland orifice near stylet base. Valved median bulb prominent (Fig. 4D). Isthmus and esophageal glands typical for the genus, but with obscure nuclei. Excretory pore two annules posterior to hemizonid, at anterior half of glands. Male gonad about 40-60% of body length. Spicules slightly curved, tips pointed, thorn-like (Fig. 4H,I), gubernaculum 8-15 μm long (Fig. 4G).
Table 2.
Morphometrics (in μm) of males and second-stage juveniles of Globodera ellingtonae from Oregon.
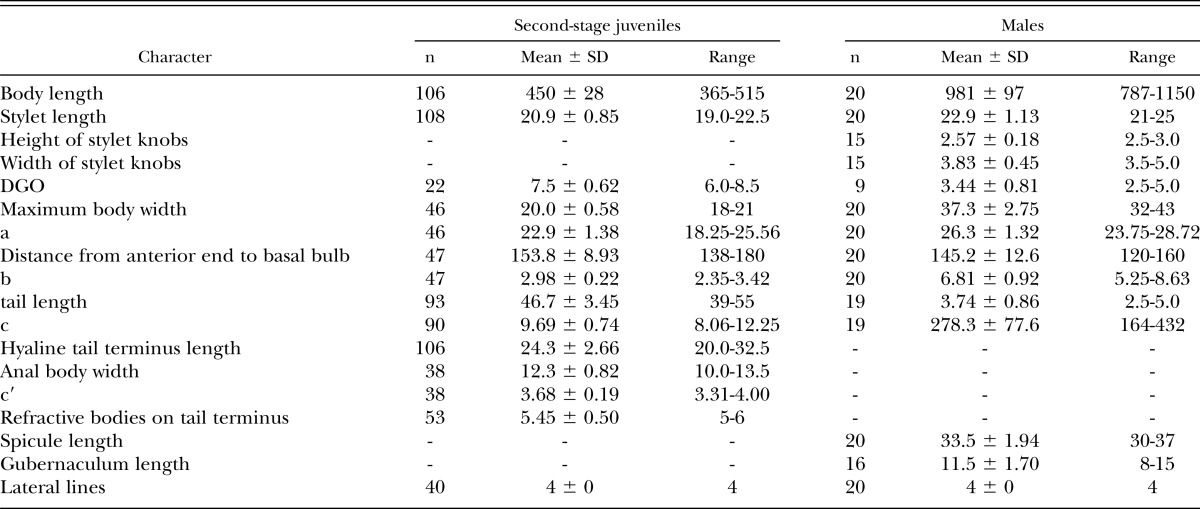
Fig. 4.
Photomicrographs of males of Globodera ellingtonae n. sp. from Oregon: A, Whole male. B-E, Anterior regions showing stylet and part of esophagus, with st = stylet in C, D, mb = median bulb in C, and k = stylet knobs in E. F, Lateral field at mid-body, single arrow = lateral lines, double arrow = areolation. G-I, Posterior regions, showing spicules (sp) with pointed thorn-like tips and g = gubernacula in G. (Scale bars: A = 250 μm; B = 100 μm; C, H, I = 25 μm).
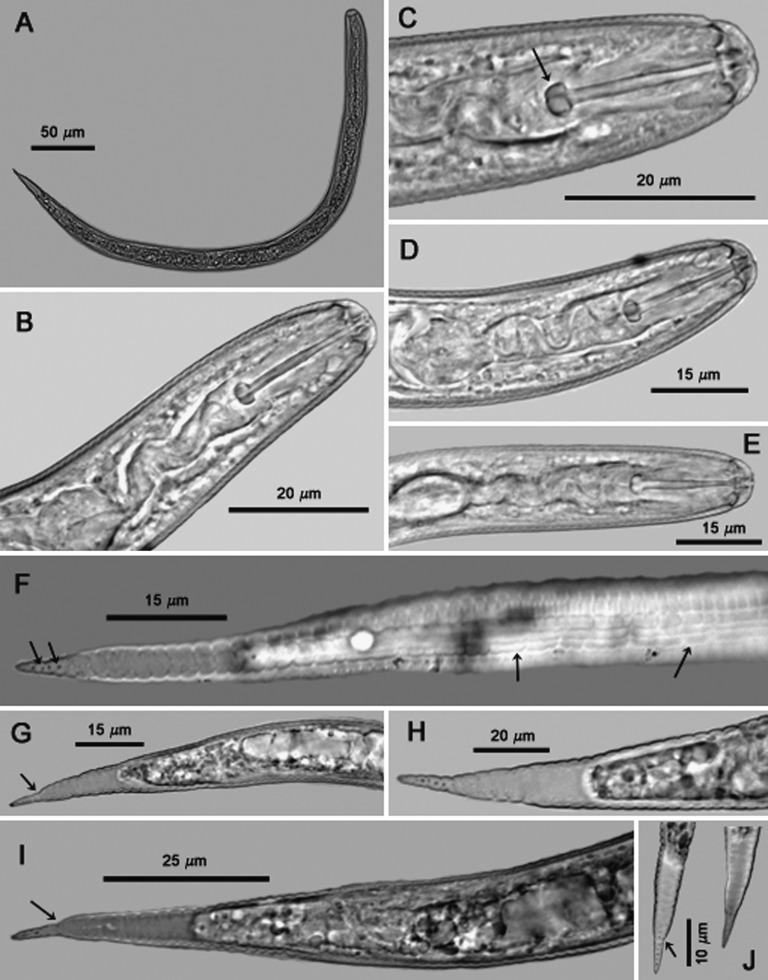
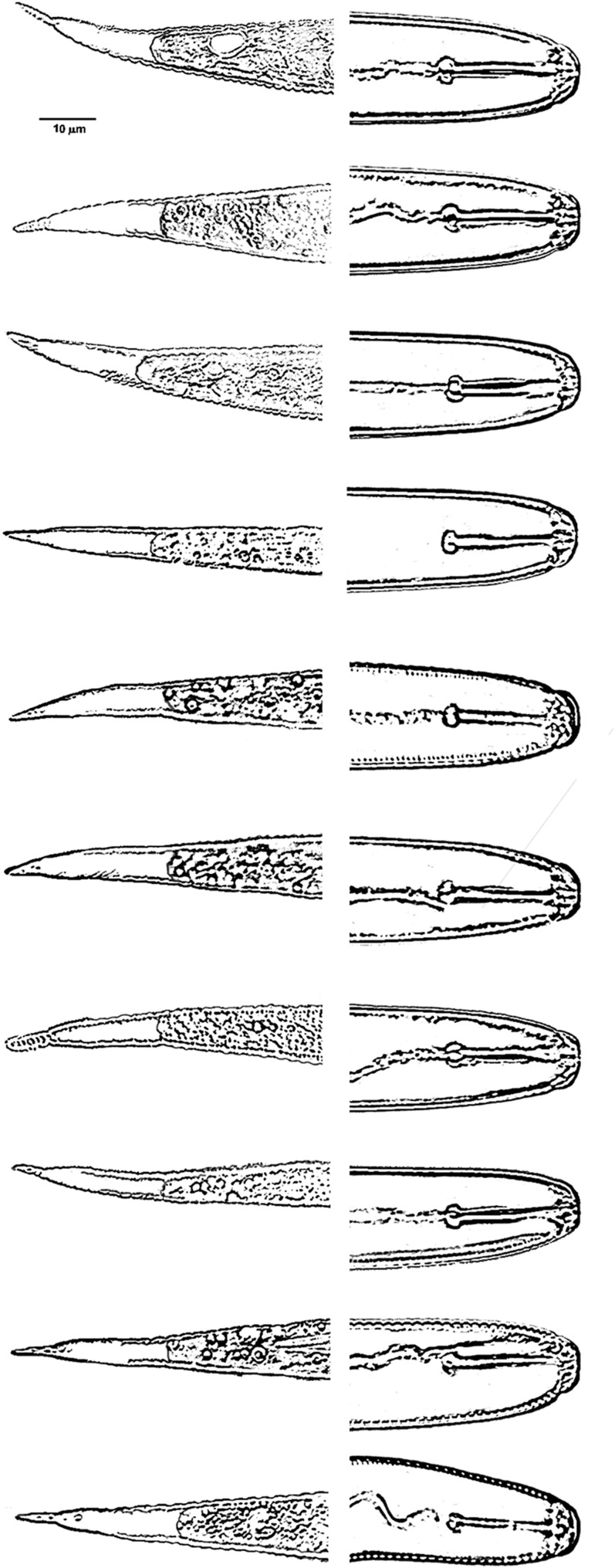
Second-stage juveniles (n = 106): Body vermiform, tapering at both extremities, more so posteriorly (Fig. 5A). Some specimens bent at four points along the body when hatched or cracked open from eggs. Head rounded, slightly set off with prominent cephalic sclerotization, bearing four to five annules (Figs. 5B-E; 6A-E). Stylet short, well developed; basal knobs rounded posteriorly, with a forward projection anteriorly (Fig. 5C), dorsal gland orifice distance from stylet base 7.5 μm (6.0-8.5) (Fig. 6A,B). Excretory pore located near anterior end of esophageal glands. Phasmids not observed. Tail tapering uniformly but abruptly narrowing with a pronounced to slight constriction near the posterior third of the hyaline portion, ending with a peg-like, finely rounded to pointed terminus (Figs. 5F-J; 6G-M). Hyaline tail terminus often with up to 7 minute refractive bodies (Figs. 5F,H,J; 6H). Cuticular annulations distinct with more prominent annules near the tail terminus (Fig. 6I). Lateral field consisting of four incisures, with occasional areolation (Figs. 5F, 6F). In a few specimens, tail and anterior end with three incisures, with outer two showing areolation, and with four incisures at mid-body. Head and tail variations are shown in Fig. 7 with head showing rounded to slightly set off lip region, rounded to anteriorly directed stylet knobs, and tail terminus bluntly pointed to peg-like.
Fig. 5.
Photomicrographs of second-stage juveniles of Globodera ellingtonae n. sp. from Oregon: A, Whole juvenile. B-E, Anterior regions showing stylet and part of esophagus, with C showing high stylet knobs (arrow). F, Tail showing areolated nature of lateral field with four lines (right arrows) and refractive bodies in tail ending with a peg-like finely rounded to pointed terminus (left arrows). G-J, tail variations, with G, I, J (arrows) showing abrupt narrowing of tail with pronounced to slight constriction. (Modified from Skantar et al., Phytopathology, 2011).
Fig. 6.
Photomicrographs of second-stage juveniles of Globodera ellingtonae n. sp. from Oregon: A-E, Anterior regions showing stylet and part of esophagus, with k in B, E showing high stylet knobs (arrow), mb = median bulb (arrow) in B, and e = esophagus (arrow) in E. F, showing areolated nature of lateral field with four lines, single arrow = lateral lines, and double arrows = areolation. G-M, tail variations, with H, M (arrows) showing refractive bodies, a = anus (arrow) in I, J-M showing abrupt narrowing of tail with pronounced to slight constriction to peg-like tail terminus. (Scale bars: A, B = 50 μm; C-M = 25 μm).
Fig. 7.
Heads and tails of second stage juveniles of Globodera ellingtonae n. sp. from Oregon.
Type host and locality: The Oregon population was collected in May 2008 during PCN surveys from a Powell Butte, Oregon, field with a history of potato and other crops. Additional specimens were from greenhouse cultures on Solanum tuberosum ‘Mazama’ established by Inga Zasada and Russell Ingham in 2009 from nematodes collected from the Oregon site.
Type specimens: Specimens from aforementioned greenhouse cultures on Solanum tuberosum ‘Mazama’: Holotype (female) on slide T-653t, paratype (females) on slides T-6050p to T-6059p, paratype (cysts) on slides T-6060p to T-6075p and in vials T-568p and T-569p, paratype (males) on slides T-6076p to T-6087p and in vial T-572p, paratype (second-stage juvenile) on slides T-6088p to T-6091p and in vials T-568p to T-571p. Specimens isolated from Oregon type host and locality: paratypes (second-stage juveniles) on slides T-5847p to T-5862p and in vials T-535p to T-541p, paratypes (cysts) on slides T-5863p to T-5870p, deposited in the U.S. Department of Agriculture Nematode Collection, Beltsville, Maryland. Additional paratype males, juveniles, and cysts deposited in the University of California-Riverside Nematode Collection, Riverside, California; the Rothamsted Nematode Collection, York, England; the University of Tennessee Nematode Collection, Knoxville, Tennessee; and the Canadian National Collection of Insects, Arachnids and Nematodes, Ottawa, Ontario, Canada.
Idaho isolate #167, Cysts (n = 2): Light brown color, spherical with a protruding neck (Fig. 8A). Cyst wall pattern ridge-like with heavy punctations (Fig. 8B). Vulval region intact or fenestrated with a single circumfenestrate opening that occupies all or part of vulval basin (Fig. 8C,D). Whole fenestra length 22.5-25 μm; distance from nearest edge of fenestra to anus 40-52 μm; number of ridges between anus and nearest edge of fenestra 10-14; Granek’s ratio 1.8-2.1.
Fig. 8.
Photomicrographs of cysts and second-stage juveniles of Globodera ellingtonae n. sp. from Idaho: A, anterior region. B-D, anal-vulval regions with B and C-D (arrows) showing cyst wall pattern and anal areas respectively. E-H, anterior and posterior regions of second-stage juveniles with E-H (arrows) showing stylet knobs, with DIC images of E,G,H. I-M, tails with variable termini, DIC image of L. Tail in I shows anal area (arrow). (Modified from Skantar et al., Phytopathology, 2011).
Second-stage juveniles (n = 10): Body vermiform, specimens were bent at four points along the body when hatched or cracked open from eggs. Tail tapering uniformly and usually bluntly rounded to pointed at the terminus (Fig. 8I-M). Tail of one specimen similar to tail typical of the Oregon population, i.e., tail abruptly narrowing with a slight constriction near the posterior third of the hyaline portion, ending with a finely rounded to pointed terminus (Fig. 8I). Lateral field with four incisures. Head rounded, slightly set off with prominent cephalic sclerotization, bearing four annules. Stylet well-developed; basal knobs rounded and with a forward projection on anterior side. Excretory pore located near the anterior end of the esophageal glands. Phasmids not observed. Length 425-485 μm (451.7 ± 23.74); stylet 21-23 μm (22.3 ± 0.52); tail 42-50 μm (48 ± 3.09); hyaline tail terminus 20-30 μm (24.6 ± 3.26).
Host and locality: Collected during PCN surveys of soil from an agricultural field with unknown cropping history in Caribou County, Idaho.
Specimens: Second-stage juveniles on slides T-5871p to T-5873p and in vial T-542p, cysts on slides T-5874p to T-5877p deposited in the U.S. Department of Agriculture Nematode Collection, Beltsville, Maryland.
Idaho isolate #347, Cysts (n = 2): Light brown color, spherical with a protruding neck. Cyst wall pattern ridge-like with heavy punctations. Vulval region intact or fenestrated, with a single circumfenestrate opening occupying all part of vulval basin. Whole fenestra length 25 μm; distance from nearest edge of fenestra to anus 30-55 μm; number of ridges between anus and nearest edge of fenestra 8-14; Granek’s ratio 1.2-2.2.
Second-stage juveniles (n = 8): Body vermiform, specimens were degraded and bent at four points along the body when hatched or cracked open from eggs. Tail tapering uniformly and usually bluntly rounded to pointed at the terminus. Lateral field with four incisures. Head rounded, slightly set off with prominent cephalic sclerotization, bearing four annules. Stylet well-developed; basal knobs rounded and with a forward projection on anterior side (Fig. 8F). Length 421-435 μm (428 ± 9.89); stylet 22-23 μm (22.5 ± 0.32); tail 48 μm; hyaline tail terminus 22.5-30 μm (25.7 ± 2.52).
Host and locality: Collected during PCN surveys of soil from an agricultural field with unknown cropping history in Teton County, Idaho.
Specimens: Cysts on slides T-5878p and T-5879p, second-stage juveniles in vial T-543p deposited in the U.S. Department of Agriculture Nematode Collection, Beltsville, Maryland.
Diagnosis and Relationships: Globodera ellingtonae n. sp. is characterized by J2 with body length of 450 μm (365-515); stylet length 20.9 μm (19-22.5) with rounded to high basal knobs with a forward projection on anterior side; areolated lateral field with 4 incisures; tail 46.7 μm (39-55) long, tapering uniformly but abruptly narrowing, with a pronounced to slight constriction near the posterior third of the hyaline portion, ending with a peg-like, finely rounded to pointed terminus (Figs. 5G,I; 6G-M); hyaline tail terminus 24.3 μm (20-33). Females are characterized by a body length excluding neck of 480 μm (312-618); stylet length of 21.3 μm (20-22.5) with 1 head annule and labial disc; heavy punctations on the cuticle; irregular arrangement of bullae-like vulval bodies and short vulval slit 7.8 μm (7.5-8). Males have a stylet length of 22.9 μm (21-25); spicule measures 33.5 μm (30-37) with a pointed thorn-like tip. Cysts are spherical to sub-spherical; light to dark brown with heavy punctations on the cuticle; circumfenestrate; number of cuticular ridges between vulva and anus 13 (10-18) and Granek’s ratio 2.4 (1.7-3).
Globodera ellingtonae n. sp. shares similarities with G. pallida (Stone, 1973) Behrens, 1975, G. rostochiensis (Wollenweber, 1923) Skarbilovich, 1959, G. mexicana Subbotin, Mundo-Ocampo and Baldwin, 2010, and G. tabacum (Lownsbery and Lownsbery, 1954) Behrens, 1975 sensu Stone, 1983 (comparative data in Table 3 and differentiation given below for closely related species). It differs from all these species in the shape of the J2 tail, which tapers uniformly but abruptly narrows with a constriction near the posterior third of the hyaline portion and ends with a peg-like, finely rounded to pointed terminus. In other Globodera spp. the tail tapers gradually to a bluntly rounded to pointed tip without any constriction or peg-like shape in the posterior third of the hyaline portion. The new species is further distinguished from G. pallida in having a shorter stylet mean in J2, females, and males [length 20.9 μm (19-22.5 vs. 23.5 μm (22.5-25.0) in J2, length 21.3 μm (20-22.5) vs. 27.4 ± 1.1 in females, length 22.9 μm (21-25) vs. 27.5 ± 1.0 in males]. The new species differs from G. rostochiensis in having J2, females, and males with a shorter stylet mean [length 20.9 μm (19-22.5) vs. 21.5 μm (18.9-23.5), length 21.3 μm (20-22.5) vs. 23.0 (22-24), length 22.9 μm (21-25) vs. 25.9 μm (25-27), respectively]; cysts have a longer mean fenestra [length 27.4 μm (20-42.5) vs. 16.3 μm (8-20.7)], smaller mean Granek’s ratio [2.3 (1.6-3.0) vs. 3.9 (2-7)], and fewer number of mean cuticular ridges between the anus and vulva [13 (10-18) vs. 18 (16-24)]; the male spicules have a pointed, thorn-like tip vs. a bluntly rounded tip, and the J2 hyaline tail terminus has a large number of refractive bodies (4-7) vs. (3-4). The new species differs from G. mexicana in having J2, females and males with a shorter mean stylet [length 20.9 μm (19-22.5) vs. 23.3 μm (20-27), length 21.3 μm (20-22.5) vs. 24.7 μm (23.2-28.8), and length 22.9 μm (21-25) vs. 26.7 μm (22-29.5), respectively]; cysts have more cuticular ridges between the anus and vulva [13 (10-18) vs. 7 (5-9)], and longer mean fenestra [length 27.4 μm (20-42.5) vs. 20.9 μm (15-28.5)]; and spicules in males have a thorn-like tip vs. a finely rounded tip.
Table 3.
Morphological and morphometric characters useful for identification of selected Globodera species.a
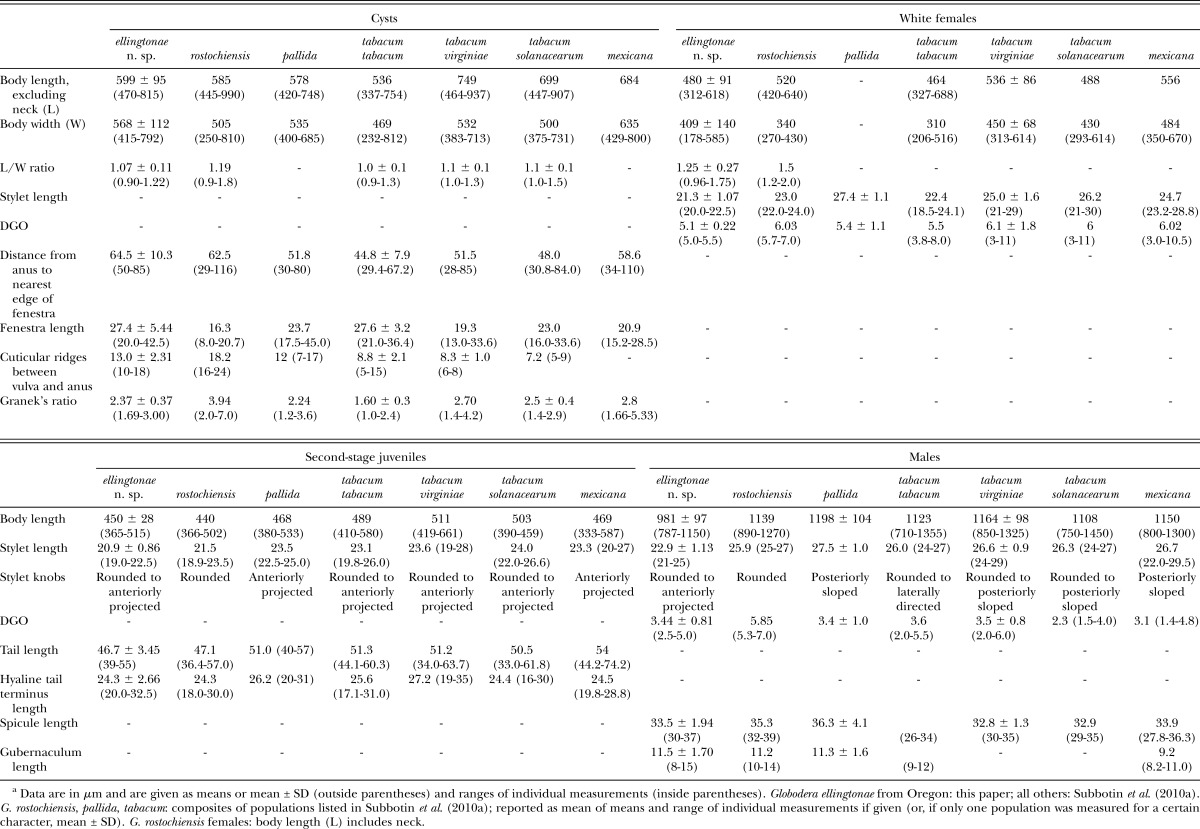
Globodera ellingtonae n. sp. differs from G. tabacum tabacum in possessing cysts with a mean Granek’s ratio of more than 2.3 vs. 1.6, and a mean of more cuticular ridges between vulva and anus [13 (10-18) vs. 8 (5-15)], and with a cyst wall lacking a network-like pattern vs. a network-like pattern with parallel anal-fenestral ridges; the J2 have a mean stylet length of less than 21 μm vs. 23.1 μm; female stylet length is 21.3 μm vs. 22.4 μm; males have shorter mean stylet lengths 22.9 μm (21-25), vs. 26 μm (24-27)]; spicules have a pointed, thorn-like tip vs. a finely rounded tip. The new species differs from G. tabacum solanacearum in that cysts have more cuticular ridges between vulva and anus [13 (10-18) vs. 7 (5-9)], the cyst wall lacks a network-like pattern or ridges are close and irregular around fenestra vs. more widely separated ridges and grooves of cyst wall that form a circular network-like pattern around the fenestra. The J2, females and males have a shorter mean stylet length of 20.9 μm vs. 24.0, 21.3 μm vs. 26.2, and 22.9 μm vs. 26.3, respectively; spicules have a pointed, thorn-like tip vs. a finely rounded tip; and perineal tubercles in females are clumped vs. solitary. The new species differs from G. tabacum virginiae by possessing cysts with shorter mean body length excluding neck (599 μm vs. 749 μm), more cuticular ridges between vulva and anus [13 (10-18) vs. 7 (6-8)], irregular anal-fenestral ridges vs. a maze-like pattern, anus distinct vs. small and indistinct, and slightly smaller mean Granek’s ratio [2.3 (1.6-3.0) vs. 2.7 (1.4-4.2)]; the J2, females and males have a shorter mean stylet length (20.9 μm vs. 23.6, 21.3 μm vs. 25.0, and 22.9 μm vs. 26.6 respectively); perineal tubercles in females are clumped vs. solitary; spicules have a pointed, thorn-like tip vs. a finely rounded tip.
Etymology: The species is named for Donna Ellington in honor of 40 years of outstanding service to the taxonomy program of the ARS Nematology Laboratory at Beltsville, Maryland.
DNA sequence analysis: The initial nematode samples obtained from Powell Butte, Oregon showed a high degree of sequence variation in the ITS-rRNA region, initially indicated by the fact that sequencing directly from PCR products gave many ambiguous base calls. Examination of additional cloned amplicons was carried out in order to further explore the extent of ITS rRNA copy variation within this species. A total of 14 clones were obtained from four juveniles of G. ellingtonae n. sp. originally isolated from Oregon. Subsequently, 28 clones were generated from five juveniles that exhibited variations in tail morphology, although no correlation between the type of ITS sequence and tail morphology was found (data not shown). Twenty-seven clones were sequenced from eight juveniles originating from four cysts (two J2 from each) that had been obtained from greenhouse cultures. A total of 11 clones, averaging two per specimen, were sequenced from 5 juveniles of the Idaho isolate #347. As described previously (Skantar et al., 2011), one identical sequence was obtained from direct sequencing of four ITS PCR products from Idaho isolate #167. The sequences ranged in size from 923 to 978 bp, with intraspecific divergence as high as 2.8% (Skantar et al., 2011). The most significant difference among them was a 27 bp deletion detected in three clone sequences amplified from separate juveniles of the original isolate as well as a greenhouse-reproduced cyst.
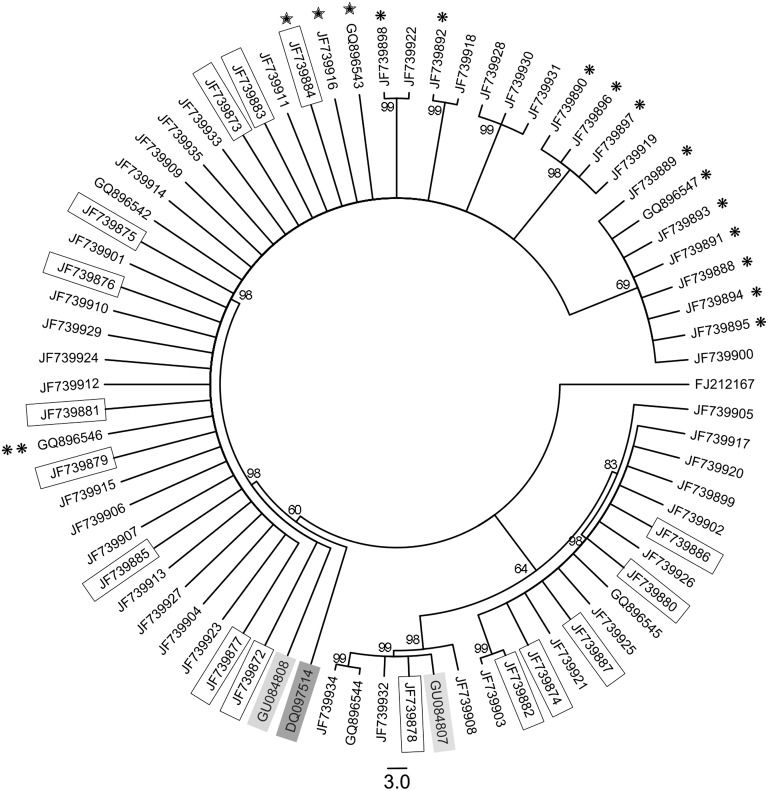
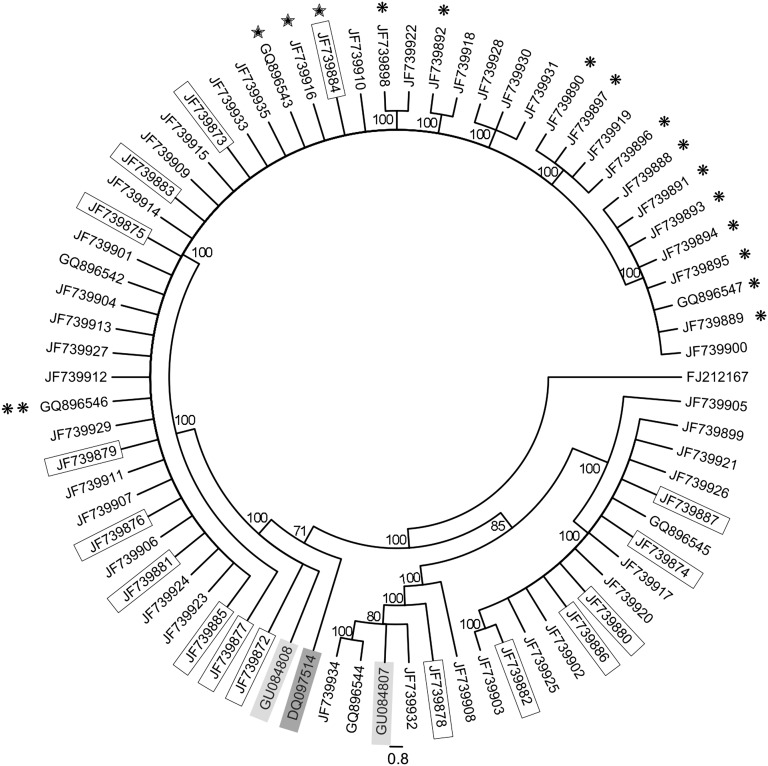
Phylogenetic analysis: The distinct phylogenetic position of G. ellingtonae relative to an extensive array of G. rostochiensis, G. pallida, and G. tabacum populations was established in two previous studies (Skantar et al., 2011; Subbotin et al., 2011). The present, more focused analysis was undertaken to determine whether ITS rRNA sequence variation present within isolates of G. ellingtonae or closely related South American populations could be segregated according to any discernible pattern. Relationships among the cloned ITS rRNA gene sequences were inferred using Bayesian Interference and Maximum Parsimony as given in Figs. 9 and 10, respectively. The BI and MP trees were congruent. The majority of clones fit in a highly supported clade (posterior probability, PP = 98; bootstrap support, BS = 100) that also included a sequence from Antofagasta, Chile (GU084808); grouping of a sequence from Argentina (DQ097514) with this clade was only moderately supported (PP = 60; BS = 71). This clade included sequences from both Idaho isolates (marked by single or double asterisks), and the three sequences containing the deletion (marked by stars). The second most common clade (PP = 98; BS = 100) was comprised of a mixture of sequences obtained from the original Oregon G. ellingtonae n. sp. isolate or greenhouse cultured J2. The smallest clade (PP = 98; BS = 100) was comprised of six sequences, including another one from Antofagasta, Chile (GU084807). Notably less intraspecific variation was found among the clone sequences from the Idaho isolate #347 than the Oregon population. The limited genetic material available for Idaho isolate #167 yielded four PCR amplicons with identical sequence. Clone types from any isolate generally did not segregate by the cyst or juvenile from which they came; nor was there any association found between juvenile tail shape (pointed or blunt) and the type of ITS rRNA sequence within (not shown). Likewise, sequences from greenhouse-reared juveniles were distributed throughout the trees.
Fig. 9.
The 50% majority rule consensus tree from Bayesian analysis generated from analysis of the ITS-rRNA gene under the model GTR + I + G for species of Globodera ellingtonae n. sp with G. rostochiensis sequence FJ212167 used as the outgroup. Posterior probability values are given for appropriate clades. Globodera sp. sequences from Argentina and Chile are shaded in dark and light boxes, respectively. Cloned ITS rRNA gene sequences from the initial Oregon sample are in regular type; sequences obtained from juveniles reproduced in the laboratory are boxed; from Idaho isolate 347 are marked by a single asterisk; a single PCR amplicon sequence from Idaho isolate 167 is marked by two asterisks. Sequences containing a 27 bp deletion are marked with stars.
Fig. 10.
Maximum parsimony tree of aligned ITS-rRNA gene sequences for species of Globodera ellingtonae n. sp with G. rostochiensis sequence FJ212167 used as the outgroup. Bootstrap support values from 1000 replicates are indicated on the branches. Globodera sp. sequences from Argentina and Chile are shaded in dark and light boxes, respectively. Cloned ITS rRNA gene sequences from the initial Oregon sample are in regular type; sequences obtained from juveniles reproduced in the laboratory are boxed; from Idaho isolate 347 are marked by a single asterisk; a single PCR amplicon sequence from Idaho isolate 167 is marked by two asterisks. Sequences containing a 27 bp deletion are marked with stars.
Cloned ITS-rRNA sequence analysis and in silico PCR-RFLP: ITS-rRNA sequences from cloned PCR products were analyzed with Sequencher 5.0 to generate virtual restriction fragments by enzymes Bsh 1236I, Hinf I, and Rsa I (Table 4). Sequences representing all three Globodera ellingtonae n. sp. populations and the three main ITS types were compared to Globodera sequences from Chile and Argentina populations, G. tabacum, G. mexicana, G. rostochiensis, and G. pallida (European and South American subclades 1 – 5). For G. ellingtonae n. sp., Bsh 1236I digestion patterns were of two types. Clone sequences with type A patterns were shared with Globodera sp. sequences from Chile (GU084808) and G. rostochiensis. The Idaho #167 sequence was slightly shorter at either end, due to the lack of reliable base calls close to ends of the amplicons, but the in silico patterns otherwise matched Type A. Type B patterns of G. ellingtonae n. sp. were the same as for Globodera sp. Chile (GU084807) and G. pallida subclades 1, 3, and 6. Globodera tabacum shared only individual bands with Bsh 1236I patterns A or B. Virtual Rsa I digestion of G. ellingtonae n. sp. also gave 2 types of patterns, with types A and B shared with Globodera sp. sequences from Chile. The Hinf I digestion products predicted for G. ellingtonae n. sp. fell into three possible patterns. Type A was only found in G. ellingtonae n. sp. and Globodera sp. from Chile; Type B was shared with G. pallida, G. mexicana, and other Globodera sp.; Type C matched G. tabacum and G. rostochiensis.
Table 4.
Approximate sizes (in bp) of restriction fragments estimated from in silico digestion of Globodera spp. ITS rDNA sequences, including the amplified region between primers TW81 and AB28.
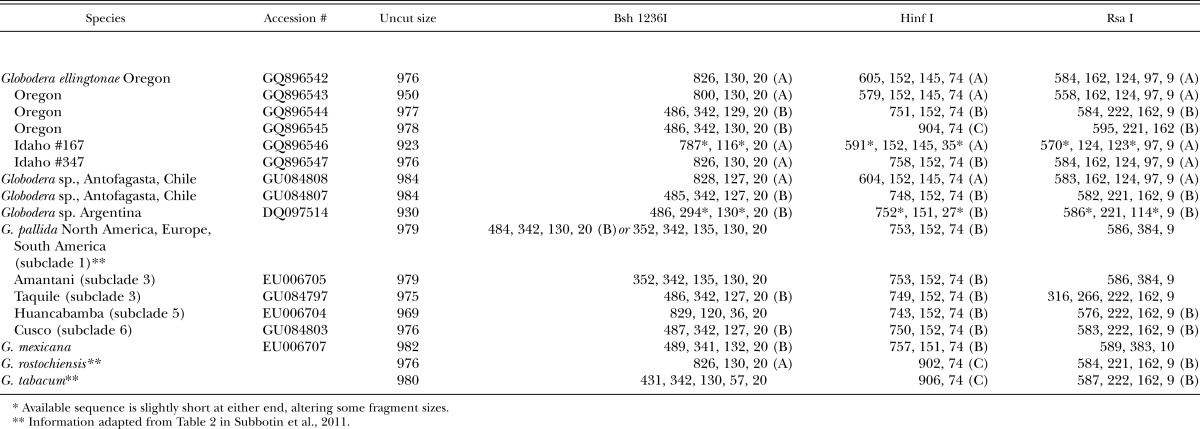
PCR-RFLP of the ITS rRNA region is an inexpensive, commonly used diagnostic assay for distinguishing PCN species. Bsh 1236I was previously shown to discriminate G. pallida, G. rostochiensis, and G. tabacum (Thiéry and Mugniéry, 1996; Skantar et al., 2007). Previous RFLP analysis of the Powell Butte, OR samples showed visible Bsh 1236I fragment sizes of approximately 820, 500, 342, and 130 bp (Skantar et al., 2011 and data not shown), matching some of the sizes predicted for A or B type patterns by the in silico analysis (Table 4). Rsa I fragments of 610, 223, 161, 123, and 96 bp were also observed in gels (not shown), and closely match the larger sizes predicted from the sequence analysis. Fragments not observed in the gel experiments may have been present at levels too low to be detected. While some of the sequence variation seen in the cloned PCR amplicons of G. ellingtonae could reasonably be attributed to errors introduced during PCR, cloning, or sequencing, the patterns representing major and minor sequence types are clearly present in the J2 and can be detected by PCR-RFLP. The presence of ITS haplotypes in G. pallida samples from subclade I was also reported by Subbotin et al. (2011). It was proposed that a higher level of rRNA gene heterogeneity may exist for some Globodera isolates, or that natural hybrids may underlie unusual diagnostic RFLP profiles. As more populations are examined from South America and elsewhere, it seems likely that this phenomenon will become more commonplace.
A number of real time (RT) PCR methods have been developed for diagnostics of PCN (Madani et al., 2008; Quader et al., 2008). A multiplex RT-PCR assay with species-specific TaqMan primer-probe sets was recently employed for the simultaneous detection and identification of G. pallida, G. rostochiensis, and G. tabacum (Nakhla et al., 2010). When G. ellingtonae n. sp. samples from Oregon were subjected to this test, no fluorescence was detected by any of the primer-probe combinations. This assay, which has become a standard protocol for screening PCN survey samples from throughout the U.S., has thus far resulted in no false positive detections involving G. ellingtonae n. sp. The higher degree of specificity conferred by the presence of species-specific TaqMan probes as well as primers may make this test more robust than the conventional PCR-RFLP.
Analysis of the 28S rRNA D2-D3 expansion segment: The sequence for 28S large subunit rRNA D2-D3 expansion regions obtained from G. ellingtonae n. sp. was 749 bp in length (JN712217). While relatively few such sequences from Globodera species were available for comparison, an alignment of 663 bp was made from 20 sequences, including G. pallida, G. rostochiensis, G. tabacum, and G. millefolii (not shown). The greatest similarity of G. ellingtonae n. sp. was with G. tabacum from Connecticut, U.S. (GQ294492), differing by only 2 bp. Compared with a limited number of available G. pallida sequences, Oregon G. ellingtonae n. sp. differed by 4bp from a Newfoundland, Canada, population (GQ294489), by 8 bp from the original G. pallida population isolated from Idaho (JN712221), and by 14 bp from sequence AY592991, representing a G. pallida population from the Netherlands. Compared to G. rostochiensis, the Oregon population of G. ellingtonae n. sp. differed by 4 bp from identical sequences originating from Quebec and Newfoundland, Canada, (GQ294487, GQ29488); and 9 bp from sequences from Netherlands populations (AY592987, A7592988). There were 12 bp separating G. ellingtonae n. sp. from G. millefolii (DQ328700). Madani et al. (2010) previously concluded that for Globodera spp., low diversity within the D2-D3 expansion segment of 28S rRNA did not allow robust phylogenetic reconstruction within this genus. The >99% similarity of G. ellingtonae n. sp. to G. tabacum sequences is consistent with this conclusion, and furthers the presumption that this marker also lacks sufficient variability to be useful as a molecular diagnostic for distinguishing G. pallida, G. rostochiensis, and G. tabacum.
The possible existence of an additional species of PCN, particularly one originating from South America, has been the subject of study and speculation for some time (Phillips and Trudgill, 1998; Blok et al., 1998; Subbotin et al., 2000; Rumpenhorst and Ayub, 2001; Picard et al., 2007; Plantard et al., 2008; Pylypenko et al., 2008). Based on phylogenetic analysis of ITS rRNA and mitochondrial cytochrome b (cytb) sequences, Subbotin and colleagues (Madani et al., 2010; Subbotin et al., 2011) have proposed possible candidates from among South American populations of G. pallida or undescribed species, appearing in ITS rRNA trees within subclades 3, 5, and 6 (see also Table 4, this study). They concluded that the pale potato cyst nematode, G. pallida, may represent a species complex, and agreed with Grenier et al. (2001) who emphasized the need to augment molecular data with morphological and biological studies. Based upon molecular data presented here and elsewhere (Skantar et al., 2011; Subbotin et al., 2011), G. ellingtonae n. sp. is molecularly more similar to G. tabacum and G. rostochiensis than to G. pallida.
Material representing the two Idaho isolates was in relatively poor condition and all available specimens were used for identification, so no additional material was available for culturing. Since 2006, the USDA Animal and Plant Health Inspection Service and the States have collected and analyzed over 300,000 official samples for Globodera from potato fields in Idaho and over 100,000 samples from potato fields in 30 other states; G. ellingtonae was not detected in any of these samples (Jonathan Jones, personal communication). Therefore, we lacked the appropriate number of specimens needed for optimal replication of the morphometrics as we had for the Oregon population. This situation warrants some degree of caution with respect to the identity of these Idaho isolates as G. ellingtonae n. sp. Nonetheless, the existence of unequivocal molecular similarity between the Oregon and Idaho isolates provides strong support that the three belong to the same species, an inference further supported by the limited morphological and morphometric data from the Idaho isolates. The morphological and molecular description provided here for G. ellingtonae n. sp. lies in contrast to the still unresolved morphological status of many South American populations of Globodera, most of which have been described only molecularly. Anthoine et al. (2008) and Grenier et al. (2010) recently highlighted potential problems with the reliability of molecular diagnostics in light of variable South American populations, pointing out that the identities of variable Chilean populations have not yet been validated. Morphological descriptions of these and other South American populations would certainly strengthen the taxonomic foundation needed for traditional diagnosis, which, despite the increased use of molecular approaches, is still an essential part of PCN control programs. Notably, the sequences from the Antofagasta, Chile isolate closely match those from G. ellingtonae; however, absent any morphological details, identifying them as such may be premature. Preliminary greenhouse experiments have demonstrated that G. ellingtonae n. sp. can reproduce on potato (Solanum tuberosum), with further host range and details about virulence anticipated in a separate publication (Zasada and Ingham, personal communication). Therefore, this present description provides fundamental information that is needed for morphological and molecular diagnosis of this species to provide continued protection for the potato industry in the U.S. and throughout the world.
Literature Cited
- Agricultural Statistics Board. 2011 Potatoes 2010 Summary. United States Department of Agriculture, National Agricultural Statistics Service. [Google Scholar]
- Anthoine G, Chappé AM, Fouville D, Henriquez Flores E, Mugniéry D, Grenier E. 2008 Reliability and limits of published molecular tests for the specific identification of potato cyst nematodes (PCN) of quarantine concern. p. 125 in Proceedings of the 5th International Congress of Nematology Brisbane, Australia. (Abstr.) [Google Scholar]
- Baldwin JG, Mundo-Ocampo M. 1991 Heteroderinae, cyst- and non-cyst-forming nematodes. Pp. 275–362 in W. R. Nickle, ed. Manual of agricultural nematology. New York: Marcel Dekker. [Google Scholar]
- Baldwin JG, Schouest LP., Jr 1990 Comparative detailed morphology of the Heteroderinae Filip'ev & Schuurmans Stekhoven, 1941, sensu Luc et al. (1988): Phylogenetic systematics and revised classification. Systematic Parasitology 15:81–106. [Google Scholar]
- Blok VC, Malloch G, Harrower B, Phillips MS, Vrain TC. Intraspecific variation in ribosomal DNA in populations of the potato cyst nematode Globodera pallida. Journal of Nematology. 1998;30:262–274. [PMC free article] [PubMed] [Google Scholar]
- Brodie BB. 1998 Potato cyst nematodes (Globodera species) in Central and North America. Pp. 317–331 in R. J. Marks and B. B. Brodie, eds. Potato cyst nematodes: Biology, distribution and control. Wallingford, UK: CAB International. [Google Scholar]
- De Ley P, Tandingan De Ley I, Morris K, Abebe E, Mundo-Ocampo M, Yoder M, Heras J, Waumann D, Rocha-Olivares A, Burr AHJ, Baldwin JG, Thomas WK. An integrated approach to fast and informative morphological vouchering of nematodes for applications in molecular barcoding. Philosophical Transactions of the Royal Society B. 2005;360:1945–1958. doi: 10.1098/rstb.2005.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Nijs L, Karssen G. Diagnostic protocols for regulated pests: Globodera rostochiensis and Globodera pallida. EPPO Bulletin. 2004;34:309–314. [Google Scholar]
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris VR, Miller LI, Faghihi J, Ferris JM. Ribosomal DNA comparisons of Globodera from two continents. Journal of Nematology. 1995;27:273–283. [PMC free article] [PubMed] [Google Scholar]
- Fleming CC, Powers TO. 1998 Potato cyst nematode diagnostics: morphology, differential hosts and biochemical techniques. Pp. 91–114 in R. J. Marks and B. B. Brodie, eds. Potato cyst nematodes: Biology, distribution and control. Wallingford, UK: CAB International. [Google Scholar]
- Fraley C, Meng SX, Osterbauer NK. 2009. Detection of a new Globodera species during surveys of Oregon seed potato fields for potato cyst nematodes. Poster presented at the 2nd National Plant Diagnostic Network Meeting, Miami, Florida, December 6-10, 2009. http://www.npdn.org/webfm_send/1054.
- Golden AM. 1986 Morphology and identification of cyst nematodes. Pp. 23–45 in F. Lamberti and C. E. Taylor, eds. Cyst nematodes. New York: Plenum Press. [Google Scholar]
- Golden AM. 1990 Preparation and mounting nematodes for microscopic observations. Pp. 197–205 in B. M. Zuckerman, W. F. Mai, and L. R. Krusberg, eds. Plant nematology laboratory manual. Amherst, MA: University of Massachusetts Agricultural Experiment Station. [Google Scholar]
- Golden AM, Ellington DMS. Redescription of Heterodera rostochiensis (Nematoda: Heteroderidae) with a key and notes on closely related species. Proceedings of the Helminthological Society of Washington. 1972;39:64–78. [Google Scholar]
- Grenier E, Bossis M, Fouville D, Renault L, Mugniéry D. Molecular approaches to the taxonomic position of Peruvian potato cyst nematodes and gene pool similarities in indigenous and imported populations of Globodera. Heredity. 2001;86:277–290. doi: 10.1046/j.1365-2540.2001.00826.x. [DOI] [PubMed] [Google Scholar]
- Grenier E, Fournet S, Petit E, Anthoine G. A cyst nematode ‘species factory’ called the Andes. Nematology. 2010;12:163–169. [Google Scholar]
- Guindon S, Gascuel O. A simple, fast and accurate method to estimate large phylogenies by maximum likelihood. Systematic Biology. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- Hafez SL, Sundararaj P, Handoo ZA, Skantar AM, Carta LK, Chitwood DJ. First report of the pale cyst nematode, Globodera pallida, in the United States. Plant Disease. 2007;91:325. doi: 10.1094/PDIS-91-3-0325B. [DOI] [PubMed] [Google Scholar]
- Hesling JJ. The estimation of Granek’s ratio in round-cyst Heteroderas. Nematologica. 1973;19:119. [Google Scholar]
- Hooper DJ. 1970 Handling, fixing, staining and mounting nematodes. Pp. 59–80 in J. F. Southey, ed. Laboratory methods for work with plant and soil nematodes, 5th ed. London: Her Majesty's Stationery Office. [Google Scholar]
- Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Madani M, Subbotin SA, Moens M. Quantitative detection of the potato cyst nematode, Globodera pallida, and the beet cyst nematode, Heterodera schachtii, using real-time PCR with SYBR green I dye. Molecular and Cellular Probes. 2005;19:81–86. doi: 10.1016/j.mcp.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Madani M, Subbotin SA, Ward LJ, Li X, DeBoer SH. Molecular characterization of Canadian populations of potato cyst nematodes, Globodera rostochiensis and G. pallida using ribosomal nuclear RNA and cytochrome b genes. Canadian Journal of Plant Pathology. 2010;32:252–263. [Google Scholar]
- Manduric S, Olsson E, Englund J-E, Andersson S. Separation of Globodera rostochiensis and G. pallida (Tylenchida: Heteroderidae) using morphology and morphometrics. Nematology. 2004;6:171–182. [Google Scholar]
- Miller LI. 1983 Diversity of selected taxa of Globodera and Heterodera and their interspecific and intergeneric hybrids. Pp. 207–220 in A. R. Stone, H. M. Pratt, and L. F. Khalil, eds. Concepts in nematode systematics. New York: Academic Press. [Google Scholar]
- Miller LI, Gray BJ. Heterodera solanacearum n. sp., a parasite of solanaceous plants. Nematologica. 1972;18:404–413. [Google Scholar]
- Mota M, Eisenback JD. Morphology of second-stage juveniles and males of Globodera tabacum tabacum, G. t. virginiae, and G. t. solanacearum (Nematoda: Heteroderinae) Journal of Nematology. 1993a;25:27–33. [PMC free article] [PubMed] [Google Scholar]
- Mota M, Eisenback JD. Morphology of females and cysts of Globodera tabacum tabacum, G. t. virginiae, and G. t. solanacearum (Nematoda: Heteroderinae) Journal of Nematology. 1993b;25:148–160. [PMC free article] [PubMed] [Google Scholar]
- Mota M, Eisenback JD. Morphometrics of Globodera tabacum tabacum, G. t. virginiae, and G. t. solanacearum (Nematoda: Heteroderinae) Journal of Nematology. 1993c;25:136–147. [PMC free article] [PubMed] [Google Scholar]
- Mulvey RH. Morphology of the terminal areas of the white females and cysts of the genus Heterodera (s.g. Globodera) Journal of Nematology. 1973;5:303–311. [PMC free article] [PubMed] [Google Scholar]
- Mulvey RH, Golden AM. An illustrated key to the cyst-forming genera and species of Heteroderidae in the Western Hemisphere with species morphometrics and distribution. Journal of Nematology. 1983;15:1–59. [PMC free article] [PubMed] [Google Scholar]
- Nakhla MK, Owens K, Li W, Wei G, Skantar AM, Levy L. Multiplex real-time PCR assays for the identification of the potato cyst and tobacco cyst nematodes. Plant Disease. 2010;94:959–965. doi: 10.1094/PDIS-94-8-0959. [DOI] [PubMed] [Google Scholar]
- Nicholas KB, Nicholas HB, Jr, Deerfield DW., II 1997. GeneDoc: Analysis and visualization of genetic variation. EMBNEW.NEWS 4:1-4. http://www.nrbsc.org/gfx/genedoc/ebinet.htm.
- Nylander JAA, Wilgenbusch JC, Warren DL, Swofford DL. AWTY (are we there yet?): a system for graphical exploration of MCMC convergence in Bayesian phylogenetics. Bioinformatics. 2008;24:581–583. doi: 10.1093/bioinformatics/btm388. [DOI] [PubMed] [Google Scholar]
- Phillips MS, Trudgill DL. Variation of virulence, in terms of quantitative reproduction of Globodera pallida populations, from Europe and South America, in relation to resistance from Solanum vernei and S. tuberosum ssp. andigena CPC 2802. Nematologica. 1998;44:409–423. [Google Scholar]
- Picard D, Sempere T, Plantard O. A northward colonization of the Andes by the potato cyst nematode during geological times suggests multiple host-shifts from wild to cultivated potatoes. Molecular Phylogenetics and Evolution. 2007;42:308–316. doi: 10.1016/j.ympev.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Plantard O, Picard D, Valette S, Scurrah M, Grenier E, Mugniéry D. Origin and genetic diversity of Western European populations of the potato cyst nematode (Globodera pallida) inferred from mitochondrial sequences and microsatellite loci. Molecular Ecology. 2008;17:2208–2218. doi: 10.1111/j.1365-294X.2008.03718.x. [DOI] [PubMed] [Google Scholar]
- Posada D. jModelTest: Phylogenetic model averaging. Molecular Biology and Evolution. 2008;25:1253–1256. doi: 10.1093/molbev/msn083. [DOI] [PubMed] [Google Scholar]
- Posada D, Crandall KA. Selecting the best-fit model of nucleotide substitution. Systematic Biology. 2001;50:580–601. [PubMed] [Google Scholar]
- Pylypenko LA, Phillips MS, Blok VC. Characterization of two Ukrainian populations of Globodera pallida in terms of their virulence and mtDNA, and the biological assessment of a new resistant cultivar Vales Everest. Nematology. 2008;10:585–590. [Google Scholar]
- Quader M, Nambiar L, Cunnington J. Conventional and real-time PCR-based species identification and diversity of potato cyst nematodes (Globodera spp.) from Victoria, Australia. Nematology. 2008;10:471–478. [Google Scholar]
- Rambaut A. 2009. FigTree. Version 1.3.1, available from http://tree.bio.ed.ac.uk/software/figtree/.
- Rumpenhorst HJ, Ayub M. Gibt e seine dritte Art beim Kartoffelnematoden? Die 29 Tagung des Arbeitskreises Nematologie. Monheim. 2001:14–15. Marz, 2001. [Google Scholar]
- Seinhorst JW. A rapid method for the transfer of nematodes from fixative to anhydrous glycerin. Nematologica. 1959;4:67–69. [Google Scholar]
- Siddiqi MR. 2000 Tylenchida: Parasites of plants and insects, 2nd ed. Wallingford, UK: CAB International. [Google Scholar]
- Skantar AM, Handoo ZA, Carta LK, Chitwood DJ. Morphological and molecular identification of Globodera pallida associated with potato in Idaho. Journal of Nematology. 2007;39:133–144. [PMC free article] [PubMed] [Google Scholar]
- Skantar AM, Handoo ZA, Zasada IA, Ingham RE, Carta LK, Chitwood DJ. Morphological and molecular characterization of Globodera populations from Oregon and Idaho. Phytopathology. 2011;101:480–491. doi: 10.1094/PHYTO-01-10-0010. [DOI] [PubMed] [Google Scholar]
- Stone AR. Heterodera pallida n. sp. (Nematoda: Heteroderidae), a second species of potato cyst nematode. Nematologica. 1973;18:591–606. [Google Scholar]
- Stone AR. 1983 Three approaches to the status of a species complex, with a revision of some species of Globodera (Nematoda: Heteroderidae). Pp. 221–233 in A. R. Stone, H. M. Pratt, and L. F. Khalil, eds. Concepts in nematode systematics. New York: Academic Press. [Google Scholar]
- Sturhan D. 1983 The use of subspecies and superspecies categories in nematode taxonomy. Pp. 41–53 in A. R. Stone, H. M. Pratt, and L. F. Khalil, eds. Concepts in nematode systematics. New York: Academic Press. [Google Scholar]
- Subbotin SA, Halford PD, Warry A, Perry RN. Variations in ribosomal DNA sequences and phylogeny of Globodera parasitizing solanaceous plants. Nematology. 2000;2:591–604. [Google Scholar]
- Subbotin SA, Mundo-Ocampo M, Baldwin JG. 2010a. Systematics of cyst nematodes (Nematoda: Heteroderinae), vol. 8 part A. Leiden, The Netherlands: Brill. [Google Scholar]
- Subbotin SA, Mundo-Ocampo M, Baldwin JG. 2010b. Systematics of cyst nematodes (Nematoda: Heteroderinae), vol. 8 part B. Leiden, The Netherlands: Brill. [Google Scholar]
- Subbotin SA, Cid Del Prado Vera I, Mundo-Ocampo M, Baldwin JG. Identification, phylogeny and phylogeography of circumfenestrate cyst nematodes (Nematoda: Heteroderidae) as inferred from analysis of ITS-rDNA. Nematology. 2011;13:805–824. [Google Scholar]
- Swofford DL. 2003 PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4. Sunderland, Massachusetts, USA: Sinauer Associates. [Google Scholar]
- Szalanski AL, Sui DD, Harris TS, Powers TO. Identification of cyst nematodes of agronomic and regulatory concern by PCR-RFLP of ITS1. Journal of Nematology. 1997;29:255–267. [PMC free article] [PubMed] [Google Scholar]
- Thiéry M, Mugniéry D. Interspecific rDNA restriction fragment length polymorphism in Globodera species, parasites of Solanaceous plants. Fundamental and Applied Nematology. 1996;19:471–479. [Google Scholar]
- Thomas WK, Vida JT, Frisse LM, Mundo M, Baldwin JG. DNA sequences from formalin-fixed nematodes: Integrating molecular and morphological approaches to taxonomy. Journal of Nematology. 1997;29:250–254. [PMC free article] [PubMed] [Google Scholar]
- Wouts WM. Globodera zealandica n. sp. (Nematoda: Heteroderidae) from New Zealand, with a key to the species of the genus. New Zealand Journal of Zoology. 1984;11:129–135. [Google Scholar]
- Wouts WM, Baldwin JG. 1998 Taxonomy and identification. Pp. 83–122 in S. B. Sharma, ed. The cyst nematodes. Dordrecht, The Netherlands: Kluwer Academic Publishers. [Google Scholar]
- Ye W, Giblin-Davis RM, Davies KA, Purcell MF, Scheffer SJ, Taylor GS, Center TD, Morris K, Thomas WK. Molecular phylogenetics and the evolution of host plant associations in the nematode genus Fergusobia (Tylenchida: Fergusobiinae) Molecular Phylogenetics and Evolution. 2007;45:123–141. doi: 10.1016/j.ympev.2007.02.027. [DOI] [PubMed] [Google Scholar]